Abstract
Speech sound disorders (SSD) are the largest group of communication disorders observed in children. One explanation for these disorders is that children with SSD fail to form stable phonological representations when acquiring the speech sound system of their language due to poor phonological memory (PM). The goal of this study was to examine PM in individuals with histories of SSD employing functional MR imaging (fMRI). Participants were 6 right-handed adolescents with a history of early childhood SSD and 7 right-handed matched controls with no history of speech and language disorders. We performed an fMRI study using an overt non-word repetition (NWR). Right lateralized hypoactivation in the inferior frontal gyrus and middle temporal gyrus was observed. The former suggests a deficit in the phonological processing loop supporting PM, while the later may indicate a deficit in speech perception. Both are cognitive processes involved in speech production. Bilateral hyperactivation observed in the pre and supplementary motor cortex, inferior parietal, supramarginal gyrus and cerebellum raised the possibility of compensatory increases in cognitive effort or reliance on the other components of the articulatory rehearsal network and phonologic store. These findings may be interpreted to support the hypothesis that individuals with SSD may have a deficit in PM and to suggest the involvement of compensatory mechanisms to counteract dysfunction of the normal network.
1. Introduction
Speech sound disorders (SSD) are the largest group of communication disorders observed in children requiring special education services (“IDEA,” Data updated as of August 3, 2009). Individuals with SSD have a reduced capacity to accurately and intelligibly produce the sounds of their native language (Peterson, McGrath, Smith, & Pennington, 2007) and often fail to apply linguistic rules for combining sounds to form words. Children with SSD have deficits on a number of phonologic tasks including phonological memory (PM) (Peterson et al., 2007) that may persist into adulthood (Kenney, Barac-Cikoja, Finnegan, Jeffries, & Ludlow, 2006). It is believed that individuals with SSD possess poorly formed and unstable underlying phonological representations that lead to speech sound errors (Pennington & Bishop, 2009). The goal of the present study was to use functional MRI (fMRI) to examine the neurological processing of individuals with a history of SSD during overt speech. We hypothesized that children with SSD fail to form stable phonological representations when acquiring the speech sound system of their language due to poor PM. While most adults with histories of SSD no longer present with overt speech sound errors, tasks such as NWR may reveal persistent PM deficits. Using functional imaging (fMRI), we expected to find neuroimaging evidence to support this supposition.
PM has been proposed as the component of short term memory that holds a temporary store of phonological information, a process believed to be essential to the formation of stable phonologic representations. The most widely accepted model of working memory is that introduced in by Baddeley and colleagues (Baddeley, 1986). The model consists of several interacting components including a domain general control system referred to as the central executive and several modality specific maintenance subsystems (e.g. verbal and visuo-spatial). The central executive is posited to coordinate the activity of the maintenance subsystems and “to mediate the allocation of attention, the inhibition of task irrelevant processes and the coding of contextual and temporal order information associated with the representations held in memory” ((Chein & Fiez, 2001), pg. 1004). The verbal maintenance subsystem (e.g. verbal working memory) is referred to as the phonological loop and supports the short term maintenance of verbal information (Baddeley, 1986, 1992, 2003). The phonological loop is postulated to be composed of two components, a phonological store and an articulatory rehearsal process, which act in concert to enable representations of verbal material to be kept in an active state (Chen & Desmond, 2005).
Nonword repetition (NWR) tasks mimic the process of forming a phonologic representation for a new word. Multiple language processes are required to successfully perform NWR, including speech perception, phonological encoding, phonological memory, phonetic encoding (transforming the linguistic codes to articulatory codes), and articulation (Coady & Evans, 2008). “It requires a robust representation of underlying speech units, and sufficient memory both to temporarily store and operate on the novel phonological string” ((Coady & Evans, 2008)pg.2). Gathercole and Baddeley and others have employed NWR to specifically measure PM (Susan E. Gathercole & Baddeley, 1989, 1990; Graf Estes, Evans, & Else-Quest, 2007). These researchers have demonstrated significant correlations between NWR accuracy and other measures of PM such as digit span (Coady & Evans, 2008). Further evidence for the utility of NWR in tapping PM is provided by (McGrath et al., 2007)) who found that a NWR task loaded heavily on PM in children with SSD age 5-7 years. In addition, (Bishop, North, & Donlan, 1996) also reported that deficits in nonword repetition persist into adolescence in individuals with a history of inherited language impairment even after the disorder has resolved.
Recent neuropsychological and neuroimaging studies have generated data that provide insight into the neural correlates of the phonological loop supporting PM. Collectively, the finding of these studies suggest that a network of areas typically associated with speech production, including the left dominant inferior frontal gyrus (Brodmann area [BA] 44/45), premotor area (lateral BA6), supplementary motor area (medial BA6), and bilateral (but right dominant) superior cerebellar hemisphere (Lobule V1/Crus I), are involved in the articulatory rehearsal system of the phonological loop (Chein & Fiez, 2001; Chen & Desmond, 2005). In contrast, the data suggest that the phonologic store resides in the left inferior parietal and supramarginal gyrus (BA 40) and that the right inferior cerebellum (VIIB) is also involved in this process(Chen & Desmond, 2005).
To date, the neural correlates of the behavioral deficits associated with SSD have not been investigated or validated by neuroimaging methods. We performed a fMRI study using an overt NWR task in a group of adolescents with a history of SSD and in a group of age matched typical speech and language (TSL) controls. Our objective was to examine group differences in neural activation consistent with the hypothesis that individuals with SSD may have a deficit in PM. We expected to observe functional differences between the two groups in brain regions known to support PM including the inferior frontal gyrus (IFG), premotor cortex, supplementary motor area, inferior parietal cortex, supramarginal gyrus and cerebellum.
