Abstract
Purpose
To provide an overview of the methodologies involved in the field of hair cell regeneration. First, a tutorial on the biotechnological foundations of this field will be provided in order to assist the reader in the comprehension and interpretation of the research involved in hair cell regeneration. Next, a review of stem cell and gene therapy will be presented and a critical appraisal of their application to hair cell regeneration will be provided. The methodologies used in these approaches will be highlighted.
Method
Narrative review of the fields of cellular, molecular, and developmental biology, tissue engineering, and stem cell and gene therapy using the PubMed database.
Results
The use of biotechnological approaches to the treatment of hearing loss, such as stem cell and gene therapy, has led to new methods of regenerating cochlear hair cells in mammals.
Conclusions
There have been incredible strides made in assembling important pieces of the puzzle that comprise hair cell regeneration. However, mammalian hair cell regeneration using stem cell and gene therapy are years if not decades away from being clinically feasible. If the goals of the biological approaches are met, these therapies may represent the future treatments for hearing loss.
Keywords: Stem cell, gene therapy, hearing loss, hair cell, regeneration, development
INTRODUCTION
The fields of Communication Disorders encompass a wide range of clinical disciplines that draw people from diverse educational backgrounds such as education, linguistics, psychology, engineering, physics, and biology. The intellectual diversity in this field is advantageous because it provides a tremendous degree of flexibility when designing more effective treatments for the clients whom we aim to serve. One drawback in such an expansive field is that it may be difficult for experts in a given specialty to interpret new findings in a closely related field.
The purpose of this paper is to present a broad overview of biotechnological applications for the treatment of hearing loss. The goal is to provide basic information to both clinicians and researchers in the fields of communicative disorders that will assist in the interpretation of the current work in stem cell and gene therapy. This information is important for researchers because it may help in the assessment of the current literature and perhaps inspire future collaborations, and is important to clinicians because it will also help to answer queries from clients who are seeking information about novel treatments for hearing loss.
SIGNIFICANCE OF BIOLOGICAL APPROACHES FOR THE TREATMENT OF HEARING LOSS
As many as 3 persons out of every 1,000 in the United States are born deaf or exhibit a hearing loss (National Institute on Deafness and Other Communicative Disorders, 2008), however, as a person advances in age, their chance of developing a hearing loss increases. It is estimated that 17% of adults in the United States exhibit some degree of hearing loss (NIDCD, 2003). Thirty percent of people over the age of 65 exhibit a hearing loss, and this percentage increases to 47% of persons 75 and older (NIDCD, 2008). Regardless of the etiology, the death or dysfunction of mechanosensory hair cells located within the organ of Corti of the cochlea is the primary cause of sensorineural deafness (Figure 1). For example, overexposure to noise (Wang, Hirose, & Liberman, 2002) or aminoglycoside antibiotics (Forge & Schacht, 2000), results in hair cell loss and subsequent sensorineural hearing loss (SNHL). In some instances, the supporting cells, which act to maintain the ionic balance within the organ of Corti and provide structural support for hair cells (reviewed in Raphael & Altschuler, 2003), remain intact after damage. In other instances, such as a focal lesion caused by exposure to narrow-band noise, specific regions of the organ of Corti become ablated and the remaining cells form a flat epithelium that spans the basilar membrane (Kim & Raphael, 2007). Therefore, SNHL can be viewed as a biological disorder caused by death or dysfunction of particular cell types within the cochlea. The current treatments for SNHL are been based on electronic technologies such as amplification of the speech signal using hearing aids and electrical stimulation of the surviving spiral ganglion neurons using cochlear implants. Many cases of postlingual SNHL, such as presbycusis, are treated using amplification provided by hearing aids. While hearing aids do not restore normal hearing, they do provide significant communication benefit for those who exhibit mild to moderate hearing loss and exhibit a good ability to discriminate speech stimuli (Vuorialho, Karinen, & Sorri, 2006). However, there is a significant population with severe-to-profound SNHL who receive minimal communicative benefit from hearing aids (Cohen, Labadie, Dietrich, & Haynes, 2004).
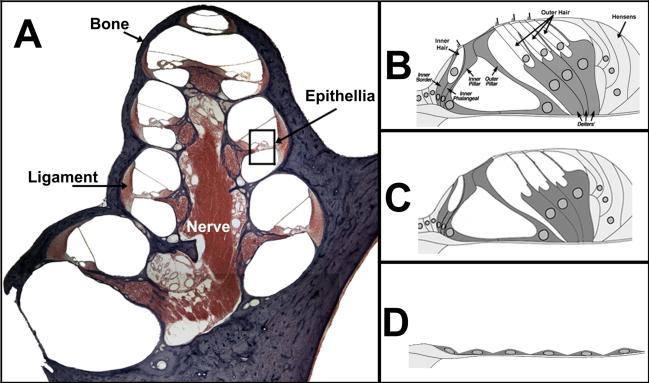
Figure 1. Gross Anatomy of the Mammalian Cochlea.
A) The cochlea is composed of many cell types including bone (purple), ligament, sensory epithelia, and nerve that enables communication between the sensory epithelia and the brain. B) Close-up of the sensory epithelium (boxed region in A) illustrates the organ of Corti, which consists of inner and outer hair cells and their corresponding supporting cells. Hair cells are responsible for transducing the mechanical motion of the basilar membrane into neural impulses that can be interpreted as sound by the brain. The supporting cells act to provide structural and nutritional support for hair cells and possibly act as hair cell progenitors as well. C) Ototoxic damage, such as exposure to noise or aminoglycoside antibiotics, results in hair cell loss and subsequent hearing loss. In some instances, the supporting cells remain intact after damage and may act as a target for gene therapy. D) It is also possible for ototoxic damage to completely ablate the organ of Corti. In such an instance, the remaining cells form a flat epithelium that spans the basilar membrane.
Severe-to-profound SNHL is commonly treated using cochlear implants, which directly stimulate the surviving auditory nerve fibers (Clark, 1975). In terms of speech recognition in quiet environments, cochlear implants have proven to be more effective than hearing aids for those who exhibit severe-to-profound SNHL (Cohen et al., 2004; Parving, 2003). In the absence of any other disabilities, a significant number of children who have received cochlear implants can be expected to acquire speech and language at a similar rate as their hearing peers and attain satisfactory educational and academic performance (Uziel et al., 2007). Furthermore, longitudinal studies suggest that the long term benefits in oral communication continue long after implantation (Beadle et al., 2005).
As encouraging as the results of cochlear implants appear, their use does not restore normal hearing. For instance, some social activities diminish the effectiveness of cochlear implants, such as eating in crowded restaurants that contain a high degree of background conversational noise, or that preclude use of the device, such as swimming or participation in contact sports. While it appears that in the best cases, cochlear implant recipients can be expected to learn speech and language at a similar rate as their hearing pears (Geers, Nicholas, & Sedey, 2003), there is a wide variation on performance even when controlling for age of implantation, time between deafness and implantation, insertion depth, implant device, and speech processor settings (reviewed in B. S. Wilson & Dorman, 2008a, 2008b; B. S. Wilson, Lawson, Muller, Tyler, & Kiefer, 2003). Along with the high degree of variability in performance, implant users in general also perform poorly in the presence of background noise, a difficulty which is exacerbated when the competing noise consists of speech stimuli (Donaldson et al., 2009; Plyler, Bahng, & von Hapsburg, 2008; Turner, Reiss, & Gantz, 2008). Cochlear implant recipients also exhibit a relatively poor ability to localize sound, an effect that is particularly noted in patients with unilateral implants (B. S. Wilson & Dorman, 2008b). Although bilateral implantation or bimodal stimulation using a contralateral hearing aid has been shown to improve the ability to detect speech in noise and perhaps to localize sound, there is large subject variability for these tasks as well (reviewed in Das & Buchman, 2005; Johnston, Durieux-Smith, Angus, O'Connor, & Fitzpatrick, 2009; Olson & Shinn, 2008). Finally, cochlear implant recipients exhibit poor pitch perception, which interferes with perception of music even with optimal programming aimed to restore music perception (Gfeller et al., 2002). Similarly, cochlear implant recipients exhibit poor representation of tonal languages such as Punjabi of India, and Chinese languages such as Mandarin, Cantonese, and Taiwanese (Fu, Hsu, & Horng, 2004; Fu, Zeng, Shannon, & Soli, 1998; Luo & Fu, 2004; Peng, Tomblin, Cheung, Lin, & Wang, 2004). Therefore, a significant population of deaf people who would communicate using tonal languages are underserved with current implant technologies.
While implant technology has advanced significantly since the development of the first devices, there is some evidence that the benefits derived from the current implant has plateaued (reviewed in Clark, 2008, 2009; B. S. Wilson & Dorman, 2008b). Recent attempts to address the deficiencies of current implant technology have focused on improved speech processing capabilities, such as amplitude modulation of the speech signal (Luo & Fu, 2004), bimodal stimulation using a hearing aid simultaneously (Blamey, Dooley, James, & Parisi, 2000; Mok, Grayden, Dowell, & Lawrence, 2006; Waltzman, Cohen, & Shapiro, 1992), and integration of implant technology with biological approaches aimed to preserve the endogenous auditory neurons (Chikar et al., 2008; Rejali et al., 2007; Yagi et al., 2000). These areas of research are promising, but some are at the basic level at this time.
In contrast to the use of amplification or electrical stimulation to treat SNHL, there is a growing body of work aimed at using biotechnology for this aim (reviewed in Atar & Avraham, 2005; Batts & Raphael, 2007; Beisel, Hansen, Soukup, & Fritzsch, 2008; Brigande & Heller, 2009; Coleman, de Silva, & Shepherd, 2007; Cotanche, 2008; Groves, 2010; Holley, 2002; Parker & Cotanche, 2004; Patel, Mhatre, & Lalwani, 2004; Raphael, Kim, Osumi, & Izumikawa, 2007). Biological approaches, such as stem cell and gene therapy, attempt to restore hearing by repairing the damaged structures of the auditory system. In particular, regenerating cochlear hair cells has been the main focus of these biological approaches. While the initial research on cochlear implants started in the 1950's (Djourno, Eyries, & Vallancien, 1957), exploration of biological approaches for the treatment of SNHL didn’t start until after the discovery that birds possessed the capacity to repair their auditory organs after damage (Corwin & Cotanche, 1988; Ryals & Rubel, 1988). When compared to the use of hearing aids and cochlear implants, biological treatments are in their infancy and will not be ready for human trials for many years. However, there has been significant progress in the identification of the mechanisms responsible for hair cell regeneration and the use of biotechnological approaches have led to new methods of regenerating hair cells. The methodologies of the biological approaches are based on the fields of cellular and molecular biology, developmental biology, and tissue regeneration. Therefore, a review of the current state of biotechnology as it is applied to the treatment of SNHL, particularly stem cell therapy and gene therapy, will be provided.
CELLULAR AND MOLECULAR BIOLOGY OF HEARING RESEARCH
The current research on hair cell regeneration is primarily based on cellular and molecular biology. Therefore, an understanding of the basic principles in these fields will assist the reader in the comprehension of the methodologies and interpretation of the research involved in hair cell regeneration. Additionally, this overview will provide the reader with an introduction to common experimental tools used to examine hair cell regeneration.
The cell is the basic unit of study for cellular biologists (Figure 2). The outer plasma membrane is hydrophobic and acts as a semi-permeable barrier between the cytoplasm within the cell and the extracellular fluid (for reference, please see Ch 1, Alberts, Johnson, Lewis, & Raff, 2007). The plasma membrane contains many different types of proteins, some of which can act as either receptors for extracellular signals (also called ligands) or as channels through which ions can enter or exit. One example of a ligand-receptor interaction is the neurotransmitter of the nervous system whereby a neurotransmitter, such as glutamate, is secreted from nearby neurons and will bind to a glutamate receptor on the cellular membrane (for reference, please see Ch 10, Kandel, Schwartz, & Jessell, 2000).
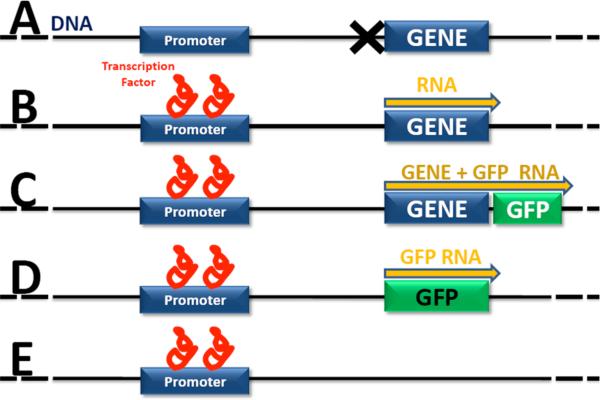
Figure 2. Molecular Genetics in Hearing Research.
A) Gene is expression is tightly regulated. Typically, genes are quiescent and not transcribed until transcription factors bind to a region of DNA call a promoter (B). Each gene has a specific promoter and several factors may be required to bind to the promoter before transcription of the gene into RNA may begin (yellow arrow). C) A common method to measure gene expression is to insert an easily detectable gene, such as the gene for GFP, into the DNA just after the gene-of-interest. In this model, the GFP gene will be transcribed and then translated into protein at the same time as the gene-of-interest. The GFP protein is easily detected using a fluorescent microscope. D) In some cases, such as when the control of gene expression is to be measured, the molecular tag is inserted into the DNA after the promoter rather than after the gene-of-interest. E) In a gene knock-out organism, the gene-of-interest has been deleted or otherwise mutated to render it dysfunctional. Sometimes, the gene-of-interest is deleted and replaced by a molecular marker such as GFP (D).
