Abstract
A sucrose (Suc) transporter cDNA has been cloned from Alonsoa meridionalis, a member of the Scrophulariaceae. This plant species has an open minor vein configuration and translocates mainly raffinose and stachyose in addition to Suc in the phloem (C. Knop, O. Voitsekhovskaja, G. Lohaus [2001] Planta 213: 80–91). These are typical properties of symplastic phloem loaders. For functional characterization, AmSUT1 cDNA was expressed in bakers' yeast (Saccharomyces cerevisiae). Substrate and inhibitor specificities, energy dependence, and Km value of the protein agree well with the properties measured for other Suc transporters of apoplastic phloem loaders. A polyclonal antiserum against the 17 N-terminal amino acids of the A. meridionalis Suc transporter AmSUT1 was used to determine the cellular localization of the AmSUT1 protein. Using fluorescence labeling on sections from A. meridionalis leaves and stems, AmSUT1 was localized exclusively in phloem cells. Further histological characterization identified these cells as companion cells and sieve elements. p-Chloromercuribenzenesulfonic acid affected the sugar exudation of cut leaves in such a way that the exudation rates of Suc and hexoses decreased, whereas those of raffinose and stachyose increased. The data presented indicate that phloem loading of Suc and retrieval of Suc in A. meridionalis are at least partly mediated by the activity of AmSUT1 in addition to symplastic phloem loading.
Assimilates produced during photosynthesis in mature leaves are distributed by the phloem system to support the growth of heterotrophic organs. The flow of assimilates such as Suc starts with symplastic cell-to-cell transport through mesophyll cells and bundle sheath cells, after which they are loaded into the sieve element (SE)-companion cell (CC) complex.
Plasmodesmal frequencies between the SE-CC complex and the adjacent cells in minor veins have been studied in many plant species. Gamalei (1989) defined two types of minor veins, an open type 1 with numerous plasmodesmal connections between the SE-CC complex and the adjacent cells and a closed type 2 with a reduced number of plasmodesmal connections. The minor vein phloem of angiosperms is composed of phloem parenchyma cells, SEs, and CCs. In type 2 species, the CCs are often specialized as transfer cells. These are defined as cells with cell wall ingrowths and an enlarged plasma membrane surface area (Gunning and Pate, 1969) increasing the capacity to take up photo-assimilates from the apoplast. On the other hand, minor veins of type 1 species often contain two types of CCs: two large, peripherally located intermediary cells and also some smaller, usually more internal, ordinary CCs (Turgeon et al., 1975, 1993; Fisher, 1986; Hoffmann-Thoma et al., 2001). This is also the case for Alonsoa meridionalis (Knop et al., 2001). Intermediary cells contain enzymes for the synthesis of raffinose oligosaccharides (α 1–6 galactosyln-Suc) in Cucumis melo, Coleus blumei, and Ajuga reptans (Holthaus and Schmitz, 1991; Turgeon and Gowan, 1992; Sprenger and Keller, 2000), and those plants translocate these sugars in addition to Suc.
The minor vein structure is correlated with the mode of phloem loading. Based on the plasmodesmal connections between the phloem and the surrounding cells and the presence of intermediary cells, transfer cells, or ordinary CCs, two types of phloem loading have been proposed: the apoplastic (Giaquinta, 1983) and the symplastic (Turgeon, 1991) phloem loading mode.
The apoplastic phloem loading mode is based on: (a) the steep uphill Suc gradient between the phloem sap and the cytosol of the surrounding cells (Geiger et al., 1973; Riens et al., 1991; Lohaus et al., 1995), (b) the relative symplastic isolation of the SE-CC complex from adjacent cells, (c) the active Suc transport at the plasma membrane of the SE-CC complex (Sovonick et al., 1974; Komor et al., 1977; Delrot, 1981), and (d) the inhibition of Suc export by p-chloromercuribenzenesulfonic acid (PCMBS) treatment (Giaquinta, 1983; Turgeon and Gowan, 1990; van Bel et al., 1992, 1994). PCMBS acts as a membrane-impermeable SH modifier in the leaf apoplast and impairs the Suc transporters involved in apoplastic phloem loading (Giaquinta, 1983).
In apoplastic phloem loaders, the long-distance transport form of carbohydrates is almost exclusively Suc. In the last decade, several genes of the proton-coupled Suc uptake transporter (SUT) family have been cloned and characterized from apoplastic phloem loaders (Riesmeier et al., 1992; Gahrtz et al., 1994; Williams et al., 2000). Expressed in bakers' yeast (Saccharomyces cerevisiae), they have been shown to catalyze the energy-dependent uptake of Suc across yeast plasma membranes. In several plant species, such Suc transporters are localized in CCs (Stadler et al., 1995; Stadler and Sauer, 1996) or in SEs (Kühn et al., 1997; Barker et al., 2000; Weise et al., 2000) of the phloem.
In symplastic phloem loaders, such as members of the Cucurbitaceae, Lamiaceae, or several species of the Scrophulariaceae, carbohydrates are translocated in form of raffinose and stachyose (Zimmermann and Ziegler, 1975). However, Suc is also still present in the sieve tube sap at a significant level (Knop et al., 2001). To explain symplastic uphill transport of assimilates into the SE-CC complex, a “polymer trap model” has been proposed by Turgeon (1991). According to this model, synthesis of raffinose oligosaccharides may take place within the intermediary cells from mesophyll-derived galactinol and Suc. These precursors are thought to be delivered to the intermediary cells via the plasmodesmata between these cells and the mesophyll. It is assumed that raffinose and stachyose are too large to diffuse back due to the size exclusion limit of the mesophyll cell-intermediary cell plasmodesmata. As a result, they can only diffuse through the plasmodesmata between intermediary cells and SEs that have a larger size exclusion limit; this causes an accumulation of raffinose and stachyose in the phloem cells. So far, however, no proof for the plasmodesmal ability to discriminate between Suc (0.34 kD) and raffinose (0.50 kD) has been provided.
Until now, the existence of Suc transporters has been much less investigated in plant species with open minor vein configuration than in those with closed minor vein configuration. Therefore, we analyzed a member of the Scrophulariaceae, A. meridionalis. In a previous study, we have shown that the minor vein phloem of this species contains intermediary cells and some ordinary CCs (Knop et al., 2001). Raffinose oligosaccharides were shown to be the main carbohydrate transport form with an aggregated concentration of raffinose and stachyose in the phloem sap of about 600 mm (Knop et al., 2001). These properties mark A. meridionalis as a typical symplastic phloem loader. Surprisingly, we were still able to isolate Suc transporter cDNAs from leaves and phloem sap of this plant species (Knop et al., 2001), although Suc transporters should not be involved in phloem loading in symplastic phloem loaders.
The aim of the present study was to elucidate the functional properties of the Suc transporter from A. meridionalis (AmSUT1) by expression of the AmSUT1 cDNA in yeast. Furthermore, we studied the localization of the AmSUT1 protein with polyclonal antisera. The protein could be localized in the SEs and CCs of the collection and transport phloem of A. meridionalis. Therefore, the functions of the Suc transporter in this putative symplastic phloem loader will be discussed.
