Abstract
Background
Both delayed sleep phase syndrome (DSPS) and seasonal affective disorder (SAD) may manifest similar delayed circadian phase problems. However, the relationships and co-morbidity between the two conditions have not been fully studied. The authors examined the comorbidity between DSPS and SAD.
Methods
We recruited a case series of 327 DSPS and 331 controls with normal sleep, roughly matched for age, gender, and ancestry. Both DSPS and controls completed extensive questionnaires about sleep, the morningness-eveningness trait, depression, mania, and seasonality of symptoms, etc.
Results
The prevalences of SAD and subsyndromal SAD (S-SAD) were higher in DSPS compared to controls (χ2=12.65, p=0.002). DSPS were 3.3 times more likely to report SAD (odds ratio, 3.34; 95% CI, 1.41–7.93) compared to controls as defined by the Seasonal Pattern Assessment Questionnaire (SPAQ). Correspondingly, DSPS showed significantly higher seasonality scores compared to controls in mood, appetite, and energy level subscores and the global seasonality score (t=3.12, t=0.002; t=2.04, p=0.041; t=2.64, p=0.008; and t=2.15, p=0.032, respectively). Weight fluctuation during seasons and winter-summer sleep length differences were also significantly higher in DSPS than controls (t=5.16, p<0.001 and t=2.64, p=0.009, respectively). SAD and S-SAD reported significantly higher eveningness, higher depression self-ratings, and more previous mania symptoms compared to non-seasonal subjects regardless of whether they were DSPS or controls.
Conclusions
These cases suggested that DSPS is partially comorbid with SAD. These data support the hypothesis that DSPS and SAD may share a pathophysiological mechanism causing delayed circadian phase.
Keywords: delayed sleep phase syndrome, seasonal affective disorder, comorbidity
1. Introduction
Delayed sleep-phase syndrome (DSPS) is a circadian rhythm sleep disorder. DSPS patients tend to fall asleep some hours after midnight and have difficulty waking up in the morning. Not only their timing of sleep but also their peak times of performance, body temperature and hormonal rhythms are much delayed from societal norms. When allowed to choose their preferred schedules, DSPS patients are said to exhibit normal sleep quality and duration and maintain a delayed, but stable, phase of entrainment to the 24-h sleep–wake pattern (American Academy of Sleep Medicine, 2005). However, some studies suggest that sleep quality is poorer even when sleep and wake times have been self-selected (Campbell and Murphy, 2007). A prevalence in the adult population, equally distributed among women and men, has been reported at approximately 0.15% (Schrader et al., 1993; Yazaki et al., 1999), but Ando et al. (2002) found that the prevalence of mild DSPS symptoms might be much higher. Light therapy and melatonin administration have been reported to be effective treatments (Barion and Zee, 2007; Mundey et al., 2005).
Seasonal affective disorder (SAD), which is usually synonymous with winter depression, is a mood disorder in which people experience recurrent depressive symptoms in the winter while they are asymptomatic throughout much of the year. It has been estimated that 1.5–9% of adults in the US experience SAD (Modell et al., 2005). An excellent Canadian study estimated that the seasonal subtype of depression represents 11% of all subjects with major depression yielding a point prevalence of 2.9% (Levitt et al, 2000). However, a milder form, so-called subsyndromal SAD (S-SAD), is much more common than SAD. Rosenthal (1989) estimated that for the United States about 6.1% have SAD and about 14.3% have S-SAD, which represents a higher estimate than Levitt’s for a Canadian sample.
SAD patients may report sleeping too much, display little energy, and may also feel depressed during winter, though there may be more lethargy about getting out of bed than objective hypersomnia. Many different treatments for SAD have been recommended, including light therapy with sunlight or bright lights, antidepressant medications, cognitive-behavioral therapy, and timed administration of the hormone melatonin. (Lavoie et al., 2009; Lam et al., 2006; Rohan et al., 2007; Rohan et al., 2009; Terman and Terman, 2010; Lewy et al., 2009) DSPS and SAD might share similar pathophysiological mechanisms, since both manifest problems of delayed circadian rhythm phase and both are treated by morning light therapy and melatonin before bedtime. However, as far as we know, there are no large scale studies of the relationships between DSPS and SAD yet except one case report. (Uruha et al., 1991)
Although the dim light melatonin onset (DLMO) is delayed in relation to the timing of their sleep in most SAD patients (Lewy et al., 2006), there also appears to be a subgroup of SAD patients in which symptom severity correlates with a DLMO advance in reference to sleep, suggesting internal circadian misalignment. Interestingly, DSPS has also been reported associated with internal circadian misalignment which can be either a phase delay or a phase advance (Hughes and Lewy, 1998). Therefore, the relationships and comorbidity of these two conditions are interesting research topics in chronobiology and psychiatry. The purpose of this report was to investigate the relationship between DSPS and SAD in a DSPS case and control sample.
2. Methods
2.1. DSPS sample recruitment
The study subjects were recruited for a case-control genetic study of DSPS. In the initial part of the study, recruitment was limited to the Southern California region, particularly San Diego County, to enable the investigators to make home visits when necessary. The study was later expanded to other states of the US. Recruitment of the sample took place between June, 2004 and September, 2008. Some data from the early portion of the sample was published previously (Kripke et al., 2008). Recruitment of DSPS participants utilized contacts with sleep physicians, word of mouth, media contacts, newspaper advertising, late night radio advertising, UCSD minority outreach programs, paid context-based internet ads, and free internet advertising. A web site was constructed to facilitate contacts, provide potential volunteers with information, and allow potential volunteers to self-screen themselves versus the Horne-Östberg questionnaire: those with scores < 30 were encouraged to volunteer for the full study.
