Abstract
Background
Renin-angiotensin system blockade reduces inflammation in several organ systems. Having found a fourfold increase in angiotensin II type Ia receptor expression in a dextran sodium sulfate colitis model, we targeted blockade with angiotensin II type Ia receptor antagonists to prevent colitis development. Because hypotension is a major complication of angiotensin II type Ia receptor antagonists use, we hypothesized that use of angiotensin II type Ia receptor antagonists compounds which lack cell membrane permeability, and thus enteric absorption, would allow for direct enteral delivery at far higher concentrations than would be tolerated systemically, yet retain efficacy.
Methods
Based on the structure of the angiotensin II type Ia receptor antagonist losartan, deschloro-losartan was synthesized, which has extremely poor cell membrane permeability. Angiotensin II type Ia receptor antagonist efficacy was evaluated by determining the ability to block NF-κB activation in vitro. Dextran sodium sulfate colitis was induced in mice and angiotensin II type Ia receptor antagonist efficacy delivered transanally was assessed.
Results
In vitro, deschloro-losartan demonstrated near equal angiotensin II type Ia receptor blockade compared to losartan as well as another angiotensin II type Ia receptor antagonist, candesartan. In the dextran sodium sulfate model, each compound significantly improved clinical and histologic scores and epithelial cell apoptosis. Abundance of TNF-α, IL-1β, and IL6 mRNA were significantly decreased with each compound. In vitro and in vivo intestinal drug absorption, as well as measures of blood pressure and mucosal and colonic blood flow, showed significantly lower uptake of deschloro-losartan compared to losartan and candesartan.
Conclusions
This study demonstrated efficacy of high-dose angiotensin II type Ia receptor antagonists in this colitis model. We postulate that a specially designed angiotensin II type Ia receptor antagonist with poor oral absorption may have great potential as a new therapeutic agent for inflammatory bowel disease in the future.
Keywords: Angiotensin II type Ia receptor, Dextran sodium sulfate, Colitis, Angiotensin II type Ia receptor antagonist, Nuclear factor κB
Introduction
The renin-angiotensin system (RAS) is well known to have various physiologic roles [1–3]. In this system, angiotensin II (ATII), produced through the enzymatic cleavage of angiotensin I (ATI) by ATI-converting enzyme (ACE), exerts a number of physiological actions, including effects on vascular tone, hormone secretion, tissue growth, and neuronal activities [4, 5]. There are two major subtypes of ATII receptors; ATII type 1 receptor (type 1a and 1b in mice, and type 1a in humans) and ATII type 2 receptor. ATII type 1 receptor (AT1R) mediates vasoconstriction and AT1R is also involved in the mediation of apoptosis, vascular remodeling, and inflammation [6–9]. Therefore, ACE inhibitors and AT1R antagonists (AT1R-A), commonly used as therapeutic agents for treating hypertension, have additional therapeutic actions.
Recently, in vitro experiments have shown that ligation of the AT1aR will lead to nuclear factor κB (NF-κB) activation[10], and the subsequent production of inflammatory mediators such as tumor necrosis factor α (TNF-α)[11], transforming growth factor β1, interleukin 1β (IL-1β) and monocyte chemotactic protein-1 (MCP-1) [12, 13]. ATII activation of NF-κB has been shown in human monocytes [14] and in cultured vascular smooth muscle cells [15]. Based on this, the NF-κB activation pathway has emerged as an extremely attractive target for the development of anti-inflammatory drugs [9, 16–18]. Although the function of ATII in the intestine is not well understood, our laboratory has previously shown that ACE is expressed in the intestinal epithelium, increases in a rodent colitis model, and is critically important in promoting the development of intestinal epithelial cell apoptosis [3, 19–21]. Interestingly, receptors for ATII are also found in the intestinal mucosa [22]. Furthermore, recent studies using pharmacologic ACE inhibitors (ACE-I) have shown that ACE-I significantly reduces epithelial cell (EC) apoptosis and decreases the severity of inflammation in a dextran sodium sulfate (DSS) model [19, 21]. Other investigators have reported that ACE mediation of apoptosis is via ATII signaling through its respective receptors in a number of organs and cells such as lung, prostate, and endothelial cells [23–25]. Although ATII may signal via several receptors, the AT1aR predominates, and appears to be the critical mediator of pro-inflammatory and pro-apoptotic signaling [3, 26–28]. In fact, use of angiotensinogen knockout mice, AT1aR knockout mice or use of AT1aR-As has led to a marked improvement in the severity of a dextran sodium sulfate (DSS) and trinitrobenzene sulphonic acid induced colitis [6, 29]. Additionally, AT1aR-As have been successfully used via parenteral administration to decrease gastric inflammation [30]. Taken together, this suggests that blockade of ATII signaling may have a useful role in the treatment of inflammatory conditions of the gastrointestinal tract. However, because a major consequence of the use these antagonists is hypotension, with resultant diminished local and systemic blood flow, we hypothesized that the use of an AT1aR-A which lacks cell membrane permeability, and thus has poor mucosal absorption, would allow for direct enteral delivery at far higher concentrations than would be tolerated systemically, yet retain local efficacy. In this study, we used three AT1aR-A compounds to determine the role of the AT1aR in a DSS-induced colitis model in mice. We demonstrated efficacy for high-dose AT1aR-A in preventing histologic changes, colonic apoptosis, and increased pro-inflammatory cytokines in this colitis model. We further showed that synthesis of an analogue of the conventional antihypertensive losartan, with poor oral absorption, may have equal local efficacy, but lacks systemic absorption and hemodynamic changes.
Materials and Methods
Animals
Specific pathogen-free male, 8-week-old C57BL/6 mice (Taconic Farms Inc, Germantown, NY) were maintained in a 12-h night rhythm at 23°C and a relative humidity of 40–60%. Animals were fed standard rodent chow (LabDiet 5001Rodent Diet, PMI Nutrition International, LLC, Brentwood, MO) ad libitum. All experiments were approved by the University Committee on Use and Care of Animals at the University of Michigan.
Induction of Colitis
Colitis was induced by 2.5% (W/V) reagent-grade dextran sulfate sodium (DSS; molecular weight; 36,000–50,000, ICN Biomedicals, Inc, Aurora, OH) dissolved in drinking water that was ingested ad libitum.
Angiotensin II Type I Receptor Antagonists
Suspensions of chemical inhibitors in doubly distilled water were prepared immediately before use. Commercially available inhibitors investigated included: losartan (2-butyl-4-chloro-1-[[2′-(2H-tetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-1H-imidazole-5-methanol, mono-potassium salt; C22H22ClKN6O; molecular weight; 461.00 (purchased from Hallochem Pharma Co., Chongqing, China) and candesartan (2-ethoxy-1-[[2′-(2H-tetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-1H-benzimidazole-7-carboxylic acid; C33H34N6O6, molecular weight; 440.45 (also purchased from Hallochem Pharma Co.). A new synthetic method was developed to access deschloro-losartan (DCL; 2-butyl-1-[[2′-(2H-tetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-1H-imidazole-5-methanol) directly from commercially available losartan potassium salt (Fig. 1). Deschloro-losartan, previously synthesized by a longer route, had been shown to have retained ATIaR-A activity, yet possess poor oral absorption [31]. The synthesis was carried out as follows: Losartan monopotassium salt (647 mg, 1.4 mmol) was dissolved in 10 ml of absolute ethanol in a dry flask under nitrogen. Ammonium formate (443 mg, 7.0 mmol) was added followed by palladium black catalyst (75 mg, 0.7 mmol). The mixture was stirred for 18–24 h at 25°C while monitoring by silica gel thin-layer chromatography for maximum conversion of starting material to product before any side-products began to form. The reaction mixture was vacuum filtered through a wet pad of Celite (2 g), and the filtrate was concentrated to a white foam that was purified by silica gel flash chromatography, eluting with a gradient of 5–20% methanol in dichloromethane. Product fractions were combined and concentrated to a residue that was crystallized from 2-propanol to yield 391 mg (72% yield) of product as a crystalline white power; mp 173–174°C, 1H NMR (DMSO-d6, 500 MHz): δ 0.80 (t, J = 7.2 Hz, 3H), 1.23 (m, 2H), 1.48 (m, 2H), 2.52 (t, J = 7.7 Hz, 2H), 4.34 (s, 2H), 5.24 (s, 2H), 6.88 (s, 1H), 6.95 (d, J = 8.0 Hz, 2H), 7.06 (d, J = 8.0 Hz, 2H), 7.45 (d, J = 7.6 Hz, 1H), 7.50 (t, J = 7.5 Hz, 1H), 7.60 (m, 2H). Anal. Calcd. for C22H24N6O (molecular weight: 388.47): C, 68.02; H, 6.23; N, 21.63. Found: C, 67.70; H, 6.17; N, 21.39.