RESULTS
Characterization of AmSUT1 in Yeast
Previous physiological studies indicated that A. meridionalis is a putative symplastic phloem loader (Knop et al., 2001). However, we were able to clone a Suc transporter cDNA from source leaves of this plant species. The clone encodes an open reading frame of 1,506 bp that corresponds to a protein of 502 amino acids and a calculated molecular mass of 52.3 kD (Knop et al., 2001). The sequence of AmSUT1 used in this study slightly differs from the original sequence (Knop et al., 2001), which turned out to contain several PCR artifacts. The sequence has now been updated in the GenBank (accession no. AF191025) and shows 96.4% identity on the amino acid level to the first sequence.
For functional analysis and for studies of the kinetic parameters, AmSUT1 was expressed in yeast. The AmSUT1 cDNA was cloned into the Escherichia coli/yeast shuttle vector pNEV-E in sense or antisense orientation downstream of the strong promoter of the PMA1 plasma membrane ATPase from yeast and transformed into the yeast strain DBY2617 (Kaiser and Botstein, 1986).
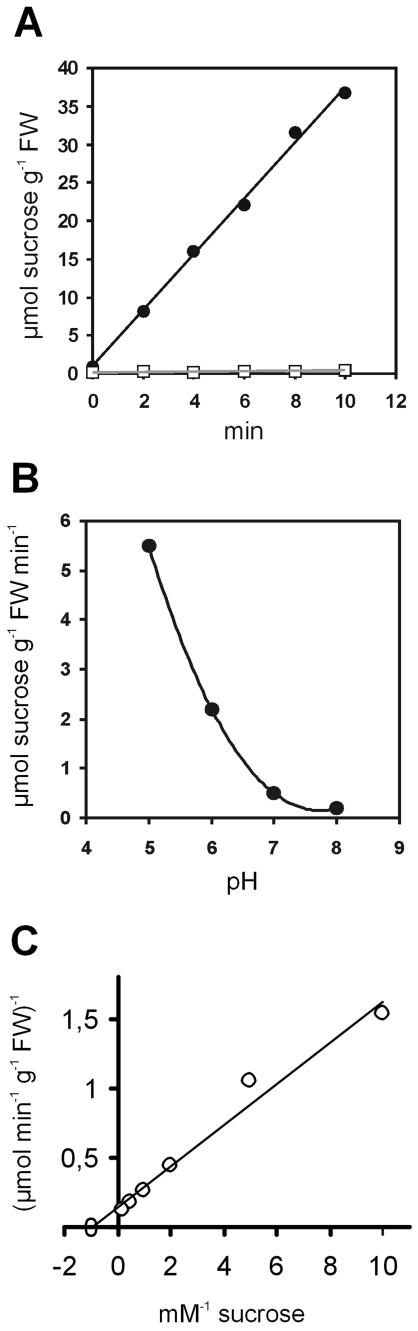
Yeast cells harboring the sense construct (CKY-Am1s) transported 14C-Suc at high rates, whereas the Suc transport into yeast cells harboring the antisense construct (CKY-Am1as) was negligible (Fig. 1A). These results indicate that the AmSUT1 protein is a transporter for Suc. The pH dependence of Suc transport by AmSUT1 was determined in the range of pH 5 to 8 (Fig. 1B). Transport rates increased continuously with decreasing external pH. At pH 8, AmSUT1 was almost completely inactive. Kinetic analysis of 14C-Suc uptake by yeast cells expressing AmSUT1 revealed an apparent Km value for Suc of 1.8 mm at pH 5.5 (Fig. 1C).
Figure 1.
Kinetic analysis of 14C-Suc uptake by transgenic yeast cells. A, Uptake of Suc into yeast cells transformed with AmSUT1 in sense orientation (yeast strain CKY-Am1s; •) and Suc uptake into control cells transformed with AmSUT1 in antisense orientation (yeast strain CKY-Am1as; □). Transport assays were performed with an initial outside Suc concentration of 1 mm at pH 5.5. B, pH dependence of Suc uptake by yeast cells expressing AmSUT1 (yeast strain CKY-Am1s). C, Lineweaver-Burk plot of Suc uptake as a function of concentration in yeast cells expressing AmSUT1 (yeast strain CKY-Am1s). Reciprocal uptake rates are plotted against reciprocal Suc concentrations in the assays.
To determine the substrate specificity of AmSUT1, transport of 14C-labeled Suc was studied in the 10-fold excess of other sugars that might be potential substrates for this transporter like raffinose and stachyose because these are the predominant translocated sugars in A. meridionalis. The only sugar competing with Suc uptake was maltose, whereas the trisaccharide raffinose and the tetrasaccharide stachyose had no effect on the transport rate (Table I).
Table I.
Uptake of 1 mm14C-labeled Suc in the absence or presence of various compounds by yeast cells (CKY-Am1s) expressing AmSUT1
Shown are mean values of n = 3 to 4 independent measurements ± sd.
| Compound Added | Residual Transport Rate |
|---|---|
| % | |
| None | 100 |
| 10 mm Maltose | 45 ± 9 |
| 10 mm Raffinose | 99 ± 3 |
| 10 mm Stachyose | 96 ± 10 |
| 50 μmm-Chlorophenyl hydrazone (CCCP) | 12 ± 6 |
| 25 μm PCMBS | 81 ± 11 |
| 50 μm PCMBS | 51 ± 7 |
| 100 μm PCMBS | 19 ± 5 |
| 250 μm PCMBS | 7 ± 1 |
14C-Suc uptake via AmSUT1 was almost completely inhibited by the protonophore carbonyl cyanide CCCP (Table I). These data are consistent with a proton-coupled Suc uptake mechanism. All Suc transporters from higher plants characterized so far are highly sensitive to the SH-modifying agent PCMBS (Giaquinta, 1983; Lemoine, 2000; Williams et al., 2000). The relative activity of AmSUT1 was substantially influenced by the applied PCMBS concentration. Inhibitor concentrations above 100 μm strongly inhibited the Suc uptake in yeast cells, whereas a concentration of 25 μm only resulted in a very weak inhibition (Table I).
Specificity of the Anti-AmSUT1 Antiserum
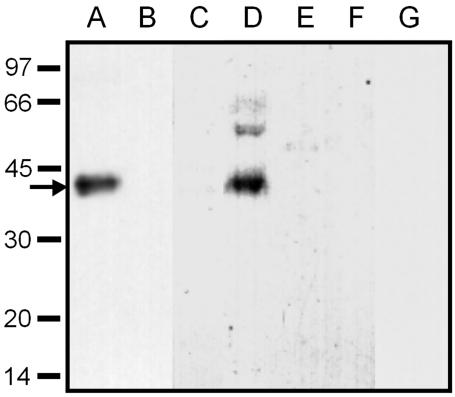
Polyclonal antisera were raised in rabbits by using synthetic peptides corresponding to the 17 N-terminal amino acids of AmSUT1. Protein extracts from plasma membranes of transgenic yeast cells expressing AmSUT1 and from yeast cells transformed with AmSUT1 in antisense direction were separated by SDS-PAGE, blotted, and treated with anti-AmSUT1 antiserum. A single specific band at an apparent molecular mass of 43 kD, which corresponds to the AmSUT1 protein, was recognized only in lane A of Figure 2, and the corresponding band was absent from plasma membrane preparations of yeast cells transformed with AmSUT1 in antisense orientation (Fig. 2, lane B). The difference of 9 kD between the molecular mass calculated from the DNA sequence (52.3 kD) and from SDS gels (43 kD) agrees well with the results from other plant Suc transporters such as AtSUC2 and PmSUC2 (Gahrtz et al., 1994; Sauer and Stolz, 1994) and is typical for lipophilic membrane proteins (Sauer and Tanner, 1984). Peptide competition experiments were performed to test antibody specificity. Pre-incubation of anti-AmSUT1 antibodies with the peptide used for immunization prevented the signal in western blots with yeast plasma membrane proteins of AmSUT1 expressing yeast cells (Fig. 2, lane C). This indicates the specificity of the anti-AmSUT1 antiserum against the 17 N-terminal amino acids of AmSUT1.