2. Results
2.1 Longitudinal neurobehavioral assessments of SSD participants
The six individuals (5 male) with a history of moderate to severe SSD recruited for the fMRI study were participants in a large longitudinal ongoing study of SSD (Lewis, Freebairn, & Taylor, 2000). These individuals had completed behavioral speech and language testing and developmental history questionnaires following their initial diagnosis at early childhood (ages 4-7 years) and were followed longitudinally with the most recent assessment occurring 0.5-2 years prior to the neuroimaging study. None of the participants had a history of a developmental disorder other than speech and language delay. Participants presented with a wide range of scores on the performance IQ (PIQ). Specifically, all but one of the SSD participants scored average or above on PIQ; while one scored in the low average range at assessment time points 1 and 3 and below the normal range at time point 2 (Figure 1A). Similarly, the SSD participants presented with a wide range of scores on language and reading (Figure 1A). Three of the SSD participants consistently performed low or below average on language across assessment time points. Reading scores were low or below average at time points 1 and 2, reaching average at time point 3 for all but one of the SSD participants (Figure 1A). Five out of the 6 SSD participants achieved normal adult articulation with no errors by behavioral testing at school-age. Notably, as a group, the SSD participants consistently performed below or low average on verbal memory tests (Figure 1B and 1C) (Lewis et al., 2007). There was however, variability among participants on the PM measures at every assessment time point. Two participants scored poorly at every assessment on all measures of PM. The remainder of the SSD subjects scored poorly on one or more PM measures at at least one time point. Specifically, with the exception of one SSD individual who scored above average at assessment time point 3, all 6 SSD participants scored below or low average on the Digit Span across the assessment time points tested (Figure 1B). Similarly, all but one SSD participant scored below or low average on Sentence Repetition for at least one of the assessment time points and 3 SSD participants performed very poorly or below average across all assessment time points (Figure 1B). Finally, all but one (a male) of the SSD participants made no speech errors in conversation at the time of the fMRI study.
Figure 1.
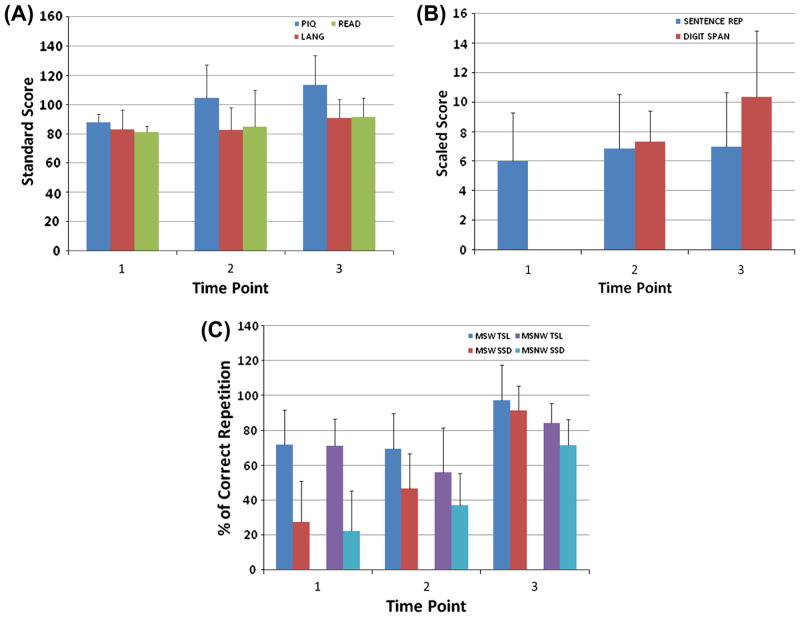
Longitudinal neurobehavioral data for the SSD participants (N=6) collected at three different time points. The Mean(SD) age in years of the SSD participants at Time Point 1, 2 and 3 was 6(2), 10(2) and 17(2) respectively. For the TOLD sentence repetition and the Wisc Digit span, the scaled scores have a mean of 10 and a SD of 3. They are interpreted as follows: 1-4 very poor, 5-7 below average or weakness, 8-12 average,13-15 above average or strength; 16-19 superior. For the standard scores (PIQ, reading and language), 90-110 average, 80-89 low average, 70-79 borderline or poor; below 70 is very poor or mentally retarded. (A) Standard scores for Performance IQ (PIQ), Language (LANG) and Reading (READ). Language is the standard total score on either the Test of Language Development-Primary 2nd Edition (TOLD-P:2), the Test of Language Development-Primary 3rd Edition (TOLD-P:3) or the Clinical Evaluation of Language Fundamentals-3rd Edition (CELF-3). Reading (READ) is the Word Identification and Word Attack Subtests of the Woodcock Reading Mastery Test-Revised (WRMT-R). (B) Scaled scores for the working memory measures: Sentence Repetition and Digit Span Subtests. (C) Performance on the working memory measures: Multisyllabic Word (MSW) and Multisyllabic Nonword (MSNW) Repetition reported as percent correct repetition (% Correct Rep) for a control group composed of individuals with TSL development and the SSD subjects evaluated in this study. Both groups were recruited from a large cohort of individuals enrolled in an ongoing longitudinal study of speech and language development. Note that the normal TSL controls enrolled in the fMRI study were not recruited from the ongoing longitudinal study cohort.
2.2 Verbal response
Repetition accuracy for the stimulus non-words was high for both groups and a Wilcoxon rank sum test failed to reveal a significant difference between groups (p=0.51). The results suggest that the discrepancy in the brain activation patterns identified for the SSD and TSL cohorts reflects an inherent difference in the functional neural network supporting phonologic processing associated with speech production in these two groups rather than a difference in skill level in the articulation of nonwords.
2.3 FMRI data
Compared to their TSL peers, participants with a history of SSD demonstrated regional differences in activation in response during the NWR vs. baseline conditions. Figure 2A presents axial slices illustrating the primary brain regions of hypoactivation for the SSD group (Control > SSD). The regions of hypoactivation were exclusively right lateralized and were found in the IFG (BA 45 / BA 46) and middle temporal gyrus (BA21/22).
Figure 2.
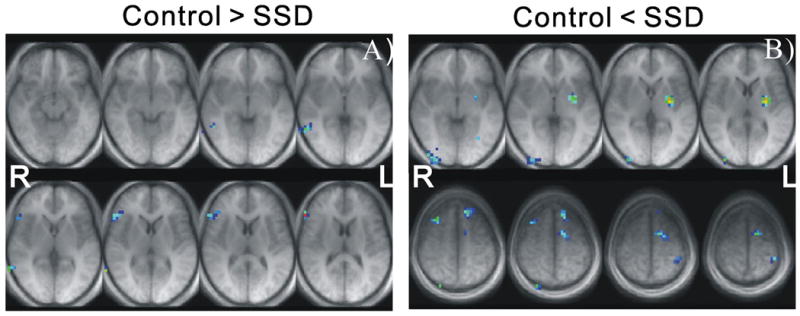
Group differential maps illustrated in transverse AC-PC orientation in Talairach (TLRC) space. (A) Eight consecutive axial slices from TLRC z=-9mm to z=+13mm, demonstrating the hypoactivation identified in the SSD group; (B) Axial slices from TLRC z=-3mm to z=+7mm (top row), and TLRCz = +48 mm to z = +58 mm (bottom row), demonstrating the hyperactivation identified in the SSD group.