Fig. 1.
Structure of study compounds losartan, deschloro-losartan, and candesartan
In Vitro Assessment of AT1aR Antagonism
A variation of an in vitro model established by McAllister-Lucas et al. [10] was used to measure the degree of antagonism on AT1aR signaling. This model makes use of the fact that AT II-dependent stimulation of the AT1aR results in rapid activation of the NF-κB transcription factor. In this model, HepG2-AR cells, which stably express the AT1aR, were transiently transfected with an NF-κB-luciferase reporter plasmid and a control Renilla plasmid (to correct for transfection efficiency). Cells were then treated with or without ATII (1 μM) for 16 h in the presence or absence of varying doses of either losartan or deschloro-losartan. Cells were harvested and the luciferase/Renilla ratio determined using the Promega Dual Luciferase Assay Kit. Cells were treated (in triplicate) with either media alone (negative control), ATII (1 μM), ATII plus losartan (0.1–100 μM), or ATII plus deschloro-losartan (4-log range of concentration). Loss of ATII-dependent luciferase induction (measured by luminometer; LMax; Molecular Devices), due to the presence of receptor blockade was then calculated and used to indicate the extent of successful blockade of the AT1aR signaling pathway.
Experimental Colitis Design
Mice were randomly divided into five groups. One group consisted of naive mice as a control group (n = 6), which received plain drinking water ad libitum, and received only water (0.25 ml) via the transanal route without AT1aR-A. In the remaining four groups, DSS was administered through drinking water for 7 days. In the DSS + placebo group, mice were given transanal water (total volume 0.25 ml) each day as a control (n = 13). Three DSS + AT1aR antagonists (AT1aR-A) groups were studied consisting of losartan (n = 10), DCL (n = 6), and candesartan (n = 9) suspended in ddH2O at a total volume of 0.25 ml. As we have previously shown that the administration of a very high dose (tenfold higher than typically given systemically) of an ACE-I had little to no systemic side-effects, yet improved outcome in a DSS acute and chronic colitis model [32], a similar high-dose strategy was selected. AT1aR-A were given at 100-fold higher doses (losartan and DCL: 100 mg/kg/day, candesartan: 10 mg/kg/day) than typically given systemically, and daily dosing was continued for the entire 7 days of the study. Study drugs were administered using a blunt needle via the transanal route. Preliminary testing confirmed that this amount of drug evenly coated the entire colon. Furthermore, to ensure retention of AT1aR-A within the entire colon, mice were held by the tail in a vertical position for 30 s after the transanal administration.
Harvesting
The mice were killed 7 days after DSS by carbon dioxide asphyxiation. A 0.5-cm segment taken from the distal half of the colon was excised and placed into 10% formaldehyde. Formalin-preserved sections of distal colon were preserved in paraffin, sectioned transversely (5 μm) and stained with hematoxylin and eosin (H&E). The remaining colon was immediately processed for mucosal cell isolation.
Assessment of Colitis
The body weight of each mouse, stool characteristics, and intestinal bleeding were recorded. All animals were evaluated daily. Occult bleeding was tested using a Hemoccultcard test (Beckman Coulter Inc, Fullerton, CA). Histologic grading of colitis was performed in a masked fashion (investigator blinded to the study group) according to previously described methods [21]. Crypt shortening and distortion, together with inflammatory infiltrative thickening of the lamina propria, were assigned a score 0 (normal) through 4 (complete loss of crypt, ulceration, and severe thickening of lamina propria). The individual colitis score (0–4) from four quadrants of a left-sided colonic section were summed, such that the maximum score for a given section was 16, and the minimum score was 0. The mean of at least two sections were assessed in this manner for each mouse.
Epithelial Cell Apoptosis Assays
A terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL) staining method was used to detect apoptosis, according to the manufacturer’s instructions (ApopTag Plus Peroxidase InSitu Apoptosis Detection Kit, Chemicon International Inc, Temecula, CA), with slight modification. Assessment of apoptosis consisted of separate counting of all TUNEL-positive EC in all well-oriented crypts separately, and dividing the total number of counted apoptotic cells per number of analyzed crypts and the number represents the Apoptotic Index.
Mucosal Cell Isolation and Purification
Isolation of mucosal cells was performed using a previously described protocol [33]. Colonic tissue, not including the cecum, was placed in RPMI cell culture medium on ice, and fecal contents were gently flushed out. Colonic epithelium was isolated for RNA as described previously [21]. Briefly, the colon was opened longitudinally and rinsed with fresh cold RPMI, then the colonic mucosa was mechanically scraped off on a glass slide, and epithelial cells (EC) collected in fresh RPMI with glutamine. These EC were then immediately snap-frozen in liquid nitrogen and processed for RNA extraction.
Real-Time Polymerase Chain Reaction (RT-PCR)
Mucosal scrapings were placed in TRIzol (Invitrogen), homogenized, RNA extracted and purified as previously described [21]. All primers for selected gene sequences were designed using proprietary software (Lasergene, DNA star Inc, Madison, WI). Real-time PCR (RT-PCR) was performed using a Rotor-Gene 6000 (Corbett Life Science, Sydney, Australia) and β-actin was used as an internal control for normalization. Fold changes of target genes were calculated using comparative quantification to β-actin.
Assessment of Drug Uptake From Gastrointestinal Tract
Several methods were used to determine the uptake of each compound from the gastrointestinal tract, including in vitro assessment with Ussing chambers, in vivo assessment of serum levels and local and systemic physiologic changes.
Ussing-Chamber Experiments
Methods of assessing the in vitro passage of each compound across the intestinal wall were similar to those previously described [34]. Briefly, a Ussing chamber setup was utilized with freshly isolated jejunum and left-sided colon from naive and DSS-treated mice (after 7 days) using standard techniques [35]. Intestinal tissues with an exposed tissue surface area of 0.3 cm2 were incubated in 5 ml of preheated 37°C Krebs buffer in each half membrane (serosal side and mucosal side), and pH was adjusted to 7.4. Each chamber was continuously oxygenated with O2/CO2 (95%/5%) and stirred by gas flow in the chambers. An AT1a antagonist was added to the mucosal side of each sample (final concentration 1 mg/ml). Samples (0.3 ml) were taken from the serosal side at time points 0, 20, 40, 60, 80, 100, and 120 min in triplicate and tandem mass spectroscopy (liquid chromatography with mass spectroscopy/mass spec, LCMSMS; see below) was performed. The permeability of each AT1aR-A across the excised mouse tissues in the Ussing chamber was calculated using the following equation and published method [34]:
where dC/dt is the slope of AT1aR-A concentration in the serosal side (mg/ml-s), V is the volume of the serosal side (5 ml), A is the exposed surface area (0.3 cm2) of the membrane, and Co the starting concentration in the mucosal side (1 mg/ml).
Plasma Levels of Orally Administered AT1a Antagonists
In a separate set of experiments, 7-day DSS-treated mice were given the oral administration of each AT1aR-A at a dose of 10 mg/kg, and then killed 2 h later. Plasma samples were obtained from cardiac puncture and analyzed by LCMSMS.
Tandem Mass Spectroscopy
Mass spectroscopy experimental methods were performed as follows using previously published techniques [36, 37]. Samples were diluted tenfold with the mobile phase and injected to the mass detector using a Hewlett Packard HP1100 HPLC system with a Gemini® (C18, 150 × 2.0 mm) column with flow rate of 0.2 ml/min. The mobile phase consisted of 50% acetonitrile and 50% of water containing 0.1% formic acid. A Waters Micromass Quattro II mass spectrometer in electrospray ionization mode was used for the detection of the AT1aR-A. Sample acquisitions were performed with multiple reaction monitoring (MRM) mode using the mass to charge transitions: losartan: 423 > 207, candesartan: 441 > 192 and DCL: 389 > 207. Integration and quantification of the acquired data were performed using the MassLynx software, version 4.1 (Waters Corp., Milford, MA).
