Abstract
Stroke being the third leading cause of death and foremost cause of disability, if potential diagnostic utility of blood borne protein biomarkers in predicting acute stroke is established, it would be a substantial adjunct to computerized tomography and magnetic resonance imaging which have their own limitations. This study was done to correlate serum Interleukin 6, high sensitivity C reactive protein at the time of admission with neurological worsening assessed by NIHSS at the time of admission and 7 days after admission. 46 Patients admitted in neurology department SAIMS, Indore with first ever ischemic stroke within 72 h of onset were included in the study. All patients with history of stroke of more than 72 h onset, Infection & peripartum stroke were excluded from the study. Disability scoring was done by NIHSS and their serum samples assayed for hsCRP, IL6 by commercially available quantitative sandwich enzyme-linked immunoadsorbent assay kits. Serum samples of 50 control cases which included healthy volunteers and staff from SAIMS were also analyzed for hsCRP, IL6 for comparative study. A significant correlation was observed between NIHSS scoring and serum hsCRP and IL6 at the time of admission. Patients with initial high serum IL6 and hsCRP also showed significant clinical deterioration as assessed by NIHSS scoring 7 days after admission. Elevated hsCRP and IL6 within 72 h of admission strongly correlated with functional disability in study population in India and may serve as useful adjunct to CT Scan in emergency setting.
Keywords: Ischemic stroke, High sensitive C-Reactive Protein, Interleukin-6, National Institute of Health Stroke Scale, Disability score
Introduction
Like all developing countries stroke is fast emerging as a major public health problem in India [1]. The age standardized average annual incidence rate to world standard population of first-ever-in-a-lifetime stroke is 145.30 (95% CI, 120.39 to 174.74) per 100000 persons per year [2]. Stroke is the third most common cause of death in the developed countries [3].
Over the past few years, a body of evidence has stressed the role of inflammation in the pathophysiology of acute brain ischemia [4]. Most inflammatory reactions are mediated by cytokines, small glycoproteins expressed by many cell types in response to acute cerebral ischemia. Cytokine release results in up regulation of adhesion molecules, recruitment and activation of leukocytes, promotion of leukocyte-endothelium interaction, and conversion of the local endothelium to a prothrombotic state [4]. Increases of proinflammatory cytokines (interleukin [IL]-1, tumor necrosis factor [TNF]-a, and IL-6) have been detected in the ischemic cortex 1 h after middle cerebral artery (MCA) occlusion in experimental models of brain ischemia [5]. In this study we analyzed the relationship between proinflammatory cytokines in serum and the disability as assessed by NIHSS scoring at the time of admission and 7 days after admission. hs-CRP, an acute-phase reactant, is significantly increased in inflammatory disorders, and it has been shown to enhance immune reactivity [6]. Several studies have reported that higher levels of inflammatory markers such as C-reactive protein (CRP) and interleukin-6 (IL-6) are associated with worse outcome after both ischemic [7] and hemorrhagic [8, 9] strokes. Early clinical deterioration results in increased mortality and functional disability [10–12].
Subjects and Methods
A group of 46 patients admitted in neurology ward of SAIMS INDORE with first-ever acute ischemic stroke, diagnosed according to clinical history, physical examination and computed tomography (CT) brain scan. CT scan was performed within 12 h of admission to exclude patients with stroke mimic or primary intracerebral hemorrhage. All patients were submitted to standardized neurological evaluation on admission using the National Institutes of Health Stroke Scale.
The venous blood samples were drawn within 72 h from the onset of symptoms in 5 ml serum separator tubes, centrifuged at 3000 g for 15 min and then aliquotted in 2 ml tubes. Samples were stored at −20°C until assays were run to evaluate hsCRP and IL6 markers of stroke progression. Simultaneously blood samples from 50 healthy volunteers and SAIMS staff were collected and evaluated for IL6 and hsCRP levels. To avoid the confounding effect on inflammatory markers of clinical conditions that can be associated with ischemic stroke, subjects with inflammatory or infectious diseases, cancer, peripartum stroke or severe renal or liver failure, or current daily treatment with anti-inflammatory drugs were not included in the study. The protocol was approved by the local Ethics Committee; Informed consent was given by patients themselves or by relatives as legally required.