Figure 2B presents axial slices demonstrating the primary brain regions of hyperactivation for the SSD group (Control < SSD) during the NWR task. Compared to their TSL peers, the SSD participants demonstrated increased activation in the left supplementary motor area (BA 6), bilateral premotor cortex (BA 6) and left inferior frontal cortex (BA 47). Increased activation for the SSD group was also noted in the left superior temporal pole (BA 38), left anterior cingulate gyrus (BA 24), left ventral post central gyrus (BA 2), left angular gyrus (BA 39) and the left parietal cortex (BA 40) including inferior parietal lobule and supramarginal gyrus. Greater brain activation was also observed for the SSD participants in the frontal eye fields (BA 8) and visual occipital areas (BA 18/19) bilaterally and in the left putamen and hypothalamus. Relative brain activation differences were also noted in the cerebellum. Individuals with a history of SSD exhibited hyperactivation in the declive (i.e. Lobule VI) bilaterally as well as in the right culmen (i.e. Lobules IV and V; see (Schmahmann et al., 1999)) for nomenclature). Table 1 summarizes the coordinates in Talairach (TLRC) space, the corresponding anatomical label of the hypo and hyperactivation regions and their Z scores.
Table 1. Summary of brain activation differences between the SSD and control groups in NWR.
Maximum Z scores and the Talairach (TLC) coordinates (x, y, z) of the primary brain regions of demonstrating hypo and hyperactivation for the SSD group as compared to the TSL controls during NWR. R= Right; L=Left.
| Brain Region | Brodmann Area | Side | Control >SSD
|
Control < SSD
|
||
|---|---|---|---|---|---|---|
| x,y,z | Maxima Z | x,y,z | Maxima Z | |||
| Postcentral Gyrus | BA 2 | L | -52,-26,36 | 2.71 | ||
| R | - | - | ||||
|
| ||||||
| Superior Frontal Gyrus | BA 6 | L | -12,26,52 | 2.36 | ||
| R | - | - | ||||
| BA 8 | L | -16,30,49 | 2.49 | |||
| R | - | - | ||||
|
| ||||||
| Middle Frontal Gyrus | BA 6 | L | -20,-2,58 | 2.20 | ||
| R | 28,14,49 | 2.24 | ||||
| BA 8 | L | - | - | |||
| R | 28,22,45 | 2.52 | ||||
|
| ||||||
| Medial Frontal Gyrus | BA 6 | L | -12,2,55 | 2.11 | ||
| R | - | - | ||||
|
| ||||||
| Inferior Frontal Gyrus | BA 45 | L | - | - | ||
| R | 56,26,13 | 2.99 | ||||
| BA 46 | L | - | - | |||
| R | 56,30,13 | 2.36 | ||||
| BA 47 | L | - | - | -36,18,-19 | 2.51 | |
| R | - | - | - | - | ||
|
| ||||||
| Sub-Gyral Frontal lobe | BA 6 | L | - | - | -20,-6,55 | 2.27 |
| R | - | - | - | - | ||
|
| ||||||
| Superior Temporal Gyrus | BA 38 | L | -36,14,-22 | 2.88 | ||
| R | - | - | ||||
|
| ||||||
| Middle Temporal Gyrus | BA 21/22 | L | - | - | ||
| R | 64,-54,7 | 2.66 | ||||
|
| ||||||
| Angular Gyrus | BA 39 | L | -56,-58,36 | 2.55 | ||
| R | - | - | ||||
|
| ||||||
| Inferior Parietal Lobule | BA 40 | L | -52,-30,39 | 2.51 | ||
| R | - | - | ||||
|
| ||||||
| Supramarginal Gyrus | BA 40 | L | -56, -54,36 | 2.42 | ||
| R | - | - | ||||
|
| ||||||
| Cingulate Gyrus | BA 24 | L | -12,-2,49 | 2.05 | ||
| R | - | - | ||||
|
| ||||||
| Inferior Occipital Gyrus | BA 18/19 | L | -44,-82,12 | 2.33 | ||
| R | 36,-86,-9 | 3.01 | ||||
|
| ||||||
| Cuneus | BA 18 | L | - | - | ||
| R | 28,-94,-2.6 | 2.30 | ||||
|
| ||||||
| Lingual Gyrus | BA 18 | L | - | - | ||
| R | 24,-78,-9 | 2.23 | ||||
|
| ||||||
| Middle Occipital Gyrus | BA 18/19 | L | -48,-78,-9 | 2.11 | ||
| R | 36,-82,-9 | 2.84 | ||||
|
| ||||||
| Putamen | L | -28,-6,4 | 2.97 | |||
| R | - | - | ||||
|
| ||||||
| Hypothalamus | L | -4,-6,-9 | 2.38 | |||
| R | - | - | ||||
|
| ||||||
| Declive | L | -32,-58,-9 | 2.35 | |||
| R | 28,-82,-15 | 2.19 | ||||
|
| ||||||
| Culmen | L | - | - | |||
| R | 24,-42,-15 | 2.31 | ||||
3. Discussion
In the present study, we used fMRI to test the hypothesis of differential activation of brain areas related to PM during a NWR task in individuals with histories SSD compared with typically developing controls. All 6 of the SSD participants evaluated in this fMRI study had been found to perform below the normative mean on standardized tests (i.e. Sentence Repetition of the Test of Language Development-Primary 2nd Edition (TOLD-P:2, (P. Newcomer & Hammill, 1988)), the Test of Language Development-Primary 3rd Edition (TOLD-P:3, (P. Newcomer & Hammill, 1997)) or the Clinical Evaluation of Language Fundamentals-3rd Edition (CELF-3, (Semel, Wiig, & Secord, 1995)) and Digit Span Subtests of the Wechsler Intelligence Scale for Children- 3rd Edition (WISC-III, (Wechsler, 1991)) or the Wechsler Adult Intelligence Scale-Revised (WAIS-R, (Wechsler, 1981)) believed to tap PM (Figure 1B). In addition, the mean percent correct repetition of the SSD fMRI cohort on the Multisyllabic Word (MSW) and Multisyllabic Nonword (MSNW) Repetition (Kamhi, Catts, Mauer, Apel, & Gentry, 1988) neurobehavioral working memory measures was below that of a control group composed of age matched individuals with TSL development (Figure 1C). We sought to ensure that the in-scanner performance was similar between SSD and Control participants on the NWR task so that the potential confound of skill level in interpreting differences in brain activation could be avoided. Therefore we selected short (1-2 syllable) nonwords as stimuli for this fMRI study. The nonword stimuli used were taken from the Word Attack Subtest in Form G of the Reading Mastery Test-Revised (WRMT-R, (Woodcock, 1987)). These the nonwords follow phonetic and structural rules of English and simulate the real-life task of encountering an unknown but real word (Example nonword, zirdent). Differences in the pattern of brain activation associated were identified in overt speech production in SSD individuals as compared to their TSL peers examined in this study. Regions of both hypo and hyperactivation were revealed. The hypoactivation pattern was exclusively right sided and limited to the IFG and middle temporal gyrus (MTG). The hyperactivation pattern was bilateral and diffuse. The region of hypoactivation may suggest a functional impairment. Alternatively, the regions of hyperactivation revealed in this study may reflect the recruitment of compensatory networks to counter dysfunction within the normal phonologic loop, the use of an alternative behavioral strategy, or simply extra cognitive effort or attention. Below we consider these interpretations of our neuroimaging findings.