Only participants 25 years of age or older were accepted (with a few exceptions), to reduce the likelihood that participants might acquire late hours entirely through the social influences of youth culture or a college environment. Elderly participants were accepted if there was no suggestion of dementia or other illness which might distort circadian rhythms, and if there was a life-long history of delayed sleep.
Potential participants were provided written and verbal information about the study and signed written informed consent under the supervision of the UCSD Human Research Protection Program. Subjects referred or self-referred as potential DSPS cases completed a series of questionnaires, contributed a sample of blood or saliva for DNA, and the initial half of DSPS participants underwent a 2-week wrist activity and illumination recording, using a wrist-activity recorder (Kripke et al., 2008). DSPS participants were reimbursed $100 for their time and effort, which included completing the questionnaires, providing a blood or saliva sample, wearing the wrist actigraph for 2 weeks, completing sleep logs, and associated travel.
2.2. Control recruitment
While the DSPS case series was being assembled, the investigators recruited normal controls who claimed to have healthy and normal sleep. Control volunteers were recruited by word-of-mouth, outreach at health fairs and community meetings, the internet, and by a campus poster seeking volunteers who “sleep like a baby.” Control recruitment was targeted, so far as possible, to match the ancestry of the case series. Each control received an explanation of the study and signed written informed consent. They completed the questionnaires and contributed a sample of blood or saliva, but they were not asked to wear the actigraphs or to provide sleep logs. Since control participation usually required less than 2 hours, they were reimbursed $25. The principal investigator (DFK) reviewed the record of each participant volunteering as a control. Based on their questionnaires, control volunteers were retrospectively rated as 1) certain DSPS, 2) possible DSPS, 3) neither, 4) possible ASPS, or 5) certain ASPS. Only those with ratings 3 or 4 were included among the normal controls in this analysis.
2.3. Measures
2.3.1. Horne-Östberg questionnaire
An U.S.-idiom rephrasing was adopted for the Horne-Östberg morningness-eveningness questionnaire (Horne and Östberg, 1976; Terman et al., 2001a). This is a 19-item form yielding Horne-Östberg scores (HOS) from 16–30 (extreme eveningness) to above 70 (extreme morningness), as diagnostic ranges were originally defined.
2.3.2. BALM
The Basic Language Morningness scale (BALM) (Brown, 1993) was administered as a simplified-language revision of the 13-item Composite Scale (CS) (Smith et al., 1989). The CS was reported to have superior psychometric properties to the HOS as well as greater brevity.
2.3.3. SPAQ
All subjects completed a modified version of the Seasonal Pattern Assessment Questionnaire (SPAQ) (Rosenthal et al., 1987). The SPAQ is a retrospective, self-rating questionnaire that assesses seasonality of mood and behaviors. The first part of the SPAQ inquires “To what degree do the following change with the seasons?” for six items (sleep length, social activity, mood, weight, appetite, and energy level). For each item, responses were 0 (none), 1 (mild), 2 (moderate), 3 (marked), or 4 (extreme). The sum of these six items is the Global Seasonality Score (GSS), which therefore ranges from 0 (no seasonality) to 24 (extreme seasonality). Another section of the SPAQ requests that respondents fill in the month or months of the year (if any) when they feel best, feel worst, eat least, eat most, etc. The severity of problems with seasonality was also queried by “How much of a problem are these seasonal changes?” with choices being 0 (none), 1 (mild), 2 (moderate), 3 (marked), 4 (severe), or 5 (disabling). Another SPAQ section evaluated the weight fluctuation during the course of the year in 6 levels (1, 0–3 lbs; 2, 4–7 lbs; 3, 8–11 lbs; 4, 12–15 lbs; 5, 16–20 lbs; 6, Over 20 lbs). Sleep duration including napping in each season, and changes of food preference during the seasons were also reported by subjects. We calculated the sleep duration difference between winter and summer and included this difference in our analyses.
2.3.4. Other measures
Participants completed the Mood Disorders Questionnaire (MDQ), a lifetime screening scale for history of mania with very high specificity (Hirschfeld et al, 2000). They also completed the Quick Inventory of Depression Symptomatology Self Report (QIDS-SR), a self-rating scale for current major depression with high correlation to the psychiatrist-administered Hamilton Depression rating scale (Rush et al., 2003; Rush et al., 2006). Residence of subjects and test date were recorded. Subjects completed other questionnaires which will be reported in separate papers.
2.4. Clinical Evaluation
2.4.1. DSPS
Once all data were assembled, the principal investigator (DFK) reviewed the record of each participant who had volunteered as a DSPS case and recorded the participant’s DSPS classification as 1) absolutely certain, 2) fairly certain, 3) questionable, 4) unlikely, or 5) very doubtful. The main criterion for classification was the HOS, recognizing that the criterion for definite evening type of < 30 was too strict for the San Diego population (Kripke et al., 2008). Confirmatory classification criteria included the score on the BALM, reported prior-week and adult-life bedtimes and awakening times, the actigraphic recordings, and whether the participant reported going to sleep “somewhat later” or “much later” than most people their age, both as a child and as an adult. Whether the participant reported distress about falling asleep, reported related social or vocational problems, or had sought medical attention for a sleep problem was also considered. The consistency of the data supporting a classification of DSPS was evaluated, together with the presence of depression, other mental illnesses, or other sleep disorders which might confuse the classification. However, if depression or other disorders had their first incidence after the onset of delayed sleep and did not appear to be causing the delay, these disorders were not considered exclusionary. As many DSPS patients cannot consistently report to work by 8–9 AM, evening or night shift work was not considered exclusionary if the history indicated that the delay in sleep occurred before shift work was adopted, and the delay tended to persist when the participant was off work.
