Abstract
We investigated sirolimus and mycophenolate mofetil (MMF) as graft-versus-host disease (GVHD) prophylaxis in patients with advanced hematologic malignancies receiving myeloablative hematopoietic cell transplantation (HCT) from HLA-identical sibling donors. Based on pre-study stopping rules, the trial was closed to accrual after enrollment of 11 adult patients. Seven of the 11 patients received busulfan-containing preparative regimens. Sirolimus was discontinued in 3 patients due to toxicity-related events of severe sinusoidal obstructive syndrome, portal vein thrombosis, altered mental status and in 1 patient due to risk of poor wound healing. Six of the 11 patients developed grade II-IV acute GVHD (AGVHD) a median of 15.5 days post-HCT. Two of 3 patients with grade IV AGVHD had sirolimus discontinued by 9 days post-HCT. All patients responded to AGVHD therapy without GVHD-related deaths. There were 2 nonrelapse- and 2 relapse-related deaths. At a median follow-up of 38 months (2–47 months), 7 of 11 patients were alive without disease. MMF and sirolimus GVHD prophylaxis did not reduce the risk of acute GVHD, however, there were no GVHD-related deaths. The severe toxicities in the patients receiving the busulfan-containing preparative regimens limited the continued use of sirolimus and MMF for the prevention of AGVHD.
Keywords: sirolimus, mycophenolate mofetil, GVHD prophylaxis, T regulatory cell
Introduction
Sirolimus (rapamycin, rapamune) is an agent utilized as an alternative immunosuppressive therapy in solid organ as well as HCT1–3. Sirolimus inhibits cytokine-driven signaling pathways of the T cell via blockade of the mammalian target of rapamycin (mTOR) and specifically inhibits the progression of cells from the G1 phase to the S phase4. In combination with FK506 in the HCT setting, sirolimus may reduce the incidence of acute GVHD while decreasing the severity and incidence of mucositis and cytomegalovirus reactivation as compared to methotrexate (MTX)-containing regimens3, 5, 6. Unfortunately, these encouraging results have been diminished by significant risks of renal failure, thrombotic microangiopathy (TMA) and sinusoidal obstructive syndrome (SOS) observed with the sirolimus and calcineurin inhibitor (CNI) combination in GVHD prophylactic, acute and chronic GVHD treatment trials2, 7–10.
Mycophenolate mofetil is a prodrug of mycophenolic acid (MPA), an inhibitor of inosine-5'-monophosphate dehydrogenase, the enzyme controlling the rate-limiting step in de novo purine nucleotide synthesis in T and B lymphocytes11. As with the use of sirolimus, MMF has been incorporated into the solid organ transplant immunosuppressive regimens with the goals of maintaining and/or improving graft and patient survival while minimizing or eliminating the use of CNI's and corticosteroids12–14. MMF has been utilized in HCT as treatment and prevention of acute and chronic GVHD with historical comparisons as well as a randomized trial supporting reduced mucositis and less cytopenias compared to MTX-containing prophylactic regimens and similar acute GVHD incidence15–20.
With the goals of reducing the incidence and severity of acute GVHD and minimizing the toxicities seen with CNI-containing GVHD prophylactic regimens, we conducted a phase II trial of GVHD prophylaxis of sirolimus and MMF. In addition, intriguing evidence suggests sirolimus and MMF may conserve the inhibition of T cell proliferation compared to cyclosporine in an acute GVHD murine model as well as reports supporting sirolimus' preservative or augmentative effects on regulatory T (CD4+CD25+FoxP3+; Treg) cells in animals and humans in comparison to cyclosporine21. Hence, our study also included the prospective analysis of the Treg cell populations in the peripheral blood of the patients receiving the sirolimus and MMF GVHD prophylactic regimen.
Materials and Methods
Patient Eligibility
Patients 2–61 years of age with a diagnosis of high risk hematologic malignancy were eligible, however, at the closure of this trial, there were no pediatric patients enrolled. The diagnoses included AML and ALL beyond first complete remission (CR) or with relapsed or refractory disease; patients age 51–60 in ≥ CR1 or with relapsed or refractory disease; patients age 2–61 years with MDS World Health Organization (WHO)-1, WHO-2 or secondary MDS; NHL ≥ CR1 receiving a myeloablative HCT with an identified 6/6 HLA-A, B and DRB1 matched related donor (MRD) were eligible for this phase II clinical trial. Patients were required to have a Karnofsky Performance Status (KPS) of ≥ 70%, no evidence of uncontrolled infections, adequate organ function, fasting cholesterol ≤ 300 mg/dl and triglycerides ≤ 300 mg/dl while on lipid lowering agents and no prior myeloablative HCT. Pregnant or HIV positive patients were not eligible. The study protocol was approved by the Institutional Review Board at Stanford University and all patients gave written informed consent.
A control group of fifteen adult HCT recipients of myeloablative, MRD peripheral blood progenitor cell transplantation was chosen based on receiving non-sirolimus containing GVHD prophylaxis. These control patients underwent HCT via IRB-approved clinical trials after written informed consent.
Treatment Regimen
Eligible patients received one of three myeloablative preparative regimens based on their age and hematologic malignancy: 1) Patients age 18–60 years with lymphoma: BCNU, 15 mg/kg (maximum dose 550 mg/m2 actual body weight) on day −6, etoposide, 60 mg/kg on day −4 and cyclophosphamide, 100 mg/kg on day −2;22 2) Patients age 18–50 years with AML, ALL or CML: total body irradiation 1320 centigray (cGy) delivered in eleven 120 cGy fractions over four days on days −8 thru −5, etoposide 60 mg/kg on day −4, cyclophosphamide 60 mg/kg on day −2;23 3) Patients age 51–60 years with MDS, AML or ALL or patients age 18–60 with MDS, secondary AML or non-CML myeloproliferative disease received intravenous (IV) busulfan 1 mg/kg every 6 hours × 14 doses on days −9 thru −6 with target concentration at steady state of ≥ 800 ng/ml based on first dose pharmacokinetics, etoposide 60 mg/kg on day −5, cyclophosphamide 45 mg/kg/day × 2 days on day −3 and day −224.
