SUMMARY
Substance abuse increases an individual's vulnerability to stress-related illnesses, which is presumably mediated by drug-induced neural adaptations that alter subsequent responses to stress. Here, we identify repressive histone methylation in nucleus accumbens (NAc), an important brain reward region, as a key mechanism linking cocaine exposure to increased stress vulnerability. Repeated cocaine administration prior to chronic social defeat stress potentiated depressive-like behaviors in mice through decreased levels of histone H3 lysine 9 dimethylation in NAc. Cre-mediated reduction of the histone methyltransferase, G9a, in NAc promoted increased susceptibility to social stress, similar to that observed with repeated cocaine. Conversely, G9a overexpression in NAc after repeated cocaine protected mice from the consequences of subsequent stress. This resilience was mediated, in part, through repression of BDNF-TrkB-CREB signaling, which was induced after repeated cocaine or stress. Identifying such common regulatory mechanisms may aid in the development of new therapies for addiction and depression.
Keywords: Social stress, cocaine, vulnerability, histone methylation, G9a, GLP, depression, affective disorders, CREB, Ras, nucleus accumbens, BDNF, TrkB
INTRODUCTION
Neuroadaptations to chronic cocaine, in brain areas critical for reward, persist long after the cessation of drug intake and are associated with drug relapse and with emotional signs of withdrawal, including depression-like symptoms (Der-Avakian and Markou, 2010; Nestler, 2005; Shaham and Hope, 2005). The convergence of aversive and rewarding symptoms suggests shared neural mechanisms, a hypothesis supported by high rates of comorbid mood and substance abuse disorders in humans (Ford et al., 2009). Indeed, it is well known that a mood disorder increases an individual's risk for substance abuse, and this has been widely corroborated in animal models, which illustrate that stress, like cocaine itself, can directly promote subsequent drug taking (Covington et al., 2005; Prasad et al., 1998; Schindler et al., 2010; Shaham et al., 2000; Sorg and Kalivas, 1991; Tidey and Miczek, 1997). Conversely, clinical evidence shows that substance abuse can increase an individual's risk for a mood disorder, however, the neurobiological mechanisms underlying this phenomenon remain largely unexplored.
Chromatin regulation, whereby post-mitotic neurons incorporate changes in transcriptional activity without altering DNA sequence, has received increasing attention for its role in mediating the lasting effects of drugs of abuse and stress on brain function (Borrelli et al., 2008; Grayson et al., 2010; Tsankova et al., 2007). One prominent type of chromatin modification, histone H3 lysine 9 dimethylation (H3K9me2), is controlled by a complex interaction between two histone methyltransferases, G9a and G9a-like protein (GLP) (Rice and Allis, 2001; Tachibana et al., 2001). Recent evidence has demonstrated that decreased expression of G9a in nucleus accumbens (NAc), a key brain reward region, along with corresponding reductions in H3K9me2, are important in mediating heightened levels of transcriptional and behavioral responses to repeated cocaine (Maze et al., 2010). Data obtained from postmortem human brain tissue, as well as from animal models, have further indicated a role for histone methylation in the promulgation of depressive-like behaviors and other psychiatric syndromes (Akbarian et al., 2005; Gupta et al., 2010; Schaefer et al., 2009; Tsankova et al., 2006). However, the specific genes at which cocaine- or stress-induced alterations in H3K9me2 influence addiction or depression symptoms have not yet been identified.
Here, we examine a possible role for H3K9me2 in mediating the effects of repeated cocaine on vulnerability to stress-induced depressive-like behaviors. We first show that, as seen in humans, cocaine increases the susceptibility of mice to chronic social defeat stress, an ethologically valid model of depression (Berton et al., 2006; Kudryavtseva et al., 1991; Rygula et al. 2005). We go on to show that cocaine-induced downregulation of G9a and H3K9me2 in NAc is a key mechanism by which the drug renders the animals more vulnerable to social stress. Subsequent investigation of G9a-dependent molecular mechanisms common to both cocaine- and stress-induced behavioral phenotypes uncovered an essential role for BDNF-TrkB-CREB signaling, which has been implicated in NAc in mediating both addiction- and depression-related phenomena (see Discussion). We show that chronic cocaine induces the small G protein, Ras, which in turn promotes BDNF-TrkB signaling and its subsequent activation of CREB, in NAc and thereby increases vulnerability to social defeat stress. Together, these results provide fundamentally novel insight into how prior exposure to a drug of abuse enhances vulnerability to depression and other stress-related disorders.
RESULTS
Cocaine Promotes Enhanced Susceptibility to Social Stress
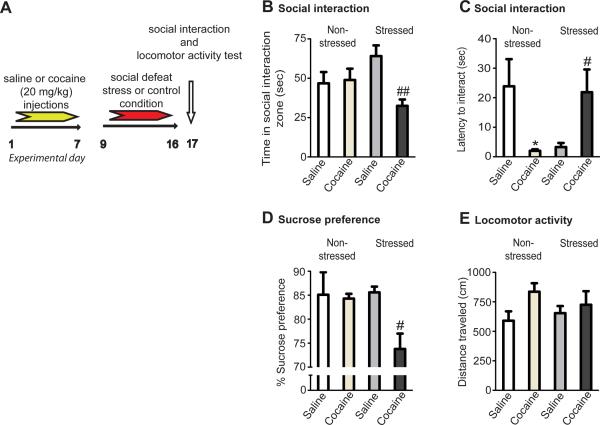
To investigate the impact of repeated cocaine on stress vulnerability, we utilized a sub-maximal version of social defeat. Previous work has shown that 10 days of defeat stress induces several cardinal depressive-like behaviors, such as social avoidance and reduced sucrose preference (Berton et al., 2006; Krishnan et al., 2007). Here, only 8 days of defeat stress were used, which in initial studies did not induce these symptoms. Next, either saline or a sensitizing regimen of cocaine was administered prior to initiating 8 days of defeat stress (Figure 1A). Seven days of repeated cocaine (20 mg/kg/day), immediately followed by 8 days of defeat stress, revealed social avoidance (Figure 1B) and diminished sucrose preference (Figure 1D). This is in contrast to control animals receiving saline prior to chronic stress, which showed no such deleterious behavioral responses. To further verify the potential long-lasting effects of cocaine on behavioral deficits observed after 8 days of defeat stress, animals were re-exposed to a low dose of cocaine (5 mg/kg) 24 hrs after the social interaction test (Figure S1A). Both stressed and non-stressed cocaine-treated animals displayed sensitized locomotor responses to cocaine. The social stress did not, however, potentiate cocaine-induced locomotor activity in cocaine-naïve mice (Figure S1B). Animals exposed to cocaine, in the absence of later social stress, displayed more rapid social interaction (i.e., decreased latency to interact) - an effect of cocaine that was completely reversed by exposure to 8 days of defeat stress (Figure 1C). The effects of cocaine, stress, or the combination of both stimuli had no impact on general levels of locomotor activity (Figure 1E).
Figure 1. Cocaine Enhances Vulnerability to Social Stress.
(A) Time-course of saline or cocaine (20 mg/kg/injection) treatment prior to control handling or sub-maximal (8 days) social defeat stress. (B) Repeated cocaine prior to sub-maximal social stress increases social avoidance during a social interaction test. (C) Repeated cocaine treatment decreases an animal's latency to interact with a target under non-stressed conditions, whereas prior drug administration increases an animal's latency to interact after social stress. (D) Repeated cocaine prior to sub-maximal social stress decreases sucrose preference. (E) Neither repeated cocaine nor social defeat affects locomotor activity in an open field. #, ## indicates significant differences from saline injections prior to defeat stress, p<0.05, or 0.01, respectively. * indicates significant differences from non-stressed controls injected with saline, p<0.05. Data are presented as average ± SEM, n = 8–10/group.
Cocaine, when self-administered during binges, increases thresholds for intra-cranial self-stimulation, indicating a withdrawal syndrome characterized by anhedonia (Markou and Koob, 1991). However, as shown in Figure 1B&D, we did not observe an effect of cocaine alone on social interaction or sucrose preference.
