Abstract
Background
Nicotine is one of the world’s most addictive substances and the primary reason that humans inhale tobacco smoke. The pharmacological effects of nicotine can be investigated in planarians, aquatic flatworms that possess an integrated neural network including cephalic ganglia that some consider the earliest “brain” and spinal cord. Here, we tested the hypothesis that nicotine exposure elicits mammalian-like behaviors in planarians.
Methods
Planarian motility and stereotypy (C-shape hyperkinesias) were quantified following acute nicotine exposure. During repeated nicotine exposure, we investigated the presence of withdrawal, tolerance, behavioral sensitization, and environmental place conditioning.
Results
Acute nicotine exposure increased stereotypical activity and elicited biphasic effects on motility. A low concentration (0.01 mM) increased motility whereas higher concentrations (0.3 – 10 mM) elicited the opposite effect. Planarians exposed to nicotine (0.03 mM) for 60 min and then tested in water displayed reduced motility that was not observed during exposure to water, acute nicotine, or continuous nicotine. Nicotine-treated planarians withdrawn from the drug for 3 days before being challenged with nicotine displayed behavioral sensitization at low concentrations (0.1, 0.3 mM) but tolerance at higher concentrations (1, 3 mM). Planarians conditioned with nicotine in the ambient light (non-preferred environment) displayed a reduction in their natural preference for a dark environment.
Conclusions
The present results suggest nicotine elicits mammalian-like effects in planarians, including decreased motility and increased stereotypy following acute administration and abstinence-induced withdrawal, behavioral sensitization, tolerance, and place conditioning during repeated exposure.
Keywords: nicotine, planaria, sensitization, tolerance, withdrawal, place preference
1. Introduction
Nicotine addiction is characterized by compulsive tobacco use, loss of control over tobacco consumption despite its harmful effects, the appearance of withdrawal symptoms upon cessation of tobacco smoking, and relapse after periods of abstinence (McLellan et al., 2000). Most smokers wish to discontinue tobacco consumption, but relapse rates are as high as 80% one year after the quit date, even with the use of pharmacological and non-pharmacological therapies (Dwoskin et al., 2009). When administered to rodents, nicotine produces pharmacological effects that are consistent with most other addictive substances. Acute nicotine administration produces hyperactivity in rats (Jerome and Sanberg, 1987). Rats exposed repeatedly to nicotine, then a period of abstinence, and then reintroduced to nicotine, display an enhanced hyperactivity compared to the increase in activity produced by initial exposure (Schoffelmeer et al., 2002). The augmented behavioral response is defined as behavioral sensitization (reverse tolerance) (Vanderschuren and Kalivas, 2000; Narendran and Martinez, 2008). Following repeated nicotine administration in rats, a withdrawal syndrome can be evoked by abrupt discontinuation of nicotine exposure or administration of a nicotinic antagonist (Kenny and Markou, 2001). The rewarding and reinforcing effects of nicotine in rats are commonly studied in the conditioned place preference and self-administration procedures (Le Foll and Goldberg, 2005).
As part of our ongoing investigation into drug action and abuse issues using the planarian model (Raffa and Rawls, 2008), the present study investigated the pharmacological effects of nicotine in planarians, flatworms that possess a primitive, yet centralized, nervous system (cephalic ganglia and spinal processes) and utilize neurotransmitter systems, including glutamate, dopamine, serotonin, acetylcholine, and GABA (Eriksson and Panula, 1994; Vyas et al., 2010; Nishimura et al., 2010). Planarians display mammalian-like behaviors during exposure to addictive substances that include enhanced motility and stereotypical activity; abstinence-related withdrawal; behavioral sensitization to cocaine; cross-sensitization to cocaine and glutamate; and conditioned place preference to methamphetamine (Kusayama and Watanabe, 2000; Raffa et al., 2008; Raffa and Valdez, 2001; Raffa and Rawls, 2008; Rawls et al., 2006, 2007, 2008, 2009; Venturini et al., 1989; Umeda et al., 2005; Palladini et al., 1996; Pagán et al., 2008, 2009; Rowlands and Pagán, 2008). Two studies demonstrated that acute nicotine exposure alters stereotypical activity and motility and produces quantifiable withdrawal effects following cessation of exposure (Buttarelli et al., 2000; Pagán et al., 2009). Here, we used multiple behavioral assays to further characterize nicotine withdrawal in planarians and to test the hypothesis that nicotine produces behavioral sensitization and place conditioning in planarians.
2. Methods
2.1 General Methodology
Planarians (Dugesia dorotocephala) were purchased from Carolina Biological Supply (Burlington, NC, USA), acclimated to room temperature (21 °C), and tested within three days of receipt. Nicotine hydrogen tartrate salt was purchased from Sigma-Aldrich (St. Louis, MO). Nicotine solutions were prepared daily in tap water containing AmQuel® water conditioner (sodium hydroxymethanesulfonate) (1 ml Amquel per 1 gallon of water). All behavioral experiments were conducted in plastic petri dishes (5.5 cm diameter) containing water or nicotine under room temperature conditions. Each experiment used independent groups of planarians that were not reused for additional experiments. Behavioral observations were conducted by a well-trained experimenter who was blinded to treatment. All experiments were conducted between 1 PM and 5 PM.
2.2 Behavioral experiments
Experiment 1: Does acute nicotine exposure affect planarian motility and stereotypical activity?