When a ligand binds to its receptor, a biochemical signaling cascade may be initiated within the cell depending upon the type of receptor that is activated (for reference, please see Ch 10, Kandel et al., 2000). In this process, called signal transduction, an extracellular ligand may cause many different biochemical reactions to occur within the cytoplasm. For example, ligand binding may cause molecule A to be activated, which in turn will activate molecule B, and so on. In some cases, the signaling cascade will influence other biochemical reactions within the plasma membrane or the cytoplasm. In other cases, such as in the activation of proteins called transcription factors, activation will cause the molecule to be transported into the nucleus of a cell where it may influence the expression of certain genes (Wilhelm, Johnson, Haselkorn, & Geiduschek, 1972). Some biotechnological approaches to the treatment of SNHL exploit the endogenous signaling pathways involved in hair cell regeneration in order to study their therapeutic effects on hearing loss. For example, the addition of retinoic acid (Kelley, Xu, Wagner, Warchol, & Corwin, 1993) or DAPT (Woods, Montcouquiol, & Kelley, 2004) to the embryonic organ of Corti of mice stimulates signaling pathways that lead to production of extra (supernumerary) hair cells.
Typically, the signaling pathways involved in hair cell regeneration include the activation of pro-hair cell genes which lead to hair cell differentiation. A gene is a specific region of DNA which codes for the production of a specific protein (for reference, please see Ch 6, Alberts et al., 2007). Genes are typically quiescent and not expressed until transcription factors bind to a region of DNA called a promoter (Figure 3). Promoters, therefore, are small regions of DNA that are responsible for activating a gene-of-interest. Each gene has a specific promoter and several transcription factors may be required to bind to the promoter before a gene is expressed. Once the appropriate signaling molecules are bound to its promoter, the gene may be copied into an RNA molecule in a process termed transcription (Kidson & Kirby, 1964; Somerville & Yanofsky, 1964). While the DNA molecule is restricted to the nucleus, RNA molecules may be shuttled out of the nucleus into the cytoplasm where they may be copied into a protein in a process called (translation for reference, please see Ch 6, Alberts et al., 2007). Proteins play crucial roles in normal cell physiology. For example, proteins are responsible for initiating cell division and cell death, comprising receptors, enabling the structural morphology of a cell, catalyzing cytoplasmic biochemical reactions, acting as signals in the signaling cascade, and acting as transcription factors which bind to the promoter region of DNA and activate gene transcription. Proteins are also used as molecular markers for different types of cells. For example, hair cells are the only cell types in the cochlea which express the Myosin 7a protein (Gillespie, 1995). Therefore, expression of the myosin 7a gene, RNA, or protein can be used as a molecular marker for cochlear hair cells.
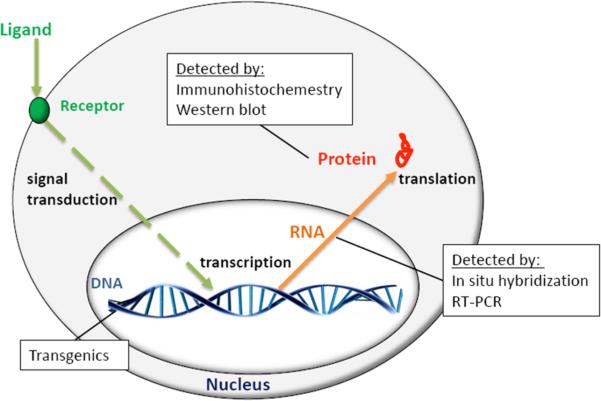
Figure 3. Cellular and Molecular Biology in Hearing Research.
Gene expression requires a molecular signal to initiate the transcription of DNA into an RNA molecule and then translation of the RNA into a protein. Common assays used to measure these cellular processes include the generation of transgenic animals, in situ hybridization, RT-PCR, and Western blot analysis. See text for details.
The identification of pro-hair cell genes and the regulation of their expression is a growing area of research in hair cell regeneration (see Development below). Methods developed in the field of molecular genetics are used to observe gene expression in the live animal (in vivo), or in live tissue cultured in an incubator (in vitro). Individual genes, RNA, and many proteins are too small to be visualized with microscopes. However, the use of transgenic technology has led to new uses of DNA as a tool to study cell biology in vitro. Transgenics refers to the addition of a gene from one animal into the DNA of another (Gordon & Ruddle, 1982). A common method to measure gene expression is to insert an easily detectable gene, known as a reporter gene, into the host DNA just after the gene-of-interest. For example, the jelly fish Aequorea victoria expresses a gene that codes for a protein which emits a green fluorescent protein (GFP) in the presence of blue light (Shimomura, Johnson, & Saiga, 1962). GFP is widely used as a visible marker that can be inserted into target genes in order to help determine their modes of expression. If the GFP gene is inserted in the proper manner into the DNA of a host, the GFP gene will be transcribed along with the gene-of-interest (Chalfie, Tu, Euskirchen, Ward, & Prasher, 1994; Prasher, McCann, & Cormier, 1985) (Figure 3C). The resulting translated protein will exhibit a green fluorescence which is easily detectable when viewed in a fluorescence microscope. Auditory researchers have applied similar transgenics to their research as well. For example, transgenic mice have been bred that exhibit fluorescent labeling of the myosin 7a protein which allows for the analysis of hair cells in vitro (Boeda, Weil, & Petit, 2001). Similarly, strains of transgenic mice have been engineered to exhibit cell-specific GFP labeling of supporting cells (Rio, Dikkes, Liberman, & Corfas, 2002) and neurons (Feng et al., 2000).
Another way to measure gene expression is to perform assays to detect specific RNA molecules that are transcribed from a gene-of-interest. A procedure called in situ hybridization (ISH) is commonly used to detect or localize RNA expression in tissue during development (for reference, please see Ch 8, Alberts et al., 2007). In this procedure, animal tissue is fixed and a probe that will bind to a RNA molecule of interest is added to the tissue. The RNA probe typically contains a molecular tag such as a fluorescent marker in order to determine which cell types express the RNA molecule. Another common method used to detect RNA expression is called reverse transcriptase polymerase-chain reaction (RT-PCR) (for reference, please see Ch8, Alberts et al., 2007). In this procedure, all of the intracellular RNA is isolated from the tissue and then is converted back into DNA fragments (also known as complimentary DNA or cDNA) using a technique called as reverse transcription. The resulting pool of cDNA represents all of the genes being expressed at a particular time. To determine the expression of a specific gene within this cDNA library, probes are used to generate copies of a gene-of-interest in a technique called a polymerase chain reaction. These genes are then identified by loading them in an agarose gel and applying an electrical current that separates the genes by their size.
A procedure called immunohistochemistry (or immunofluorescence) is commonly used to detect the presence of proteins such as myosin 7a that may act as cell-specific markers (for reference, please see Ch8, Alberts et al., 2007). In this procedure, an antibody that binds specifically to the myosin 7a protein is used as a molecular tag for the native myosin 7a proteins present in the hair cells. Next, a fluorescent label is added to the antibody so that the antibody/myosin 7a protein complex can then be viewed using a fluorescent microscope. Immunohistochemestry (immunolabeling) is used routinely to label specific cells in the cochlea such as spiral ganglion neurons (e.g. βIII-tubulin (Molea, Stone, & Rubel, 1999), neurofilament (Anniko, Thornell, Gustavsson, & Virtanen, 1986), supporting cells (e.g. p27kip1 (Chen & Segil, 1999), prox1 (Bermingham-McDonogh et al., 2006), or hair cells (e.g. myosin 6 (Hasson et al., 1997), myosin 7a (Gillespie, 1995)).
The biotechnological approaches to the treatment of SNHL use a combination of these approaches to study the cellular and molecular mechanisms required for hair cell regeneration. Current research has become more focused on the identifying the essential signal transduction pathways, gene regulation, and gene expression required for normal hair cell development and hair cell regeneration after damage. Next, a review of hair cell development and regeneration will be presented. Current cellular and molecular biological techniques used in these fields will be highlighted.
DEVELOPMENTAL BIOLOGY OF THE AUDITORY SYSTEM
An understanding of the mechanisms that control the normal development of the cochlea is crucial to the goal of repairing the damaged cochlea. There is a growing body of research on the developmental biology of the inner ear that describes the normal signaling required for hair cell genesis during embryonic development (reviewed in Alsina, Giraldez, & Pujades, 2009; Kelly & Chen, 2009; Morsli, Choo, Ryan, Johnson, & Wu, 1998; Schwander, Kachar, & Muller, 2010). Hypothetically, the repair processes involved in hair cell regeneration could be based on similar mechanisms.
Most of the developmental studies of the cochlea use the mouse for the animal model. The facts that mice are mammals with similar cochlear morphology as humans (albeit on a smaller scale), they breed relatively quickly which facilitates the pace of research, and the sequence of their genome is known and can therefore be modified (see gene knock-out below) make them an optimal mammalian animal model. In the mouse, normal cochlear development begins approximately 8.5 days after fertilization (embryonic day [E] 8.5) when cells on the lateral wall of the fetus thicken to form the otic placode (Hartman, Reh, & Bermingham-McDonogh, 2010)(Figure 4). Next these cells begin to divide and proliferate, invaginate from the lateral wall, and then pinch off to form the otic vesicle (otocyst) by E9 (Anniko & Wikstrom, 1984). Between E12 and E16, a portion of the otocyst called the prosensory domain, which will develop into the organ of Corti, begins to express a gene called p27kip1 which inhibits these cells from dividing (Chen & Segil, 1999). Once the cells within the pro-sensory domain stop dividing, the maturation of these cells will begin. Hair cell development begins within the prosensory domain at E13 (reviewed in Barald & Kelley, 2004; Driver & Kelley, 2009) and the developing hair cells signal their surrounding cells to differentiate into supporting cells (likely through lateral inhibition via the Notch signaling pathway) (Zine, Van De Water, & de Ribaupierre, 2000) approximately one day later (Anniko, 1983). Individual pillar cells are the first supporting cells to differentiate (Thelen, Breuskin, Malgrange, & Thiry, 2009) and appear in mouse as early as E15 (Anniko, 1983). By E18, most of the supporting cells types are present in the lateral sensory epithelium including Hensen's cells, and Dieters’ cells (Bermingham-McDonogh et al., 2006). However, the supporting cells of the inner hair cell region will not develop until after birth in the mouse (Pujol & Hilding, 1973). Instead, the space between the spiral limbus and the row of inner hair cells consists of columnar epithelial cells collectively called Kölliker's organ (also known as the greater epithelial ridge [GER]) (Hinojosa, 1977; Kikuchi & Hilding, 1965). At birth, all of the cell types lateral to the inner hair cells are present, however development of the inner sulcus, tunnel of Corti, tectorial membrane, and hair cell innervation is incomplete until postnatal day 16 (P16) (Pujol & Hilding, 1973; Tritsch, Yi, Gale, Glowatzki, & Bergles, 2007), which is approximately the time that the organ of Corti begins to produce adult-like responses in mice (Alford & Ruben, 1963).
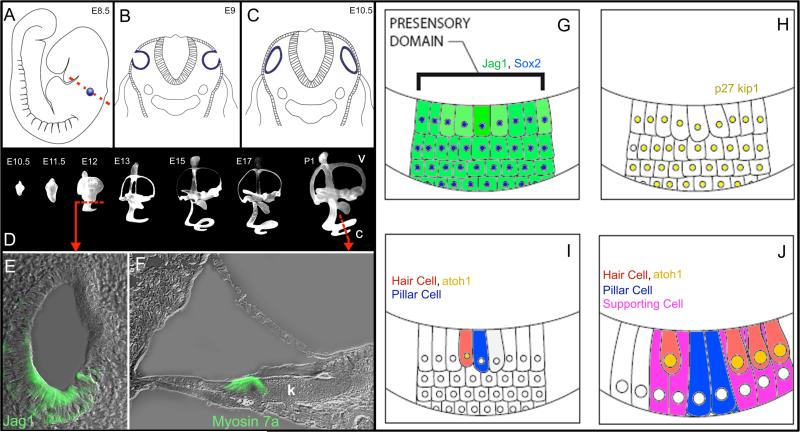
Figure 4. Development of the murine cochlea.
A) Cochlear development starts at approximately E8.5 with a thickening of a group of cells on the lateral wall of the developing fetus called the otic placode (blue). The cells of the otic placode invaginate (B) and pinch off from the surface of the embryo to form the otocyst by E10 (C). The red line in A indicates the cross section illustrated in B and C. D) Experiments where latex paint was injected into the developing scala media illustrate the development of the inner ear. The dorsal portion of the otocyst develops into the vestibular system (v) and the ventral portion develops into the cochlea (c). The scala media of the cochlea begins to elongate from the vestibule and spirals apically. Images adapted with permission from Morsli et al., (1998); Society for Neuroscience Publisher. E) Cross section through the E12 otocyst (left dashed line in D) highlights a group of cells that immunolabel for the Jagged-1 protein (Jag1; green), which labels the prosensory domain in the developing cochlea which will develop into the organ of Corti. F) Cross section through the postnatal day 1 (P1) organ of Corti (right dashed line in D) shows most of the adult cells are present at birth, including hair cells that immunolabel for the myosin 7a protein (green). However, the tunnel of Corti has not developed and Kölliker's organ (k) remains in place of the inner sulcus. G) The prosensory domain, identified by jag1 and sox2 expression, begins to express p27kip1 between E12 and E16 which initiates maturation of the sensory epithelium (H). I) Hair cell development begins within the prosensory domain at E13 when hair cells express atoh1 and signal their surrounding cells to differentiate into supporting cells. J) By E18, most of the supporting cells types are present in the sensory epithelium, however; the auditory system is not fully developed into 2 weeks after birth in mice.
Early research on the development of the cochlea was performed by anatomists who described the morphological changes that occur in the fetus which lead to the development of the inner ear (Friedmann, 1959; F. W. Jones, 1952; Wislocki & Ladman, 1955). More recently, developmental biologists have adopted techniques from cell and molecular biology to identify essential signaling pathways required for cochlear development and hair cell genesis. Developmental biologists use ISH (to localize or detect RNA expression) and immunohistochemistry (to localize or detect protein expression) in order to identify which genes are expressed during cochlear development. In some cases, particularly during early development, genes are up-regulated for a period of time, and are then down-regulated, never to be expressed again (Srinivasan et al., 2007). In other cases, genes such as such as myosin 7a are initially quiescent and are then expressed throughout the lifespan (Sahly, El-Amraoui, Abitbol, Petit, & Dufier, 1997). These experimental methods are important in the identification of the temporal pattern of gene expression required for hair cell development, but they do not always indicate the function of the gene.