Histone Methylation is Similarly Regulated in NAc by Cocaine and Social Stress
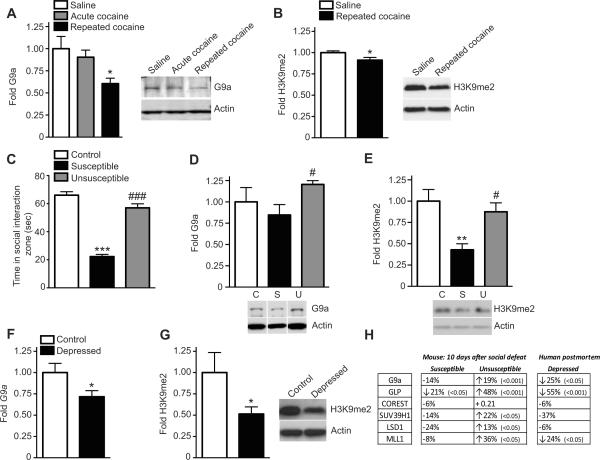
We have shown recently that, following repeated (not acute) cocaine, the repressive histone modification, H3K9me2, and its associated `writer' enzymes, G9a and GLP, are reduced in NAc, leading to the activation of numerous synaptic plasticity-related transcripts, increased dendritic spine density on medium spiny neurons (MSNs), and enhanced cocaine reward (Maze et al., 2010). To validate these earlier findings, animals were treated with either saline or cocaine (20 mg/kg/day) for 7 days. At 24 hrs after the final injection, NAc dissections were collected and analyzed for global alterations in G9a and H3K9me2 expression. Consistent with previous data, levels of G9a mRNA (saline vs. repeated cocaine, t10 = 2.559, p < 0.05) and protein (Figure 2A) and of H3K9me2 (Figure 2B) were reduced in NAc of animals treated with repeated, but not acute, cocaine. Such changes were absent in dorsal striatum (Figure S2A&B). Next, to examine potential similarities between cocaine- and stress-induced regulation of histone methylation in this brain region, the standard 10-day chronic social defeat stress protocol was used (Berton et al., 2006). As previously reported (Krishnan et al., 2007; Vialou et al., 2010), two distinguishable groups of defeated mice, susceptible and unsusceptible, were observed based on a measure of social avoidance, in which susceptible mice displayed decreased social interaction compared to both control and unsusceptible mice (Figure 2C). Ten days after the final defeat, susceptible and unsusceptible mice, as well as non-defeated controls, were analyzed for G9a protein levels in NAc. G9a expression was significantly decreased in NAc of susceptible mice compared to unsusceptible animals (Figure 2D), which were no different from non-stressed controls. These effects were not observed in dorsal striatum (Figure S2C). Consistent with a reduction in G9a expression in susceptible animals, global levels of H3K9me2 were decreased in NAc of these mice compared to both control and unsusceptible mice (Figure 2E). Additionally, Glp mRNA, similar to G9a mRNA, was significantly reduced in NAc of susceptible mice, a molecular response that was absent in unsusceptible mice (Figure 2H). Numerous other repressive chromatin modifiers, such as Suv39h1, many of which form multimeric binding complexes with G9a and GLP (Fritsch et al., 2010), were also significantly induced in NAc of unsusceptible mice (Figure 2H), indicating that increased repressive chromatin regulation may contribute to pro-adaptive responses to stressful stimuli. In dorsal striatum, in contrast, H3K9me2 was significantly increased after social stress in both susceptible and unsusceptible mice (Figure S2D). Regulation of H3K9me2 in NAc after chronic social stress is specific for this mark, which is euchromatic, as global levels of the associated heterochromatic mark H3K9me3 were unaltered in NAc of both susceptible and unsusceptible mice (Figure S3A).
Figure 2. Cocaine, Social Stress, and Clinical Depression Similarly Regulate Repressive Histone Methylation in NAc.
Repeated cocaine decreases (A) G9a and (B) H3K9me2 protein levels in NAc. (C) Mice subjected to chronic (10 days) social stress can be grouped into susceptible and unsusceptible subpopulations. (D) G9a shows a trend for reduced levels in susceptible mice compared to controls, but is significantly reduced in susceptible animals compared to unsusceptible mice. (E) H3K9me2 expression is reduced in susceptible animals, an effect not seen in unsusceptible animals. For all experiments, n=6–7/group. (F) G9a and (G) H3K9me2 protein are reduced in NAc of human postmortem depressed subjects, n = 8–10/group. *, ** and *** indicates significant differences from control condition, p<0.05, p<0.01 and p<0.001, respectively. #, ##, and ### indicate significant differences from susceptible mice, p<0.05, p<0.01 and p<0.001, respectively. Data are presented as average ± SEM. (H) mRNA expression levels for chromatin modifying enzymes were quantified in the NAc of control, susceptible, and unsusceptible mice after 10 days of social stress, and compared between clinically depressed and control individuals using postmortem tissue. Significant changes are noted by an arrow, and a p value. Increased expression of several genes was observed in unsusceptible mice, as compared to susceptible animals. Likewise, many of these genes were significantly decreased in clinical depression. For mouse work, n = 8/group, and for human analysis, n = 8–10/group.
To extend these findings in mice to clinical depression, we evaluated G9a and Glp levels, as well as other repressive chromatin modifiers, in NAc of postmortem human depressed patients (all symptomatic at their time of death – see Supplemental Materials for detailed methods on tissue collection). Similar to results observed in mice susceptible to social stress, G9a (Figure 2F) and Glp (Figure 2H) mRNA levels were significantly reduced in these patients. Numerous other enzymes involved in transcriptional repression—shown to be significantly induced in NAc of unsusceptible mice–were also downregulated in NAc of human depressed subjects (Figure 2H). Consistent with decreased G9a and Glp expression in NAc of depressed humans, global levels of H3K9me2 were also significantly reduced (Figure 2G). Unexpectedly, H3K9me3 protein expression was decreased in NAc of human depressed cases (Figure S3B), a finding inconsistent with data from our social defeat model, where no changes occurred in susceptible or unsusceptible mice.
G9a in NAc Promotes Resilience to Chronic Social Stress
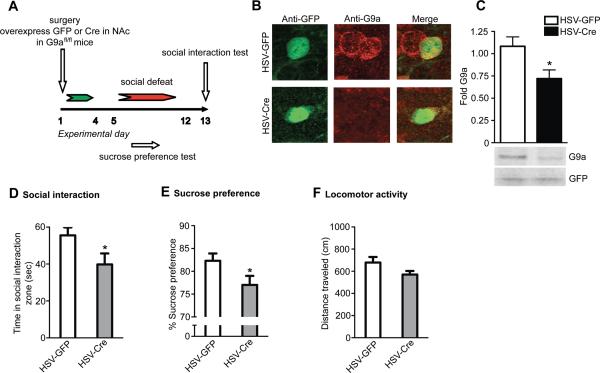
We next investigated the role of stress-induced alterations in G9a/GLP and H3K9me2 in NAc in controlling vulnerability to social stress. Animals were subjected to 10 days of chronic social defeat and, 24 hrs after the final stress episode following a social interaction test, randomly selected susceptible mice received intra-NAc injections of herpes simplex virus (HSV) vectors expressing either wildtype G9a-GFP, which has previously been demonstrated to induce H3K9me2 in this brain region (Maze et al., 2010), or GFP as a control (Figure 3A). Mice were tested for social interaction four days after viral surgery, a time at which maximal transgene expression is seen (Figure 3B), as verified by western blotting (Figure 3C) and quantitative PCR (qPCR) (Figure S4A). As expected, stressed animals overexpressing GFP displayed reduced social interaction compared to GFP-expressing non-stressed controls. In contrast, G9a overexpression, which mimics induction of endogenous G9a in NAc of unsusceptible animals, blocked the chronic stress-induced deficits in social interaction (Figure 3D). GFP and G9a overexpression in both stressed and non-stressed animals had no effect on general locomotor activity (Figure 3E). These data support a role for G9a and H3K9me2 in mediating resilience to chronic social stress.
Figure 3. G9a Overexpression in NAc Reverses Social Defeat-induced Social Deficits.
(A) Time-course of chronic (10 days) social defeat stress and HSV-GFP/HSV-G9a overexpression in NAc. (B) Immunohistochemical analysis of HSV-G9a overexpression in NAc. (C) Western blot analysis of G9a protein in NAc four days after HSV infection. (D) Chronic social defeat stress reduces time spent in the social interaction zone during a social interaction test, an effect that can be reversed by viral overexpression of G9a in NAc. (E) Neither social stress nor G9a overexpression affects locomotor activity in an open field. * indicates a significant difference from control condition (non-defeated mice infected with GFP), p<0.05, and # indicates a significant difference from defeated mice infected with GFP, p<0.05. Data are presented as average ± SEM, n = 6 group.