Figure 2.
Western-blot analysis of the specificity of the anti-AmSUT1 antiserum in transgenic yeast cells and in leaves, stems, and plasma membranes of A. meridionalis. Plasma membrane proteins from yeast strains expressing AmSUT1 in sense (CKY-Am1s; A) or antisense (CKY-Am1as; B) orientation were tested for their reaction with anti-AmSUT1 antiserum. Only in AmSUT1 expressing yeast cells a single band of 43 kD could be detected. Plasma membrane proteins from transgenic yeast strains expressing AmSUT1 in sense orientation were tested for their reaction with anti-AmSUT1 antiserum which had been pre-incubated with the antigenic peptide. This preincubation resulted in a complete loss of antibody binding (C). SDS solubilized proteins from plasma membranes (D) of source leaves from A. meridionalis were tested for their reaction with anti-AmSUT1 antiserum. A strong band corresponding to AmSUT1 was detectable. SDS-solubilized proteins from source leaves (E) or stems (F) of A. meridionalis were also tested for their reaction with anti-AmSUT1 antiserum showing no signal on the western blot. Plasma membrane proteins from transgenic yeast strains expressing AmSUT1 in sense orientation (G) were tested for their reaction with pre-immune serum showing no signal. Each lane contains 5 μg of protein. The apparent molecular masses of the size markers are given at the left in kilodaltons. The arrow indicates the AmSUT1 band at an apparent molecular mass of 43 kD.
Does the anti-AmSUT1 antiserum recognize proteins other than AmSUT1 in A. meridionalis? To test this possibility, proteins were solubilized from A. meridionalis leaves and petioles. Western-blot analysis of these fractions gave no bands, probably as a result of low and cell-specific expression of AmSUT1 (Fig. 2, lanes E and F). Only in plasma membrane proteins isolated from A. meridionalis leaves was AmSUT1 detected as a band at a molecular mass of 43 kD (Fig. 2, lane D). Some weaker bands could also be detected at higher molecular masses (Fig. 2, lane D). Controls in which the anti-AmSUT1 antibody was replaced by pre-immune serum showed no bands (Fig. 2, lane G).
Immunolocalization of AmSUT1 in the Phloem of Leaves and Stems
AmSUT1 was localized at the cellular level by using anti-AmSUT1 antiserum. Sections from stems or leaves of A. meridionalis were incubated with the anti-AmSUT1 antiserum, stained with a fluorescein isothiocyanate (FITC) isomer 1-conjugated secondary antibody, and investigated under fluorescent light.
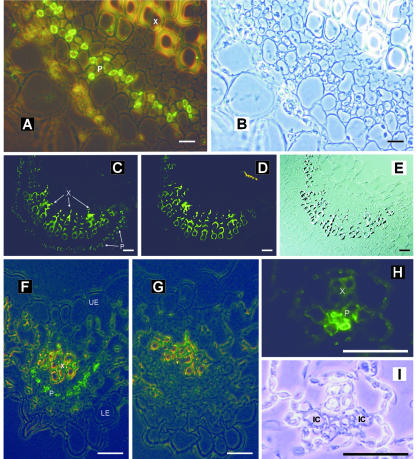
Cross sections of the A. meridionalis midrib are presented in Figure 3, A to E. In these sections, the cells labeled with the anti-AmSUT1 antiserum are located within the phloem (Fig. 3, A and C). The labeled cells have a dense cytoplasm as can be seen in white light (Fig. 3B). Controls in which the anti-AmSUT1 antibody was omitted showed no FITC labeling (Fig. 3D). The yellow-green staining of the xylem vessels is due to the phenolic compounds in the cell wall of the respective cells.
Figure 3.
Immunolocalization of AmSUT1 in midrib and major and minor-sized veins of A. meridionalis leaves. A, Cross section through the midrib of an A. meridionalis leaf treated with anti-AmSUT1 antiserum; antibody binding was detected with an anti-rabbit IgG-FITC conjugate. The fluorescence of the antibody-labeled cells was localized to the phloem (P). The yellow-green autofluorescence of xylem (X) vessels results from phenolic compounds in the walls of these cells. Mixed light (fluorescence and phase contrast) was used. Scale bar = 25 μm. B, Same section as shown in A in transmission light. C, Cross section of the midrib treated with anti-AmSUT1 antiserum. Scale bar = 100 μm. D, Control section of the midrib in which the anti-AmSUT1 antiserum was omitted. Scale bar = 100 μm. E, Same section as shown in C in transmission light. F, Localization of AmSUT1 in a third order vein of an A. meridionalis leaf (X, xylem; P, phloem; LE, lower epidermis; UE, upper epidermis). Scale bar = 25 μm. G, Control section of the third order vein in which the anti-AmSUT1 antiserum was omitted. Scale bar = 25 μm. H, Localization of AmSUT1 in a minor vein of an A. meridionalis leaf (X, xylem; P, phloem). Scale bar = 10 μm. I, Same section as shown in H in transmission light. IC indicates intermediary cell.
Figure 3, F to I, show sections of veins of the third order (major veins) and minor veins that were also labeled with anti-AmSUT1 antiserum and stained with FITC. Fluorescence microscopy of labeled sections shows green, anti-AmSUT1 antiserum-dependent fluorescence in defined cells opposite of the xylem cells (Fig. 3, F and H), indicating localization of the AmSUT1 protein in phloem cells of major (third order, Fig. 3F) or minor veins (Fig. 3H). In Figure 3H, the internal part of the phloem of the minor veins is more heavily labeled, probably the CCs and sieve tubes, than the laterally positioned cells of the minor vein, probably the intermediary cells (Fisher, 1986; Knop et al., 2001). Controls in which the anti-AmSUT1 antiserum was omitted showed no FITC labeling (Fig. 3G).
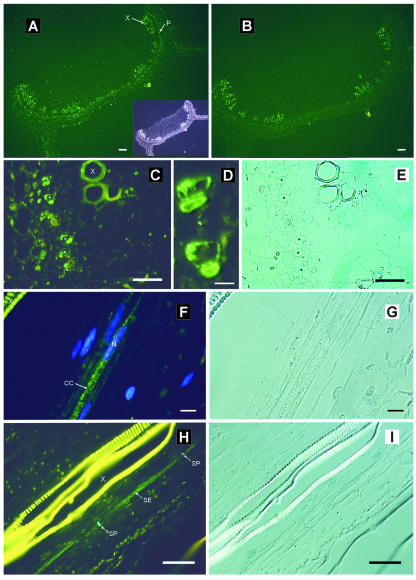
Immunofluorescent detection of AmSUT1 was also performed on stem tissue of A. meridionalis. Sections incubated with anti-AmSUT1 antiserum show again that the AmSUT1 protein was detected in cells of the transport phloem (Fig. 4A). Control sections incubated with the secondary antibody alone gave no immunofluorescence signal (Fig. 4B).