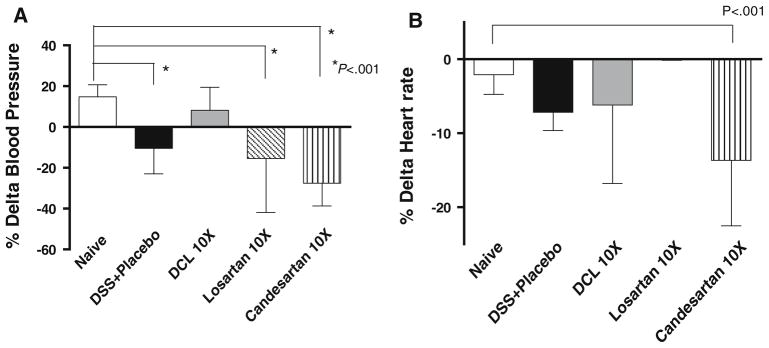
Measurements of Blood Pressure and Heart Rate
Blood pressure and heart rate were measured using a noninvasive computerized tail-cuff system (IITC Life Science Inc., Woodland Hills, CA) at 1 h before transanal treatment (Pre) and 2 h later after treatment (Post) on day 6 of DSS intake. The system was designed to perform all functions automatically, including a programmable routine of cuff inflation and deflation, analysis and assignment of pulse rate and blood pressure, and recording of data electronically. We expressed these results using % Delta Blood Pressure (percent change in systolic pressures prior to and after treatment) and the mean heart rate.
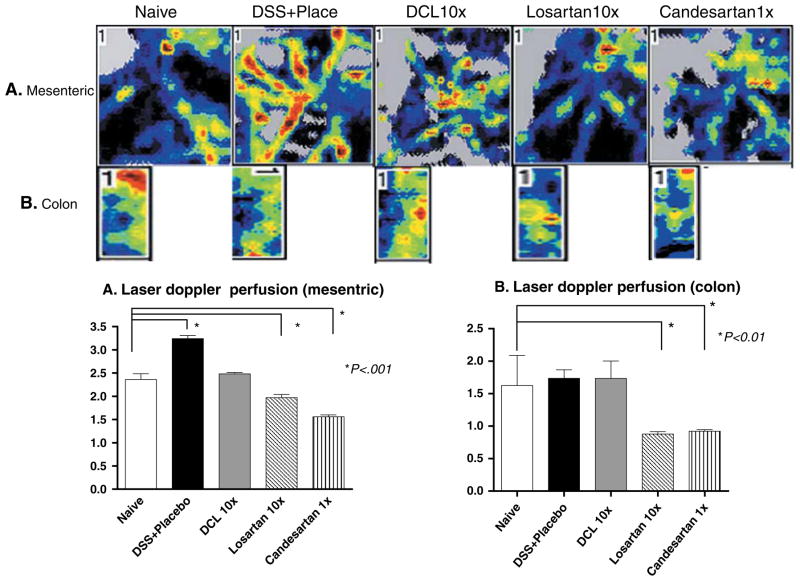
Measurements of Colonic Mucosa and Mesenteric Blood Flow
Because a major consequence of the use of AT1aR-A is hypotension which could compromise intestinal blood flow, colonic mucosa and mesenteric blood flow were also evaluated at 7 days of DSS administration, just before harvesting tissues using laser Doppler perfusion imaging (LDPI, Perimed Inc., North Royalton, OH), as previously reported [21]. Mesenteric, as well as distal colonic mucosal blood flows were measured in anesthetized mice (n = 6, in each group). A median laparotomy was performed and the mesentery exposed. A LDPI 670-nm helium–neon laser beam was placed 12 cm above the mesentery and sequentially scanned the surface of the mesentery, as well as colonic mucosa over a 2-cm length. Maximum, minimum, and mean percent perfusion was normalized to total pixel area. At the end of the measurements the mice were killed and tissue harvested.
Statistical Analysis
Data are reported as mean ± standard deviation (SD). Results were analyzed using the t test for comparison of two means, and a one-way analysis of variance (ANOVA) for comparison of multiple groups. A post-hoc Bonferroni test was used to assess statistical difference between groups. The Chi-square test was used for categorical data (Prism software; GraphPad Software, Inc., San Diego, CA). A value of p < 0.05 was considered to be statistically significant.
Results
In Vitro Assessment of AT1aR Antagonism
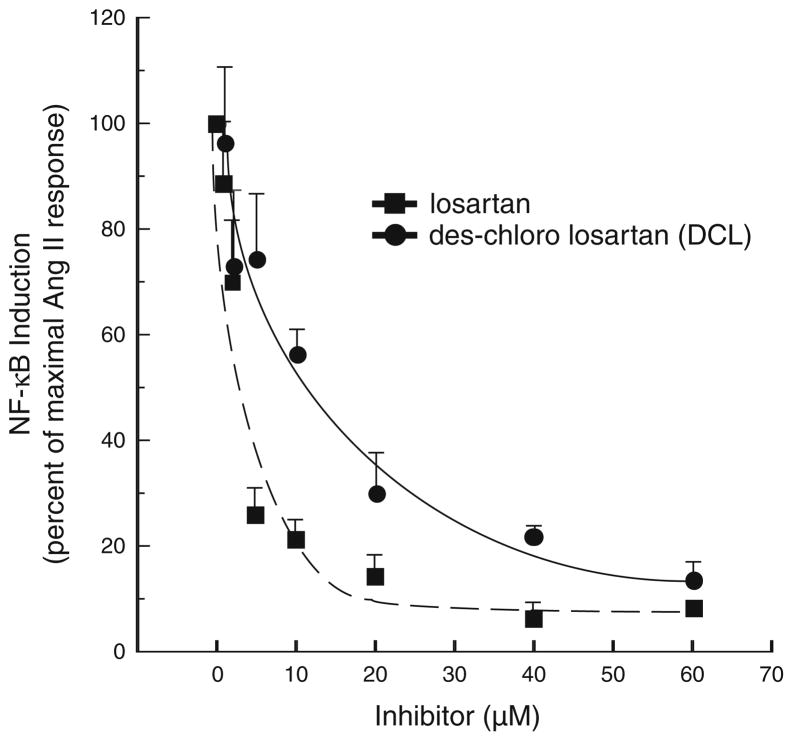
To determine if our synthesized analogue of losartan, DCL, had similar antagonism to AT1aR compared to losartan, in vitro assessment was determined by the ability of each compound to prevent NF-κB activation in vitro. Figure 2 shows the extent of both compound’s ability to block NF-κB activation. The average maximal NF-κB induction seen with incubation with angiotensin II alone was 5.95-fold, and this value was set to a level of 100% induction. DCL demonstrated near equal AT1aR antagonism compared to losartan (85 vs. 90% inhibition of NF-κB activation at 60 μM dosing, respectively). Thus, DCL came within 10% of the inhibitory capacity of losartan, and was felt to have comparable antagonistic behavior.
Fig. 2.
NF-κB-induction, as detected by a luciferase reporter, is shown for HepG2-AR cells treated with or without Ang II (1 μM) for 16 h, in the presence or absence of varying doses of either losartan or DCL. The average maximal NF-κB induction seen with Ang II alone was 5.95-fold, and this value was set as 100% induction. Note that DCL demonstrated near equal AT1aR blockade compared to losartan (85 vs. 90% inhibition of NF-κB activation at 60 μM dosing, respectively)
Effect of AT1aR-A on Clinical Parameters
After DSS administration, mice developed colitis, which was manifested by loose stools, intestinal bleeding, and weight loss.