2.4.2. SAD
SAD and subsyndromal SAD were diagnosed by Kasper’s criteria based on SPAQ scores (Kasper et al., 1989). To be diagnosed with SAD, subjects must have met the following criteria: (1) GSS of 11 or more; (2) a ‘problem’ rating with seasonal mood changes of at least a moderate degree (2 or above); and (3) feeling worst during one of the winter months. To be categorized as having subsyndromal SAD (S-SAD), the subjects would either have a GSS of 11 or more with a ‘problem’ rating of not more than mild (0 or 1), or have a GSS of 9 or 10 with a ‘problem’ rating of at least mild (1 or above). Furthermore, the S-SAD subject had to feel worst in the winter months. We classified both SAD and S-SAD as a winter “seasonals” category and all other participants were classed as “non-seasonals”.
2.5. Statistical Analysis
We compared the differences of basic, clinical and seasonal characteristics between DSPS and controls by t-tests for continuous data or by χ2 tests for categorical data. We also performed comparisons among SAD, S-SAD, and non-seasonals by analyses of variance (ANOVAs) for continuous data or by χ2 tests for categorical data. All of the analyses were performed using SPSS for Windows version 11 (SPSS, Chicago, Ill), with the cutoff for statistical significance set at P < 0.05.
3. Results
3.1. Subjects
By the recruitment process described above, we recruited a case series of 359 DSPS along with 389 controls. Out of these subjects, 658 participants (327 DSPS and 331 controls) who completed the SPAQ were included in our analysis. Their mean age was 37.84 (SD=11.94). The gender distribution was 69.4% female and 30.6% male. Among 658 participants, 29 (4.4%) were categorized as a SAD, 44 (6.7%) were categorized as a S-SAD, and 585 (88.9%) were categorized as non-seasonals according to Kasper’s criteria (Kasper et al., 1989). Residents of California were 75.4% and the others were residents of 42 different states. The seasonal distribution of questionnaire completions was 20.8% winter, 21.0% spring, 28.6% summer, 26.9% fall, and 2.7% unknown.
3.2. DSPS and SAD
Figure 1 shows the difference in prevalence of SAD and S-SAD between DSPS and controls. The prevalence of SAD and S-SAD in DSPS were 6.7% and 8.6% respectively, which were significantly higher than the prevalence in controls (SAD, 2.1%; S-SAD, 4.8%) (χ2=12.65, p=0.002). DSPS were 3.3 times more likely to report SAD (odds ratio, 3.34; 95% CI, 1.41–7.93) and 2.4 times more likely to be seasonals (SAD + S-SAD) (odds ratio, 2.42; 95% CI, 1.44–4.07) compared with controls.
Figure 1.
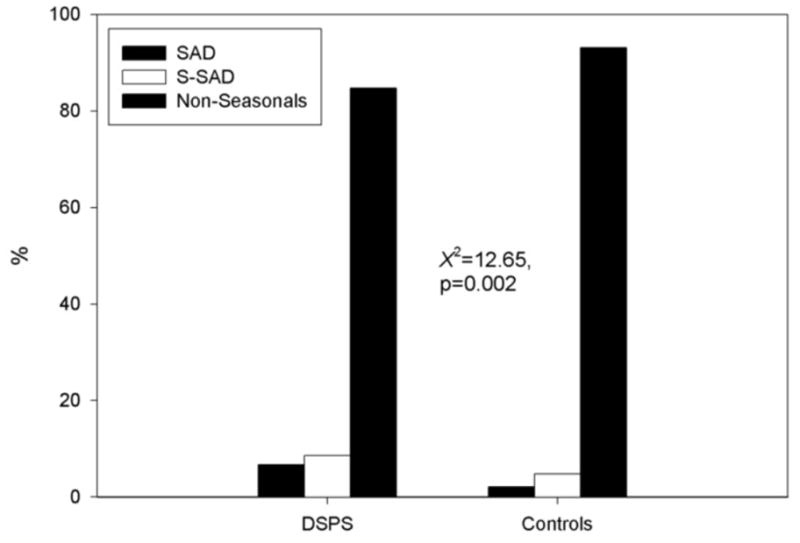
Differences in prevalence of SAD and S-SAD between DSPS and controls.
Abbreviation; DSPS, delayed sleep phase syndrome; SAD, seasonal affective disorder; S-SAD, subsyndromal SAD
Table 1 shows comparisons of basic, clinical and circadian characteristics between DSPS and controls. DSPS showed significantly higher seasonality scores compared to controls in mood, appetite, and energy level subscores and global seasonality scores (t=3.12, t=0.002; t=2.04, p=0.041; t=2.64, p=0.008; and t=2.15, p=0.032, respectively). Although 3 seasonality subscores (weight, sleep length, and social activity) were not significantly different between DSPS and controls, other SPAQ items concerning weight fluctuation during seasons and sleep duration variation between winter and summer were significantly higher in DSPS (t=5.16, p<0.001; t=2.64, p=0.009, respectively). Thus among 6 seasonality characteristics, all except social activity were significantly different between DSPS and controls.
