Abstract
Narrative discourse is an essential component of day-to-day communication, but little is known about narrative in Lewy Body spectrum disorder (LBSD), including Parkinson's disease (PD), Parkinson's disease with dementia (PDD), and dementia with Lewy bodies (DLB). We performed a detailed analysis of a semi-structured speech sample in 32 non-aphasic patients with LBSD, and we related their narrative impairments to gray matter (GM) atrophy using voxel-based morphometry. We found that patients with PDD and DLB have significant difficulty organizing their narrative speech. This was correlated with deficits on measures of executive functioning and speech fluency. Regression analyses associated this deficit with reduced cortical volume in inferior frontal and anterior cingulate regions. These findings are consistent with a model of narrative discourse that includes executive as well as language components and with an impairment of the organizational component of narrative discourse in patients with PDD and DLB.
Keywords: Parkinson's disease, discourse, speech, language, Dementia with Lewy bodies
Introduction
Parkinson's disease (PD) is considered to be primarily a motor disorder, but it is also known to affect cognition. Cognitive deficits in mild PD may include impaired memory, executive dysfunction, and visuospatial deficits (Bosboom, Stoffers, & Wolters, 2004; Brown & Marsden, 1990; Levin, Tomer, & Rey, 1992). PD is not thought to affect language per se (Bayles, 1990), although there is an accumulation of evidence that cognitive deficits in PD may extend to language as well (Bastiaanse & Leenders, 2009; Colman et al., 2009; Grossman, 1999; Hochstadt, 2009; Pereira et al., 2009). Hypothesized mechanisms implicate depletion of dopaminergic neurons in the substantia nigra. This causes impaired functioning of the basal ganglia, an area that may mediate cognitive functioning through its rich cortical connections, particularly involving frontal cortex, or may lead to impaired frontal and anterior temporal lobe functioning more directly through compromised projections from the ventral tegmental portion of the substantia nigra to these anterior regions of the cerebrum.
Over time, a progressive reduction in cognitive functioning in a proportion of PD patients reaches the status of dementia (PDD). This is estimated to occur in about 20% of PD patients (Brown & Marsden, 1984; Ebmeier et al., 1991; Grossman, 1999; Mayeux et al., 1988), with estimates ranging from 11% to 36% (Giladi et al., 2000; Girotti et al., 1988; Lees, 1985; Parashos, Johnson, Erickson-Davis, & Wielinski, 2009). Dementia in PD is associated with a proliferation of Lewy bodies in the cerebral cortex. This histopathologic picture is identical to that seen in dementia with Lewy bodies (DLB), a condition that is said to differ clinically from PDD in that there is a later onset of a motor disorder in DLB compared to PDD (McKeith et al., 2005). Thus, there exists a spectrum of cognitive disorders associated with extrapyramidal features, unified by the presence of histopathologic Lewy bodies, varying in the relative onset of motor and cognitive features, and including PD patients potentially converting to clear dementia. We refer to this family of conditions as Lewy body spectrum disorder (LBSD). This includes nondemented patients (PD), cognitively impaired patients with a relatively early onset motor disorder (PDD), and demented patients with minimal or late onset motor disorder (DLB). We acknowledge that this view of PD, PDD, and DLB as a spectrum of cognitive disorders is not universally accepted. Other researchers have identified both similarities and differences in the cognitive consequences of these diseases (Aarsland et al., 2003; Downes et al., 1998). In general, however, both the cognitive and brain atrophy differences that have been found among the groups are interpretable as quantitative differences in degree of cognitive impairment and atrophy in specified brain regions, rather than qualitative differences in the nature of these conditions (Double et al., 1996; Harrington et al., 1994). The shared features of proliferation of Lewy bodies in cerebral cortex and a similar range of cognitive deficits are the grounds for our regarding these conditions as a spectrum of disorders.
Consistent with the hypothesized role of the frontal lobe in the cognitive difficulties of LBSD, these patients appear to have prefrontal disease quantified by prefrontal cortical atrophy (Whitwell et al., 2007). Most studies of language in LBSD are limited to nondemented patients, although there are exceptions (Parashos et al., 2009; Piatt, Fields, Paolo, Koller, & Troster, 1999). In this report, we examine the organization of narrative speech in a semi-structured speech sample from non-aphasic patients with LBSD. We test the hypothesis that LBSD patients are impaired in their narrative organization and that these deficits are due in part to an executive disorder mediated by prefrontal cortical disease.
Language-specific domains such as phonology, lexicon, and grammar certainly contribute to successful narrative discourse, and we examine the role of these domains in the narrative performance of LBSD patients. Recent theories suggest that higher-level cognitive processes also play a crucial role in conveying the meaning of a narrative. This includes sustaining a story's theme through working memory and maintaining story coherence through top-down planning and organization (Farag et al., 2010; Mar, 2004). Thus, non-aphasic patients with LBSD may have a disorder of narrative discourse even if their speech and language are relatively preserved. Executive limitations on tasks requiring working memory as well as planning and organizational skills have been described in LBSD (Calderon et al., 2001; Kraybill et al., 2005; Lambon Ralph et al., 2001; Libon et al., 2001). From this perspective, deficits in executive resources such as these may interfere with the capacity for effective communication in LBSD, not necessarily at the level of an isolated word or sentence, but at the higher level of planning and organizing a narrative. These higher-level organizational functions allow non-adjacent events of a narrative to be related to each other and support communicative coherence by maintaining the theme of the narrative. Consistent with a model of narrative production that involves executive functioning, narrative discourse deficits have been associated with limited executive resources in non-aphasic patients with behavioral variant frontotemporal dementia (Ash et al., 2006). In our prior work, a detailed analysis of performance in the narration of a wordless picture story showed a limited grasp of the story's overall theme and poor connectedness between specific events in the patients' stories, even though lexical and grammatical aspects of word and sentence use were relatively preserved. In addition, impaired discourse cohesion correlated with cortical atrophy in an anatomic distribution that included right prefrontal cortex, an area associated with executive resources.
Additional evidence consistent with the contribution of prefrontal regions to narrative speech comes from studies of regional brain activation in healthy young adults. In one influential study, regional brain activity was monitored with positron emission tomography (PET) during production of an extended speech sample (Braun, Guillemin, Hosey, & Varga, 2001). A broad range of brain regions was activated during subjects' extemporaneous accounts of personal experiences. Among these were inferior, dorsolateral, and medial frontal regions, more prominently in the left hemisphere than the right hemisphere. More recently, an fMRI study assessed the neuroanatomic basis for narrative speech production during presentation of pictures from a children's wordless picture story. Prefrontal activation was seen during production of the entire story compared to descriptions of single pictures from the story (Troiani et al., 2008). These prefrontal activations overlap with the recruitment seen in fMRI studies of young adults investigating executive resources such as working memory (Botvinick, Braver, Barch, Carter, & Cohen, 2001; Ramnani & Owen, 2004; Smith, Marshuetz, Geva, & Grafman, 2002). Prefrontal recruitment during narrative discourse also overlaps with activations seen in several studies involving increasingly complex decisions about visual-perceptual stimuli that require higher levels of organizational abstraction (Badre & D'Esposito, 2007; Badre & Wagner, 2004; Braver & Bongiolatti, 2002; Koechlin & Jubault, 2006; Ramnani & Owen, 2004). These findings are relevant to the observation of prefrontal atrophy in patients with LBSD (Burton et al., 2009; Burton, McKeith, Burn, Williams, & O'Brien, 2004; Sauer, ffytche, Ballard, Brown, & Howard, 2006; Tam, Burton, McKeith, Burn, & O'Brien, 2005; Whitwell et al., 2007).
In the present study, we investigated whether executive resource limitations in non-aphasic patients with LBSD are associated with narrative discourse deficits. We elicited semi-structured speech samples in the narration of a wordless children's picture story, and we assessed performance on measures of executive functioning. We analyzed narrative speech to characterize discourse impairments in PD and DLB/PDD, and we related these impairments to the neuropsychological and neuroanatomical underpinnings of discourse deficits with volumetric MRI. We hypothesized that LBSD patients would be impaired in their ability to organize a narrative and that this deficit would be related in part to their executive resource limitations but minimally to other aspects of language and cognitive functioning. We also hypothesized that limited organizational attributes of LBSD narratives would be related to cortical atrophy affecting prefrontal cortex.
Methods
Subjects
We studied 32 non-aphasic patients with LBSD, diagnosed in the Cognitive Neurology or Movement Disorders clinics of the Department of Neurology at the University of Pennsylvania by experienced neurologists (RGG, AS, MG) according to published criteria (Hughes, Daniel, Kilford, & Lees, 1992; McKeith et al., 2005; McKeith et al., 1996; McKeith, O'Brien, & Ballard, 1999). Fourteen patients exhibited evidence of dementia (DLB/PDD), and 18 were not demented (PD). Patients were assigned to DLB/PDD or PD subgroups using a consensus evaluation based on modifications of published criteria that entailed two independent raters reviewing a semi-structured neurologic history, a complete neurologic exam, and a detailed mental status exam. In conjunction with clinical criteria, patients were classified as having dementia if (1) the Mini-Mental State Exam (MMSE) score was less than or equal to 24, or (2) the MMSE was greater than 24 but the patient performed in the demented range on the Mattis Dementia Rating Scale (DRS-2; age-adjusted score less than or equal to 5) (Folstein, Folstein, & McHugh, 1975; Lucas et al., 1998; Mattis, Jurica, & Leitten, 2001). This latter criterion was implemented for patients judged clinically to be demented who had a predominantly dysexecutive syndrome that was not detected by the MMSE, an instrument that is relatively insensitive to executive deficits. Of the 14 demented patients, 8 had a diagnosis of DLB and 6 had a diagnosis of PDD. In determining the diagnosis, the convention recommended by the Third Report of the DLB Consortium (McKeith et al., 2005) was followed: a diagnosis of PDD was made when motor symptoms preceded the onset of dementia by at least one year, and a diagnosis of DLB was made when dementia preceded the development of motor symptoms by at least one year. Features of DLB recognized in the Third Report of the DLB Consortium (McKeith et al., 2005) such as fluctuating cognition, variations in attention and alertness, and visual hallucinations were mild and did not interfere with performance at the time of testing.