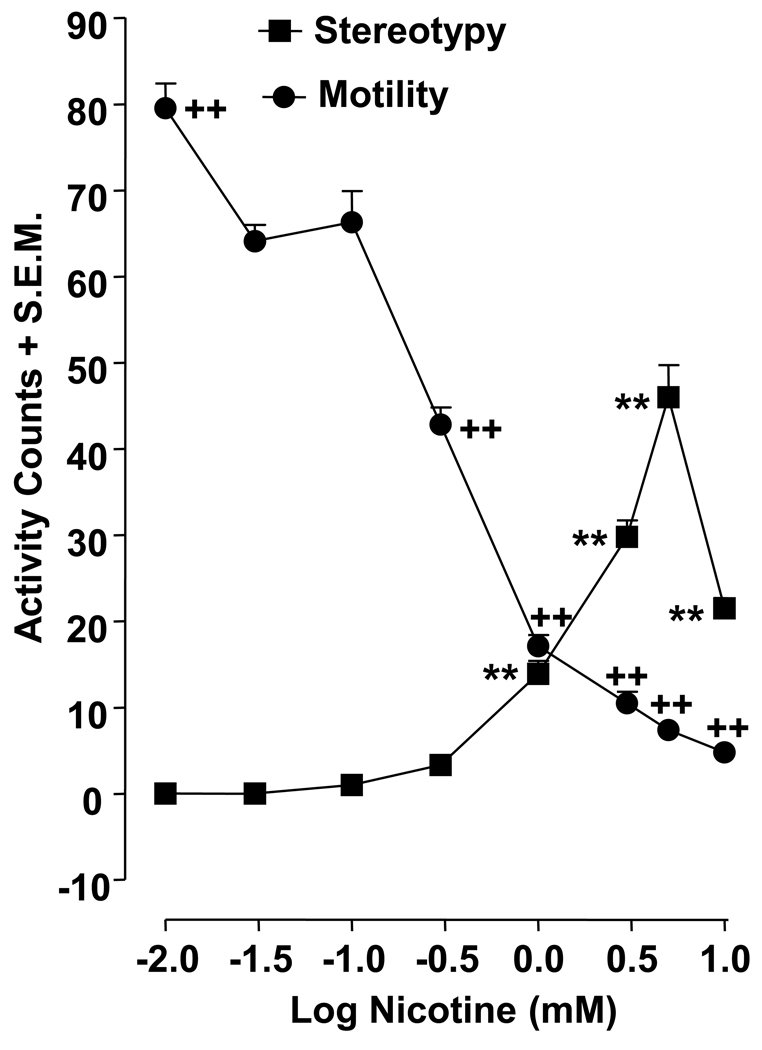
Individual planarians were placed into a petri plastic dish (5.5 cm diameter) containing different concentrations of nicotine solution (0, 0.01, 0.03, 0.1, 0.3, 1, 3, 10 mM). The dish was placed over paper with gridlines spaced 0.5 cm apart. Motility counts were quantified as the number of gridlines crossed or re-crossed over a 5-min observation interval (Raffa and Valdez, 2001). Stereotypical counts were defined as the number of C-like hyperkinesias during the same 5-min interval (Rawls et al., 2009). Prior work has demonstrated that motility and C-shape hyperkinesias displayed by planarians are not caused by changes in the pH or osmolarity of the solution (Rawls et al., 2009).
Experiment 2: Does the condition of nicotine abstinence produce withdrawal in planarians?
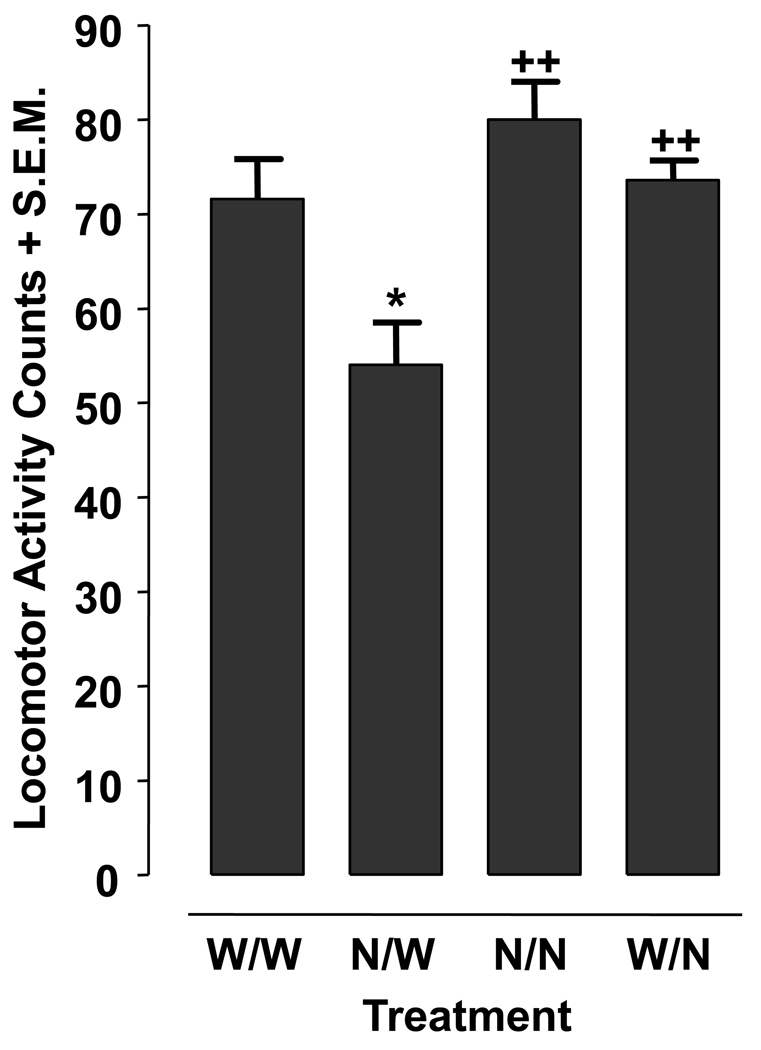
Physiological dependence is present when the withdrawal of a drug produces symptoms and signs that are frequently the opposite of those produced when the drug is present (O’Brien, 2006). It is thought that the organism adjusts to a new level of homeostasis during the period of drug exposure and reacts in opposite fashion when the new equilibrium is disturbed by removal of the drug. The presence of a withdrawal symptom or sign is the hallmark characteristic of physical dependence. Prior work has demonstrated that planarians display a reduction in motility (grid crossings) following spontaneous discontinuation of extended exposure to addictive substances such as cocaine, amphetamine, methamphetamine, cannabinoids, opioids, and benzodiazepines (Raffa and Rawls, 2008). We routinely use this endpoint (reduced motility) to quantify the condition of abstinence-induced withdrawal. In the present experiments, individual planarians were tested in nicotine (0.03 mM) or water for 60 min. Planarians from both the nicotine- and water-pretreated groups were then placed into a petri dish containing nicotine (0.03 mM) or water for 5 min, and motility counts during this 5-min exposure were quantified as described above.
