Abstract
The Simian Virus large T antigen (SVLT) induces replication of plasmids bearing the SV40 origin of replication (SV40 ori) within mammalian cells. The internal ribosomal entry site (IRES) is an element that allows for the co-translation of proteins from one polycistronic mRNA. Through the combination of these elements, IRES-dependent co-expression of a protein of interest and the SVLT, either constitutive or regulated, on plasmids bearing the SV40 ori generates a positive feedback loop, resulting in enhanced expression. A vector linking red florescent protein (RFP) to the IRES-SVLT element enhances florescence ~10-fold over that demonstrated from a vector lacking this element. In transfection-resistant CV-1 cells, the RFP-IRES-SVLT vector substantially increases the number of cells expressing detectable levels of RFP. Furthermore, inclusion of the IRES-SVLT/SV40 ori elements in standard luciferase-based reporter gene constructs and associated effectors results in marked increases in luminescent output and sensitivity, using the β-catenin/TCF pathway and the mammalian two-hybrid assay as models. Ultimately, vector systems combining these well-established elements (IRES-SVLT/SV40 ori) will increase the utility of transient transfection for the production of recombinant proteins, the use of transfection-resistant cell lines and the effectiveness of luciferase-based high throughput screening assays.
Keywords: SV40 large T antigen, IRES, luciferase, β-catenin, mammalian two-hybrid
Introduction
The Simian Virus 40 large T antigen (SVLT) regulates viral genome replication and expression in the host cell upon infection (1). When expressed alone, the SVLT functions as a proto-oncogene capable of inducing cellular transformation through direct actions on the retinoblastoma and p53 genes (2). A lesser-known function of the SVLT is as a model helicase capable of unwinding double-stranded DNA and initiating replication through direct binding to the SV40 origin of replication (SV40 ori) (1). The helicase properties of the SVLT have been exploited in the COS cell overexpression system. COS cells are CV-1 (African Green monkey kidney) cells stably expressing a replication-deficient SV40 genome, including the SVLT (3). Upon transient transfection, plasmids bearing the SV40 ori undergo SVLT-dependent replication on the order of 50 to 100 thousand copies per cell, resulting in markedly enhanced expression of the gene of interest on the plasmid. Calos and co-workers (4) also stably expressed the SVLT in the standard HEK293 cell line, generating HEK293T cells. In combination with shuttle vectors containing the SV40 ori, HEK293T cells are routinely used to generate high-titer retroviruses through SVLT-mediated replication of transiently transfected plasmids expressing the viral constituents, as originally described by Baltimore and co-workers (5). The requirement for stable expression of the SVLT limits the use of this overall process to these specific cell lines; raising the possibility that linked transient co-expression of the SVLT with a gene of interest may overcome this limitation.
Another element derived from viruses is the internal ribosomal entry site (IRES), which allows for the expression of two or more proteins from a single, polycistronic mRNA transcript by providing an internal binding site for ribosomes (6). The IRES sequence from the encephalomyocarditis virus (ECMV) has been used to generate mammalian vectors capable of linking expression of a gene of interest to an accessory protein in a biscistronic message. These accessory proteins include the green florescent protein and associated variants (7) and various antibiotic resistance genes (8), allowing for identification of transfected cells and the generation of stable cell lines expressing the gene of interest, respectively. Herein, the utility of transient expression of the SVLT directly linked to a gene of interest or to luciferase reporter gene constructs in conjunction with the SV40 ori is investigated. Mechanistically, one can envision that transient expression of the SVLT, either constitutively or under the control of an inducible response element, will initiate replication of plasmids bearing the SV40 ori, resulting in a positive feedback loop and enhanced expression of linked constituents. Thus, an over-expression vector and reporter gene constructs linking the SVLT to genes of interest using the IRES element are described.
Materials and Methods
Generation of constructs
The cDNA for the SV40 large T antigen (SVLT) was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Using the high fidelity pfu turbo polymerase (Agilent Technologies, Santa Clara, CA, USA) and PCR-mediated cloning, the SVLT was cloned downstream of the CMV promoter in the pcDNA3.1 vector (Invitrogen, Carlsbad, CA, USA) using EcoRI and XbaI. This vector also contains the SV40 ori. Restriction enzymes were either from Promega (Madison, WI, USA) or New England Biolabs (Ipswich, MA, USA). To generate an origin-less vector, the CMV-SVLT portion was cloned into the pIRESpuro2 plasmid (Clontech, Mountain View, CA, USA) using the BglII and XbaI sites. Using the pIRESpuro2 plasmid as a substrate, the IRES element was PCR-cloned into the pcDNA3.1-SVLT vector using EcoRI sites with the addition of NotI and XhoI on the 5’ side. A red florescent protein (RFP) variant (monomeric cherry, a generous gift for Dr. Roger Tsien) was cloned upstream of the IRES element using NheI and HindIII to yield the 3.1RFP-IRSV construct. The pBIND-Id and pACT-MyoD vectors were purchased from Promega, components of the Checkmate Mammalian Two-Hybrid system that function as positive controls due to the interaction between Id and MyoD. The pBIND vector expresses in-frame fusions with the DNA-binding domain of the yeast gal4 transcription factor, while the pACT vector directs fusions with the VP16 transactivation domain of the herpes simplex virus. Both pBIND and pACT contain an SV40 ori.