Because LBSD is a spectrum disorder, means are presented for each patient subgroup separately and also for the combined subgroups. Demographic and clinical characteristics are summarized in Table 1. Clinical features include Unified Parkinson's Disease Rating Scale (UPDRS) motor assessments (Fahn, Elton, & UPDRS Program Members, 1987), Hoehn & Yahr staging (Hoehn & Yahr, 1967), and dopaminergic medication use. Dopaminergic medication use is expressed as levodopa equivalents. In accordance with Hobson, et al. (2002), the following dosages of medication are taken as equivalent: 100 mg levodopa; 130 mg controlled-release levodopa; 70 mg levodopa in conjunction with catechol-O-methyl transferase (COMT) inhibitor; 1 mg pergolide; 1 mg pramipexole; 5 mg ropinirole. Other PD medications (e.g., anticholinergics and monoamine oxidase inhibitors) were not included in the determination of levodopa equivalent dose. Exclusionary criteria included other causes of dementia, such as metabolic, endocrine, vascular, structural, nutritional, and infectious etiologies and primary psychiatric disorders. The DLB/PDD patients were mildly impaired according to the Mini Mental State Exam (MMSE) (Folstein et al., 1975). One-way ANOVAs indicated that control, PD, and DLB/PDD subject groups were matched for age and education. Disease duration, medication dosage, UPDRS motor disorder, and Hoehn & Yahr stage did not differ significantly across LBSD subgroups. Sixteen healthy seniors were evaluated as control subjects. All subjects completed an informed consent procedure in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the University of Pennsylvania.
Table 1. Mean ± standard deviation of demographic and clinical characteristics of patients and controls1, 2.
| Lewy Body Spectrum Disorder | DLB/PDD subgroup | PD subgroup | Controls | |
|---|---|---|---|---|
| N (male/female) | 21 / 11 | 10 / 4 | 11 / 7 | 5 / 11 |
| Age (yrs) | 71.9 ±8.5 | 72.6 ±9.4 (14) | 71.4 ±7.9 (18) | 68.6 ±6.8 (16) |
| Education (yrs) | 15.7 ±2.8 (32) | 15.6 ±3.0 (14) | 15.8 ±2.7 (18) | 15.6 ±2.6 (16) |
| MMSE (max=30) | 25.0 ±4.7** (29) | 20.9 ±4.7**++ (12) | 27.9 ±1.5* (17) | 29.1 ±1.2 (13) |
| Disease duration (yrs) | 6.3 ± 2.8 (30) | 6.6 ±2.3 (14) | 6.1 ±3.2 (16) | -- |
| Levadopa equivalent dose | 405 ±389 (23) | 287 ±377 (12) | 534 ±376 (11) | -- |
| UPDRS total motor Score | 22.6 ±11.4 (24) | 24.1 ±13.3 (13) | 20.9 ±9.1 (11) | -- |
| Hoehn & Yahr stage | 2.5 ±.07 (24) | 2.7 ±0.6 (13) | 2.2 ± 0.7 (11) | -- |
| Handedness (right/left) | 28 / 3 | 13 / 0 | 15 / 3 | 16 / 0 |
NOTES
Pairwise statistical differences between groups
differs from controls, p<.05
differs from controls, p<.01
differs from PD, p<.05
differs from PD, p<.01. Because of technical limitations in recovering some demographic and clinical features, we provide the numbers of participants ascertained for each characteristic in parentheses.
For all statistically significant comparisons of DLB/PDD patients to Controls, the effect size is “large” (Cohen's d > .8).
Materials
The subjects' task was to tell the story of the wordless children's picture book, Frog, Where Are You (Mayer, 1969). An outline of the story is given elsewhere (Ash et al., 2006). Briefly, the story begins with a boy and his dog admiring a frog that they keep in a large jar, as they prepare to go to bed for the night. The following morning, the boy and his dog find that the window is open and the frog is gone. The story illustrates the adventures of the boy and his dog as they search for the frog in the forest behind their house. Ultimately, they find their frog with a lady frog and a brood of baby frogs. The book's sequence of 24 drawings elicited an extended speech sample with a known target that was comparable in content across subjects and gave patients an opportunity to demonstrate the full breadth of their language production capability. We elected to study speech production in this manner to minimize the interruptions of turn-taking that occur in free conversation. We used a longer story rather than the description of a single picture in order to elicit a reasonably lengthy speech sample that was representative of the patient's speech and language abilities. We used a relatively unknown story rather than a fairy tale to avoid the intrusion of previously learned material.
Procedure
Each subject was asked to look through the book to become familiar with the story. When ready, the subject was asked to start at the beginning and narrate the story as if telling it to a child. Since the task was not intended to be a test of memory, participants looked at the story's pictures as they produced their narratives. Due to the nature of the protocol, there was no influence of the examiner on the time taken by the subjects to tell the story. Seventeen narrations were recorded on a Macintosh Powerbook G3 laptop computer using the Macintosh external microphone (part #590-0670) and the computer program SoundEdit 16, v. 2, with 16-bit recording at a sampling frequency of 44.1 kHz. Twenty-three were recorded on a Dell Inspiron 2200 PC using the signal processing software Praat (Boersma & Weenink, 1992-2005) with 16-bit recording at a sampling rate of 22.05 kHz, using a Radio Shack omnidirectional lavaliere electret condenser microphone. Eight were recorded on a Marantz PMD 670 digital recorder with 16-bit recording at a sampling frequency of 32 kHz, using a Sennheiser MKE2 omnidirectional lavaliere condenser microphone.
The recordings of the narratives were transcribed in detail by trained transcribers using the signal processing software Praat. The transcription conventions used to capture the irregularities in patients' speech are defined elsewhere (Ash et al., 2006). The narratives were scored from the transcripts by trained judges, referring to the original speech files as needed. All coding was checked by a linguist (SA) with expertise in phonetic, grammatical, and discourse analysis.
Narrative organization
To assess the hypothesis that LBSD patients have a disorder of narrative organization, we evaluated several aspects of narrative in the patients' productions:
Local connectedness
An event was scored as locally connected if the narrative gave a relationship between it and the preceding material. This was accomplished by rhetorical devices such as sequencing adverbials, pronominal reference to preceding events, reference by definite as opposed to indefinite determiners (Given vs. New information), and statements of cause and effect. Alternatively, the requirements of local connectedness are violated if a new element in the story is referred to in terms that are only appropriate for an element that has already been mentioned, as with definite determiners or pronominal reference when there is no immediately preceding noun antecedent. An event was scored as not connected if appropriate connecting devices were not present and the reported event did not follow logically from the preceding utterances.
The extract in (1) illustrates a failure of local connectedness. The speaker, an 80-year-old man with a 10-year history of DLB, is talking about the bees that are coming out of their hive, which is on the ground, since the dog has shaken it down from the tree where it was hanging. Then he turns his attention to the boy, who has climbed partway up a different tree and is looking in a hole in the trunk:
-
(1) (a)
It's a … it's an ug- bees, from- from the one hive, I guess.
-
(b)
Oh! By golly there's another one.
-
(c)
Uh that's t- about midway the- halfway up the tree, where the tree is- the base is broken.
In the third line, there is no explicit reference to the boy at all, although he has been newly introduced into the narrative and should have nominal reference. In addition, the tree that the boy is climbing is new to the story and should have an indefinite determiner. Thus line (c) represents a failure of local connectedness.
Search theme
The essence of the story is that a boy and his dog have a pet frog which escapes from the boy's room while he and the dog are asleep. They search for the frog, first in the boy's room and then outdoors in the woods near his house. Ultimately, they find the frog. Thus, the search for the frog is the central theme of the story, and keeping the theme in mind unifies the narrative and maintains its coherence. Search theme is scored from 0 to 4 by counting points accrued according to these criteria: one point for noting that the frog is missing, one point for noting that the boy is searching for the frog, one point for one or two further mentions of the search theme, and one point for any additional mentions of the search theme (Reilly, Losh, Bellugi, & Wulfeck, 2004).
Global connectedness
The resolution of the story is that the boy and dog find their frog. A narrative achieves global connectedness if the speaker acknowledges that the frog found at the end is the one that escaped from the boy's room at the beginning.
An extract of the speech of a representative DLB patient is given in (2). This illustrates the disconnected nature of the narrative that may be found in the speech of these patients. Lines (a) - (c) set the scene. Line (d) describes the action from which the entire rest of the story follows, but then, in (e), the speaker switches attention back to the boy, making no connection to the escape of the frog, only adding the detail of the presence of the dog on the bed. In (f), on the next page, the mention of the boy and dog being on the bed is repeated. Line (g) is an inaccurate description of the scene: it is plainly morning, and the boy is getting up, not preparing to go to sleep, and he is expressing surprise at the disappearance of the frog. Describing the next page, line (h) does not relate the action to the previous scene, since the boy is actually looking in his boot for the frog, not “playing with” his boot, as the speaker states. Line (i) redirects attention to the dog but does not express any connection between the dog's behavior and the preceding events. Line (j) switches reference back to the boy again, but still with no explicit or implicit connection to the preceding events.