Experiment 3: Does repeated nicotine exposure elicit sensitization or tolerance in planarians?
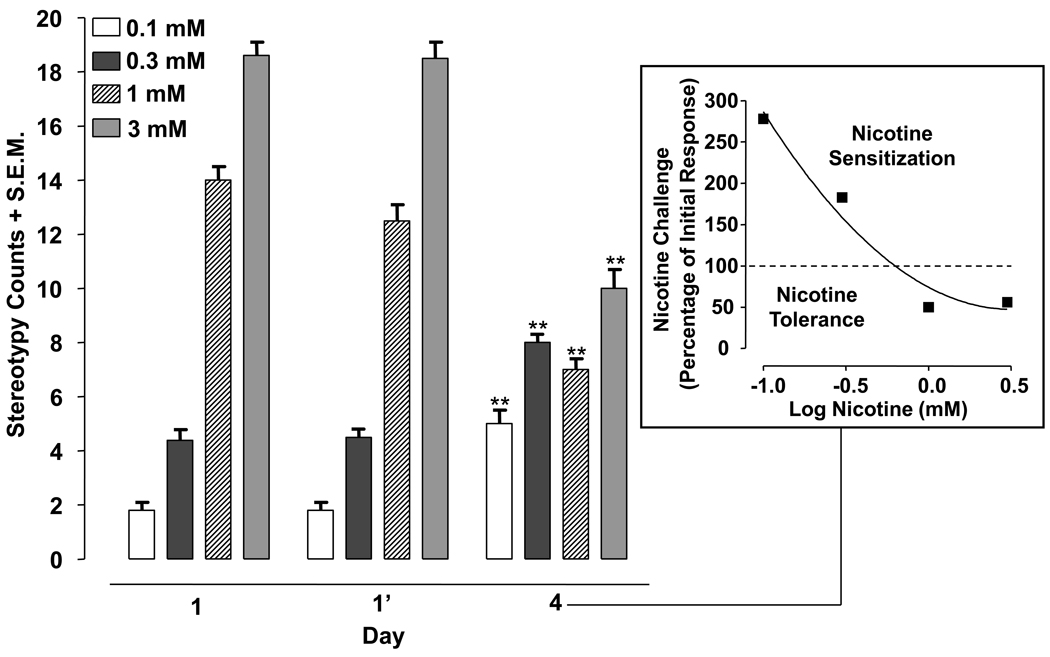
Individual planarians were treated with nicotine for 5 min (0.1, 0.3, 1, 3 mM) in three different test sessions: initial exposure (day 1); second exposure, 120 min later on day 1 (day 1’); and challenge exposure on day 4. C-shape hyperkinesias were quantified over each 5-min interval.
Experiment 4: Does nicotine induce environmental place conditioning in planarians?
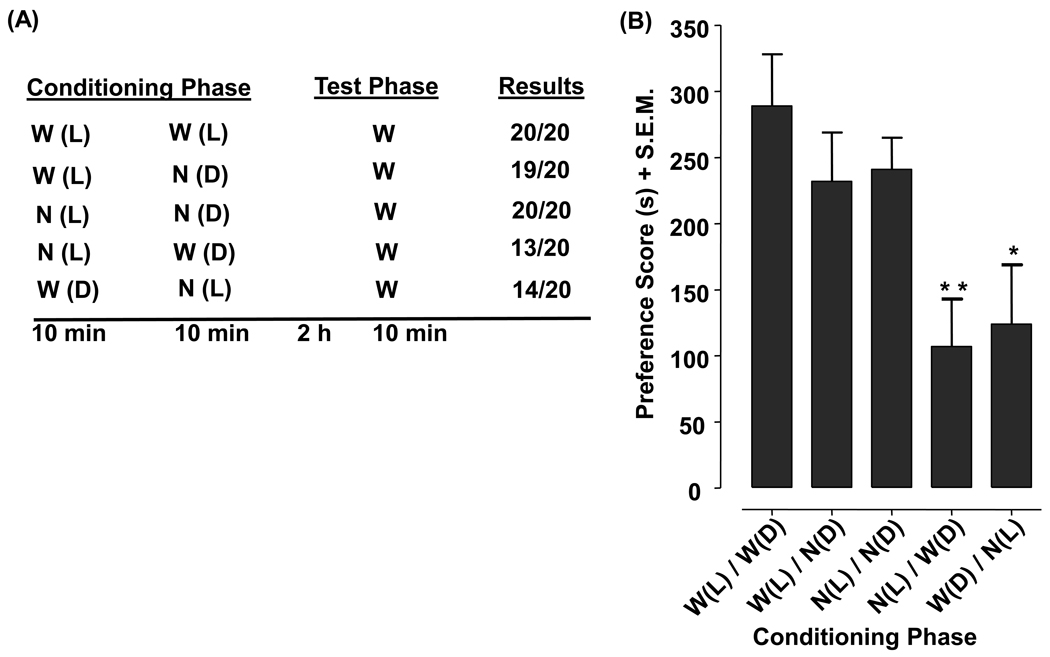
It is well established that planarians prefer a dark versus a light environment. When given a choice between the two environments, they will typically spend about 90% of the time in the dark. (Raffa et al., 2003). Therefore, we used a biased placed conditioning design to test the hypothesis that planarians conditioned with nicotine in the ambient light (non-preferred environment) – but not in the dark (preferred environment) – display a subsequent shift in their normal preference for the dark. The experimental design consisted of conditioning and test phases (see Fig. 4 for description). During conditioning, petri dishes containing nicotine (0.1 mM) or water were placed in an ambient light or dark environment. We covered the top and bottom of each petri dish with paper to create a dark environment. Planarians were conditioned with water in the ambient light for 10 min and then immediately conditioned with either water or nicotine in the dark for 10 min [W(L)/W(D) group and W(L)/N(D) group]. Separate groups of planarians were conditioned with nicotine in the ambient light for 10 min and then immediately conditioned with either water or nicotine for 10 min [N(L)/W(D) group and N(L)/N(D) group]. In a final group of planarians, water was first paired with the dark environment for 10 min followed immediately by the pairing of nicotine with ambient light for 10 min [W(D)/N(L)].