National Institute of Health Stroke Scale
The severity of stroke was scored on admission and after 7 days by the neurologist, using the National Institute of Health Stroke Scale (NIHSS). The NIHSS score consists of 15 items and a total score of 42 points. Score of 0 = no stroke, 1–4 = minor stroke, 5–20 = moderate stroke, 21–42 = severe stroke. Early neurological worsening was diagnosed as an increase in National Institute of Health Stroke Score (NIHSS) by two or more points (or stroke-related death) between admission and day 5 [13] and who remained stable or improved in the same period were classified as no worsening.
Detection of serum IL-6 and hsCRP is based on the principle of a solid phase enzyme linked immunosorbent assay [14].
Result
Patient Population
The baseline characteristics of the study and the control group were found to be similar (age 58.5 ± 8.9 vs. 55.5 ± 7.7 years).The clinical characteristics of stroke patients and control group included in the study is shown in Table 1.
Table 1.
Demographic profile of stroke cases and controls
| Demographic variables | Frequency (%) Study group |
Frequency (%) Control group |
|---|---|---|
| Age (in years) | ||
| 35–45 | 6.5 | 12 |
| 46–55 | 21.7 | 34 |
| 56–65 | 54.3 | 48 |
| 66–75 | 17.4 | 6 |
| Hypertensive | 76.1 | 51.5 |
| Smokers | 28.3 | 20.2 |
| Atrial fibrillation | 10.9 | 2 |
| Diabetes mellitus | 30.4 | 15.7 |
| No risk factor | – | 10 |
hsCRP levels of >4.0 mg/dl was found in 45.6% cases and IL 6 levels >16 pg/ml was found in 67.4% cases. In controls hsCRP levels >0.5 mg/dl was found in 16% cases and in patient’s hsCRP levels >0.5 mg/dl was found in 84% cases.
The probability value of Chi-Square is 60.90 at 6 degrees of freedom, which shows a highly significant value (P < 0.001, two-tailed) and high significant association between Inflammatory Marker hs-CRP Score and type of stroke at the time of admission. The probability value of Chi-Square is 41.60 at 4 degrees of freedom, which shows a highly significant value (P < 0.001, two-tailed) and high significant association between Inflammatory Marker IL-6 Score and type of stroke at the time of admission (Tables 2, 3).
Table 2.
hsCRP and IL 6 levels in cases and controls
| Stroke cases | Control | P value | |
|---|---|---|---|
| hsCRP levels(mg/dl) | 3.74 ± 2.22 | 0.40 ± 0.084 | <0.001 |
| IL 6 levels (pg/ml) | 19.34 ± 7.35 | 4.81 ± 1.12 | <0.001 |
Table 3.
Association between hs-CRP score and IL-6 score with types of stroke
| Type of stroke at the time of admission | N | Minimum | Maximum | Mean | Std. deviation |
|---|---|---|---|---|---|
| Mild | |||||
| hs-CRP score | 13 | 0.53 | 3.40 | 0.94 | 0.753 |
| IL-6 score | 0.74 | 15.20 | 10.92 | 3.903 | |
| Moderate | |||||
| hs-CRP score | 15 | 1.30 | 5.60 | 3.65 | 1.114 |
| IL-6 score | 13.20 | 23.20 | 18.37 | 3.511 | |
| Severe | |||||
| hs-CRP score | 18 | 3.90 | 7.20 | 5.83 | 1.005 |
| IL-6 score | 20.20 | 32.40 | 26.23 | 4.104 | |
The probability value of Chi-Square is 35.08 at 3 degrees of freedom, which shows a highly significant value (P < 0.001, two-tailed) and high significant association between Inflammatory Marker hs-CRP Score and Cases of Neurological worsening. The probability value of Chi-Square is 30.19 at 2 degrees of freedom, which shows a highly significant value (P < 0.001, two-tailed) and high significant association between Inflammatory Marker IL-6 Score and Cases of Neurological worsening (Tables 4, 5).