Body Weights
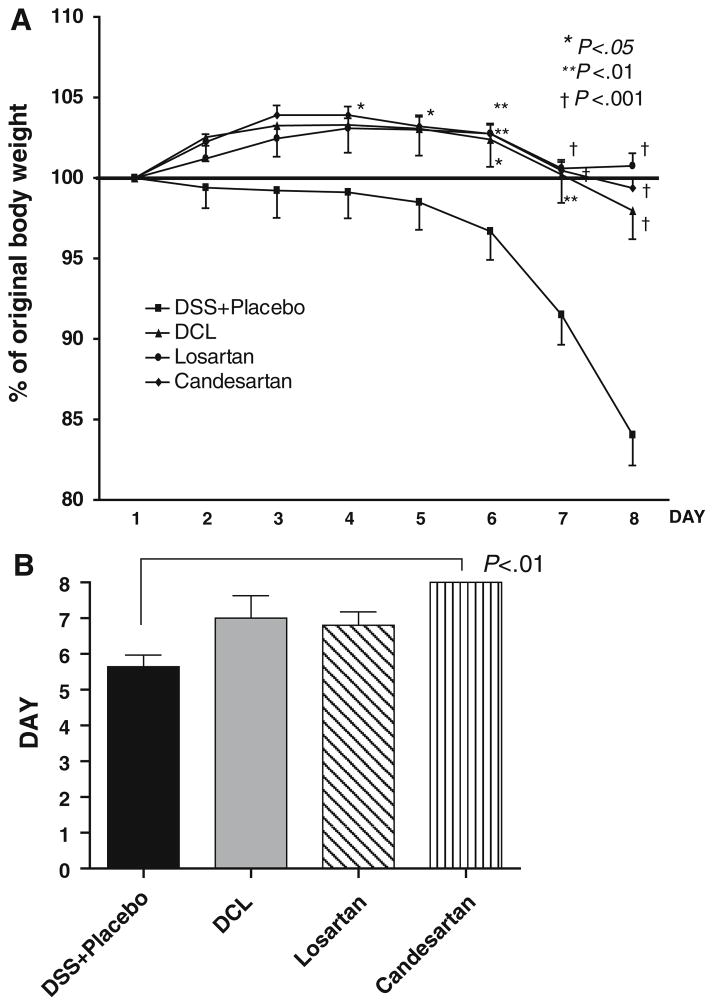
Body weight change (reported as percentage change from baseline body weight on day 1) is shown in Fig. 3a. As shown by others [32], significant weight loss occurred toward the end of 1 week of DSS. However, AT1aR-A treatment significantly protected against this weight loss (Fig. 3a). The difference between the placebo and the AT1aR-A-treated groups became significant after day 5 of DSS. After 1 week, weight loss was severe in the placebo group (15.8 ± 6.5% weight loss); however, this was significantly attenuated in all AT1aR-A groups (DCL: −2.02 ± 4.34%, losartan: 0.07 ± 2.50%, candesartan: −0.63 ± 1.63%; p < 0.001 versus placebo; p > 0.050 between AT1aR-A groups, respectively).
Fig. 3.
a Time course of changes in the body weight loss. All mice received 2.5% DSS in drinking water. DSS was administrated during the 7 days. All mice were evaluated daily for weight loss, stool consistency, and occult or gross intestinal bleeding. The groups were: DSS + placebo (n = 13) mice, deschloro-losartan 10 × (2,500 μg per dose) treated (n = 6), losartan 10 × (2,500 μg per dose) treated (n = 10) mice, and candesartan 1 × (250 μg per dose) treated (n = 9) mice. b Days to heme-positive stool as compared with the DSS + placebo group. Results are expressed as mean ± SD, with ANOVA used to compare with the DSS + placebo group
Fecal Blood
The onset of heme-positive stools corresponded closely to the development of weight loss (Fig. 3b). Mice in AT1aR-A groups experienced longer periods before developing heme-positive stools (DCL: 7.00 ± 1.4 days, N.S, losartan: 6.8 ± 2.7 days, N.S, candesartan: 8.0 ± 0.0 days), compared to mice in the placebo group (5.64 ± 1.22 days); and the change became significant for candesartan (p < 0.01).
Effect of AT1aR-A on Histopathology
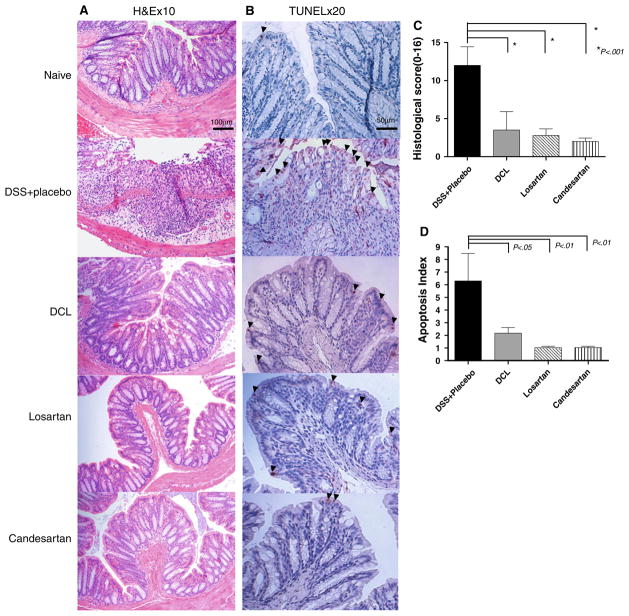
To evaluate if transanal treatment with AT1aR-A was associated with a reduction in the severity of colitis a blinded histological score at day 7 was assessed. In the DSS + Placebo group mice consistently developed severe ulcerative lesions in the distal colon. These histologic changes were significantly attenuated in all of the AT1aR-A-treated mice (histopathology in Fig. 4a, histologic scores reported in Fig. 4c). The colon of all ATa1R-A-treated mice showed nearly normal mucosal architecture.
Fig. 4.
a Representative histologic sections of distal colon are shown after undergoing H&E staining (×10 magnification). In the DSS + placebo group, diffuse cellular destruction was noted. In AT1aR-A-treated mice, the colon showed almost normal mucosa architecture and mild edema located in the submucosa. b Representative histologic sections of distal colon are shown after undergoing TUNEL staining (×20 magnification) after 5 days of DSS. Note the prominent epithelial cell apoptosis in the DSS + placebo group as represented by a dense brown staining (arrowheads). Whereas, only mild apoptosis is noted in AT1aR-A-treated mice. c Histologic scoring of each study group, the naive group is not shown, as the score is 0. Note that the histologic score was markedly increased in DSS + placebo mice, and significantly decreased in each of the AT1aR-A groups. Data (mean ± SD; N a minimum of six per group) were analyzed using ANOVA compared with DSS + placebo. d Apoptosis index scores are shown. Note that the apoptosis index was markedly increased in DSS + placebo mice, and indices significantly decreased in each of the AT1aR-A groups. Data (mean ± SD; N a minimum of six per group) were analyzed using ANOVA compared with DSS + placebo, # p < 0.01 compare with DSS + placebo
Effect of AT1aR-A on Epithelial Cell Apoptosis
TUNEL staining was performed after 5 days of DSS. Enterocyte apoptosis rates were significantly higher in the DSS + placebo group compared to all AT1aR-A treatment groups. Administration of an AT1aR-A resulted in a significant decline in EC apoptosis rates in DSS colitis (representative TUNEL staining in Fig. 4b, apoptosis rates are reported in Fig. 4d).
Ussing-Chamber Experiments
Table 1 shows the results of permeation of each AT1aR-A in both naive (normal) and DSS-treated mice. Passage of AT1aR-A for colonic and small intestinal tissue was moderately high for losartan- and candesartan-treated segments. Interestingly, the permeability coefficient was threefold to sixfold lower for DCL-treated segments compared to other AT1aR-antagonists. This was true for both naive- and DSS-treated specimens.
Table 1.
Permeability (Peff) of AT1a antagonists across the excised mouse intestinal segments in Ussing chamber (× 106 cm/s)
| Drug | Peff (× 106 cm/s)
|
||
|---|---|---|---|
| Normal or DSS | Colon (mean ± SD) | Small intestine (mean ± SD) | |
| Losartan | Normal | 6.40 ± 5.60 | 4.21 ± 1.67 |
| DSS | 5.77 ± 3.37 | 5.24 ± 1.40 | |
| Candesartan | Normal | 4.13 ± 3.04 | 7.85 ± 5.91 |
| DSS | 5.94 ± 4.41 | 3.85 ± 2.03 | |
| DCL | Normal | 1.58 ± 1.24 | 1.37 ± 0.15 |
| DSS | 2.66 ± 0.96 | 1.52 ± 1.31 | |
| Enalaprilata | Normal rat jejunum | 6.0 | |
| Metoprolola | Normal rat jejunum | 35.0 | |
Enalaprilat and metoprolol permeability in Ussing chambers are listed for comparison from a previous publication [34]
Oral Absorption of AT1aR-A
Plasma levels of AT1a-antagonists 2 h after oral administration are shown in Table 2. Although standard deviations were high, losartan and candesartan administration led to very high plasma levels; whereas, DCL levels were threefold lower than losartan, and over tenfold lower than candesartan.