Fig. 4.
Nicotine elicits environmental place conditioning in planarians. Table) The conditioning phase paired nicotine (N) (0.1 mM) or water (W) with the ambient light (L) or dark (D) for 10 min. Preference testing was conducted 2 h later when planarians were placed for 10 min at the center of a petri dish containing water (half of dish was covered on the top and bottom by paper to create a dark side and ambient light side). Data are presented as the (A) number of planarians (out of 20) that spent a greater amount of time in the dark and (B) mean preference score (s) + S.E.M. **p < 0.01, *p < 0.05 compared to W(L)/W(D). N = 20 planarians per group.
Two hours following conditioning, preference testing was conducted. Half of a petri dish was covered on the top and bottom by paper to create a dark side and ambient light side. Planarians were then placed at the midpoint of the petri dish (i.e., in-between the dark and ambient light environments) containing water and the amount of time spent on the dark and light sides of the dish during a 10-min test interval was determined. Results from the place conditioning experiments were presented as a preference score. The preference score was defined as the difference between the amount of time spent in the preferred environment (dark) during the test phase and the amount of time spent in the non-preferred environment (ambient light) during the test phase.
2.3. Data analysis
Comparisons of group means (± S.E.M.) were evaluated by one-way ANOVA followed by a Dunnett's post-hoc analysis or, for the behavioral sensitization/tolerance, two-way ANOVA (treatment, day) followed by a Bonferroni test for multiple comparisons. Values of p < 0.05 were considered statistically significant.
3. Results
3.1. Acute nicotine alters planarian motility and stereotypical activity
Planarians exposed to water (nicotine naïve) did not display C-like hyperkinesias (i.e., stereotypical activity counts) (Fig. 1). The lowest nicotine concentration that produced significant C-like hyperkinesias was 1 mM and concentrations ranging from 1 to 5 mM concentration-dependently increased stereotypical activity (p < 0.01 for 1, 3, 5 mM). The highest nicotine concentration, 10 mM, increased C-like hyperkinesias compared to the water control group (p <0.01), but the effect was less pronounced than that observed with nicotine concentrations in the 1 – 5 mM range. For motility experiments, planarians exposed to water displayed 62.8 ± 1.7 motility counts over the 5-min observation interval. One-way ANOVA revealed a significant drug effect for the motility data [F(8, 63) = 218.6, p < 0.0001] (Fig. 1). Compared to the water control group, only the lowest nicotine concentration, 0.01 mM, significantly increased motility counts (79.5 ± 2,5) (p < 0.01). Higher nicotine concentrations (0.3, 1, 3, 5, 10 mM) concentration-dependently decreased planarian motility counts compared to water (p < 0.01).
Fig. 1.
Acute nicotine administration increases planarian motility and stereotypy. Planarians were exposed to different concentrations of nicotine (0.01, 0.03, 0.1, 0.3, 1, 3, 5, 10 mM). Motility and stereotypical activity was quantified during 5 min of nicotine exposure and presented as mean activity counts + S.E.M in 5 min. **p < 0.01 compared to water control for stereotypical activity and ++p < 0.01 compared to water control for motility. N = 8 planarians per group.
3.2. The condition of nicotine abstinence results in a withdrawal response
Planarian motility counts following nicotine abstinence, during continuous water or nicotine exposure, and during acute nicotine exposure are presented in Fig. 2. One-way ANOVA indicated a significant main effect for the data set [F(3, 28) = 8.447, p = 0.0004]. Planarians pretreated with nicotine (0.03 mM) for 60 min and then tested in water (N/W) displayed lower motility counts compared to: 0.03 mM nicotine-naïve planarians tested in water (W/W) (p < 0.05); 0.03 mM nicotine-pretreated planarians tested in 0.03 mM nicotine (N/N) (p < 0.01); and water-pretreated planarians tested in 0.03 mM nicotine (W/N) (p < 0.01). Nicotine-naïve planarians tested in water (W/W) displayed motility counts that were not significantly different than the motility counts displayed by nicotine-pretreated planarians tested in nicotine (N/N) or water-pretreated planarians tested in nicotine (p > 0.05).
