Abstract
The unicellular soil-freshwater alga Chlamydomonas reinhardtii was found to secrete substances that mimic the activity of the N-acyl-l-homoserine lactone (AHL) signal molecules used by many bacteria for quorum sensing regulation of gene expression. More than a dozen chemically separable but unidentified substances capable of specifically stimulating the LasR or CepR but not the LuxR, AhyR, or CviR AHL bacterial quorum sensing reporter strains were detected in ethyl acetate extracts of C. reinhardtii culture filtrates. Colonies of C. reinhardtii and Chlorella spp. stimulated quorum sensing-dependent luminescence in Vibrio harveyi, indicating that these algae may produce compounds that affect the AI-2 furanosyl borate diester-mediated quorum sensing system of Vibrio spp. Treatment of the soil bacterium Sinorhizobium meliloti with a partially purified LasR mimic from C. reinhardtii affected the accumulation of 16 of the 25 proteins that were altered in response to the bacterium's own AHL signals, providing evidence that the algal mimic affected quorum sensing-regulated functions in this wild-type bacterium. Peptide mass fingerprinting identified 32 proteins affected by the bacterium's AHLs or the purified algal mimic, including GroEL chaperonins, the nitrogen regulatory protein PII, and a GTP-binding protein. The algal mimic was able to cancel the stimulatory effects of bacterial AHLs on the accumulation of seven of these proteins, providing evidence that the secretion of AHL mimics by the alga could be effective in disruption of quorum sensing in naturally encountered bacteria.
The production and exchange of specific signal substances between individual cells enables many bacterial species to coordinate their gene expression in a population density-dependent manner (Miller and Bassler, 2001; von Bodman et al., 2003). This kind of regulation, known as quorum sensing, affects the expression of many genes and behaviors in various bacteria (Whitehead et al., 2001; Schuster et al., 2003; Wagner et al., 2003). Bacterial pathogens and symbionts of both animals and plants require quorum sensing to colonize and invade their eukaryotic hosts (Eberl et al., 1996; Tan et al., 1999; Visick and Ruby, 1999; de Kievit and Iglewski, 2000; Loh et al., 2002).
In view of the bacterial dependence on quorum sensing for infection of hosts, it makes good evolutionary sense that eukaryotes have acquired the ability to recognize and respond to bacterial quorum sensing signals (Telford et al., 1998; Smith et al., 2002; Mathesius et al., 2003) and the ability to actively interfere with bacterial quorum sensing through the production of compounds that mimic the bacterium's own signals (Kjelleberg et al., 1997; Bauer and Teplitski, 2001). Givskov et al. (1996) were the first to show that a eukaryote, the marine red macroalga Delisea pulchra, secretes compounds that act as mimics of bacterial N-acyl-l-homoserine lactone (AHL) quorum sensing signals. AHLs are the best studied of the known bacterial quorum sensing signals and have been detected in over 50 species of protobacteria (Eberhard et al., 1981; Eberl, 1999; Fuqua et al., 2001). The AHL signal mimics of D. pulchra are halogenated furanones. These compounds are structurally similar to bacterial AHLs and inhibit quorum sensing regulation in various bacteria (de Nys et al., 1993; Givskov et al., 1996). The concentration of furanone mimics at the algal surface was found to be effective in disrupting colonization of the algal thalli by gram-negative bacteria (Kjelleberg et al., 1997; Dworjanyn et al., 1999; Kjelleberg and Steinberg, 2002). Biochemical studies indicate that the D. pulchra furanone AHL mimics are able to bind to a bacterial AHL receptor (LuxR; Manefield et al., 1999) and that such binding alters the stability of the protein-ligand complex, leading to rapid proteolytic turnover of the receptor (Manefield et al., 2002). In addition to the halogenated furanones of D. pulchra, a variety of bacteria and eukaryotes have been shown to produce cyclic dipeptides (diketopiperazines) that can act as AHL mimics to affect quorum sensing-regulated behaviors in bacteria, apparently through interactions with the ligand-binding sites of AHL receptor proteins (Holden et al., 1999).
More recently, various higher plants were also shown to secrete AHL signal mimic substances (Teplitski et al., 2000; Daniels et al., 2002; Gao et al., 2003; Mathesius et al., 2003). The active compounds from plants have not been chemically identified, but most of them partition differently in organic solvents than do bacterial AHLs (Teplitski et al., 2000; Gao et al., 2003). Many of the AHL mimic compounds from plants stimulate quorum sensing-regulated responses in bacteria, in contrast to the halogenated furanones from D. pulchra, which all act to inhibit quorum sensing-regulated responses.
In this study, we have investigated the production of AHL mimic substances by the unicellular green alga Chlamydomonas reinhardtii. In contrast to D. pulchra, C. reinhardtii is well suited to genetic and molecular genetic analysis of AHL signal mimic production. The alga has been used as a model organism for the molecular genetic and biochemical analysis of photosynthesis and other processes (Harris, 1989, 2001). Its genomic sequence is now available (http://www.biology.duke.edu/chlamy_genome/). C. reinhardtii is also haploid for most of its life cycle, which facilitates genetic studies to identify relevant mutants (Harris, 2001). Our present study shows that C. reinhardtii produces a variety of AHL mimics. A purified preparation of one of the active mimics quite specifically affected diverse AHL-regulated behaviors in the plant symbiotic soil bacterium Sinorhizobium meliloti.
RESULTS
Production of Substances Affecting Quorum Sensing by Colonies of C. reinhardtii and Chlorella Spp.
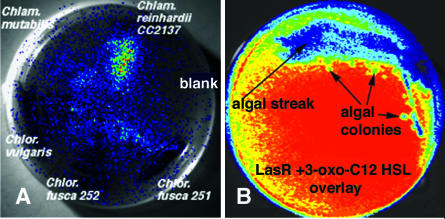
Colonies of C. reinhardtii and Chlorella spp. growing on agar plates were overlaid with suspensions of quorum sensing reporter strains (Table I), and their luminescence responses were monitored with a CCD camera. As shown in Figure 1A, colonies of C. reinhardtii, C. mutablis, Chlorella vulgaris, and Chlorella fusca all stimulated luminescence responses over a period of 24 h in the Vibrio harveyi BB170 reporter strain, which selectively responds to the furanosyl borate diester AI-2 quorum sensing signal (Chen et al., 2002) of this bacterium. Colonies of these algal species also stimulated luminescence in the V. harveyi 404 wild type (data not shown). In contrast, as shown in Figure 1B, colonies of C. reinhardtii strongly inhibited the quorum sensing-regulated luminescence response of the Escherichia coli LasRI′::luxCDABE reporter of Winson et al. (1998) to its cognate AHL (3-oxo-C12-HSL). The luminescence responses of the LuxRI′::luxCDABE or AhyRI′::luxCDABE AHL reporters to their cognate AHLs (3-oxo-C6-HSL and C4-HSL) were also inhibited (data not shown). These results suggest that C. reinhardtii secretes substances that inhibit AHL-mediated induction of luminescence. The luminescence of a constitutively luminescent Tn5::luxCDABE isolate of E. coli was not inhibited by C. reinhardtii streaks or colonies in similar overlays (data not shown), indicating that the observed inhibition of AHL-induced luminescence in the E. coli AHL reporters by C. reinhardtii colonies was not due to the secretion of toxic or luminescence-blocking compounds.