Table 1.
Contrast between DSPS and controls.
| DSPS (n=327) | Controls (n=331) | |||
|---|---|---|---|---|
| Sex (male/female) | 90/237 | 110/221 | X2=2.54 | p=0.11 |
| Age (yrs) | 37.37±11.13 | 38.31±12.68 | t=−1.00 | p=0.32 |
| Horne Östberg score | 25.40±5.73 | 55.56±10.77 | t=−44.91 | p<0.001 |
| BALM | 17.86±4.11 | 38.28±7.96 | t=−41.44 | p<0.001 |
| QIDS-SR | 5.72±3.90 | 3.75±3.37 | t=6.94 | p<0.001 |
| MDQ | 3.66±3.81 | 3.36±3.60 | t=1.02 | p=0.31 |
| SPAQ | ||||
| Global Seasonality Score | 5.48±4.90 | 4.71±4.23 | t=2.15 | p=0.032 |
| Sleep length seasonality | 0.79±1.01 | 0.67±0.97 | t=1.53 | p=0.13 |
| Social activity seasonality | 1.07±1.15 | 1.00±1.03 | t=0.83 | p=0.41 |
| Mood seasonality | 1.16±1.16 | 0.90±1.01 | t=3.12 | p=0.002 |
| Weight seasonality | 0.67±0.89 | 0.65±0.82 | t=0.35 | p=0.73 |
| Appetite seasonality | 0.66±0.90 | 0.50±1.09 | t=2.04 | p=0.041 |
| Energy level seasonality | 1.12±1.11 | 0.91±0.95 | t=2.64 | p=0.008 |
| Problem Severity of Seasonality | 0.61±1.06 | 0.30±0.72 | t=4.50 | p<0.001 |
| Seasonal weight fluctuation* | 2.28±1.21 | 1.85±0.94 | t=5.16 | p<0.001 |
| Sleep duration-winter (hrs) | 8.18±1.95 | 7.94±1.35 | t=1.88 | p=0.061 |
| Sleep duration-spring (hrs) | 7.57±1.70 | 7.54±1.25 | t=0.26 | p=0.80 |
| Sleep duration-summer (hrs) | 7.43±1.71 | 7.44±1.30 | t=−0.072 | p=0.94 |
| Sleep duration-fall (hrs) | 7.73±1.66 | 7.68±1.29 | t=0.46 | p=0.65 |
| Sleep duration difference (winter – summer) (hrs) | 0.75±1.37 | 0.50±1.05 | t=2.64 | p=0.009 |
| Food preference change (yes/no) | 121/206 | 127/204 | X2=0.13 | p=0.72 |
SPAQ, Seasonal Pattern Assessment Questionnaire; BALM, Basic Language Morningness Scale; QIDS-SR, Quick Inventory of Depression Symptomatology-Self Report; MDQ, Mood Disorders Questionnaire
1, 0–3 lbs; 2, 4–7 lbs; 3, 8–11 lbs; 4, 12–15 lbs; 5, 16–20 lbs; 6, Over 20 lbs
Depression scores evaluated by QIDS-SR were significantly higher in DSPS than controls (t=6.94, p<0.001), however, the life-time mania history evaluated by MDQ was not different between them (t=1.02, p=0.31).
3.3. Comparison among SAD, S-SAD and non-seasonals
Table 2 shows contrasts of basic, clinical and circadian characteristics among three groups (SAD, S-SAD and non-seasonals) divided by SPAQ seasonality criteria irrespective of DSPS or control status. The BALM scores and Horne-Östberg scores (HOS), which evaluate morningness-eveningness, were significantly lower in SAD and S-SAD groups than non-seasonals (HOS, F(2,655)=5.20, p=0.006; BALM, F(2,655)=6.26, p=0.002). This indicated that SAD and S-SAD groups tended to report less morningness (more eveningness) than non-seasonals. Depression scores evaluated by QIDS-SR were significantly higher in SAD and S-SAD than non-seasonals (F(2,655)=47.50, p<0.001), Furthermore, scores in MDQ, a lifetime screening scale for mania, were significantly higher in SAD and S-SAD than non-seasonals (F(2,655)=15.63, p<0.001). Sleep duration during the seasons highlighted interesting contrasts, in which SAD reported sleeping less in summer but more in winter compared to non-seasonals (summer, F(2,652)=4.90, p=0.008; winter, F(2,652)=6.32, p=0.002). The mean sleep length difference between winter and summer of SAD subjects was 2.24 hours, and this was significantly longer than S-SAD and non-seasonals (F(2,652)=40.42, p<0.001). SAD and S-SAD showed more food preference changes during the seasons (χ2=16.45, p<0.001). Most frequently they reported more carbohydrate intake during winter. Due to the previous reports that SPAQ scores are influenced by the latitude and season, we analyzed the relationships between seasonality, questionnaire completion dates, and latitude. The latitudes were not significantly different among SAD, S-SAD and non-seasonals (SAD, 33.89±3.29; S-SAD, 34.12±3.46; non-seasonals, 33.55±3.58; F(2,637)=0.60, p=0.55). The seasonal distribution of questionnaire completions were also not different among three groups (χ2=8.1, p=0.23).