One valuable method used to determine gene function is to engineer an animal (mouse, fruit fly, etc.) in which a particular gene has been deleted or otherwise mutated so as to render it dysfunctional (Figure 3E). Called “knock-out”, the animal with the deleted gene will be bred, and any morphological changes in the developing animal will be analyzed in order to determine the function of the mutated gene (Bernstein & Breitman, 1989; Breitman et al., 1987). In terms of audition, gene knock-out organisms have identified the function of several genes important to the development of the inner ear in general and hair cells in particular. Notably, a when a gene called atonal is knocked-out in a fruit fly, their hearing organs fail to develop (Akazawa, Ishibashi, Shimizu, Nakanishi, & Kageyama, 1995; Shimizu, Akazawa, Nakanishi, & Kageyama, 1995). Furthermore, when the atonal knock-out animals were given a new copy of the gene, their auditory organs developed normally (Jarman, Grau, Jan, & Jan, 1993). Lineage studies, in which transgenic mice express a molecular marker (similar to GFP) to label which cell types express the mammalian form of atonal (atonal homolog 1, or atoh1), indicate that the cells in the developing mouse cochlea that express atoh1 will differentiate into hair cells (Ben-Arie et al., 2000; Gazit, Krizhanovsky, & Ben-Arie, 2004; Krizhanovsky, Golenser, & Ben-Arie, 2004). Additionally, knock-out mice that lack the atoh1 gene fail to develop hair cells (Bermingham et al., 1999). These data indicate that the expression of atoh1 is required for hair cell formation and has suggested the use of atoh1 as a source of gene therapy to treat SNHL (see discussion below).
The study of the cochlear development is a cornerstone of the applied biological approaches to the treatment of hearing loss. As shall be discussed, the knowledge of the molecular signals required for the cellular development of the organ of Corti is crucial for both stem cell and gene therapy used in the treatment of SNHL. Next, a review of tissue regeneration, which is another basis for the applied approaches for hair cell regeneration, will be presented.
TISSUE REGENERATION
The applied approaches to the treatment of SNHL also look to the field of tissue regeneration for models of hair cell regeneration. Many animals exhibit the ability to regenerate body parts after damage. Sea stars (reviewed in Michael, Wei-Chung, Philip, & Marco, 2001), hydra (reviewed in Hoffmeister-Ullerich, 2007), lizards (reviewed in Alibardi, 2010), and amphibians such as axolotl salamanders (reviewed in Roy & Gatien, 2008) can spontaneously regenerate entire limbs, including skin, muscle, nerve, and bone (in the case of vertebrates), after amputation. In mammals, several adult organs such as the skin (Fukuda et al., 1978), blood (Nossal & Makela, 1962), placenta (Pattillo, Gey, Delfs, & Mattingly, 1968), and intestine (Leblond, Clermont, & Nadler, 1967) maintain a limited ability to regenerate. For instance, a scab will form on the epidermal layer of the skin after an abrasion, and eventually the epidermis will repair itself. The field of tissue regeneration attempts to characterize the morphological, cellular and molecular mechanisms responsible for tissue repair.
The field of tissue regeneration is also a rich source of information for the biological approaches for the treatment of SNHL. The first evidence that hair cells could be created after normal embryogenesis was published in 1981 when it was found that sharks were able to add new hair cells to their vestibular system as they grew in age (Corwin, 1981). While this example is not illustrative of a complete regeneration of the organ, this study was the first to demonstrate that hair cells of some animals could appear after embryonic development. Later in that decade, Douglas Cotanche discovered that bird ears could regenerate their tectorial membrane (Cotanche, 1987a) and cilia of auditory hair cells (Cotanche, 1987b) after noise damage. Additionally, researchers discovered that in birds, the auditory hair cells themselves could regenerate after drug (Cruz, Lambert, & Rubel, 1987) or noise damage (Corwin & Cotanche, 1988; Ryals & Rubel, 1988) (Figure 5). It was later discovered that regenerated hair cells were able to re-establish their neural connections (Ryals & Westbrook, 1994; Stone & Rubel, 2000) and normal behavioral hearing thresholds hearing could be restored after ototoxic insult (Saunders, Salvi, & Miller, 1995).
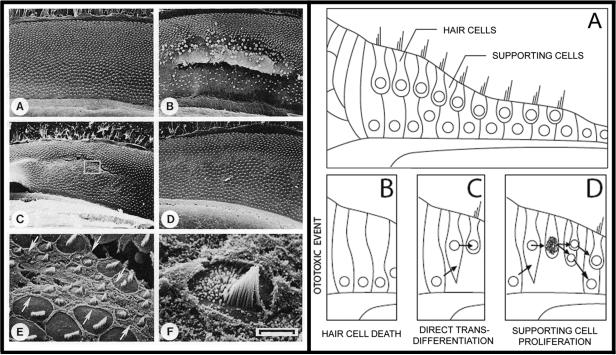
Figure 5. Spontaneous Regeneration of Auditory Hair Cells the Chick.
LEFT PANEL. A) Unlike the mammalian organ of Corti, the auditory sensory epithelium in the chick is covered in many rows of hair cells. B) Exposure to loud sound results in significant damage to sensory epithelia including loss of hair cells. C, E and F) Small ciliated cells spontaneously appear on the surface of the epithelium by six days after noise exposure. These cells maintain morphological similarities to developing hair cells. D) A significant number of hair cells appear on the epithelia by 10 days after the noise damage. These cells appear morphologically similar to adult hair cells with the exception that the orientations of their cilia are often askew. Image taken from Corwin and Cotanche (1988) reprinted with permission from AAAS and authors. RIGHT PANEL. The current model of hair cell regeneration holds that there are two mechanisms of hair cell regeneration in chicks (Stone and Cotanche, 2007). In each of these models, hair cells are regenerated from supporting cells that survived the ototoxic stimulus (A, B). C) Initially, supporting cells differentiate into a hair cell in a process called direct transdifferentiation. D) Later in the regenerative process, supporting cells enter into the mitotic cycle and undergo cell division. Of the two daughter cells, one will regenerate into a supporting cell and one will differentiate into a regenerated hair cell.
Current theory proposes that there are two mechanisms of hair cell regeneration in birds (reviewed in Stone & Cotanche, 2007). An ototoxic stimulus such as sound or drug damage may cause a hair cell to die. Next, the dying hair cell becomes ejected from the sensory epithelium that may trigger hair cell regeneration by the following mechanisms. The a process termed direct transdifferentiati on (Roberson, Alosi, & Cotanche, 2004). The second possibility is that a cochlear supporting cell will undergo cellular division (Stone & Cotanche, 1994) and produce one daughter cell that becomes a regenerated supporting cell and one daughter cell that becomes a regenerated hair cell. Both of these mechanisms are thought to occur in the regenerating chicken cochlea and highlight the critical role of supporting cells in hair cell regeneration.
Further research has demonstrated that hair cell regeneration is not limited to birds, but is exhibited by other non-mammalian vertebrates such as fish (Harris et al., 2003; Lombarte, Yan, Popper, Chang, & Platt, 1993), frogs (Baird, Torres, & Schuff, 1993), axolotls (J. E. Jones & Corwin, 1993) , and lizards (Avallone et al., 2003) . Notably, the zebrafish has become an attractive animal model for hair cell regeneration for a few reasons. The zebrafish exhibits a rapid rate of hair cell regeneration with regenerated hair cells appearing 24-48 hours after neomycin treatment (Harris et al., 2003). Additionally, the hair cells within its lateral line organ are readily visible using a microscope from the outside of the body, therefore hair cell regeneration can be observed in vivo using time lapse videography (Harris et al., 2003). Finally, the zebrafish is a good model for the study of gene function because of the relative ease in manipulating their gene expression during development and because they produce many progeny which may be easily screened for genetic mutations that could affect hair cell regeneration (Brignull, Raible, & Stone, 2009). Considering the disparate species involved, hair cell regeneration is not uncommon, because many vertebrates maintain the capacity to repair their peripheral auditory organs after damage. The obvious exception to this finding is that mammals do not possess this capability after normal embryonic development.
The lack of spontaneous hair cell regeneration in the mammalian cochlea does not necessarily mean that mammals lack the capacity for hair cell regeneration. Similar to the vestibular system in sharks, humans exhibit a low level of vestibular hair cell regeneration as we age (Warchol, Lambert, Goldstein, Forge, & Corwin, 1993). Additionally, a low level of spontaneous hair cell regeneration is possible in murine embryonic organ of Corti when cultured in vitro (Kelley, Talreja, & Corwin, 1995) and some signaling molecules involved in fate determination during embryological development such as retinoic acid are able to induce extra hair cell production in murine embryonic organ of Corti explant cultures (Kelley et al., 1993) (Figure 6). Finally, it has been show that cochlear supporting cells in knock-out mice which lack the p27kip1 gene are able to proliferate and become new hair cells (Lowenheim et al., 1999). However, spontaneous cochlear hair cell regeneration and the ability to induce hair cell proliferation using morphogens (signals involved in the initial stages of organogenesis) mitogens (agents that induce cellular proliferation), growth factors (agents that stimulate cell growth), or inhibition of the cell cycle inhibitor gene p27kip1 are lost perinatally in mice. Therefore, mammals do possess the capability to regenerate cochlear hair cells, but this ability is lost around the time of birth in mice and earlier in human fetal development. While researches are continuing their investigations into the mechanisms of hair cell generation in chickens, fish, and other non-mammalian animals, new avenues of research have advanced the field using biotechnological approaches, such as stem cell and gene therapy, to regenerate mammalian hair cells.
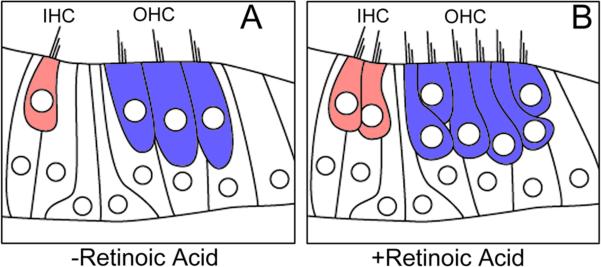
Figure 6. Plasticity in the Mammalian Organ of Corti.
The ability of the mammalian cochlea to produce hair cells after normal cochlear development suggests that the organ of Corti maintains the proper cell types required for hair cell regeneration. A) In the mouse cochlea, hair cells are normally developed by 14 days after fertilization. Red cell=one row of inner hair cells, blue cells = three rows of outer hair cells. B) Culturing the organs of Corti in the presence of retinoic acid, a mitotic agent that induces cellular proliferation, results in the production of supernumerary hair cells (Kelley et al., 1993).
STEM CELL THEARPY FOR THE TREATMENT OF SNHL
Stem cell therapy, where exogenous stem cells are used to replace dead or damaged endogenous cell types, is a relatively new technique used to treat many forms of human disease. Stem cell therapy is used clinically in the treatment of certain types of leukemia (Kessinger et al., 1989), for example. Research investigating the possible use of stem cell therapy for the treatment of hearing loss has started within the past decade (Ito, Kojima, & Kawaguchi, 2001; Parker et al., 2003), and based on promising initial research, this field has expanded and represents a cutting edge form of therapy. This section will first provide a background regarding stem cell biology and then move to the current body of work using stem therapy in the treatment of SNHL using in vivo animal models.
During embryonic development, a sperm fertilizes an egg and the resulting zygote is the only cell that is totipotent because it maintains the ability to develop into extra-embryonic tissue (such as the umbilical cord) and any cell type within the embryo (for reference, please see Ch4, Gilbert, 2010). Within a few hours after fertilization, the zygote will divide into two daughter cells. These two cells will then divide, and so on until a (mostly) hollow ball of cells is formed called a blastula. Inside of the blastula is a mass of cells called the inner cell mass (or embryonic stem cells).
Embryonic stem cells are pluripotent cells that maintain the ability to develop into any cell type in the developing embryo (Figure 7 see Ch8, Gilbert, 2010). As embryonic stem cells further divide, they become more restricted in the types of cells in which they can differentiate (reviewed in Anderson, Gage, & Weissman, 2001; Weissman, Anderson, & Gage, 2001). Near the end of organogenesis, progenitor cells give rise to a small number of cell types within a given organ until a terminal stage of cell division occurs. In the organ of Corti, a common progenitor cell gives rise to both hair cells and supporting cells (reviewed in Driver & Kelley, 2009; Kelley, 2006). As noted above, the development of the hair cell signals surrounding supporting cells to differentiate into supporting cells. Therefore, hair and supporting cells share the same developmental signaling and differentiation patterns until the final stages of terminal differentiation. Evidence of the close relationship between these cell types comes from various studies that describe supporting cell to hair cell differentiation in the chick (described above) and mammal (White, Doetzlhofer, Lee, Groves, & Segil, 2006).
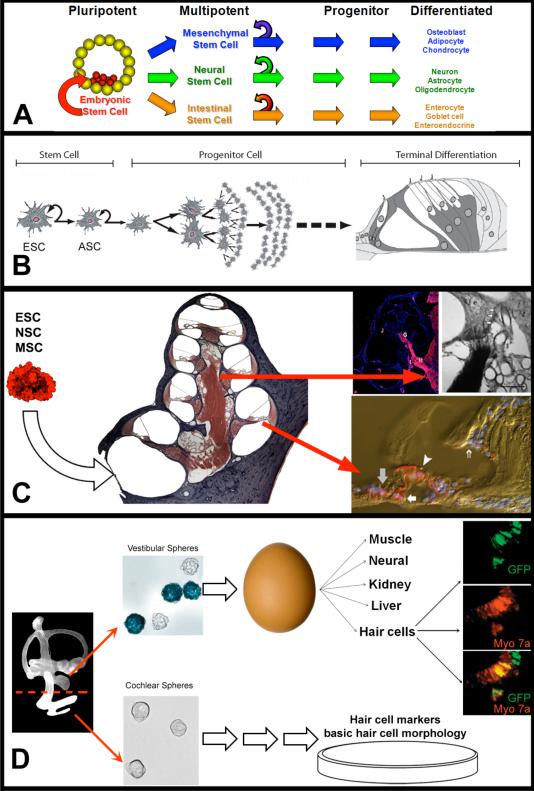
Figure 7. Stem Cell Replacement Therapy in the Cochlea.