Table I.
Quorum sensing reporter strains
| Bacterium | Receptor | Cognate Signal | Source |
|---|---|---|---|
| C. violaceum CV026 | CviR | C6-HSL | (McClean et al., 1997) |
| P. putida pAS-C8 | CepR | C3-HSL | (Steidle et al., 2001) |
| V. harveyi BB170 | LuxP | Al-2 furanosyl borate ester | (Bassler et al., 1993) |
| E. coli JM109 | (Winson et al., 1998) | ||
| p(SB401) | LuxR (Vibrio fisheri) | 3-oxo-C6-HSL | |
| p(SB536) | AhyR (Aeromonas hydrophila) | C4-HSL | |
| p(SB1075) | LasR (P. aeruginosa) | 3-oxo-C12-HSL |
Figure 1.
Effects of C. reinhardtii and Chlorella sp. colonies on quorum sensing responses in bacterial reporters. Streaks of the algae were overlayed with soft agar containing a quorum sensing reporter, then later examined for induced luminescence with a Hamamatsu C2400 intensified CCD camera (Hamamatsu Photonic Systems, Bridgewater, NJ). False color images were superimposed on the digital images of the algal streaks. Increasing luminescence intensity is indicated by red > yellow > green > blue. A, Stimulation of the AI-2-dependent luminescence in V. harveyi BB170 after 9 h. The three Chlorella spp. luminesced strongly at 7 h (not shown) but show dark areas corresponding to inhibited background luminescence at 9 h. B, Inhibition of LasRI'::luxCDABE reporter responses to its cognate AHL, 3-oxo-C12-HSL, by C. reinhardtii colonies after 5 h.
Purification and Detection of AHL Signal Mimic Substances in Culture Filtrates of C. reinhardtii
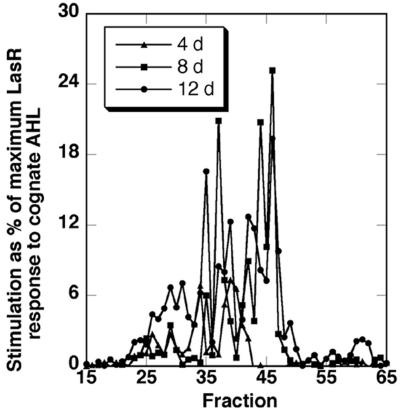
Culture filtrates from C. reinhardtii cells grown phototrophically in mineral salts medium were extracted with ethyl acetate and the concentrated extracts were fractionated by reverse-phase C18 HPLC. As shown in Figure 2, the E. coli LasR AHL reporter strain, most responsive to 3-oxo-C12-HSL, detected a number of substances in the extracts that stimulated strong quorum sensing-regulated responses. Two major and perhaps two minor, partially separated peaks with LasR stimulatory activity were detected in extracts from 4-d-old cultures, whereas extracts from 12-d-old cultures had perhaps six major and two or three minor peaks of LasR stimulatory activity. The activity peaks were quite consistent in duplicate cultures, although some variation occurred in activity level for individual peaks. Peaks of LasR activity with similar retention times were detected in extracts from cultures grown on Tris-acetate phosphate (TAP) medium containing acetate as a carbon source, but the activity levels in these peaks were generally 5 to 10 times lower (data not shown).
Figure 2.
LasR stimulatory substances from C. reinhardtii culture filtrates. Samples of HPLC fractions equivalent to approximately 22 mL of algal culture filtrate from cultures harvested after 4, 8, and 12 d were assayed with the LasR AHL reporter as described in “Materials and Methods.” Responses are reported as percentage of the maximum response obtained with the reporter to saturating levels of the cognate AHL, 3-oxo-C12-HSL. Results are from a single experiment representative of three independent trials.
Solvent-only injection controls to test for contaminating activities released from the column during fractionation were negative. Experiments to detect active compounds present in the HS medium and the ethyl acetate solvent were conducted with 1 L of HS medium extracted with 2 × 300 mL ethyl acetate. HPLC fractions obtained after injection of the dried solvent residue had no activity detectable with the LasR or CepR reporters.
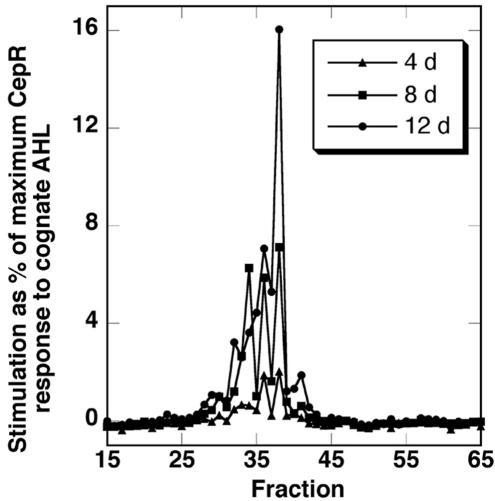
As illustrated in Figure 3, when the HPLC fractions of Figure 2 were assayed with the Pseudomonas putida CepRI′::GFP reporter strain of Steidle et al. (2001), which is most responsive to C8-HSL, a set of perhaps six partially separated peaks of CepR stimulatory activity were detected, with increasing activity in filtrates from older cultures.
Figure 3.
CepR stimulatory substances from C. reinhardtii culture filtrates. Samples of HPLC fractions equivalent to approximately 33 mL of algal culture filtrate from cultures harvested after 4, 8, and 12 d were assayed with the CepR AHL reporter as described in “Materials and Methods.” Responses are reported as percentage of the maximum response obtained with the reporter to saturating levels of the cognate AHL, C8-HSL. Results are from a single experiment representative of at least three independent trials.
No substances that stimulated either the E. coli LuxR or AhyR reporters, which are most responsive to 3-oxo-C6-HSL and C4-HSL, respectively, were detected in any of the HPLC fractions of the ethyl acetate extracts (data not shown), nor was there any evidence of substances that affected violacein synthesis in the Chromobacterium violaceum CV026 reporter strain, most responsive to C6-HSL, or that inhibited the responses of the LasR, LuxR, or CepR reporters to partially inducing concentrations of their cognate AHLs (data not shown). Storage of the crude extracts for 6 months at -20 C in 50% (v/v) acetonitrile resulted in substantial loss of the original activity for most of the LasR and CepR stimulatory substances (data not shown). When freeze-dried culture filtrate from C. reinhardtii was extracted first with methanol and then 1:1 (v/v) methanol:water, HPLC fractions obtained after injection of the combined extracts contained no detectable LasR, LuxR, or CepR stimulatory substances when tested at the same levels used to test fractions in Figures 2 and 3 (data not shown). This is in contrast to pea (Pisum sativum) and Medicago truncatula, where most of the AHL mimic activities were recovered in the methanol and 50% (v/v) methanol extracts, not the ethyl acetate extract (Teplitski et al., 2000; Gao et al., 2003). The CepR and LasR reporters were neither stimulated nor inhibited by 100 nm to 10 μm concentrations of several fatty acid methyl esters (myristic, palmitic, palmitoleic, lauric, and oleic) identical or similar to those detected by gas chromatography-mass spectrometry in LasR active fractions from culture filtrates of C. reinhardtii (A. Eberhard, M. Teplitski, P. Rubinelli, and W.D. Bauer, unpublished data).
