Abstract
Betalains are pigments that replace anthocyanins in the majority of families of the plant order Caryophyllales. Betalamic acid is the common chromophore of betalains. The key enzyme of the betalain biosynthetic pathway is an extradiol dioxygenase that opens the cyclic ring of dihydroxy-phenylalanine (DOPA) between carbons 4 and 5, thus producing an unstable seco-DOPA that rearranges nonenzymatically to betalamic acid. A gene for a 4,5-DOPA-dioxygenase has already been isolated from the fungus Amanita muscaria, but no homolog was ever found in plants. To identify the plant gene, we constructed subtractive libraries between different colored phenotypes of isogenic lines of Portulaca grandiflora (Portulacaceae) and between different stages of flower bud formation. Using in silico analysis of differentially expressed cDNAs, we identified a candidate showing strong homology at the level of translated protein with the LigB domain present in several bacterial extradiol 4,5-dioxygenases. The gene was expressed only in colored flower petals. The function of this gene in the betalain biosynthetic pathway was confirmed by biolistic genetic complementation in white petals of P. grandiflora genotypes lacking the gene for color formation. This gene named DODA is the first characterized member of a novel family of plant dioxygenases phylogenetically distinct from Amanita sp. DOPA-dioxygenase. Homologs of DODA are present not only in betalain-producing plants but also, albeit with some changes near the catalytic site, in other angiosperms and in the bryophyte Physcomitrella patens. These homologs are part of a novel conserved plant gene family probably involved in aromatic compound metabolism.
Betalains are vacuolar pigments that entirely replace anthocyanins in most plants of the order Caryophyllales, with the exception of Caryophyllaceae and Molluginaceae (for review, see Strack et al., 2003). Betalains are also present in some basidiomycete fungi belonging to the Amanita and Hygrocybe genera. Betalains provide a key taxonomic criterion in our understanding of Caryophyllales evolution (Cuenoud et al., 2002); the origin of the alternative pigment biosynthetic pathway is still unknown (Clement and Mabry, 1996), and the purpose of our work is to provide, through the characterization of a key biosynthetic enzyme, one element of the answer to this problem.
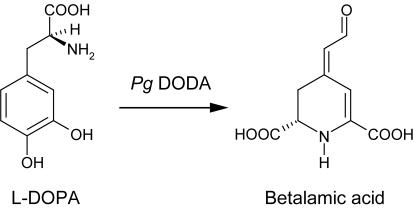
Information on the biochemistry and genetics of the plant betalain pathway is scarce. Genetic analysis of the betalain biosynthetic pathway in the ornamental plant Portulaca grandiflora (Pg) indicates that four structural genes would be sufficient to account for the different color phenotypes observed in petals (Trezzini and Zrÿd, 1990). A tyrosinase (Mueller et al., 1996; Steiner et al., 1999) is needed to convert the amino acid Tyr to DOPA, and a ring-opening extradiol DOPA-4,5-dioxygenase is crucial for the synthesis of the chromophore betalamic acid (see Fig. 1; Girod and Zrÿd, 1991a; Hinz et al., 1997; Schliemann et al., 1998). The formation of cyclo-DOPA is needed for the building of violet betanidin and betacyanins (Kobayashi et al., 2001). Betaxanthins are formed spontaneously (Schliemann et al., 1999) in presence of the required amines (amino acids); genetic analysis indicates, nevertheless, that a gene (with a dominant allele I) selectively inhibits the accumulation of yellow betaxanthins in P. grandiflora (Trezzini and Zrÿd, 1990), leading to a switch from deep yellow to pale yellow flowers.
Figure 1.
Substrate and product of the Pg DODA: l-DOPA (dihydroxy-Phe) is a widespread product of plant secondary metabolism. Betalamic acid is the chromophore of betalains (betacyanins and betaxanthins) and is formed spontaneously from the 4,5-seco-DOPA intermediate resulting from the enzymatic activity (see Fig. 5).
Some enzymes from the betalain biosynthetic pathway in plants have been partly characterized. A glucosyltransferase catalyzing the formation of betacyanins from betanidin has been sequenced (Vogt et al., 1999; Vogt, 2002). A tyrosinase was partially purified from callus cultures from P. grandiflora and red beet (Beta vulgaris [Bv]; Steiner et al., 1996, 1999). More recently, a Tyr hydroxylase activity, catalyzing the formation of DOPA from Tyr, was separated from a polyphenol-oxidase activity catalyzing specifically the oxidation of DOPA to dopaquinone (Yamamoto et al., 2001).
The extradiol ring-cleaving enzyme DOPA-4,5-dioxygenase required for the formation of betalamic acid (Terradas and Wyler, 1991) escaped many attempts of isolation and purification from higher plants. In the mean time, a DOPA-dioxygenase was purified from Amanita muscaria (Girod and Zrÿd, 1991a), and an Amanita sp. DODA gene was isolated and characterized (Hinz et al., 1997; Mueller et al., 1997b). The fungal enzyme possesses a unique double 2,3- and 4,5-aromatic ring-cleaving activity, allowing the simultaneous biosynthesis of betalain and muscaflavin. Biochemical complementation of the betalain biosynthetic pathway in plants was demonstrated by using transformation by particle bombardment of white P. grandiflora petals with DODA cDNA (Mueller et al., 1997a). The fungal gene is still an orphan with only partial homology with some bacterial sequences; in silico analysis and antibody screening failed to show homologs in higher plants. The evolution of the plant enzyme seems therefore to follow a completely different phylogenetic pathway. To isolate plant genes, we initiated a subtractive library strategy using the different isogenic lines of P. grandiflora.
In this work, we describe the isolation and functional identification of a P. grandiflora gene (DODA) coding for a higher plant DOPA-dioxygenase. This gene encodes for a novel 4,5-plant extradiol ring-cleaving dioxygenase that is part of a well-conserved higher plant gene family. The identification of a gene coding for the key enzyme of betalain biosynthesis in plants will allow us to better understand the evolution of betalains and sheds some light on a family of yet uncharacterized plant genes.