Figure 4.
Immunolocalization of AmSUT1 in sections of stems from A. meridionalis. A, Cross section of the stem treated with anti-AmSUT1 antiserum; antibody binding was detected with an anti-rabbit IgG-FITC conjugate. The inset shows the same cross section in transmission light. Antibody-labeled cells are marked with an arrow and P (phloem). The large xylem (X) vessels show yellow-green autofluorescence. Scale bar = 50 μm. B, Control section of the stem in which the anti-AmSUT1 antiserum was omitted. Scale bar = 50 μm. C, Higher magnification of the vascular bundle shown in A. The asterisks in C mark five fluorescence-labeled cell pairs. Scale bar = 50 μm. D, Higher magnification of the upper two of the labeled cell pairs shown in C. Scale bar = 10 μm. E, Same section as shown in C in transmission light. F, Longitudinal section of the stem. Histochemical staining of nuclei with 4′,6′-diamidino-2-phenylindole (DAPI) in cells treated with the anti-AmSUT1 antiserum/FITC conjugate. Scale bar = 10 μm. G, Same section as shown in F in transmission light. H, Longitudinal section of the vascular bundle of the stem. Immunofluorescent detection of AmSUT1 in long cells colocalized with the aniline blue-stained callose in the sieve plate. Scale bar = 50 μm. I, Same section as shown in H in transmission light.
To determine in which cell types of the phloem AmSUT1 was located, the fluorescence and light microscopic pictures were compared (Fig. 4, C and E; stars mark the same cells). At higher magnification, pairs of labeled cells were visible (Fig. 4D). One cell type had dense cytoplasm that showed green fluorescence, probably the CCs, and was adjacent to the other AmSUT1 expressing cell type that had a less dense cytoplasm and non-labeled parts, probably the SEs or young SEs; here, the green fluorescence was limited to the outer parts of the cells.
For a more precise characterization of the cell type, it was necessary to look at additional properties of the labeled cells. One of the characteristics of mature SEs is denucleation during development. Double staining of longitudinal sections with the anti-AmSUT1 antiserum/FITC conjugate and with DAPI, which binds to DNA and results in a blue fluorescent staining of the nucleus, revealed that one type of the AmSUT1-containing cells has a well-developed nucleus. The cells are specifically located in the phloem and are very long in size, suggesting that these cells are CCs or very young SEs (Fig. 4F).
In addition, double staining of longitudinal sections was performed using anti-AmSUT1 antiserum/FITC conjugate and aniline blue, which is SE specific, because it binds to callose that is deposited in the sieve plates of the SEs. The green FITC and the blue anilin label the same cell (Fig. 4H). The fact that both labels marked the same cell revealed that the other AmSUT1-containing cell type is the SE (Fig. 4H).
Effects of PCMBS on Sugar Contents in Phloem Exudates
To gain further information on the mode of phloem loading in A. meridionalis, the effect of PCMBS on sugar exudation of cut leaves was tested. The extent of PCMBS sensitivity of Suc translocation has been used to distinguish between apoplastic and symplastic phloem loaders in several studies (Turgeon and Gowan, 1990; van Bel et al., 1992, 1994) because Suc transporters should only be involved in apoplastic, not in symplastic, phloem loading.
In previous studies, we collected phloem sap of A. meridionalis with the laser-aphid-stylet technique (Knop et al., 2001). Because of the difficulties with this technique to collect several samples of phloem sap at a defined time, we used a different method, the EDTA exudation method (King and Zeevaart, 1974). To assess the reliability of the inhibitor application method, parallel experiments were performed with potato (Solanum tuberosum) that had been identified previously as a typical apoplastic phloem loader (Heineke et al., 1992).
In Table II, the effects of PCMBS on A. merdionalis and potato are shown. The apoplastic PCMBS concentration was determined as follows (van Bel et al., 1994): The amount of PCMBS absorbed by the leaf was calculated from the volume transpired by the leaf during the incubation period. The aqueous apoplastic space in leaves of potato (Leidreiter et al., 1995) and A. meridionalis (Knop et al., 2001) occupied about 5% of the leaf fresh weight. The apoplastic volume of the leaf together with the amount of PCMBS absorbed per leaf make it possible to calculate the average PCMBS concentration in the apoplastic space, based on the premise that the absorbed PCMBS is only in the apoplastic space. These calculations yielded similar apoplastic PCMBS concentrations in A. meridionalis and potato, 5.2 ± 0.8 and 4.6 ± 0.7 mm, respectively. Previous results of van Bel et al. (1994) have shown that apoplastic PCMBS concentrations in this range have no effect on the rate of photosynthesis of leaves.
Table II.
Effects of PCMBS treatment on sugar exudation from cut leaves of A. meridionalis and potato
The PCMBS concentration before leaf preincubation was 1 mm. The calculated apoplastic PCMBS concentration after preincubation was 5.2 ± 0.8 mm in A. meridionalis and 4.6 ± 0.7 mm in potato. Controls were treated with dimethyl sulfoxide (DMSO) only. Data are represented in nanomoles per gram fresh wt per hour and represent the mean ± sd from 16 independent measurements. Those determined by Fisher's protected lsd test to be significantly different (significance level 0.05) from controls are in bold.
| Sugars
|
A. meridionalis
|
Potato
|
||||
|---|---|---|---|---|---|---|
| Control PCMBS | % of control | Control | PCMBS | % of control | ||
| Glc | 34 ± 12 | 15 ± 6 | 44 | 316 ± 106 | 21 ± 10 | 7 |
| Fru | 26 ± 10 | 8 ± 3 | 31 | 333 ± 89 | 22 ± 10 | 7 |
| Suc | 35 ± 10 | 28 ± 6 | 80 | 867 ± 245 | 16 ± 9 | 2 |
| Raffinose | 10 ± 3 | 16 ± 6 | 160 | n.d.a | n.d. | — |
| Stachyose | 18 ± 8 | 43 ± 12 | 239 | n.d. | n.d. | — |
| Sum of sugars | 123 ± 35 | 110 ± 28 | 89 | 1,516 ± 361 | 59 ± 26 | 4 |
n.d., Not detectable
With control leaves, the exudation rate of sugars was much higher in potato than in A. meridionalis. PCMBS treatment had different effects on sugar exudation of the two plant species: Although the exudation rate was strongly reduced in potato (4% of control), it was nearly the same in A. meridionalis (89% of control). Although the exudation rate of the sum of sugars was almost unchanged in A. meridionalis, the exudation rates of single sugars changed in different directions. Although the exudation rates of raffinose and stachyose significantly increased, the rates of Glc, Fru, and Suc significantly decreased. However, the exudation rate of hexoses decreased more pronounced than that of Suc. In contrast to the exudate of cut petioles (Table II; Heineke et al., 1992; Bachmann et al., 1994; Zuther et al., 2003), phloem sap normally does not contain Glc and Fru (Knop et al., 2001). These hexoses in the exudate mainly represent artificially hydrolyzed Suc (Heineke et al., 1992). The fact that the ratio of Glc to Fru is not exactly one (Table II; Flora and Madore, 1996; Zuther et al., 2003) might be caused in part by the activities of monosaccharide metabolizing enzymes from the cut surface. Taking into account that monosaccharide transporters of plants, in contrast to Suc transporters analyzed so far, are insensitive to PCMBS (M. Büttner, personal communication), and the hexoses in the phloem exudate (Table II) represent mainly artificially hydrolyzed Suc, the overall content of Suc decreased in the phloem by about 40%.