The Effects of a Purified C. reinhardtii Mimic on Protein Accumulation in S. meliloti
To test whether physiological concentrations of AHL mimic substances from C. reinhardtii might be able to affect important functions in wild-type bacteria, we exposed low-density cell cultures of S. meliloti to a purified preparation of one of the LasR stimulatory substances from the alga and looked for mimic-induced changes in protein accumulation in the bacterial cells. Like C. reinhardtii, S. meliloti is a common inhabitant in soils. S. meliloti was selected as a suitable model for such testing because a previous study showed global proteome responses to added AHLs in low-density cultures of the wild type (Chen et al., 2003).
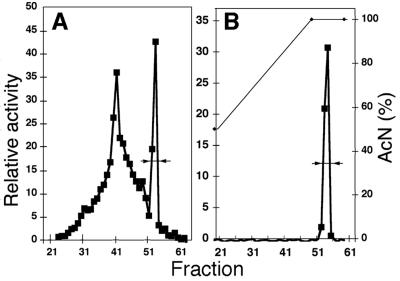
To obtain a highly purified AHL mimic from the alga, substances from approximately 10 L of C. reinhardtii culture filtrate were extracted, fractionated, and assayed with the LasR reporter strain (Fig. 4A). Fractions 52 to 55, corresponding to the last, most clearly separated peak of LasR stimulatory activity in Figure 4A, were pooled and refractionated to obtain a highly purified preparation for testing of responses in bacteria (fractions 52-54, Fig. 4B).
Figure 4.
Purification of a LasR stimulatory mimic from C. reinhardtii. Culture filtrate from approximately 10 L of 7-d-old HS algal cultures were extracted, purified, and assayed with the LasR reporter as described in “Materials and Methods.” Relative activity responses to samples of HPLC fractions equivalent to about 22 mL of the original culture are given as x-fold over reporter-only controls. A, Initial purification. B, Repurification of fractions 51 to 54 from the initial HPLC fractionation. The gradient of increasing acetonitrile used to elute the column is indicated by a solid thin line.
Proteome responses in S. meliloti to this purified substance were determined by redissolving the putative signal mimic substance in 1 mL of TA medium and adding portions to duplicate, washed, early log phase (A600 = 0.03) cultures of S. meliloti 1021 at a concentration calculated to be approximately equivalent to its concentration in the original C. reinhardtii cultures. After 2 h, the bacteria were collected by centrifugation, freeze dried, and later extracted to recover proteins for two-dimensional gel separation. A second set of duplicate cultures of the bacterium was treated with a mixture of the AHLs extracted from filtrates of an early stationary phase, defined medium culture of S. meliloti 1021. These AHLs were added to the early log culture of the bacterium at approximately the same concentration present in the original early stationary phase bacterial cultures. A third set of duplicate cultures was treated with a mixture of the bacterial AHLs and the C. reinhardtii LasR stimulatory mimic substance. Duplicate control cultures were treated with the residue from LasR inactive HPLC fractions 48 to 50 (Fig. 4B).
Table II lists the S. meliloti proteins that accumulated to significantly different levels in response to either the purified C. reinhardtii LasR mimic or the bacterium's own AHLs or to the mixture of bacterial AHLs and C. reinhardtii AHL mimic. Thirty-two of the 34 differentially accumulated proteins were identified by peptide mass fingerprint comparison with peptides predicted from the genomic sequence (Galibert et al., 2001). Sixteen of the 25 proteins that accumulated in response to the bacterium's own AHL quorum sensing signals were also affected by the putative LasR AHL signal mimic from the alga (Table II). About one-half of the proteins affected by the algal mimic, the bacterial AHLs, or both were accumulated to levels at least 3-fold higher or lower than in the controls. One would expect such changes to have substantial effects on the functions carried out by these proteins.
Table II.
S. meliloti proteins differentially accumulated in response to purified LasR stimulatory mimic compound(s) from C. reinhardtii and/or AHLs from S. meliloti
| Spot No. | Predicted Protein | Gene Identificationab | PMF Match Ratingc | AHLs | Protein Levels (% of Control)d: AHLs + Mimic | Mimic |
|---|---|---|---|---|---|---|
| 44 | GroEL2 chaperonin | SMa0744 | 3 | — | — | 60 |
| 30 | Hypothetical protein | SMa0967 | 1 | 420 | — | 350 |
| 35 | Conserved hypothetical protein | SMa1821 | 3 | 10 | 30 | 20 |
| 1 | Hypothetical protein | SMb20552 | 3 | 60 | 40 | — |
| 7 | Putative urea/short-chain amide or branched-chain amino acid uptake ABC transporter ATP-binding protein | SMb20602 | 3 | 270 | — | 240 |
| 4 | Putative heat shock protein groEL | SMb21566 | 3 | 860 | — | 480 |
| 26 | 3-Hydroxydecanoyl-acyl-carrier-protein dehydratase | SMc00328 | 1 | 330 | — | 530 |
| 3 | 30s Ribosomal protein S1 | SMc00335 | 3 | 210 | 30 | 150 |
| 25 | DnaK suppressor protein | SMc00469 | 3 | 470 | — | 350 |
| 17 | Hypothetical protein | SMc00496 | 3 | 250 | 140 | 230 |
| 48 | Hypothetical protein | SMc00530 | 3 | — | — | 240 |
| 34 | Adenylosuccinate synthetase IMP-Asp ligase | SMc00643 | 3 | 40 | 30 | 40 |
| 16 | Conserved hypothetical protein | SMc00791 | 3 | 280 | — | — |
| 5 | Putative octaprenyl-diphosphate synthase protein | SMc00860 | 3 | 250 | — | — |
| 2 | Molybdenum cofactor biosynthesis protein B | SMc00863 | 2 | 1040 | Missing | Missing |
| 21 | Nitrogen regulatory protein PII | SMc00947 | 3 | — | New | New |
| 38 | Pyruvate dehydrogenase beta2 subunit | SMc01031 | 3 | 50 | — | — |
| 9 | Conserved hypothetical protein | SMc01119 | 3 | 220 | — | — |
| 27 | Putative peptidyl-prolyl cis-trans isomerase B protein | SMc01208 | 3 | 220 | — | 70 |
| 13 | Outer membrane protein | SMc01342 | 1 | 250 | — | — |
| 14 | Biotin carboxyl carrier protein of acetyl-CoA carboxylase | SMc01344 | 3 | 210 | 340 | — |
| 22 | Conserved hypothetical protein | SMc01833 | 3 | — | — | 570 |
| 6 | Transmembrane hypothetical | SMc01876 | 3 | 280 | — | 280 |
| 43 | Transmembrane outer membrane protein | SMc02094 | 3 | — | — | 50 |
| 20 | Conserved hypothetical protein (isoform I) | SMc02111 | 3 | 260 | 220 | — |
| 24 | Conserved hypothetical protein (isoform II) | SMc02111 | 3 | 790 | — | 230 |
| 46 | ABC transporter branched-chain amino acid-binding periplasmic protein | SMc02356 | 3 | — | — | 180 |
| 41 | Transmembrane hypothetical | SMc02634 | 3 | — | — | 40 |
| 28 | 50s Ribosomal protein L25 | SMc02692 | 3 | 310 | 230 | 190 |
| 15 | Thioredoxin | SMc02761 | 3 | 190 | 30 | |
| 45 | Heat shock protein 70 chaperone | SMc02857 | 3 | — | — | 80 |
| 47 | GTP-binding protein (Tyr phosphorylated protein A) | SMc03242 | 3 | — | — | 490 |
| 28 | No good match | — | — | 310 | 230 | 190 |
| 37 | No good match | — | — | 170 | 140 | 170 |
Based on the S. meliloti 1021 genomic database (Galibert et al., 2001)
Smc, Sma, and Smb, Products of ORFs located on the chromosome and symbiotic plasmids A and B, respectively
Confidence ratings were assigned based on the criteria described in “Materials and Methods”
Differential accumulation of proteins is expressed as the average percentage difference in spot volume between the treated and corresponding untreated cultures. Values are based on statistical analysis of at least three gels per treatment and two cultures per treatment. Missing/New, Polypeptides not detectable in the treated/untreated gels, respectively. Protein levels not significantly different than the untreated controls are indicated by “—”