Table 2.
Cmax of oral AT1a antagonist in DSS-treated mice
| AT1a antagonist | DSS mice Cmax (ng/ml) |
|---|---|
| Losartan | 117 ± 87 |
| DCL | 37.8 ± 26.4 |
| Candesartan | 569 ± 188 |
Serum values (mean ± SD; n = 5) from DSS-treated mice dosed with each study compound (10 mg/kg) and killed 2 h after oral dosing. Samples determined from cardiac puncture
Blood Pressure and Heart Rate Measurements
To test the systemic effect of each compound, blood pressure Delta BP (post systolic–pre systolic) was determined. Losartan and candesartan resulted in a decline in systolic BP (as shown by a large delta BP in the negative direction); however, DCL did not significantly change the BP compared to naive (non-treated) mice (Fig. 5a). Heart rates were not significantly different between the placebo, naive, DCL, and the losartan groups; however, heart rate did decline significantly in the candesartan group compared to the naive group (Fig. 5b).
Fig. 5.
BP and heart rates via tail cuff measurements 7 days after treatment. Data (mean ± SD; n = 6 per group) were analyzed using ANOVA compared with the DSS + placebo group. Note that losartan and candesartan resulted in a decline in BP, but DCL did not significantly change BP
Colonic Mucosal and Mesenteric Blood Flow Measurements
Mesenteric blood flow measurements showed significantly lower levels in losartan- and candesartan-treated groups compared to the naïve group (1.97 ± 0.58, 1.56 ± 0.33 vs. 2.82 ± 0.23, respectively, p < 0.001). On the other hand, in the DSS + placebo group blood flow was significantly elevated compared to naive mice (3.24 ± 0.30 vs. 2.82 ± 0.23, p < 0.001), which suggested a greater inflammatory response (Fig. 6a). Further, laser Doppler measurements of colonic blood flow at the mucosal level showed a significant decrease for losartan- and candesartan-treated groups compared to naive mice (0.88 ± 0.32, 0.92 ± 0.20 vs. 1.91 ± 0.26, p < 0.001; Fig. 6b). Interestingly, treatment of mice with DCL failed to change either mesenteric or mucosal blood flow; supporting a lack of systemic or even local–regional vascular dilation, which would be associated with AT1aR-A action.
Fig. 6.
Mesenteric and mucosal distal colon blood flow as measured by laser Doppler perfusion imager. Mesenteric flow was significantly increased in the DSS + placebo group vs. the naive group. Losartan and Candesartan resulted in a decline in mesenteric and mucosal flows compared to the naive group, but use of DCL did not significantly change flow at either site. The scores were compared to the naive cohort for significance. Data (mean ± SD; n = 6 per group) were analyzed using ANOVA
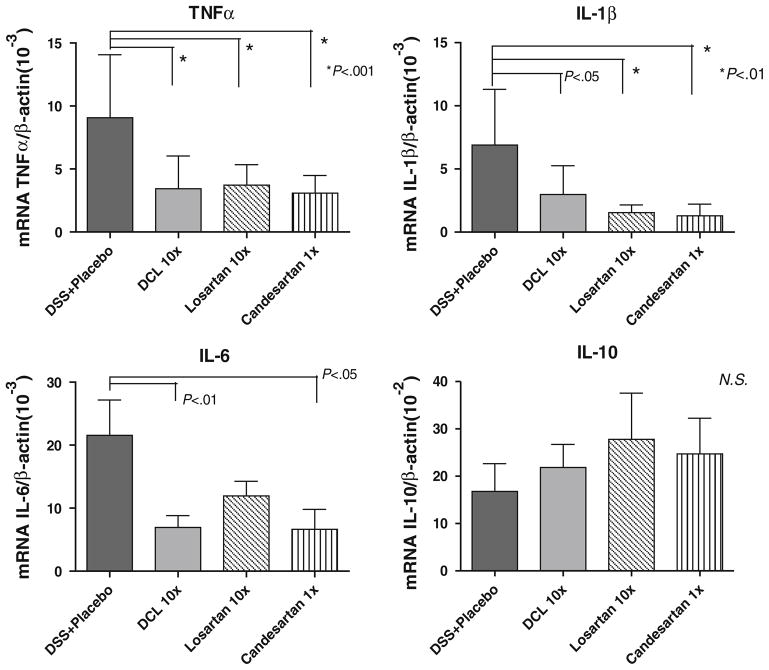
Effect of AT1aR-A on Pro-inflammatory Cytokine Expression
Expression of TNF-α, IL-1β, and IL-6 are pro-inflammatory cytokines that were investigated based on previous work that showed a reduction in the abundance of these cytokines with ACE-I treatment [21]. Further, these cytokines are known to be up-regulated in the DSS colitis model, and may be responsible for acute tissue injury formation. Therefore, these were quantified as independent biochemical markers of inflammation. Figure 7 shows mRNA expression of TNF-α, IL-1β, and IL-6 as compared to placebo-treated DSS mice. DSS + placebo mice showed significantly increased levels of TNF-α, IL-1β and IL6 mRNA. However, each AT1aR-A compound significantly decreased the mRNA abundance of these cytokines.
Fig. 7.
Abundance of mucosal cytokines TNF-α, IL-1β, IL-6, and IL-10, as detected by real-time PCR. TNF-α, IL-1β, and IL-6 mRNA were significantly lower in the AT1aR-A-treated groups compared to the DSS + placebo group. There were no significant differences in IL-10 expression between study groups. Results are mean ± SD, of a minimum of six samples from each group. Comparisons were made using ANOVA with post hoc Bonferroni test
Additionally, IL-10 abundance was examined, as IL-10 can down-regulate or completely inhibit the expression of several pro-inflammatory cytokines. There was a trend toward higher IL-10 abundance on day 7 in AT1aR-A treatment groups compared to placebo; however, this difference was not statistically significant (Fig. 7).
Discussion
The present study hypothesized that the use of AT1aR-A compounds which lack cell membrane permeability, and thus mucosal absorption, would allow for direct enteral delivery at far higher concentrations than would be tolerated systemically, yet retain efficacy. To achieve this, we modified the structure of the conventional anti-hypertensive drug losartan. In vitro assessment of AT1aR blockade confirmed that this analog, DCL, had nearly the same ability to block this receptor. Each AT1aR-antagonist, losartan, DCL, and candesartan decreased the percent of weight loss and time to the development of heme-positive stools. The histopathologic grade of colitis and level of EC apoptosis were also significantly lower in AT1aR-A-treated mice. Finally, the study showed that treatment with AT1aR-A led to a decline in the abundance of several pro-inflammatory cytokines.
There are two major ATII receptors, the AT1R (including the 1a and 1b sub-types) and AT2R. The AT1aR is best known to mediate vasoconstriction, and AT1aR-As are therefore used as therapeutic agents for treating hypertension. In addition to its anti-hypertensive actions, AT1aR-A have been shown to strongly suppress the generation of reactive oxygen species induced by ATII in activated leukocytes [27, 38–40] and reduce production of pro-inflammatory cytokines and adhesion molecules induced by ATII. These RAS actions are also mediated via the function of ADAMs (a disintegrin and metalloproteinases). In fact, ADAM 17 may function to cleave receptors for both AT1a and TNF-α, and may significantly modulate downstream signaling in many cell types [41]. Interestingly, use of pharmacologic blockade of ADAMs is becoming a potential clinical approach to block TNF-α signaling [42]; however, it will be interesting to better understand the implications of the blockade of ADAM on AT1aR signaling, where such action may actually adversely affect a patient with inflammatory bowel disease. Initial interest in the RAS in the gastrointestinal tract is based on the fact that several components of the RAS are highly expressed in the small and large intestine of rodents and humans [3, 19, 21, 32, 43–45]. It has been previously shown either through the blockade AT1aR or via inhibition of ACE that colitis can be palliated in rodent models [6, 21].