RESULTS
Isolation and Expression Analysis of Betalain-Specific cDNAs from P. grandiflora Petals
The expression of the three genes C, R, and I determine P. grandiflora flower color. Flowers from plants containing at least one copy of gene C are colored, and flowers from cc plants are white (Trezzini and Zrÿd, 1990). C is therefore expected to code for the key enzyme protein 4,5-DOPA-dioxygenase that catalyzes the conversion of DOPA into betalamic acid. Pigments and precursor composition of white (cc, -, -), yellow (C-, rr, ii), and violet (C-, R-, I-) petals at different stages of bud development and pigment content of violet and green stems and green leaves have been previously quantified (Trezzini, 1990). Biosynthetic activity is only present in pigmented tissues (C-genotype) and is at its maximum during the early stages of their development (young flower buds). A cDNA subtractive hybridization strategy between pigmented (C-) and white (cc) genotypes was used. Amplified young petal cDNAs were produced for the construction of the subtractive cDNA libraries. Two subtractive cDNA libraries were built with RsaI digested cDNAs from white and yellow immature petals, respectively, from white and violet immature petals from P. grandiflora using the PCR-Select cDNA Subtraction kit (BD Biosciences Clontech, Palo Alto, CA). Subtracted cDNAs were ligated in pBlueScript SK plasmid and transformed in Escherichia coli. We expected the subtraction library to yield predominantly genes involved in betalain metabolism.
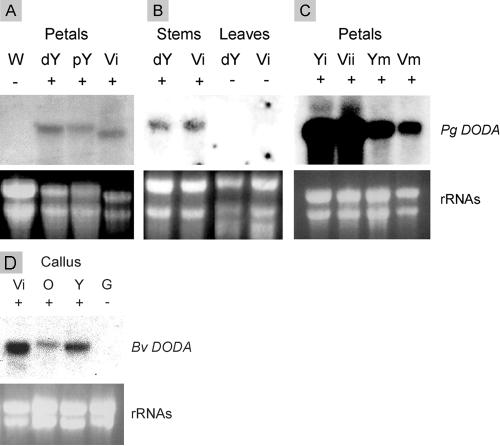
Individual clones were amplified by PCR and applied in duplicate to a membrane, using a slot blotter. In this way, we screened differently 750 clones with yellow and white specific probes and 950 clones with violet specific probes. Color-specific candidates were sequenced and compared with each other to eliminate most of the redundant clones. Nonredundant candidates were further analyzed for their expression in yellow, violet, and white immature petals by northern analysis. Two different yellow-specific clones and five clones specific to both violet and yellow-pigmented tissues were isolated. To assess their specific expression, clones were submitted to further transcription analysis in violet and green stems and leaves. Their levels of expression in immature and mature yellow and violet petals were also compared together to verify the expected lower amount of transcript in mature pigmented tissues. One of the promising candidates labeled L.6 (Zaiko, 2000) had transcripts present in all pigmented tissues and absent from white petals, green stems, and leaves; its level of expression is very high in immature colored petals and decreases in mature colored petals (see Fig. 2 A, B, and C). Detection of transcripts in violet stems was particularly difficult due to the restricted localization of pigment in the thin epidermal layer, and we had to use a higher amount of total RNA. Pooling these results together raised the expectation that this cDNA could well originate from the color gene C necessary for the biosynthesis of the chromophore betalamic acid. We then tentatively assigned L.6 as DODA.
Figure 2.
RNA gel-blot analysis of DODA gene expression in P. grandiflora flowers. Presence or absence of betalain pigments (no Pg DODA) in plant tissue is indicated by + or - signs. A, Pg petals of different genotypes (W, white cc, -, -; dY, deep yellow C-, rr, ii; pY, pale yellow C-, rr, I-; Vi, violet C-, R-, ii). B, Pg stems and leaves. C, Expression at different stages of bud development (with yellow immature [Yi], yellow mature [Ym], violet immature [Vii], and violet mature [Vim]). D, RNA gel-blot analysis of expression of the putative red beet (Bv) DODA in Bv colored calli containing betalains and green calli lacking betalain (Vi, violet; O, orange; Y, yellow; and G, green).
Similar results were obtained by northern-blot analysis of DODA homolog in red beet callus; green callus do not show any expression, but positive results were obtained with yellow, orange, and red calli (Fig. 2 D).
Cloning and in Silico Functional Identification of Full-Length Pg DODA cDNA Encoding an Extradiol Ring-Opening Dioxygenase
DNA adaptors containing the annealing sites for two long primers that follow one another (Genome Walker kit, BD Biosciences Clontech) were ligated to the partial putative Pg DODA cDNA. Two gene-specific primers were designed in the same way, allowing the RACE amplification of the missing 3′ and 5′ cDNA ends and the reconstitution of the full-length cDNA. Pg DODA cDNA sequence (GenBank entry AJ580598) is 1,249 bp long with an open reading frame predicted to encode a 271-amino acid protein with a theoretical molecular mass of 29.9 kD and an acidic pI of 5.6. PSORTII tool (Nakai and Horton, 1999) predicts a clear cytoplasmic localization of the protein. PCR-amplified Pg DODA genomic DNA had the same size as the open reading frame, suggesting the absence of intron. A Southern-blot analysis performed on genomic DNA from P. grandiflora, using a radiolabeled NcoI-digested 789-bp fragment from Pg DODA cDNA as a probe, confirmed the presence of a single gene. Genomic DNAs were individually digested with EcoRI and NheI. They were then separated on agarose gel and transferred to a nylon membrane. We observed two bands for NheI due to the presence of an internal NheI site in Pg DODA cDNA and one band for EcoRI as expected in the case of a single gene (Fig. 3). Intensity of the obtained bands was corresponding to a one-copy gene equivalent from the plasmid. Pg DODA cDNA is therefore a single copy gene.
Figure 3.

Southern-blot analysis of the Pg DODA gene. Washes in SSC buffer were done to exclude the hybridization of sequences with less than 95% of homology.
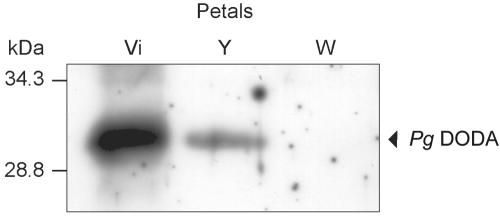
To verify the betalain-specific expression of the Pg DOPA-dioxygenase, specific polyclonal antibodies were produced against a Pg DODA synthetic peptide fragment. Western-blot analysis of the crude protein extract from different Pg colored petals showed the presence of a 30-kD band in violet and yellow petals corresponding to the size expected for Pg DODA (Fig. 4). This result confirms the correlation of the expression of Pg DODA with betalain biosynthesis. Because the size of the protein is equivalent to that predicted, we conclude that there is no major size-altering post-translational modification.
Figure 4.
Western-blot analysis of DODA in P. grandiflora petals of different colors (Vi, violet; Y, yellow; and W, white). Pg DODA (29.9 kD) is present in high amount in violet petals, less in yellow petals, and absent from non-pigmented white petals.