(2) Male DLB patient, age 76
page 1: a) There's a boy, his little dog and his frog sitting up by the boy's bed.
page 1 or 2: b) And it's nighttime.
page 2: c) Boy's fallen asleep.
page 2: d) The frog is getting out of his … container.
page 2: e) and the dog is with the boy, I believe.
page 3: f) Yep, then uh there's a boy, in the bed with the dog on top of him.
page 3: g) and he's about ready to fall asleep I believe.
page 4: h) Boy's playing with his boots.
page 4: i) The dog's crawling into the.. container.
page 4: j) The boy's looking in the boots.
Speech analysis
To assess the hypothesis that speech and language difficulties contributed minimally to narrative deficits in LBSD, we evaluated lexical, grammatical and phonological aspects of the patients' stories (Table 2). To evaluate fluency and lexical aspects of the subjects' speech, we measured the total number of complete words spoken; the number of words per minute; the percentage of open class (content) words; and the mean length of utterance in words, where an utterance is defined as an independent clause and all clauses or phrases dependent on it (Hunt, 1965). Thus a series of independent clauses conjoined by and was counted as the number of utterances equal to the number of independent clauses. An incomplete sentence was also counted as an utterance if it stood alone in the flow of speech.
Table 2. Mean ± standard deviation for measures of language and speech 1, 2.
| Lewy Body Spectrum Disorder | DLB/PDD subgroup | PD subgroup | Controls | |
|---|---|---|---|---|
| Words and sentences | ||||
| Total word count | 534 ±228 | 452 ±291*+ | 598 ±212 | 609 ±228 |
| Words per minute | 106 ±45* | 73 ±39** | 131 ±32 | 140 ±22 |
| Open class words (%) | 41.1 ±3.3 | 41.1 ±4.0 | 41.1 ±2.8 | 42.8 ±3.1 |
| Mean length of utterance (words) | 10.2 ±3.2 | 9.5 ±3.9 | 10.7 ±2.6 | 10.8 ±2.1 |
| Well-formed utterances (%) | 87 ±18 | 77 ±24**++ | 95 ±6 | 99 ±2 |
| Utterances with complex structures (%) | 29 ±17 | 24 ±16* | 33 ±16 | 36 ±11 |
|
| ||||
| Speech sounds | ||||
| Phonetic errors/100 words | .117 ±.230* | .249 ±.301*+ | .014 ±.042 | .000 ±.000 |
| Phonemic errors/100 words | .75 ±1.58** | 1.58 ±2.15**++ | .109 ±.194 | .080 ±.165 |
NOTES
Pairwise statistical differences between groups:
differs from controls, p<.05
differs from controls, p<.01
differs from PD, p<.05
differs from PD, p<.01.
For all statistically significant comparisons of DLB/PDD patients to Controls, the effect size is “large” (Cohen's d > .8).
The percentage of utterances that were grammatically well formed was assessed. A well-formed utterance was one that was complete, with a subject and predicate, and free of grammatical errors, whether or not it was appropriate to the story. In addition, the percentage of utterances with complex structures was calculated. Complex structures included dependent clauses and phrasal adjuncts, defined elsewhere (Ash et al., 2009).
As a measure of phonology and articulatory performance, phonetic and phonemic errors were counted (Ash et al., 2010).
Characteristics of the subjects' speech output are summarized in Table 2. Speech rate was significantly diminished in LBSD compared to controls [U=141.0; p<0.05], and phonetic and phonemic speech errors were significantly more frequent in LBSD than in the control group [for phonetic errors, U=184.0; p<.05; for phonemic errors, U=143.0; p<0.01]. The two measures of syntax considered here, the proportion of well-formed utterances and syntactic complexity, did not differ significantly from those of controls [U=186.0; p>0.1 and U=180.5; p>0.09, respectively].
Inspection of patient subgroups revealed that DLB/PDD patients are impaired relative to controls on fluency, syntax, and phonology. Their overall rate of speech was about half that of healthy seniors [U=19.0; p<0.001], and it was also significantly less than that of PD patients [U=31.0; p<0.001], who speak at about the same rate as controls. DLB/PDD patients were impaired in comparison to both controls [U=46.5; p<0.01] and PD patients [U=54.5; p<0.01] on the proportion of well-formed sentences, and they were impaired in comparison to controls on the proportion of sentences with complex structures [U=60; p<0.05]. DLB/PDD patients made both phonetic and phonemic errors in the production of speech sounds [compared to controls, for phonetic errors, U=72.0; p<0.05; for phonemic errors, U=20.0; p<0.001]. However, they exhibited good access to the lexicon in terms of frequency of open class (content) words [U=123.0; p>0.9] and normal utterance length [U=88.0; p>0.1], compared to controls.
For the measures of language production within the DLB/PDD group, there was a significant difference between DLB and PDD only on words per minute [U=8.0; p<.05]. DLB patients produced an average of 53 words per minute, and PDD patients spoke at an average rate of 100 words per minute.
The speech output of PD patients, in contrast, was consistently similar to that of controls. They did make some phonetic errors, while control subjects made none, but the frequency of these errors did not differ significantly from that of controls [U=128.0; p>0.5].
Neuropsychological evaluation
To assess the hypothesis that executive functioning contributed to a narrative deficit, but memory, semantic, or visuospatial functioning did not, the patients underwent neuropsychological testing within an average of 88 (± 66) days of the date of narrative recording. Comparisons were made to performance on these tests using a panel of 25 healthy seniors matched for age and education.
Executive functioning was assessed by letter-guided category-naming fluency (FAS – averaged total number of non-repeated words in 1 minute for each letter), a test of mental search and planning; category-naming fluency for animals (total number of non-repeated animal names in 1 minute), a test of mental planning needed to search a semantic field; reverse digit span (total number of digits correctly repeated in reverse order), a test of working memory; time taken to complete Trails B (up to 180 sec), a test of planning and mental flexibility; and time taken to complete an 80-item color-word Stroop interference test (up to 180 sec), a test of inhibitory control. Episodic memory was tested by a three-trial verbal list-learning task with subsequent delayed free recall (maximum score = 10) and by delayed free recall of a modified Rey-Osterreith figure (maximum score = 36). Semantics was tested by an abbreviated form of the Boston Naming Test (% correct), and by the Pyramids and Palm Trees test (maximum score = 52), a test of object associative knowledge. Visuospatial functioning was tested by asking subjects to copy the Rey-Osterreith complex figure (maximum score = 36).
The results of the neuropsychological testing are listed in Table 3. The LBSD cases differed significantly from controls on most neuropsychological measures. Examination of demented and non-demented subgroups revealed that the differences were due largely to deficits within the DLB/PDD subgroup. Within the DLB/PDD group, we compared the 8 DLB and 6 PDD patients on these neuropsychological measures. There was no statistically significant difference between the two groups on 9 out of the 10 neuropsychological tests listed in Table 3. A difference was found only in performance on the FAS test [mean of DLB = 13.9, mean of PDD = 27.2 (for the 4 patients with data available), U=4.0; p<0.05]. The one test on which non-demented PD patients differed statistically from controls--the Pyramid and Palm Tree measure of semantic memory--exhibits a minimal deficit in absolute score and thus the difference may be due in part to a ceiling effect in controls.
Table 3. Mean ± standard deviation of neuropsychological characteristics of patients and controls1, 2.
| Lewy Body Spectrum Disorder | DLB/PDD Subgroup | PD subgroup | Controls | |
|---|---|---|---|---|
| Memory | ||||
| Word list recall | 4.9 ±3.2 (15) | 2.4 ±1.7**++ (8) | 7.8 ±1.6 (7) | 6.9 ±2.3 (18) |
| Rey recall (max=36) | 12.8 ±8.3* (22) | 6.7 ±5.7**++ (11) | 18.8 ±5.6 (11) | 20.2 ±7.4 (10) |
|
| ||||
| Executive function | ||||
| Letter-guided fluency (FAS) | 31.2 ±17.6* (29) | 18.3 ±10.8**++ (12) | 40.4 ±15.7 (17) | 44.1 ±9.6 (19) |
| Category fluency (animals) | 13.0 ±7.7** (29) | 7.1 ±3.6**++ (12) | 17.1 ±7.1 (17) | 21.3 ±5.2 (21) |
| Reverse digit span | 4.1 ±1.5* (27) | 3.0 ±0.8**++ (11) | 4.9 ±1.4 (16) | 5.5 ±1.6 (13) |
| Trails B time (sec) | 137.6 ±48.6 (26) | 173.6 ±15.7**++ (11) | 111.2 ±47.6 (15) | 108.6 ±44.2 (10) |
| Stroop time (sec) | 112.6 ±52.4 (22) | 159.6 ±32.7**++ (9) | 80.2 ±35.9 (13) | 76.2 ±18.5 (10) |
|
| ||||
| Semantics | ||||
| Boston Naming Test (% correct) | 89.7 ±8.8 (24) | 84.4 ±8.0*+ (12) | 91.4 ±8.6 (12) | 91.9 ±10.1 (19) |
| Pyramids & Palm Trees (max=52) | 46.9 ±5.5** (16) | 45.2 ±6.8** (9) | 49.0 ±2.1* (7) | 51.7 ±0.6 (13) |
|
| ||||
| Visuospatial function | ||||
| Rey copy (max=36) | 23.3 ±11.9** (22) | 16.8 ±12.1**++ (11) | 29.8 ±7.7 (11) | 34.2 ±3.1 (10) |
NOTES
Pairwise statistical differences between groups
differs from controls, p<.05
differs from controls, p<.01
differs from PD, p<.05
differs from PD, p<.01. Since not all participants were available for testing on all neuropsychological measures, we provide the numbers of participants ascertained for each characteristic in parentheses.
For all statistically significant comparisons of DLB/PDD patients to Controls, the effect size is “large” (Cohen's d > .8).