The parent vector for the firefly luciferase reporter genes was pTK-luc, which contains an enhancer-less, minimal thymidine kinase promoter with low basal activity (9,10). For the TK-TCF-Luc vector, four copies of the TCF/LEF response element were cloned upstream of the thymidine kinase promoter using BamHI and BglII cloning sites. The TK-TCF-Luc-IRSV plasmid was generated by sub-cloning the IRES-SVLT element into the TK-TCF-Luc vector using NotI on the 5’ side and PmeI/HpaI blunt cloning on the 3’ side. The gal4-responsive reporter gene was generated in the same manner except for the inclusion of four copies of the gal4 DNA-binding element cloned upstream of the TK promoter.
Hirt extraction
In order to estimate the extent of plasmid replication within transfected cells, episomally expressed plasmids were extracted from HEK293 cells using the method of Hirt (11) with modifications described by Seed and Aruffo (12). Briefly, HEK293 cells were transiently transfected with 100 ng of either pcDNA3.1-RFP or pcDNA3.1-RFP-IRSV in 6-well plates using Fugene 6 (Roche, Indianapolis, IN, USA). Forty-eight hours post-transfection, cells were washed in PBS, pelleted in microfuge tubes and extracted with a solution containing 0.6% SDS, and 10 mM EDTA, buffered with 25 mM HEPES, pH 7.4, for 10 minutes on ice. A final concentration of 1 M NaCl was added and tubes were mixed by inversion and placed on ice for eight hours. Lysates were spun down and supernatants were extracted with phenol:chloroform:isoamyl alcohol and ethanol precipitated after the addition of 10 µg of glycogen. Pellets were resuspended, transformed into chemically competent E. coli and the number of colonies generated per µg of the initially tranfected DNA was determined.
Immunoblotting
The effect of the SV40 ori during transient SVLT protein expression was analyzed in HEK293 cells. Cells were transiently transfected using Fugene 6 for forty-eight hours. Post-transfection, cells were pelleted and lysed in a buffer containing 25 mM HEPES, pH 7.4, 1% Triton-X 100 and 0.1% SDS, supplemented with a protease inhibitor cocktail (Sigma, Saint Louis, MO, USA). Whole cell lysates were analyzed by SDS-PAGE and immunoblotting to a PVDF membrane. Membranes were probed with antibodies specific to SVLT (Pab 108; sc-148) from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and bands detected using Western Lightning Plus enhanced chemiluminescence (Perkin-Elmer, Boston, MA, USA).
Florescence and luminescence measurements
HEK293 and CV-1 cells were plated at 80% confluence in culture slides (BD Falcon, Bedford, MA, USA), and transiently transfected with the RFP expression constructs using Fugene 6. Forty-eight hours later, cells were fixed with 4% paraformaldehyde and analyzed using a Radiance 2100 confocal microscope and the associated LaserSharp 2000 software (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Images of cells expressing the 3.1-RFP and 3.1-RFP-IRSV constructs were collected using the same laser intensity and gain. For florescence quantification, 10 cm dishes of HEK293 cells were transiently transfected for forty-eight hours and lifted from the plates using calcium- and magnesium-free hank’s balanced salt solution supplemented with 2 mM EDTA. Cells were placed in a clear, four sided cuvette and emission scans analyzed using a PTI Deltascan dual-wavelength fluorometer (Photon Technologies Incorporated, Lawrenceville, NJ, USA) with an excitation at 560 nm and emission scan between 580 and 690 nm. Florescence was also quantified using an EnVision Multilabel Reader (Perkin-Elmer, Boston, MA, USA) of HEK293 cells transiently transfected in black, 96-well plates using appropriate filter sets.
For quantification of luciferase activity, HEK293 cells were transiently transfected in 96-well plates with the noted combinations of effectors and reporter genes for forty-eight hours at 25 µg per well for effectors and reporters using Fugene6. Luciferase activity was analyzed using the Steady-Glo luciferase assay system (Promega) and a Victor 2 plate reader (Perkin-Elmer) in solid white 96-well assay plates.
Data Analysis
For all of the data shown, experiments were replicated a minimum of three independent investigations.