DISCUSSION
Production of Substances by C. reinhardtii That Affect Bacterial Quorum Sensing
The present study provides evidence that C. reinhardtii CC2137 is capable of producing and secreting at least a dozen chromatographically separable substances capable of specifically stimulating LasR- and CepR-mediated quorum sensing functions in well-characterized reporters (Figs. 2 and 3). For comparison, the model legume M. truncatula was found to produce about 15 to 20 separable AHL mimics (Gao et al., 2003), some stimulatory and some inhibitory, whereas D. pulchra was found to produce about 25 to 30 different halogenated furanone AHL mimics (Kjelleberg and Steinberg, 2002), all inhibitory. When individual HPLC fractions were assayed with both the LasR and CepR reporters, none of the peaks of CepR stimulatory activity (Fig. 3) were found to match with LasR stimulatory peaks (Fig. 2), indicating that the two reporters are probably responding to different substances in the ethyl acetate extracts. The production of diverse AHL mimic compounds by D. pulchra, higher plants, and C. reinhardtii seems reasonable if the biological aim of these eukaryotes is to disrupt AHL-mediated quorum sensing regulation in many different bacterial strains. The production of multiple LasR active and CepR active substances by C. reinhardtii may reflect the usefulness of mimics that cover the range of binding specificities of relevant AHL receptors in different bacteria.
Because the LasR and CepR active substances from C. reinhardtii did not elicit responses in several other AHL reporter strains (LuxR, AhyR, and CviR), it appears that the algal compounds are acting in a receptor-specific manner. We speculate that the algal compounds are most likely interacting directly with the LasR or CepR receptor polypeptides in a manner similar to the interactions reported between the D. pulchra furanone AHL mimics and the LuxR receptor (Manefield et al., 1999, 2002). With the LasR and CepR reporters, direct ligand-receptor interactions are perhaps the only plausible mechanism for receptor-specific stimulation of luminescence or fluorescence by added compounds (Winson et al., 1998; Steidle et al., 2001). Because the HPLC fractions that stimulated the E. coli LasR reporter failed to stimulate either the E. coli AhyR or LuxR reporters, the observed stimulation of luminescence cannot be an artifact of increased reporter growth and background luminescence arising from consumption of algal metabolites. The LasR, LuxR, and AhyR reporter strains are identical except for the AHL receptor gene and synthase promotor sequence (Table I) and, thus, would respond similarly to any added nutrient.
The chemical identities of the C. reinhardtii compounds responsible for activation of quorum sensing-regulated responses in the LasR and CepR reporters are still unknown. Because the active substances all stimulated the reporters, they are unlikely to be halogenated furanones like those made by D. pulchra, which all have inhibitory effects on bacterial quorum sensing. Because they partition into ethyl acetate in the same manner as bacterial AHLs, it is possible that the active compounds from C. reinhardtii are identical or very similar to bacterial AHLs. However, an initial mass spectral analyses of purified LasR active fractions from the alga using the methods described by Marketon et al. (2002) failed to detect any signature peaks corresponding to known AHLs (A. Eberhard, M. Gronquist, M. Teplitski, P. Rubinelli, and W.D. Bauer, unpublished data), and examination of the genomic sequence for C. reinhardtii failed to reveal any homologs of known bacterial AHL synthases. Thus, the algal compounds may prove to have structures related to but different from bacterial AHLs. Studies to determine the structures of these compounds are in progress. In this paper, we tentatively refer to the LasR/CepR active compounds as AHL mimics. If they prove to have structures identical to those of known bacterial AHLs, their production by C. reinhardtii would be the first known instance of AHL synthesis by a eukaryote.
Both culture age and growth conditions were important factors in production of AHL mimics by C. reinhardtii. The levels of the LasR and CepR mimic substances were considerably higher when the alga was cultured phototrophically than when cultured on medium containing acetate as a carbon source, despite the fact that cell number did not increase appreciably during phototrophic culture. The LasR mimic activities, but not the CepR mimic activities, changed markedly with length of culture (Figs. 2 and 3). The observed changes in LasR activities with culture age suggest that the LasR active mimic compounds are not simply accumulating in the culture medium but may be subject to biotic or abiotic inactivation and changes in regulation of production or secretion.
No LasR inhibitory mimic compounds were detected in ethyl acetate or methanol extracts of C. reinhardtii culture filtrate. Nonetheless, the LasR reporter's responses to its cognate AHL were strongly inhibited by algal streaks or colonies in overlays (Fig. 1B), as were the LuxR and AhyR reporters (not shown). This inhibition does not appear to be due to nonspecific toxicity or to an inhibition of luminescence by algal substances because a constitutively luminescent derivative of E. coli was not inhibited in similar overlays. These results suggest that that the algal colonies are secreting specific inhibitors of AHL quorum sensing and that these inhibitory substances are probably more hydrophylic than the stimulatory mimics, not readily extracted in organic solvents. This possibility is supported by the observation that LasR stimulatory substances could be detected on polyvinylidene difluoride membranes after passing C. reinhardtii culture filtrate through the membrane but only after rinsing the membranes with water, presumably removing the more hydrophilic inhibitory compounds (data not shown). Further studies are clearly needed to isolate, purify, and characterize these putative inhibitory AHL mimics.
Both the V. harveyi wild-type and BB170 reporter strains were stimulated to luminesce in overlays of C. reinhardtii and Chlorella spp. colonies (Fig. 1A), providing evidence that these algal isolates secrete compounds that are identical to, or capable of mimicking, the quorum sensing signals of V. harveyi. Quorum sensing-regulated luminescence in V. harveyi is complex, stimulated both independently and synergistically by an AHL (3-OH-C4-HSL = AI-1) and by a furanosyl borate diester (AI-2), and is mediated by pairs of two-component phosphorelay proteins rather than typical AHL receptors (Mok et al., 2003). Several putative AI-2 mimic substances were recently detected in exudates from M. truncatula seedlings (Gao et al., 2003). Thus, it appears that higher plants and unicellular green algae like C. reinhardtii and Chlorella sp. may share a common ability to affect AI-2-mediated quorum sensing in bacteria that have suitable receptors. Further studies are in progress to isolate and identify the algal substances responsible for stimulating V. harveyi quorum sensing-regulated responses.