Statistical considerations
Levene's test of homogeneity of variance indicated that some measures of language and neuropsychological test scores did not meet the requirement of homogeneity of variance for parametric statistical tests. Therefore we used nonparametric tests to assess the differences between subject groups, which were calculated by the Mann-Whitney U statistic. Correlations were calculated using Spearman's rho. Global connectedness is a categorical variable; therefore eta, an unsigned statistic, was calculated to assess its association with neuropsychological variables, and chi-square was calculated to evaluate differences between groups. Effect sizes were measured by Cohen's d. All comparisons were motivated by a priori hypotheses; thus corrections for multiple comparisons were not performed.
Imaging methods
Eleven LBSD patients, including 7 patients with PD and 4 patients with PDD/DLB, had a volumetric brain MRI scan within one year of the narrative task [mean (SD) = 219 (176) days]. These 11 patients did not differ statistically from the larger set of 32 LBSD patients on any neuropsychological or language measures. (See Appendix, Table 1).
Ten patients had MRI scans acquired using a SIEMENS 1.5T scanner with 1.2-mm slice thickness and a 144 × 256 matrix. For one patient and 45 age-matched controls, images were collected using a SIEMENS Trio 3.0T scanner with 1-mm slice thickness and a 195 × 256 matrix. Images from both scanners were deformed into a standard local template space with a 1-mm3 resolution using PipeDream (https://sourceforge.net/projects/neuropipedream/) and Advanced Normalization Tools (ANTS, http://www.picsl.upenn.edu/ANTS/). These tools have been validated as stable and reliable for performing multivariate normalization (Avants, Epstein, Grossman, & Gee, 2008; Klein et al., 2009). Both PipeDream and ANTS mapped T1 structural MRI images to an optimal template space, using diffeomorphic and symmetric registration methods (Avants & Gee, 2004; Avants et al., 2010). The registered images were segmented into gray matter probability maps using template-based priors and then registered to MNI-template space for statistical comparisons. Gray matter probability images were smoothed in SPM5 (http://www.fil.ion.ucl.ac.uk/spm/sortware/spm5) using a 4-mm full-width half-maximum Gaussian kernel to minimize individual gyral variations.
In SPM5, a two-sample t-test contrasted gray matter probability between patients with LBSD and healthy controls to identify regions of significant cortical atrophy. For this atrophy analysis, an explicit mask was defined by generating a mean gray matter image from the healthy controls in order to limit the analysis to voxel-wise comparisons within gray matter. We used a p<0.05 (uncorrected) height threshold, 100-voxel extent, and accepted clusters with a peak voxel Z-score > 3.09 (p<0.001).
The regression module in SPM5 was used to relate gray matter atrophy to local connectedness, a measure of narrative organization. We performed a whole-brain analysis but then used an explicit mask so that we could examine the relationship between narrative performance and gray matter atrophy in brain areas known to be significantly atrophied from the prior analysis of whole brain gray matter atrophy. We related local connectedness to gray matter atrophy in the regression analysis because this variable is critical to the coherence of the narrative and it varies over a wide range (from 0 to 30), which makes it amenable to a meaningful regression analysis. The other two variables that relate to coherence of the narrative are restricted in their ranges: search theme varies only from 0 to 4, and global connectedness is only either present or absent. Finally, local connectedness was highly correlated with search theme (s=0.74), suggesting that local connectedness is representative of narrative organization performance. We interpreted only regions where narrative performance was related to atrophied gray matter areas because it would be difficult to explain with confidence significant associations between non-atrophied regions and patients' performance. For the regression analysis, we used a statistical height threshold of p<0.05 (uncorrected) and accepted clusters containing a peak with Z-score > 2.95 (p<0.002) and an extent greater than 50 voxels. Coordinates for all accepted clusters were converted to Talairach space (Talairach & Tournaux, 1988).
Results
Narrative organization
LBSD patients were impaired relative to controls on all measures of narrative organization. Performance on measures of narrative organization is summarized in Table 4. Inspection of performance in the patient subgroups revealed that these deficits are due to the subgroup of patients with DLB/PDD: these patients were impaired relative to both controls and PD patients on all measures of narrative structure. They conveyed a connection of one event to the next only about half the time [for DLB/PDD vs. controls, U=8.0; p<0.001; for DLB/PDD vs. PD, U=37.5; p<0.001]; they maintained the theme of searching for the frog to a very limited extent [for DLB/PDD vs. controls, U=16.0; p<0.001; for DLB/PDD vs. PD, U=43.0; p<0.01] and fewer than one-third of the patients understood the point of the story, as reflected by their low level of global connectedness [for DLB/PDD vs. controls, χ2 (1) =18.3, p<0.001; for DLB/PDD vs. PD, χ2 (1) =9.1, p<0.005]. Within the DLB/PDD group, the DLB and PDD patients differed in their performance on local connectedness [mean of DLB = 11.9, mean of PDD = 22.7, U=4.0; p<0.01] and search theme [mean of DLB = .25, mean of PDD = 2.3, U=6.0; p<0.05]. The PD patients did not differ from controls on any of the three measures of narrative organization.
Table 4. Mean ± standard deviation for measures of narrative discourse1, 2.
| Lewy Body Spectrum Disorder | DLB/PDD subgroup | PD subgroup | Controls | |
|---|---|---|---|---|
| Local connectedness (max=30) | 21.8 ±8.1** | 16.5 ±8.7**++ | 25.9 ±4.6 | 27.9 ±2.5 |
| Search theme maintenance (max=4) | 2.4 ±1.8** | 1.1 ±1.5**++ | 3.3 ±1.4 | 4.0 ±0.0 |
| Global connectedness (% patients) | 58 ±50** | 29 ±47**++ | 82 ±39 | 100 ±00 |
NOTES
Pairwise statistical differences between groups
differs from controls, p<.05
differs from controls, p<.01
differs from PD, p<.05
differs from PD, p<.012.
For all statistically significant comparisons of DLB/PDD patients to Controls, the effect size is “large” (Cohen's d > .8).
Correlations of cognitive, language and motor measures with narrative organization
We focused on narrative discourse measures in the subgroup of LBSD patients with DLB/PDD because PD patients were relatively unimpaired in their language and neuropsychological performance. We examined correlations of DLB/PDD patients' impaired narrative discourse performance with motor, neuropsychological, and language measures to investigate the basis for the narrative structure impairment (Table 5).
Table 5. Significant correlations of narrative discourse with motor, neuropsychological, and language measures in DLB/PDD1.
| Local connectedness | Search theme maintenance | Global connectedness | |
|---|---|---|---|
| Disease severity | |||
| Hoehn-Yahr stage (13) | -.61* | .70** | |
| Total motor score (13) | -.69** | ||
|
| |||
| Executive function | |||
| Reverse digit span (11) | .61* | ||
| Letter-guided fluency (12) | .70* | .60* | |
| Category fluency (12) | .62* | .64* | |
| Stroop time (9) | -.70* | ||
|
| |||
| Speech | |||
| Words per minute (14) | .72** | .67** | |
NOTES
Significant correlations
p<.05
p<.01. Since not all participants were available for testing on all neuropsychological measures, and because of technical limitations in recovering some clinical features, we provide the numbers of participants ascertained for each characteristic in parentheses.
Difficulty with local connectedness correlated with deficits on measures of executive functioning, including working memory and mental search. Similarly, difficulty maintaining the theme of the search for the frog also correlated with impaired executive functioning, including mental search and inhibitory control.
With respect to measures of speech production, local connectedness and search theme maintenance correlated with words per minute, the basic measure of fluency. We found no significant correlations of measures of narrative organization with impairments of syntax or phonology.
Local connectedness correlated with the motor score. Local connectedness and global connectedness also correlated with the Hoehn & Yahr stage of motor disease progression. It is noteworthy that the DLB/PDD patients' score of motor dysfunction also correlated with two aspects of speech production that depend intimately motor performance, the frequency of phonological errors (s=0.68; p<0.05) and speech rate (words per minute; s=-0.63; p<0.05).
Imaging
The structural images for 11 LBSD patients exhibited extensive gray matter atrophy compared to healthy seniors (Figure 1). The coordinates of atrophy peaks are given in Table 6. Atrophy was observed bilaterally in anterior, dorsolateral, and middle frontal regions. There was also atrophy in left ventrolateral, ventromedial, cingulate, and insula frontal regions, as well as right medial, inferior, lateral, and precentral frontal regions. In addition, there was atrophy in inferior, middle, superior, and posterolateral temporal regions bilaterally, and in left postcentral and inferior parietal regions. Further, there was atrophy in right hippocampal, lingual, middle occipital, and precuneus regions.
Figure 1. Cortical atrophy in Lewy body spectrum disorder patients1.
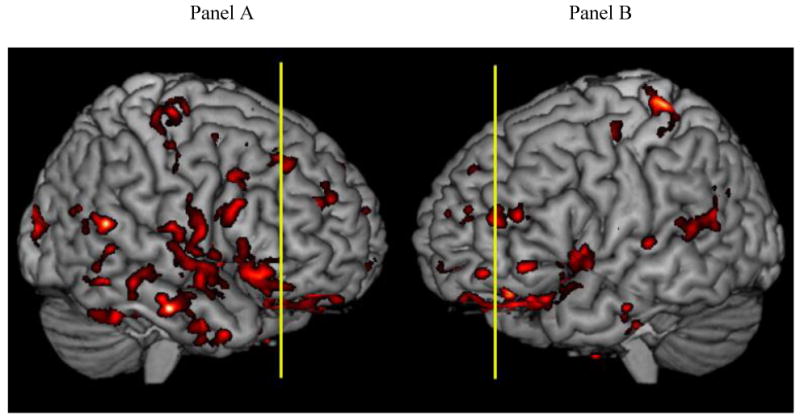
Note
1. Significant gray matter atrophy is shown in red. The vertical lines show the locations of the coronal slices displayed in Figure 2. In Panel A, the vertical slice is at y=38. In Panel B, the vertical slice is at y=48.