Table 4.
Association between NIHSS score (0 and 7 day) and neurological worsening
| Disability scores | 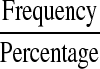 |
Cases of neurological worsening 0 day | Total | |
|---|---|---|---|---|
| No | Yes | |||
| 1–4 (Mild) | Frequency | 12 | 0 | 12 |
| % | 41.4% | 0% | 26.1% | |
| 5–20 (Moderate) | Frequency | 13 | 3 | 16 |
| % | 44.8% | 17.6% | 34.8% | |
| 21–31 (Severe) | Frequency | 4 | 14 | 18 |
| % | 13.8% | 82.4% | 39.1% | |
| Total | Total Frequency | 29 | 17 | 46 |
| % | 100.0% | 100.0% | 100.0% | |
Table 5.
Association of hsCRP and IL6 Score with Neurological Worsening
| No neurological worsening | No. | Minimum | Maximum | Mean | Std. deviation |
|---|---|---|---|---|---|
| NIHSS score 0 day | 29 | 3 | 21 | 11.83 | 7.37 |
| hs-CRP score | 29 | 0.53 | 4.90 | 2.35 | 1.52 |
| IL-6 score | 29 | 0.74 | 22.50 | 14.84 | 4.90 |
| Neurological worsening | No. | Minimum | Maximum | Mean | Std. deviation |
|---|---|---|---|---|---|
| NIHSS score 0 day | 17 | 17 | 24 | 21.65 | 1.62 |
| hs-CRP score | 17 | 4.90 | 7.20 | 6.10 | 0.64 |
| IL-6 score | 17 | 22.40 | 32.40 | 27.01 | 3.29 |
Discussion
This study was hospital-based. Therefore patients with very minor deficits (not hospitalized whatever the reason), those who refused admission, those with severe deficits who died before admission, and those admitted to private hospitals were not included in our cohort.
In our study we found higher baseline levels of IL-6 in plasma of patients with acute ischemic stroke as compared to controls and more specifically found a direct association between IL-6 level and severity of stroke as well with early neurological deterioration compared with patients who remained clinically stable or improved. Our data on the elevated level of IL-6 are consistent with those already reported in the literature [15, 16]. There is ample evidence in the literature supporting a role of inflammatory response in acute phase of ischemic stroke. Although cytokines such as IL-1, IL-6 and TNF-α appear to be the main mediators of that response, the exact role of inflammatory markers in pathogenesis of ischemic stroke is still unclear [17]. Moreover, correlation of serum IL-6 levels with stroke severity, clinical outcome (assessed by SSS and BI) and extent of brain damage was also found in some earlier studies [17, 18]. IL-6 seems to be a robust early marker for outcome in acute ischemic stroke [19]. We were able, in the present study, to confirm these reports on the association of early IL-6 levels (before 72 h after stroke onset) with both stroke severity and neurological deterioration.
IL-6 which is a major circulating cytokine produced in response to cerebral ischemia is also a potent stimulus for production of hepatic acute phase protein [20]. In the present study, hsCRP levels were significantly higher as compared to their age and sex matched controls. Elevated levels of CRP have been shown in multiple cohort studies to correlate with increased incidence of stroke [21–23]. Our study supported the previous findings that hsCRP levels at the time of admission significantly correlated with functional disability as well as further clinical worsening [24] and our observations are consistent with results from similar studies in the west [25, 26].