A) Pluripotent ESCs are derived from the developing blastocyst and have the capacity to differentiate into any cell type. When these cells divide, the daughter cells maintain the capacity for self renewal (curved arrow) and the potential to develop into any cell type within the developing fetus. ASCs, such as mesenchymal, neural, and intestinal stem cells, are multipotent cells found in the end-organs of adult tissue that maintain the ability for self renewal and produce daughter cells that can differentiate into many of the cell types that comprise the organ in which they reside. By contrast, progenitor cells exhibit a more limited potential and differentiate into a restricted number of cell types. B) The goals of stem cell replacement therapy are to use ESC, ASCs, or cochlear progenitor cells to replace dead or damaged cell types which result in hearing loss. C) Initial experimenters aiming to achieve this goal injected various types of stem cells into the deafened cochlea and later examined the differentiated fates of the transplanted stem cells. As discussed in the text, most stem cells types were able to survive transplantation and engraft into the cochlea, but the terminal cell type and their degree of differentiation depended on the specific type of injected stem cell. Images adapted with permission from TOP LEFT ((Shi et al., 2007) John Wiley and Sons; TOP RIGHT (Corrales et al., 2006) John Wiley and Sons; BOTTOM (Parker et al., 2007) Elsevier. D) Investigators are trying to determine whether the mammalian inner ear may harbor a population of dormant stem cells. Li et al (2003) isolated cells with stem cell properties from the developing vestibular system (vestibular spheres), injected them into a chicken egg, incubated the egg, and then assayed for terminal differentiation of the transplanted cells (which expressed GFP). The authors suggest that the vestibular spheres are pluripotent stem cells because they differentiated into many different tissue types, including hair cells. Reprinted by permission from Macmillan Publishers Ltd: Nature Medicine, (Li et. al. 2003), copyright (2003). In addition, several laboratories are investigating the ability of similar cochlear spheres to differentiate into hair cells in vitro.
Many of the mammalian organs that exhibit regeneration in the adult (i.e. blood, skin, intestine) have been found to contain a niche of stem cells that contribute to regeneration of the organ (Leblond et al., 1967; Shihabuddin, Ray, & Gage, 1999; Weissman, 2000). Adult stem cells (ASC) are multipotent cells found in the end-organs of adult tissue. In these organs, when an ASC divides, one of the resulting daughter cells will be a renewed ASC and the other daughter cell will acts as a progenitor cell that will differentiate into cell types of a particular organ (reviewed in Gage, 2000). In this way, ASCs act to repopulate both themselves and lost or damaged tissue. ASCs, therefore, act in a self-repair mechanism. In 1992, it was discovered that the brain possessed a small population of cells that maintained the functional definition of a stem cell; the ability to self renew after cell division, and ability for the daughter cells to differentiate into multiple cells types, such as neurons and astrocytes (Reynolds & Weiss, 1992). This study was important because it was the first evidence that the brain contained a population of cells that could differentiate into neurons after the critical period of brain development. Further studies indicated that exogenously transplanted neural stem cells could be used to replace the damaged nervous tissue such as neurons (E. Y. Snyder, Yoon, Flax, & Macklis, 1997) and glia (Cao, Benton, & Whittemore, 2002), which highlighted their potential in cell replacement therapy. Additional data have shown that neural stem cells which have been transplanted into the sectioned spinal cord were able to replace damaged nervous tissue and facilitate a recovery of motor function as well (Teng et al., 2002).
There is speculation that the inner ear may contain a niche of stem cells. In the mouse cochlea, both hair and supporting cells are developed by 14 days after fertilization (Ruben, 1967). The ability for the mammalian perinatal cochlea to regenerate hair cells after this point suggests that the developing cochlea contains cells that are able to develop into hair cells. One group of researchers have reported that the vestibular system, which does exhibit a low level of supporting cell proliferation after damage that continues into adulthood (Warchol et al., 1993), contains pluripotent stem cells that maintain the additional ability to differentiate into non-cochlear tissue such as muscle and nervous tissue (Li, Liu, & Heller, 2003) (Figure 7D). In addition to this group, other groups have reported the ability to culture cochlear progenitor cells using the same methods utilized to culture neural stem cells (as floating spheres in serum free media containing growth factors) which suggests that the perinatal cochlea contains a stem cell niche (Oshima et al., 2007; Oshima, Senn, & Heller, 2009; Senn, Oshima, Teo, Grimm, & Heller, 2007; Zhai et al., 2005; Zhang et al., 2007).
Despite these data, it is not certain that the adult cochlea contains a population of ASCs. For example, the cochlear progenitor cells described above can only be cultured from embryonic and perinatal cochleas, not from mature ears. Additionally, in other ASC niches, such as the subventricular zone of the forebrain (Halliday & Cepko, 1992) or endothelial cells in the intestine (Halliday & Cepko, 1992; Leblond et al., 1967), stem cells undergo a low rate of cell division and replace dead cells within the organ. There is no documentation that similar cellular proliferation and replacement rates occur in the adult mammalian cochlea.
However, data suggests that the cochlear supporting cells may function as hair cell progenitors during development, which differentiate into a more restricted number of cell types, rather than bona fide multi-potent stem cells capable of self-renewal. When isolated from the organ of Corti and cultured in vitro, cochlear supporting cells are capable of expressing hair cell markers such as myosin 7a, suggesting that they differentiate into hair cells (White et al., 2006). However, once the supporting cells differentiate into hair cells, they do not re-populate themselves (Doetzlhofer et al., 2009). Therefore, supporting cells lack the ability to self-renew, which is a defining characteristic of a stem cell. Regardless of whether the cochlea contains a stem cell or progenitor niche, there must be some factors that inhibit the ability for stem/progenitor cells to differentiate into hair cells in the adult mammal.
Instead of stimulating endogenous stem cell/progenitor cell differentiation, other groups of investigators have examined the ability of transplanted exogenous stem cells to replace damaged cells of the cochlea (Figure 7 C). Although numerous studies have shown that transplanted stem cells are able to survive within the cochlea for several weeks, the cell types into which they differentiate vary widely and are dependent upon the type of stem cell used for the transplants. For instance, embryonic stem cells that have been transplanted into the spiral ganglion of the cochlea were found to express neural markers (Hu, Andang, Ni, & Ulfendahl, 2005; Lang et al., 2008) and develop cellular processes similar to axons that extend towards the organ of Corti (Corrales et al., 2006; Martinez-Monedero, Corrales, Cuajungco, Heller, & Edge, 2006; Okano et al., 2005; Shi, Corrales, Liberman, & Edge, 2007). These data suggest that embryonic stem cells have the ability to differentiate into neuronal cell types when transplanted into the spiral ganglion. Similarly, neural stem cells that have been transplanted into the scala tympani migrated to the spiral ganglion and then expressed characteristics of neurons and glial cells (Hakuba et al., 2005; Iguchi et al., 2003; Tateya et al., 2003). In some instances, a small number of transplanted neural stem cells migrated to the scala media (Ito et al., 2001) and expressed organ of Corti markers, including hair cell markers (Parker et al., 2007; Wei et al., 2008). An additional study indicated that neural stem cells which migrated to the vestibular system expressed hair cell markers as well (Tateya et al., 2003). These data suggest that neural stem cells more readily express cochlear markers than then embryonic stem cells. For example, the ability of transplanted cells to express hair cell markers in the cochlea, specifically myosin 7a, has only been demonstrated using neural stem cells (Parker et al., 2007; Tateya et al., 2003; Wei et al., 2008). So far, it has been found that embryonic stem cells (Sakamoto et al., 2004), and the two stem cell populations present in the blood (mesenchymal and hematopoietic stem cells) are unable to express hair cell markers when transplanted into the cochlea (Tan, Lee, & Ruan, 2008). One explanation for the ability of neural stem cells to more readily express cochlear markers than other stem cell types is that neural stem cells may be more closely related to cochlear tissue than other stem cell types. For instance, the cochlea and brain share similar signaling pathways during development. In fact, cochlear development is dependent upon several signals secreted from the developing central nervous system (reviewed in Abello & Alsina, 2007; Schneider-Maunoury & Pujades, 2007; Streit, 2007; Whitfield & Hammond, 2007). Likewise, neural stem cells and cochlear tissue express many of the same molecular markers such as GFAP, connexin 26, and myosin 7a (Parker et al., 2007). Neural stem cells may more readily express these markers after transplantation than mesenchymal or embryonic stem cells because they have differentiated along more similar developmental pathways. Therefore, a shorter amount of reprogramming is required for a NSC to express markers of cochlear tissue.
Taken together, it can be said that the cochlea provides a permissive environment for stem cell incubation and that certain types of stem cells, especially neural and mesenchymal stem cells, maintain the ability to migrate throughout the damaged cochlea. The ability for the transplanted cell to migrate is important because a migrating cell presents an advantage in that it may be injected into the cochlea and then work its way to the site of lesion (reviewed in Parker & Cotanche, 2004). Additionally, it appears that embryonic and neural stem cells maintain the potential to differentiate into neural cell types of the cochlea, which suggests that they may be useful in replacing lost spiral ganglion neurons. These data are encouraging for therapies aimed at replacing the cell types lost in neurodegenerative diseases involving the spiral ganglion such as auditory neuropathy and the spiral ganglion degeneration secondary to long term SNHL (Berlin, Morlet, & Hood, 2003; Lang et al., 2008; Shi et al., 2007).
It should be noted, however, that stem cell transplantation into the cochlea exhibit a limited potential for widespread differentiation into organ of Corti cell types. Importantly, even though the aforementioned studies show that transplanted stem cells adopt some molecular markers of cochlear cell types, the expression of a few molecular markers does not necessarily indicate that these cells fully develop into mature neural or sensory tissue. More troubling than the inability to completely differentiate into cochlear cell types, is that some stem cell types have the propensity to develop tumors when transplanted into the cochlea (Lang et al., 2008). Finally, the fact that transplanted ESCs extend processes towards hair cells is encouraging, but not conclusive evidence of their utility in treating SNHL. For example, it is yet to be determined whether these processes form functional synaptic contacts with hair cells or extend processes centrally into the cochlear nucleus of the brainstem, as do the endogenous spiral ganglion neurons. Therefore, these experiments represent a promising initial wave of investigations regarding the use of stem cells in cell replacement therapy for hearing loss, but much more work is required before this technology is clinically feasible.
This limited potential may be due to a lack of control over the stem cell differentiation after transplantation into the cochlea. Once injected, stem cells have been left to differentiate by their own devices and there has been little attempt to drive stem cell differentiation along a cochlear pathway (Parker & Cotanche, 2004). Rather than inject naive stem cells into the adult cochlea, it may be more beneficial to lead the exogenous stem cells along a hair cell pathway by sequentially exposing them signals that are present in the developing cochlea (Oshima et al., 2010). This can be accomplished by exposing stem cells to these signals in vitro prior to transplantation, genetically engineering the stem cells to sequentially express these signals after transplantation, or a combination of both of these techniques. Current research in both developmental and stem cell biology is focusing on identifying the complete list of morphogens, growth factors, and signaling ligands involved in hair cell development. Once the essential signaling pathways and gene expression patterns are determined, they will be used to drive stem cell-to-hair cell differentiation. Alternatively, inner ear stem cells may be used in cell replacement therapy. Inner ear stem cells possess an advantage over other stem cell types because they are derived from the cochlear or vestibular tissue and have been exposed to the aforementioned signals during normal development. Currently, there are several groups actively pursuing these avenues of research.
GENE THERAPY FOR THE TREATMENT OF SNHL
Gene therapy involves the up-regulation or down-regulation of specific genes in order to treat human disease (reviewed in B. R. Snyder, Boulis, & Federici, 2010; D. R. Wilson, 2002; Young, Searle, Onion, & Mautner, 2006). One technicality with gene therapy in general concerns the method of injecting the gene, which consists of a segment of DNA, into the target cell. Genes can be inserted in to cells using electric pulses (Neumann, Schaefer-Ridder, Wang, & Hofschneider, 1982; Zheng & Gao, 2000), encasement in lipid-like spheres (Fassati, Takahara, Walsh, & Dickson, 1994), or by packaging into viruses (Aposhian, Barr, & Keck, 1977; Han et al., 1999; Zheng, Lewis, & Gao, 1998) (Figure 8). For example, viruses are efficient at infecting a host cell with their own DNA and have been used successfully as vehicles for gene delivery in many different systems. In a viral-mediated gene delivery system, most of the viral DNA is deleted and replaced with a gene-of-interest before it is re-packaged back into the virus. In this system, any cell that comes in contact with the virus may be infected with the gene-of-interest.
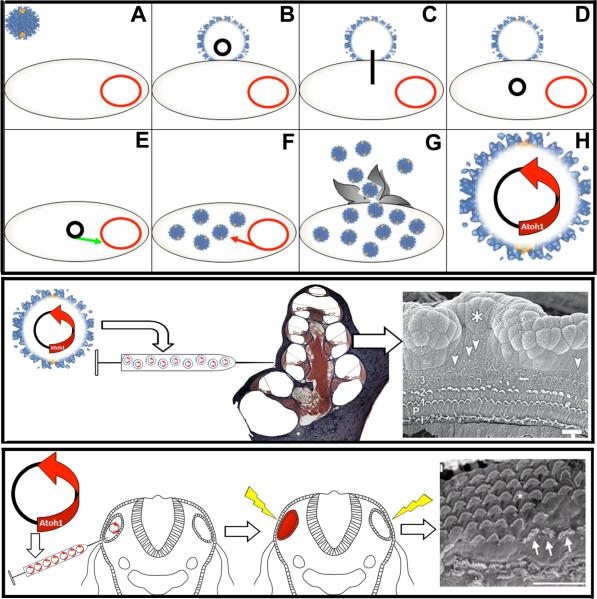
Figure 8. Gene Delivery in Hearing Research.