Patients with active Crohn’s disease and ulcerative colitis show a decreased expression of circulating levels of ACE [46–48]. These depressed serum levels of ACE may be a response to increased local expression of ACE. This concept is supported by Jaszewski et al., wherein colonic mucosal expression of ATI and ATII were shown to be markedly increased in patients with active Crohn’s disease and ulcerative colitis [49]. Interestingly, genetic mutations in ACE and ATII [50] show a significant association of the angiotensinogen-6 AA genotype with Crohn’s disease. Another more recent study analyzed for genetic mutations in I and D alleles of the ACE gene in ulcerative colitis and Crohn’s disease patients. These investigators found an increase in the DD homozygous pattern of ACE for those ulcerative colitis patients with extra-intestinal diseases [51].
A clear obstacle to the successful use of the AT1aR-A class of drugs is that most of these drugs were designed for optimal gastrointestinal absorption. As such, adequate dosing for the local gastrointestinal treatment of inflammatory bowel disease could lead to both hypotension as well as diminished gastrointestinal blood flow. Losartan was one of the first AT1aR-A, and remains a standard to which other antagonists are compared. Interestingly, during the development of losartan much of this effort centered on improving the oral bio-availability of the early analogs, many of which were devoid of systemic activity. A natural but unintended consequence of these investigations was the identification of highly potent AT1aR antagonists that completely lacked oral activity, including DCL, the dechlorinated form of losartan. Previous reports have recognized that in vitro DCL has a 50% inhibitory action on aortic contractility (IC50) following ATII stimulation of 0.019 μM; and this concentration of DCL compares quite closely to losartan’s IC50 of 0.019 μM [31]. The bio-availability characteristics of these two drugs differ considerably. Whereas the oral 50% effective dose (ED50, blood pressure lowering) for losartan in rats was determined to be 0.59 mg/kg, DCL was virtually undetected (>100 mg/kg)[31]. Such very early evidence strongly suggested that we could capitalize on this poor mucosal permeability to approach our unique treatment of colitis. Another AT1aR-A selected was candesartan due to its strong efficacy and known long-acting half-life [52, 53]. Whereas, the half-life for release of losartan from the AT1 receptor is only 5 min [54], candesartan has one of the longest reported “insurmountable” antagonisms, as is demonstrated by a 152 min half-life for ligand-receptor dissociation [54]. In vivo studies have shown that candesartan is able to reduce tissue injury induced by myocardial ischemia–reperfusion injury, liver ischemia [55, 56] and cerebral ischemia [57, 58]. In addition, it was demonstrated that candesartan protects against ischemia–reperfusion injury of the small intestine in rats [4]. Because Candesartan has only moderate oral absorption, a cyclohexyl 1-hydroxyethyl carbonate ester (cilexetil) prodrug is used commercially to improve intestinal absorption. Because of this, we elected to use the purified free carboxylic acid form of Candesartan in this study to minimize absorption.
We confirmed poor permeation of DCL both in our in vitro assessment with Ussing-chamber studies, as well as with assessment of plasma levels of the three compounds. Both of these showed a several-fold-lower permeation of DCL compared to losartan and candesartan. Permeability of these agents were compared (in Table 1) for enalaprilat, an angiotensin converting enzyme inhibitor with poor enteral absorption, and was seen to be at a similar range to these two compounds; whereas metoprolol had a fivefold-higher permeability. The fact that mesenteric and colonic mucosal blood flow measurements significantly declined and delta BP increased in both losartan and candesartan groups compared to naive mice strongly confirms that local and systemic absorption were negatively impacting upon the mice. The fact that our synthesized analog DCL failed to significantly impact local blood flow or blood pressure further supports our hypothesis that a modified AT1aR-A could be developed with poor enteral absorption yet still have the ability to impart a significant reduction in a DSS colitis model. It is also important to emphasize that this was achieved despite delivery of DCL at a tenfold-higher concentration than conventionally delivered in rodent models. This opens up a wide area of potential investigation, which may allow future novel analogs which are designed to avoid mucosal absorption, yet retain AT1aR-A function. Importantly, such a class of drugs has not been shown to compromise the immune system, which may make such compounds quite desirable as either an adjunct to current immunosuppressive therapies, or as a stand-alone treatment.
Although AT1aR-A produced significant clinical and histological improvements, it is important to note that both the abundance of pro-inflammatory cytokines, heme-positive stools, and the histopathology were not completely prevented with the use of high dose AT1aR-As. It is probable that other mechanisms are responsible for inflammatory formation that are not affected by AT1aR-A. It may also suggest that a wider range of drug dosing may need to be explored to determine the full efficacy of this class of drugs.
In summary, the present study indicates that the use of AT1aR-A can significantly protect against colonic epithelial apoptosis, epithelial destruction, and pro-inflammatory cytokine abundance in a DSS colitis model. These results suggest that the interaction between angiotensin II and angiotensin II type I receptor might be implicated in the pathogenesis of DSS colitis. Use of specially designed AT1R-A compounds with poor oral absorption, such as DCL, may have great potential as a new therapeutic approach to treating inflammatory bowel disease.
Acknowledgments
We wish to thank Janet Hoff of the University of Michigan Coordinator Center for support via the Integrative Genomics Cancer center grant. This research was funded by NIH, 1R43DK083074-01A1 _DIG10, NIH UL1-RR024986, and R01 AI044076-12.
Contributor Information
Manabu Okawada, Email: manabuo@med.umich.edu, Section of Pediatric Surgery, Department of Surgery, The University of Michigan Medical School, Mott Children’s Hospital, F3970, Ann Arbor, MI 48109-0245, USA.
Hiroyuki Koga, Email: h-koga@juntendo.ac.jp, Section of Pediatric Surgery, Department of Surgery, The University of Michigan Medical School, Mott Children’s Hospital, F3970, Ann Arbor, MI 48109-0245, USA.
Scott D. Larsen, Email: sdlarsen@umich.edu, Department of Medicinal Chemistry, College of Pharmacy, University of Michigan, Ann Arbor, MI 48109-1065, USA
Hollis D. Showalter, Email: showalh@umich.edu, Department of Medicinal Chemistry, College of Pharmacy, University of Michigan, Ann Arbor, MI 48109-1065, USA
Anjanette J. Turbiak, Email: koritnik@umich.edu, Department of Medicinal Chemistry, College of Pharmacy, University of Michigan, Ann Arbor, MI 48109-1065, USA
Xiaohong Jin, Email: xhjin@umich.edu, Department of Pathology, The University of Michigan Medical School, Ann Arbor, MI 48109, USA.
Peter C. Lucas, Email: plucas@umich.edu, Department of Pathology, The University of Michigan Medical School, Ann Arbor, MI 48109, USA
Elke Lipka, Email: elipka@tsrlinc.com, Therapeutic Systems Research Laboratories, Inc, Ann Arbor, MI 48108, USA.
John Hillfinger, Email: jhilfinger@tsrlinc.com, Therapeutic Systems Research Laboratories, Inc, Ann Arbor, MI 48108, USA.
Jae Seung Kim, Email: jkim@tsrlinc.com, Therapeutic Systems Research Laboratories, Inc, Ann Arbor, MI 48108, USA.