G9a Repression in NAc Promotes Susceptibility to Social Stress
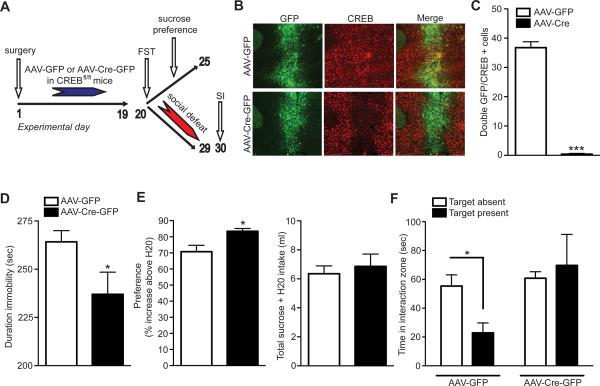
To directly test whether G9a and H3K9me2 repression in response to repeated cocaine mediates the increased susceptibility to social defeat stress observed under these conditions, we examined the influence of knocking down G9a in NAc (Maze et al., 2010) on the development of stress-induced depressive-like behaviors. G9afl/fl mice were injected intra-NAc with HSV vectors expressing GFP or Cre-GFP before being subjected to sub-maximal (8 days) defeat stress (Figure 4A). G9a knockdown in NAc, which was confirmed immunohistochemically (Figure 4B) and quantitatively via western blotting (Figure 4C) and qPCR (Figure S4B), promoted increased susceptibility to social stress, similar to the effect of repeated cocaine (Figure 4D). This G9a knockdown also reduced sucrose preference after social defeat (Figure 4E), but had no effect on baseline locomotor activity (Figure 4F).
Figure 4. G9a Repression in NAc Promotes Susceptibility to Stress.
(A) Time-course of Cre-mediated G9a knockdown in NAc and subsequent sub-maximal (8 days) social defeat stress. (B) Immunohistochemical and (C) western blot validation of G9a knockdown in NAc using HSV-Cre-GFP viruses in G9afl/fl mice. (D) G9a repression in NAc enhances social avoidance in a social interaction test. (E) G9a depletion in NAc promotes anhedonia as measured by sucrose preference. * indicates a significant difference from control condition (defeated mice infected with GFP), p<0.05. Data are presented as average ± SEM, n = 6–7/group. (E) G9a knockdown does not affect locomotor activity in an open field.
G9a Overexpression in NAc Prevents Cocaine-Induced Vulnerability to Social Stress
The findings that G9a repression in NAc, which occurs after repeated cocaine, increases an animal's vulnerability to subsequent stress, and that G9a overexpression, which occurs in unsusceptible mice, reverses the behavioral deficits observed in susceptible animals, support the interpretation that cocaine-induced repression of G9a and H3K9me2 in this brain region renders animals more vulnerable to future stress experiences. To directly examine this possibility, we increased G9a expression in NAc after repeated cocaine to test whether such gene `replacement' was sufficient to prevent cocaine-induced vulnerability to subsequent stress episodes. A compressed sub-maximal social defeat protocol was employed to accommodate the time-course of HSV expression: animals were injected daily with saline or cocaine (20 mg/kg/day) for 7 days, followed by 8 defeats over a course of 4 days of (2×/day) (Figure 5A). Using this compressed protocol, animals receiving prior cocaine still exhibited increased susceptibility to repeated social stress, as evidenced by increased levels of social avoidance (Figure 5B), without deficits in general locomotor activity (Figure 5C), similar to observations using the 8-day sub-maximal protocol.
Figure 5. G9a Overexpression in NAc Prevents Cocaine-induced Vulnerability to Stress.
(A) Time-course of saline or cocaine (20 mg/kg/injection) prior to control handling or compressed sub-maximal (8 defeats over 4 days) social defeat stress. (B) Repeated cocaine prior to compressed social stress increases social avoidance during a social interaction test. (C) Repeated cocaine does not affect locomotor activity in an open field following compressed social stress. * indicates significant differences from mice injected with saline, p<0.05, n = 5–6/group. (D) Time-course of saline or cocaine (20 mg/kg/injection) prior to G9a overexpression, followed by compressed social defeat stress. (E) Overexpression of G9a in NAc following repeated cocaine significantly reduces cocaine-induced social avoidance during a social interaction test. (F) Neither prior cocaine, social stress, nor G9a overexpression affects locomotor activity in an open field. * indicates a significant difference from control condition (non-defeated mice infected with GFP), p<0.05, and # indicates a significant difference from defeated mice infected with GFP, p<0.05, n = 10 mice/group. Data are presented as average ± SEM.
To test the role of G9a in cocaine-induced vulnerability to social stress, animals were injected once a day for 7 days with cocaine (20 mg/kg/day), before being injected intra-NAc with HSV-GFP or HSV-G9a-GFP, followed by 8 social defeats over 4 days (Figure 5D). As expected, cocaine-treated HSV-GFP animals displayed increased vulnerability to social stress, as measured by social avoidance behavior. In contrast, cocaine-treated HSV-G9a-GFP animals did not display such deficits (Figure 5E), indicating that increasing G9a expression in NAc after repeated cocaine - and thereby opposing the cocaine-induced repression of endogenous G9a/GLP - is sufficient to prevent drug-induced vulnerability to subsequent stressful experiences without affecting baseline locomotor activity (Figure 5F).
G9a Repression by Cocaine Promotes Increased Vulnerability to Stress through Enhanced BDNF-TrkB Signaling in NAc
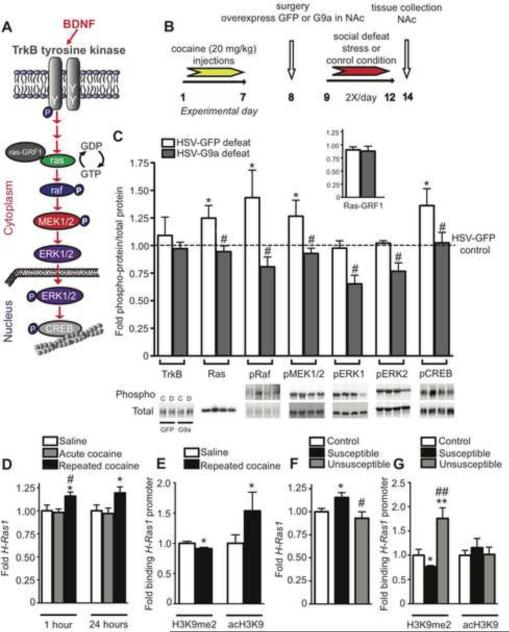
To gain insight into the molecular mechanisms by which alterations in G9a/GLP and H3K9me2 in NAc controls cocaine-induced vulnerability to stress, we focused on BDNF-TrkB signaling, given the considerable overlap between the regulation of this pathway (Figure 6A) in the development of addictive- and depressive-like behaviors (see Discussion). Animals were treated×X/day) (Figure 6B). As was shown in Figure 5, such G9a `replacement' in NAc after repeated cocaine reversed cocaine's enhancement of stress vulnerability. At 48 hrs after the final defeat experience, virally-infected NAc tissue was analyzed for alterations in BDNF-TrkB signaling. Consistent with increased BDNF-TrkB signaling observed in NAc after chronic social defeat stress (see Krishnan et al., 2007), numerous other components of this signaling cascade, not previously examined, were upregulated by social stress in cocaine-experienced animals: including increased levels of phospho-Raf, phospho-MEK1/2, and phospho-CREB (Figure 6C and Figure S5C,E&G). Although ERK1/2 has previously been demonstrated to display robust phosphorylation/activation after chronic social stress (Krishnan et al., 2007), such phosphorylation was not observed using our modified defeat protocol, possibly the result of an increased baseline level of phospho-ERK1/2 in NAc due to prior cocaine in these animals, as reported for cocaine previously (Valjjent et al., 2000). Alternatively, such differences in ERK1/2 phosphorylation may result from the sub-maximal defeat protocol used. We also observed induction of total levels of Ras in NAc of mice treated chronically with cocaine followed by repeated social defeat (Figure 6C). In contrast, total levels of other proteins in this cascade, including BDNF, TrkB, Raf, MEK1/2, ERK1/2, and CREB were not altered. Also unaltered was Ras-GRF1, a guanine nucleotide exchange factor which activates Ras (Figure 6C inset). These results suggest that induction of Ras expression per se may be responsible for activating downstream components of this signaling pathway necessary for the cocaine enhancement of depressive-like behaviors (Figure 6C).