Table 2.
Contrasts between SAD, S-SAD and non-seasonals irrespective of DSPS or control status
| SAD (n=29) | S-SAD (n=44) | Non-seasonals (n=585) | |||
|---|---|---|---|---|---|
| Sex (male/female) | 5/24 | 13/31 | 182/403 | X2=2.53 | p=0.28 |
| Age (yrs) | 41.59±12.86 | 35.75±10.38 | 37.81±11.98 | F(2,655)=2.11 | p=0.12 |
| Horne-Östberg score | 33.86±13.31a | 34.82±14.81 | 41.34±17.61a | F(2,655)=5.20 | p=0.006 |
| BALM | 22.55±8.68a | 24.18±10.43b | 28.70±12.17a,b | F(2,655)=6.26 | p=0.002 |
| QIDS-SR | 9.55±5.00 a | 7.68±4.72 b | 4.26±3.34 a,b | F(2,655)=47.50 | p<0.001 |
| MDQ | 6.52±4.08a | 5.07±3.85b | 3.24±3.59a,b | F(2,655)=15.63 | p<0.001 |
| SPAQ | |||||
| Global Seasonality Score | 15.79±3.31a,c | 11.41±0.47b,c | 4.09±3.62a,b | F(2,655)=227.79 | p<0.001 |
| Sleep length seaonality | 2.52±1.02 a,c | 1.61±0.99 b,c | 0.58±0.86 a,b | F(2,655)=90.89 | p<0.001 |
| Social activity seasonality | 2.76±0.99 a | 2.30±0.77 b | 0.86±0.98 a,b | F(2,655)=93.97 | p<0.001 |
| Mood seasonality | 3.10±0.67 a,c | 2.48±0.66 b,c | 0.82±0.94 a,b | F(2,655)=146.75 | p<0.001 |
| Weight seasonality | 2.21±0.90 a,c | 1.43±1.04 b,c | 0.52±0.72 a,b | F(2,655)=93.45 | p<0.001 |
| Appetite seasonality | 2.21±0.94 a,c | 1.39±0.89 b,c | 0.44±0.91 a,b | F(2,655)=70.76 | p<0.001 |
| Energy level seasonality | 3.00±0.76 a,c | 2.20±0.67 b,c | 0.82±0.90 a,b | F(2,655)=127.06 | p<0.001 |
| Problem Severity of Seasonality | 2.93±1.16 a,c | 0.84±0.96 b,c | 0.30±0.69 a,b | F(2,655)=181.48 | p<0.001 |
| Seasonal weight fluctuation* | 3.21±1.24a,c | 2.41±1.30 b,c | 1.98±1.04 a,b | F(2,655)=20.62 | p<0.001 |
| Sleep duration-winter (hrs) | 9.07±2.24 a | 8.30±1.86 | 7.99±1.61 a | F(2,652)=6.32 | p=0.002 |
| Sleep duration-spring (hrs) | 7.76±1.38 | 7.50±1.64 | 7.55±1.48 | F(2,652)=0.31 | p=0.74 |
| Sleep duration-summer (hrs) | 6.83±1.37 | 6.98±1.66 | 7.50±1.51 | F(2,652)=4.90 | p=0.008 |
| Sleep duration-fall (hrs) | 8.00±1.46 | 7.59±1.62 | 7.70±1.47 | F(2,652)=0.71 | p=0.49 |
| Sleep duration difference (winter – summer) (hrs) | 2.24±1.86 a,c | 1.32±1.39 b,c | 0.49±1.09 a,b | F(2,652)=40.42 | p<0.001 |
| Food preference change (yes/no) | 20/9 | 22/22 | 206/379 | X2=16.45 | p<0.001 |
SAD, seasonal affective disorder; S-SAD, subsyndromal SAD; SPAQ, Seasonal Pattern Assessment Questionnaire; BALM, Basic Language Morningness Scale; QIDS-SR, Quick Inventory of Depression Symptomatology-Self Report; MDQ, Mood Disorders Questionnaire
1, 0–3 lbs; 2, 4–7 lbs; 3, 8–11 lbs; 4, 12–15 lbs; 5, 16–20 lbs; 6, Over 20 lbs
means significantly different 2 × 2 according to Bonferroni post hoc tests
4. Discussion
The pathophysiologic mechanisms of DSPS remain to be clarified. Genetic factors, especially in circadian oscillator genes, may be associated with this syndrome (Archer et al., 2010; Takano et al., 2004). Our group is performing circadian genetic association studies on DSPS using this sample and will report results separately. In addition to genetic factors, environmental factors such as exposure to light are important in the development of DSPS. Decreased light exposure in the morning can exacerbate DSPS.
SAD has been regarded as an ideal model for understanding the role of circadian rhythms in human mood and behavior, because of predictable depression in winter and predictable recovery as days lengthen in the spring. SAD patients become depressed in the winter at least in part because of a phase delay with respect to the sleep/wake cycle (Lewy et al., 1987) and bright light should usually be scheduled in the morning in order to provide a corrective phase advance. However, studies of melatonin administration suggest a more complicated etiology (Lewy et al., 2006; Rahman et al., 2010).
Both DSPS and SAD have been considered to arise from delayed circadian phases; however, this study was the first to demonstrate comorbidity in a large number of cases. This comorbidity supports the hypothesis that DSPS and SAD share similar pathophysiological mechanisms of delayed circadian phase and low mood.