TOP PANEL. Viral mediated gene delivery utilizes virus particles to deliver a gene-of-interest into a cell. A) A virus (blue) attaches to a cell (B). Once attached, the DNA of the virus (black) will be inserted into the cytoplasm of the host cell (C-D) where the viral DNA will incorporate into the DNA of the host cell (red) (E). Viral DNA consists of self-replicating genes and will cause the host cell to manufacture more viral particles (F). G) Eventually, the host cell will release vial particles back into the environment. H) In a viral-mediated gene delivery system, the viral DNA that codes for self-replication is replaced with a gene-of-interest, such as atoh1. Cells that are infected with the engineered virus will express the atoh1. Image adapted from (Stewart, Fuller, & Burnett, 1993). MIDDLE PANEL. Izumikawa et al. (2005) injected an adenovirus that carried the atoh1 gene into the cochleas of chemically deafened adult guinea pigs. The results indicated that the guinea pigs injected with the atoh1 gene had better ABR threshold and more hair cells than controls injected with virus particles lacking the atoh1 gene. Arrow heads in the far right panel highlight extra streocilia bundles. Asterisk marks the site of injection into the scala media. Adapted by permission from Macmillan Publishers Ltd: Nature Neuroscience (Izumikawa et al., 2005), copyright (2005). BOTTOM PANEL. Genes can also be delivered into the inner ear using pulse trains of electric charge (electroporation). Gubbels et al. (2008) injected atoh1 DNA into the developing otocysts of E11.5 mice in utero, electroporated the embryos, allowed them to mature, and then analyzed the cochleas at various time points for hair cell markers. These results indicated that the overexpression of atoh1 during embryogenesis resulted in extra rows of functional hair cells into adulthood. Arrows in far right point to hair cells that exhibit abnormal stereocilia. Reprinted by permission from Macmillan Publishers Ltd: Nature (Gubbels et al., 2008), copyright (2008).
While research into gene therapy had begun in earnest during the early 1970s (Aposhian, 1970; Osterman, Waddell, & Aposhian, 1970), its application to SNHL is relatively recent, having started about a decade ago with the discovery of the pro-hair cell gene atoh1 (Bermingham et al., 1999) (Figure 9). Further studies have indicated that expressing atoh1 in the mammalian cochlea is sufficient (i.e. no other signals are required) for hair cell genesis in the developing (Bermingham et al., 1999; Zheng & Gao, 2000) and adult cochlea (Izumikawa et al., 2005; Kawamoto, Ishimoto, Minoda, Brough, & Raphael, 2003). Misexpression studies, where genes are expressed in atypical patterns (such as over-expression) or expression in atypical cell types, have shown that cells which up-regulate atoh1 within the prenatal (Brigande, Gubbels, Woessner, Jungwirth, & Bresee, 2009; Gubbels, Woessner, Mitchell, Ricci, & Brigande, 2008) and perinatal organ of Corti (Zhai et al., 2005; Zheng & Gao, 2000) will also up-regulate hair cell markers such as myosin 7a and adopt morphological characteristics of hair cells such as cilia (Figure 9 B,C). Taken together, these data indicate the expression of atoh1 in cells within the prenatal and perinatal mammalian cochlea is sufficient for hair cell genesis and has led to the use of this gene as an experimental therapy for the treatment of SNHL in mammalian animal models.
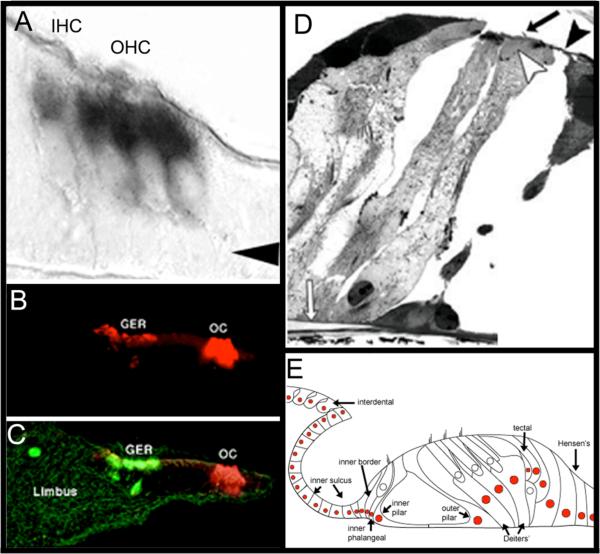
Figure 9. Expression of the atoh1 Gene in the organ of Corti is Sufficient for Hair Cell Genesis.
A) ISH shows that hair cells express RNA for atoh1 gene (black) by E17. Arrow indicates Deiters’ cell layer. Image adapted from (Lanford, Shailam, Norton, Ridley, & Kelley, 2000). B and C) Organs of Corti that were isolated from perinatal rats, then electroporated with the atoh1 gene tagged with a GFP marker (green) expressed the hair cell marker myosin 7a (red) when assayed by immunohistochemestry, indicating that these cells expressed hair cell specific markers. Most of the regenerated hair cells were seen in Kölliker's organ (a.k.a. the greater epithelial ridge, (GER)). Image in C is the overlay of B and both were adapted by permission from Macmillan Publishers Ltd: Nature Neuroscience (Zheng & Gao, 2000), copyright (2000). D) Adult guinea pigs were deafened, and then their cochleas were infected with a virus that had been engineered to deliver atoh1. Numerous regenerated hair cells appeared in the infected cochleas. In some cases, the regenerated hair cells maintained the morphology of ciliated supporting cells (nucleus close to basilar membrane with a cellular process that reached the reticular lamina). White arrow: basilar membrane; black arrow: cilia, black arrowhead: reticular lamina. Adapted by permission from Macmillan Publishers Ltd: Nature Neuroscience (Izumikawa et al., 2005), copyright (2005). E) Viral-mediated delivery of atoh1 results in random infection of cells within the cochlea. Each colored cell nucleus indicates a cell type that exhibited a hair cell phenotype after infection of atoh1 into the deafened cochlea. Image adapted with permission from Kawamoto et al. (2003); Society for Neuroscience Publisher.
One group of researchers have engineered a virus to express atoh1 and then injected this virus into the cochleas of drug-deafened adult guinea pigs (Kawamoto et al., 2003) (Figure 8, Middle Panel). The data showed that the infected animals exhibited more hair cells compared to the sham infected controls. The structure of some of the regenerated hair cells was surprising because they maintained the general morphology of a supporting cell, with a nucleus close to the basilar membrane, but had grown cilia on their apical surface similar to a hair cell (Figure 9 D). This result suggests that atoh1 may allow supporting cells to directly transdifferentiat e into hair cells as observed in chickens. Furthermore, the infected animals had demonstrated an improvement in auditory brainstem response thresholds relative to sham-infected controls, indicating that atoh1 infected animals were able to recover some hearing function that would have been lost due to the ototoxic insult (Izumikawa et al., 2005). Interestingly, similar experiments showed that forced expression of atoh1 failed to produce hair cells when infected into cochlea's lacking any supporting cells (Izumikawa, Batts, Miyazawa, Swiderski, & Raphael, 2008), which suggests that the presence of supporting cells is a prerequisite for atoh1-induced hair cell regeneration in mammals. This datum is also noteworthy, because it is the first time that hair cell regeneration has been documented in an adult mammal and suggests that the use of atoh1 as gene therapy may be useful in treating SNHL in adults.
However, it is important to note that this research is in its infancy and will not be ready for clinical use for several years. There are several obstacles that remain to be surmounted before gene therapy can be used effectively and safely in the clinic. Foremost, is that these results have yet to be verified by an independent laboratory and therefore remain somewhat controversial. Perhaps the greatest obstacle for gene therapy involves the cell-specificity of gene delivery systems. Most gene delivery vectors, such as viruses, will infect cells randomly. Therefore, the gene-of-interest will be expressed in random cell types. In the case of viral-mediated delivery of atoh1, ectopic hair cells were produced in unexpected regions of the organ of Corti, such as the spiral limbus, inner sulcus, and Hensen's cells (Figure 9 E). While this datum provides important information regarding the identities of the specific supporting cell types capable of hair cell differentiation, the formation of ectopic hair cells is problematic from a clinical standpoint. Additionally, the viral vectors themselves may present a significant health risk (Connolly, 2002). Furthermore, safe injection of virus particles into the scala media requires a complicated surgical procedure that has not yet been optimized. Other common gene delivery systems such as lipophilic vectors and electroporation result in similar roadblocks. Nonetheless, current research being conducted in several laboratories is addressing these shortcomings. For example, multiple laboratories are developing new generations of gene delivery systems aimed to provide more safety to a clinical population (Pathak, Patnaik, & Gupta, 2009; B. R. Snyder et al., 2010). Additionally, several laboratories are developing cell specific delivery systems for genes such as atoh1 (Parker, Jiang, Adams, & Edge, 2010). This research is continuing at a rapid rate and is being conducted by teams of talented researchers.
CONCLUSION
Even with the acknowledgement of their current limitations, biological approaches may represent the future for the treatment of SNHL. Currently, hearing aids remain the treatment of choice for mild to severe SNHL and cochlear implants remain the gold-standard for the treatment of profound deafness. However, it is unclear whether current amplification and implant technology has plateaued. Given a need for improvement in the cochlear implant performance, it may be that biological approaches will eventually result in more effective treatments for SNHL than electronic approaches. Alternatively, it may be that a biological approach combined with a cochlear implant system will improve its efficacy.
Currently, mammalian hair cell regeneration using stem cell and gene therapy for the treatment of SNHL is years if not decades away from being clinically feasible. However significant progress has been made in this area since the discovery of hair cell regeneration 30 years ago. For instance, it is possible to regenerate hair cells in the mammalian cochlea after their critical period of development using gene therapy. Furthermore, it may possible to replace damaged auditory neurons using stem cells. However, there are limitations to each of these approaches and the efficacy of these treatments needs to be methodically investigated. It may be that a combination of approaches will be the most effective means of accomplishing the goal of cochlear repair. While hearing aids and cochlear implants remain the standard treatment for SNHL and deafness, if the goals of the biological approaches are met, to structurally repair the damaged cochlea, these therapies may represent the future treatments for SNHL.
ACKNOWLEDGEMENTS
The author would like to thank M. Charles Liberman and Albert S. Edge at the Massachusetts Eye and Ear Infirmary; and William L. Parker and Joshua Merritt Parker at the Parker Hearing and Speech Institute for their feedback on previous drafts of this paper, and Amy Osborne for her artwork. This work was supported by a grant from the National Institute of Deafness and other Communicative Disorders (R03DC010065), a division of the National Institute of Health.