Fig. 2.
Nicotine (0.03 mM) produces abstinence-induced withdrawal behavior in planarians. Planarians pre-treated with nicotine (N) or water (W) for 60 min were then tested in N or water (W) for 5 min. Data are presented as mean motility counts + S.E.M in 5 min. *p < 0.05 compared to W/W and ++p < 0.01 compared to N/W. N = 8 planarians per group.
3.3. Repeated, intermittent nicotine administration produces sensitization and tolerance
Stereotypy of planarians exposed to nicotine (0.1, 0.3, 1, 3 mM) twice on day 1 (1, 1’) and then treated with the same nicotine concentration on day 4 are displayed in Fig. 3. Two-way ANOVA revealed a significant drug effect [F(3, 28) = 447.6, p < 0.0001], day effect [F(2, 56) = 25.11, p < 0.0001], and interaction [F(6, 56) = 60.71, p < 0.0001]. A within-subjects ANOVA comparing group means across days for the 0.1 mM [F(2, 21) = 23.81, p < 0.0001] and 0.3 mM [F(2, 21) = 37.31, p < 0.0001] nicotine experiments indicated significant main effects. Post-hoc analysis revealed that 0.1 mM nicotine caused greater stereotypy on day 4 than on day 1 (initial exposure) (p < 0.001). A similar increase in stereotypical activity was observed in planarians treated with 0.3 mM nicotine (p < 0.001). For higher nicotine concentrations, a within-subjects ANOVA comparing group means across days for the 1 mM [F(2, 21) = 52.92, p < 0.0001] and 3 mM [F(2, 21) = 66.46, p < 0.0001] nicotine experiments indicated significant main effects. Post-hoc analysis revealed that 1 or 3 mM nicotine produced less stereotypy on day 4 than on day 1 (initial exposure) (p < 0.001).
Fig. 3.
Low concentrations of nicotine produce sensitization of stereotypical activity and high concentrations of nicotine produce tolerance to stereotypical activity. Planarians were exposed to nicotine (0.1, 0.3, 1, 3 mM) twice on day 1 (120 min apart) and then re-exposed to the same concentration of nicotine for 5 min on day 4. Data are expressed as mean stereotypy counts + S.E.M. during 5 min of nicotine exposure versus day (1, 1’, 4). **p < 0.01 compared to the stereotypy counts produced by initial drug exposure (day 1). N = 8 planarians per group. Box) The percentage of the initial nicotine response (day 1) produced by nicotine challenge on day 4 is plotted versus log nicotine concentration.
3.4. Nicotine produces environmental place conditioning in planarians
During the test phase, as expected, 100% of nicotine-naïve planarians [W(L)/W(D)] spent a greater amount of time on the dark side (Fig. 4, schematic). Similarly, 100% of planarians conditioned with 0.1 mM nicotine in both dark and ambient light environments [N(L)/N(D)] spent more time in the dark during subsequent testing, and 95% of planarians conditioned with nicotine in only the dark environment [W(L)/N(D)] spent more time in the dark. However, only 70% of planarians in which nicotine exposure was paired with just the non-preferred environment (ambient light) during conditioning [W(D)/N(L)] spent more time in the dark during testing. The reversed pairing during conditioning [N(L)/W(D)] produced similar effects with 65% of those planarians spending more time on the dark side.
For each group we also calculated a preference score, the difference between time spent in the preferred (dark) and non-preferred (light) environments during testing (Fig. 4). One-way ANOVA comparing mean preference scores indicated a significant main effect [F(4, 96) = 4.644, p = 0.0019]. Planarians in which nicotine conditioning was paired with ambient light during conditioning displayed a significant reduction in preference for the dark upon subsequent testing [p < 0.01 for N(L)/W(D) and p < 0.05 for W(D)/N(L)]. Preference scores were not affected by the order in which nicotine was paired with the ambient light during conditioning (p >0.05) [i.e., preference scores were not significantly different in the N(L)/W(D) and W(D)/N(L) groups].
4. Discussion
These results provide the first evidence that nicotine produces behavioral sensitization, tolerance, and place conditioning in planarians. We confirm prior evidence that spontaneous discontinuation of nicotine exposure elicits a withdrawal syndrome and acute nicotine administration increases motility and stereotypical activity in planarians (Buttarelli et al., 2000; Pagán et al., 2009). The nicotine effect on acute stereotypy was concentration-dependent, with lower concentrations producing minimal effects and higher concentrations eliciting marked enhancements. We quantified planarian stereotypical activity as the frequency of C-shape hyperkinesias (Rawls et al., 2009, 2010a,b). The relation between C-shape hyperkinesias displayed by planarians and stereotypy displayed by rodents is unclear, but drugs that possess abuse liability in humans often enhance stereotypical activity in rodents (Koob, 1992).
Acute nicotine exposure elicited bimodal effects on planarian motility. Consistent with previous work in planarians and C. elegans (Pagán et al., 2009; Feng et al., 2006), higher concentrations (0.3 to 10 mM) inhibited motility. Concentrations that produced the greatest suppression in motility also caused the most pronounced increase in C-shape hyperkinesias. It is interesting to note that the lowest nicotine concentration (0.01 mM) caused a slight, but significant, increase (about 126% of control) in motility that was not observed in the Pagán et al. (2009) study. Variations in experimental design may account for the difference. For instance, Pagán and colleagues (2009) measured planarian motility following a 15-min incubation period with nicotine and then measured motility over an 8-min test interval whereas we measured motility over a 5-min interval without the utilization of an incubation period.