Proteome Analysis of S. meliloti Responses to a Purified C. reinhardtii AHL Mimic
S. meliloti was used here as a representative soil bacterium to test whether its quorum sensing regulation could be disrupted by exposure to physiological levels of one of the AHL signal mimics from C. reinhardtii. The present study should be regarded as preliminary because it involved treatment with an algal mimic compound that is not chemically identified, only partially purified and added at undefined concentrations. Nonetheless, a comparison of the S. meliloti proteins affected by a mixture of its own AHL signals with those affected by the purified AHL mimic from the alga shows a high degree of overlap (16 of 25 proteins, Table II). This provides evidence that the purified AHL mimic from C. reinhardtii, identified initially through its specific stimulation of responses in the E. coli LasR reporter strain, can affect diverse aspects of quorum sensing regulation in a representative wild-type soil bacterium.
The furanone AHL mimics of D. pulchra rather specifically target quorum sensing-regulated functions in bacteria and do not appreciably disrupt other aspects of bacterial metabolism or behavior (Manefield et al., 1999; Hentzer et al., 2002). The C. reinhardtii AHL mimic also appears to rather specifically target AHL-regulated functions (Table II). However, nine of the 34 differentially accumulated proteins were significantly affected by the addition of the C. reinhardtii mimic but not by the AHLs (Table II). Some of these nine proteins may prove to have roles in the metabolism or transport of the algal mimic compound. It is also possible that impurities in the AHL mimic preparation were responsible for the differential accumulation of some of these proteins.
Many of the AHL/mimic responsive proteins correspond to open reading frames (ORFs) listed as hypothetical, with unknown function. However, several proteins with potentially interesting functions were identified based on the S. meliloti 1021 annotated genome database, available at http://sequence.toulouse.inra.fr/meliloti.html. The PII nitrogen regulatory protein encoded by glnB (Smc00947) was newly detected in response to the algal mimic. GlnB is similar to the central PII regulators of carbon and nitrogen balance in both prokaryotes and eukaryotes (Ninfa and Atkinson, 2000) and is reported to control both N assimilation and symbiotic nodule development in S. meliloti (Arcondeguy et al., 1997). The typA ORF (Smc03242) encodes a protein with strong similarity to BipA (BLAST E score = 0.0), a Tyrphosphorylated GTPase that mediates interactions between enteropathogenic E. coli and host epithelial cells (Grant et al., 2003). purA (Smc0643) encodes a homolog of adenylosuccinate ligase (EC 6.3.4.4), an enzyme catalyzing the first committed step to AMP in the purine biosynthesis pathway (Hou et al., 2002). S. meliloti dksA (Smc0469) encodes a homolog of E. coli DksA, which suppresses mutations in the DnaK/DnaJ chaperone system. A close ortholog of DksA has been found to govern the levels of the RhlI AHL synthase and the quorum sensing-regulated production of virulence factors in Pseudomonas aeruginosa (Branny et al., 2001; Jude et al., 2003). It seems noteworthy that a number of the AHL/mimic-responsive proteins identified in Table II appear to be chaperones or have roles in protein synthesis or folding, including two GroEL chaperones (Sma0744 and Smb21566), DnaK (heat shock protein70 and Smc2857), DksA (Smc0469), PpiB (peptidyl prolyl cistrans-isomerase B and Smc01208), and the ribosomal proteins S1 and L25 (Smc0335 and 02692).
When added by itself, the algal mimic had effects that were qualitatively and quantitatively similar to those of the bacterium's AHLs on the accumulation of the 16 coresponsive proteins (Table II). However, the proteins encoded by moaB (Smc0863) and ppiB (Smc01208) responded in opposite ways to the addition of bacterial AHLs and algal mimic. The MoaB homolog, which is involved in synthesis of the molybdopterin cofactor required for many oxidoreductases (McLuskey et al., 2003), increased 10-fold in response to the bacterium's AHLs but was reduced to below detectable levels in gels from bacteria exposed to the algal mimic. The mechanism underlying such opposite responses is unknown.
When added together, the algal mimic frequently cancelled the effect of the bacterial AHLs. Proteins encoded by Sma0967, Smb20602, and 21566; chromosomal genes 00328, 00469, and 01876; and isoform II encoded by Smc02111 all accumulated significantly in response to either the AHLs alone or the mimic alone. However, when the bacteria were exposed to the mixture of AHLs and mimic, there was no significant change in the level of these proteins. Because AHL receptors typically appear to be active as dimers (Qin et al., 2000; Whitehead et al., 2001), we speculate that this “canceling” effect of mixing an AHL with an AHL mimic might be due to the formation of inactive dimers between a mimic-bound receptor polypeptide and an AHL-bound receptor polypeptide. Other proteins, e.g. those encoded by Smc00496, 00643, and 02692 and the unidentified protein spots 28 and 37, were found to accumulate in the same direction and to essentially the same extent after exposure to either the AHLs alone, the mimic alone, or to the mixture of mimic and AHLs (Table II). This is what one might expect if one or more of the five putative AHL receptors in S. meliloti generated active heterodimers. In terms of benefit to a mimic-producing eukaryote, both the inhibition of quorum sensing-regulated functions by a mimic compound (e.g. Smc0335, 0863, and 1208) or the cancellation of AHL-mediated stimulation by a mimic (e.g. Smc0328 and 0469) could be useful in manipulating the ability of bacteria to colonize or infect a host.
MATERIALS AND METHODS
Organisms and Growth Conditions
Chlamydomonas reinhardtii wild-type strain 2137 (CC-1021) was maintained on TAP (mineral salts + acetate) agar under 12 h of 40 μmol photons m-2 s-1 of white light at 25°C, restreaking every 3 weeks. Individual colonies were inoculated into TAP liquid shake cultures and grown axenically in light for 5 to 6 d to A750 > 1.2. The TAP-grown starter cultures were centrifuged, and the cells were resuspended in 20 volumes of HS mineral salts medium lacking a carbon source. Cultures were incubated for 4 to 12 d on a shaker (175 rpm) under continuous white light (75 μmol photons m-2 s-1). TAP and HS media were prepared as described (Harris, 1989), adjusting the pH of the media to 6.6 to 6.8 with TRIZMA-Base (Sigma, St. Louis) before autoclaving. Bacto-agar (1.5% [w/v]) was added to solidify TAP when needed. The stock of Hunter's Trace Metals was added as a filter-sterilized solution after the media components were combined and autoclaved. Cultures were discarded if they showed contamination after plating aliquots of each shake culture on Luria-Bertani, TAP, tryptic soy medium (Difco Laboratories, Detroit), tryptone agar, high-salt autoinducer bioassay medium (Joyce et al., 2000), and corn meal agar (Sigma).
Bacterial strains used as reporters for AHL and AI-2 quorum sensing signals are listed in Table I and were cultured as described (Teplitski et al., 2000; Gao et al., 2003) except that the Pseudomonas putida CepRI′::GFP reporter was cultured in Luria-Bertani medium diluted 1:1 (v/v) with water.