Figure 6. Repeated Cocaine and Chronic Social Stress Induce Ras and Downstream BDNFTrkB Signaling in NAc.
(A) Cartoon depicting stimulus-dependent regulation of the BDNF-TrkB signaling cascade. (B) Time-course of saline or cocaine (20 mg/kg/injection) prior to G9a overexpression, followed by control handling or compressed sub-maximal (8 defeats over 4 days) social defeat stress and NAc tissue collection. N = 10 mice/group (C) Compressed social stress following repeated cocaine promotes increased expression of Ras, a member of the BDNF-TrkB signaling cascade, thereby increasing the phosphorylation state of downstream members of the pathway. G9a overexpression, following prior cocaine exposure, prevents stress-induced increases in Ras signaling, and decreases the activity (phospho levels) of downstream signaling molecules, including Raf, MEK1/2, ERK1/2 and CREB. The Ras activator, Ras-GRF1, is unaffected by social stress and G9a overexpression (inset). * indicates a significant difference from control condition (non-defeated mice infected with GFP), p<0.05, and # indicates a significant difference from defeated mice infected with GFP, p<0.05, n = 8–9/group. (D) Repeated, but not acute, cocaine treatment increases H-Ras1 mRNA expression in NAc both at 1 and 24 hrs. # indicates a significant difference from an acute cocaine, p<0.05. (E) Repeated cocaine reduces H3K9me2 association and increases acH3K9 binding to the H-Ras1 promoter, n = 4/group (5 mice pooled/n). (F) Like repeated cocaine, chronic (10 days) social stress increases H-Ras1 mRNA expression in NAc of susceptible mice only, n = 7/group. # indicates a significant difference from susceptible mice, p<0.05. (E) Susceptibility to social stress reduces H3K9me2 binding to the H-Ras1 promoter, whereas this repressive mark is increased at the H-Ras1 promoter in unsusceptible mice (no changes were observed in acH3K9 binding after social defeat stress), n = 4/group (5 mice pooled/n). * indicates a significant difference from saline injected or control mice, p<0.05,. Data are presented as average ± SEM.
Overexpression of G9a, which blocks cocaine-induced vulnerability to stress, significantly reduced cocaine- and stress-mediated increases in Ras expression in NAc, without affecting total protein levels of any of the other members of this pathway examined (Figure 6C and Figure S5A–H). NAc-specific overexpression of G9a did, however, significantly reduce the phosphorylation state of all downstream signaling molecules, including phospho-Raf, phospho-MEK1/2, phospho-ERK1/2, and phospho-CREB, indicating that transcriptional repression of Ras activity by G9a suppresses the activity of this entire signaling cascade. It is important to note that G9a overexpression had no effect on total levels or phosphorylation state of any of the proteins examined in this pathway under control conditions (i.e., HSV-G9a in cocaine- and stress-naïve mice), suggesting that G9a acts in a stimulus-dependent manner to block BDNF-TrkB signaling.
To further investigate the possibility that cocaine's induction of Ras contributes to increased stress vulnerability, we examined expression of H-Ras1, the Ras transcript most predominately expressed in adult brain, in NAc of animals receiving either saline, acute, or repeated cocaine, either immediately after cocaine exposure (1 hr) or 24 hrs after the final drug dose. Although H-Ras1 mRNA expression was unaffected by a single dose of cocaine, it was significantly induced by repeated cocaine at 1 hr, an effect that persisted for 24 hrs (Figure 6D). A substantial literature indicates functional differences between NAc and dorsal striatum in the development of stress- and drug-induced behaviors (Saka et al. 2004; Di Ciano et al. 2008; Dias-Ferreira et al. 2010). We thus investigated Ras, ERK, and CREB in dorsal striatum in response to repeated cocaine or stress (Figure S6A–G). Phospho-CREB was decreased in dorsal striatum by acute cocaine, and increased by repeated cocaine, similar to our observations in NAc (Figure S6D). Cocaine had no effect on Ras or ERK in dorsal striatum. Ras protein levels were increased in dorsal striatum after chronic social stress, but in unsusceptible mice (Figure S6E), whereas Ras induction in NAc was seen in susceptible mice only. These data highlight the importance and potential divergence of Ras-ERK-CREB signaling in NAc vs. dorsal striatum in chronic drug and stress models (Berton et al., 2006; Krishnan et al., 2007).
In NAc, the expression of two related GTPases, Cdc42 and Rac1, which can also be activated in response to TrkB signaling, were unaltered after acute or repeated cocaine (Figure S7A&B). Since G9a overexpression in NAc selectively repressed Ras induction after social stress in cocaine-experienced animals, we investigated whether H-Ras1 represents a direct target of G9a, and whether H-Ras1 expression correlates with changes in H3K9me2 promoter binding after either repeated cocaine or social defeat stress. Chromatin immunoprecipitation (ChIP) was performed using anti-G9a, anti-H3K9me2, or anti-acH3K9 antibodies to examine their binding to the H-Ras1 gene promoter 24 hrs after repeated cocaine or social stress. Consistent with changes in Ras expression, H3K9me2 displayed reduced (complemented by increased acH3K9) binding to the H-Ras1 promoter following repeated (Figure 6E), but not acute (data not shown, p > 0.05), cocaine; such reduced binding of H3K9me2 was associated with a similar reduction in G9a binding to the H-Ras1 promoter after repeated cocaine (t6 = 1.960, p < 0.05).
To verify that Ras regulation in NAc influences the development of both addictive- and depressive-like behaviors, mice were socially defeated for 10 days and their NAc were analyzed for H-Ras1 expression. H-Ras1 mRNA was significantly induced in NAc of susceptible, but not unsusceptible, mice 10 days after the last defeat episode (Figure 6F). Like repeated cocaine exposure, social stress reduced H3K9me2 binding to the H-Ras1 promoter in susceptible mice only, whereas unsusceptible mice displayed increased H3K9me2 binding with no changes observed in acH3K9 promoter association(Figure 6G).
CREB Activity in NAc Promotes Pro-Depressive Behavioral Phenotypes
To verify that G9a-dependent alterations in Ras-CREB signaling after repeated cocaine or chronic social defeat directly affects behavioral responses to stress, we examined the effects of manipulating CREB on the development of depressive-like behaviors. Although CREB activity in NAc has been implicated in depressive-like behavior in routine assays such as the forced swim and sucrose preference tests (Carlezon et al., 2005), it has not to date been examined in the social defeat paradigm. Moreover, this previous work relied solely on the use of overexpression systems, which are prone to artifact. We thus generated a conditional Crebfl/fl mouse line (see Figure S8 and Supplemental Materials for detailed methods) to directly study the role of endogenous CREB in depression-like behavior. Following generation and validation of the line, adult Crebfl/fl mice were injected intra-NAc with adeno-associated virus (AAV) vectors expressing GFP or Cre-GFP. At 18 days after viral injections (a time at which transgene expression is maximal), separate cohorts of animals were subjected to a battery of tests of depressive-like behaviors (Figure 7A). Knockdown of CREB was verified immunohistochemically and by counting CREB+ cells in NAc of AAV-Cre-GFP injected Crebfl/fl mice (Figure 7B&C). Consistent with our hypothesis that G9a induction mediates behavioral resilience to chronic social stress in part through downregulation of CREB activity in NAc, AAV-Cre-GFP expressing Crebfl/fl mice displayed consistent antidepressant-like behavioral responses in the social defeat paradigm (i.e., decreased social avoidance), forced swim test (i.e., decreased immobility), and sucrose preference test (i.e., increased sucrose preference), compared to AAV-GFP expressing animals (Figure 7D–F).
Figure 7. Local Knockdown of Endogenous CREB in NAc Promotes Antidepressant-like Behavioral Phenotypes.