3.1 Neuroimaging Findings
Hypoactivation
Of particular relevance to this study, was the hypoactivation that was observed in the right IFG in the SSD participants examined. (Chein & Fiez, 2001) conducted an fMRI study designed to identify the neural correlates of the maintenance, encoding, and retrieval processes sub serving verbal working memory. Their findings revealed significant activation of bilateral inferior frontal regions, the supplementary motor area, left premotor cortex and the right dorsolateral prefrontal cortex (BA 9/46) during the maintenance phase of the verbal working memory task. Similarly, (Chen & Desmond, 2005) reported significant activity in bilateral regions of the IFG including BA 47/BA45, BA46 and BA9 as well as in other regions of the brain. Although the verbal stimuli were presented visually in the two aforementioned studies, their findings are still relevant to and compliment those of the present study. Guided by the findings of (Chein & Fiez, 2001; Chen & Desmond, 2005), the hypoactivaiton in the right IFG that we observed in our SSD cohort during the NWR task may suggest that the functional integrity of this component of the phonological loop is compromised in these individuals. This interpretation compliments the neurobehavioral data collected on the SSD participants on standardized measures of PM (Figure 1B) and provides support for our hypothesis.
Hypoactivation was also observed in the right MTG of the SSD participants. Evidence from lesion and imaging studies suggests that the right temporal lobe plays an important role in speech perception (Hickok & Poeppel, 2004). Specifically, a meta-analysis of 82 fMRI studies found that with the exception of the posterior right MTG, all regions of the MTG bilaterally were reliably activated in tasks of passive listening to words and non-words (Indefrey & Levelt, 2004). Hence, the decreased activation in the right MTG revealed for SSD participants may suggest that speech perception, another cognitive process supporting successful NWR, is compromised in these individuals. Notably, the operation of the MRI scanner was suspended during auditory stimulus delivery and subject response, so that the overt repetition was accomplished in the absence of any background noise. Consequently, the reduced activity observed in the right MTG of the SSD individuals could also be associated with the auditory perception of one’s own voice, an important component in self-monitoring in speech production (Indefrey & Levelt, 2004). The addition of a passive listening condition to the experimental paradigm would allow the dissociation between the possible effect of speech perception and the effect that is related to self-monitoring of speech production.
Hyperactivation
The hyperactivation identified in this study in the SSD group was bilateral and diffuse. Of particular interest to this study was the hyperactivation identified in the pre and supplementary motor areas, superior cerebellum and in the left inferior parietal lobe (BA 40), and left supramarginal gyrus and BA 47 of the SSD participants compared to their TSL peers. As noted previously, these brain regions have been implicated to be part of the phonologic loop (Chein & Fiez, 2001; Chen & Desmond, 2005). In particular, the left inferior parietal lobe (BA40) and the supramarginal gyrus have been implicated to support the phonologic store. As such, these findings may suggest that in order to achieve comparable NWR task performance, there is a compensatory enhancement in the activity (i.e. increases in cognitive effort and/or reliance on) of these components of the phonologic loop to counteract dysfunction in others (e.g. right IFG).
The hyperactivity observed in the superior cerebellum could also in part reflect an increased reliance on a compensatory mechanism, namely an increased reliance on error-detection and correction of internal speech. The superior cerebellum has been implicated to play a role in error-driven adjustments of motor commands (Ben-Yehudah, Guediche, & Fiez, 2007; Bohland & Guenther, 2006; Guenther, Ghosh, & Tourville, 2006). Many models of speech production include a monitoring process that detects errors and adjust the planned articulation prior to its execution, a process critical to fluid and error free speech (Levelt, 1999; Postma, 2000). (Desmond, Gabrieli, Wagner, Ginier, & Glover, 1997) incorporated such a cerebellum based monitoring function into a model of verbal working memory.
In addition to being implicated to be a component of the phonologic store, one of the widely-acknowledged roles of the parietal cortex in speech production relates to the integration of somatosensory information. In the Directions into Velocities of Articulation (DIVA) model, the tactile and proprioceptive representations of the articulators (referred to as the somatosensory state map in the DIVA model) are hypothesized to lie along the inferior postcentral gyrus and the somatosensory error map to lie immediately posterior, within the inferior parietal cortex along the anterior supramarginal gyrus (Guenther, 2006).Within this framework, the hyperactivation revealed in the left ventral post-central gyrus (BA 2) and the left parietal cortex (BA 40) including inferior parietal lobe and supramarginal gyrus intimates a greater dependence on the somatosensory feedback control for the SSD group relative to the TSL controls. Interpreted as such, these findings provide additional support for the postulated increased reliance on the error-detection and correction of internal speech by the SSD individuals for the successful performance of the NWR task.
In addition, the cerebellum has been implicated as an internal timing device (Ben-Yehudah et al., 2007; Bohland & Guenther, 2006; R. Ivry, 1997; R. B. Ivry & Spencer, 2004). As such, based on the review of neuropsychological and neuroimaging studies, (Ackermann, Mathiak, & Ivry, 2004) postulated that the cerebellum plays a critical role in the temporal organization of internal speech. Hence, the increased cerebellar activation observed in this study in the SSD participants could in part reflect a greater reliance on or cognitive effort to meet the timing demands of articulatory rehearsal system to compensate for dysfunction in other components (e.g. IFG) of the phonologic loop.