Daniel H. Teitelbaum, Email: dttlbm@umich.edu, Section of Pediatric Surgery, Department of Surgery, The University of Michigan Medical School, Mott Children’s Hospital, F3970, Ann Arbor, MI 48109-0245, USA
References
- 1.Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez-Vita J, Sanchez-Lopez E, Esteban V, et al. Angiotensin II activates the Smad pathway in vascular smooth muscle cells by a transforming growth factor-beta-independent mechanism. Circulation. 2005;111:2509–2517. doi: 10.1161/01.CIR.0000165133.84978.E2. [DOI] [PubMed] [Google Scholar]
- 3.Koga H, Yang H, Haxhija EQ, et al. The role of angiotensin II type 1a receptor on intestinal epithelial cells following small bowel resection in a mouse model. Pediatr Surg Int. 2008;24:1279–1286. doi: 10.1007/s00383-008-2277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takagi T, Yoshida N, Isozaki Y, et al. CV-11974, angiotensin II type I receptor antagonist, protects against ischemia-reperfusion injury of the small intestine in rats. Eur J Pharmacol. 2006;535:283–290. doi: 10.1016/j.ejphar.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Dzau VJ, Gibbons GH, Pratt RE. Molecular mechanisms of vascular renin-angiotensin system in myointimal hyperplasia. Hypertension. 1991;18:II100–II105. doi: 10.1161/01.hyp.18.4_suppl.ii100. [DOI] [PubMed] [Google Scholar]
- 6.Inokuchi Y, Morohashi T, Kawana I, et al. Amelioration of 2, 4, 6-trinitrobenzene sulphonic acid induced colitis in angiotensinogen gene knockout mice. Gut. 2005;54:349–356. doi: 10.1136/gut.2003.036343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossig L, Dimmeler S, Zeiher AM. Apoptosis in the vascular wall and atherosclerosis. Basic Res Cardiol. 2001;96:11–22. doi: 10.1007/s003950170073. [DOI] [PubMed] [Google Scholar]
- 8.Wesselman JP, De Mey JG. Angiotensin and cytoskeletal proteins: role in vascular remodeling. Curr Hypertens Rep. 2002;4:63–70. doi: 10.1007/s11906-002-0055-9. [DOI] [PubMed] [Google Scholar]
- 9.Wolf G, Wenzel U, Burns KD, et al. Angiotensin II activates nuclear transcription factor-kappaB through AT1 and AT2 receptors. Kidney Int. 2002;61:1986–1995. doi: 10.1046/j.1523-1755.2002.00365.x. [DOI] [PubMed] [Google Scholar]
- 10.McAllister-Lucas L, Ruland J, Siu K, et al. CARMA3/Bcl10/MALT1-dependent NF-κB activation mediates angiotensin II-responsive inflammatory signaling in nonimmune cells. PNAS. 2007;104:139–144. doi: 10.1073/pnas.0601947103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizushima T, Sasaki M, Ando T, et al. Blockage of angiotensin II type 1 receptor regulates TNF-gamma-induced MAdCAM-1 expression via inhibition of NF-κB translocation to the nucleus and ameliorates colitis. Am J Physiol Gastrointest Liver Physiol. 2009;298:G255–G266. doi: 10.1152/ajpgi.00264.2009. [DOI] [PubMed] [Google Scholar]
- 12.Ruiz-Ortega M, Bustos C, Hernandez-Presa MA, et al. Angiotensin II participates in mononuclear cell recruitment in experimental immune complex nephritis through nuclear factor-kappa B activation and monocyte chemoattractant protein-1 synthesis. J Immunol. 1998;161:430–439. [PubMed] [Google Scholar]
- 13.Klahr S, Morrissey J. Angiotensin II and gene expression in the kidney. Am J Kidney Dis. 1998;31:171–176. doi: 10.1053/ajkd.1998.v31.pm9428470. [DOI] [PubMed] [Google Scholar]
- 14.Kranzhofer R, Browatzki M, Schmidt J, et al. Angiotensin II activates the proinflammatory transcription factor nuclear factor-kappaB in human monocytes. Biochem Biophys Res Commun. 1999;257:826–828. doi: 10.1006/bbrc.1999.0543. [DOI] [PubMed] [Google Scholar]
- 15.Ruiz-Ortega M, Lorenzo O, Ruperez M, et al. Angiotensin II activates nuclear transcription factor kappaB through AT(1) and AT(2) in vascular smooth muscle cells: molecular mechanisms. Circ Res. 2000;86:1266–1272. doi: 10.1161/01.res.86.12.1266. [DOI] [PubMed] [Google Scholar]
- 16.May MJ, Marienfeld RB, Ghosh S. Characterization of the IkappaB-kinase NEMO binding domain. J Biol Chem. 2002;277:45992–46000. doi: 10.1074/jbc.M206494200. [DOI] [PubMed] [Google Scholar]
- 17.Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 18.Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wildhaber B, Yang H, Haxhija E, et al. Intestinal intraepithelial lymphocyte derived angiotensin converting enzyme modulates epithelial cell apoptosis. Apoptosis. 2005;10:1305–1315. doi: 10.1007/s10495-005-2138-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haxhija E, Yang H, Spencer A, et al. Modulation of mouse intestinal epithelial cell turnover in the absence of angiotensin converting enzyme. Am J Physiol Gastrointest Liver Physiol. 2008;295:G88–G98. doi: 10.1152/ajpgi.00589.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spencer AU, Yang H, Haxhija EQ, et al. Reduced severity of a mouse colitis model with angiotensin converting enzyme inhibition. Dig Dis Sci. 2007;52:1060–1070. doi: 10.1007/s10620-006-9124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirasawa K, Sato Y, Hosoda Y, et al. Immunohistochemical localization of angiotensin II receptor and local renin-angiotensin system in human colonic mucosa. J Histochem Cytochem. 2002;50:275–282. doi: 10.1177/002215540205000215. [DOI] [PubMed] [Google Scholar]
- 23.Wang R, Ibarra-Sunga O, Verlinski L, et al. Abrogation of bleomycin-induced epithelial apoptosis and lung fibrosis by captopril or by a caspase inhibitor. Am J Physiol Lung Cell Mol Physiol. 2000;279:L143–L151. doi: 10.1152/ajplung.2000.279.1.L143. [DOI] [PubMed] [Google Scholar]
- 24.de Resende MM, Greene AS. Effect of ANG II on endothelial cell apoptosis and survival and its impact on skeletal muscle angiogenesis after electrical stimulation. Am J Physiol Heart Circ Physiol. 2008;294:H2814–H2821. doi: 10.1152/ajpheart.00095.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu W, Zhao YY, Zhang ZW, et al. Angiotensin II receptor 1 blocker modifies the expression of apoptosis-related proteins and transforming growth factor-beta1 in prostate tissue of spontaneously hypertensive rats. BJU Int. 2007;100:1161–1165. doi: 10.1111/j.1464-410X.2007.07150.x. [DOI] [PubMed] [Google Scholar]
- 26.Li X, Rayford H, Uhal BD. Essential roles for angiotensin receptor AT1a in bleomycin-induced apoptosis and lung fibrosis in mice. Am J Pathol. 2003;163:2523–2530. doi: 10.1016/S0002-9440(10)63607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okuda T, Yoshida N, Takagi T, et al. CV-11974, angiotensin II type I receptor antagonist, reduces the severity of indomethacin-induced rat enteritis. Dig Dis Sci. 2008;53:657–663. doi: 10.1007/s10620-007-9931-0. [DOI] [PubMed] [Google Scholar]
- 28.Santiago O, Rivera E, Ferder L, et al. An angiotensin II receptor antagonist reduces inflammatory parameters in two models of colitis. Regul Pept. 2008;146:250–259. doi: 10.1016/j.regpep.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sasaki M, Wada T, Mizushima T, et al. Amelioration of Dextran Sulfate Sodium Induced Colitis in Angiotensin II Type 1 Receptor Knockout Mice. Gastroenterology. 2007;131:M1671. [Google Scholar]
- 30.Suzuki T, Kuroda M, Yoshida N, et al. Angiotensin II Type 1A receptor blockade attenuates gastric inflammation and fibrosis in experimental ulcer healing. Gastroenterology. 2006;130:M1979. [Google Scholar]