Fractionation and Detection of Quorum Sensing Mimics from C. reinhardtii
C. reinhardtii cultures maintained in HS medium for 4, 8, or 12 d were tested for bacterial contamination and uncontaminated batches were centrifuged, filtered through 0.8-μm nitrocellulose membranes, and partitioned twice with 0.3 volumes of ethyl acetate. The ethyl acetate extracts were rotary evaporated at 32°C, and the dried extracts were resuspended in 50% (v/v) acetonitrile:water in 1 μL mL-1 of ethyl acetate. This solution was fractionated on a reverse-phase C18 analytical HPLC column (5 μm, 250-4 LiChrospher100, Agilent Technologies, Palo Alto, CA) mounted with a guard column by injecting 200-μL samples, eluting for 5 min with water, then for 40 min with a linear gradient of increasing acetonitrile to 100% (v/v), and maintaining 100% (v/v) acetonitrile elution for an additional 15 min. A flow rate of 0.75 mL min-1 was maintained throughout the sample run.
Bioassays to detect algal compounds with the ability to affect bacterial AHL reporter strains were conducted as described previously (Teplitski et al., 2000; Gao et al., 2003), measuring luminescence or fluorescence for 0.1 s well-1 in a Wallac Victor2 multimode plate reader (Perkin Elmer, Gaithersburg, MD). Assays with the P. putida CepR reporter were similar except that 100 μL rather than 80 μL of suspension was used, and the plates were incubated at 30°C. The negative control wells contained only the reporter suspension, providing a measure of background luminescence/fluorescence not induced by AHLs. The positive control wells contained sufficient levels of the cognate AHL to give a maximal response for that reporter. For assays to measure inhibition of AHL-induced responses by algal substances, sufficient levels of the cognate AHL to induce partial (approximately 10%-30% maximal) responses were added with the reporter.
Purification of an AHL Mimic from C. reinhardtii for Proteome Analysis
TAP-grown cultures of C. reinhardtii CC-2137 (A750 = 0.8-1.0) were subcultured into 3 volumes of fresh HS medium and incubated in light at 25°C for a week (A750 = 0.3-0.4). The algae were then removed by centrifugation, and the culture filtrates (pH 6.4-6.8) were collected. The cell-free culture filtrates were extracted twice with an equal volume of ethyl acetate, and the extract was rotary evaporated to dryness over a 40°C water bath and stored in glass vials at -20°C. For HPLC analysis, the residue from about 10 L of culture was brought up in 1 mL of acetonitrile, centrifuged, and the precipitate was extracted with 1 mL of acetonitrile:water (1:1 [v/v]). The supernatants were combined and injected onto a 40% (v/v) acetonitrile:60% (v/v) water-equilibrated semipreparatory C18 column (Whatman Partisil 10, ODS-3, Whatman, Clifton, NJ) fitted with a guard column. The column was eluted at 2 mL min-1 with a linear water:acetonitrile gradient starting at 40% (v/v) acetonitrile and increasing to 100% (v/v) acetonitrile over 70 min, followed by an additional 10 min with 100% (v/v) acetonitrile. One-minute fractions were assayed with the LasR AHL reporter.
Proteome Analysis of Sinorhizobium meliloti after Addition of a Purified LasR Stimulatory Mimic from C. reinhardtii
S. meliloti 1021 cells were subcultured and washed several times to reduce the concentration of endogenous AHLs as much as possible as described previously (Chen et al., 2003). Cells from replicate 300-mL portions of the third low-density subculture of strain 1021 grown in TA medium (A600 = 0.05) were inoculated into flasks containing 1.5 L of TA medium supplemented with the highly purified algal AHL mimic. Duplicate sets of cells were treated with the mixture of crude AHLs recovered from an early stationary phase culture of S. meliloti 1021 grown in NM, a defined Glcnitrate medium as described by Pellock et al. (2002). A third duplicate set of cells was treated with a combination of the purified algal AHL mimic and the crude bacterial AHLs. As a negative control, the bacterial cultures were exposed to residue from HPLC fractions (fractions 48-50, Fig. 4B) that did not have any detectable LasR mimic activity. The treated and control cultures were incubated for 2 h at 28°C and 200 rpm and had a final A600 = 0.03 to 0.04, yielding 0.1 g of dry cells per 1.5 L of culture. All centrifugations were conducted at room temperature to minimize stress. All treatments and controls were duplicated.
Protein Extraction, Separation, Quantification, and Identification
Proteins were extracted from freeze-dried cells as described (Chen et al., 2000a, 2000b) and quantified based on a modified Bradford protein assay (Guerreiro et al., 1999). Protein concentrations were normalized, and the samples were subjected to two-dimensional gel separation as described (Chen et al., 2000b). Preparative gels were stained with Coomassie Brilliant Blue in a step-wise colloidal staining procedure (Neuhoff et al., 1988). Digitized images (600 dots per inch) of the stained gels were quantified using MELANIE 3 image analysis software (Bio-Rad, Hercules, CA). Protein spot locations were compared with 10 landmark proteins and matched against a specialized proteomic database for S. meliloti 1021 (Weiller et al., 2001). Optical density of each spot over its area (volume) as a percentage of the relative optical density of the gel image (percentage volume) was used to quantify each spot. Digitized spot images were statistically analyzed using GenStat 4.2 software as described by Mathesius et al. (2003). A polypeptide was deemed differentially accumulated if χ2 was less than 0.05.
Proteins were identified by tryptic digestion of the polypeptides isolated from the Coomassie-stained control gels followed by peptide mass fingerprinting with matrix-assisted laser-desorption ionization time of flight mass spectrometry performed on a Micromass TofSpec 2E Time of Flight Mass Spectrometer (Waters Corporation, Milford, MA) at the Australian Proteome Analysis Facility (Macquarie University, Sydney). Peptide mass fingerprints were identified by comparison with the S. meliloti 1021 proteomic database using Mascot software (Micromass; Waters Corp) as described previously (Weiller et al., 2001). Scoring of peptide mass fingerprint matches was based on the following criteria: (a) a minimum three peptides matched within 100 ppm to the theoretical mass of the polypeptide without any protein modification, (b) good agreement between the actual and predicated molecular mass and pI, and (c) the absence of other polypeptides that match. A confidence rating of 3 was assigned if all criteria were met and if the probability-based Mowse scores were greater than 50; a score of 2 was assigned if one criterion was not met and if the probability-based Mowse scores were between 40 and 50. A confidence score of 1 means that at least one of the criteria was met and probability-based Mowse score was between 25 and 39.
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes. However, no supplies of the purified LasR mimic or other active compounds are presently available, and their isolation requires considerable effort.
Acknowledgments
We thank Brian Ahmer for use of his CCD camera, James Metzger for generous help with HPLC; Simon Swift, Bonnie Bassler, and Leo Eberl for providing AHL reporter strains; and Anatol Eberhard for providing synthetic AHLs.
Article, publication date, and citation information can be found at http://www.plantphysiol.org/cgi/doi/10.1104/pp.103.029918.
This work was supported by the U.S. Department of Agriculture National Research Initiatives (grant no. 2002-3531911559 to B.G.R., J.B.R., and W.D.B.), by the Ohio Plant Biotechnology Consortium (grant no. to J.B.R. and W.D.B), by the Ohio State University Office of International Education Travel (grants to W.D.B and M.T.), by the Ohio Agricultural Research and Development Center (presidential fellowship to M.T.), by the Ohio State University (Research Enhancement grant and Extension Sustainable Agriculture grant to M.T.), and by state and federal funds appropriated to the Ohio Agricultural Research and Development Center in partial salary and research support to W.D.B. This is contribution no. 03-14 of Department of Horticulture and Crop Science.