Table 6. Regional distribution of significant atrophy in Lewy body spectrum disorder patients.
| Anatomic locus (Brodmann area) | Coordinates | Z-score | Cluster size (voxels) | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Left anterior frontal (10) | -14 | 56 | 12 | 3.13 | 135 |
| Left anterior frontal (10) | -32 | 52 | -9 | 3.40 | 287 |
| Left dorsolateral prefrontal (46) | -45 | 41 | 16 | 3.34 | 474 |
| Left ventral lateral prefrontal (47) | -51 | 40 | -5 | 3.49 | 183 |
| Left ventral medial prefrontal (11) | -32 | 37 | -16 | 5.83 | 74087 |
| Left cingulate (33) | -4 | 21 | 19 | 3.48 | 717 |
| Left middle frontal (6) | -25 | -2 | 43 | 3.62 | 575 |
| Left insula | -35 | 9 | 4 | 3.33 | 2092 |
| Left insula | -40 | -7 | -6 | 3.46 | 1770 |
| Left inferior temporal (20) | -43 | -2 | -39 | 3.53 | 112 |
| Left inferior temporal (20) | -53 | -12 | -20 | 3.65 | 151 |
| Left inferior temporal (20) | -61 | -15 | -28 | 3.69 | 230 |
| Left middle temporal (21) | -50 | -48 | -1 | 3.40 | 244 |
| Left superior temporal (42) | -66 | -17 | 7 | 3.12 | 129 |
| Left superior temporal (22) | -62 | -49 | 21 | 3.13 | 640 |
| Left posterolateral temporal (39) | -42 | -52 | 29 | 3.60 | 617 |
| Left postcentral (1) | -33 | -33 | 67 | 4.38 | 647 |
| Left postcentral (3) | -21 | -37 | 60 | 3.32 | 138 |
| Left inferior parietal (40) | -36 | -30 | 35 | 3.18 | 105 |
| Right anterior frontal (10) | 3 | 49 | 14 | 4.54 | 293 |
| Right anterior frontal (10) | 25 | 49 | 19 | 3.53 | 472 |
| Right dorsolateral prefrontal (8) | 30 | 33 | 43 | 3.10 | 340 |
| Right dorsolateral prefrontal (9) | 34 | 14 | 28 | 4.22 | 928 |
| Right medial frontal (8) | 19 | 28 | 38 | 3.96 | 705 |
| Right inferior frontal (44) | 53 | 22 | 24 | 3.44 | 426 |
| Right middle frontal (6) | 26 | 0 | 41 | 3.92 | 766 |
| Right lateral frontal (6) | 36 | -15 | 63 | 3.45 | 864 |
| Right precentral (4) | 46 | -10 | 44 | 3.12 | 185 |
| Right inferior temporal (20) | 59 | -13 | -20 | 4.90 | 805 |
| Right middle temporal (21) | 46 | 9 | -32 | 3.49 | 954 |
| Right middle temporal (21) | 66 | -28 | -9 | 3.28 | 510 |
| Right middle temporal (21) | 55 | -46 | -8 | 3.66 | 276 |
| Right superior temporal (22) | 51 | 4 | -5 | 3.54 | 1696 |
| Right superior temporal (22) | 62 | -39 | 5 | 3.22 | 165 |
| Right superior temporal (22) | 63 | -41 | 17 | 4.58 | 362 |
| Right posterolateral temporal (39) | 41 | -57 | 29 | 3.43 | 568 |
| Right hippocampal (36) | 27 | -35 | -9 | 4.30 | 177 |
| Right precuneus (7) | 11 | -45 | 48 | 3.35 | 152 |
| Right precuneus | 18 | -66 | 24 | 3.67 | 238 |
| Right lingual (19) | 16 | -71 | -3 | 3.22 | 181 |
| Right cuneus/middle occipital (18/31) | 23 | -79 | 20 | 5.16 | 1226 |
| Right middle occipital (19) | 42 | -83 | 13 | 3.43 | 554 |
| Right middle occipital (18) | 28 | -83 | 3 | 4.07 | 290 |
We performed a whole brain regression analysis to relate local connectedness, one of the primary features of narrative discourse coherence, to gray matter atrophy. Figure 2 displays two coronal slices illustrating regions of significant atrophy that are related to local connectedness and that also overlap with areas of gray matter atrophy. In Figure 2, Panel A shows the left ventrolateral prefrontal (BA 47) and left ventromedial (BA 11) areas of atrophy that were significantly related to local connectedness, and Panel B illustrates the right ventromedial (BA 11) region that was significantly related to local connectedness. The coordinates of the peak voxels of the significant clusters in the regression analysis are given in Table 7. We observed correlations of atrophy with local connectedness bilaterally in ventromedial and ventrolateral prefrontal cortex and also in left cingulate, putamen, and temporal regions.
Figure 2.
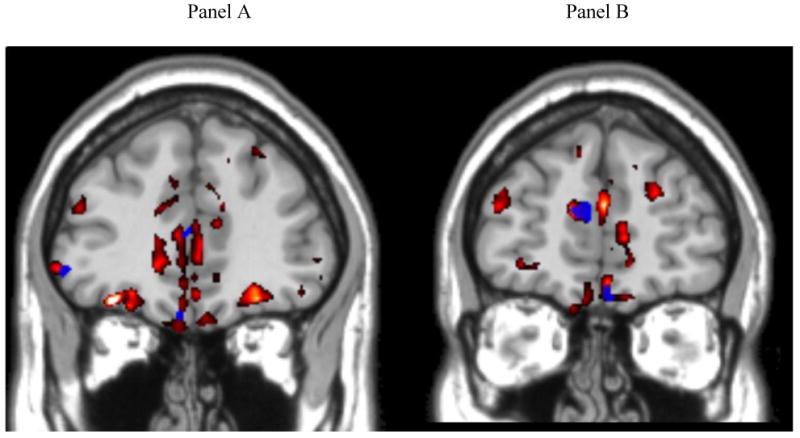
Regression analysis relating Local Connectedness to gray matter atrophy in brain regions that have significant gray matter atrophy. 1
Note
1. Significant gray matter atrophy is shown in red. Areas of correlation with local connectedness are shown in blue. Increasing impairment in local connectedness is related to cortical thinning. Panel A: y-axis = 38; Panel B: y-axis = 48. Coordinates of the atrophy peaks are given in Table 7.
Table 7. Regional distribution of significant atrophy in Lewy body spectrum disorder patients related to narrative discourse (local connectedness).
| Anatomic locus (Brodmann area) | Coordinates | Z-score | Cluster size (voxels) | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Left ventral lateral prefrontal (47) | -52 | 38 | -7 | 2.99 | 63 |
| Left ventral medial prefrontal (11) | -5 | 34 | -19 | 3.08 | 58 |
| Left anterior cingulate (32) | -5 | 46 | 9 | 2.97 | 477 |
| Left putamen | -25 | -7 | 6 | 3.37 | 78 |
| Left superior temporal (22) | -63 | -37 | 11 | 2.96 | 62 |
| Left middle temporal (21/37) | -48 | -47 | -5 | 3.84 | 186 |
| Right ventral lateral prefrontal (47) | 48 | 36 | -10 | 2.95 | 138 |
| Right ventral medial prefrontal (11) | 6 | 50 | -18 | 3.12 | 216 |
Discussion
We studied 32 patients with LBSD to determine whether and to what extent this syndrome disrupts the organization of narrative discourse. We assessed the ability of speakers to communicate a narrative effectively in the task of telling a complex story. The story was presented to the subject as a series of detailed drawings that were easily and accurately interpreted by healthy seniors, and the narrative was performed while the subjects were looking at the pictures to minimize the impact of memory difficulty on narrative performance. A detailed quantitative analysis revealed a significant narrative production deficit in non-aphasic patients with LBSD. This deficit was most evident in the DLB/PDD subgroup of LBSD patients. Difficulty with narrative organization in the DLB/PDD subgroup was related to impairments on measures of executive functioning and speech fluency, while other measures of language and cognitive functioning were unrelated to their narrative production. A regression analysis related the deficit for narrative organization in LBSD patients to gray matter atrophy in ventral frontal and anterior cingulate regions. We discuss each of these issues below.
We examined the narratives produced by LBSD patients and healthy seniors to assess the communicative competence of the patients, and we related the organization of their narratives to language-specific attributes of their speech, neuropsychological measures of executive functioning, and imaging studies in order to elucidate the basis for their narrative impairment. LBSD patients demonstrated significant deficits in their narratives. These included an impairment in connecting one scene to the next over the course of the story, poor ability to maintain the search theme throughout the narrative, and difficulty appreciating the resolution of the story. These aspects of the narrative do not involve processing at the level of a single word or sentence, but instead appear to depend largely on a higher-level organizational component (Farag et al., 2010; Mar, 2004). Several previous studies have emphasized the contribution of executive resources to organizational aspects of a narrative. Non-aphasic patients with behavioral variant frontotemporal dementia have shown a significant deficit in the production of an organized narrative (Ash et al., 2006). A study of narrative comprehension revealed that these patients were impaired at judging errors in the ordering of events in a brief script, although they were relatively unimpaired at identifying semantically anomalous single events in a script (Cosentino, Chute, Libon, Moore, & Grossman, 2006). In a follow-up study, we examined whether there was a higher-level organizational deficit or difficulty ordering events in a narrative, and we found that non-aphasic behavioral variant frontotemporal dementia patients were impaired at assessing the top-down organization of brief narratives (Farag et al., 2010). In all of these studies, impairments on measures of narrative organization were related to deficits on neuropsychological measures of executive control.