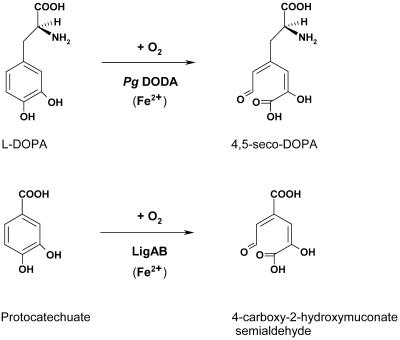
The National Center for Biotechnology Information Conserved Domain Database domain search tool (Marchler-Bauer et al., 2002) revealed a homology of Pg DODA translated protein (34% of identity, 48% of similarity) with the LigB domain (pfam02900) present in several bacterial extradiol 4,5-dioxygenases. This domain was first identified on the β-subunit of a bacterial heterotetrameric class III extradiol-type 4,5-dioxygenase (GenBank accession no. AAA17728) of Sphingomonas paucimobilis (Noda et al., 1990; Spence et al., 1996). The crystal structure 1B4U of this LigAB protein (Sugimoto et al., 1999) revealed the three amino acids coordinating the non-heme Fe2+ cofactor and the catalytic residues interacting with the protocatechuate (PCA) substrate. In-depth analysis of the Pg DODA amino acid sequence alignment with LigB-containing proteins and other plant homologs, allowed us to identify clearly Pg DODA as an extradiol 4,5-dioxygenase. The conservation of the iron-binding amino acid and of three of five of the catalytic site amino acids (see Supplemental Data, available in the online version of this article at http://www.plantphysiol.org) is the main feature of interest. Referring to P. grandiflora 4,5-dioxygenase, the Ser-16 (position A), the His-121 (position D), and the His-177 (position E) that orient the aromatic substrate are conserved. Referring to PCA 4,5-dioxygenase structure, the Ser-269 and Asn-270 linked by hydrogen bonds to the two oxygen of the PCA lateral carboxylate group (Fig. 5) are conserved only in monocotyledons and tomato (Lycopersicon esculentum) 1. Substrates used by most plant homologs of the bacterial enzyme may not have a lateral carboxy group or a modified one. Pg DODA substrate dihydroxy-Phe possesses a longer (one carbon) lateral chain containing a -NH3 group (Fig. 5). Thus, these two amino acids probably play a major role in substrate recognition. The His-17 (position B), His-55 (position C), and Glu-231 (position F) coordinating the ferrous cofactor are conserved except that the latter is shifted one position and a conserved His-232 (position G) is present in its place, allowing as an alternative a possible triple His coordination of the Fe2+ ion as already observed in a soybean (Glycine max) lipoxygenase (Boyington et al., 1993). Secondary structure predictions realized with PROF tool (Rost and Sander, 1993) show a high degree of conservation between the Pg DODA and the PCA LigAB dioxygenase secondary structure, confirming our hypothesis (see Appendix). There is no structural analogy of Pg DODA with the A. muscaria protein DODA.
Figure 5.
The l-DOPA substrate of Pg 4,5-DOPA extradiol dioxygenase DODA and the PCA substrate for the bacterial LigAB differ in the complexity of the chain containing the carboxyl group (position 1).
Complementation of the Betalain Biosynthetic Pathway by Pg DODA DOPA-Dioxygenase in P. grandiflora White Petals
White petals lack the Pg DODA enzyme; successful biochemical complementation through transformation by a construct containing a putative gene candidate would constitute a proof that this gene encodes a specific dioxygenase. In a previous paper, we demonstrated that shot gun (biolistic) transformation of white petals with a construct containing the Amanita sp. DOPA-dioxygenase gene complemented pigment production (Mueller et al., 1997a). Depending on the plant genetic background, (cc, rr, ii) or (cc, R-, I-), transformed cells produced yellow or violet pigments.
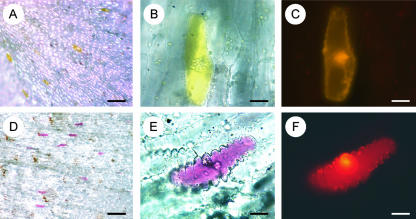
To confirm Pg DODA protein as an extradiol dioxygenase responsible for the conversion of DOPA into betalamic acid, Pg DODA cDNA was subcloned into a cauliflower mosaic virus (CaMV) 35S pNco plant expression vector (double-sequenced to control its sequence integrity). Transient overexpression of Pg DODA gene by shot gun transformation of white Pg petals produced, after 18 h of incubation, bright yellow betaxanthin spots (Fig. 6A) or deep violet betacyanin spots (Fig. 6D) according to the plant genetic background. Each spot is due to one individual cell (Fig. 6, B and E); we observed no diffusion of the pigment during the next 2 d. We counted five to 80 spots per petal, depending on the shot, petal size, and maturity. We observed more spots on fully developed petals. We used a p35S DsRed2 expression construct as a positive control in the same transformation. All violet- and yellow-transformed cells also produced the DsRed2 red fluorescent protein, whereas some cells only emitted the red fluorescence. White petals transformed by DsRed2 alone, as a negative control, did not show any yellow- or violet-colored spot.
Figure 6.
The specificity of the Pg DODA gene is demonstrated by biolistic complementation of the betalain pathway in the white petals of P. grandiflora plants deficient in DODA. A pNco DODA expression vector containing full-length Pg DODA driven by a CaMV promoter has been used with pDsRed2 vector as a positive control. A, Yellow spots revealed after biolistic transformation of a white petal from a plant with yellow genetic background. B, Close-up of a cell accumulating betaxanthins in its vacuole. C, The same cell displaying the DsRed2 fluorescent protein modified to an orange one by the fluorescence of the betaxanthins. D, Violet spots revealed in a white petal from a plant with a violet genetic background. E, Close-up of a cell accumulating betacyanins. F, The same cell displaying the DsRed2 fluorescent protein. The red fluorescence hue from DsRed2 was slightly modified by filtration through yellow betaxanthin or violet betacyanin pigments (allowing for color differences between C and F). Bars = 200 μm in A and D and 20 μm in B, C, E, and F.