Immunosuppression
Sirolimus was begun on day −3 with a 12 mg oral loading dose followed by 4 mg/day orally for adults. Sirolimus doses were adjusted to achieve the target serum trough level of 3–12 ng/ml with tablet or liquid formulations of the drug. MMF was begun IV on day 0 at 15 mg/kg (based on actual body weight, unless ≥ 15 kg above ideal body weight, then adjusted IBW was used) twice daily ≥ 2 hours after the completion of the donor cell infusion. MMF was changed to oral dosing upon recovery of regimen-related gastrointestinal toxicities. MMF doses were not adjusted based on serum MPA levels. Tapering of sirolimus and MMF began after day 100 post-HCT with the target day of discontinuation of each drug by 6 months post-HCT. In the absence of GVHD or relapse of hematologic disease, sirolimus and MMF were ideally tapered in an alternate fashion every other week until simultaneous discontinuation of each drug.
Hematopoietic Cell Collection and Infusion
HLA compatibility was determined by serologic or molecular methods for HLA Class I antigens and at least low-resolution molecular typing for HLA class II antigens. HLA-matched donors received G-CSF at a dose of 16 mcg/kg/day subcutaneously beginning on day −5 with apheresis begun on day −1. The cell goal was 5 × 106 CD34+ cells/kg of recipient weight with a minimum dose of 2 × 106 CD34+ cells/kg of recipient weight required for the allografting procedure.
Supportive Care
Supportive care was administered by our institutional guidelines as previously described22. Antifungal therapy with fluconazole 400 mg/day was planned as previously described, however, with the marked elevation of sirolimus drug levels soon after fluconazole administration in the first two patients, the routine antifungal prophylaxis was changed to ambisome 5 mg/kg three times a week beginning day +1 until day +75. All patients received ursodeoxycholic 6 mg/kg/day as hepatic sinusoidal obstructive syndrome (SOS) prophylaxis until day +90.
Toxicity and Study Definitions
The diagnosis of TMA was based on the BMT Clinical Trials Network Toxicity Committee Consensus Summary on TMA25. Hyperlipidemia was monitored via fasting lipid panel prior to and at one-month intervals for the first 3 months after transplantation. The diagnosis of SOS was based on the Baltimore Criteria26. The time to white blood cell engraftment was defined by the first of three consecutive days when the absolute neutrophil count (ANC) reached 0.5 × 109/L. Platelet engraftment was defined as the first of seven days of platelets > 20,000/μl without transfusional support. The diagnosis and grading of acute GVHD was based on the consensus criteria27. Chronic GVHD was diagnosed based on the criteria recommended by the National Institute of Health Consensus Conference28. Nonrelapse mortality (NRM) was defined as death due to any cause other than relapse. Disease-free survival (DFS) was determined from day of transplantation (day 0) to date of relapse of disease. Overall survival (OS) was determined from day of transplantation to date of death or last follow-up.
Pharmacokinetics of MMF
MPA area under the curve (AUC) was measured for each patient on Day +2 and Day +21. With IV administration, samples were obtained at predose, 1, 2, 4, 6, 8, 10 and 12 hours after morning dose and with PO administration, samples were obtained at predose, 1, 3, 4, 6 and 8 hours after morning dose. The 8 hour sample on Day +21 was excluded if not feasible to obtain while the patient was an outpatient. The MPA concentrations were carried out via Mayo Clinic laboratories, Rochester, Minnesota. Noncompartmental analysis of the concentration-time data was performed using WinNonlin, as previously described29. The MPA AUC was calculated if 4 or more concentration-time points were above the limit of quantitation, which occurred in 7 of the 11 patients.
Analysis of FoxP3 expressing CD4+CD25+ Treg cells
Analysis of FoxP3 expressing CD4+ cells was carried out on G-CSF mobilized peripheral blood of the donor as well as the peripheral blood of the recipients post-transplant at months 1 and 2, 100 days, months 6, 9, 12 and 24. CD4+ FoxP3+ Treg cell enumeration was carried out by immunofluorescent staining of intracellular FoxP3. Fresh peripheral blood mononuclear cells(PBMC) were prepared by Ficoll-Hypaque density-gradient centrifugation and frozen in Fetal Calf serum + 10% DMSO. PBMC were thawed at 37°C, washed once in RPMI and resuspended in cold staining buffer at a concentration of 1×107 cells/ml. The following antibodies were added to each sample: CD45-FITC, CD4-PE, CD19-PECy5, CD3-PECy7 and CD8 APC-Cy7 (Becton Dickenson, San Jose, CA). The cells were incubated in the dark on ice for 30 minutes and 1 ml of staining buffer was added. The cells were collected by centrifugation and resuspended in 1 ml of cold Fix/Perm buffer, vortexed and incubated at 4 °C for 30–60 minutes in the dark. The fixed cells were collected by centrifugation and resuspended in 1 ml of permeablization buffer. Blocking was performed with 2% normal rat serum in approximately 100 μl permeablization buffer for 15 minutes. 20μl of APC-conjugated FoxP3 antibody or the APC-conjugated rat IgG2a isotype control antibody (eBiosciences, San Deigo, CA) was added and incubated for 30–60 minutes at 4°C in the dark. Cells were washed in permeablization buffer, centrifuged and resuspended in staining buffer for flow cytometric analysis. Stained cells were analyzed on a Becton-Dickinson Influx flow cytometer. The number of FoxP3 expressing CD4+ cells were assessed serially and compared to the absolute number of CD4+ and CD8+ conventional Tcon cells. Both absolute numbers and the ratio of Treg:Tconv were enumerated and analyzed according to GVHD prophylactic protocol administered as well as clinical responses.