The observed hyperactivation might also indicate the adoption of an alternative strategy as opposed to simply suggesting extra cognitive effort. Of particular interest, was the increased activation that was observed in the left angular gyrus (BA39). This increased activity may suggest that the SSD individuals are less efficient in processing nonwords. The results of both lesion (Hart & Gordon, 1990) and neuroimaging (Nakai et al., 1999; Newman & Twieg, 2001) studies implicate the involvement of the angular gyrus in semantic processing. Thus, as a consequence of poorly formed and unstable underlying phonological representations, this finding may suggest that SSD participants are adopting a strategy (i.e. searching their lexicon) typically used for processing real words, to process nonwords. Alternatively, (S. E. Gathercole, 1995) demonstrated that repetition of nonword-like nonwords most heavily tapped PM processes, while repetition of wordlike items was also influenced by long-term lexical knowledge. Hence, the increased activity observed in the left angular gyrus (BA39) of the SSD participants could also be interpreted to simply reflect increased cognitive effort in searching their lexicon for the processing of the “wordlike” nonword stimuli in these individuals.
3.2 Relation to previous fMRI studies of SSD
Our findings are somewhat discrepant from those of (Liegeois et al., 2003) who conducted a neuroimaging study to examine language in an extended family known as the KE family in which half of the members are affected by a severe inherited speech and language disorder characterized by verbal dyspraxia resulting from a mutation of the FOXP2 gene. In their study, an overt real word repetition fMRI experiment revealed underactivation in the left inferior frontal gyrus, pars triangularis in the affected as compared to unaffected family members. Significant over activation was observed only in the left anterior insular cortex. These findings are discrepant with those for the SSD cohort examined in the present study. This is not surprising as there are significant differences in the phenotype of the KE family (i.e. verbal and oral facial dyspraxia, dysmorphology, cognitive deficits), the underlying etiology of the disorder (i.e. FOXP2 gene variant), the persistence of the disorder in the KE family despite therapy and the presence of co-morbid conditions. In addition, the studies differed in the specific fMRI task and analysis employed.
3.3 Critique of Current Methodology and Future Directions
To our knowledge, this is the first fMRI study of the neural correlates of a speech-based phonological processing task in a cohort of unrelated adolescents with a history of an SSD. The fMRI methodology employed in this study was effective in demonstrating differences in the brain networks supporting phonologic processing associated with speech production in SSD individuals as compared to their TSL peers. Nevertheless, several methodological limitations were identified for this initial fMRI investigation of speech production in SSD. For example, the experimental paradigm used in this fMRI study did not include a passive listening condition. Consequently, it was not possible to differentiate brain activation that relates to the encoding of auditory stimulus from that due to the production of the spoken response. Future studies employing the overt NWR task should include a passive listening condition so that the two can be distinguished.
The individuals with a history of SSD who particpated in this fMRI experiment were adolescents who had been enrolled in a large on-going longitudinal study upon diagnosis at age 4-7 years. Hence, compensatory mechanisms reflecting speech therapy or natural recovery may also contribute to the functional abnormalities observed in the neural networks supporting speech production in SSD. This speculation is supported by the fact that all but one of the SSD participants enrolled in this imaging study demonstrated abnormal FMRI findings, but no longer displayed speech production deficits. A number of plausible compensatory mechanisms consistent with the brain activation findings of this study were presented. However, within the constraints of the current study, the “compensatory mechanism” interpretation is difficult to test and verify. With this in mind, future fMRI studies should be designed to examine the development of these networks longitudinally, from an early age to explore the origins of both dysfunctional and compensatory brain systems.
The exact etiology of SSD remains unknown. Neurobehavioral data suggests that a deficit in PM may contribute to the disorder. In this study, we used an event-related HUSH fMRI paradigm in combination with an overt NWR task to identify functional differences in the neural substrates underlying the speech production impairment for SSD that would support our hypothesis that PM is impaired in this population. Our data demonstrated that this methodology was effective in identifying differences in the brain networks supporting phonologic processing associated with speech production in SSD individuals as compared to their TSL peers. The fact that these findings may be interpreted to support the hypothesis that individuals with SSD may have a deficit in PM and to suggest the involvement of compensatory mechanisms to counteract dysfunction of the normal network was not totally unexpected for the age range of the participants evaluated in this study.
4. Methods
4.1 Participants
The participants (Mean(SD) = 17(2.6) years) were 6 right-handed adolescents with history of early childhood SSD and 7 age matched controls (Mean(SD) = 18(3.1) years) with no history of speech and language disorders. The individuals (5 male) with a history of moderate to severe SSD were participants in a large longitudinal ongoing study of SSD. Individuals with SSD had been enrolled in speech-language therapy as children and were required to have: (1) normal hearing (2) normal intelligence as defined by a prorated Performance IQ of at least 80 (3) normal peripheral speech mechanism (Robbins & Klee, 1987), (4) moderate-severe speech-sound production deficits in single words as sampled in the Goldman-Fristoe Test of Articulation (GFTA, (Goldman & Fristoe, 1986)) and the Khan-Lewis Phonological Analysis (KLPA, (Kahn & Lewis, 1986)) including four phonological process errors.
All participants with SSD completed neurobehavioral speech and language testing and developmental history questionnaires following their initial diagnosis at early childhood (ages 4-7 years) and were followed longitudinally with the most recent assessment occurring 0.5-2 years prior to the neuroimaging study. As shown in Figure 1A, participants completed tests assessing Performance IQ, receptive and expressive language and reading at three time points (early childhood, school-age, and adolescence). Performance IQ was assessed using the age-appropriate version of the Wechsler Preschool and Primary Scale of Intelligence-Revised (WPPSI-R, (Wechsler, 1989)), WISC-III (Wechsler, 1991) or Wechsler Adult Intelligence Scale-Revised (WAIS-R, (Wechsler, 1981)). Language was evaluated using the age-appropriate version of the TOLD-P:2 (P. Newcomer & Hammill, 1988)), TOLD-P:3 (P. Newcomer & Hammill, 1997) or CELF-3 (Semel et al., 1995). Reading was assessed using the Word Identification and Word Attack subtests of the Woodcock Reading Mastery Test-Revised (WRMT-R, (Woodcock, 1987)). Articulation was also assessed at the same three time points using the GFTA (Goldman & Fristoe, 1986). Five out of the 6 SSD participants achieved normal adult articulation with no errors by neurobehavioral testing at school-age. In addition, PM was examined at each of the three time points by the Digit Span Subtest of the WISC-III (Wechsler, 1991) or WAIS-R (Wechsler, 1989) and the Sentence Repetition Subtest of the TOLD-P:2 (P. Newcomer & Hammill, 1988), the TOLD-P:3 (P. Newcomer & Hammill, 1997) or the CELF-3 (Semel et al., 1995) (Figure 2A). PM was also assessed at each of the three time points by the MSW and the MSNW Repetition task and compared to the performance on this task of TSL (Figure 1C) (Kamhi et al., 1988). All but one (a male) of the SSD participants made no speech errors in conversation at the time of the fMRI study. The 7 right handed control individuals were matched to the participants with SSD for age and gender and reported no history of speech, language, or reading disorders. Participants completed a laterality questionnaire to insure that they were right handed. None of the participants had a history of developmental disorder other than speech and language delay.