However, there must be further factors explaining why DSPS experience fluctuating SAD symptoms in winter, if their circadian phases are delayed throughout the year. Two possibilities are that 1) the timing of bright light exposures may change in reference to internal rhythms and 2) changes may occur in the internal phase-angles between melatonin-related circadian rhythms and the sleep-wake cycle. These alternatives resemble the internal coincidence and external coincidence models of photoperiodic responses and the phenomena of photoperiodic sensitivity to changing photoperiod independent of absolute photoperiod duration (Lewy et al., 2006; Terman et al., 2001b; Lewy, 2010).
Patients with major depression which does not meet SAD criteria apparently tend to display delayed melatonin in reference to sleep timing throughout the year (Sekula et al., 1997; Tuunainen et al., 2002; Emens et al., 2009). The internal phase malsynchronization is hypothesized to have a causal role in the depression. In contrast, patients with DSPS, in the original description, have no malsynchronization between sleep and internal rhythms (Weitzman et al., 1981). However, careful study showed that patients with DSPS may display melatonin rhythms which are either early or late in reference to their sleep times, and vice versa (Uchiyama et al. 2000; Cole et al., 2002). In DSPS, both melatonin and sleep are usually late, but sometimes melatonin might display timing within the normal range or even somewhat advanced (Hughes and Lewy, 1998). The possibility of subgroups of different pathophysiology in DSPS and SAD may be able to explain why most DSPS did not have seasonality and why some seasonals do not have DSPS in our sample. Patients with overlap of SAD and DSPS have a delay in circadian rhythms with respect to the sleep/wake cycle. These patients should preferentially respond to the morning bright light treatment so as to provide a corrective phase advance and restore optimum alignment between the endogenous circadian rhythms and those rhythms that are related to the sleep/wake cycle. Therefore, for the overlap patients, it is recommended to undergo bright light treatment as soon after the usual awakening time as possible.
Another interesting finding is that SAD is related to bipolar disorder. The MDQ is a brief, self-report screening instrument that can be used to identify patients most likely to have bipolar disorder (Hirschfeld et al. 2000). The mean MDQ score of SAD was 6.52 (SD=4.08), which was significantly higher than non-seasonals (3.24±3.59) and close to the score of ‘7’ that was chosen as the optimal cutoff of MDQ for bipolar disorder in the previous study (Hirschfeld et al. 2000). This is another indication that SAD is often related to bipolar disorder, as was reported in the original description (Rosenthal et al., 1984).
There are some limitations in this study. First, as a diagnostic tool, the SPAQ discriminates poorly between SAD and S-SAD, and hence it has a poor case-finding ability for SAD (Magnusson, 1996). Furthermore, it is a self-report scale which does not always agree well with longitudinal observations (Lund and Hansen, 2001; Christensen et al., 2003). Second, the Southern California concentration of the subjects is a limitation. This restricted our opportunity to observe an association between latitude and SAD prevalence and may have produced a relatively low prevalence of SAD and S-SAD compared to the U.S. average. Nevertheless, the large scale of the DSPS and well-matched control sample are appropriate to the aim of study. Further studies for the molecular mechanisms and similar pathophysiology of DSPS and SAD are needed.
Acknowledgments
The authors do not have any acknowledgements related to the development of present investigation.
Role of funding source
This research was supported by the National Institutes of Health’s NHLBI HL071123 (D.F.K.), and H.J.L. was supported by Kil Chung-Hee Fellowship Grant.
Footnotes
Conflict of interest
All authors declare no conflict of interest that could influence their work.
Contributors
Each author contributed to the conceptualization and writing of the present investigation. Additionally, H.J.L. made statistical analysis, managed literature search, interpreted the data, and wrote the manuscript. D.F.K. designed the study, collected samples, interpreted the data and wrote the manuscript. Each author contributed to and has approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, Diagnostic and Coding Manual. 2. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- Ando K, Kripke DF, Ancoli-Israel S. Delayed and advanced sleep phase symptoms. Israel Journal of Psychiatry & Related Sciences. 2002;39:11–8. [PubMed] [Google Scholar]
- Archer SN, Carpen JD, Gibson M, Lim GH, Johnston JD, Skene DJ, von Schantz M. Polymorphism in the PER3 promoter associates with diurnal preference and delayed sleep phase disorder. Sleep. 2010;33:695–701. doi: 10.1093/sleep/33.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barion A, Zee PC. A clinical approach to circadian rhythm sleep disorders. Sleep Medicine. 2007;8:566–77. doi: 10.1016/j.sleep.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown FM. Psychometric equivalence of an improved Basic Language Morningness (BALM) Scale using industrial population within comparisons. Ergonomics. 1993;36:191–7. doi: 10.1080/00140139308967872. [DOI] [PubMed] [Google Scholar]