DISCUSSION
Comparison of the Biochemical Characteristics of AmSUT1 with Those of Other Suc Transporters
The cDNA of a Suc transporter was isolated from the putative symplastic phloem loader A. meridionalis (Knop et al., 2001). In this paper, the function of the transporter was proven by heterologous expression of the full-length AmSUT1 cDNA in yeast. Suc uptake in yeast expressing AmSUT1 is extremely pH sensitive and is also sensitive to the uncoupler CCCP. These data indicate that AmSUT1 works as a Suc/H+ cotransporter like the other Suc transporters analyzed so far. The apparent Km for Suc is 1.8 mm (Fig. 1C). Similar Km values have been published for Suc transporters from several other plant species when expressed in yeast: 1.5 mm for SoSUT1 from spinach (Spinacia oleracea; Riesmeier et al., 1992), 1.0 mm for StSUT1 from potato (Riesmeier et al., 1993), 0.5 mm for AtSUC2 from Arabidopsis (Sauer and Stolz, 1994), 1.0 mm for PmSUC2 from Plantago major (Gahrtz et al., 1994), and 2 mm for RcSCR1 from Ricinus communis (Weig and Komor, 1996). The kinetic of pH-dependent proton-Suc uptake was examined earlier using isolated plasma membrane vesicles of sugar beet (Beta vulgaris) leaves. The apparent Km for Suc derived from these experiments was also approximately 1 mm (Bush, 1989; Lemoine and Delrot, 1989).
The SH-modifying agent PCMBS is an inhibitor of Suc transporters. Also, the Suc transporter from A. meridionalis is inhibited by this agent. In the presence of 50 or 100 μm PCMBS, Suc uptake in yeast expressing AmSUT1 was decreased to 51% and 19% (Table I), respectively, which is similar to the inhibition of other Suc transporters tested by heterologous expression in yeast cells (SoSUT1, 100 μm PCMBS, 21% residual activity, Riesmeier et al., 1992; StSUT1, 100 μm, 20% residual activity, Riesmeier et al., 1993; PmSUC2, 50 μm, 27% residual activity, Gahrtz et al., 1994; and AtSUC2, 100 μm, 49% residual activity, Sauer and Stolz, 1994).
The substrate specificity observed for AmSUT1 is in agreement with the already described characteristics of Suc transporters in typical apoplastic phloem loaders, like spinach (Riesmeier et al., 1992), potato (Riesmeier et al., 1993), Arabidopsis (Sauer and Stolz, 1994), P. major (Gahrtz et al., 1994), and several others (Lemoine, 2000). Several sugars have been tested for substrate recognition by AmSUT1 (Table I), but only maltose partially inhibits the transport of Suc. Neither raffinose nor stachyose, typical transport sugars in A. meridionalis, showed inhibitory effects on Suc uptake by AmSUT1 (Table I).
Localization of AmSUT1
To study the cell-specific localization of AmSUT1, antibodies were raised against the N terminus of the protein. The antiserum recognized a single polypeptide of 43 kD in plasma membranes from yeast expressing AmSUT1 and some weaker bands in addition to the 43-kD polypeptide in plasma membranes from A. meridionalis leaves. By immunofluorescence, AmSUT1 was localized in the phloem of leaves and stems of A. meridionalis. Additional use of histochemical techniques showed that the antiserum/FITC conjugate-labeled cells were CCs and either mature and/or young SEs (Fig. 4, F–I). In several other plant species, Suc transporters were only localized either in the CCs, e.g. in Arabidopsis and P. major (PmSUC2, Stadler et al., 1995; and AtSUC2, Stadler and Sauer, 1996) or in the sieve tubes, e.g. in solanaceous species such as potato, tomato (Lycopersicon esculentum), and tobacco (Nicotiana tabacum; SUT1, Kühn et al., 1997; and SUT4, Weise et al., 2000). The localization of AmSUT1 to SEs and CCs was also corroborated by an earlier study, in which AmSUT1 mRNA could be detected in pure phloem sap via semiquantitative RT-PCR (Knop et al., 2001).
It is possible that A. meridionalis contains more than one Suc transporter and that the anti-AmSUT1 antiserum cross-reacted with these proteins. This could be the reason for the label in two cell types, the CCs and the sieve tubes. However, cross-reaction is unlikely because the antibodies were raised against the N terminus of the protein, which is a highly variable region of Suc transporters (Sauer and Stolz, 1994; Lemoine, 2000). Even if cross-reaction with other Suc transporters had occurred, it would be irrelevant to the question addressed, namely the role of Suc transporters in the phloem of plant species with an open vein anatomy.
What Is the Function of the AmSUT1 Protein in the Phloem of the Putative Symplastic Phloem Loader A. meridionalis?
The polymer trap model (Turgeon, 1991) of symplastic phloem loading does not require the participation of Suc or other metabolite transporters. However, the putative symplastic phloem loader A. meridionalis contains a Suc transporter in the phloem of minor- and medium-sized veins of the leaves (Fig. 3) and in the phloem of the midrib (Fig. 3) and stems (Fig. 4). Indications for the existence of Suc transporters in putative symplastic phloem loaders and oligosaccharide-translocating species come also from electrophysiological experiments (van Bel et al., 1996). In this study, leaf cells of symplastic phloem loaders showed depolarization responses to Suc and to raffinose.
The minor- and medium-sized veins are the major sites of primary phloem loading. Similar to other herbaceous putative symplastic phloem loaders (e.g. squash [Cucurbita pepo], Turgeon et al., 1975; C. blumei, Fisher, 1986; Verbascum chaixii, Turgeon et al., 1993; and A. reptans, Hoffmann-Thoma et al., 2001) in A. meridionalis, these veins contain two types of CCs, intermediary cells and ordinary CCs (Knop et al., 2001). Perhaps these two types of CCs facilitate Suc uptake in a dual fashion. Either Suc diffuses through plasmodesmata from the mesophyll cells into the intermediary cells or Suc might be taken up from the apoplast into ordinary CCs by the activity of a Suc transporter. Figure 3H shows that the internal part of the minor vein, which contains the SEs and the ordinary CCs (Knop et al., 2001), is stronger labeled than the two larger, laterally positioned cells, probably the intermediary cells. This does not exclude that the plasma membrane of intermediary cells also contains Suc transporters. The assumption of mixed phloem loading of Suc in A. meridionalis is also supported by the effect of PCMBS on sugar exudation of cut petioles. The exudation of Suc and hexoses was only slightly reduced in comparison with the typical apoplastic phloem loader potato (Table II). This excludes that the activity of the Suc transporter is the only possibility for Suc uptake into the SE-CC complex. van Bel et al. (1992) have shown a strong reduction of phloem loading by PCMBS in Acanthus mollis. In this plant species, intermediary cells and transfer cells, connected to different SEs, occurred within the same vein. This supports the idea that the existence of intermediary cells is not inevitably correlated with exclusive symplastic phloem loading.