4.2 Stimuli and Design
The stimuli were composed of 6 monosyllabic and 4 bi-syllabic (bim, plip, quiles, roo, shab, tweb, bufty, sigbet, zirdent, vunhip) non-words taken from the Word Attack Subtest in Form G of the WRMT-R (Woodcock, 1987). These words were chosen because of their high word-likedness. All stimuli were professionally recorded at a digitized sampling rate of 22.05 Hz and had an average duration of 736 ms with a standard deviation of 82.5 ms.
The fMRI experiment consisted of an overt NWR task and a baseline condition in which the participant rested quietly. Trials corresponding to each condition had a fixed duration of 12 s. Ten repetition trials alternated with ten baseline trials, leading to a duration of 20 × (12 s/trial) = 4 minutes for the functional run.
Each repetition trial began with the auditory presentation of one non-word stimulus. Participants were instructed to repeat the stimulus non-word aloud immediately following the completion of the stimulus presentation and then to rest quietly. The word “SAY” was displayed as visual instruction during the 6s interval of stimulus presentation/repeat and was then replaced with the word “REST” during the data acquisition that began 6 seconds after the onset of the stimulus presentation. In a baseline trial, no auditory stimuli were presented. Participants were asked to rest quietly and the visual instruction “REST” was displayed throughout the trial.
The auditory stimuli were transmitted with equalized sound spectrum through an MRI compatible audio system (Avotec SS3100) with acoustically padded headphones to reduce fMRI acoustic noise by ~ 30dB. (Silent Scan; Avotec, Stuart FL, USA). The presentation of both auditory stimuli and the visual instruction was controlled by a paradigm implemented in E-Prime 1.2 (Psychology Software Tools, Pittsburgh, PA, USA). Using output from a microphone built into the headphones, verbal responses were scored online as correct or incorrect and audiotape recorded for later review.
4.3 Data Acquisition
All MRI scanning was performed on a 4 Tesla Bruker/Siemens Whole Body Medspec MR system using an 8 channel transmit/receive head coil. Corresponding to the experiment design, Blood Oxygen Level Dependent (BOLD) fMRI data were obtained using a clustered acquisition paradigm named HUSH(Schmithorst & Holland, 2004). In each trial, the operation of the scanner was suspended and no data was acquired during the first 6s, allowing the auditory stimulus presentation and overt response to occur in completely silent gradient intervals. Three consecutive whole-head volumes [time of repetition (TR)=2s] were then acquired beginning 6s after trial onset. The functional acquisitions were timed to include the peak of the hemodynamic response associated with speech production, which has previously been estimated to occur 4-7 s after the onset of articulation (Birn, Bandettini, Cox, Jesmanowicz, & Shaker, 1998; Birn, Cox, & Bandettini, 2004) (Engelien et al., 2002). A time efficient fMRI paradigm, HUSH has a fixed inter-cluster-interval (ICI) of 12s, which is sufficiently long to allow the hemodynamic response associated with the speech production to decay to baseline prior to the control data acquisition. This HUSH paradigm offered several advantages over traditional block and event-related methods: (a) the task was performed in a more natural, quiet-speaking environment, (b) BOLD responses in auditory areas were not compromised by the imaging noise, (c) artifacts due to head movement and changing oral cavity volumes were avoided, and (d) monitoring participant responses for proper pronunciation of the stimuli was facilitated.
Functional volumes were acquired using a single shot gradient-echo echo planar imaging (EPI) sequence. Each volume consisted of 44 contiguous 3mm T2*-weighted slices covering the entire brain [TR = 2 s, time of echo (TE) = 20 ms, flip angle = 76.8°, field of view (FOV) = 240×240 mm, matrix 64×64]. Slices were oriented parallel to an imaginary plane passing through the anterior and posterior commissures and perpendicular to the sagittal midline and had a spatial resolution of 3.75 × 3.75 × 3 mm.
A total of 63 fMRI volumes were collected, including 60 volumes from the 20 trials (10 repetition trials alternated with 10 baselines) plus 3 dummy scans collected at the beginning of the run. Prior to the fMRI run, a high-resolution T1-weighted 3D data set was acquired to aid in the localization of functional data. A 3D MPRAGE sequence was used to acquire the T1 volume with the following parameters: TR = 2.5 s, time of inversion (TI) = 1.1 s, TE = 3.52 ms, flip angle = 12°, FOV= 256 ×192 × 176mm and matrix = 256×192×176, resulting in a 3D spatial resolution of 1 × 1 × 1 mm.
4.4 Data Analysis
4.4.1 Verbal response
For each participant, verbal responses to the stimulus non-words were scored online as correct or incorrect and the repetition accuracy was calculated as the percentage of correct repetitions. Group comparison of repetition accuracy was made using the Wilcoxon rank sum test.
4.4.2 FMRI data
For each participant, a multi-echo reference scan was initially used to correct for Nyquist ghosts and geometric distortion due to B0 field in homogeneity during image reconstruction (Schmithorst, Dardzinski, & Holland, 2001). The first 3 volumes were eliminated and the fMRI data were corrected for motion using a pyramid iterative co-registration algorithm (Thevenaz, Ruttimann, & Unser, 1998). All data sets met the criterion of median voxel displacement at the center of the brain <2 mm. The data were subsequently spatially smoothed using a Gaussian filter with Full Width Half Maximum (FWHM) of 4 mm. Because of the low sampling frequency in HUSH, temporal low-pass filtering was not applied. The corrected volumes were then grouped according to 1st, 2nd, or 3rd volume of each data acquisition period, and further subdivided into rest and task subsets. A paired t-test was computed on a voxel wise basis between the condition pair. Because the two points in each pair are only 12 s apart, low frequency drift removal was not applied.