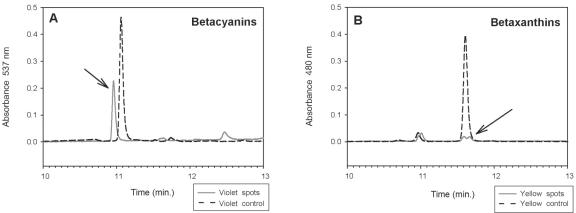
To check the identity of the pigments responsible for cell coloration, we collected about 500 yellow- and 500 violet-transformed cells. We extracted the pigments in acidified methanol. HPLC analysis of these pigments showed a strict identity with the betalain pigments present naturally in violet and deep yellow P. grandiflora petals (Fig. 7, A and B). We identified the violet pigment as betanin and the yellow pigment as dopaxanthin by comparison with standards. The quantity of dopaxanthin measured in the yellow-transformed cells was lower per cell than that present in untransformed cells of the yellow phenotype. This could be due to the synthesis of other betaxanthins in minor quantities, which were not measurable. We did not detect the presence of muscaflavin, which would indicate a 2,3-dioxygenase activity. These results demonstrate the biochemical complementation of the missing C gene product in Pg white petals by the Pg DODA gene product. Our identification of Pg DODA in silico as an extradiol 4,5-dioxygenase is thus confirmed, and we have shown that, in contrary to the Amanita sp. dioxygenase, the plant dioxygenase does not have a significant 2,3-ring-cleaving activity. DODA represents a novel and previously unidentified family of plant protein and the first example of a non-heme ring-opening dioxygenase from plant.
Figure 7.
HPLC analysis of betalain pigments extracted from violet-(A) and yellow-transformed (B) cells in the white P. grandiflora background. These pigments were identified by comparing their elution profile with elution profile of the pigments extracted from violet and deep yellow P. grandiflora petals, respectively. Arrows indicate the major peaks of the violet betanin (A) and of the yellow dopaxanthin (B). The minor 3-s shift observed between the two betanin peaks is due to a slight inaccuracy of the injection process.
Identification of a Pattern Specific to Betalain Plants and Analysis of Its Influence on the Protein Functionality
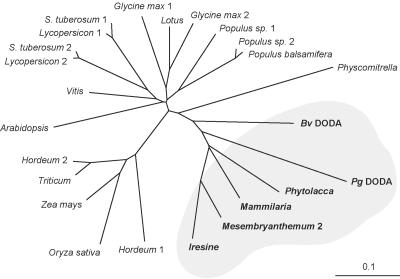
Protein sequences from betalain and non-betalain plants, bacteria and archea have been used for sequence alignments and construction of a phylogenetic tree for plant homologs (Fig. 8). Caryophyllales sequences are clustered with 60% to 62% of identity with Pg DODA, whereas homology with other plant genes was lower with 52% of identity with Arabidopsis, 50% with major monocotyledons and 45% with the bryophyte Physcomitrella patens (Pp). Novel putative proteins having a higher level of homology with Pg DODA than Sphingomonas sp. LigAB dioxygenase have been also identified in numerous bacteria and archeabacteria species. Pseudomonas spp. and Xanthomonas spp. are the closest bacteria species with 38% of identity and 55% of similarity.
Figure 8.
Phylogenetic analysis of Pg DODA homologs in plants. Multiple alignments from Pg DODA homologous fragment (Met-22 to Lys-163) were done with ClustalW, and the tree was created with PHYLIP. The moss (bryophyte) P. patens corresponds to the root. Betalain-producing species in the gray surface clearly form a cluster distinct from other plants. For GenBank accession numbers, see Table I.
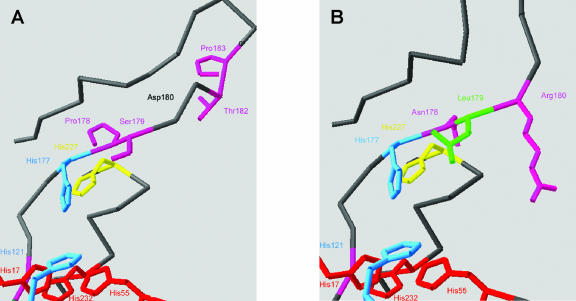
Alignment with ClustalW tools allowed the identification of a conserved motif (HNL-R/G) present in all organisms except in plants synthesizing betalains. In these plants, a completely different conserved motif is present (see Fig. 9). Both motifs begin with the strictly conserved His-177, which is essential for the catalytic activity of extradiol dioxygenase class III enzyme like P. grandiflora DODA (Sugimoto et al., 1999). Preliminary three-dimensional modeling of the enzyme based on the crystal structure from the PCA 4,5-dioxygenase LigAB revealed that the amino acids following His-177 could be involved in the substrate specificity of 4,5-DOPA dioxygenase by controlling access to the catalytic site (Fig. 10).
Figure 9.
Identification of one conserved pattern specific to DODA proteins from betalain-producing plants by alignment of the Pg DODA homologous sequences from different kingdoms. The conserved catalytic amino acid His-177 is followed by the pattern P-(S,A)-(N,D)-x-T-P in all homologs of betalain-producing plants, whereas at the same place, a H-N-L pattern is conserved in all archeabacteria, and a H-N-L-R pattern is conserved in all plant homologs not producing betalains. For GenBank accession numbers, see Table I.
Figure 10.
Preliminary modeling with SwissPdbViewer of the catalytic site of Pg DODA (A) and the moss P. patens DODA homolog (B) based on the three-dimensional structure 1B4U of Sphingomonas sp. LigAB. Referring to Pg DODA sequence, catalytic His-177 is conserved (blue), whereas the three following residues conserved in betalain plants Pro-178-Ser-179-Asp-180 are replaced in all non-betalain plants by Asn-178-Leu-179-Arg-180, which reduce clearly the access to the catalytic site represented by the three conserved His linking the iron cofactor (red) and the two conserved His making hydrogen bonds with the substrate (blue). Pro-178-Ser-179-Asp-180 and Thr-182-Pro-183 are conserved only in betalain plants potentially participating to the substrate recognition.
DISCUSSION
The in silico identification of a higher plant 4,5-dioxygenase and demonstration that this protein could efficiently complement plants deficient in the corresponding gene, opens a new window in our understanding of the evolution of the betalain biosynthetic pathway. We hypothesize that this pathway originated by recruitment of an existing metabolic pathway compensating for loss(es) in the capacity of synthesizing colored anthocyanins in plants of the order Caryophyllales. The fact that the Pg DODA belongs to a family of highly conserved proteins present in all land plants from bryophytes to angiosperms will allow the study of the function of those proteins that until now are cataloged as “unknown function protein.” This class of proteins codes for conserved proteins that should be important for broad plant fitness. We could postulate, from our knowledge about the conserved structural properties of the catalytic site, that those proteins are involved in the metabolism of aromatic compounds either in a degradation step or in a biosynthetic one. We have evidence that tagged libraries of Arabidopsis contain interesting entries that could perhaps allow the isolation of a null mutant. As a fast and easiest alternative, we have already started the construction of knockout and overexpressing mutants in the bryophyte P. patens.