Inspection of the data in the present study revealed that the narrative deficit in LBSD is attributable largely to the DLB/PDD subgroup of LBSD patients. This subgroup has a progressive dementia involving executive functioning, memory, and visuospatial processing (Emre et al., 2007; McKeith et al., 2005). Indeed, the DLB/PDD patients participating in this study were impaired on neuropsychological measures in these cognitive domains. It is important to point out, however, that non-specific dementia may not be an adequate explanation for the discourse deficit in DLB/PDD. While some measures showing impaired neuropsychological functioning were correlated with deficits in the organization of the narrative, other measures were not correlated, such as visual-perceptual functioning. Patients with Alzheimer's disease appear to have difficulty with lengthy narratives because of their memory impairment, but a study investigating brief narratives showed that Alzheimer's disease patients do not differ from controls in their processing of narrative organization (Farag et al., 2010).
Our findings support the contention that executive resources play a central role in the ability of DLB/PDD patients to organize their production of a narrative. The two features of narrative performance that entail the greatest executive resource demands are local connectedness and maintenance of the search theme. These require the storyteller to keep the present and immediately past elements of the story in working memory, to keep in mind the continuing theme of the story, and to embed these events in an appropriate context that is governed by top-down organization. The demand on executive resources is shown by the correlations of local connectedness and search theme maintenance in DLB/PDD with tests of mental organization, working memory, and inhibitory control (Table 5). As far as we are aware, this is the first study to demonstrate a role for executive resources in the narrative discourse deficits of patients with DLB/PDD.
We also found that narrative organization is more impaired in DLB than PDD with regard to both local connectedness and search theme. Although only small numbers of patients were examined with each of these clinical phenotypes, this observation is in line with the findings of previous reports of poorer executive functioning in mild DLB than in mild PDD (Aarsland et al., 2003; Downes et al., 1998). These authors interpret their findings as evidence that involvement of the frontal cortex occurs earlier in DLB than in PDD. This is consistent with our view that while DLB and PDD may differ in the progression of impairment, they represent a single spectrum of disease. Longitudinal studies of these patients are needed to evaluate this hypothesis empirically.
The DLB/PDD patients were not clinically aphasic, but they were significantly impaired on some language-specific measures of speech production, including the amount and rate of speech output, the proportions of grammatical and syntactically complex utterances, and the frequency of speech sound errors. The only correlation of a language measure with features of narrative production was the correlation of speech rate, in words per minute, with local connectedness and search theme. Speech rate is a very general measure that depends on many factors, such as motor control, attention, grammatical facility, and access to the lexicon. Speech rate may be reduced for different reasons in different patient groups (Ash et al., 2009), so by itself, this finding only signals some unspecified disruption of speech production. Speech rate in DLB/PDD appears to be correlated with motor functioning as well. This observation merits further study, though it is outside the scope of the present report.
Previous work has demonstrated a deficit of grammatical processing in LBSD, although this work has concentrated on non-demented LBSD patients with PD. This prior work has also emphasized the role of executive resources in processing grammatically demanding sentences. For example, correlation studies have related the comprehension of complex grammatical sentences to measures of executive functioning such as working memory and information processing speed, and on-line studies of grammatical processing have demonstrated the contribution of executive resources to grammatical processing in PD that is independent of task-related performance demands (Grossman, 1999). The present study found a deficit in grammatical competence in the narrative production of DLB/PDD patients, but we did not find a significant correlation between syntax and narrative organization. Previous studies of grammatical processing in LBSD have focused largely on comprehension, while the present study assessed the production of syntax and narrative, which may draw on different resources from those involved in comprehension. While executive resources may contribute independently to grammatical comprehension, grammatical production, and narrative production, the nature of these resources may differ depending on the specific aspect of language that is being processed.
Phonological features of speech production were impaired in these patients as well, but this did not correlate with the narrative deficit. Speech sound errors in LBSD may be due in part to a motor disorder that interferes with articulatory clarity (Skodda, Visser, & Schlegel, 2010), although additional work is needed to determine whether all errors of speech sound production are due to a motor disorder in these patients. Taken together, these observations imply that deficits in the structuring of narrative speech in LBSD are not related to an undifferentiated impairment of speech and language, but depend in part on working memory and other executive resources which appear to play a role in organizing a narrative.
We found further that organizational components of narrative discourse are correlated with motor functioning. As an extension of the motor theory of speech perception, recent work based on the hypothesized mirror neuron system has implicated a motor disorder in deficits of many aspects of speech and language (Hauk, Johnsrude, & Pulvermuller, 2004; Liberman, Cooper, Shankweiler, & Studdert-Kennedy, 1967; Liberman & Mattingly, 1985). Other work has related greater difficulty with action verbs than object nouns in PD to the motor deficit of these patients (Boulenger et al., 2008). However, this line of reasoning must be interpreted cautiously (Hickok, 2010). Evidence against a role for the mirror neuron system in narrative organization comes from the finding that, although the subgroup of patients with PD has an equally prominent motor disorder, PD patients have minimal difficulty with narrative discourse. Additional work is needed to determine the basis for the association of a motor impairment with impaired organization of narrative discourse in DLB/PDD.
Non-demented PD patients do not appear to have a significant impairment of narrative organization. They also have fewer language and cogntive deficits than the DLB/PDD subgroup of patients. Many studies have reported minimal speech deficits in non-demented PD patients, although this has rarely been quantified (Bayles, 1990; Piatt et al., 1999). The present report supports this generalization of reasonably preserved language production in PD. In particular, the organization of the narratives of the 18 PD patients in this study did not differ substantially from those of healthy seniors (Table 4) on either local or global connectedness of the narrative or on maintenance of the search theme. Furthermore, the PD patients did not differ from healthy seniors on several basic measures of linguistic competence in expression, such as speech output, speech rate, syntactic competence, or speech sound errors (Table 2). In addition, the PD patients were relatively unimpaired in their performance on neuropsychological measures, including measures of executive resources. One reason for the discrepancy relative to prior work in PD may be that previous assessments of language in PD have focused on comprehension, and the present study examined expression. Another possibility is that a subset of PD patients assessed in prior work demonstrated an executive deficit but were not otherwise demented, while the present study carefully separated LBSD patients with a cognitive deficit from those with essentially normal cognitive performance. With these caveats in mind, we did find a possible deficit in semantic memory in non-demented patients with PD. This has been observed previously (Copland, 2003, 2006) and may be related to factors such as selection and information processing speed that are mediated in part by changes in dopaminergic functioning in the basal ganglia (Chenery, Angwin, & Copland, 2008; Copland, McMahon, Silburn, & de Zubicaray, 2009). One study has related a semantic deficit to narrative discourse impairments in PD (Godbout & Doyon, 2000), although we did not find a correlation between semantic difficulty and narrative impairments. Godbout and Doyon (2000) did not characterize the narrative impairment in LBSD quantitatively, and additional work is needed to specify the precise contribution of semantic limitations to the narrative deficits of LBSD patients. The imaging results emphasize the contribution of frontal brain regions to narrative organization. Cortical atrophy in LBSD is seen bilaterally in frontal, temporal, and parietal/occipital regions (Burton et al., 2009; Burton et al., 2004; Sauer et al., 2006; Tam et al., 2005; Whitwell et al., 2007). We replicated these findings in the present study. Moreover, within this area of atrophy, we observed a specific pattern of gray matter thinning related to narrative organization. While we know that regression analyses related local connectedness to cortical thickness in regions that are not significantly atrophied, we did not interpret these regressions because neither their presence nor their absence can be explained in any clear manner. For example, such a finding in areas of no associated significant atrophy might suggest that the area may become atrophied in the future, that the component of the task related to the area is not central to the task, or that the area plays a marginal role in task performance. Local connectedness was related to atrophy bilaterally in ventral prefrontal regions. This observation suggests a role for ventral frontal cortex in processing narrative production by contributing to maintenance of cohesion within the narrative. In an fMRI study of comprehension, healthy young adults showed greater bilateral ventral frontal activation in judgments of more closely related events compared to less closely related events taken from short scripts (Farag et al., 2010). In the same study, patients with progressive non-fluent aphasia and behavioral variant frontotemporal dementia (bvFTD) did not distinguish between more and less closely related events, and structural MRI analysis showed that these patients had significantly more atrophy relative to healthy seniors in a region of interest corresponding to the left ventral frontal activation seen in the fMRI study of healthy young adults (Farag et al., 2010). These results implicate ventral frontal regions in the processing of discourse cohesion. In a study of language production using the same procedure as the present report, significant cortical atrophy was found in right ventral frontal regions in bvFTD patients, and this correlated with poor local connectedness (Ash et al., 2006). Another investigation used arterial spin labeling perfusion fMRI in healthy young adults performing a story-telling task with the same stimuli. This study found activation bilaterally in ventral frontal regions on the task of narrating a continuous story relative to describing single, unconnected pictures (Troiani et al., 2008). Independent evidence that these frontal brain regions contribute to executive resources comes from a variety of imaging studies of healthy adults that show activation of these areas during performance of planning, working memory, and decision-making tasks (Ramnani & Owen, 2004). The involvement of brain regions in the right hemisphere not typically associated with core elements of language processing is consistent with the hypothesis that the executive resource component of narrative organization is not necessarily linguistic. These converging results imply that impaired discourse cohesion in DLB/PDD patients is related to executive and organizational deficits associated with frontal lobe atrophy.