Planarians displayed abstinence-induced withdrawal following spontaneous discontinuation of nicotine exposure (Pagán et al., 2009). The response to the abstinence condition observed here was decreased motility, an endpoint we have used to quantify planarian withdrawal to cocaine, amphetamines, benzodiazepines, and opioids (Raffa and Valdez, 2001; Rawls et al., 2007, 2009). We opted to test a nicotine concentration (0.03 mM) that lacked acute effects on basal motility. Experiments revealed that only those planarians spontaneously withdrawn from 0.03 mM nicotine exposure displayed reduced motility. No other overt withdrawal responses were observed, but, on the basis of prior work, it can be predicted that such responses would be observed following withdrawal from higher nicotine concentrations. Pagán et al. (2009) demonstrated that 0.1 mM nicotine produced four distinct planarian withdrawal responses: “HeadBops” (“nodding”-like movements while gliding at the bottom of the dish), “HeadSwings” (head rotation in the absence of gliding while the tail is fixed to the bottom of the dish), “TailTwists” (bending of the tail tip) and “Corkscrews” (spiral rotation while floating/swimming). The presence of a withdrawal symptom or sign is the hallmark feature of physical dependence, but the relevance of nicotine withdrawal responses in planarians to nicotine physical dependence in mammals is unknown. One attractive feature of the planarian assay is sensitivity as a quantifiable effect is present during the condition of nicotine abstinence. It is often difficult to detect withdrawal signs or symptoms following spontaneous discontinuation of nicotine administration in mammals, although a well-documented withdrawal syndrome comprised of somatic effects (e.g. forelimb tremor, head twitches, jumps and piloerection) and affective signs (e.g. anhedonia) is precipitated by administration of cholinergic antagonists to nicotine-dependent rats (Kenny and Markou, 2001; Malin, 2001). It is also possible that the reduced motility displayed by planarians during the condition of nicotine abstinence reflects a “depressive-like state”, similar to the immobility displayed by nicotine-withdrawn rats in the forced swim assay (Chae et al., 2008).
Nicotine sensitization and tolerance in planarians have not been reported previously. Low concentrations produced sensitized stereotypical responses in planarians exposed to nicotine twice on day 1 and challenged with nicotine after 48 h of drug abstinence. Planarians challenged with nicotine 120 min after initial exposure did not display enhanced stereotypy, suggesting that detectable sensitization was dependent on a minimum period of nicotine abstinence or a minimum number of nicotine exposures. Tolerance was detected following exposure to higher nicotine concentrations (1, 3 mM). Related work in C. elegans has demonstrated that repeated, intermittent exposure to low nicotine concentrations elicits behavioral sensitization whereas continuous, uninterrupted nicotine exposure produces tolerance (Feng et al., 2006). Although nicotine accumulation in planarians during repeated exposure may have contributed to the sensitization and tolerance, this possibility is not supported by the observation that neither sensitized nor tolerant responses were observed until planarians were withdrawn from nicotine for 48 h. Further evidence that nicotine accumulation was not a primary factor is that the opposing phenomena of sensitization and tolerance resulted from an identical experimental protocol. Future neurochemical and pharmacokinetic studies are planned to investigate nicotine accumulation and metabolism in planarians.
The correlation between sensitization in planarians and mammals is unclear. Nicotine sensitization occurs in both rats and mice (Vanderschuren and Kalivas, 2000). Nicotine doses as low as 0.1 mg/kg produce behavioral sensitization in rats but doses in the 0.4 to 0.6 mg/kg produce a more robust sensitized response (Domino, 2001; Villégier et al., 2003). These doses also fall within the range used to demonstrate the rewarding effects of nicotine through self-administration or conditioned place preference. Higher doses of 0.8 mg/kg or greater elicit profound locomotor depression, toxicity, seizures, and death in rats (Collins and Izenwasser, 2004). Interestingly, doses of 0.4 mg/kg or 0.5 mg/kg, which are optimal for producing sensitization in rats, failed to induce sensitization in mice whereas mice did sensitize to a much smaller dose of 0.05 mg/kg (Itzhak and Martin, 1999; Kim and Kim, 1999). These findings generally suggest that a more robust sensitized response in rats and mice is favored by low doses of nicotine, a finding that is consistent with the observed effects in planarians. Evidence that humans develop sensitization to nicotine is quite limited. Part of the difficulty in determining if sensitization occurs in humans is a lack of knowledge about which physiologic or behavioral phenomena would be affected. In the only study that we could find in which nicotine sensitization was investigated in humans, nonsmokers exposed to three doses of nicotine daily for 8 days did not display sensitization to gross motor coordination, working memory, attention, euphoria, drug liking, dysphoria, sedation, nausea, psychomimetic symptoms, blood pressure, heart rate, or skin temperature (Heishman and Henningfield, 2000). One application of the present finding is that planarians may offer an attractive model to study nicotine sensitization.
Environmental place conditioning was elicited by a concentration of nicotine (0.1 mM) that produced behavioral sensitization. Because planarians have a natural preference for a dark environment, we used a biased place conditioning paradigm in which nicotine exposure was paired with the non-preferred (ambient light) environment. The hypothesis was that nicotine conditioning in the non-preferred environment would attenuate the planarians natural preference for the dark. Indeed, this is what we found. Not surprisingly, all planarians that were either naïve to nicotine or conditioned with nicotine in both the ambient light and dark spent a greater amount of time in the dark. A similar result was displayed by planarians conditioned with nicotine in the dark, as 95% of those worms spent more time in the dark during testing. Planarians displayed a reduction in their natural preference for the dark in the case in which nicotine was paired with ambient light during conditioning. Only 65–70% of these planarians spent more time in the dark during testing. Preference was not dependent on the order in which nicotine was paired with ambient light during conditioning (i.e., nicotine/light and water/dark pairings versus water/dark and nicotine/light pairings produced shifts in preference). Thus, it is unlikely that the shift in preference was due to prior association with the condition of nicotine withdrawal [N(L)/W(D)]. It is worth noting that nicotine does induce conditioned place preference in rats (Le Foll and Goldberg, 2005), and that methamphetamine produces environmental place conditioning in planarians (Kusayama and Watanabe, 2000).