Images from individual participants were spatially normalized to Talairach (TLRC) space and then entered into a second level analysis to investigate the group difference between the SSD and TSL controls. A general linear model (GLM) was used in the second level analysis to generate a random effect group differential map and the threshold was set at Z=2.0 (uncorrected p=0.023) and limited to clusters of size greater than or equal to 5 contiguous voxels. All image processing was completed using Cincinnati Children’s Hospital Image Processing Software abbreviated as CCHIPS© (http://irc.cchmc.org/software/cchips.php). Anatomical location of the local brain activation maxima was determined by means of the software tool Talairach Daemon using the nearest grey matter option (http://www.talairach.org/).
Acknowledgments
We thank Drs. H. Gerry Taylor and Lawrence D. Shriberg for their helpful comments. We would also like to extend our sincere gratitude to Mr. John Jesberger for his assistance in data collection and Dr. Prasana Karunanayaka and Ms. Akila Rajagopal for their technical support in data analysis. This research was supported in part by the National Institutes on Deafness and Other Communication Disorders (NIDCD) Grant DC00528 (Barbara Lewis, Ph.D, Principal Investigator).
References
- Ackermann H, Mathiak K, Ivry RB. Temporal organization of “internal speech” as a basis for cerebellar modulation of cognitive functions. Behav Cogn Neurosci Rev. 2004;3(1):14–22. doi: 10.1177/1534582304263251. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working Memory. Oxford: Claredon; 1986. [Google Scholar]
- Baddeley A. Working memory. Science. 1992;255(5044):556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory and language: an overview. J Commun Disord. 2003;36(3):189–208. doi: 10.1016/s0021-9924(03)00019-4. [DOI] [PubMed] [Google Scholar]
- Ben-Yehudah G, Guediche S, Fiez JA. Cerebellar contributions to verbal working memory: beyond cognitive theory. Cerebellum. 2007;6(3):193–201. doi: 10.1080/14734220701286195. [DOI] [PubMed] [Google Scholar]
- Birn RM, Bandettini PA, Cox RW, Jesmanowicz A, Shaker R. Magnetic field changes in the human brain due to swallowing or speaking. Magn Reson Med. 1998;40(1):55–60. doi: 10.1002/mrm.1910400108. [DOI] [PubMed] [Google Scholar]
- Birn RM, Cox RW, Bandettini PA. Experimental designs and processing strategies for fMRI studies involving overt verbal responses. Neuroimage. 2004;23(3):1046–1058. doi: 10.1016/j.neuroimage.2004.07.039. [DOI] [PubMed] [Google Scholar]
- Bishop DV, North T, Donlan C. Nonword repetition as a behavioural marker for inherited language impairment: evidence from a twin study. J Child Psychol Psychiatry. 1996;37(4):391–403. doi: 10.1111/j.1469-7610.1996.tb01420.x. [DOI] [PubMed] [Google Scholar]
- Bohland JW, Guenther FH. An fMRI investigation of syllable sequence production. Neuroimage. 2006;32(2):821–841. doi: 10.1016/j.neuroimage.2006.04.173. [DOI] [PubMed] [Google Scholar]
- Chein JM, Fiez JA. Dissociation of verbal working memory system components using a delayed serial recall task. Cereb Cortex. 2001;11(11):1003–1014. doi: 10.1093/cercor/11.11.1003. [DOI] [PubMed] [Google Scholar]
- Chen SH, Desmond JE. Cerebrocerebellar networks during articulatory rehearsal and verbal working memory tasks. Neuroimage. 2005;24(2):332–338. doi: 10.1016/j.neuroimage.2004.08.032. [DOI] [PubMed] [Google Scholar]
- Coady JA, Evans JL. Uses and interpretations of non-word repetition tasks in children with and without specific language impairments (SLI) Int J Lang Commun Disord. 2008;43(1):1–40. doi: 10.1080/13682820601116485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond JE, Gabrieli JD, Wagner AD, Ginier BL, Glover GH. Lobular patterns of cerebellar activation in verbal working-memory and finger-tapping tasks as revealed by functional MRI. J Neurosci. 1997;17(24):9675–9685. doi: 10.1523/JNEUROSCI.17-24-09675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelien A, Yang Y, Engelien W, Zonana J, Stern E, Silbersweig DA. Physiological mapping of human auditory cortices with a silent event-related fMRI technique. Neuroimage. 2002;16(4):944–953. doi: 10.1006/nimg.2002.1149. [DOI] [PubMed] [Google Scholar]
- Gathercole SE. Is nonword repetition a test of phonological memory or long-term knowledge? It all depends on the nonwords. Mem Cognit. 1995;23(1):83–94. doi: 10.3758/bf03210559. [DOI] [PubMed] [Google Scholar]
- Gathercole SE, Baddeley AD. Evaluation of the role of phonological STM in the development of vocabulary in children: A longitudinal study. Journal of Memory and Language. 1989;28(2):200–213. [Google Scholar]
- Gathercole SE, Baddeley AD. Phonological memory deficits in language disordered children: Is there a causal connection? Journal of Memory and Language. 1990;29(3):336–360. [Google Scholar]
- Goldman R, Fristoe M. The Goldman-Fristoe Test of Articulation (GFTA) Circle Pines, MN: American Guidance Services, Inc; 1986. [Google Scholar]
- Graf Estes K, Evans JL, Else-Quest NM. Differences in the nonword repetition performance of children with and without specific language impairment: a meta-analysis. J Speech Lang Hear Res. 2007;50(1):177–195. doi: 10.1044/1092-4388(2007/015). [DOI] [PubMed] [Google Scholar]
- Guenther FH. Cortical interactions underlying the production of speech sounds. J Commun Disord. 2006;39(5):350–365. doi: 10.1016/j.jcomdis.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Guenther FH, Ghosh SS, Tourville JA. Neural modeling and imaging of the cortical interactions underlying syllable production. Brain Lang. 2006;96(3):280–301. doi: 10.1016/j.bandl.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart J, Jr, Gordon B. Delineation of single-word semantic comprehension deficits in aphasia, with anatomical correlation. Ann Neurol. 1990;27(3):226–231. doi: 10.1002/ana.410270303. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92(1-2):67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- IDEA. U.S Department of Education, Office of Special Education Programs, Data Analysis System (DANS), OMB #1820-0043: “Children with Disabilities Receiving Special Education Under Part B of the Individuals with Disabilities Education Act,” 2008 Data updated as of August 3, 2009 [Google Scholar]