Mixed apoplastic and symplastic phloem loading also has been assumed for other plants with an open vein anatomy (Turgeon et al., 1975, 1993; Fisher, 1986; Oparka and Turgeon, 1999). In squash tissue cultures, SEs accumulate solutes, but their associated CCs do not (Lackney and Sjolund, 1991). Oparka and Turgeon (1999) interpreted these data suggesting that in squash phloem, loading proceeds in a dual fashion by symplastic transfer of assimilates into the intermediary cells and also by an apoplastic transfer into the SEs. Turgeon and Gowan (1990) observed an inhibitory effect of PCMBS on assimilate translocation in the symplastic phloem loader C. blumei. As in A. meridionalis, the minor veins of C. blumei contain both CCs and intermediary cells. The authors discussed that the reduction of translocation by PCMBS is due to the inhibition of Suc-proton cotransport from the apoplast into the ordinary CCs. Fisher (1986) also proposed that the ordinary CCs in the veins of C. blumei might be loaded from the apoplast, whereas intermediary cells are loaded symplastically.
Although the phloem of the midrib and the stem is primarily responsible for long-distance transport and allocation, phloem loading of Suc is also required along this path (retrieval) because the high concentration of Suc and other sugars within the sieve tubes causes a permanent passive leakage of Suc into the apoplast. Moreover, the apoplastic Suc concentration in A. meridionalis (2.1 mm, Knop et al., 2001) is similar to those in typical apoplastic phloem loaders, like spinach or barley (Hordeum vulgare; 1.0 and 1.3 mm, respectively; Lohaus et al., 1995). To maintain a high Suc concentration and an optimal mass flow within the sieve tubes, it is necessary to re-import this lost Suc into the SE-CC complex. The activity of retrieval pumps located in the transport phloem has been demonstrated earlier (Willenbrink, 1980; Minchin and Thorpe, 1987; Grimm et al., 1990). AmSUT1, localized in the SEs (Fig. 4, C, D, and H) could be active in Suc retrieval along the transport path. This assumption is supported by the decreased Suc and hexose exudation from PCMBS-treated cut petioles (Table II). Turgeon and Gowan (1990) also observed a loss of 14C along the major leaf veins and a reduction of translocation in PCMBS-treated leaves of C. blumei, a putative symplastic phloem loader. This observation could be explained by decreased Suc retrieval as a consequence of the partial inhibition of the Suc transporters.
The results presented here show that phloem loading of Suc or Suc retrieval in A. meridionalis, a putative symplastic phloem loader (Knop et al., 2001), could occur at least partially via the apoplast. It is not clear which environmental conditions influence the extent of the two types of phloem loading. In summary, it is obvious that more information is needed on the structure/function of plasmodesmata and transporters in assimilate transport of plant species with an open minor vein anatomy.
MATERIALS AND METHODS
Plant Material
Alonsoa meridionalis O. Kuntze and potato (Solanum tuberosum cv Désirée) were grown in compost soil under greenhouse conditions as described by Knop et al. (2001). Two-month-old plants were taken for the experiments.
Expression of AmSUT1 in Yeast (Saccharomyces cerevisiae) and Uptake Experiments
For heterologous expression of AmSUT1, the Escherichia coli/yeast shuttle vector pNEV-E (Sauer and Stolz, 1994) was used. A full-length cDNA clone of AmSUT1 containing the 5′-untranslated region of PmSUC2 (5′-AAGCTTGTAAAAGAA-3′; Gahrtz et al., 1994) was cloned into the EcoRI site of the vector. This vector allows expression of full-length cDNAs under control of the yeast PMA1 promoter. The 5′-untranslated region of PmSUC2 was introduced because it allows a high-level expression of Suc transporters in yeast (Sauer and Stolz, 2000).
Plasmids with inserts in sense and antisense orientations (pCK-Am1s and pCK-Am1as) were used to transform yeast strain DBY 2617 (Kaiser and Botstein, 1986), resulting in the transgenic yeast strains CKY-Am1s and CKY-Am1as, respectively. Uptake was analyzed as described by Gahrtz et al. (1994).
Preparation of Plasma Membranes and SDS-PAGE
Plasma membrane proteins from yeast cells were enriched (Winzer, 1999) and separated on SDS-polyacrylamide gels (Laemmli, 1970). Plant plasma membranes were isolated using a two-phase system according to Robinson and Hinz (2001).
Production of Anti-AmSUT1 Antiserum
Synthetic peptides corresponding to the N terminus of AmSUT1 (MEVGNEAKSTALPPAQA) were synthesized and coupled to keyhole limpet hemocyanin via the C terminus. These proteins were used to immunize three rabbits (Dr. Julio Pineda, Pineda-Antikörper-Service, Berlin). Immunization was repeated every month until d 120. The quality of the antisera was tested using protein extracts of plasma membranes from transgenic yeast cells expressing AmSUT1 in sense (CKY-Am1s) or antisense (CKY-Am1as; negative control) orientation (Fig. 2). The specificity of the antiserum was also tested by reaction of plasma membrane proteins from transgenic yeast strains expressing AmSUT1 in sense orientation with anti-AmSUT1 antiserum that had been pre-incubated overnight with the antigenic peptide for saturating the antiserum. The final concentration of the antigenic N-terminal peptide was 3.5 mg mL-1. The serum was used directly for western blots at a dilution of 1:500 (v/v) and for immunocytochemistry at a dilution of 1:500 (v/v) to 1:5,000 (v/v).
Preparation and Fixation of A. meridionalis Sections for Light Microscopy
The fixation of the material was carried out according to Stadler et al. (1995). Parts of leaves and stems (2 × 2 × 5 mm) of A. meridionalis were briefly degassed in 3 mL of fixing solution (3:1 [v/v] ethanol:acetic acid), and the tissues were fixed at room temperature for 1 h. After three washing steps with 70% (v/v) ethanol, 1 mm dithiothreitol (DTT) for each 30 min, and one overnight step, the tissues were dehydrated with 80%, 85%, 90%, and 95% (v/v) ethanol, 1 mm DTT for each 20 min on ice, and finally two times with 99.8% (v/v) ethanol and 10 mm DTT for each 20 min. The tissue was infiltrated with methacrylate in three sequential incubations in methacrylate mix (75% [v/v] butyl methacrylate, 25% [v/v] methyl methacrylate, 0.5% [v/v] benzoine ethyl ether, and 10 mm DTT) at 4°C with increasing methacrylate mix:ethanol ratios (first incubation overnight, 1:2 [v/v]; second incubation for 6 h, 1:1 [v/v]; and third incubation overnight, 2:1 [v/v]). After one additional 6-h incubation and two final overnight incubations at 4°C (all in 100% [v/v] methacrylate mix) samples were transferred to ultrathin PCR tubes. Methacrylate was polymerized during incubation at 4°C for 15 h under UV light (310 nm) in 100% (v/v) methacrylate mix. Semithin sections (about 2 μm) were prepared with an ultramicrotome (Ultracut R, Leica, Bensheim, Germany) and placed on poly-l-Lys-coated coverslips.