Conclusion
The availability of a rapid diagnostic assay i.e. proinflammatory serum biomarkers (hsCRP, IL-6) for acute stroke would be highly beneficial in assessing the care pathway for patients and their treatment options as the most important challenge facing physicians globally is to reduce the unacceptable burden of stroke.
As from our study we observed that disability was associated with higher concentrations of IL-6 and hsCRP in plasma and early neurological deterioration was too observed in cases with high levels of hsCRP and IL-6. Thus development of new neuroprotective therapies if targeted to modulate cytokine induced inflammation could be a promising way to prevent early deterioration in acute ischemic stroke.
References
- 1.Poungvarin N. Stroke in the developing countries. Lancet. 1998;352(SIII):19–22. doi: 10.1016/S0140-6736(98)90090-3. [DOI] [PubMed] [Google Scholar]
- 2.Das SK, Banerjee TK, Biswas A, et al. A prospective community based study of stroke in Kolkata, India. Stroke. 2007;38:906. doi: 10.1161/01.STR.0000258111.00319.58. [DOI] [PubMed] [Google Scholar]
- 3.Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics—a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 4.Clark WM. Cytokines and reperfusion injury. Neurology. 1997;49(suppl 4):10–14. doi: 10.1212/wnl.49.5_suppl_4.s10. [DOI] [PubMed] [Google Scholar]
- 5.Yamasaki Y, Matsuura N, Shozuhara H, Onodera H, Itoyama Y, Kogure K. Interleukin-1 as a pathogenic mediator of ischemic brain damage in rats. Stroke. 1995;26:676–681. doi: 10.1161/01.STR.26.4.676. [DOI] [PubMed] [Google Scholar]
- 6.Tanne D, Benderly M, Goldbourt U, Hain M, Tenenbaum A, et al. C-reactive protein as a predictor of incident ischemic stroke among patients with preexisting cardiovascular disease. Stroke. 2006;37:1720–1724. doi: 10.1161/01.STR.0000227004.08182.bf. [DOI] [PubMed] [Google Scholar]
- 7.Whiteley W, Chong WL, Sengupta A, Sandercock P. Blood markers for the prognosis of ischemic stroke: a systematic review. Stroke. 2009;40:e380–e389. doi: 10.1161/STROKEAHA.108.528752. [DOI] [PubMed] [Google Scholar]
- 8.Castellanos M, Leira R, Tejada J, Gil-Peralta A, Davalos A, et al. Predictors of good outcome in medium to large spontaneous supratentorial intracerebral haemorrhages. J Neurol Neurosurg Psychiatry. 2005;76:691–695. doi: 10.1136/jnnp.2004.044347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castillo J, Davalos A, Alvarez-Sabin J, Pumar JM, Leira R, et al. Molecular signatures of brain injury after intracerebral hemorrhage. Neurology. 2002;58:624–629. doi: 10.1212/wnl.58.4.624. [DOI] [PubMed] [Google Scholar]
- 10.Dávalos A, Cendra E, Teruel J, Martinez M, Genis D. Deteriorating ischemic stroke: risk factors and prognosis. Neurology. 1990;40:1865–1869. doi: 10.1212/wnl.40.12.1865. [DOI] [PubMed] [Google Scholar]
- 11.Toni D, Fiorelli M, Gentile M, Bastianello S, Sacchetti ML, Argentino C, et al. Progressing neurological deficit secondary to acute ischemic stroke: a study on predictability, pathogenesis, and prognosis. Arch Neurol. 1995;52:670–675. doi: 10.1001/archneur.1995.00540310040014. [DOI] [PubMed] [Google Scholar]
- 12.Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS. Effect of blood pressure and diabetes on stroke in progression. Lancet. 1994;344:156–159. doi: 10.1016/S0140-6736(94)92757-X. [DOI] [PubMed] [Google Scholar]