Statistical Methods
The study was designed as a phase II trial with the original accrual goal of 38 patients based on the projection that the combination of sirolimus and MMF would reduce the rate of Grade II-IV acute GVHD from the historical expectation of ≥ 40% to ≤ 20%, with a two-sided alpha of 0.1 and 90% power. With the use of the Simon 2-stage design30 the study was planned in two stages with discontinuation of the study if ≥ 5 of the first 11 patients enrolled were diagnosed with grade II-IV acute GVHD. The patient characteristics and outcomes of the study and control groups were statistically analyzed via the Fischer Exact Test for categorical variables and via the Mann Whitney U Test for continuous variables. The statistical analysis of the CD4FoxP3:CD4 ratios of study patients compared to control patients was a test by linear mixed effects (LME, Laird-Ware) model.
Results
Patient Characteristics
Eleven adult patients were enrolled in this clinical protocol from October 2006 through July 2007. All patients had high-risk disease at the time of HCT based on the age, status or type of disease, with a median age of 51 years (range 26–59 years). Eight of eleven patients had persistent evidence of their primary hematologic malignancy at the time of transplant. The study and control patient characteristics and outcomes are summarized in Table 1. The study and control patient groups differed significantly in their median age (p = 0.0017), type of preparative regimen (p = 0.0016) and number of MDS patients (p = 0.007).
Table 1.
Patient Characteristics and Outcomes of the Study (Sirolimus and Mycophenolate mofetil) versus Control (Cyclosporin-containing) Cohorts
| GVHD Prophylaxis | ||
|---|---|---|
| Sirolimus/MMF | CSP-containing | |
|
|
||
| Number of Patients | 11 | 15 |
| Median Age, years* | 51 (26–59) | 27 (20–56) |
| Disease Characteristics | ||
| AML/ALL- CR1/2 | 2 | 7 |
| AML/ALL- Relapsed/Refractory | 1 | 3 |
| CML | 2 | |
| Non-CML Myeloproliferative Disease | 1 | |
| MDS* | 5 | |
| NHL-CR1 | ||
| >CR1 | 3 | 2 |
| Preparative regimen* | ||
| TBI/VP | 7 | |
| BU/CY | 2 | |
| BU/VP/CY | 7 | 2 |
| BCNU/VP/CY | 3 | 3 |
| TBI/VP/CY | 1 | 1 |
| GVHD prophylaxis | ||
| CSP/Methotrexate | 9 | |
| CSP/prednisone | 6 | |
| Sirolimus/MMF | 11 | |
| Median CD34 dose, 106/kg (range) | 7.3 (5.1–15.3) | 6.9 (2.2–20) |
| Median CD3 dose, 108/kg (range) | 2.5 (1.2–4.1) | 2.6 (1.3–4.6) |
| Grade II–IV acute GVHD incidence | 6/11 | 7/15 |
| Onset post HCT (range) | day 15.5 (11–18) | day 23 (9–106) |
| Relapse Rate | 2/11 | 4/15 |
| Median Follow-up, months | 41 (34–47) | 34 (24–49) |
| Overall Survivors | 7/11 | 6/15 |
Statisitically significant difference in characteristic between study and control groups
GVHD – graft versus host disease; MMF- mycophenolate mofetil; CSP – cyclosporine; AML/ALL- acute myelogenous leukemia/acute lymphoblastic leukemia; CR1/2 – first or second complete remission; CML- chronic myelogenous leukemia; MDS – myelodysplasia; NHL – nonhodgkin lymphoma; TBI/VP – total body irradiation/VP16; BU/CY – busulfan/cyclophosphamide; BCNU/VP/CY – carmustine, VP16, cyclophosphamide; HCT – hematopoietic cell transplantation
Engraftment
Median time to ANC > 500/ul was 12 days (range 10–19 days) in eleven patients and median time to platelet engraftment was 16 days (14–66 days) in 10 patients with platelet engraftment not reached at the time of death in one patient.
Toxicity
Table 2 summarizes the study patients' treatment courses and outcomes. Four patients required discontinuation of sirolimus due to presumed sirolimus-related toxicities (study patient number (SPN) 3704 and 3888), risk of poor wound healing (SPN 3717) or inability to continue oral medication due to altered mental status (SPN 3783). Sirolimus was replaced with FK506 in three of the four cases with MMF continued in all cases. All of the patients requiring discontinuation of sirolimus had received a busulfan-containing preparative regimen.
Table 2.