The Pg DODA protein shares no homology with the Amanita sp. DODA protein already studied in our laboratory. Furthermore, the catalytic activity is that of a genuine 4,5-extradiol dioxygenase with no 2,3-activity (Mueller et al., 1997b). The origins of both the plant and the fungal protein are therefore distinct and should be traced to the evolution of different gene families. The production of betalain by Amanita sp. and Hygrocybe sp. and by Caryophyllales is an interesting case of convergent evolution. If we can easily hypothesize about the evolutionary forces behind the establishment of a betalain biosynthetic pathway in plants (Ehrendorfer, 1976), it is more difficult to understand the function of those pigments in fungi.
Only a few mutations are needed to switch from non-betalain-producing plants to betalain-producing ones, they involve mainly a six amino acid stretch that follows the His-177 highly conserved amino acid of the catalytic site. In most plants, this stretch is homologous to the bacterial sequence (H-N-L-x), and in the few Caryophyllales species analyzed up to now, it is replaced by a P-(S,A)-(N,D)-x-T-P sequence.
Due to the availability of a functional complementation test in P. grandiflora white petals, we can easily verify the importance of each of the supposed catalytic amino acid in the dioxygenase reaction by building point-mutated DODA genes. In the same manner, it will be interesting to modify a DODA homolog from a non-Caryophyllales plant in such a way to evaluate its capacity to use DOPA as a substrate.
The appearance of substrate specificity for DOPA occurred probably during the evolution of early betalain-synthesizing plants of the order Caryophyllales. Only an extensive analysis across the phylogenetic tree will answer the question. We could expect to find a few plants with intermediate sequences, at the interface of betalain-producing and nonproducing plants, which have escaped current investigations; those plants could help to solve some critical aspects of the molecular phylogeny of Caryophyllales (Cuenoud et al., 2002). Examination of the protein phylogenetic tree puts Chenopodiaceae (red beet) at the base of the branch of betalain-producing plants. The Portulacaceae (with DODA) branch is followed by two groups formed by Phytollacaceae and Cactaceae followed by Aizoaceae and Amaranthaceae at the top. This feature, if confirmed by including cDNA from more species, could reflect the evolution of Pg DODA-related genes.
The P. grandiflora gene is a single-copy gene, but in some plants, it appears that more than one sequence is present. Subtle differences exist between the sequences of homologous proteins of betalain-producing plants that will require further studies. For example, the Bv DODA (Chenopodiaceae) sequence has an Asn and a Gly instead of two Asp residues in the catalytic domain. Some plants like common ice plant (Mesembryanthemum crystallinum [Aizoaceae]) seem to possess two different proteins. This could mean that a similar protein, perhaps with a function distinct from the production of betalamic acid is present in this species.
We have not yet succeeded in demonstrating a significant expression and activity of the Pg DODA protein in Escherichia coli. The protein enzymatic activity has yet escaped all attempts of in vitro detection; this can be due to its instability in this physiological context, to a missing cofactor, or to both. We are currently trying to express Pg DODA in a plant expression system by transformation of the moss P. patens.
The identification of the plant DOPA-dioxygenase DODA, catalyzing the last enzymatic reaction of the betalamic acid biosynthesis, opens the way to study the architecture of the betaxanthins and betacyanins pathways. Those pathways could be engineered to further produce high amounts of natural water-soluble yellow or violet pigments in beet root and could provide visual markers in genetic transformation. Markers based on the DODA sequences could contribute to the development of new strongly pigmented beet varieties by marker-assisted selection.
MATERIALS AND METHODS
Plant and Cell Culture Cultivation
We grew Portulaca grandiflora, Mammilaria sp., and Iresine sp. in a greenhouse under normal daylight conditions. Pg buds (6-8 mm) were collected from vegetatively propagated clones obtained from quasi-isogenic lines established in our lab (Trezzini and Zrÿd, 1990), their basal part were cut-off, and petals were extracted and frozen in liquid N2. Beet root calli cell cultures displaying different colors were selected from red beet (Beta vulgaris) Bikores monogerm (Girod and Zrÿd, 1991b) and cultivated on a maintenance medium at 26°C with a 16-h photoperiod (light from GROLUX WSSYLVANIA, 6 mW m-2). We collected samples for the RNA extraction by vacuum filtration of suspension cultures; these samples were stored frozen at -80°C and ground in liquid N2 just before extraction. The moss Physcomitrella patens was grown in the same conditions as beet cell cultures on solid minimal medium (Ashton et al., 1979) supplemented with 2.7 mm ammonium tartrate and 50 mm Glc.
RNA Isolation and Construction of a Subtractive cDNA Library
Total RNA from Pg was isolated from immature petals (1 g) using the hot phenol method (Rochester et al., 1986), except that we used 5× larger volumes throughout to reduce the high viscosity of the extracts. mRNA was purified using the PolyATract mRNA isolation system (Promega, Madison, WI) according to the suppliers' protocol. The Smart PCR cDNA Library Construction kit (BD Biosciences Clontech) was used to amplify a sufficient amount of cDNA from Pg violet, yellow, and white immature petals for the construction of a subtractive cDNA library. cDNAs were purified on a Chromaspin-1000 column (BD Biosciences Clontech), digested by RsaI enzyme, and purified on a PCR purification column (Qiagen USA, Valencia, CA). Four subtraction experiments were performed using PCR-Select cDNA Subtraction kit (BD Biosciences Clontech) according to manufacturer's instructions: two direct subtractions where yellow and violet cDNA were used as testers and white cDNA as a driver, and two reverse subtractions with white cDNA as a tester. After PCR-based subtractive hybridization, cDNA fragments were cloned into pGEM-T-easy vector (Promega) and transformed in Escherichia coli DH5α strain.
For comparative studies, we isolated Bv DODA cDNA by reverse transcriptase-PCR from yellow red beet callus total RNA with nested primers designed from the 5′-untranslated region of a partial expressed sequence tag (EST; gi14522284) and nested primers annealing with the added adaptors from Genome walker kit (BD Biosciences Clontech).