References
- Arcondeguy T, Huez I, Tillard P, Gangneux C, de Billy F, Gojon A, Truchet G, Kahn D (1997) The Rhizobium meliloti PII protein, which controls bacterial nitrogen metabolism, affects alfalfa nodule development. Genes Dev 11: 1194-1206 [DOI] [PubMed] [Google Scholar]
- Bassler BL, Wright M, Showalter RE, Silverman MR (1993) Intercellular signalling in Vibrio harveyi: sequence and function of genes regulating expression of luminescence. Mol Microbiol 9: 773-786 [DOI] [PubMed] [Google Scholar]
- Bauer WD, Teplitski M (2001) Can plants manipulate bacterial quorum sensing? Aust J Plant Physiol 28: 913-921 [Google Scholar]
- Branny P, Pearson JP, Pesci EC, Kohler T, Iglewski BH, Van Delden C (2001) Inhibition of quorum sensing by a Pseudomonas aeruginosa dksA homologue. J Bacteriol 183: 1531-1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Higgins J, Kondorosi E, Kondorosi A, Djordjevic MA, Weinman JJ, Rolfe BG (2000a) Identification of nolR-regulated proteins in Sinorhizobium meliloti using proteome analysis. Electrophoresis 21: 3823-3832 [DOI] [PubMed] [Google Scholar]
- Chen H, Higgins J, Oresnik IJ, Hynes MF, Natera S, Djordjevic MA, Weinman JJ, Rolfe BG (2000b) Proteome analysis demonstrates complex replicon and luteolin interactions in pSyma-cured derivatives of Sinorhizobium meliloti strain 2011. Electrophoresis 21: 3833-3842 [DOI] [PubMed] [Google Scholar]
- Chen H, Teplitski M, Gao M, Robinson JB, Rolfe BG, Bauer WD (2003) Proteomic analysis of wild type Sinorhizobium meliloti responses to N-acyl homoserine lactone quorum sensing signals. J Bacteriol 185: 5029-5036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Schauder S, Potier N, Van Dorsselaer A, Pelczer I, Bassler BL, Hughson FM (2002) Structural identification of a bacterial quorum-sensing signal containing boron. Nature 415: 545-549 [DOI] [PubMed] [Google Scholar]
- Daniels R, De Vos DE, Desair J, Raedschelders G, Luyten E, Rosemeyer V, Verreth C, Schoeters E, Vanderleyden J, Michiels J (2002) The cin quorum sensing locus of Rhizobium etli CNPAF512 affects growth and symbiotic nitrogen fixation. J Biol Chem 277: 462-468 [DOI] [PubMed] [Google Scholar]
- de Kievit TR, Iglewski BH (2000) Bacterial quorum sensing in pathogenic relationships. Infect Immunol 68: 4839-4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nys R, Wright A, Konig GM, Sticher O (1993) New halogenated furanones from the marine alga Delisea pulchra (cf. fimbriata). Tetrahedron 49: 11213-11220 [Google Scholar]
- Dworjanyn SA, de Nys R, Stieinberg PD (1999) Localization and surface quantification of secondary metabolites in the red alga Delisea pulchra. Marine Biol 133: 727-736 [Google Scholar]
- Eberhard A, Burlingame AL, Eberhard C, Kenyon GL, Nealson KH, Oppenheimer NJ (1981) Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry 20: 2444-2449 [DOI] [PubMed] [Google Scholar]
- Eberl L (1999) N-acyl homoserinelactone-mediated gene regulation in gram-negative bacteria. Syst Appl Microbiol 22: 493-506 [DOI] [PubMed] [Google Scholar]
- Eberl L, Winson MK, Sternberg C, Stewart GS, Christiansen G, Chhabra SR, Bycroft B, Williams P, Molin S, Givskov M (1996) Involvement of N-acyl-l-homoserine lactone autoinducers in controlling the multicellular behaviour of Serratia liquefaciens. Mol Microbiol 20: 127-136 [DOI] [PubMed] [Google Scholar]
- Fuqua C, Parsek MR, Greenberg EP (2001) Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu Rev Genet 35: 439-468 [DOI] [PubMed] [Google Scholar]
- Galibert F, Finan TM, Long SR, Puhler A, Abola P, Ampe F, Barloy-Hubler F, Barnett MJ, Becker A, Boistard P et al. (2001) The composite genome of the legume symbiont Sinorhizobium meliloti. Science 293: 668-672 [DOI] [PubMed] [Google Scholar]
- Gao M, Teplitski M, Robinson JB, Bauer WD (2003) Production of substances by Medicago truncatula that affect bacterial quorum sensing. Mol Plant-Microbe Interact 16: 827-834 [DOI] [PubMed] [Google Scholar]
- Givskov M, Nys Rd, Manefield M, Gram L, Maximilien R, Eberl L, Molin S, Steinberg PD, Kjelleberg S (1996) Eukaryotic interference with homoserine lactone-mediated prokaryotic signalling. J Bacteriol 178: 6618-6622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant AJ, Farris M, Alefounder P, Williams PH, Woodward MJ, O'Connor CD (2003) Co-ordination of pathogenicity island expression by the BipA GTPase in enteropathogenic Escherichia coli (EPEC). Mol Microbiol 48: 507-521 [DOI] [PubMed] [Google Scholar]
- Guerreiro N, Djordjevic MA, Rolfe BG (1999) Proteome analysis of the model microsymbiont Sinorhizobium meliloti: isolation and characterisation of novel proteins. Electrophoresis 20: 818-825 [DOI] [PubMed] [Google Scholar]
- Harris EH (2001) Chlamydomonas as a model organism. Annu Rev Plant Physiol Plant Mol Biol 52: 363-406 [DOI] [PubMed] [Google Scholar]
- Harris EH (1989) The Chlamydomonas Sourcebook: A Comprehensive Guide to Biology and Laboratory Use. Academic Press, San Diego [DOI] [PubMed]
- Hentzer M, Riedel K, Rasmussen TB, Heydorn A, Andersen JB, Parsek MR, Rice SA, Eberl L, Molin S, Hoiby N et al. (2002) Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology 148: 87-102 [DOI] [PubMed] [Google Scholar]
- Holden MT, Ram Chhabra S, de Nys R, Stead P, Bainton NJ, Hill PJ, Manefield M, Kumar N, Labatte M, England D et al. (1999) Quorum-sensing cross talk: isolation and chemical characterization of cyclic dipeptides from Pseudomonas aeruginosa and other gram-negative bacteria. Mol Microbiol 33: 1254-1266 [DOI] [PubMed] [Google Scholar]
- Hou Z, Wang W, Fromm HJ, Honzatko RB (2002) IMP alone organizes the active site of adenylosuccinate synthetase from Escherichia coli. J Biol Chem 277: 5970-5976 [DOI] [PubMed] [Google Scholar]
- Joyce EA, Bassler BL, Wright A (2000) Evidence for a signaling system in Helicobacter pylori: detection of a luxS-encoded autoinducer. J Bacteriol 182: 3638-3643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jude F, Kohler T, Branny P, Perron K, Mayer MP, Comte R, van Delden C (2003) Posttranscriptional control of quorum-sensing-dependent virulence genes by DksA in Pseudomonas aeruginosa. J Bacteriol 185: 3558-3566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelleberg S, Steinberg P (2002) Defenses against bacterial colonisation of marine plants. In SE Lindow, E Poinar, V Elliott, eds, Phyllosphere Microbiology. American Phytopathological Society Press, Minneapolis