- 13.Kwan J, Hand P. Early neurological deterioration in acute stroke: clinical characteristics and impact on outcome. QJM. 2006;99:625–633. doi: 10.1093/qjmed/hcl082. [DOI] [PubMed] [Google Scholar]
- 14.Ramadori G, Christ B. Cytokines and the hepatic acute-phase response. Semin Liver Dis. 1999;19:141–155. doi: 10.1055/s-2007-1007106. [DOI] [PubMed] [Google Scholar]
- 15.Orion D, Schwammenthal Y, Reshef T, et al. Interleukin-6 and soluble intercellular adhesion molecule-1 in acute brain ischaemia. Eur J Neurol. 2008;15:323–328. doi: 10.1111/j.1468-1331.2008.02066.x. [DOI] [PubMed] [Google Scholar]
- 16.Shenhar-Tsarfaty S, Assayag EB, Bova I, et al. Early signaling of inflammation in acute ischemic stroke: clinical and rheological implications. Thromb Res. 2008;122(2):167–173. doi: 10.1016/j.thromres.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 17.Smith CJ, Emsley HCA, Gavin CM, et al. Peak plasma interleukin-6, other peripheral markers of inflammation in the first week of ischemic stroke correlate with brain infarct volume, stroke severity, long-term outcome. BMC Neurol. 2004;4:2. doi: 10.1186/1471-2377-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrarese C, Mascarucci P, Zoia C, et al. Increased cytokine release from peripheral blood cells after acute stroke. J Cerebr Blood Flow Metab. 1999;19:1004–1009. doi: 10.1097/00004647-199909000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Waje-Andreassen U, Kråkenes J, Ulvestad E, et al. IL-6: an early marker for outcome in acute ischemic stroke. Acta Neurol Scand. 2005;112(4):273–274. doi: 10.1111/j.1600-0404.2005.00481.x. [DOI] [PubMed] [Google Scholar]
- 20.Cao JJ, Thach C, Manolio TA, Psaty BM, Kuller LH, Chaves PHM, et al. C-reactive protein, carotid intima media thickness and incidence of stroke in elderly: the Cardiovascular Health Study. Circulation. 2003;108:166–170. doi: 10.1161/01.CIR.0000079160.07364.6A. [DOI] [PubMed] [Google Scholar]
- 21.Di Napoli M, Papa F, Bacola V. Prognostic influence of increased C Reactive Protein and fibrinogen levels in ischaemic stroke. Stroke. 2001;32:133–138. doi: 10.1161/01.STR.32.1.133. [DOI] [PubMed] [Google Scholar]
- 22.Di Napoli M, Papa F, Bocola V. C-reactive protein in ischemic stroke: an independent prognostic factor. Stroke. 2001;32:917–924. doi: 10.1161/01.STR.32.4.917. [DOI] [PubMed] [Google Scholar]
- 23.Winbeck K, Poppert H, Etgen T, Conrad B, Sander D. Prognostic relevance of early serial C Reactive protein measurements after first ischemic stroke. Stroke. 2002;33:2459–2464. doi: 10.1161/01.STR.0000029828.51413.82. [DOI] [PubMed] [Google Scholar]
- 24.Kim JS, Kim YI, Lee KS. Relationship between high-sensitivity C-Reactive Protein and clinical functional outcome after acute ischemic stroke in a Korean Population. Cerebrovasc Dis. 2009;28:545–550. doi: 10.1159/000247597. [DOI] [PubMed] [Google Scholar]
- 25.Panicker JN, Thomas M, Pavithran K, Nair D, Sarma PS. Morbidity predictors in ischaemic stroke. Neurol India. 2003;51:49–51. [PubMed] [Google Scholar]
- 26.Angerio AD, Bialko MF, White BM. C-reactive protein, stroke, statins. Cardiac Emergencies. Crit Care Nurs Q. 2007;30(2):161–165. doi: 10.1097/01.CNQ.0000264259.82875.c0. [DOI] [PubMed] [Google Scholar]