Patient Characteristics and Outcomes for Patients Receiving Sirolimus and Mycophenolate Mofetil Graft-versus-Host Disease Prophylaxis
| Patient | Diagnosis | Age (years) | Regimen | Toxicity | Sirolimus stopped early | Sirolimus level median (range) | AGVHD (Grade, organ) Day of Onset | OS (months) |
|---|---|---|---|---|---|---|---|---|
| 3704 | MDS, RAEB2 | 47 | BU/VP16/CY | Severe SOS | Yes, Day 9 | 9.4 (3.6–>30) | 4, skin D25 | 46.6 |
| 3717 | CMML | 56 | BU/VP16/CY | Colonic ulceration | Yes, Day 39 | 10.6 (3.9–27.3) | None | 1.8 =, sepsis |
| 3737 | NHL-CR2 | 26 | BCNU/VP16/CY | None | No | 10.8 (3.6–27.6) | 2, skin D18 | 43.3 |
| 3783 | AML-CR1 | 57 | BU/VP16/CY | Altered mental status | Yes, Day 9 | 5.5 (2.9–17.5) | 4, gut/liver D24 | 10 =, relapse |
| 3805 | NHL-PR1 | 44 | BCNU/VP16/CY | None | No | 10 (3.5–18.7) | 2, skin D13 | 41.3 |
| 3822 | AML-Refractory | 49 | TBI/VP16/CY | None | No | 11 (3.6–19.5) | 1, skin D15 | 23.4=, 2nd CA |
| 3838 | NHL-PR1 | 42 | BCNU/VP16/CY | None | No | 12 (5–28.6) | 1, skin D10 | 41.6 |
| 3852 | MDS, RAEB2 | 51 | BU/VP16/CY | None | No | 10.8 (4.4–19.9) | None | 3.4 =, relapse |
| 3878 | AML-CR1 | 55 | BU/VP16/CY | None | No | 8 (2.6–13.2) | 2, skin D12 | 38.5 |
| 3888 | MDS, therapy-related | 54 | BU/VP16/CY | Portal vein thrombosis | Yes, Day 61 | 8.25 (3.5–26.6) | None | 34.3 |
| 3889 | MDS,RA | 59 | BU/VP16/CY | None | Yes, Day 39 | 5.75 (3.1–8.6) | 4, skin D11 | 38.2 |
=- Died
Sirolimus level ng/ml; AGVHD- acute graft-versus-host disease; OS- overall survival; NHL- nonhodgkin lymphoma; PR- partial remission; BCNU- carmustine, CY-cyclophosphamide; CR- complete remission; AML-acute myelocytic leukemia; TBI- total body irradiation; RAEB-refractory anemia with excess blasts; RA- refractory anemia; CMML-chronic myelomonocytic leukemia;
The median and range of sirolimus levels of each patient are included in Table 2. There was no correlation between maximum or median sirolimus levels and development of the toxicities requiring discontinuation of sirolimus or the diagnosis of acute GVHD. The median sirolimus level on day 0 was 6.2 ng/ml (range < 2.5 – 27.3) in all patients. The two patients requiring discontinuation of sirolimus due to liver toxicity had levels on day 0 of 24.1 ng/ml and < 2.5 ng/ml, respectively. There were no cases of TMA identified. Maximum triglyceride levels post-HCT ranged from 99–621 mg/dl, responsive to lipid-lowering agents when appropriate.
The median MPA AUC of all patients was 12.8 mcg*hr/mL (range: 6.4–17.4) at day +2 and 17.9 mcg*hr/mL at day +21 (range 8.0–26.6). The median MPA-glucuronide AUC was 324.8 mcg*hr/mL (range: 176–463.7) at day +2 and 553.6 mcg*hr/mL (range: 72–1311) at day +21.
Graft versus Host Disease
Six of the eleven patients developed grade II-IV acute GVHD at a median onset of 15.5 days (range 11–18 days) post-HCT. The fifth case of grade ≥ 2 acute GVHD (SPN 3878) occurred after the tenth (SPN 3888) and eleventh (SPN 3889) patients had begun their preparative regimens with the eleventh patient subsequently developing grade 4 acute GVHD. All patients received solumedrol at 2 mg/kg/day as primary acute GVHD therapy with three patients receiving extracorporeal photopheresis (ECP) as second- or third-line acute GVHD therapy. Two of the patients (SPN 3822 and 3838) that developed grade I acute GVHD of the skin responded to a less than one week course of corticosteroid therapy. Two of the three patients with grade IV acute GVHD (SPN 3704 and 3783) required sirolimus discontinuation by day 9 post-HCT due to severe SOS and progressive altered mental status, respectively. In both cases, sirolimus was replaced by FK506 with subsequent development of acute GVHD on days 25 and 24 post-HCT, respectively. The third patient (SPN 3889) was switched to FK506 from sirolimus after the onset of grade IV acute GVHD, as a second-line treatment for acute GVHD without evidence of sirolimus toxicity. All patients with acute GVHD had partial or complete responses to GVHD therapy without GVHD-related deaths. Eight of the nine patients surviving beyond 100 days developed chronic GVHD a median of 224 days (range 135–668 days) post-HCT, two with extensive and six with limited chronic GVHD. Seven of the eight patients with chronic GVHD remain alive at a median follow-up of 40 months (range 10–46.6 months) with one dying of AML relapse 304 days post-HCT. The median KPS of the surviving patients was 90% (range 80–100%) at last follow-up.
Analysis of FoxP3 expressing CD4+ T cells
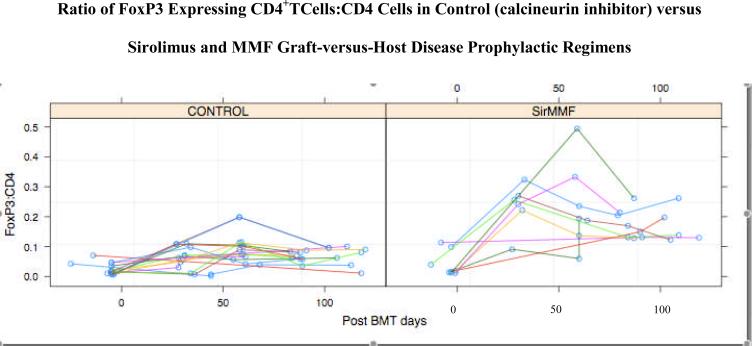
FoxP3+ cell populations in the peripheral blood (PB) were analyzed in the G-CSF mobilized graft prior to HCT and after HCT in nine of the eleven study patients receiving sirolimus and MMF as well as in the fifteen control patients receiving cyclosporin-containing GVHD prophylaxis. There was a highly statistically significant increase in the ratio of CD4+FoxP3+ Treg cells to CD4+Foxp3− conventional T cells between the MMF/sirolimus study patients and the CNI-containing GVHD prophylaxis control patients (p< 0.0001; Figure 1). A test by linear mixed effects (LME, Laird-Ware) model yielded a highly significant divergence in average within-patient slope over time between the two GVHD prophylaxis groups. By 50 days post-HCT the divergence was 7.5% with a standard error of 1%. The linear mixed effects analysis corrected for within-subject correlation by including a random intercept for patients. A more conservative analysis was based on taking a summary of the trajectory for each patient and comparing those summaries by two-group t-test. Analyses of both the within patient maximum and mean were consistent with the LME analysis, and yielded statistically significant differences. The incidences of acute GVHD for the study and control patients are summarized in Table 1. The absolute number of CD4+FoxP3+ T cells was also greater in the sirolimus/MMF patients compared with the control patients, median of 40/ul (2–317) and 14/ul (range 0.4–53), respectively. In the sirolimus/MMF group, the absolute number of CD4+FoxP3+ T cells was greater in the patients without grade 2–4 acute GVHD (54, range 11–317) compared to the patients with grade 2–4 acute GVHD (28.5, range 2–58) however, this difference was not seen in the control group patients nor when both patient groups were combined. There was no clear relationship between the ratios of CD4+FoxP3+ T cells to CD4+Foxp3− conventional T cells and the development of GVHD in either cohort of patients.