REFERENCES
- Abello G, Alsina B. Establishment of a proneural field in the inner ear. Int J Dev Biol. 2007;51(6-7):483–493. doi: 10.1387/ijdb.072343ga. [DOI] [PubMed] [Google Scholar]
- Akazawa C, Ishibashi M, Shimizu C, Nakanishi S, Kageyama R. A mammalian helix-loop-helix factor structurally related to the product of Drosophila proneural gene atonal is a positive transcriptional regulator expressed in the developing nervous system. J Biol Chem. 1995;270(15):8730–8738. doi: 10.1074/jbc.270.15.8730. [DOI] [PubMed] [Google Scholar]
- Alberts B, Johnson A, Lewis J, Raff M. Molecular Biology of the Cell. 5 ed. Garland Science; New York: 2007. [Google Scholar]
- Alford BR, Ruben RJ. Physiological, behavioral and anatomical correlates of the development of hearing in the mouse. Ann Otol Rhinol Laryngol. 1963;72:237–247. doi: 10.1177/000348946307200119. [DOI] [PubMed] [Google Scholar]
- Alibardi L. Morphological and cellular aspects of tail and limb regeneration in lizards. A model system with implications for tissue regeneration in mammals. Adv Anat Embryol Cell Biol. 2010;207:iii, v–x, 1–109. [PubMed] [Google Scholar]
- Alsina B, Giraldez F, Pujades C. Patterning and cell fate in ear development. Int J Dev Biol. 2009;53(8-10):1503–1513. doi: 10.1387/ijdb.072422ba. [DOI] [PubMed] [Google Scholar]
- Anderson DJ, Gage FH, Weissman IL. Can stem cells cross lineage boundaries? Nat Med. 2001;7(4):939–935. doi: 10.1038/86439. [DOI] [PubMed] [Google Scholar]
- Anniko M. Cytodifferentiation of cochlear hair cells. Am J Otolaryngol. 1983;4(6):375–388. doi: 10.1016/s0196-0709(83)80043-x. [DOI] [PubMed] [Google Scholar]
- Anniko M, Thornell LE, Gustavsson H, Virtanen I. Intermediate filaments in the newborn inner ear of the mouse. ORL J Otorhinolaryngol Relat Spec. 1986;48(2):98–106. doi: 10.1159/000275854. [DOI] [PubMed] [Google Scholar]
- Anniko M, Wikstrom SO. Pattern formation of the otic placode and morphogenesis of the otocyst. Am J Otolaryngol. 1984;5(6):373–381. doi: 10.1016/s0196-0709(84)80051-4. [DOI] [PubMed] [Google Scholar]
- Aposhian HV. The use of DNA for gene therapy--the need, experimental approach, and implications. Perspect Biol Med. 1970;14(1):98–108. doi: 10.1353/pbm.1970.0011. [DOI] [PubMed] [Google Scholar]
- Aposhian HV, Barr SM, Keck K. Experimental gene delivery systems for mammalian cells-the polyoma pseudovirus system. Adv Enzyme Regul. 1977;16:275–288. doi: 10.1016/0065-2571(78)90078-x. [DOI] [PubMed] [Google Scholar]
- Atar O, Avraham KB. Therapeutics of hearing loss: expectations vs reality. Drug Discov Today. 2005;10(19):1323–1330. doi: 10.1016/S1359-6446(05)03618-4. [DOI] [PubMed] [Google Scholar]
- Avallone B, Porritiello M, Esposito D, Mutone R, Balsamo G, Marmo F. Evidence for hair cell regeneration in the crista ampullaris of the lizard Podarcis sicula. Hear Res. 2003;178(1-2):79–88. doi: 10.1016/s0378-5955(03)00040-6. [DOI] [PubMed] [Google Scholar]
- Baird RA, Torres MA, Schuff NR. Hair cell regeneration in the bullfrog vestibular otolith organs following aminoglycoside toxicity. Hear Res. 1993;65(1-2):164–174. doi: 10.1016/0378-5955(93)90211-i. [DOI] [PubMed] [Google Scholar]
- Barald KF, Kelley MW. From placode to polarization: new tunes in inner ear development. Development. 2004;131(17):4119–4130. doi: 10.1242/dev.01339. [DOI] [PubMed] [Google Scholar]
- Batts SA, Raphael Y. Transdifferentiation and its applicability for inner ear therapy. Hear Res. 2007;227(1-2):41–47. doi: 10.1016/j.heares.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Beadle EAR, McKinley DJ, Nikolopoulos TP, Brough J, O'Donoghue GM, Archbold SM. Long-Term Functional Outcomes and Academic-Occupational Status in Implanted Children After 10 to 14 Years of Cochlear Implant Use. Otology & Neurotology. 2005;26(6):1152–1160. doi: 10.1097/01.mao.0000180483.16619.8f. [DOI] [PubMed] [Google Scholar]
- Beisel K, Hansen L, Soukup G, Fritzsch B. Regenerating cochlear hair cells: quo vadis stem cell. Cell Tissue Res. 2008;333(3):373–379. doi: 10.1007/s00441-008-0639-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Arie N, Hassan BA, Bermingham NA, Malicki DM, Armstrong D, Matzuk M, et al. Functional conservation of atonal and Math1 in the CNS and PNS. Development. 2000;127(5):1039–1048. doi: 10.1242/dev.127.5.1039. [DOI] [PubMed] [Google Scholar]
- Berlin CI, Morlet T, Hood LJ. Auditory neuropathy/dyssynchrony: its diagnosis and management. Pediatr Clin North Am. 2003;50(2):331–340. vii–viii. doi: 10.1016/s0031-3955(03)00031-2. [DOI] [PubMed] [Google Scholar]
- Bermingham-McDonogh O, Oesterle EC, Stone JS, Hume CR, Huynh HM, Hayashi T. Expression of Prox1 during mouse cochlear development. Journal of Comparitive Neurology. 2006;496(2):172–186. doi: 10.1002/cne.20944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, et al. Math1: An Essential Gene for the Generation of Inner Ear Hair Cells. Science. 1999;284(5421):1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- Bernstein A, Breitman M. Genetic ablation in transgenic mice. Mol Biol Med. 1989;6(6):523–530. [PubMed] [Google Scholar]
- Blamey PJ, Dooley GJ, James CJ, Parisi ES. Monaural and binaural loudness measures in cochlear implant users with contralateral residual hearing. Ear Hear. 2000;21(1):6–17. doi: 10.1097/00003446-200002000-00004. [DOI] [PubMed] [Google Scholar]
- Boeda B, Weil D, Petit C. A specific promoter of the sensory cells of the inner ear defined by transgenesis. Hum. Mol. Genet. 2001;10(15):1581–1589. doi: 10.1093/hmg/10.15.1581. [DOI] [PubMed] [Google Scholar]
- Breitman ML, Clapoff S, Rossant J, Tsui LC, Glode LM, Maxwell IH, et al. Genetic ablation: targeted expression of a toxin gene causes microphthalmia in transgenic mice. Science. 1987;238(4833):1563–1565. doi: 10.1126/science.3685993. [DOI] [PubMed] [Google Scholar]
- Brigande JV, Gubbels SP, Woessner DW, Jungwirth JJ, Bresee CS. Electroporation-mediated gene transfer to the developing mouse inner ear. Methods Mol Biol. 2009;493:125–139. doi: 10.1007/978-1-59745-523-7_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigande JV, Heller S. Quo vadis, hair cell regeneration? Nat Neurosci. 2009;12(6):679–685. doi: 10.1038/nn.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brignull HR, Raible DW, Stone JS. Feathers and fins: Non-mammalian models for hair cell regeneration. Brain Research. 2009;1277:12–23. doi: 10.1016/j.brainres.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q, Benton RL, Whittemore SR. Stem cell repair of central nervous system injury. Journal of Neuroscience Research. 2002;68(5):510–510. doi: 10.1002/jnr.10240. [DOI] [PubMed] [Google Scholar]
- Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC. Green fluorescent protein as a marker for gene expression. Science. 1994;263(5148):802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126(8):1581–1590. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- Chikar JA, Colesa DJ, Swiderski DL, Di Polo A, Raphael Y, Pfingst BE. Over-expression of BDNF by adenovirus with concurrent electrical stimulation improves cochlear implant thresholds and survival of auditory neurons. Hear Res. 2008;245(1-2):24–34. doi: 10.1016/j.heares.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark GM. A surgical approach for a cochlear implant: an anatomical study. J Laryngol Otol. 1975;89(1):9–15. doi: 10.1017/s0022215100080038. [DOI] [PubMed] [Google Scholar]
- Clark GM. Personal reflections on the multichannel cochlear implant and a view of the future. J Rehabil Res Dev. 2008;45(5):651–693. doi: 10.1682/jrrd.2007.05.0064. [DOI] [PubMed] [Google Scholar]
- Clark GM. The multi-channel cochlear implant: past, present and future perspectives. Cochlear Implants Int. 2009;10(Suppl 1):2–13. doi: 10.1179/cim.2009.10.Supplement-1.2. [DOI] [PubMed] [Google Scholar]
- Cohen SM, Labadie RF, Dietrich MS, Haynes DS. Quality of life in hearing-impaired adults: the role of cochlear implants and hearing aids. Otolaryngol Head Neck Surg. 2004;131(4):413–422. doi: 10.1016/j.otohns.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Coleman B, de Silva MG, Shepherd RK. Concise review: the potential of stem cells for auditory neuron generation and replacement. Stem Cells. 2007;25(11):2685–2694. doi: 10.1634/stemcells.2007-0393. [DOI] [PubMed] [Google Scholar]
- Connolly JB. Lentiviruses in gene therapy clinical research. Gene Ther. 2002;9(24):1730–1734. doi: 10.1038/sj.gt.3301893. [DOI] [PubMed] [Google Scholar]
- Corrales CE, Pan L, Li H, Liberman MC, Heller S, Edge AS. Engraftment and differentiation of embryonic stem cell-derived neural progenitor cells in the cochlear nerve trunk: growth of processes into the organ of Corti. J Neurobiol. 2006;66(13):1489–1500. doi: 10.1002/neu.20310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin JT. Postembryonic production and aging in inner ear hair cells in sharks. J Comp Neurol. 1981;201(4):541–553. doi: 10.1002/cne.902010406. [DOI] [PubMed] [Google Scholar]
- Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science. 1988;240(4860):1772–1774. doi: 10.1126/science.3381100. [DOI] [PubMed] [Google Scholar]
- Cotanche DA. Regeneration of the tectorial membrane in the chick cochlea following severe acoustic trauma. Hear Res. 1987a;30(2-3):197–206. doi: 10.1016/0378-5955(87)90136-5. [DOI] [PubMed] [Google Scholar]
- Cotanche DA. Regeneration of hair cell stereociliary bundles in chick cochlea following severe acoustic trauma. Hearing Research. 1987b;30:181–196. doi: 10.1016/0378-5955(87)90135-3. [DOI] [PubMed] [Google Scholar]
- Cotanche DA. Genetic and pharmacological intervention for treatment/prevention of hearing loss. J Commun Disord. 2008;41(5):421–443. doi: 10.1016/j.jcomdis.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz RM, Lambert PR, Rubel EW. Light microscopic evidence of hair cell regeneration after gentamicin toxicity in chick cochlea. Arch Otolaryngol Head Neck Surg. 1987;113(10):1058–1062. doi: 10.1001/archotol.1987.01860100036017. [DOI] [PubMed] [Google Scholar]
- Das S, Buchman CA. Bilateral cochlear implantation: current concepts. Curr Opin Otolaryngol Head Neck Surg. 2005;13(5):290–293. doi: 10.1097/01.moo.0000179808.00638.ab. [DOI] [PubMed] [Google Scholar]
- Djourno A, Eyries C, Vallancien B. Electric excitation of the cochlear nerve in man by induction at a distance with the aid of micro-coil included in the fixture. C R Seances Soc Biol Fil. 1957;151(3):423–425. [PubMed] [Google Scholar]
- Doetzlhofer A, Basch ML, Ohyama T, Gessler M, Groves AK, Segil N. Hey2 regulation by FGF provides a Notch-independent mechanism for maintaining pillar cell fate in the organ of Corti. Dev Cell. 2009;16(1):58–69. doi: 10.1016/j.devcel.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson GS, Chisolm TH, Blasco GP, Shinnick LJ, Ketter KJ, Krause JC. BKB-SIN and ANL Predict Perceived Communication Ability in Cochlear Implant Users. Ear Hear. 2009 doi: 10.1097/AUD.0b013e3181a16379. [DOI] [PubMed] [Google Scholar]
- Driver EC, Kelley MW. Specification of cell fate in the mammalian cochlea. Birth Defects Res C Embryo Today. 2009;87(3):212–221. doi: 10.1002/bdrc.20154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassati A, Takahara Y, Walsh FS, Dickson G. Production of high titre helper-free recombinant retroviral vectors by lipofection. Nucleic Acids Res. 1994;22(6):1117–1118. doi: 10.1093/nar/22.6.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, et al. Imaging Neuronal Subsets in Transgenic Mice Expressing Multiple Spectral Variants of GFP. Neuron. 2000;28(1):41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Forge A, Schacht J. Aminoglycoside antibiotics. Audology and Neurootology. 2000;5(1):3–22. doi: 10.1159/000013861. [DOI] [PubMed] [Google Scholar]
- Friedmann I. Electron microscope observations on in vitro cultures of the isolated fowl embryo otocyst. J Biophys Biochem Cytol. 1959;5(2):263–268. doi: 10.1083/jcb.5.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu QJ, Hsu CJ, Horng MJ. Effects of speech processing strategy on Chinese tone recognition by nucleus-24 cochlear implant users. Ear Hear. 2004;25(5):501–508. doi: 10.1097/01.aud.0000145125.50433.19. [DOI] [PubMed] [Google Scholar]
- Fu QJ, Zeng FG, Shannon RV, Soli SD. Importance of tonal envelope cues in Chinese speech recognition. J Acoust Soc Am. 1998;104(1):505–510. doi: 10.1121/1.423251. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Okamura K, Fujita S, Bohm N, Rohrbach R, Sandritter W. The different stem cell populations in mouse epidermis and lingual epithelium. Pathol Res Pract. 1978;163(3):205–227. doi: 10.1016/S0344-0338(78)80015-6. [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287(5457):1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Gazit R, Krizhanovsky V, Ben-Arie N. Math1 controls cerebellar granule cell differentiation by regulating multiple components of the Notch signaling pathway. Development. 2004;131(4):903–913. doi: 10.1242/dev.00982. [DOI] [PubMed] [Google Scholar]
- Geers AE, Nicholas JG, Sedey AL. Language skills of children with early cochlear implantation. Ear Hear. 2003;24(1 Suppl):46S–58S. doi: 10.1097/01.AUD.0000051689.57380.1B. [DOI] [PubMed] [Google Scholar]
- Gfeller K, Turner C, Mehr M, Woodworth G, Fearn R, Knutson JF, et al. Recognition of familiar melodies by adult cochlear implant recipients and normal-hearing adults. Cochlear Implants Int. 2002;3(1):29–53. doi: 10.1179/cim.2002.3.1.29. [DOI] [PubMed] [Google Scholar]
- Gilbert SF. Developmental Biology. 9 ed. Sunderland (MA): Sinauer Associates, Inc.: 2010. [Google Scholar]
- Gillespie PG. Molecular machinery of auditory and vestibular transduction. Current Opinion in Neurobiology. 1995;5(4):449–455. doi: 10.1016/0959-4388(95)80004-2. [DOI] [PubMed] [Google Scholar]
- Gordon JW, Ruddle FH. Germ line transmission in transgenic mice. Prog Clin Biol Res. 1982;85(Pt B):111–124. [PubMed] [Google Scholar]