Staining of Sections with Fluorescent Dyes
For removal of methacrylate from the semithin sections, coverslips were incubated for 2 min in 100% (v/v) acetone. Rehydration proceeded in a series of ethanol (100%, 70%, and 30% [v/v]) for each 30 s. The coverslips were washed with Tris-buffered saline buffer (50 mm Tris-HCl [pH 7.5] and 150 mm NaCl) for 30 s and incubated in blocking buffer (1% [w/v] skimmed milk powder in Tris-buffered saline) for 45 min. After overnight incubation with anti-AmSUT1 antiserum (diluted 1:500–1:5,000 [v/v] in blocking buffer), the coverslips were washed three times with blocking buffer and incubated for 1 h with anti-rabbit IgG-FITC isomer 1 conjugate (diluted 1:300 [w/v] in blocking buffer, Sigma, St. Louis). For control sections, the anti-AmSUT1 antiserum was omitted. After five final washes with blocking buffer for 5 min each, the coverslips were rinsed with water and mounted in 10 μL of ProLong-Antifade Kit (Molecular Probes, Leiden, The Netherlands). Photographs were taken with a fluorescence phase microscope (Carl Zeiss, Göttingen, Germany) with an excitation light of 450 to 490 nm.
For double staining of the AmSUT1 protein with antiserum/FITC conjugate and of nuclei with DAPI (Serva, Heidelberg), sections were treated as described above. After the final rinsing with water, coverslips were incubated for 1 h at room temperature in DAPI (0.2 μg mL-1). DAPI fluorescence was detected with an excitation light of 365 nm.
For double staining of the AmSUT1 protein with antiserum/FITC conjugate and of sieve plates with aniline blue (Water Blue, Fluka, Buchs, Switzerland), the coverslips were incubated for 5 min in aniline blue (0.5% [w/v] in 200 mm NaPO4 buffer [pH 7.2]). Aniline blue fluorescence was detected with an excitation light of 365 nm.
Collection of Phloem Exudates
Exudates were collected by the EDTA-facilitated exudation method described by King and Zeevaart (1974). Leaves were detached from the A. meridionalis and potato. The petioles were cut again in 10 mm EDTA (pH 6.0) and shaken to remove sugars from damaged petiole cells. The petiole of each leaf was immediately passed through a slit in a Parafilm cover into an Eppendorf cup (Eppendorf Scientific, Westbury, NY) containing 0.5 mL of fresh EDTA solution. Exudation of leaves took place in climate chambers for 4 h.
To study the effect of PCMBS, the cut petioles of leaves were placed in 0.5 mL of tap water with or without 1 mm PCMBS and dissolved in 0.1% (v/v) DMSO for 1 h before exudation. Control assays were performed with the same concentration of DMSO. The Eppendorf cups along with their contents were preweighed. After 1 h, the leaves were removed, and the cups were weighed again. The difference in weight before and after the transpiration period enabled calculation of the volume transpired and the corresponding amount of PCMBS absorbed by the treated leaves.
Determination of Sugars
Sugars in phloem exudates were assayed by HPLC with pulsed amperometric detection using a CarboPAC10 (Dionex, Sunnyvale, CA) column and precolumn and an NaOH eluent as described previously (Lohaus et al., 1995).
Acknowledgments
We thank Katharina Pawlowski and Jens Tilsner for critical reading of the manuscript and Carola Schröder for excellent technical assistance.
Article, publication date, and citation information can be found at http://www.plantphysiol.org/cgi/doi/10.1104/pp.103.029264.
This work was supported by the Deutsche Forschungsgemeinschaft.
References
- Bachmann M, Matile P, Keller F (1994) Metabolism of the raffinose family oligosaccharides in leaves of Ajuga reptans L. Plant Physiol 105: 1335-1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker L, Kühn C, Weise A, Schulz A, Gebhardt C, Hirner B, Hellmann H, Schulze W, Ward JM, Frommer WB (2000) SUT2, a putative sucrose sensor in sieve elements. Plant Cell 12: 1153-1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush DR (1989) Proton-coupled sucrose transport in plasmalemma vesicles isolated from sugar beet leaves. Plant Physiol 89: 1318-1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delrot S (1981) Proton fluxes associated with sugar uptake in Vicia faba leaf tissue. Plant Physiol 68: 706-711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher DG (1986) Ultrastructure, plasmodesmata frequency, and solute concentration in green areas of variegated Coleus blumei Benth. leaves. Planta 169: 141-152 [DOI] [PubMed] [Google Scholar]
- Flora LL, Madore MA (1996) Significance of minor-vein anatomy to carbohydrate transport. Planta 198: 171-178 [Google Scholar]
- Gahrtz M, Stolz J, Sauer N (1994) A phloem-specific sucrose-H+ symporter from Plantago major L. supports the model of apoplastic phloem loading. Plant J 6: 697-706 [DOI] [PubMed] [Google Scholar]
- Gamalei Y (1989) Structure and function of leaf minor veins in trees and herbs. Trees 3: 96-110 [Google Scholar]
- Geiger DR, Giaquinta RT, Sovonick SA, Fellow RJ (1973) Solute distribution in sugar beet leaves in relation to phloem loading and translocation. Plant Physiol 52: 585-589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta RT (1983) Phloem loading of sucrose. Annu Rev Plant Physiol 34: 347-387 [Google Scholar]
- Grimm E, Bernhardt G, Rothe K, Jacob F (1990) Mechanism of sucrose retrieval along the phloem path: a kinetic approach. Planta 182: 480-485 [DOI] [PubMed] [Google Scholar]
- Gunning BES, Pate JS (1969) “Transfer cells” plant cells with wall ingrowths, specialized in relation to short distance transport of solutes: their occurrence, structure, and development. Protoplasma 37: 107-133 [Google Scholar]
- Heineke D, Sonnewald U, Büssis D, Günter G, Leidreiter K, Wilke I, Raschke K, Willmitzer L, Heldt HW (1992) Apoplastic expression of yeast-derived invertase in potato. Plant Physiol 100: 301-308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann-Thoma G, van Bel AJE, Ehlers K (2001) Ultrastructure of minorvein phloem and assimilate export in summer and winter leaves of the symplastically loading evergreens Ajuga reptans L., Aucuba japonica Thunb, and Hedera helix L. Planta 212: 231-242 [DOI] [PubMed] [Google Scholar]
- Holthaus U, Schmitz K (1991) Distribution and immunolocalization of stachyose synthase in Cucumis melo L. Planta 162: 283-288 [DOI] [PubMed] [Google Scholar]
- Kaiser CA, Botstein D (1986) Secretion-defective mutations in the signal sequence for Saccharomyces cerevisiae invertase. Mol Cell Biol 6: 2382-2391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King RW, Zeevaart JAD (1974) Enhancement of phloem exudation from cut petioles by chelating agents. Plant Physiol 53: 96-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop C, Voitsekhovskaja O, Lohaus G (2001) Sucrose transporters in two members of the Scrophulariaceae with different types of transport sugar. Planta 213: 80-91 [DOI] [PubMed] [Google Scholar]
- Komor E, Rotter M, Tanner W (1977) A proton-cotransport system in a higher plant: sucrose transport in Ricinus communis. Plant Sci Lett 9: 153-162 [Google Scholar]