Figure 1.
Analyses of FoxP3 expressing CD4+ T cells on the peripheral blood of ten study (sirolimus and mycophenolate mofetil) graft-versus-host disease (GVHD) prophylaxis patients and 15 control (calcineurin inhibitor-containing) GVHD prophylaxis patients at time points post-transplant. The pretransplant analyses are on the GCSF-mobilized peripheral blood of the patient donors.
Survival
Seven of the eleven study patients remain alive (Table 2). Four patients have died, two due to relapse at 104 and 304 days post-HCT, one due to sepsis associated with colonic perforation 54 days post-HCT and one due to uterine leiomyosarcoma 713 days post-HCT, diagnosed approximately 530 days post HCT. The median DFS and OS of all living patients is 41 months (34–47 months). The survival data of the study and control patients are summarized in Table 1.
Discussion
Sirolimus and MMF is a novel GVHD prophylactic regimen with a compilation of toxicities projected to be less limiting than CNIs or methotrexate. This prophylactic regimen also has the possible benefits of maintaining or increasing the FoxP3 expressing regulatory T cell population compared with CNIs31. The use of sirolimus and MMF as GVHD prophylaxis in this pilot trial of myeloablative HCT patients was associated with acute GVHD in six of the eleven patients enrolled. Although there was not a reduced incidence of acute GVHD compared to expectations with standard GVHD prophylactic regimens, remarkably, seven of these 11 high risk patients remain alive and relapse-free at a median follow-up of over 3.5 years.
The expected toxicities associated with sirolimus from the non-HCT experience include hyperlipidemias, mild cytopenias, interstitial pneumonitis and poor wound healing32,33, 34. Renal insufficiency has not been associated with the use of sirolimus alone, however, there is evidence sirolimus potentiates the nephrotoxicity of CSP and FK50635,36–40. In fact, a notable incidence of renal insufficiency and TMA with the combination of sirolimus and CNIs has been reported in the setting of prophylaxis and treatment of steroid refractory acute and chronic GVHD2, 7–9, 41. The more recent use of sirolimus and FK506 as GVHD prophylaxis in myeloablative HCT has shown a low incidence of grade II-IV acute GVHD of 20.5% with minimal need for drug removal due to toxicities3. These investigators, however, reported an increased risk of TMA with sirolimus and FK506 when compared historically to FK506 and MTX8. In addition there was an increased risk of SOS, most marked with the combination of sirolimus and busulfan-containing myeloablative preparative regimens10, similar to our experience.
In our current study, sirolimus was discontinued after transplantation in four patients, three due to toxicities including severe SOS, hepatic vein thrombosis and unexplained altered mental status. There was no evidence of TMA and only one episode of reversible acute renal failure in association with severe SOS, supporting the expectation of minimal risk of TMA or renal toxicity when sirolimus is administered without a CNI. Of interest, severe acute GVHD occurred in two of four patients after early discontinuation of sirolimus, likely raising their risk of developing acute GVHD. Based on our current experience of severe toxicities leading to sirolimus removal only in the patients receiving the busulfan-containing preparative regimen and the recent evidence from other investigators reporting an increased risk of SOS with sirolimus or everolimus and busulfan combinations10, 42, the use of sirolimus in conjunction with busulfan should be contraindicated. Supratherapeutic sirolimus levels were not clearly predictive of toxicity in our patient cohort, however, close monitoring of sirolimus levels are likely important in preventing sirolimus-associated toxicities as well.
Sirolimus and less so MMF have been shown to augment or maintain regulatory T cell populations in animal models and human studies43, 44. In our study, the Treg:CD4 ratios in the GCSF mobilized peripheral blood of the donors and peripheral blood of the control group patients were similar to what has been previously reported from unmobilized peripheral blood of donors and post-HCT patients (0–10%)47, 48. There was a statistically significant increase in the Treg:CD4 ratio in the peripheral blood of patients receiving sirolimus-MMF versus CSP-containing GVHD prophylactic regimens. Of note, the study and control groups, chosen based on their GVHD prophylaxis regimens, differed significantly in their median age, number of patients with MDS and the preparative regimens. Hence, one cannot definitively state that the GVHD prophylactic regimen was the only factor effecting the difference in the Treg:CD4+ ratios between the patient groups. The Treg:CD4+ ratios between and within each patient group did not clearly correlate with the development or severity of acute GVHD, perhaps explained by the limited number of patients available for analysis and/or the differences in the patient characteristics. It is possible, monitoring the Treg:CD4+ ratios more frequently than monthly would have allowed a more ideal opportunity for intrapatient comparison, i.e. before and after onset of acute GVHD, or during acute GVHD treatment and response. Interestingly, patients requiring sirolimus removal had persistently increased Treg:CD4+ ratios, however, these patients were also receiving ECP as acute GVHD therapy. There are now multiple reports in animal and human settings of ECP affecting the T regulatory cell population in the peripheral blood45, 46, hence the use of ECP may have impacted the Treg:CD4+ ratio in our patients.