- Groves AK. The challenge of hair cell regeneration. Exp. Biol. Med. 2010;235(4):434–446. doi: 10.1258/ebm.2009.009281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbels SP, Woessner DW, Mitchell JC, Ricci AJ, Brigande JV. Functional auditory hair cells produced in the mammalian cochlea by in utero gene transfer. Nature. 2008;455(7212):537–541. doi: 10.1038/nature07265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakuba N, Hata R, Morizane I, Feng G, Shimizu Y, Fujita K, et al. Neural stem cells suppress the hearing threshold shift caused by cochlear ischemia. Neuroreport. 2005;16(14):1545–1549. [PubMed] [Google Scholar]
- Halliday AL, Cepko CL. Generation and migration of cells in the developing striatum. Neuron. 1992;9(1):15–26. doi: 10.1016/0896-6273(92)90216-z. [DOI] [PubMed] [Google Scholar]
- Han JJ, Mhatre AN, Wareing M, Pettis R, Gao WQ, Zufferey RN, et al. Transgene expression in the guinea pig cochlea mediated by a lentivirus-derived gene transfer vector. Hum Gene Ther. 1999;10(11):1867–1873. doi: 10.1089/10430349950017545. [DOI] [PubMed] [Google Scholar]
- Harris JA, Cheng AG, Cunningham LL, MacDonald G, Raible DW, Rubel EW. Neomycin-Induced Hair Cell Death and Rapid Regeneration in the Lateral Line of Zebrafish ( Danio rerio). JARO - Journal of the Association for Research in Otolaryngology. 2003;4(2):219–234. doi: 10.1007/s10162-002-3022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman BH, Reh TA, Bermingham-McDonogh O. Notch signaling specifies prosensory domains via lateral induction in the developing mammalian inner ear. Proceedings of the National Academy of Sciences. 2010;107(36):15792–15797. doi: 10.1073/pnas.1002827107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson T, Gillespie PG, Garcia JA, MacDonald RB, Zhao Y.-d., Yee AG, et al. Unconventional Myosins in Inner-Ear Sensory Epithelia. J. Cell Biol. 1997;137(6):1287–1307. doi: 10.1083/jcb.137.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinojosa R. A note on development of Corti's organ. Acta Otolaryngol. 1977;84(3-4):238–251. doi: 10.3109/00016487709123963. [DOI] [PubMed] [Google Scholar]
- Hoffmeister-Ullerich SA. Hydra--ancient model with modern outfit. Cell Mol Life Sci. 2007;64(23):3012–3016. doi: 10.1007/s00018-007-7204-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley MC. Application of new biological approaches to stimulate sensory repair and protection. British Medical Bulletin. 2002;63:157–169. doi: 10.1093/bmb/63.1.157. [DOI] [PubMed] [Google Scholar]
- Hu Z, Andang M, Ni D, Ulfendahl M. Neural cograft stimulates the survival and differentiation of embryonic stem cells in the adult mammalian auditory system. Brain Res. 2005;1051(1-2):137–144. doi: 10.1016/j.brainres.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Iguchi F, Nakagawa T, Tateya I, Kim TS, Endo T, Taniguchi Z, et al. Trophic support of mouse inner ear by neural stem cell transplantation. Neuroreport. 2003;14(1):77–80. doi: 10.1097/00001756-200301200-00015. [DOI] [PubMed] [Google Scholar]
- Ito J, Kojima K, Kawaguchi S. Survival of Neural Stem Cells in the Cochlea. Acta Otolaryngol. 2001;121:140–142. doi: 10.1080/000164801300043226. [DOI] [PubMed] [Google Scholar]
- Izumikawa M, Batts SA, Miyazawa T, Swiderski DL, Raphael Y. Response of the flat cochlear epithelium to forced expression of Atoh1. Hear Res. 2008;240(1-2):52–56. doi: 10.1016/j.heares.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, et al. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nature Medicine. 2005;11(3):271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- Jarman AP, Grau Y, Jan LY, Jan YN. atonal is a proneural gene that directs chordotonal organ formation in the Drosophila peripheral nervous system. Cell. 1993;73(7):1307–1321. doi: 10.1016/0092-8674(93)90358-w. [DOI] [PubMed] [Google Scholar]
- Johnston JC, Durieux-Smith A, Angus D, O'Connor A, Fitzpatrick E. Bilateral paediatric cochlear implants: A critical review. Int J Audiol. 2009:1–16. doi: 10.1080/14992020802665967. [DOI] [PubMed] [Google Scholar]
- Jones FW. The mandible and its relation to the otocyst and auditory organ. J Laryngol Otol. 1952;66(10):473–479. doi: 10.1017/s0022215100047964. [DOI] [PubMed] [Google Scholar]
- Jones JE, Corwin JT. Replacement of lateral line sensory organs during tail regeneration in salamanders: identification of progenitor cells and analysis of leukocyte activity. J Neurosci. 1993;13(3):1022–1034. doi: 10.1523/JNEUROSCI.13-03-01022.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel E, Schwartz J, Jessell T. Principles of Neural Science. McGraw-Hill Medical; New York: 2000. [Google Scholar]
- Kawamoto K, Ishimoto S-I, Minoda R, Brough DE, Raphael Y. Math1 Gene Transfer Generates New Cochlear Hair Cells in Mature Guinea Pigs In Vivo. J. Neurosci. 2003;23(11):4395–4400. doi: 10.1523/JNEUROSCI.23-11-04395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley MW. Hair cell development: commitment through differentiation. Brain Res. 2006;1091(1):172–185. doi: 10.1016/j.brainres.2006.02.062. [DOI] [PubMed] [Google Scholar]
- Kelley MW, Talreja DR, Corwin JT. Replacement of hair cells after laser microbeam irradiation in cultured organs of corti from embryonic and neonatal mice. Journal of Neuroscience. 1995;15(4):3013–3026. doi: 10.1523/JNEUROSCI.15-04-03013.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley MW, Xu X, Wagner M, Warchol M, Corwin JT. The developing organ of Corti contains retinoic acid and forms supernumerary hair cells in response to exogenous retinoic acid in culture. Development. 1993;119(4):1041–1053. doi: 10.1242/dev.119.4.1041. [DOI] [PubMed] [Google Scholar]
- Kelly MC, Chen P. Development of form and function in the mammalian cochlea. Current Opinion in Neurobiology. 2009;19(4):395–401. doi: 10.1016/j.conb.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessinger A, Smith DM, Strandjord SE, Landmark JD, Dooley DC, Law P, et al. Allogeneic transplantation of blood-derived, T cell-depleted hemopoietic stem cells after myeloablative treatment in a patient with acute lymphoblastic leukemia. Bone Marrow Transplant. 1989;4(6):643–646. [PubMed] [Google Scholar]
- Kidson C, Kirby KS. Selective Alterations of Mammalian Messenger-RNA Synthesis: Evidence for Differential Action of Hormones on Gene Transcription. Nature. 1964;203:599–603. doi: 10.1038/203599a0. [DOI] [PubMed] [Google Scholar]
- Kikuchi K, Hilding D. The development of the organ of Corti in the mouse. Acta Otolaryngol. 1965;60(3):207–222. doi: 10.3109/00016486509127003. [DOI] [PubMed] [Google Scholar]
- Kim YH, Raphael Y. Cell division and maintenance of epithelial integrity in the deafened auditory epithelium. Cell Cycle. 2007;6(5):612–619. doi: 10.4161/cc.6.5.3929. [DOI] [PubMed] [Google Scholar]
- Krizhanovsky V, Golenser E, Ben-Arie N. Genotype identification of Math1/LacZ knockout mice based on real-time PCR with SYBR Green I dye. J Neurosci Methods. 2004;136(2):187–192. doi: 10.1016/j.jneumeth.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Lanford PJ, Shailam R, Norton CR, Ridley T, Kelley MW. Expression of Math1 and HES5 in the Cochleae of Wildtype and Jag2 Mutant Mice. JARO - Journal of the Association for Research in Otolaryngology. 2000;1(2):161–171. doi: 10.1007/s101620010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang H, Schulte BA, Goddard JC, Hedrick M, Schulte JB, Wei L, et al. Transplantation of mouse embryonic stem cells into the cochlea of an auditory-neuropathy animal model: effects of timing after injury. J Assoc Res Otolaryngol. 2008;9(2):225–240. doi: 10.1007/s10162-008-0119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblond CP, Clermont Y, Nadler NJ. The pattern of stem cell renewal in three epithelia: (esophagus, intestine and testis). Proc Can Cancer Conf. 1967;7:3–30. [PubMed] [Google Scholar]
- Li H, Liu H, Heller S. Pluripotent stem cells from the adult mouse inner ear. Nature Medicine. 2003;9(10):1293–1299. doi: 10.1038/nm925. [DOI] [PubMed] [Google Scholar]
- Lombarte A, Yan HY, Popper AN, Chang JS, Platt C. Damage and regeneration of hair cell ciliary bundles in a fish ear following treatment with gentamicin. Hear Res. 1993;64(2):166–174. doi: 10.1016/0378-5955(93)90002-i. [DOI] [PubMed] [Google Scholar]
- Lowenheim H, Furness DN, Kil J, Zinn C, Gultig K, Fero ML, et al. Gene disruption of p27(Kip1) allows cell proliferation in the postnatal and adult organ of corti. Proc Natl Acad Sci U S A. 1999;96(7):4084–4088. doi: 10.1073/pnas.96.7.4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Fu Q-J. Enhancing Chinese tone recognition by manipulating amplitude envelope: Implications for cochlear implants. The Journal of the Acoustical Society of America. 2004;116(6):3659–3667. doi: 10.1121/1.1783352. [DOI] [PubMed] [Google Scholar]
- Martinez-Monedero R, Corrales CE, Cuajungco MP, Heller S, Edge AS. Reinnervation of hair cells by auditory neurons after selective removal of spiral ganglion neurons. J Neurobiol. 2006;66(4):319–331. doi: 10.1002/neu.20232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael CT, Wei-Chung C, Philip WB, Marco P. Molecular approach to echinoderm regeneration. Microscopy Research and Technique. 2001;55(6):474–485. doi: 10.1002/jemt.1192. [DOI] [PubMed] [Google Scholar]
- Mok M, Grayden D, Dowell RC, Lawrence D. Speech perception for adults who use hearing aids in conjunction with cochlear implants in opposite ears. J Speech Lang Hear Res. 2006;49(2):338–351. doi: 10.1044/1092-4388(2006/027). [DOI] [PubMed] [Google Scholar]
- Molea D, Stone JS, Rubel EW. Class III beta-tubulin expression in sensory and nonsensory regions of the developing avian inner ear. J Comp Neurol. 1999;406(2):183–198. [PubMed] [Google Scholar]
- Morsli H, Choo D, Ryan A, Johnson R, Wu DK. Development of the mouse inner ear and origin of its sensory organs. J Neurosci. 1998;18(9):3327–3335. doi: 10.1523/JNEUROSCI.18-09-03327.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann E, Schaefer-Ridder M, Wang Y, Hofschneider PH. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982;1(7):841–845. doi: 10.1002/j.1460-2075.1982.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIDCD Hearing Loss Defined. [October 7, 2009];NIH Senior Health. 2009 June 01; 2003. from http://nihseniorhealth.gov/hearingloss/hearinglossdefined/01.html.
- NIDCD Quick Statistics. [October 7, 2009];Statistics and Epidemiology. 2008 from http://www.nidcd.nih.gov/health/statistics/quick.htm.
- Nossal GJ, Makela O. Autoradiographic studies on the immune response.I. The kinetics of plasma cell proliferation. J Exp Med. 1962;115:209–230. doi: 10.1084/jem.115.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano T, Nakagawa T, Endo T, Kim TS, Kita T, Tamura T, et al. Engraftment of embryonic stem cell-derived neurons into the cochlear modiolus. Neuroreport. 2005;16(17):1919–1922. doi: 10.1097/01.wnr.0000187628.38010.5b. [DOI] [PubMed] [Google Scholar]
- Olson AD, Shinn JB. A systematic review to determine the effectiveness of using amplification in conjunction with cochlear implantation. J Am Acad Audiol. 2008;19(9):657–671. doi: 10.3766/jaaa.19.9.2. quiz 735. [DOI] [PubMed] [Google Scholar]
- Oshima K, Grimm CM, Corrales CE, Senn P, Martinez Monedero R, Géléoc GSG, et al. Differential Distribution of Stem Cells in the Auditory and Vestibular Organs of the Inner Ear. Journal of the Association for Research in Otolaryngology. 2007;8(1):18–31. doi: 10.1007/s10162-006-0058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima K, Senn P, Heller S. Isolation of sphere-forming stem cells from the mouse inner ear. Methods Mol Biol. 2009;493:141–162. doi: 10.1007/978-1-59745-523-7_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima K, Shin K, Diensthuber M, Peng AW, Ricci AJ, Heller S. Mechanosensitive Hair Cell-like Cells from Embryonic and Induced Pluripotent Stem Cells. Cell. 2010;141(4):704–716. doi: 10.1016/j.cell.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterman JV, Waddell A, Aposhian HV. DNA and gene therapy: uncoating of polyoma pseudovirus in mouse embryo cells. Proc Natl Acad Sci U S A. 1970;67(1):37–40. doi: 10.1073/pnas.67.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker MA, Corliss DA, Anderson JK, Liberman MC, Snyder EY, Cotanche DA. Neural Stem Cell Transdifferentiation Into Cochlear Cell Types. Association for Research in Otolaryngology Abstracts. 2003:1529. [Google Scholar]
- Parker MA, Corliss DA, Gray B, Anderson JK, Bobbin RP, Snyder EY, et al. Neural stem cells injected into the sound-damaged cochlea migrate throughout the cochlea and express markers of hair cells, supporting cells, and spiral ganglion cells. Hear Res. 2007;232(1-2):29–43. doi: 10.1016/j.heares.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker MA, Cotanche DA. The potential use of stem cells for cochlear repair. Audiology and Neurootology. 2004;9(2):72–80. doi: 10.1159/000075998. [DOI] [PubMed] [Google Scholar]
- Parker MA, Jiang K, Adams J, Edge AS. SUBMITTED MANUSCRIPT: TAK1 Expression in the Cochlea: A Specific Marker for Supporting Cells. 2010. Unpublished manuscript. [DOI] [PMC free article] [PubMed]
- Parving A. The hearing aid revolution: fact or fiction? 2003;123(2):245–248. doi: 10.1080/00016480310001088. [DOI] [PubMed] [Google Scholar]
- Patel NP, Mhatre AN, Lalwani AK. Biological therapy for the inner ear. Expert Opin Biol Ther. 2004;4(11):1811–1819. doi: 10.1517/14712598.4.11.1811. [DOI] [PubMed] [Google Scholar]
- Pathak A, Patnaik S, Gupta KC. Recent trends in non-viral vector-mediated gene delivery. Biotechnol J. 2009;4(11):1559–1572. doi: 10.1002/biot.200900161. [DOI] [PubMed] [Google Scholar]
- Pattillo RA, Gey GO, Delfs E, Mattingly RF. In vitro identification of the trophoblastic stem cell of the human villous placenta. Am J Obstet Gynecol. 1968;100(4):582–588. doi: 10.1016/s0002-9378(15)33497-9. [DOI] [PubMed] [Google Scholar]