- Campbell SS, Murphy PJ. Delayed sleep phase disorder in temporal isolation. Sleep. 2007;30:1225–8. doi: 10.1093/sleep/30.9.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen EM, Larsen JK, Gjerris A. The stability of the Seasonal Pattern Assessment Questionnaire score index over time and the validity compared to classification according to DSM-III-R. Journal of Affective Disorders. 2003;74:167–72. doi: 10.1016/s0165-0327(02)00009-5. [DOI] [PubMed] [Google Scholar]
- Cole RJ, Smith JS, Alcalá YC, Elliott JA, Kripke DF. Bright-light mask treatment of delayed sleep phase syndrome. Journal of Biological Rhythms. 2002;17:89–101. doi: 10.1177/074873002129002366. [DOI] [PubMed] [Google Scholar]
- Emens J, Lewy A, Kinzie JM, Arntz D, Rough J. Circadian misalignment in major depressive disorder. Psychiatry Research. 2009;168:259–61. doi: 10.1016/j.psychres.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Hirschfeld RM, Williams JB, Spitzer RL, Calabrese JR, Flynn L, Keck PE, Jr, et al. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. American Journal of Psychiatry. 2000;157:1873–5. doi: 10.1176/appi.ajp.157.11.1873. [DOI] [PubMed] [Google Scholar]
- Horne JA, Östberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. International Journal of Chronobiology. 1976;4:97–110. [PubMed] [Google Scholar]
- Hughes RJ, Lewy AJ. Light and melatonin treatment of circadian phase sleep disorders. In: Lam RW, editor. Seasonal Affective Disorder and Beyond: Light Treatment for SAD and Non-SAD Conditions. Washington, DC: American Psychiatric Press; 1998. p. p221. [Google Scholar]
- Kasper S, Wehr TA, Bartko JJ, Gaist PA, Rosenthal NE. Epidemiological findings of seasonal changes in mood and behavior. A telephone survey of Montgomery County, Maryland. Archives of General Psychiatry. 1989;46:823–33. doi: 10.1001/archpsyc.1989.01810090065010. [DOI] [PubMed] [Google Scholar]
- Kripke DF, Rex KM, Ancoli-Israel S, Nievergelt CM, Klimecki W, Kelsoe JR. Delayed sleep phase cases and controls. Journal of Circadian Rhythms. 2008;6:6. doi: 10.1186/1740-3391-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam RW, Levitt AJ, Levitan RD, Enns MW, Morehouse R, Michalak EE, Tam EM. The Can-SAD study: a randomized controlled trial of the effectiveness of light therapy and fluoxetine in patients with winter seasonal affective disorder. American Journal of Psychiatry. 2006;163:805–12. doi: 10.1176/ajp.2006.163.5.805. [DOI] [PubMed] [Google Scholar]
- Lavoie MP, Lam RW, Bouchard G, Sasseville A, Charron MC, Gagné AM, et al. Evidence of a biological effect of light therapy on the retina of patients with seasonal affective disorder. Biological Psychiatry. 2009;66:253–8. doi: 10.1016/j.biopsych.2008.11.020. [DOI] [PubMed] [Google Scholar]
- Levitt AJ, Boyle MH, Joffe RT, Baumal Z. Estimated prevalence of the seasonal subtype of major depression in a Canadian community sample. Canadian Journal of Psychiatry. 2000;45:650–4. doi: 10.1177/070674370004500708. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Emens JS, Songer JB, Sims N, Laurie AL, Fiala SC, Buti AL. Winter Depression: Integrating mood, circadian rhythms, and the sleep/wake and light/dark cycles into a bio-psycho-social-environmental model. Sleep Medicine Clinics. 2009;4:285–99. doi: 10.1016/j.jsmc.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewy AJ, Lefler BJ, Emens JS, Bauer VK. The circadian basis of winter depression. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:7414–9. doi: 10.1073/pnas.0602425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewy AJ, Sack RL, Singer CM, White DM. The phase shift hypothesis for bright light’s therapeutic mechanism of action: theoretical considerations and experimental evidence. Psychopharmacology Bulletin. 1987;23:349–53. [PubMed] [Google Scholar]
- Lewy AJ. Depressive disorders may more commonly be related to circadian phase delays rather than advances: time will tell. Sleep Medicine. 2010;11:117–8. doi: 10.1016/j.sleep.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Lund E, Hansen V. Responses to the seasonal pattern assessment questionnaire in different seasons. American Journal of Psychiatry. 2001;158:316–8. doi: 10.1176/appi.ajp.158.2.316. [DOI] [PubMed] [Google Scholar]
- Magnusson A. Validation of the Seasonal Pattern Assessment Questionnaire (SPAQ) Journal of Affective Disorders. 1996;40:121–9. doi: 10.1016/0165-0327(96)00036-5. [DOI] [PubMed] [Google Scholar]
- Modell J, Rosenthal NE, Harriett AE, Krishen A, Asgharian A, Foster VJ, et al. Seasonal affective disorder and its prevention by anticipatory treatment with bupropion XL. Biological Psychiatry. 2005;58:658–67. doi: 10.1016/j.biopsych.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Mundey K, Benloucif S, Harsanyi K, Dubocovich ML, Zee PC. Phase-dependent treatment of delayed sleep phase syndrome with melatonin. Sleep. 2005;28:1271–8. doi: 10.1093/sleep/28.10.1271. [DOI] [PubMed] [Google Scholar]