Northern-Blot Analysis
We blotted 5 to 20 μg of total RNA per lane onto a Zeta-Probe GT membrane (Bio-Rad Laboratories, Munich). For hybridization, we used [32P]dCTP-labeled DNA probes in 0.5 m phosphate buffer (pH7.2), 7% (w/v) SDS, and 1 mm EDTA at 60°C. We washed the blots under stringent conditions and exposed them using X-OMAT AR film (Eastman Kodak, Rochester, NY).
Full-Length cDNA Isolation
Undigested Pg yellow-specific total cDNA amplified using capFinder cDNA Construction Kit (BD Biosciences Clontech) was ligated to DNA adaptors from Universal Genome Walking kit (BD Biosciences Clontech) containing annealing sites for two long primers (AP1, AP2) that follow one another. Two gene-specific primers were designed in the same manner from the partial Pg DODA cDNA (PgDODAp2, 5′-CTTCCTTCTAGGAGAGCATCCTC-3′; PgDODAp3, 5′-TTCACATCTTCGTACCTTCCTTCTAGGAGAG-3′), thus allowing a two-step amplification of the missing 5′ regions of Pg DODA cDNA with annealing and extension performed at 68°C in the presence of Expand High-Fidelity Taq polymerase mix (Roche Diagnostics, Mannheim, Germany). We reconstituted the full-length cDNA from the extremities by high-fidelity PCR amplifications and sequenced on both strands.
Southern-Blot Analysis
We extracted Pg genomic DNA from young leaves and stems ground in liquid N2 with the DNeasy Plant Maxi kit (Qiagen). We digested 4 μg of DNA with the restriction enzymes EcoRI and NheI (Invitrogen, Carlsbad, CA) and separated them by electrophoresis on a 0.7% (w/v) agarose gel. A [32P]dCTP probe was made from a NcoI-digested 789 bp-fragment of Pg DODA cDNA. Blotting and hybridization conditions were the same as described for the northern-blot analysis. We washed the filters progressively in SSC buffer from 70% to 95% of homology and exposed using X-OMAT AR film (Eastman Kodak).
Production of a Pg DODA-Specific Antibody and Western-Blot Analysis
Rabbit Pg DODA polyclonal antibodies were produced by injection of a 16-amino acid peptide (H2N-CRYEDVNNYQTKAPEG-CONH2) selected from Pg DODA sequence for its antigenic properties and specificity, and followed by an affinity purification of the obtained serum on a protein A column (Eurogentec, Seraing, Belgium). Crude plant proteins extracts were obtained by grinding tissues in the CCLR lysis buffer (Promega). Proteins were quantified by the Bradford method (Bradford, 1976). SDS-PAGE was performed on a Miniprotean II (Bio-Rad Laboratories) according to Laemmli (1970) with the Low Range Molecular Weight marker (Bio-Rad Laboratories). Proteins were blotted on a Trans-Blot nitrocellulose membrane by electrotransfer according to Bio-Rad Laboratories' manual instruction. Transferred proteins were revealed by incubating the membrane 1 min in Red Ponceau solution followed by a wash with water. Immunodetection was done with the Immun-Star horseradish peroxidase (HRP) kit (Bio-Rad Laboratories), with Pg DODA-specific antibody as primary antibody (diluted 3,500×) and an anti-rabbit IgG HRP-conjugate antibody (Promega) as secondary antibody (diluted 2,500×). Detection of the HRP-conjugate antibody was performed by 5-min incubation of the membrane in the chemiluminescent immune-Star HRP reagents mix (Bio-Rad Laboratories), and it was exposed using Hyperfilm ECL film (Amersham Biosciences, Uppsala).
Expression Study by Particle Bombardment and Pigment Analysis
Pg DODA full-length cDNA was amplified with high-fidelity Taq polymerase and modified primers. Sense primers contained XbaI and antisense PstI restriction sites to facilitate cloning. The sequence before the start codon was also corrected according to Kozak sequence to facilitate the initiation of the translation (Kozak, 1991). Amplified cDNA was digested with XbaI and PstI and purified. The plant expression vector pNco Pg DODA was constructed by placing the XbaI/PstI-digested Pg DODA cDNA in pNco vector (Mueller et al., 1997a). The cloned cDNA was constitutively expressed under the control of a CaMV 35S promoter and terminated by CaMV polyadenylation signal. We submitted the double-stranded DNA of this construct for sequencing at Microsynth Gmbh (CH 9436 Balgach, Switzerland) facilities.
We performed ballistic transformations of young white petals from flower buds with the pNco construct on gold particles (Mueller et al., 1997a). Petals from plants homozygous for the cc locus (white phenotype) with different genetic background for gene R and I (hidden violet or yellow phenotypes) were tested (for details, see Trezzini, 1990). We counted the number of colored spots per petal after 24 h of incubation at 26°C under light. A positive control of transformation was done in parallel and simultaneously with a construct containing the cDNA encoding the red fluorescent protein DsRed2 (BD Biosciences Clontech) driven by 35S promoter and terminated by Nos polyadenylation signal (p35S DsRed2; A. Finka, unpublished data).
Violet and yellow spots synthesized in pNco Pg DODA-transformed petals were extracted with acidified methanol and analyzed by HPLC (Waters, Milford, MA) using a Hypersil column (Bischoff, Leonberg, Germany) as described previously (Trezzini and Zrÿd, 1991). We compared for identification the spectra and retention time from the pigments produced by ballistic transformation with the naturally occurring betalain pigments from yellow and violet P. grandiflora petals.
Homologous Genes and Partial Genomic Sequence Isolation
The Arabidopsis homolog for Pg DODA(AtL.6) was isolated by PCR amplification with primers (AtL.6p1rev, 5′-TCTTGAAATTCACATGACATTCA-3′; AtL.6p2rev, 5′-TGTATTCGTATACAAGACAGGAT-3′) designed from the complete mRNA AY050948 on the 5′ sequenced homolog EST N65678 (Newman et al., 1994) from the Arabidopsis Biological Resource Center Lambda-PRL2 EST library. The P. patens homolog for Pg DODA(Pp DIOXA) was amplified by nested PCR strategy on Lambda-UniZAP cDNA library (Girod et al., 1999) with long primers (DIOXAp5, 5′-ACCAACAACTACCATACTAGTTTTGAGGAT-3′; DIOXAp4, 5′-GTGAGCATCCACTCAAGCAGCACA-3′) designed from the partially sequenced EST BJ195116 corresponding to the 5′ region of the gene (Nishiyama et al., 2003). The red beet homolog for Pg DODA was isolated from yellow callus total RNA by reverse transcriptase-PCR amplification strategy. DNA adaptors from Universal Genome Walking kit (BD Biosciences Clontech) containing annealing sites for two long primers (AP1, AP2) were ligated to the amplified cDNA according to the manufacturer's prescriptions. Gene-specific nested primers (BvDODAp1, 5′-TACTTAATATGATACTTTCGTGCCA-3′; BvDODAp4, 5′-GCTCAAATCTGAAAATGGGTAGTGAAGA) were designed on the 5′-untranslated region from EST sequence BI095902 and used in combination with nested primer AP1 and AP2 to amplify the full-length cDNA.