The MMF pharmacokinetics of our study revealed low AUC's of the active metabolite of MMF, MPA, compared to reports in the HCT literature19, 49, 50. Although there is no clear correlation between MPA AUC or MPA trough levels and efficacy, these low levels may have contributed to the moderate incidence of acute GVHD in this trial. There were no definitive MMF-associated toxicities appreciated in this trial, although the one case of cecal ulceration and perforation is a reported toxicity of MMF described in the solid organ transplantation literature51. Of note, the combination of sirolimus and MMF did not appear to delay ANC recovery in this trial. Given the low MPA levels and lack of definitive MMF toxicity in this preliminary trial, it may be beneficial to incorporate a higher daily dose of MMF via an every 8 hour regimen.
Offering the patient with high risk hematologic malignancy a curative therapy is severely limited by the risk of relapse of disease, regimen- and GVHD-related morbidity and mortality. Despite our preliminary experience showing lack of reduction of acute GVHD and regimen-related toxicity of MMF and sirolimus with the preparative regimens utilized, there remained remarkably low NRM and seven of eleven patients were disease-free at a median follow-up of over 3.5 years. Further study of the MMF and sirolimus combination could be pursued with the use of increased dosing of MMF but with the need to avoid busulfan-containing preparative regimens. Continuing the prospective monitoring of Treg:CD4+ ratios in GVHD studies are necessary to further understand the T regulatory cell's impact on GVHD.
Acknowledgments
Grant Support: NIH Program Project Grant P01CA49605; Grant from the Doris Duke Charitable Foundation
Footnotes
The authors have declared there is no conflict of interest to disclose.
References
- 1.Kahan BD, Camardo JS. Rapamycin: clinical results and future opportunities. Transplantation. 2001;72(7):1181–93. doi: 10.1097/00007890-200110150-00001. [DOI] [PubMed] [Google Scholar]
- 2.Benito AI, Furlong T, Martin PJ, Anasetti C, Appelbaum FR, Doney K, et al. Sirolimus (rapamycin) for the treatment of steroid-refractory acute graft-versus-host disease. Transplantation. 2001;72(12):1924–9. doi: 10.1097/00007890-200112270-00010. [DOI] [PubMed] [Google Scholar]
- 3.Cutler C, Li S, Ho VT, Koreth J, Alyea E, Soiffer RJ, et al. Extended follow-up of methotrexate-free immunosuppression using sirolimus and tacrolimus in related and unrelated donor peripheral blood stem cell transplantation. Blood. 2007;109(7):3108–14. doi: 10.1182/blood-2006-09-046219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chao NJ. Pharmacology and use of immunosuppressive agents after hematopoietic cell transplantation. In: Thomas E, Blume K, Forman S, editors. Hematopoietic Cell Transplantation. 2nd edn. Blackwell; Malden, MA: 1999. pp. 176–185. [Google Scholar]
- 5.Cutler C, Li S, Kim HT, Laglenne P, Szeto KC, Hoffmeister L, et al. Mucositis after allogeneic hematopoietic stem cell transplantation: a cohort study of methotrexate- and non-methotrexate-containing graft-versus-host disease prophylaxis regimens. Biol Blood Marrow Transplant. 2005;11(5):383–8. doi: 10.1016/j.bbmt.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Marty FM, Bryar J, Browne SK, Schwarzberg T, Ho VT, Bassett IV, et al. Sirolimus-based graft-versus-host disease prophylaxis protects against cytomegalovirus reactivation after allogeneic hematopoietic stem cell transplantation: a cohort analysis. Blood. 2007;110(2):490–500. doi: 10.1182/blood-2007-01-069294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Couriel DR, Saliba R, Escalon MP, Hsu Y, Ghosh S, Ippoliti C, et al. Sirolimus in combination with tacrolimus and corticosteroids for the treatment of resistant chronic graft-versus-host disease. Br J Haematol. 2005;130(3):409–17. doi: 10.1111/j.1365-2141.2005.05616.x. [DOI] [PubMed] [Google Scholar]
- 8.Cutler C, Henry NL, Magee C, Li S, Kim HT, Alyea E, et al. Sirolimus and thrombotic microangiopathy after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11(7):551–7. doi: 10.1016/j.bbmt.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Johnston LJ, Brown J, Shizuru JA, Stockerl-Goldstein KE, Stuart MJ, Blume KG, et al. Rapamycin (sirolimus) for treatment of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2005;11(1):47–55. doi: 10.1016/j.bbmt.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Cutler C, Stevenson K, Kim HT, Richardson P, Ho VT, Linden E, et al. Sirolimus is associated with veno-occlusive disease of the liver after myeloablative allogeneic stem cell transplantation. Blood. 2008;112(12):4425–31. doi: 10.1182/blood-2008-07-169342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allison AC, Eugui EM. Mechanisms of action of mycophenolate mofetil in preventing acute and chronic allograft rejection. Transplantation. 2005;80(2 Suppl):S181–90. doi: 10.1097/01.tp.0000186390.10150.66. [DOI] [PubMed] [Google Scholar]
- 12.Bestard O, Cruzado JM, Grinyo JM. Calcineurin-inhibitor-sparing immunosuppressive protocols. Transplant Proc. 2005;37(9):3729–32. doi: 10.1016/j.transproceed.2005.09.129. [DOI] [PubMed] [Google Scholar]
- 13.Ciancio G, Miller J, Gonwa TA. Review of major clinical trials with mycophenolate mofetil in renal transplantation. Transplantation. 2005;80(2 Suppl):S191–200. doi: 10.1097/01.tp.0000187035.22298.ba. [DOI] [PubMed] [Google Scholar]