In summary, we report that nicotine, as in mammals, produces behavioral sensitization, tolerance, abstinence-induced withdrawal, and place conditioning in planarians. As such, they provide a valuable model to study nicotine action, issues of abuse, tolerance, and craving, and possible convenient screen for anti-nicotine agents.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boileau I, Dagher A, Leyton M, Gunn RN, Baker GB, Diksic M, Benkelfat C. Modeling sensitization to stimulants in humans: an [11C]raclopride/positron emission tomography study in healthy men. Arch. Gen. Psychiatry. 2006;63:1386–1395. doi: 10.1001/archpsyc.63.12.1386. [DOI] [PubMed] [Google Scholar]
- Buttarelli FR, Pontieri FE, Margotta V, Palladini G. Acetylcholine/dopamine interaction in planaria. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2000;125:225–231. doi: 10.1016/s0742-8413(99)00111-5. [DOI] [PubMed] [Google Scholar]
- Chae Y, Yeom M, Han JH, Park HJ, Hahm DH, Shim I, Lee HS, Lee H. Effect of acupuncture on anxiety-like behavior during nicotine withdrawal and relevant mechanisms. Neurosci. Lett. 2008;430:98–102. doi: 10.1016/j.neulet.2007.10.026. [DOI] [PubMed] [Google Scholar]
- Collins SL, Izenwasser S. Chronic nicotine differentially alters cocaine-induced locomotor activity in adolescent vs. adult male and female rats. Neuropharmacology. 2004;46:349–362. doi: 10.1016/j.neuropharm.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Domino EF. Nicotine induced behavioral locomotor sensitization. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2001;25:59–71. doi: 10.1016/s0278-5846(00)00148-2. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Smith AM, Wooters TE, Zhang Z, Crooks PA, Bardo MT. Nicotinic receptor-based therapeutics and candidates for smoking cessation. Biochem. Pharmacol. 2009;78:732–743. doi: 10.1016/j.bcp.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson KS, Panula P. gamma-Aminobutyric acid in the nervous system of a planarian. J. Comp. Neurol. 1994;345:528–536. doi: 10.1002/cne.903450405. [DOI] [PubMed] [Google Scholar]
- Feng Z, Li W, Ward A, Piggott BJ, Larkspur ER, Sternberg PW, Xu XZ. A C. elegans model of nicotine-dependent behavior: regulation by TRP-family channels. Cell. 2006;127:621–633. doi: 10.1016/j.cell.2006.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien CP. Drug Addiction and Drug Abuse (Chapter 23) In: Brunton LL, Lazo JS, Parker KL, editors. Goodman & Gilman's The Pharmacological Basis of Therapeutics. 11th edition. New York: The McGraw-Hill Companies, Inc.; 2006. pp. 611–612. [Google Scholar]
- Heishman SJ, Henningfield JE. Tolerance to repeated nicotine administration on performance, subjective, and physiological responses in nonsmokers. Psychopharmacology. 2000;152 doi: 10.1007/s002130000541. 321-133. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Martin JL. Effects of cocaine, nicotine, dizocipline and alcohol on mice locomotor activity: cocaine-alcohol cross-sensitization involves upregulation of striatal dopamine transporter binding sites. Brain Res. 1999;818:204–211. doi: 10.1016/s0006-8993(98)01260-8. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Neurobiology of the nicotine withdrawal syndrome. Pharmacol. Biochem. Behav. 2001;70:531–549. doi: 10.1016/s0091-3057(01)00651-7. [DOI] [PubMed] [Google Scholar]
- Kim HS, Kim KS. Inhibitory effects of ginseng total saponin on nicotine-induced hyperactivity, reverse tolerance and dopamine receptor supersensitivity. Behav. Brain Res. 1999;103:55–61. doi: 10.1016/s0166-4328(99)00030-3. [DOI] [PubMed] [Google Scholar]
- Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol. Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- Kusayama T, Watanabe S. Reinforcing effects of methamphetamine in planarians. Neuroreport. 2000;11:2511–2513. doi: 10.1097/00001756-200008030-00033. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Nicotine as a typical drug of abuse in experimental animals and humans. Psychopharmacology. 2006;184:367–381. doi: 10.1007/s00213-005-0155-8. [DOI] [PubMed] [Google Scholar]
- Malin DH. Nicotine dependence: studies with a laboratory model. Pharmacol. Biochem. Behav. 2001;70:551–559. doi: 10.1016/s0091-3057(01)00699-2. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O'Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- Narendran R, Martinez D. Cocaine abuse and sensitization of striatal dopamine transmission: a critical review of the preclinical and clinical imaging literature. Synapse. 2008;62:851–869. doi: 10.1002/syn.20566. [DOI] [PubMed] [Google Scholar]
- Nestby P, Schotte A, Janssen PF, Tjon GH, Vanderschuren LJ, De Vries TJ, Mulder AH, Leysen JE, Schoffelmeer AN. Striatal dopamine receptors in rats displaying long-term behavioural sensitization to morphine. Synapse. 1997;27:262–265. doi: 10.1002/(SICI)1098-2396(199711)27:3<262::AID-SYN10>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Nishimura K, Kitamura Y, Taniguchi T, Agata K. Analysis of motor function modulated by cholinergic neurons in planarian Dugesia japonica. Neuroscience. 2010;168:18–30. doi: 10.1016/j.neuroscience.2010.03.038. [DOI] [PubMed] [Google Scholar]