- Peng SC, Tomblin JB, Cheung H, Lin YS, Wang LS. Perception and production of mandarin tones in prelingually deaf children with cochlear implants. Ear Hear. 2004;25(3):251–264. doi: 10.1097/01.aud.0000130797.73809.40. [DOI] [PubMed] [Google Scholar]
- Plyler PN, Bahng J, von Hapsburg D. The Acceptance of Background Noise in Adult Cochlear Implant Users. J Speech Lang Hear Res. 2008;51(2):502–515. doi: 10.1044/1092-4388(2008/036). [DOI] [PubMed] [Google Scholar]
- Prasher D, McCann RO, Cormier MJ. Cloning and expression of the cDNA coding for aequorin, a bioluminescent calcium-binding protein. Biochem Biophys Res Commun. 1985;126(3):1259–1268. doi: 10.1016/0006-291x(85)90321-3. [DOI] [PubMed] [Google Scholar]
- Pujol R, Hilding D. Anatomy and physiology of the onset of auditory function. Acta Otolaryngol. 1973;76(1):1–10. doi: 10.3109/00016487309121476. [DOI] [PubMed] [Google Scholar]
- Raphael Y, Kim YH, Osumi Y, Izumikawa M. Non-sensory cells in the deafened organ of Corti: approaches for repair. Int J Dev Biol. 2007;51(6-7):649–654. doi: 10.1387/ijdb.072370yr. [DOI] [PubMed] [Google Scholar]
- Rejali D, Lee VA, Abrashkin KA, Humayun N, Swiderski DL, Raphael Y. Cochlear implants and ex vivo BDNF gene therapy protect spiral ganglion neurons. Hear Res. 2007;228(1-2):180–187. doi: 10.1016/j.heares.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255(5052):1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Rio C, Dikkes P, Liberman MC, Corfas G. Glial fibrillary acidic protein expression and promoter activity in the inner ear of developing and adult mice. Journal of Comparitive Neurology. 2002;442(2):156–162. doi: 10.1002/cne.10085. [DOI] [PubMed] [Google Scholar]
- Roberson DW, Alosi JA, Cotanche DA. Direct transdifferentiation gives rise to the earliest new hair cells in regenerating avian auditory epithelium. J Neurosci Res. 2004;78(4):461–471. doi: 10.1002/jnr.20271. [DOI] [PubMed] [Google Scholar]
- Roy S, Gatien S. Regeneration in axolotls: a model to aim for! Experimental Gerontology. 2008;43(11):968–973. doi: 10.1016/j.exger.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Ruben RJ. Development of the inner ear of the mouse: a radioautographic study of terminal mitoses. Acta Otolaryngol. 1967;220(Suppl):221–244. [PubMed] [Google Scholar]
- Ryals BM, Rubel EW. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science. 1988;240(4860):1774–1776. doi: 10.1126/science.3381101. [DOI] [PubMed] [Google Scholar]
- Ryals BM, Westbrook EW. TEM analysis of neural terminals on autoradiographically identified regenerated hair cells. Hear Res. 1994;72(1-2):81–88. doi: 10.1016/0378-5955(94)90208-9. [DOI] [PubMed] [Google Scholar]
- Sahly I, El-Amraoui A, Abitbol M, Petit C, Dufier JL. Expression of myosin VIIA during mouse embryogenesis. Anat Embryol (Berl) 1997;196(2):159–170. doi: 10.1007/s004290050088. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Nakagawa T, Endo T, Kim TS, Iguchi F, Naito Y, et al. Fates of mouse embryonic stem cells transplanted into the inner ears of adult mice and embryonic chickens. Acta Otolaryngol Suppl. 2004;(551):48–52. doi: 10.1080/03655230310016825. [DOI] [PubMed] [Google Scholar]
- Saunders SS, Salvi RJ, Miller KM. Recovery of thresholds and temporal integration in adult chickens after high-level 525-Hz pure-tone exposure. J Acoust Soc Am. 1995;97(2):1150–1164. doi: 10.1121/1.412228. [DOI] [PubMed] [Google Scholar]
- Schneider-Maunoury S, Pujades C. Hindbrain signals in otic regionalization: walk on the wild side. Int J Dev Biol. 2007;51(6-7):495–506. doi: 10.1387/ijdb.072345ss. [DOI] [PubMed] [Google Scholar]
- Schwander M, Kachar B, Muller U. Review series: The cell biology of hearing. J Cell Biol. 2010;190(1):9–20. doi: 10.1083/jcb.201001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senn P, Oshima K, Teo D, Grimm C, Heller S. Robust postmortem survival of murine vestibular and cochlear stem cells. J Assoc Res Otolaryngol. 2007;8(2):194–204. doi: 10.1007/s10162-007-0079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Corrales CE, Liberman MC, Edge AS. BMP4 induction of sensory neurons from human embryonic stem cells and reinnervation of sensory epithelium. Eur J Neurosci. 2007;26(11):3016–3023. doi: 10.1111/j.1460-9568.2007.05909.x. [DOI] [PubMed] [Google Scholar]
- Shihabuddin LS, Ray J, Gage FH. Stem cell technology for basic science and clinical applications. Arch Neurol. 1999;56(1):29–32. doi: 10.1001/archneur.56.1.29. [DOI] [PubMed] [Google Scholar]
- Shimizu C, Akazawa C, Nakanishi S, Kageyama R. MATH-2, a mammalian helix-loop-helix factor structurally related to the product of Drosophila proneural gene atonal, is specifically expressed in the nervous system. Eur J Biochem. 1995;229(1):239–248. doi: 10.1111/j.1432-1033.1995.tb20461.x. [DOI] [PubMed] [Google Scholar]
- Shimomura O, Johnson FH, Saiga Y. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J Cell Comp Physiol. 1962;59:223–239. doi: 10.1002/jcp.1030590302. [DOI] [PubMed] [Google Scholar]
- Snyder BR, Boulis NM, Federici T. Viral vector-mediated gene transfer for CNS disease. Expert Opin Biol Ther. 2010;10(3):381–394. doi: 10.1517/14712590903514074. [DOI] [PubMed] [Google Scholar]
- Snyder EY, Yoon C, Flax JD, Macklis JD. Multipotent neural precursors can differentiate toward replacement of neurons undergoing targeted apoptotic degeneration in adult mouse neocortex. PNAS. 1997;94(21):11663–11668. doi: 10.1073/pnas.94.21.11663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville RL, Yanofsky C. On the translation of the A gene region of tryptophan messenger RNA. Journal of Molecular Biology. 1964;8:616–619. doi: 10.1016/s0022-2836(64)80019-x. [DOI] [PubMed] [Google Scholar]
- Srinivasan RS, Dillard ME, Lagutin OV, Lin FJ, Tsai S, Tsai MJ, et al. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes and Development. 2007;21(19):2422–2432. doi: 10.1101/gad.1588407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PL, Fuller SD, Burnett RM. Difference imaging of adenovirus: bridging the resolution gap between X-ray crystallography and electron microscopy. EMBO J. 1993;12(7):2589–2599. doi: 10.1002/j.1460-2075.1993.tb05919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JS, Cotanche DA. Identification of the timing of S phase and the patterns of cell proliferation during hair cell regeneration in the chick cochlea. Journal of Comparitive Neurology. 1994;341:50–67. doi: 10.1002/cne.903410106. [DOI] [PubMed] [Google Scholar]
- Stone JS, Cotanche DA. Hair cell regeneration in the avian auditory epithelium. Int J Dev Biol. 2007;51(6-7):633–647. doi: 10.1387/ijdb.072408js. [DOI] [PubMed] [Google Scholar]
- Stone JS, Rubel EW. Cellular studies of auditory hair cell regeneration in birds. Proc Natl Acad Sci U S A. 2000;97(22):11714–11721. doi: 10.1073/pnas.97.22.11714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit A. The preplacodal region: an ectodermal domain with multipotential progenitors that contribute to sense organs and cranial sensory ganglia. Int J Dev Biol. 2007;51(6-7):447–461. doi: 10.1387/ijdb.072327as. [DOI] [PubMed] [Google Scholar]
- Tan BT, Lee MM, Ruan R. Bone-marrow-derived cells that home to acoustic deafened cochlea preserved their hematopoietic identity. J Comp Neurol. 2008;509(2):167–179. doi: 10.1002/cne.21729. [DOI] [PubMed] [Google Scholar]
- Tateya I, Nakagawa T, Iguchi F, Kim TS, Endo T, Yamada S, et al. Fate of neural stem cells grafted into injured inner ears of mice. Neuroreport. 2003;14(13):1677–1681. doi: 10.1097/00001756-200309150-00004. [DOI] [PubMed] [Google Scholar]
- Teng YD, Lavik EB, Qu X, Park KI, Ourednik J, Zurakowski D, et al. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc Natl Acad Sci USA. 2002;99(5):3024–3029. doi: 10.1073/pnas.052678899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen N, Breuskin I, Malgrange B, Thiry M. Early identification of inner pillar cells during rat cochlear development. Cell and Tissue Research. 2009;337(1):1–14. doi: 10.1007/s00441-009-0810-1. [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Yi E, Gale JE, Glowatzki E, Bergles DE. The origin of spontaneous activity in the developing auditory system. Nature. 2007;450(7166):50–55. doi: 10.1038/nature06233. [DOI] [PubMed] [Google Scholar]
- Turner CW, Reiss LA, Gantz BJ. Combined acoustic and electric hearing: preserving residual acoustic hearing. Hear Res. 2008;242(1-2):164–171. doi: 10.1016/j.heares.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uziel AS, Sillon M, Vieu A, Artieres F, Piron J-P, Daures J-P, et al. Ten-Year Follow-Up of a Consecutive Series of Children With Multichannel Cochlear Implants. Otology & Neurotology. 2007;28(5):615–628. doi: 10.1097/01.mao.0000281802.59444.02. [DOI] [PubMed] [Google Scholar]
- Vuorialho A, Karinen P, Sorri M. Effect of hearing aids on hearing disability and quality of life in the elderly. Int J Audiol. 2006;45(7):400–405. doi: 10.1080/14992020600625007. [DOI] [PubMed] [Google Scholar]
- Waltzman SB, Cohen NL, Shapiro WH. Sensory aids in conjunction with cochlear implants. Am J Otol. 1992;13(4):308–312. [PubMed] [Google Scholar]
- Wang Y, Hirose K, Liberman MC. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J Assoc Res Otolaryngol. 2002;3(3):248–268. doi: 10.1007/s101620020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warchol ME, Lambert PR, Goldstein BJ, Forge A, Corwin JT. Regenerative proliferation in inner ear sensory epithelia from adult guinea pigs and humans. Science. 1993;259(5101):1619–1622. doi: 10.1126/science.8456285. [DOI] [PubMed] [Google Scholar]
- Wei D, Levic S, Nie L, Gao WQ, Petit C, Jones EG, et al. Cells of adult brain germinal zone have properties akin to hair cells and can be used to replace inner ear sensory cells after damage. Proc Natl Acad Sci U S A. 2008;105(52):21000–21005. doi: 10.1073/pnas.0808044105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman IL. Stem Cells: Units of Development, Units of Regeneration, and Units in Evolution. Cell. 2000;100(1):157–168. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
- Weissman IL, Anderson DJ, Gage F. Stem and Progenitor cells: Origins, phenotypes, lineage commitments, and transdifferentiations. Annu. Rev. Cell Dev. Biol. 2001;17:387–403. doi: 10.1146/annurev.cellbio.17.1.387. [DOI] [PubMed] [Google Scholar]
- White PM, Doetzlhofer A, Lee YS, Groves AK, Segil N. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature. 2006;441(7096):984–987. doi: 10.1038/nature04849. [DOI] [PubMed] [Google Scholar]
- Whitfield TT, Hammond KL. Axial patterning in the developing vertebrate inner ear. Int J Dev Biol. 2007;51(6-7):507–520. doi: 10.1387/ijdb.072380tw. [DOI] [PubMed] [Google Scholar]
- Wilhelm JM, Johnson G, Haselkorn R, Geiduschek EP. Specific inhibition of bacteriophage SPO1 DNA-directed protein synthesis by the SPO1 transcription factor, TF 1. Biochemical and Biophysical Research Communications. 1972;46(5):1970–1977. doi: 10.1016/0006-291x(72)90078-2. [DOI] [PubMed] [Google Scholar]
- Wilson BS, Dorman MF. Cochlear implants: a remarkable past and a brilliant future. Hear Res. 2008a;242(1-2):3–21. doi: 10.1016/j.heares.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BS, Dorman MF. Cochlear implants: Current designs and future possibilities. Journal of Rehabilitation Research & Development. 2008b;45(5):695–730. doi: 10.1682/jrrd.2007.10.0173. [DOI] [PubMed] [Google Scholar]
- Wilson BS, Lawson DT, Muller JM, Tyler RS, Kiefer J. COCHLEAR IMPLANTS: Some Likely Next Steps. Annual Review of Biomedical Engineering. 2003;5(1):207–249. doi: 10.1146/annurev.bioeng.5.040202.121645. [DOI] [PubMed] [Google Scholar]
- Wilson DR. Viral-mediated gene transfer for cancer treatment. Curr Pharm Biotechnol. 2002;3(2):151–164. doi: 10.2174/1389201023378445. [DOI] [PubMed] [Google Scholar]
- Wislocki GB, Ladman AJ. Selective and histochemical staining of the otolithic membranes, cupulae and tectorial membrane of the inner ear. J Anat. 1955;89(1):3–12. [PMC free article] [PubMed] [Google Scholar]
- Woods C, Montcouquiol M, Kelley MW. Math1 regulates development of the sensory epithelium in the mammalian cochlea. Nat Neurosci. 2004;7(12):1310–1318. doi: 10.1038/nn1349. [DOI] [PubMed] [Google Scholar]
- Yagi M, Kanzaki S, Kawamoto K, Shin B, Shah PP, Magal E, et al. Spiral ganglion neurons are protected from degeneration by GDNF gene therapy. J Assoc Res Otolaryngol. 2000;1(4):315–325. doi: 10.1007/s101620010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LS, Searle PF, Onion D, Mautner V. Viral gene therapy strategies: from basic science to clinical application. J Pathol. 2006;208(2):299–318. doi: 10.1002/path.1896. [DOI] [PubMed] [Google Scholar]
- Zhai S, Shi L, Wang BE, Zheng G, Song W, Hu Y, et al. Isolation and culture of hair cell progenitors from postnatal rat cochleae. J Neurobiol. 2005;65(3):282–293. doi: 10.1002/neu.20190. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhai S.-q., Shou J, Song W, Sun J.-h., Guo W, et al. Isolation, growth and differentiation of hair cell progenitors from the newborn rat cochlear greater epithelial ridge. Journal of Neuroscience Methods. 2007;164(2):271–279. doi: 10.1016/j.jneumeth.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nature Neuroscience. 2000;3(6):580–586. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- Zheng JL, Lewis AK, Gao WQ. Establishment of conditionally immortalized rat utricular epithelial cell lines using a retrovirus-mediated gene transfer technique. Hear Res. 1998;117(1-2):13–23. doi: 10.1016/s0378-5955(97)00205-0. [DOI] [PubMed] [Google Scholar]
- Zine A, Van De Water TR, de Ribaupierre F. Notch signaling regulates the pattern of auditory hair cell differentiation in mammals. Development. 2000;127(15):3373–3383. doi: 10.1242/dev.127.15.3373. [DOI] [PubMed] [Google Scholar]