Three-Dimensional Preliminary Modeling
Three-dimensional preliminary models of Pg DODA and Pp DIOXA proteins were created with Swiss-PdbViewer (Guex and Peitsch, 1997) from the Sphingomonas paucimobilis crystallized structure PDB 1B4U as a template. Pg DODA and Pp DIOXA sequences were aligned manually with the structure 1B4U based on the same alignment as described previously. The longer length of the bacteria sequence generated gaps in the modeling program; we introduced a carbon chain of the same size as the bacterial amino acids sequence length to replace those gaps. We did not attempt to correct the errors that were located away from the active site amino acids.
Computational Analysis
Similarity search of amino acid and nucleotide sequences of Pg DODA was performed using the different options of the “Blast” algorithms (Altschul et al., 1990; Gish and States, 1993). The National Center for Biotechnology Information Conserved Domain Database domain search tool (Marchler-Bauer et al., 2002) was used to identify the LigB domain (pfam02900). Sequence analysis was performed with the Vector NTI software package (Invitrogen). Sequences alignments were done using ClustalW software (Thompson et al., 1994) and edited manually with Jalview tool (http://www.jalview.org). PHYLIP was used for the phylogenetic analysis (Felsenstein, 1989).
Supplementary Material
Table I.
Genbank accession numbers
| DODA Homologs | GenBank Accession No. |
|---|---|
| Arabidopsis | NM_117597 |
| Bv DODA | AJ583017 |
| Soybean 1 | BE608113, BG651843, BE191341, AW733761 |
| Soybean 2 | AW348985, BM523589 |
| Barley (Hordeum vulgare) 1 | BI956621 |
| Barley 2 | BE558845, BI780256 |
| Iresine sp. | Incomplete genomic fragment |
| Lotus corniculatus var. japonicus | AP004916 |
| Tomato 1 | AI896661, BG128676 |
| Tomato 2 | AI898647, AW222441, BF050515 |
| Mammilaria sp. | Incomplete genomic fragment |
| Medicago truncatula | BF645123 |
| Common ice plant 1 | BF480453 |
| Common ice plant 2 | BE131205 |
| Methanosarcina acetivorans str. C2A | NC_003552 |
| Rice (Oryza sativa) | AP003227 |
| P. patens | AJ583016 |
| Pokeweed (Phytolacca americana) | Incomplete genomic fragment |
| Populus balsamifera subsp. trichocarpa | BU879976, BU875430 |
| Populus tremula × Populus tremuloides 1 | BI127978, BI122325 |
| Populus tremula × Populus tremuloides 2 | BU812037, BU887517 |
| P. grandiflora DODA | AJ580598 |
| Pseudomonas putida str. KT2440 | NP_744024 |
| Pseudomonas syringae pv tomato str. DC3000 | NC_004578 |
| Potato (Solanum tuberosum) 1 | BG098818, BG886949 |
| Potato 2 | BG590982 |
| S. paucimobilis str. SYK6 LigAB | AAA17728 |
Acknowledgments
We are grateful to Andrija Finka for the gift of the pDsRed2 construct, to Marco Pagni for help in the manual edition of the multiple alignment and discussion, and to Alexander Diemand for his helpful suggestions concerning the three-dimensional modeling. We thank Edward E. Farmer for critically reading the manuscript.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.031914.
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic Local Alignment Search Tool. J Mol Biol 215: 403-410 [DOI] [PubMed] [Google Scholar]
- Ashton NW, Cove DJ, Featherstone DR (1979) Isolation and physiological analysis of mutants of the moss, Physcomitrella patens, which over-produce gametophores. Planta 144: 437-442 [DOI] [PubMed] [Google Scholar]
- Boyington JC, Gaffney BJ, Amzel LM (1993) The 3-dimensional structure of an arachidonic-acid 15-lipoxygenase. Science 260: 1482-1486 [DOI] [PubMed] [Google Scholar]
- Bradford MM (1976) Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal Biochem 72: 248-254 [DOI] [PubMed] [Google Scholar]
- Clement JS, Mabry TJ (1996) Pigment evolution in the Caryophyllales: a systematic overview. Bot Acta 109: 360-367 [Google Scholar]
- Cuenoud P, Savolainen V, Chatrou LW, Powell M, Grayer RJ, Chase MW (2002) Molecular phylogenetics of Caryophyllales based on nuclear 18S rDNA and plastid rbcL, atpB, and matK DNA sequences. Am J Bot 89: 132-144 [DOI] [PubMed] [Google Scholar]
- Ehrendorfer F (1976) Closing remarks: systematics and evolution of centrospermous families. Plant Syst Evol 126: 99-105 [Google Scholar]
- Felsenstein J (1989) Phylogeny Inference Package (version 3.2). Cladistics 5: 164-166 [Google Scholar]
- Girod PA, Fu HY, Zrÿd JP, Vierstra RD (1999) Multiubiquitin chain binding subunit MCB1 (RPN10) of the 26S proteasome is essential for developmental progression in Physcomitrella patens. Plant Cell 11: 1457-1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girod PA, Zrÿd JP (1991a) Biogenesis of betalains: purification and partial characterization of Dopa 4,5-dioxygenase from Amanita muscaria. Phytochemistry 30: 169-174 [Google Scholar]
- Girod PA, Zrÿd JP (1991b) Secondary metabolism in cultured red beet (Beta vulgaris L) cells: differential regulation of betaxanthin and betacyanin biosynthesis. Plant Cell Tiss Organ Cult 25: 1-12 [Google Scholar]
- Gish W, States DJ (1993) Identification of protein coding regions by database similarity search. Nat Genet 3: 266-272 [DOI] [PubMed] [Google Scholar]
- Guex N, Peitsch MC (1997) SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18: 2714-2723 [DOI] [PubMed] [Google Scholar]
- Hinz UG, Fivaz J, Girod PA, Zrÿd JP (1997) The gene coding for the DOPA dioxygenase involved in betalain biosynthesis in Amanita muscaria and its regulation. Mol Gen Genet 256: 1-6 [DOI] [PubMed] [Google Scholar]
- Kobayashi N, Schmidt J, Wray V, Schliemann W (2001) Formation and occurrence of dopamine-derived betacyanins. Phytochemistry 56: 429-436 [DOI] [PubMed] [Google Scholar]