(A) Time-course of CREB knockdown in Crebfl/fl mice and subsequent analyses of depression associated behaviors. (B) Immunohistochemical validation of CREB knockdown in Crebfl/fl mice using AAV-Cre-GFP viruses. (C) Quantification of double labeled GFP and CREB positive cells in NAc following knockdown in Crebfl/fl mice using AAV-Cre-GFP viruses. *** indicates a significant difference from AAV-GFP, p<0.001, n = 5/group. (D) Viral-mediated knockdown of CREB in NAc reduces social avoidance behavior following chronic (10 days) social stress. (E) Knockdown of CREB in NAc decreases the duration of immobility in the forced swim test. (F) Knockdown of CREB in NAc increases sucrose preference without affecting total levels of fluid (sucrose plus water) intake. * indicates a significant difference from control condition (defeated mice infected with GFP), p<0.05, n = 6–8/group. Data are presented as average ± SEM.
DISCUSSION
Here, we demonstrate that repeated cocaine increases the severity of depressive-like responses to social stress in mice—a phenomenon that parallels high co-morbid rates of substance abuse and mood disorders in humans. Furthermore, our data reveal a critical role for repressive histone methylation in NAc in mediating this cocaine-induced vulnerability to social stress. We show that repeated cocaine reduces global levels of H3K9me2 in this brain region, and its associated writer enzymes, G9a and GLP, which enhance susceptibility to subsequent social defeat stress. We demonstrate similar reductions in H3K9me2 and G9a/GLP levels in NAc of depressed humans and of mice subjected to chronic social defeat stress, but only in those animals susceptible to the negative consequences of repeated stress. We then establish that such downregulation of G9a and H3K9me2 in NAc mediates cocaine enhancement of stress vulnerability by demonstrating that local knockout of G9a in this brain region is sufficient to enhance an animal's vulnerability to social stress, while overexpression of G9a in NAc blocks the ability of chronic cocaine to increase stress susceptibility.
An important role for repressive H3K9me2 modifications in the regulation of both cocaine and stress responses comes from recent ChIP-chip studies which characterized altered H3K9me2 binding in NAc, genome-wide, in response to repeated cocaine or chronic social defeat stress (Renthal et al., 2009; Wilkinson et al., 2009). Interestingly, we found that a majority of changes in H3K9me2 binding observed in NAc of susceptible mice were reversed by chronic treatment with standard antidepressant treatments and were not observed in unsusceptible animals (Wilkinson et al., 2009). Although chronic cocaine and chronic social defeat stress similarly regulate repressive histone methylation in NAc, development of therapeutics to target enzymes regulating these processes would be difficult, given the ubiquitous nature of histone methyltransferases and demethylases. Therefore, it will be important to identify downstream proteins affected by such alterations in histone methylation, with the hopes that such targets may be more suitable for future drug interventions.
Previous studies from our laboratory have shown that histone acetylation is persistently increased in NAc after chronic social stress (Covington et al., 2009). This effect was interpreted to be adaptive, since mimicking the effect by locally infusing histone deacetylase (HDAC) inhibitors into NAc exerts antidepressant-like actions in several behavioral assays (Covington et al., 2009). Repeated cocaine has also been demonstrated to increase histone acetylation in this brain region, a phenomenon shown to increase the rewarding, reinforcing, and locomotor-activating properties of the drug (Kumar et al., 2005; Renthal et al., 2007; Sanchis-Segura et al., 2009; Schroeder et al., 2008; Sun et al., 2008; Wang et al., 2010). These findings indicate that, in contrast to cocaine repression of G9a and H3K9me2 in NAc, cocaine induction of histone acetylation in this brain region exerts the opposite effect and protects animals from the deleterious consequences of chronic stress.
Numerous biochemical pathways have been implicated in stress- and cocaine-induced behaviors, whereby these stimuli produce similar alterations in the expression or function of many types of signaling proteins. A striking example is the BDNF-TrkB cascade, which is upregulated in NAc by both cocaine and stress exposures and promotes addictive- and depressive-like behaviors (Bahi et al., 2008; Berglind et al., 2009; Berton et al., 2006; Cleck et al., 2008; Eisch et al., 2003; Graham et al., 2007; Green et al., 2010; Grimm et al., 2003; Horger et al., 1999). It should be noted, however, that, although G9a has previously been demonstrated to regulate Bdnf mRNA expression in NAc after repeated cocaine (Maze et al., 2010), local BDNF transcription in NAc does not affect behavioral responses to chronic stress (Krishnan et al., 2007). Therefore, it is unlikely that G9a's regulation of local BDNF expression in NAc after repeated cocaine treatment per se can fully account for the increased stress vulnerability observed in cocaine-exposed animals. Rather, our data implicate G9a regulation of Ras expression in NAc as an important mediator of this phenomenon. We show that Ras is similarly upregulated in NAc by both chronic cocaine and stress, and represents a novel mechanism through which these two stimuli act to alter cell signaling through manipulations of a common pathway (Figure 8). This is consistent with prior reports of Ras's effect on behavioral responses to cocaine (Fasano et al., 2009; Ferguson et al., 2006; Zhang et al., 2007). Importantly, H-Ras1 was one of the genes in our previous study that exhibited reduced H3K9me2 binding in NAc of susceptible mice only, with antidepressant treatment fully reversing this effect (Wilkinson et al., 2009). While we ascribe cocaine and stress regulation of Ras and CREB to the BDNF-TrkB signaling cascade, there are many other upstream pathways that could potentially be involved, including other neurotrophic factors, G-protein coupled receptors, and many Ras modulatory proteins, to name a few (Zhang et al., 2007; Flavell and Greenberg, 2008; Meitzen et al., 2011).
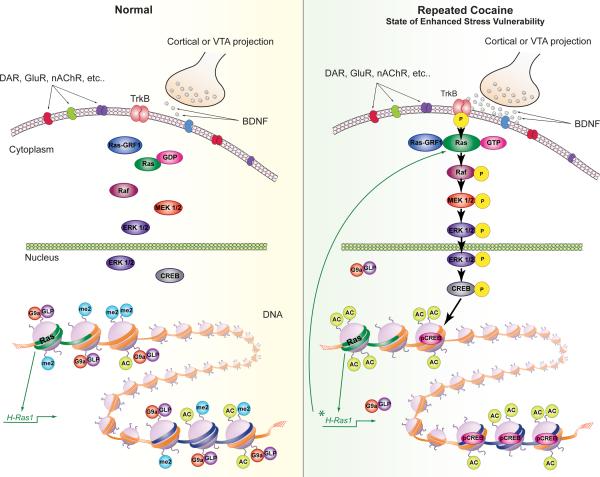
Figure 8. Enhanced Vulnerability to Stress Via Cocaine-Induced Priming of BDNF Signaling in NAc.
Repeated cocaine increases vulnerability to the depressive-like effects of social defeat stress via priming BDNF signaling through Ras induction in NAc. Under control conditions, BDNF activation of TrkB signaling is limited. However, after repeated cocaine, BDNF-TrkB signaling is elevated in NAc, causing enhanced phosphorylation and activity of downstream signaling mediators including CREB. This cocaine-initiated adaptive response occurs not only through increased BDNF signaling in NAc, but also through increased Ras expression as a result of decreased G9a binding at the H-Ras1 gene promoter. Chronic stress is associated with similar adaptations in this brain region. Ras also appears to be a target for CREB, creating a positive feed-forward loop, promoting CREB activation and Ras expression as well as depressive-like behavior.