The regression analysis also related local connectedness to atrophy in the anterior cingulate (BA 32). Previous work has suggested that this area is associated with a component of top-down organization involved in response selection (Botvinick et al., 2001; Botvinick, Cohen, & Carter, 2004), and selection has been implicated in the language-mediated deficits of PD patients (Copland, 2006). The present study suggests that response selection mediated by the anterior cingulate may contribute to decisions about narrative organization as well.
A limitation of the study is the variability of the interval between the narrative task and the MRI scan. The average interval for the 11 patients in our sample is about 7 months. Parkinson's disease progresses slowly, with survival often decades long. In this context, an average discrepancy between behavioral study and image acquisition of 7 months is acceptable. Nevertheless, a larger study is needed to help estimate the maximum acceptable difference that allows reasonable inferences about brain-behavior relationships.
In sum, our findings are consistent with a model of narrative discourse that includes both linguistic and non-linguistic components. It appears that a resource-related deficit in planning and organization interferes with narrative discourse in patients with DLB and PDD. Moreover, this pattern of impairment is related to disease affecting frontal brain regions in these patients.
Supplementary Material
*Highlights.
We examined narrative discourse in patients with Lewy body spectrum disorder. Lewy body disease and PD with dementia patients show impaired narrative organization. Their narrative organization is correlated with deficits in executive functioning. Their impaired discourse cohesion is related to reduced frontal cortical volume. Narrative discourse includes both linguistic and non-linguistic components.
Acknowledgments
This work was supported by the Morris K. Udall Parkinson's Disease Research Center of Excellence and the National Institutes of Health (AG17586, AG15116, NS44266, and NS53488).
This work was supported by the National Institutes of Health (NS53488, AG17586, AG15116, NS44266, and AG32953).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarsland D, Litvan I, Salmon D, Galasko D, Wentzel-Larsen T, Larsen JP. Performance on the dementia rating scale in Parkinson's disease with dementia and dementia with Lewy bodies: Comparison with progressive supranuclear palsy and Alzheimer's disease. Journal of Neurology, Neurosurgery and Psychiatry. 2003;74:1215–1220. doi: 10.1136/jnnp.74.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash S, McMillan C, Gunawardena D, Avants B, Morgan B, Khan A, et al. Speech errors in progressive non-fluent aphasia. Brain Lang. 2010;113(1):13–20. doi: 10.1016/j.bandl.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash S, Moore P, Antani S, McCawley G, Work M, Grossman M. Trying to tell a tale: Discourse impairments in progressive aphasia and frontotemporal dementia. Neurology. 2006;66:1405–1413. doi: 10.1212/01.wnl.0000210435.72614.38. [DOI] [PubMed] [Google Scholar]
- Ash S, Moore P, Vesely L, Gunawardena D, McMillan C, Anderson C, et al. Non-fluent speech in frontotemporal lobar degeneration. Journal of Neurolinguistics. 2009;22:370–383. doi: 10.1016/j.jneuroling.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants B, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12(1):26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants B, Gee JC. Geodesic estimation for large deformation anatomical shape and intensity averaging. Neuroimage. 2004;23:S139–S150. doi: 10.1016/j.neuroimage.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Avants B, Yushkevich P, Pluta J, Minkoff D, Korczykowski M, Detre J, et al. The optimal template effect in hippocampus studies of diseased populations. Neuroimage. 2010;49(3):2457–2466. doi: 10.1016/j.neuroimage.2009.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, D'Esposito M. Functional magnetic resonance imaging evidence for a hierarchical organization of the prefrontal cortex. J Cogn Neurosci. 2007;19(12):2082–2099. doi: 10.1162/jocn.2007.19.12.2082. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Selection, integration, and conflict monitoring: Assessing the nature and generality of prefrontal cognitive control mechanisms. Neuron. 2004;41:473–487. doi: 10.1016/s0896-6273(03)00851-1. [DOI] [PubMed] [Google Scholar]
- Bastiaanse R, Leenders KL. Language and Parkinson's disease. Cortex. 2009;45(8):912–914. doi: 10.1016/j.cortex.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Bayles KA. Language and Parkinson disease. Alzheimer Dis Assoc Disord. 1990;4(3):171–180. doi: 10.1097/00002093-199040300-00005. [DOI] [PubMed] [Google Scholar]
- Boersma P, Weenink D. Praat, v. 4.3.27: Institute of Phonetic Sciences. University of Amsterdam; 1992-2005. [Google Scholar]
- Bosboom JL, Stoffers D, Wolters E. Cognitive dysfunction and dementia in Parkinson's disease. J Neural Transm. 2004;111(10-11):1303–1315. doi: 10.1007/s00702-004-0168-1. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Boulenger V, Mechtouff L, Thobois S, Broussolle E, Jeannerod M, Nazir TA. Word processing in Parkinson's disease is impaired for action verbs but not for concrete nouns. Neuropsychologia. 2008;46(2):743–756. doi: 10.1016/j.neuropsychologia.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Braun AR, Guillemin A, Hosey L, Varga M. The neural organization of discourse: An H215O-PET study of narrative production in English and American sign language. Brain. 2001;124:2028–2044. doi: 10.1093/brain/124.10.2028. [DOI] [PubMed] [Google Scholar]
- Braver TS, Bongiolatti SR. The role of frontopolar cortex in subgoal processing during working memory. Neuroimage. 2002;15:523–536. doi: 10.1006/nimg.2001.1019. [DOI] [PubMed] [Google Scholar]
- Brown RG, Marsden CD. How common is dementia in Parkinson's disease? Lancet. 1984;2:1262–1265. doi: 10.1016/s0140-6736(84)92807-1. [DOI] [PubMed] [Google Scholar]
- Brown RG, Marsden CD. Cognitive function in Parkinson's disease: from description to theory. Trends Neurosci. 1990;13(1):21–29. doi: 10.1016/0166-2236(90)90058-i. [DOI] [PubMed] [Google Scholar]
- Burton EJ, Barber R, Mukaetova-Ladinska EB, Robson J, Perry RH, Jaros E, et al. Medial temporal lobe atrophy on MRI differentiates Alzheimer's disease from dementia with Lewy bodies and vascular cognitive impairment: a prospective study with pathological verification of diagnosis. Brain. 2009;132(Pt 1):195–203. doi: 10.1093/brain/awn298. [DOI] [PubMed] [Google Scholar]
- Burton EJ, McKeith IG, Burn DJ, Williams ED, O'Brien JT. Cerebral atrophy in Parkinson's disease with and without dementia: a comparison with Alzheimer's disease, dementia with Lewy bodies and controls. Brain. 2004;127(Pt 4):791–800. doi: 10.1093/brain/awh088. [DOI] [PubMed] [Google Scholar]
- Calderon J, Perry RJ, Erzinclioglu S, Berrios GE, Dening TR, Hodges JR. Perception, attention, and working memory are disproportionately impaired in dementia with Lewy bodies compared with Alzheimer's disease. Journal of Neurology, Neurosurgery and Psychiatry. 2001;70:157–164. doi: 10.1136/jnnp.70.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenery HJ, Angwin AJ, Copland DA. The basal ganglia circuits, dopamine, and ambiguous word processing: a neurobiological account of priming studies in Parkinson's disease. J Int Neuropsychol Soc. 2008;14(3):351–364. doi: 10.1017/S1355617708080491. [DOI] [PubMed] [Google Scholar]
- Colman KS, Koerts J, van Beilen M, Leenders KL, Post WJ, Bastiaanse R. The impact of executive functions on verb production in patients with Parkinson's disease. Cortex. 2009;45(8):930–942. doi: 10.1016/j.cortex.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Copland DA. The basal ganglia and semantic engagement: potential insights from semantic priming in individuals with subcortical vascular lesions, Parkinson's disease, and cortical lesions. J Int Neuropsychol Soc. 2003;9(7):1041–1052. doi: 10.1017/S1355617703970081. [DOI] [PubMed] [Google Scholar]
- Copland DA. Meaning selection and the subcortex: evidence of reduced lexical ambiguity repetition effects following subcortical lesions. J Psycholinguist Res. 2006;35(1):51–66. doi: 10.1007/s10936-005-9003-6. [DOI] [PubMed] [Google Scholar]
- Copland DA, McMahon KL, Silburn PA, de Zubicaray GI. Dopaminergic neuromodulation of semantic processing: a 4-T FMRI study with levodopa. Cereb Cortex. 2009;19(11):2651–2658. doi: 10.1093/cercor/bhp017. [DOI] [PubMed] [Google Scholar]
- Cosentino S, Chute D, Libon D, Moore P, Grossman M. How does the brain support script comprehension? A study of executive processes and semantic knowledge in dementia. Neuropsychology. 2006;20(3):307–318. doi: 10.1037/0894-4105.20.3.307. [DOI] [PubMed] [Google Scholar]
- Double KL, Halliday GM, McRitchie DA, Reid WG, Hely MA, Morris JG. Regional brain atrophy in idiopathic parkinson's disease and diffuse Lewy body disease. Dementia. 1996;7(6):304–313. doi: 10.1159/000106896. [DOI] [PubMed] [Google Scholar]
- Downes JJ, Priestley NM, Doran M, Ferran J, Ghadiali E, Cooper P. Intellectual, mnemonic, and frontal functions in dementia with Lewy bodies: A comparison with early and advanced Parkinson's disease. Behav Neurol. 1998;11(3):173–183. [PubMed] [Google Scholar]