- Kozak M (1991) Structural features in eukaryotic messenger-RNAs that modulate the initiation of translation. J Biol Chem 266: 19867-19870 [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during assembly of head of bacteriophage-T4. Nature 227: 680-685 [DOI] [PubMed] [Google Scholar]
- Marchler-Bauer A, Panchenko AR, Shoemaker BA, Thiessen PA, Geer LY, Bryant SH (2002) CDD: a database of conserved domain alignments with links to domain three-dimensional structure. Nucleic Acids Res 30: 281-283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller LA, Hinz U, Uze M, Sautter C, Zrÿd JP (1997a) Biochemical complementation of the betalain biosynthetic pathway in Portulaca grandiflora by a fungal 3,4-dihydroxyphenylalanine dioxygenase. Planta 203: 260-263 [Google Scholar]
- Mueller LA, Hinz U, Zrÿd JP (1996) Characterization of a tyrosinase from Amanita muscaria involved in betalain biosynthesis. Phytochemistry 42: 1511-1515 [Google Scholar]
- Mueller LA, Hinz U, Zrÿd JP (1997b) The formation of betalamic acid and muscaflavin by recombinant dopa-dioxygenase from Amanita. Phytochemistry 44: 567-569 [Google Scholar]
- Nakai K, Horton P (1999) PSORT: a program for detecting sorting signals in proteins and predicting their subcellular localization. Trends Biochem Sci 24: 34-35 [DOI] [PubMed] [Google Scholar]
- Newman T, Debruijn FJ, Green P, Keegstra K, Kende H, McIntosh L, Ohlrogge J, Raikhel N, Somerville S, Thomashow M et al. (1994) Genes galore: a summary of methods for accessing results from large-scale partial sequencing of anonymous Arabidopsis cDNA clones. Plant Physiol 106: 1241-1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama T, Fujita T, Shin-I T, Seki M, Nishide H, Uchiyama I, Kamiya A, Carninci P, Hayashizaki Y, Shinozaki K et al. (2003) Comparative genomics of Physcomitrella patens gametophytic transcriptome and Arabidopsis thaliana: implication for land plant evolution. Proc Natl Acad Sci USA 100: 8007-8012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda Y, Nishikawa S, Shiozuka KI, Kadokura H, Nakajima H, Yoda K, Katayama Y, Morohoshi N, Haraguchi T, Yamasaki M (1990) Molecular-cloning of the protocatechuate 4,5-dioxygenase genes of Pseudomonas paucimobilis. J Bacteriol 172: 2704-2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochester DE, Winer JA, Shah DM (1986) The structure and expression of maize genes encoding the major heat-shock protein, Hsp70. EMBO J 5: 451-458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rost B, Sander C (1993) Prediction of protein secondary structure at better than 70-percent accuracy. J Mol Biol 232: 584-599 [DOI] [PubMed] [Google Scholar]
- Schliemann W, Kobayashi N, Strack D (1999) The decisive step in betaxanthin biosynthesis is a spontaneous reaction. Plant Physiol 119: 1217-1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliemann W, Steiner U, Strack D (1998) Betanidin formation from dihydroxyphenylalanine in a model assay system. Phytochemistry 49: 1593-1598 [DOI] [PubMed] [Google Scholar]
- Spence EL, Kawamukai M, Sanvoisin J, Braven H, Bugg TDH (1996) Catechol dioxygenases from Escherichia coli (MhpB) and Alcaligenes eutrophus (MpcI): sequence analysis and biochemical properties of a third family of extradiol dioxygenases. J Bacteriol 178: 5249-5256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner U, Schliemann W, Bohm H, Strack D (1999) Tyrosinase involved in betalain biosynthesis of higher plants. Planta 208: 114-124 [Google Scholar]
- Steiner U, Schliemann W, Strack D (1996) Assay for tyrosine hydroxylation activity of tyrosinase from betalain-forming plants and cell cultures. Anal Biochem 238: 72-75 [DOI] [PubMed] [Google Scholar]
- Strack D, Vogt T, Schliemann W (2003) Recent advances in betalain research. Phytochemistry 62: 247-269 [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Senda T, Aoshima H, Masai E, Fukuda M, Mitsui Y (1999) Crystal structure of an aromatic ring opening dioxygenase LigAB, a protocatechuate 4,5-dioxygenase, under aerobic conditions. Struct Fold Design 7: 953-965 [DOI] [PubMed] [Google Scholar]
- Terradas F, Wyler H (1991) The Secodopas, natural pigments in Hygrocybe conica and Amanita muscaria. Phytochemistry 30: 3251-3253 [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ (1994) Clustal-W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22: 4673-4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezzini GF (1990) Génétique des bétalaines chez Portulaca grandiflora Hook. PhD thesis. University of Lausanne, Lausanne, Switzerland
- Trezzini GF, Zrÿd JP (1991) Characterization of some natural and semisynthetic betaxanthins. Phytochemistry 30: 1901-1903 [Google Scholar]
- Trezzini GF, Zrÿd J-P (1990) Portulaca grandiflora: a model system for study of the biochemistry and genetics of betalain synthesis. Acta Hortic 280: 581-585 [Google Scholar]
- Vogt T (2002) Substrate specificity and sequence analysis define a polyphyletic origin of betanidin 5- and 6-O-glucosyltransferase from Dorotheanthus bellidiformis. Planta 214: 492-495 [DOI] [PubMed] [Google Scholar]
- Vogt T, Grimm R, Strack D (1999) Cloning and expression of a cDNA encoding betanidin 5-O-glucosyltransferase, a betanidin- and flavonoid-specific enzyme with high homology to inducible glucosyltransferases from the Solanaceae. Plant J 19: 509-519 [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Kobayashi N, Yoshitama K, Teramoto S, Komamine A (2001) Isolation and purification of tyrosine hydroxylase from callus cultures of Portulaca grandiflora. Plant Cell Physiol 42: 969-975 [DOI] [PubMed] [Google Scholar]
- Zaiko M (2000) Colour-specific genes from betalain producing plants. PhD thesis. University of Lausanne, Lausanne, Switzerland. http://www.unil.ch/lpc/docs/theses/zaiko/thesemaia.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.