Since induction of Ras in NAc by chronic cocaine and chronic stress would be expected to activate CREB, and CREB in this region has previously been shown to oppose cocaine reward and promote depression-like behavior (Barrot et al., 2002; Blendy, 2006; Carlezon et al., 2005; Pliakas et al., 2001), we focused on this protein further. We show that G9a overexpression in NAc, which represses Ras expression, also reduces levels of phospho-CREB in this brain region. Moreover, we show that local knockdown of endogenous CREB in NAc exerts antidepressant actions in the social defeat and other behavioral assays, consistent with several prior studies of CREB action in addiction and depression models. We have also shown that genome-wide patterns of phospho-CREB binding to gene promoters in NAc of susceptible mice after chronic social defeat stress are reversed by chronic antidepressant treatment and not seen in unsusceptible animals (Wilkinson et al., 2009). Furthermore, microarray analyses of NAc obtained from CREB overexpressing mice revealed that CREB activity in NAc was sufficient to induce H-Ras1 expression in this brain region (McClung and Nestler, 2003), similar to that observed here with both chronic cocaine and chronic stress. Likewise, overexpression of mCREB, a dominant negative form of CREB, reduced H-Ras1 expression in NAc (McClung and Nestler, 2003) and induced antidepressant-like effects in simple behavioral tests (Barrot et al., 2002; Carlezon et al., 2005; Pliakis et al., 2001). These data indicate a role for CREB activity in the potentiation of Ras expression, in which Ras may act, through a positive feedback loop, to increase its own expression by enhancing downstream CREB phosphorylation and activity (Figure 8). Taken together, BDNF-TrkB-Ras-CREB signaling in NAc may be one pathway through which both drugs of abuse and stress trigger shared molecular, cellular, and behavioral adaptations (Nestler et al., 2002; Pierce and Bari, 2001; Thomas et al., 2008). The contribution of the core and shell subdivisions of NAc to the phenomena examined here remains unknown. While the core and shell subserve distinct functions in drug and stress models (e.g., Di Ciano et al., 2008), the viral manipulations used in the current study cannot reliably distinguish these subregions, leaving examination of this important question to future investigations.
Depressive illnesses are among the most prevalent psychiatric disorders in the U.S., afflicting ~18% of the total population (Kessler et al., 2003). Only ~40% of all individuals treated with available antidepressants experience a full remission of symptoms, underscoring the high demand for better treatments (Berton and Nestler, 2006; Covington et al., 2010). Developing newer treatments has been limited by a scarcity of knowledge concerning the molecular biology of depression (Krishnan and Nestler, 2008). Experimental probes for mechanisms of cellular function have revealed increasingly complex interactions between enzymes and structural proteins that mediate processes ranging from genomic regulation to cellular morphology to neuronal excitability and synaptic plasticity. Here, we demonstrate that cocaine generates molecular events that become further elevated in response to chronic stress. Such findings may help to explain the large incidence of co-morbidity observed for substance abuse and mood disorders, and provide insight into the molecular underpinnings of these illnesses. These studies pave the way for the elucidation of target molecules involved in these processes and the development of improved treatment agents.
EXPERIMENTAL PROCEDURES
Animals
Prior to experimentation, 9–11 week old C57Bl/6 male mice (The Jackson Laboratory, Bar Harbor, ME, USA) were group housed at five per cage in a colony room set at constant temperature (23°C) on a 12 hr light/dark cycle (lights on from 7:00 A.M. to 7:00 P.M.) with ad libitum access to food and water. All protocols involving mice were approved by the Institutional Animal Care and Use Committee (IACUC) at Mount Sinai School of Medicine.
To knockout G9a specifically in NAc neurons, we used mice homozygous for a mutant floxed G9a allele (G9afl/fl) that were fully backcrossed to C57BL/6J, as described elsewhere (Maze et al., 2010; Sampath et al., 2007; Schaefer et al., 2009). Mice were injected stereotaxically in NAc with HSV vectors bicistronically expressing GFP or Cre + GFP under distinct promoters, as described previously (Maze et al., 2010). Immunohistochemistry was used to verify Cre-mediated knockdown of G9a (see Figure 4B). HSV vectors were allowed to express in NAc for a minimum of 4 days post-surgery because recombination in G9afl/fl mice was observed to be maximal and stable at this time, consistent with published reports (Maze et al., 2010). G9a overexpression experiments were conducted similarly using HSV vectors expressing either GFP or wildtype G9a plus GFP. HSV vectors have been extensively demonstrated, in numerous previous studies, to only infect neuronal cell bodies within the injected brain area, without affecting glial cells or efferent or afferent neurons.
To induce local deletion of the Creb transcript restricted to NAc neurons, mice were sterotaxically-injected intra-NAc with AAV vectors (serotype 2) expressing GFP or Cre-GFP between the age of 7–9 weeks. Immunohistochemical analysis was used to verify the efficiency of Cre-mediated recombination (see Figure 7B). AAV injected animals 18 days post-surgery were used since recombination in Crebfl/fl mice was stable and maximal at this time-point.
Behavior
Social Defeat Stress
Mice were subjected to 10 days, sub-maximal (8 days), or compressed sub-maximal (8 defeats over 4 days) social defeat based on published reports (Berton et al., 2006; Krishnan et al., 2007). In brief, experimental mice were exposed to a novel CD1 aggressor for five min daily, and then separated from the aggressor behind a protective barrier, which was perforated to allow for sensory contact for the remainder of the day. During the five min physical defeat visible signs of subordination were observed. Non-defeated control mice were housed two per cage under the same conditions as experimental mice, but without the presence of a CD1 mouse. After the last social defeat episode, experimental and control mice were housed individually.
Social Interaction
Tests for social interaction were performed as previously described (Berton et al., 2006). Briefly, mice were placed within a novel arena that included a small animal cage at one end. Movement (distance traveled, cm) was initially monitored for 250 sec for each stressed or control mouse in the absence of a CD1 mouse, immediately followed by an additional 250 sec in the presence of a CD1 mouse, which was positioned within the small animal cage. Locomotor activity (distance traveled) and information pertaining to the duration spent in the interaction zone were obtained using Ethovision 3.0 (Noldus, Attleboro, MA, USA) software.
Open Field Test
Open-field assessments were conducted in arenas similar to those used for the social interaction tests (without small cage enclosures). Ethovision videotracking-based methods (Noldus) were used to record the distance traveled and the time spent in the open arena and a delineated “center zone” (34 cm × 34 cm).
Sucrose Preference Test
Stoppers were fitted to 50 mL tubes with ballpoint sipper tubes to prevent leakage (Ancare, Bellmore, NY, USA) were filled with solutions of either 1% sucrose (in drinking water) or drinking water. All animals were acclimatized for three days before the two-bottle choice conditions prior to 4 additional days of choice testing (noon – noon), while mice underwent social defeat. Immediately prior to each daily social defeat, fluid levels were noted and the positions of the tubes were interchanged. Sucrose preferences were calculated as the average percentage of sucrose/water consumed for each of the 4 days.
Forced Swim Test
As previously described (Krishnan et al., 2007), each forced swim test was carried out in a 4 L beaker containing approximately 3 L of tap water, at a temperature of 25±1°C. The duration of time spent immobile in the arena over a six min trial was determined using ethovision videotracking-based methods (Noldus).
Locomotor Activity
Locomotor activity was assessed in a novel cage fitted within a photocell grid device (Med Associates Inc., St. Albans, VT, USA) that counted the number of ambulatory photo beam breaks within 5 min blocks during a 1 hr long period.
Viral-mediated Gene Transfer
Expression plasmids for Cre recombinase and wildtype G9a were subcloned into HSV or AAV vectors and packaged into high-titer viral particles as previously described (Berton et al., 2006; Maze et al., 2010). Mice were positioned in small-animal stereotaxic instruments, under ketamine (100 mg/kg)/xylazine (10 mg/kg) anesthesia, and their cranial surfaces were exposed. Thirty-three gauge syringe needles were bilaterally lowered into the NAc to infuse 0.5 μl of virus at a 10° angle (AP + 1.6; ML + 1.5; DV − 4.4). Infusions occurred at a rate of 0.1 μl/min. Animals receiving HSV injections were allowed to recover for at least 24 hrs following surgery.
RNA Isolation, Reverse Transcription and Quantitative PCR
NAc punches were dissected after treatment with cocaine, social defeat, or a combination thereof post-viral surgery and frozen on dry ice. Samples were then homogenized in Trizol and processed as previously described (Maze et al., 2010). See Supplemental Materials for more details.
Western Blotting
Frozen NAc or caudate putamen (CPu) tissue were homogenized in 30 μL of homogenization buffer containing 320 mM sucrose, 5 nM Hepes buffer, 1% SDS, phosphatase inhibitor cocktails I and II (Sigma, St. Louis, MO, USA) and protease inhibitors (Roche, Basel, Switzerland) using an ultrasonic processor (Cole Parmer, Vernon Hills, Illinois, USA). Protein concentrations were determined using a DC protein assay (Bio-Rad, Hercules, CA), and 10–30 μg of protein were loaded onto 18% or 4–15% gradient Tris-HCl polyacrylamide gels for electrophoresis fractionation (Bio-Rad, Hercules, CA). See Supplemental Materials for additional methods and listing of antibodies.