- Ebmeier KP, Calder SA, Crawford JR, Stewart L, Cochrane RHB, Besson JAO. Dementia in idiopathic Parkinson's disease: prevalence and relationship with symptoms and signs of Parkinsonism. Psychological Medicine. 1991;21:69–76. doi: 10.1017/s0033291700014665. [DOI] [PubMed] [Google Scholar]
- Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord. 2007;22(12):1689–1707. doi: 10.1002/mds.21507. quiz 1837. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton R . UPDRS Program Members. Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Goldstein M, Calne D, editors. Recent Developments in Parkinson's Disease. Florham Park, NJ: Macmillan Healthcare Information; 1987. pp. 153–163.pp. 293–304. [Google Scholar]
- Farag C, Troiani V, Bonner M, Powers C, Avants B, Gee J, et al. Hierarchical organization of scripts: Converging evidence from fMRI and frontotemporal dementia. Cerebral Cortex. 2010;20(10):2453–2463. doi: 10.1093/cercor/bhp313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SF, McHugh PR. “Mini Mental State.” A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Giladi N, Treves TA, Paleacu D, Shabtai H, Orlov Y, Kandinov B, et al. Risk factors for dementia, depression and psychosis in long-standing Parkinson's disease. J Neural Transm. 2000;107(1):59–71. doi: 10.1007/s007020050005. [DOI] [PubMed] [Google Scholar]
- Girotti F, Soliveri P, Carella F, Piccolo I, Caffarra P, Musicco M, et al. Dementia and cognitive impairment in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1988;51(12):1498–1502. doi: 10.1136/jnnp.51.12.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout L, Doyon J. Defective representation of knowledge in Parkinson's disease: Evidence from a script-production task. Brain and Cognition. 2000;44:490–510. doi: 10.1006/brcg.2000.1213. [DOI] [PubMed] [Google Scholar]
- Grossman M. Sentence processing in Parkinson's disease. Brain and Cognition. 1999;40:387–413. doi: 10.1006/brcg.1999.1087. [DOI] [PubMed] [Google Scholar]
- Harrington CR, Perry RH, Perry EK, Hurt J, McKeith IG, Roth M, et al. Senile dementia of Lewy body type and Alzheimer type are biochemically distinct in terms of paired helical filaments and hyperphosphorylated tau protein. Dementia. 1994;5(5):215–228. doi: 10.1159/000106727. [DOI] [PubMed] [Google Scholar]
- Hauk O, Johnsrude I, Pulvermuller F. Somatotopic representation of action words in human motor and premotor cortex. Neuron. 2004;41:301–307. doi: 10.1016/s0896-6273(03)00838-9. [DOI] [PubMed] [Google Scholar]
- Hickok G. The role of mirror neurons in speech and language processing. Brain Lang. 2010;112(1):1–2. doi: 10.1016/j.bandl.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson DE, Lang AE, Martin WR, Razmy A, Rivest J, Fleming J. Excessive daytime sleepiness and sudden-onset sleep in Parkinson disease: a survey by the Canadian Movement Disorders Group. Jama. 2002;287(4):455–463. doi: 10.1001/jama.287.4.455. [DOI] [PubMed] [Google Scholar]
- Hochstadt J. Set-shifting and the on-line processing of relative clauses in Parkinson's disease: results from a novel eye-tracking method. Cortex. 2009;45(8):991–1011. doi: 10.1016/j.cortex.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Hoehn MD, Yahr M. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt KW. Grammatical structures written at three grade levels. Champaign, IL: National Council of Teachers of English; 1965. [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46(3):786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E, Jubault T. Broca's area and the hierarchical organization of human behavior. Neuron. 2006;50:963–974. doi: 10.1016/j.neuron.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Kraybill ML, Larson EB, Tsuang DW, Teri L, McCormick WC, Bowen JD, et al. Cognitive differences in dementia patients with autopsy-verified AD, Lewy body pathology, or both. Neurology. 2005;64:2069–2073. doi: 10.1212/01.WNL.0000165987.89198.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph MA, Powell J, Howard D, Whitworth AB, Garrard P, Hodges JR. Semantic memory is impaired in both dementia with Lewy bodies and dementia of Alzheimer's type: A comparative neuropsychological study and literature review. Journal of Neurology, Neurosurgery and Psychiatry. 2001;70:149–156. doi: 10.1136/jnnp.70.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees AJ. Parkinson's disease and dementia. Lancet. 1985;1(8419):43–44. doi: 10.1016/s0140-6736(85)90986-9. [DOI] [PubMed] [Google Scholar]
- Levin BE, Tomer R, Rey GJ. Cognitive impairments in Parkinson's disease. Neurol Clin. 1992;10(2):471–485. [PubMed] [Google Scholar]
- Liberman AM, Cooper FS, Shankweiler DP, Studdert-Kennedy M. Perception of the speech code. Psychol Rev. 1967;74(6):431–461. doi: 10.1037/h0020279. [DOI] [PubMed] [Google Scholar]
- Liberman AM, Mattingly IG. The motor theory of speech perception revised. Cognition. 1985;21:1–36. doi: 10.1016/0010-0277(85)90021-6. [DOI] [PubMed] [Google Scholar]
- Libon DJ, Bogdanoff B, Leopold N, Hurka R, Bonavita J, Skalina S, et al. Neuropsychological profiles associated with subcortical white matter alterations and Parkinson's disease: implications for the diagnosis of dementia. Arch Clin Neuropsychol. 2001;16(1):19–32. [PubMed] [Google Scholar]
- Lucas JA, Ivnik RJ, Smith GE, Bohac DL, Tangalos EG, Kokmen E, et al. Normative data for the Mattis Dementia Rating Scale. J Clin Exp Neuropsychol. 1998;20(4):536–547. doi: 10.1076/jcen.20.4.536.1469. [DOI] [PubMed] [Google Scholar]
- Mar RA. The neuropsychology of narrative: Story comprehension, story production, and their interrelation. Neuropsychologia. 2004;42:1414–1434. doi: 10.1016/j.neuropsychologia.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Mattis S, Jurica PJ, Leitten CL. Dementia Rating Scale-2: Professional Manual. Lutz, FL: Psychological Assessment Resources, Inc.; 2001. [Google Scholar]
- Mayer M. Frog, Where Are You? New York: Penguin Books; 1969. [Google Scholar]
- Mayeux R, Stern Y, Rosenstein R, Marder K, Hauser A, Cote L, et al. An estimate of the prevalence of dementia in idiopathic Parkinson's disease. Archives of Neurology. 1988;45:260–262. doi: 10.1001/archneur.1988.00520270034017. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47(5):1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- McKeith IG, O'Brien JT, Ballard C. Diagnosing dementia with Lewy bodies. Lancet. 1999;354(9186):1227–1228. doi: 10.1016/S0140-6736(99)90214-3. [DOI] [PubMed] [Google Scholar]
- Parashos SA, Johnson ML, Erickson-Davis C, Wielinski CL. Assessing cognition in Parkinson disease: use of the cognitive linguistic quick test. J Geriatr Psychiatry Neurol. 2009;22(4):228–234. doi: 10.1177/0891988709342721. [DOI] [PubMed] [Google Scholar]
- Pereira JB, Junque C, Marti MJ, Ramirez-Ruiz B, Bartres-Faz D, Tolosa E. Structural brain correlates of verbal fluency in Parkinson's disease. Neuroreport. 2009;20(8):741–744. doi: 10.1097/WNR.0b013e328329370b. [DOI] [PubMed] [Google Scholar]
- Piatt AL, Fields JA, Paolo AM, Koller WC, Troster AI. Lexical, semantic, and action verbal fluency in Parkinson's disease with and without dementia. J Clin Exp Neuropsychol. 1999;21(4):435–443. doi: 10.1076/jcen.21.4.435.885. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Owen AM. Anterior prefrontal cortex: Insights into function from anatomy and neuroimaging. Nature Reviews: Neuroscience. 2004;5:184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- Reilly J, Losh M, Bellugi U, Wulfeck B. “Frog, where are you?” Narratives in children with specific language impairment, early focal brain injury, and Williams syndrome. Brain and Language. 2004;88:229–247. doi: 10.1016/S0093-934X(03)00101-9. [DOI] [PubMed] [Google Scholar]
- Sauer J, ffytche DH, Ballard C, Brown RG, Howard R. Differences between Alzheimer's disease and dementia with Lewy bodies: an fMRI study of task-related brain activity. Brain. 2006;129:1780–1788. doi: 10.1093/brain/awl102. [DOI] [PubMed] [Google Scholar]
- Skodda S, Visser W, Schlegel U. Short- and long-term dopaminergic effects on dysarthria in early Parkinson's disease. J Neural Transm. 2010;117(2):197–205. doi: 10.1007/s00702-009-0351-5. [DOI] [PubMed] [Google Scholar]
- Smith EE, Marshuetz C, Geva A, Grafman J. Handbook of Neuropsychology. Vol. 7. New York: Elsevier Science; 2002. Working memory: Findings from neuroimaging and patient studies; pp. 55–72. [Google Scholar]
- Talairach J, Tournaux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- Tam CWC, Burton EJ, McKeith IG, Burn DJ, O'Brien JT. Temporal lobe atrophy on MRI in Parkinson disease with dementia: A comparison with Alzheimer disease and dementia with Lewy bodies. Neurology. 2005;64(5):861–865. doi: 10.1212/01.WNL.0000153070.82309.D4. [DOI] [PubMed] [Google Scholar]
- Troiani V, Fernandez-Seara MA, Wang Z, Detre JA, Ash S, Grossman M. Narrative speech production: an fMRI study using continuous arterial spin labeling. Neuroimage. 2008;40(2):932–939. doi: 10.1016/j.neuroimage.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Weigand SD, Shiung MM, Boeve BF, Ferman TJ, Smith GE, et al. Focal atrophy in dementia with Lewy bodies on MRI: a distinct pattern from Alzheimer's disease. Brain. 2007;130:708–719. doi: 10.1093/brain/awl388. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