- Rahman SA, Kayumov L, Shapiro CM. Antidepressant action of melatonin in the treatment of Delayed Sleep Phase Syndrome. Sleep Medicine. 2010;11:131–6. doi: 10.1016/j.sleep.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Rohan KJ, Roecklein KA, Lacy TJ, Vacek PM. Winter depression recurrence one year after cognitive-behavioral therapy, light therapy, or combination treatment. Behavior Therapy. 2009;40:225–38. doi: 10.1016/j.beth.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Rohan KJ, Roecklein KA, Tierney Lindsey K, Johnson LG, Lippy RD, Lacy TJ, Barton FB. A randomized controlled trial of cognitive-behavioral therapy, light therapy, and their combination for seasonal affective disorder. Journal of Consulting and Clinical Psychology. 2007;75:489–500. doi: 10.1037/0022-006X.75.3.489. [DOI] [PubMed] [Google Scholar]
- Rosenthal NE, Genhart M, Sack DA, Skwerer RG, Wehr TA. Seasonal affective disorder and its relevance for the understanding and treatment of bulimia. In: Hudson JI, Pope HG, editors. The Psychobiology of Bulimia. Washington, DC: American Psychiatric Press; 1987. pp. 205–228. [Google Scholar]
- Rosenthal NE, Sack DA, Gillin JC, Lewy AJ, Goodwin FK, Davenport Y, et al. Seasonal affective disorder. A description of the syndrome and preliminary findings with light therapy. Archives of General Psychiatry. 1984;41:72–80. doi: 10.1001/archpsyc.1984.01790120076010. [DOI] [PubMed] [Google Scholar]
- Rosenthal NE. Seasons of The Mind. New York: Bantam; 1989. [Google Scholar]
- Rush AJ, Bernstein IH, Trivedi MH, Carmody TJ, Wisniewski S, Mundt JC, et al. An evaluation of the quick inventory of depressive symptomatology and the hamilton rating scale for depression: a sequenced treatment alternatives to relieve depression trial report. Biological Psychiatry. 2006;59:493–501. doi: 10.1016/j.biopsych.2005.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, et al. The 16-item Quick Inventory of Depressive Symptomatology (QIDS), clinical rating (QIDS-C), and selfreport (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biological Psychiatry. 2003;54:573–83. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- Schrader H, Bovim G, Sand T. The prevalence of delayed and advanced sleep phase syndrome. Journal of Sleep Research. 1993;2:51–5. doi: 10.1111/j.1365-2869.1993.tb00061.x. [DOI] [PubMed] [Google Scholar]
- Sekula LK, Lucke JF, Heist EK, Czambel RK, Rubin RT. Neuroendocrine aspects of primary endogenous depression. XV: Mathematical modeling of nocturnal melatonin secretion in major depressives and normal controls. Psychiatry Research. 1997;69:143–53. doi: 10.1016/s0165-1781(96)02937-x. [DOI] [PubMed] [Google Scholar]
- Smith CS, Reilly C, Midkiff K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. Journal of Applied Psychology. 1989;74:728–38. doi: 10.1037/0021-9010.74.5.728. [DOI] [PubMed] [Google Scholar]
- Takano A, Uchiyama M, Kajimura N, Mishima K, Inoue Y, Kamei Y, et al. A missense variation in human casein kinase I epsilon gene that induces functional alteration and shows an inverse association with circadian rhythm sleep disorders. Neuropsychopharmacology. 2004;29:1901–9. doi: 10.1038/sj.npp.1300503. [DOI] [PubMed] [Google Scholar]
- Terman M, Rifkin JB, Jacobs J, White TM. Morningness-Eveningness Qeustionnaire (revised) New York, New York: New York State Psychiatric Institute; 2001a. [Google Scholar]
- Terman JS, Terman M, Lo ES, Cooper TB. Circadian time of morning light administration and therapeutic response in winter depression. Archives of General Psychiatry. 2001b;58:69–75. doi: 10.1001/archpsyc.58.1.69. [DOI] [PubMed] [Google Scholar]
- Terman M, Terman JS. Circadian rhythm phase advance with dawn simulation treatment for winter depression. Journal of Biological Rhythms. 2010;25:297–301. doi: 10.1177/0748730410374000. [DOI] [PubMed] [Google Scholar]
- Tuunainen A, Kripke DF, Elliott JA, Assmus JD, Rex KM, Klauber MR, Langer RD. Depression and endogenous melatonin in postmenopausal women. Journal of Affective Disorders. 2002;69:149–58. doi: 10.1016/s0165-0327(01)00303-2. [DOI] [PubMed] [Google Scholar]
- Uchiyama M, Okawa M, Shibui K, Liu X, Hayakawa T, Kamei Y, Takahashi K. Poor compensatory function for sleep loss as a pathogenic factor in patients with delayed sleep phase syndrome. Sleep. 2000;23:553–8. [PubMed] [Google Scholar]
- Uruha S, Jitsuzaki Y, Taniguchi M, Tanaka C, Honda H, Saito M, et al. A case with delayed sleep phase syndrome showing a significant seasonal variation in sleep-wake cycle. Japanese Journal of Psychiatry & Neurology. 1991;45:180–1. [PubMed] [Google Scholar]
- Weitzman ED, Czeisler CA, Coleman RM, Spielman AJ, Zimmerman JC, Dement W, et al. Delayed sleep phase syndrome. A chronobiological disorder with sleep-onset insomnia. Archives of General Psychiatry. 1981;38:737–46. doi: 10.1001/archpsyc.1981.01780320017001. [DOI] [PubMed] [Google Scholar]
- Yazaki M, Shirakawa S, Okawa M, Takahashi K. Demography of sleep disturbances associated with circadian rhythm disorders in Japan. Psychiatry & Clinical Neurosciences. 1999;53:267–8. doi: 10.1046/j.1440-1819.1999.00533.x. [DOI] [PubMed] [Google Scholar]