- Kjelleberg S, Steinberg P, Givskov M, Gram L, Manefield M, Nys Rd (1997) Do marine natural products interfere with prokaryotic AHL regulatory systems? Aquatic Microbial Ecol 13: 85-93 [Google Scholar]
- Loh J, Pierson EA, Pierson LS, 3rd, Stacey G, Chatterjee A (2002) Quorum sensing in plant-associated bacteria. Curr Opin Plant Biol 5: 285-290 [DOI] [PubMed] [Google Scholar]
- Manefield M, de Nys R, Kumar N, Read R, Givskov M, Steinberg P, Kjelleberg S (1999) Evidence that halogenated furanones from Delisea pulchra inhibit acylated homoserine lactone (AHL)-mediated gene expression by displacing the AHL signal from its receptor protein. Microbiology 145: 283-291 [DOI] [PubMed] [Google Scholar]
- Manefield M, Rasmussen TB, Henzter M, Andersen JB, Steinberg P, Kjelleberg S, Givskov M (2002) Halogenated furanones inhibit quorum sensing through accelerated LuxR turnover. Microbiology 148: 1119-1127 [DOI] [PubMed] [Google Scholar]
- Marketon MM, Gronquist MR, Eberhard A, Gonzalez JE (2002) Characterization of the Sinorhizobium meliloti sinR/sinI locus and the production of novel N-acyl homoserine lactones. J Bacteriol 184: 5686-5695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathesius U, Mulders S, Gao M, Teplitski M, Caetano-Anolles G, Rolfe BG, Bauer WD (2003) Extensive and specific responses of a eukaryote to bacterial quorum sensing signals. Proc Natl Acad Sci USA 100: 1444-1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClean KH, Winson MK, Fish L, Taylor A, Chhabra SR, Camara M, Daykin M, Lamb JH, Swift S, Bycroft BW et al. (1997) Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 143: 3703-3711 [DOI] [PubMed] [Google Scholar]
- McLuskey K, Harrison JA, Schuttelkopf AW, Boxer DH, Hunter WN (2003) Insight into the role of Escherichia coli MobB in molybdenum cofactor biosynthesis based on the high resolution crystal structure. J Biol Chem 278: 23706-23713 [DOI] [PubMed] [Google Scholar]
- Miller MB, Bassler BL (2001) Quorum sensing in bacteria. Annu Rev Microbiol 55: 165-199 [DOI] [PubMed] [Google Scholar]
- Mok KC, Wingreen NS, Bassler BL (2003) Vibrio harveyi quorum sensing: a coincidence detector for two autoinducers controls gene expression. EMBO J 22: 870-881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninfa AJ, Atkinson MR (2000) PII signal transduction proteins. Trends Microbiol 8: 172-179 [DOI] [PubMed] [Google Scholar]
- Neuhoff V, Arold N, Taube D, Ehrhardt W (1988) Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis 9: 255-262 [DOI] [PubMed] [Google Scholar]
- Pellock BJ, Teplitski M, Boinay RP, Bauer WD, Walker GC (2002) A LuxR homolog controls production of symbiotically active extracellular polysaccharide II by Sinorhizobium meliloti. J Bacteriol 184: 5067-5076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Luo ZQ, Smyth AJ, Gao P, Beck Von Bodman S, Farrand SK (2000) Quorum-sensing signal binding results in dimerization of TraR and its release from membranes into the cytoplasm. EMBO J 19: 5212-5221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster M, Lostroh CP, Ogi T, Greenberg EP (2003) Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J Bacteriol 185: 2066-2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RS, Harris SG, Phipps R, Iglewski B (2002) The Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxododecanoyl)homoserine lactone contributes to virulence and induces inflammation in vivo. J Bacteriol 184: 1132-1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steidle A, Sigl K, Schuhegger R, Ihring A, Schmid M, Gantner S, Stoffels M, Riedel K, Givskov M, Hartmann A et al. (2001) Visualization of N-acylhomoserine lactone-mediated cell-cell communication between bacteria colonizing the tomato rhizosphere. Appl Environ Microbiol 67: 5761-5770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan MW, Rahme LG, Sternberg JA, Tompkins RG, Ausubel FM (1999) Pseudomonas aeruginosa killing of Caenorhabditis elegans used to identify P. aeruginosa virulence factors. Proc Natl Acad Sci USA 96: 2408-2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford G, Wheeler D, Williams P, Tomkins PT, Appleby P, Sewell H, Stewart GS, Bycroft BW, Pritchard DI (1998) The Pseudomonas aeruginosa quorum-sensing signal molecule N-(3-oxododecanoyl)-l-homoserine lactone has immunomodulatory activity. Infect Immunol 66: 36-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teplitski M, Eberhard A, Gronquist M, Gao M, Robinson JB, Rolfe BG, Bauer WD (2003) Chemical identification of N-acyl homoserine lactone quorum sensing signals produced by Sinorhizobium meliloti strains in defined medium. Arch Microbiol (in press) [DOI] [PubMed]
- Teplitski M, Robinson JB, Bauer WD (2000) Plants secrete substances that mimic bacterial N-acyl homoserine lactone signal activities and affect population density-dependent behaviors in associated bacteria. Mol Plant-Microbe Interact 13: 637-648 [DOI] [PubMed] [Google Scholar]
- Visick KL, Ruby EG (1999) The emergent properties of quorum-sensing: consequences to bacteria of autoinducer signalling in their natural environment. In GM Dunny, S Winans, eds, Cell-Cell Signalling in Bacteria. ASM Press, Washington, DC, pp 333-352
- von Bodman SB, Bauer WD, Coplin DL (2003) Quorum sensing in plant-pathogenic bacteria. Annu Rev Plant Pathol 41: 455-482 [DOI] [PubMed] [Google Scholar]
- Wagner VE, Bushnell D, Passador L, Brooks AI, Iglewski BH (2003) Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: effects of growth phase and environment. J Bacteriol 185: 2080-2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiller GF, Djordjevic MJ, Caraux G, Chen H, Weinman JJ (2001). A specialised proteomic database for comparing matrix-assisted laser desorption/ionization-time of flight mass spectrometry data of tryptic peptides with corresponding sequence database segments. Proteomics 1: 1489-1494.48 [DOI] [PubMed] [Google Scholar]
- Whitehead NA, Barnard AM, Slater H, Simpson NJ, Salmond GP (2001) Quorum-sensing in Gram-negative bacteria. FEMS Microbiol Rev 25: 365-404 [DOI] [PubMed] [Google Scholar]
- Winson MK, Swift S, Fish L, Throup JP, Jorgensen F, Chhabra SR, Bycroft BW, Williams P, Stewart GS (1998) Construction and analysis of luxCDABE-based plasmid sensors for investigating N-acyl homoserine lactone-mediated quorum sensing. FEMS Microbiol Lett 163: 185-192 [DOI] [PubMed] [Google Scholar]