Immunohistochemistry
Mice were anesthetized with a lethal dose of chloral hydrate and perfused intra-cardiacally with 4% paraformaldehyde before being examined using single or double immunohistochemistry as previously described (Maze et al., 2010). See Supplemental Materials for additional methods and listing of antibodies.
Chromatin Immunoprecipitation
Freshly dissected NAc punches (14-gauge) were cross-linked with formaldehyde and prepared for ChIP as described previously (Maze et al., 2010). See Supplemental Materials for detailed methods.
Drugs
Cocaine-HCl was purchased from Sigma-Aldrich (St. Louis, MO, USA), was used at a concentration of 20 mg/kg/i.p., and mice were immediately returned to their home cage after each injection, unless otherwise noted.
Statistics
See Supplemental Materials for detailed statistical methods and statistical results.
Supplementary Material
ACKNOWLEDGEMENTS
Supported by grants from NIMH and NIDA (EJN) and NARSAD (HEC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akbarian S, Ruehl MG, Bliven E, Luiz LA, Peranelli AC, Baker SP, Roberts RC, Bunney WE, Jr., Conley RC, Jones EG, et al. Chromatin alterations associated with down-regulated metabolic gene expression in the prefrontal cortex of subjects with schizophrenia. Arch Gen Psychiatry. 2005;62:829–840. doi: 10.1001/archpsyc.62.8.829. [DOI] [PubMed] [Google Scholar]
- Bahi A, Boyer F, Chandrasekar V, Dreyer JL. Role of accumbens BDNF and TrkB in cocaine-induced psychomotor sensitization, conditioned-place preference, and reinstatement in rats. Psychopharmacology (Berl) 2008;199:169–182. doi: 10.1007/s00213-008-1164-1. [DOI] [PubMed] [Google Scholar]
- Barrot M, Olivier JD, Perrotti LI, DiLeone RJ, Berton O, Eisch AJ, Impey S, Storm DR, Neve RL, Yin JC, et al. CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc Natl Acad Sci U S A. 2002;99:11435–11440. doi: 10.1073/pnas.172091899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benus RF, Bohus B, Koolhaas JM, van Oortmerssen GA. Heritable variation for aggression as a reflection of individual coping strategies. Experientia. 1991;47:1008–1019. doi: 10.1007/BF01923336. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, Whitfield TW, Jr., LaLumiere RT, Kalivas PW, McGinty JF. A single intra-PFC infusion of BDNF prevents cocaine-induced alterations in extracellular glutamate within the nucleus accumbens. J Neurosci. 2009;29:3715–3719. doi: 10.1523/JNEUROSCI.5457-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci. 2006;7:137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- Blendy JA. The role of CREB in depression and antidepressant treatment. Biol Psychiatry. 2006;59:1144–1150. doi: 10.1016/j.biopsych.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Borrelli E, Nestler EJ, Allis CD, Sassone-Corsi P. Decoding the epigenetic language of neuronal plasticity. Neuron. 2008;60:961–974. doi: 10.1016/j.neuron.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr., Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Cleck JN, Ecke LE, Blendy JA. Endocrine and gene expression changes following forced swim stress exposure during cocaine abstinence in mice. Psychopharmacology (Berl) 2008;201:15–28. doi: 10.1007/s00213-008-1243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE, 3rd, Kikusui T, Goodhue J, Nikulina EM, Hammer RP, Jr., Miczek KA. Brief social defeat stress: long lasting effects on cocaine taking during a binge and zif268 mRNA expression in the amygdala and prefrontal cortex. Neuropsychopharmacology. 2005;30:310–321. doi: 10.1038/sj.npp.1300587. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Maze I, LaPlant QC, Vialou VF, Ohnishi YN, Berton O, Fass DM, Renthal W, Rush AJ, 3rd, Wu EY, et al. Antidepressant actions of histone deacetylase inhibitors. J Neurosci. 2009;29:11451–11460. doi: 10.1523/JNEUROSCI.1758-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE, 3rd, Vialou V, Nestler EJ. From synapse to nucleus: novel targets for treating depression. Neuropharmacology. 2010;58:683–693. doi: 10.1016/j.neuropharm.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A. Withdrawal from chronic exposure to amphetamine, but not nicotine, leads to an immediate and enduring deficit in motivated behavior without affecting social interaction in rats. Behav Pharmacol. 2010 doi: 10.1097/FBP.0b013e32833c7cc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ, Bolanos CA, de Wit J, Simonak RD, Pudiak CM, Barrot M, Verhaagen J, Nestler EJ. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: a role in depression. Biol Psychiatry. 2003;54:994–1005. doi: 10.1016/j.biopsych.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, Costa RM, Sousa N. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325:621–625. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Robbins TW, Everitt BJ. Differential effects of nucleus accumbens core, shell, or dorsal striatal inactivations on the persistence, reacquisition, or reinstatement of responding for a drug-paired conditioned reinforcer. Neuropsychopharmacology. 2008;33:1413–1425. doi: 10.1038/sj.npp.1301522. [DOI] [PubMed] [Google Scholar]
- Fasano S, D'Antoni A, Orban PC, Valjent E, Putignano E, Vara H, Pizzorusso T, Giustetto M, Yoon B, Soloway P, et al. Ras-guanine nucleotide-releasing factor 1 (Ras-GRF1) controls activation of extracellular signal-regulated kinase (ERK) signaling in the striatum and long-term behavioral responses to cocaine. Biol Psychiatry. 2009;66:758–768. doi: 10.1016/j.biopsych.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Fasano S, Yang P, Brambilla R, Robinson TE. Knockout of ERK1 enhances cocaine-evoked immediate early gene expression and behavioral plasticity. Neuropsychopharmacology. 2006;31:2660–2668. doi: 10.1038/sj.npp.1301014. [DOI] [PubMed] [Google Scholar]
- Flavell SW, Greenberg ME. Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu Rev Neurosci. 2008;31:563–590. doi: 10.1146/annurev.neuro.31.060407.125631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JD, Gelernter J, DeVoe JS, Zhang W, Weiss RD, Brady K, Farrer L, Kranzler HR. Association of psychiatric and substance use disorder comorbidity with cocaine dependence severity and treatment utilization in cocaine-dependent individuals. Drug Alcohol Depend. 2009;99:193–203. doi: 10.1016/j.drugalcdep.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch L, Robin P, Mathieu JR, Souidi M, Hinaux H, Rougeulle C, Harel-Bellan A, Ameyar-Zazoua M, Ait-Si-Ali S. A subset of the histone H3 lysine 9 methyltransferases Suv39h1, G9a, GLP, and SETDB1 participate in a multimeric complex. Mol Cell. 2010;37:46–56. doi: 10.1016/j.molcel.2009.12.017. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Flugge G. Social stress in tree shrews: effects on physiology, brain function, and behavior of subordinate individuals. Pharmacol Biochem Behav. 2002;73:247–258. doi: 10.1016/s0091-3057(02)00795-5. [DOI] [PubMed] [Google Scholar]
- Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci. 2007;10:1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- Graham DL, Krishnan V, Larson EB, Graham A, Edwards S, Bachtell RK, Simmons D, Gent LM, Berton O, Bolanos CA, et al. Tropomyosin-related kinase B in the mesolimbic dopamine system: region-specific effects on cocaine reward. Biol Psychiatry. 2009;65:696–701. doi: 10.1016/j.biopsych.2008.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson DR, Kundakovic M, Sharma RP. Is there a future for histone deacetylase inhibitors in the pharmacotherapy of psychiatric disorders? Mol Pharmacol. 2010;77:126–135. doi: 10.1124/mol.109.061333. [DOI] [PubMed] [Google Scholar]