- Pagán OR, Rowlands AL, Azam M, Urban KR, Bidja AH, Roy DM, Feeney RB, Afshari LK. Reversal of cocaine-induced planarian behavior by parthenolide and related sesquiterpene lactones. Pharmacol. Biochem. Behav. 2008;89:160–170. doi: 10.1016/j.pbb.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Pagán OR, Rowlands AL, Fattore AL, Coudron T, Urban KR, Bidja AH, Eterović VA. A cembranoid from tobacco prevents the expression of nicotine-induced withdrawal behavior in planarian worms. Eur. J. Pharmacol. 2009;615:118–124. doi: 10.1016/j.ejphar.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladini G, Ruggeri S, Stocchi F, De Pandis MF, Venturini G, Margotta V. A pharmacological study of cocaine activity in planaria. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1996;115:41–45. doi: 10.1016/s0742-8413(96)00053-9. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Valdez JM. Cocaine withdrawal in Planaria. Eur. J. Pharmacol. 2001;430:143–145. doi: 10.1016/s0014-2999(01)01358-9. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Dasrath CS, Brown DR. Disruption of a drug-induced choice behavior by UV light. Behav. Pharmacol. 2003;14:569–571. doi: 10.1097/00008877-200311000-00010. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Rawls SM. A model for drug action and abuse. Austin, TX: Landes Bioscience; 2008. [Google Scholar]
- Raffa RB, Stagliano GW, Ross G, Powell JA, Phillips AG, Ding Z, Rawls SM. The kappa-opioid receptor antagonist nor-BNI inhibits cocaine and amphetamine, but not cannabinoid (WIN 52212-2), abstinence-induced withdrawal in planarians: an instance of 'pharmacologic congruence'. Brain Res. 2008;1193:51–56. doi: 10.1016/j.brainres.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls SM, Gomez T, Stagliano GW, Raffa RB. Measurement of glutamate and aspartate in Planaria. J. Pharmacol. Toxicol. Methods. 2006;53:291–295. doi: 10.1016/j.vascn.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Rawls SM, Gomez T, Raffa RB. An NMDA antagonist (LY 235959) attenuates abstinence-induced withdrawal of planarians following acute exposure to a cannabinoid agonist (WIN 55212-2) Pharmacol. Biochem. Behav. 2007;86:499–504. doi: 10.1016/j.pbb.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Rawls SM, Cavallo F, Capasso A, Ding Z, Raffa RB. The beta-lactam antibiotic ceftriaxone inhibits physical dependence and abstinence-induced withdrawal from cocaine, amphetamine, methamphetamine, and clorazepate in planarians. Eur. J. Pharmacol. 2008;584:278–284. doi: 10.1016/j.ejphar.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Rawls SM, Thomas T, Adeola M, Patil T, Raymondi N, Poles A, Loo M, Raffa RB. Topiramate antagonizes NMDA- and AMPA-induced seizure-like activity in planarians. Pharmacol. Biochem. Behav. 2009;93:363–367. doi: 10.1016/j.pbb.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Rawls SM, Patil T, Yuvasheva E, Raffa RB. First evidence that drugs of abuse produce behavioral sensitization and cross sensitization in planarians. Behav. Pharmacol. 2010a;21:301–313. doi: 10.1097/FBP.0b013e32833b0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls SM, Karaca F, Madhani I, Bhojani V, Martinez RL, Abou-Gharbia M, Raffa RB. β-lactamase inhibitors display anti-seizure properties in an invertebrate assay. Neuroscience. 2010b;169:1800–1804. doi: 10.1016/j.neuroscience.2010.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands AL, Pagán OR. Parthenolide prevents the expression of cocaine-induced withdrawal behavior in planarians. Eur. J. Pharmacol. 2008;583:170–172. doi: 10.1016/j.ejphar.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Rowlands AL, Pagán OR. Parthenolide prevents the expression of cocaine-induced withdrawal behavior in planarians. Eur. J. Pharmacol. 2008;583:170–172. doi: 10.1016/j.ejphar.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Schoffelmeer AN, De Vries TJ, Wardeh G, van de Ven HW, Vanderschuren LJ. Psychostimulant-induced behavioral sensitization depends on nicotinic receptor activation. J. Neurosci. 2002;22:3269–3276. doi: 10.1523/JNEUROSCI.22-08-03269.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda S, Stagliano GW, Borenstein MR, Raffa RB. A reverse-phase HPLC and fluorescence detection method for measurement of 5-hydroxytryptamine (serotonin) in Planaria. J. Pharmacol. Toxicol. Methods. 2005;51:73–76. doi: 10.1016/j.vascn.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Venturini G, Stocchi F, Margotta V, Ruggieri S, Bravi D, Bellantuono P, Palladini G. A pharmacological study of dopaminergic receptors in planaria. Neuropharmacology. 1989;28:1377–1382. doi: 10.1016/0028-3908(89)90013-0. [DOI] [PubMed] [Google Scholar]
- Villégier AS, Blanc G, Glowinski J, Tassin JP. Transient behavioral sensitization to nicotine becomes long-lasting with monoamine oxidases inhibitors. Pharmacol. Biochem. Behav. 2003;76:267–274. doi: 10.1016/s0091-3057(03)00223-5. [DOI] [PubMed] [Google Scholar]
- Vyas CA, Rawls SM, Raffa RB, Shackman JG. Glutamate and aspartate measurements in individual planaria by rapid capillary electrophoresis. J. Pharmacol. Toxicol. Methods. 2010;63:119–122. doi: 10.1016/j.vascn.2010.08.002. [DOI] [PubMed] [Google Scholar]