- 14.Srinivas TR, Kaplan B, Schold JD, Meier-Kriesche HU. The impact of mycophenolate mofetil on long-term outcomes in kidney transplantation. Transplantation. 2005;80(2 Suppl):S211–20. doi: 10.1097/01.tp.0000186379.15301.e5. [DOI] [PubMed] [Google Scholar]
- 15.Basara N, Blau WI, Romer E, Rudolphi M, Bischoff M, Kirsten D, et al. Mycophenolate mofetil for the treatment of acute and chronic GVHD in bone marrow transplant patients. Bone Marrow Transplant. 1998;22(1):61–5. doi: 10.1038/sj.bmt.1701281. [DOI] [PubMed] [Google Scholar]
- 16.Bornhauser M, Schuler U, Porksen G, Naumann R, Geissler G, Thiede C, et al. Mycophenolate mofetil and cyclosporine as graft-versus-host disease prophylaxis after allogeneic blood stem cell transplantation. Transplantation. 1999;67(4):499–504. doi: 10.1097/00007890-199902270-00001. [DOI] [PubMed] [Google Scholar]
- 17.Kiehl MG, Schafer-Eckart K, Kroger M, Bornhauser M, Basara N, Blau IW, et al. Mycophenolate mofetil for the prophylaxis of acute graft-versus-host disease in stem cell transplant recipients. Transplant Proc. 2002;34(7):2922–4. doi: 10.1016/s0041-1345(02)03489-9. [DOI] [PubMed] [Google Scholar]
- 18.Neumann F, Graef T, Tapprich C, Vaupel M, Steidl U, Germing U, et al. Cyclosporine A and mycophenolate mofetil vs cyclosporine A and methotrexate for graft-versus-host disease prophylaxis after stem cell transplantation from HLA-identical siblings. Bone Marrow Transplant. 2005;35(11):1089–93. doi: 10.1038/sj.bmt.1704956. [DOI] [PubMed] [Google Scholar]
- 19.Nash RA, Johnston L, Parker P, McCune JS, Storer B, Slattery JT, et al. A phase I/II study of mycophenolate mofetil in combination with cyclosporine for prophylaxis of acute graft-versus-host disease after myeloablative conditioning and allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2005;11(7):495–505. doi: 10.1016/j.bbmt.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Bolwell B, Sobecks R, Pohlman B, Andresen S, Rybicki L, Kuczkowski E, et al. A prospective randomized trial comparing cyclosporine and short course methotrexate with cyclosporine and mycophenolate mofetil for GVHD prophylaxis in myeloablative allogeneic bone marrow transplantation. Bone Marrow Transplant. 2004;34(7):621–5. doi: 10.1038/sj.bmt.1704647. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen VH, Zeiser R, Negrin RS. Role of naturally arising regulatory T cells in hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2006;12(10):995–1009. doi: 10.1016/j.bbmt.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Law LY, Horning SJ, Wong RM, Johnston LJ, Laport GG, Lowsky R, et al. High-dose carmustine, etoposide, and cyclophosphamide followed by allogeneic hematopoietic cell transplantation for non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2006;12(7):703–11. doi: 10.1016/j.bbmt.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Long GD, Amylon MD, Stockerl-Goldstein KE, Negrin RS, Chao NJ, Hu WW, et al. Fractionated total-body irradiation, etoposide, and cyclophosphamide followed by allogeneic bone marrow transplantation for patients with high-risk or advanced-stage hematological malignancies. Biol Blood Marrow Transplant. 1997;3(6):324–30. [PubMed] [Google Scholar]
- 24.Naik S, Wong R, Arai S, Brown J, Laport G, Lowsky R, et al. Long-term outcomes in patients with high-risk myeloid malignancies following matched related donor hematopoietic cell transplantation with myeloablative conditioning of BU, etoposide and CY. Bone Marrow Transplant. 2010 doi: 10.1038/bmt.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho VT, Cutler C, Carter S, Martin P, Adams R, Horowitz M, et al. Blood and marrow transplant clinical trials network toxicity committee consensus summary: thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11(8):571–5. doi: 10.1016/j.bbmt.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Jones RJ, Lee KS, Beschorner WE, Vogel VG, Grochow LB, Braine HG, et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. 1987;44(6):778–83. doi: 10.1097/00007890-198712000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–8. [PubMed] [Google Scholar]
- 28.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–56. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Giaccone L, McCune JS, Maris MB, Gooley TA, Sandmaier BM, Slattery JT, et al. Pharmacodynamics of mycophenolate mofetil after nonmyeloablative conditioning and unrelated donor hematopoietic cell transplantation. Blood. 2005;106(13):4381–8. doi: 10.1182/blood-2005-06-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10(1):1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 31.Zeiser R, Negrin RS. Interleukin-2 receptor downstream events in regulatory T cells: implications for the choice of immunosuppressive drug therapy. Cell Cycle. 2008;7(4):458–62. doi: 10.4161/cc.7.4.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kahan BD, Podbielski J, Napoli KL, Katz SM, Meier-Kriesche HU, Van Buren CT. Immunosuppressive effects and safety of a sirolimus/cyclosporine combination regimen for renal transplantation. Transplantation. 1998;66(8):1040–6. doi: 10.1097/00007890-199810270-00013. [DOI] [PubMed] [Google Scholar]
- 33.Kuppahally S, Al-Khaldi A, Weisshaar D, Valantine HA, Oyer P, Robbins RC, et al. Wound healing complications with de novo sirolimus versus mycophenolate mofetil-based regimen in cardiac transplant recipients. Am J Transplant. 2006;6(5 Pt 1):986–92. doi: 10.1111/j.1600-6143.2006.01282.x. [DOI] [PubMed] [Google Scholar]