- Green TA, Alibhai IN, Roybal CN, Winstanley CA, Theobald DE, Birnbaum SG, Graham AR, Unterberg S, Graham DL, Vialou V, et al. Environmental enrichment produces a behavioral phenotype mediated by low cyclic adenosine monophosphate response element binding (CREB) activity in the nucleus accumbens. Biol Psychiatry. 2010;67:28–35. doi: 10.1016/j.biopsych.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Kim SY, Artis S, Molfese DL, Schumacher A, Sweatt JD, Paylor RE, Lubin FD. Histone methylation regulates memory formation. J Neurosci. 2010;30:3589–3599. doi: 10.1523/JNEUROSCI.3732-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horger BA, Iyasere CA, Berhow MT, Messer CJ, Nestler EJ, Taylor JR. Enhancement of locomotor activity and conditioned reward to cocaine by brain-derived neurotrophic factor. J Neurosci. 1999;19:4110–4122. doi: 10.1523/JNEUROSCI.19-10-04110.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Berton O, Nestler E. The use of animal models in psychiatric research and treatment. Am J Psychiatry. 2008;165:1109. doi: 10.1176/appi.ajp.2008.08071076. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudryavtseva NN, Bakshtanovskaya IV, Koryakina LA. Social model of depression in mice of C57BL/6J strain. Pharmacol Biochem Behav. 1991;38:315–320. doi: 10.1016/0091-3057(91)90284-9. [DOI] [PubMed] [Google Scholar]
- Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, Russo SJ, Laplant Q, Sasaki TS, Whistler KN, et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Lucas LR, Celen Z, Tamashiro KL, Blanchard RJ, Blanchard DC, Markham C, Sakai RR, McEwen BS. Repeated exposure to social stress has long-term effects on indirect markers of dopaminergic activity in brain regions associated with motivated behavior. Neuroscience. 2004;124:449–457. doi: 10.1016/j.neuroscience.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Markou A, Koob GF. Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology. 1991;4:17–26. [PubMed] [Google Scholar]
- Maze I, Covington HE, 3rd, Dietz DM, LaPlant Q, Renthal W, Russo SJ, Mechanic M, Mouzon E, Neve RL, Haggarty SJ, et al. Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science. 2010;327:213–216. doi: 10.1126/science.1179438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, Nestler EJ. Regulation of gene expression and cocaine reward by CREB and DeltaFosB. Nat Neurosci. 2003;6:1208–1215. doi: 10.1038/nn1143. [DOI] [PubMed] [Google Scholar]
- Meitzen J, Luoma JI, Stern CM, Mermelstein PG. β1-Adrenergic receptors activate two distinct signaling pathways in striatal neurons. J Neurochem. 2011;116:984–995. doi: 10.1111/j.1471-4159.2010.07137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Bari AA. The role of neurotrophic factors in psychostimulant-induced behavioral and neuronal plasticity. Rev Neurosci. 2001;12:95–110. doi: 10.1515/revneuro.2001.12.2.95. [DOI] [PubMed] [Google Scholar]
- Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr. Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci. 2001;21:7397–7403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad BM, Ulibarri C, Sorg BA. Stress-induced cross-sensitization to cocaine: effect of adrenalectomy and corticosterone after short- and long-term withdrawal. Psychopharmacology (Berl) 1998;136:24–33. doi: 10.1007/s002130050535. [DOI] [PubMed] [Google Scholar]
- Renthal W, Kumar A, Xiao G, Wilkinson M, Covington HE, 3rd, Maze I, Sikder D, Robison AJ, LaPlant Q, Dietz DM, et al. Genome-wide analysis of chromatin regulation by cocaine reveals a role for sirtuins. Neuron. 2009;62:335–348. doi: 10.1016/j.neuron.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renthal W, Maze I, Krishnan V, Covington HE, 3rd, Xiao G, Kumar A, Russo SJ, Graham A, Tsankova N, Kippin TE, et al. Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron. 2007;56:517–529. doi: 10.1016/j.neuron.2007.09.032. [DOI] [PubMed] [Google Scholar]
- Rice JC, Allis CD. Histone methylation versus histone acetylation: new insights into epigenetic regulation. Curr Opin Cell Biol. 2001;13:263–273. doi: 10.1016/s0955-0674(00)00208-8. [DOI] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Flugge G, Ruther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav Brain Res. 2005;162:127–134. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Saka E, Goodrich C, Harlan P, Madras BK, Graybiel AM. Repetitive behaviors in monkeys are linked to specific striatal activation patterns. J Neurosci. 2004;24:7557–7565. doi: 10.1523/JNEUROSCI.1072-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath SC, Marazzi I, Yap KL, Krutchinsky AN, Mecklenbrauker I, Viale A, Rudensky E, Zhou MM, Chait BT, Tarakhovsky A. Methylation of a histone mimic within the histone methyltransferase G9a regulates protein complex assembly. Mol Cell. 2007;27:596–608. doi: 10.1016/j.molcel.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Sanchis-Segura C, Lopez-Atalaya JP, Barco A. Selective boosting of transcriptional and behavioral responses to drugs of abuse by histone deacetylase inhibition. Neuropsychopharmacology. 2009;34:2642–2654. doi: 10.1038/npp.2009.125. [DOI] [PubMed] [Google Scholar]
- Schaefer A, Sampath SC, Intrator A, Min A, Gertler TS, Surmeier DJ, Tarakhovsky A, Greengard P. Control of cognition and adaptive behavior by the GLP/G9a epigenetic suppressor complex. Neuron. 2009;64:678–691. doi: 10.1016/j.neuron.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler AG, Li S, Chavkin C. Behavioral stress may increase the rewarding valence of cocaine-associated cues through a dynorphin/k-opioid receptor-mediated mechanism without affecting associatve learning ormemory retrieval mechanisms. Neuropsychopharmacology. 2010;35:1932–1942. doi: 10.1038/npp.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder FA, Penta KL, Matevossian A, Jones SR, Konradi C, Tapper AR, Akbarian S. Drug-induced activation of dopamine D(1) receptor signaling and inhibition of class I/II histone deacetylase induce chromatin remodeling in reward circuitry and modulate cocaine-related behaviors. Neuropsychopharmacology. 2008;33:2981–2992. doi: 10.1038/npp.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Hope BT. The role of neuroadaptations in relapse to drug seeking. Nat Neurosci. 2005;8:1437–1439. doi: 10.1038/nn1105-1437. [DOI] [PubMed] [Google Scholar]
- Sorg BA, Kalivas PW. Effects of cocaine and footshock stress on extracellular dopamine levels in the ventral striatum. Brain Res. 1991;559:29–36. doi: 10.1016/0006-8993(91)90283-2. [DOI] [PubMed] [Google Scholar]
- Sun J, Wang L, Jiang B, Hui B, Lv Z, Ma L. The effects of sodium butyrate, an inhibitor of histone deacetylase, on the cocaine- and sucrose-maintained self-administration in rats. Neurosci Lett. 2008;441:72–76. doi: 10.1016/j.neulet.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Tachibana M, Sugimoto K, Fukushima T, Shinkai Y. Set domain-containing protein, G9a, is a novel lysine-preferring mammalian histone methyltransferase with hyperactivity and specific selectivity to lysines 9 and 27 of histone H3. J Biol Chem. 2001;276:25309–25317. doi: 10.1074/jbc.M101914200. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol. 2008;154:327–342. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Acquisition of cocaine self-administration after social stress: role of accumbens dopamine. Psychopharmacology (Berl) 1997;130:203–212. doi: 10.1007/s002130050230. [DOI] [PubMed] [Google Scholar]
- Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8:355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Valjent E, Corvol JC, Pages C, Besson MJ, Maldonado R, Caboche J. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci. 2000;20:8701–8709. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialou V, Robison AJ, Laplant QC, Covington HE, 3rd, Dietz DM, Ohnishi YN, Mouzon E, Rush AJ, 3rd, Watts EL, Wallace DL, et al. DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci. 2010;13:745–752. doi: 10.1038/nn.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Lv Z, Hu Z, Sheng J, Hui B, Sun J, Ma L. Chronic cocaine-induced H3 acetylation and transcriptional activation of CaMKIIalpha in the nucleus accumbens is critical for motivation for drug reinforcement. Neuropsychopharmacology. 2010;35:913–928. doi: 10.1038/npp.2009.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson MB, Xiao G, Kumar A, LaPlant Q, Renthal W, Sikder D, Kodadek TJ, Nestler EJ. Imipramine treatment and resiliency exhibit similar chromatin regulation in the mouse nucleus accumbens in depression models. J Neurosci. 2009;29:7820–7832. doi: 10.1523/JNEUROSCI.0932-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GC, Hoffmann J, Parelkar NK, Liu XY, Mao LM, Fibuch EE, Wang JQ. Cocaine increases Ras-guanine nucleotide-releasing factor 1 protein expression in the rat striatum in vivo. Neurosci Lett. 2007;427:117–121. doi: 10.1016/j.neulet.2007.09.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.