- Kühn C, Franceschi VR, Schulz A, Lemoine R, Frommer WB (1997) Macromolecular trafficking indicated by localization and turnover of sucrose transporters in enucleate sieve elements. Science 275: 1298-1300 [DOI] [PubMed] [Google Scholar]
- Lackney VK, Sjolund RD (1991) Solute concentrations of the phloem and parenchyma cells present in squash callus. Plant Cell Environ 14: 213-220 [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680-685 [DOI] [PubMed] [Google Scholar]
- Leidreiter K, Kruse A, Heineke D, Robinson DG, Heldt HW (1995) Subcellular volumes and metabolite concentrations in potato (Solanum tuberosum cv. Désirée) leaves. Bot Acta 108: 439-444 [Google Scholar]
- Lemoine R (2000) Sucrose transporters in plants: update on function and structure. Biochim Biophys Acta 1465: 246-262 [DOI] [PubMed] [Google Scholar]
- Lemoine R, Delrot S (1989) PMF-driven sucrose uptake in sugar beet plasma membrane vesicles. FEBS Lett 249: 129-133 [Google Scholar]
- Lohaus G, Winter H, Riens B, Heldt HW (1995) Further studies of the phloem loading process in leaves of barley and spinach: comparison of metabolite concentrations in the apoplastic compartment with those in the cytosolic compartment and in the sieve tubes. Bot Acta 3: 270-275 [Google Scholar]
- Minchin PEH, Thorpe MR (1987) Measurements of loading and reloading of photo-assimilate within the stem of bean. J Exp Bot 38: 211-220 [Google Scholar]
- Oparka KJ, Turgeon R (1999) Sieve elements and companion cells: traffic control centers of the phloem. Plant Cell 11: 739-750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riens B, Lohaus G, Heineke D, Heldt HW (1991) Amino acid and sucrose content determined in the cytosolic, chloroplastic, and vacuolar compartments and in the phloem sap of spinach leaves. Plant Physiol 97: 227-233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesmeier JW, Hirner B, Frommer WB (1993) Potato sucrose transporter expression in minor veins indicates a role in phloem loading. Plant Cell 5: 1591-1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesmeier JW, Willmitzer L, Frommer WB (1992) Isolation and characterization of a sucrose carrier cDNA from spinach by functional expression in yeast. EMBO J 11: 4705-4713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DG, Hinz G (2001) Organelle isolation. In B Satiat-Jeunemaitre, C Hawes, eds, Plant Cell Biology: A Practical Approach. IRL Press, Oxford, pp 295-324
- Sauer N, Stolz J (1994) SUC1 and SUC2: two sucrose transporters from Arabidopsis thaliana; expression and characterization in baker's yeast and identification of the histidine-tagged protein. Plant J 6: 67-77 [DOI] [PubMed] [Google Scholar]
- Sauer N, Stolz J (2000) Expression of foreign transport proteins in yeast. In SA Baldwin, Membrane Transport: Practical Approach Series. Oxford University Press, Oxford, pp 79-105
- Sauer N, Tanner W (1984) Partial purification and characterization of inducible transport proteins in Chlorella. Z Pflanzenphysiol 114: 367-375 [Google Scholar]
- Sovonick SA, Geiger DR, Fellows RJ (1974) Evidence for active phloem loading in the minor veins of sugar beet. Plant Physiol 54: 886-891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger N, Keller F (2000) Allocation of raffinose family oligosaccharides to transport and storage pools in Ajuga reptans: the roles of two distinct galactinol synthases. Plant J 21: 249-258 [DOI] [PubMed] [Google Scholar]
- Stadler R, Brandner L, Schulz A, Gahrtz M, Sauer N (1995) Phloem loading by the PmSUC2 sucrose carrier from Plantago major occurs into companion cells. Plant Cell 7: 1545-1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler R, Sauer N (1996) The Arabidopsis thaliana AtSUC2 gene is specifically expressed in companion cells. Bot Acta 109: 299-308 [Google Scholar]
- Turgeon R (1991) Symplastic phloem loading and the sink-source transition in leaves: a model. In JL Bonnemain, S Delrot, WJ Lucas, J Dainty, eds, Recent Advances in Phloem Transport and Assimilate Compartmentation. Ouest Editions, Nantes, France, pp 18-22
- Turgeon R, Beebe DU, Gowan E (1993) The intermediary cell: minor-vein anatomy and raffinose oligosaccharide synthesis in the Scrophulariaceae. Planta 191: 446-456 [Google Scholar]
- Turgeon R, Gowan E (1990) Phloem loading in Coleus blumei in the absence of carrier-mediated uptake of export sugar from the apoplast. Plant Physiol 94: 1244-1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon R, Gowan E (1992) Sugar synthesis and phloem loading in Coleus blumei leaves. Planta 187: 388-394 [DOI] [PubMed] [Google Scholar]
- Turgeon R, Webb JA, Evert RF (1975) Ultrastructure of minor veins of Cucurbita pepo leaves. Protoplasma 83: 217-232 [Google Scholar]
- van Bel AJE, Ammerlaan A, Van Dijk AA (1994) A three-step screening procedure to identify the mode of phloem loading in intact leaves. Planta 192: 31-39 [Google Scholar]
- van Bel AJE, Gamalei YV, Ammerlaan A, Bik LPM (1992) Dissimilar phloem loading in leaves with symplasmic or apoplasmic minor-vein configurations. Planta 186: 518-525 [DOI] [PubMed] [Google Scholar]
- van Bel AJE, Hendriks JHM, Boon EJMC, Gamalei YV, van de Merwe AP (1996) Different ratios of sucrose/raffinose-induced membrane depolarizations in the mesophyll of species with symplasmic (Catharanthus roseus, Ocimum basilicum) or apoplasmic (Impatiens walleriana, Vicia faba) minor-vein configurations. Planta 199: 185-192 [Google Scholar]
- Weig A, Komor E (1996) An active sucrose carrier (Scr1) that is predominantly expressed in the seedling of Ricinus communis L. J Plant Physiol 147: 685-690 [Google Scholar]
- Weise A, Barker L, Kühn C, Lalonde S, Buschmann H, Frommer WB, Ward JM (2000) A new subfamily of sucrose transporters, SUT4, with low affinity/high capacity localized in enucleate sieve elements of plants. Plant Cell 12: 1345-1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenbrink J (1980) Aspects arising from the use of inhibitors in phloem transport studies. Can J Bot 58: 816-820 [Google Scholar]
- Williams LE, Lemoine R, Sauer N (2000) Sugar transporters in higher plants: a diversity of roles and complex regulation. Trends Plant Sci 5: 283-290 [DOI] [PubMed] [Google Scholar]
- Winzer T (1999) Untersuchungen zum einfluβ des phloemtransports auf das speicherverhalten von verschiedenen rübensorten (Beta vulgaris L.) unter besonderer berücksichtigung des saccharosetransporters BvSUT1. PhD thesis. University of Göttingen, Germany
- Zimmermann MH, Ziegler H (1975) List of sugars and sugar alcohols in sieve-tube exudates. In MH Zimmermann, JA Milburn, eds, Encyclopedia of Plant Physiology, Vol 1. Springer Verlag, Berlin, pp 480-505 [Google Scholar]
- Zuther E, Kwart M, Willmitzer L, Heyer AG (2003) Expression of a yeast-derived invertase in companion cells results in long distance transport of a trisaccharide in an apoplastic loader and influences sucrose transport. Planta (in press) [DOI] [PubMed]