- 31.Duncia JV, Chiu AT, Carini DJ, et al. The discovery of potent nonpeptide angiotensin II receptor antagonists: a new class of potent antihypertensives. J Med Chem. 1990;33:1312–1329. doi: 10.1021/jm00167a007. [DOI] [PubMed] [Google Scholar]
- 32.Koga H, Yang H, Adler J, et al. Angiotensin converting enzyme inhibitor prevents colonic fibrosis in a mouse colitis model: development of a unique mode of treatment. Surgery. 2008;144:259–268. doi: 10.1016/j.surg.2008.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang H, Antony PA, Wildhaber BE, et al. Intestinal intraepithelial lymphocyte gammadelta-T cell-derived keratinocyte growth factor modulates epithelial growth in the mouse. J Immunol. 2004;172:4151–4158. doi: 10.4049/jimmunol.172.7.4151. [DOI] [PubMed] [Google Scholar]
- 34.Lennernas H, Nylander S, Ungell A. Jejunal permeability: a comparison between the Ussing chamber technique and the single-pass perfusion in humans. Pharm Res. 1997;14:667–671. doi: 10.1023/a:1012121632357. [DOI] [PubMed] [Google Scholar]
- 35.Yang H, Finaly R, Teitelbaum DH. Alteration in epithelial permeability and ion transport in a mouse model of total parenteral nutrition. Crit Care Med. 2003;31:1118–1125. doi: 10.1097/01.CCM.0000053523.73064.8A. [DOI] [PubMed] [Google Scholar]
- 36.Ferreiros N, Dresen S, Alonso R, et al. Validated quantitation of angiotensin II receptor antagonists (ARA-II) in human plasma by liquid-chromatography-tandem mass spectrometry using minimum sample clean-up and investigation of ion suppression. Ther Drug Monit. 2007;29:824–834. doi: 10.1097/FTD.0b013e31815d0f66. [DOI] [PubMed] [Google Scholar]
- 37.Kolocouri F, Dotsikas Y, Apostolou C, et al. Simultaneous determination of losartan, EXP-3174 and hydrochlorothiazide in plasma via fully automated 96-well-format-based solid-phase extraction and liquid chromatography-negative electrospray tandem mass spectrometry. Anal Bioanal Chem. 2007;387:593–601. doi: 10.1007/s00216-006-0990-4. [DOI] [PubMed] [Google Scholar]
- 38.El Bekay R, Alvarez M, Monteseirin J, et al. Oxidative stress is a critical mediator of the angiotensin II signal in human neutrophils: involvement of mitogen-activated protein kinase, calcineurin, and the transcription factor NF-kappaB. Blood. 2003;102:662–671. doi: 10.1182/blood-2002-09-2785. [DOI] [PubMed] [Google Scholar]
- 39.Dandona P, Kumar V, Aljada A, et al. Angiotensin II receptor blocker valsartan suppresses reactive oxygen species generation in leukocytes, nuclear factor-kappa B, in mononuclear cells of normal subjects: evidence of an antiinflammatory action. J Clin Endocrinol Metab. 2003;88:4496–4501. doi: 10.1210/jc.2002-021836. [DOI] [PubMed] [Google Scholar]
- 40.Raiden S, Nahmod K, Nahmod V, et al. Nonpeptide antagonists of AT1 receptor for angiotensin II delay the onset of acute respiratory distress syndrome. J Pharmacol Exp Ther. 2002;303:45–51. doi: 10.1124/jpet.102.037382. [DOI] [PubMed] [Google Scholar]
- 41.Ohtsu H, Dempsey P, Eguchi S. ADAMs as mediators of EGF receptor transactivation by G protein-coupled receptors. Am J Physiol Cell Physiol. 2006;291:C1–C10. doi: 10.1152/ajpcell.00620.2005. [DOI] [PubMed] [Google Scholar]
- 42.Moss M, Sklair-Tavron L, Nudelman R. Drug Insight: tumor necrosis factor-converting enzyme as a pharmaceutical target for rheumatoid arthritis. Nat Clin Practice Rheumatol. 2008;4:300–309. doi: 10.1038/ncprheum0797. [DOI] [PubMed] [Google Scholar]
- 43.Bruneval P, Hinglais N, Alhenc-Gelas F, et al. Angiotensin I converting enzyme in human intestine and kidney. Ultrastructural immunohistochemical localization. Histochemistry. 1986;85:73–80. doi: 10.1007/BF00508656. [DOI] [PubMed] [Google Scholar]
- 44.Danilov SM, Faerman AI, Printseva O, et al. Immunohistochemical study of angiotensin-converting enzyme in human tissues using monoclonal antibodies. Histochemistry. 1987;87:487–490. doi: 10.1007/BF00496822. [DOI] [PubMed] [Google Scholar]
- 45.Wildhaber B, Teitelbaum D. Intestinal intraepithelial lymphocytes express angiotensin converting enzyme—a role for controlling epithelial cell apoptosis. Gastroenterology. 2003;124:A597. [Google Scholar]
- 46.Takeuchi N, Fukushima T, Sugita A, et al. Angiotensin-converting-enzyme (ACE) in Crohn’s disease. Nippon Shokakibyo Gakkai Zasshi. 1992:89. [PubMed] [Google Scholar]
- 47.D’Onofrio G, Levitt S, Ilett K. Serum angiotensin converting enzyme in Crohn’s disease, ulcerative colitis and peptic ulceration. Aust N Z J Med. 1984;14:27–30. doi: 10.1111/j.1445-5994.1984.tb03580.x. [DOI] [PubMed] [Google Scholar]
- 48.Matsuda T, Suzuki J, Furuya K, et al. Serum angiotensin I-converting enzyme is reduced in Crohn’s disease and ulcerative colitis irrespective of genotype. Am J Gastroenterol. 2001;96:2705–2710. doi: 10.1111/j.1572-0241.2001.03945.x. [DOI] [PubMed] [Google Scholar]
- 49.Jaszewski R, Tolia V, Ehrinpreis M, et al. Increased colonic mucosal angiotensin I and II concentrations in Crohn’s colitis. Gastroenterology. 1990;98:1543–1548. doi: 10.1016/0016-5085(90)91088-n. [DOI] [PubMed] [Google Scholar]
- 50.Hume G, Fowler E, Lincoln D, et al. Angiotensinogen and transforming growth factor beta1: novel genes in the pathogenesis of Crohn’s disease. J Med Genet. 2006;43:e51. doi: 10.1136/jmg.2005.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saibeni S, Spina L, Virgilio T, et al. Angiotensin-converting enzyme insertion/deletion gene polymorphism in inflammatory bowel diseases. Eur J Gastroenterol Hepatol. 2007;19:976–981. doi: 10.1097/MEG.0b013e3282efa3fc. [DOI] [PubMed] [Google Scholar]
- 52.Burnier M, Brunner HR. Angiotensin II receptor antagonists. Lancet. 2000;355:637–645. doi: 10.1016/s0140-6736(99)10365-9. [DOI] [PubMed] [Google Scholar]
- 53.Shibouta Y, Inada Y, Ojima M, et al. Pharmacological profile of a highly potent and long-acting angiotensin II receptor antagonist, 2-ethoxy-1-[[2′-(1H-tetrazol-5-yl)biphenyl-4- yl]methyl]-1H-benzimidazole-7-carboxylic acid (CV-11974), and its prodrug, (±)-1-(cyclohexyloxycarbonyloxy)-ethyl 2-ethoxy-1-[[2′-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl]-1H-benzimidazole-7-carboxylate (TCV-116) J Pharmacol Exp Ther. 1993;266:114–120. [PubMed] [Google Scholar]
- 54.Unger T. Pharmacology of AT1-receptor blockers. Blood Press. 2001;10:5–10. doi: 10.1080/08037050152518302. [DOI] [PubMed] [Google Scholar]
- 55.Masuko H, Jin MB, Horiuchi H, et al. Protective effect of angiotensin II type I receptor antagonist, CV-11974, on ischemia and reperfusion injury of the liver. Transplantation. 2001;71:1034–1039. doi: 10.1097/00007890-200104270-00003. [DOI] [PubMed] [Google Scholar]
- 56.Araya J, Tsuruma T, Hirata K, et al. TCV-116, an angiotensin II type 1 receptor antagonist, reduces hepatic ischemia-reperfusion injury in rats. Transplantation. 2002;73:529–534. doi: 10.1097/00007890-200202270-00006. [DOI] [PubMed] [Google Scholar]
- 57.Engelhorn T, Goerike S, Doerfler A, et al. The angiotensin II type 1-receptor blocker candesartan increases cerebral blood flow, reduces infarct size, and improves neurologic outcome after transient cerebral ischemia in rats. J Cereb Blood Flow Metab. 2004;24:467–474. doi: 10.1097/00004647-200404000-00012. [DOI] [PubMed] [Google Scholar]
- 58.Groth W, Blume A, Gohlke P, et al. Chronic pretreatment with candesartan improves recovery from focal cerebral ischaemia in rats. J Hypertens. 2003;21:2175–2182. doi: 10.1097/00004872-200311000-00028. [DOI] [PubMed] [Google Scholar]