- Indefrey P, Levelt WJ. The spatial and temporal signatures of word production components. Cognition. 2004;92(1-2):101–144. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- Ivry R. Cerebellar timing systems. Int Rev Neurobiol. 1997;41:555–573. [PubMed] [Google Scholar]
- Ivry RB, Spencer RM. The neural representation of time. Curr Opin Neurobiol. 2004;14(2):225–232. doi: 10.1016/j.conb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Kahn L, Lewis N. Khan-Lewis Phonological Analysis (KLPA) American Guidance Services, Inc.; Circle Pines, MN: 1986. [Google Scholar]
- Kamhi AG, Catts HW, Mauer D, Apel K, Gentry BF. Phonological and spatial processing abilities in language- and reading-impaired children. J Speech Hear Disord. 1988;53(3):316–327. doi: 10.1044/jshd.5303.316. [DOI] [PubMed] [Google Scholar]
- Kenney MK, Barac-Cikoja D, Finnegan K, Jeffries N, Ludlow CL. Speech perception and short-term memory deficits in persistent developmental speech disorder. Brain Lang. 2006;96(2):178–190. doi: 10.1016/j.bandl.2005.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levelt WJ. Models of word production. Trends Cogn Sci. 1999;3(6):223–232. doi: 10.1016/s1364-6613(99)01319-4. [DOI] [PubMed] [Google Scholar]
- Lewis BA, Freebairn LA, Hansen AJ, Miscimarra L, Iyengar SK, Taylor HG. Speech and language skills of parents of children with speech sound disorders. Am J Speech Lang Pathol. 2007;16(2):108–118. doi: 10.1044/1058-0360(2007/015). [DOI] [PubMed] [Google Scholar]
- Lewis BA, Freebairn LA, Taylor HG. Follow-up of children with early expressive phonology disorders. J Learn Disabil. 2000;33(5):433–444. doi: 10.1177/002221940003300504. [DOI] [PubMed] [Google Scholar]
- Liegeois F, Baldeweg T, Connelly A, Gadian DG, Mishkin M, Vargha-Khadem F. Language fMRI abnormalities associated with FOXP2 gene mutation. Nat Neurosci. 2003;6(11):1230–1237. doi: 10.1038/nn1138. [DOI] [PubMed] [Google Scholar]
- McGrath LM, Pennington BF, Willcutt EG, Boada R, Shriberg LD, Smith SD. Gene x Environment interactions in speech sound disorder predict language and preliteracy outcomes. Dev Psychopathol. 2007;19(4):1047–1072. doi: 10.1017/S0954579407000533. [DOI] [PubMed] [Google Scholar]
- Nakai T, Matsuo K, Kato C, Matsuzawa M, Okada T, Glover GH, et al. A functional magnetic resonance imaging study of listening comprehension of languages in human at 3 tesla-comprehension level and activation of the language areas. Neurosci Lett. 1999;263(1):33–36. doi: 10.1016/s0304-3940(99)00103-2. [DOI] [PubMed] [Google Scholar]
- Newcomer P, Hammill D. Test of Language Development - Primary Second Edition (TOLD-P2) Austin, TX: PRO-ED, Inc; 1988. [Google Scholar]
- Newcomer P, Hammill D. Test of Language Development - Primary Third Edition (TOLD-P3) Austin, TX: PRO-ED, Inc; 1997. [Google Scholar]
- Newman SD, Twieg D. Differences in auditory processing of words and pseudowords: an fMRI study. Hum Brain Mapp. 2001;14(1):39–47. doi: 10.1002/hbm.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington BF, Bishop DV. Relations among speech, language, and reading disorders. Annu Rev Psychol. 2009;60:283–306. doi: 10.1146/annurev.psych.60.110707.163548. [DOI] [PubMed] [Google Scholar]
- Peterson RL, McGrath LM, Smith SD, Pennington BF. Neuropsychology and genetics of speech, language, and literacy disorders. Pediatr Clin North Am. 2007;54(3):543–561. vii. doi: 10.1016/j.pcl.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Postma A. Detection of errors during speech production: a review of speech monitoring models. Cognition. 2000;77(2):97–132. doi: 10.1016/s0010-0277(00)00090-1. [DOI] [PubMed] [Google Scholar]
- Robbins J, Klee T. Clinical assessment of oropharyngeal motor development in young children. J Speech Hear Disord. 1987;52(3):271–277. doi: 10.1044/jshd.5203.271. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Doyon J, McDonald D, Holmes C, Lavoie K, Hurwitz AS, et al. Three-dimensional MRI atlas of the human cerebellum in proportional stereotaxic space. Neuroimage. 1999;10(3 Pt 1):233–260. doi: 10.1006/nimg.1999.0459. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Dardzinski BJ, Holland SK. Simultaneous correction of ghost and geometric distortion artifacts in EPI using a multiecho reference scan. IEEE Trans Med Imaging. 2001;20(6):535–539. doi: 10.1109/42.929619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK. Event-related fMRI technique for auditory processing with hemodynamics unrelated to acoustic gradient noise. Magn Reson Med. 2004;51(2):399–402. doi: 10.1002/mrm.10706. [DOI] [PubMed] [Google Scholar]
- Semel E, Wiig EH, Secord WA. Clinical Evaluation of Language Fundamentals -Third Edition (CELF-3) Pearson, Psychological Corporation; San Antonio, TX: 1995. [Google Scholar]
- Thevenaz P, Ruttimann UE, Unser M. A pyramid approach to subpixel registration based on intensity. Image Processing, IEEE Transactions on. 1998;7(1):27–41. doi: 10.1109/83.650848. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler D Wechsler Adult Intelligence Scale - Revised (WAIS-R) San Antonio, TX: The Psychological Corporation; 1981. [Google Scholar]
- Wechsler D. Wechsler Preschool and Primary Scale of Intelligence-Revised (WPPSI-R) The Psychological Corporation; San Antonio, TX: 1989. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children- Third Edition (WISC-III) The Psychological Corporation; San Antonio, TX: 1991. [Google Scholar]
- Woodcock R. Woodcock Reading Mastery Test-Revised (WRMT-R) American Guidance Services, Inc.; Cricle Pines, MN: 1987. [Google Scholar]