- 34.Champion L, Stern M, Israel-Biet D, Mamzer-Bruneel MF, Peraldi MN, Kreis H, et al. Brief communication: sirolimus-associated pneumonitis: 24 cases in renal transplant recipients. Ann Intern Med. 2006;144(7):505–9. doi: 10.7326/0003-4819-144-7-200604040-00009. [DOI] [PubMed] [Google Scholar]
- 35.Kahan BD. Sirolimus: a comprehensive review. Expert Opin Pharmacother. 2001;2(11):1903–17. doi: 10.1517/14656566.2.11.1903. [DOI] [PubMed] [Google Scholar]
- 36.Groth CG, Backman L, Morales JM, Calne R, Kreis H, Lang P, et al. Sirolimus (rapamycin)-based therapy in human renal transplantation: similar efficacy and different toxicity compared with cyclosporine. Sirolimus European Renal Transplant Study Group. Transplantation. 1999;67(7):1036–42. doi: 10.1097/00007890-199904150-00017. [DOI] [PubMed] [Google Scholar]
- 37.Kreis H, Cisterne JM, Land W, Wramner L, Squifflet JP, Abramowicz D, et al. Sirolimus in association with mycophenolate mofetil induction for the prevention of acute graft rejection in renal allograft recipients. Transplantation. 2000;69(7):1252–60. doi: 10.1097/00007890-200004150-00009. [DOI] [PubMed] [Google Scholar]
- 38.Kahan BD. Efficacy of sirolimus compared with azathioprine for reduction of acute renal allograft rejection: a randomised multicentre study. The Rapamune US Study Group. Lancet. 2000;356(9225):194–202. doi: 10.1016/s0140-6736(00)02480-6. [DOI] [PubMed] [Google Scholar]
- 39.MacDonald AS. A worldwide, phase III, randomized, controlled, safety and efficacy study of a sirolimus/cyclosporine regimen for prevention of acute rejection in recipients of primary mismatched renal allografts. Transplantation. 2001;71(2):271–80. doi: 10.1097/00007890-200101270-00019. [DOI] [PubMed] [Google Scholar]
- 40.Podder H, Stepkowski SM, Napoli KL, Clark J, Verani RR, Chou TC, et al. Pharmacokinetic interactions augment toxicities of sirolimus/cyclosporine combinations. J Am Soc Nephrol. 2001;12(5):1059–71. doi: 10.1681/ASN.V1251059. [DOI] [PubMed] [Google Scholar]
- 41.Furlong T, Kiem HP, Appelbaum FR, Carpenter PA, Deeg HJ, Doney K, et al. Sirolimus in combination with cyclosporine or tacrolimus plus methotrexate for prevention of graft-versus-host disease following hematopoietic cell transplantation from unrelated donors. Biol Blood Marrow Transplant. 2008;14(5):531–7. doi: 10.1016/j.bbmt.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Platzbecker U, von Bonin M, Goekkurt E, Radke J, Binder M, Kiani A, et al. Graft-versus-host disease prophylaxis with everolimus and tacrolimus is associated with a high incidence of sinusoidal obstruction syndrome and microangiopathy: results of the EVTAC trial. Biol Blood Marrow Transplant. 2009;15(1):101–8. doi: 10.1016/j.bbmt.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 43.Zeiser R, Nguyen VH, Beilhack A, Buess M, Schulz S, Baker J, et al. Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood. 2006;108(1):390–9. doi: 10.1182/blood-2006-01-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strauss L, Czystowska M, Szajnik M, Mandapathil M, Whiteside TL. Differential responses of human regulatory T cells (Treg) and effector T cells to rapamycin. PLoS One. 2009;4(6):e5994. doi: 10.1371/journal.pone.0005994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gatza E, Rogers CE, Clouthier SG, Lowler KP, Tawara I, Liu C, et al. Extracorporeal photopheresis reverses experimental graft-versus-host disease through regulatory T cells. Blood. 2008;112(4):1515–21. doi: 10.1182/blood-2007-11-125542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Biagi E, Di Biaso I, Leoni V, Gaipa G, Rossi V, Bugarin C, et al. Extracorporeal photochemotherapy is accompanied by increasing levels of circulating CD4+CD25+GITR+Foxp3+CD62L+ functional regulatory T-cells in patients with graft-versus-host disease. Transplantation. 2007;84(1):31–9. doi: 10.1097/01.tp.0000267785.52567.9c. [DOI] [PubMed] [Google Scholar]
- 47.Rezvani K, Mielke S, Ahmadzadeh M, Kilical Y, Savani BN, Zeilah J, et al. High donor FOXP3-positive regulatory T-cell (Treg) content is associated with a low risk of GVHD following HLA-matched allogeneic SCT. Blood. 2006;108(4):1291–7. doi: 10.1182/blood-2006-02-003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mielke S, Rezvani K, Savani BN, Nunes R, Yong AS, Schindler J, et al. Reconstitution of FOXP3+ regulatory T cells (Tregs) after CD25-depleted allotransplantation in elderly patients and association with acute graft-versus-host disease. Blood. 2007;110(5):1689–97. doi: 10.1182/blood-2007-03-079160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jacobson P, Rogosheske J, Barker JN, Green K, Ng J, Weisdorf D, et al. Relationship of mycophenolic acid exposure to clinical outcome after hematopoietic cell transplantation. Clin Pharmacol Ther. 2005;78(5):486–500. doi: 10.1016/j.clpt.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 50.Jacobson P, Green K, Rogosheske J, Brunstein C, Ebeling B, DeFor T, et al. Highly variable mycophenolate mofetil bioavailability following nonmyeloablative hematopoietic cell transplantation. J Clin Pharmacol. 2007;47(1):6–12. doi: 10.1177/0091270006295064. [DOI] [PubMed] [Google Scholar]
- 51.Golconda MS, Valente JF, Bejarano P, Gilinsky N, First MR. Mycophenolate mofetil-induced colonic ulceration in renal transplant recipients. Transplant Proc. 1999;31(1–2):272–3. doi: 10.1016/s0041-1345(98)01531-0. [DOI] [PubMed] [Google Scholar]