Results and Discussion
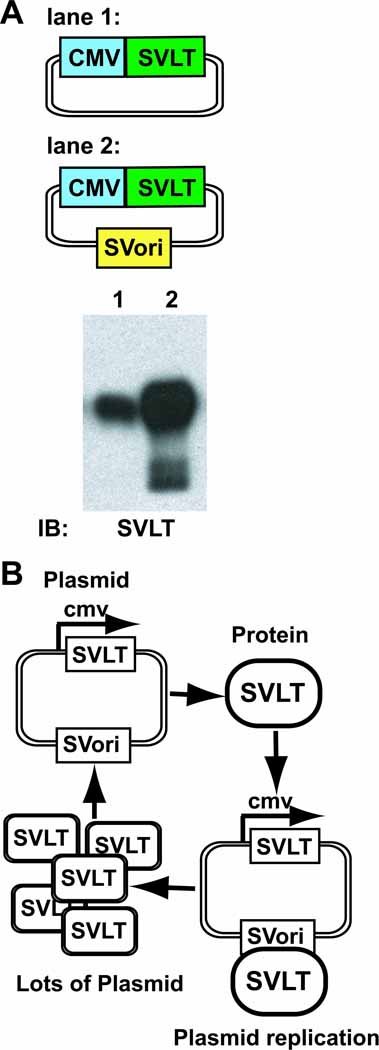
As previously stated, the stably expressed SVLT in COS7 and HEK293T cells markedly enhances transient expression of proteins on plasmids bearing the SV40 ori through vector replication. Vectors expressing the SVLT under the control of the constitutively active cytomegalovirus promoter (CMV), in the absence or presence of the SV40 ori, were generated (Figure 1A). The presence of the SV40 ori markedly enhances transient SVLT protein expression when compared to the vector lacking this element (Figure 1A). This finding demonstrates that transiently expressed SVLT induces replication of its own plasmid, resulting in super-enhanced expression due to a positive feedback loop, shown diagrammatically in Figure 1B. Thus transiently expressed SVLT is capable of inducing replication similar to that demonstrated for the stably expressed antigen in COS7 and HEK293T cells.
Figure 1.
Immunoblot analysis of SVLT expression in the absence and presence of the SV40 ori and a schematic illustrating the SVLT/SV40 ori positive feedback loop. (A) HEK293 cells were transiently transfected with SVLT expression vectors (0.5 µg) in the absence (lane 1) or presence (lane 2) of a linked SV40 ori. Whole cell lysates (50 µg) were analyzed by SDS-PAGE and immunoblotted with SVLT-specific antibodies. The blot shown is representative of three independent experiments. (B) A diagram depicting the SVLT/SV40 ori positive feedback loop responsible for the enhanced protein expression.
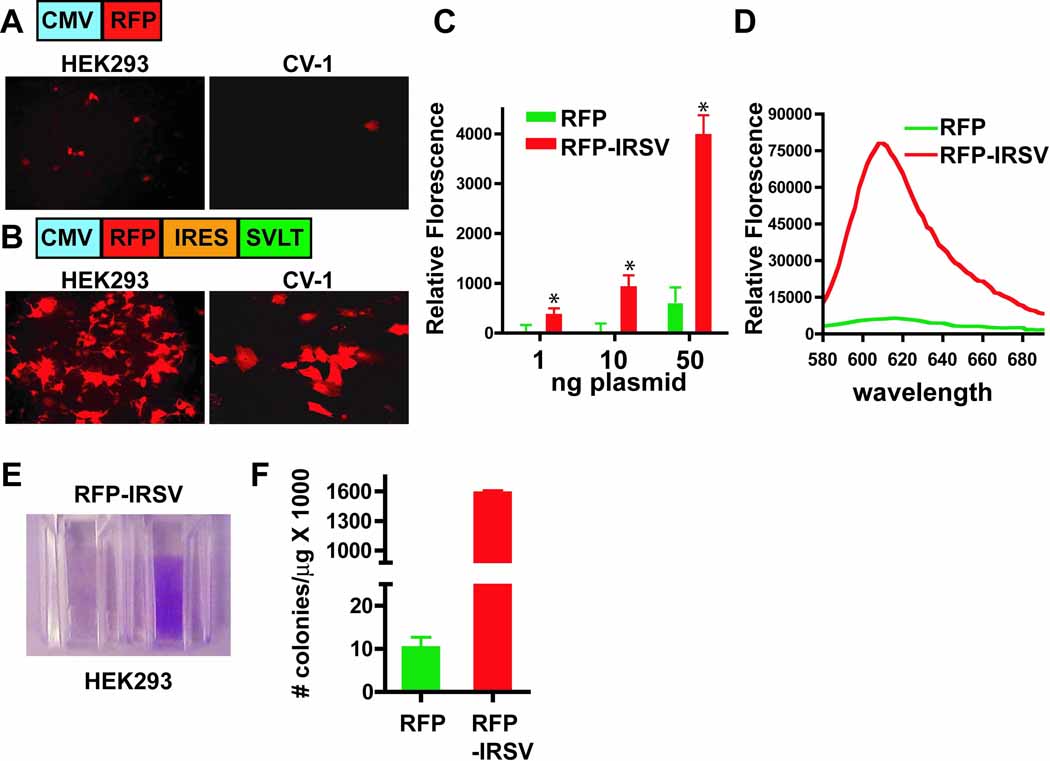
Through incorporation of the IRES element, enhanced expression of a gene of interest can be achieved via the SVLT/SV40 ori positive feedback loop. As a model protein, a red florescent protein (RFP) was cloned under the control of the CMV promoter in the absence (Figure 2A) or presence of a bicistronic message linked to the SVLT via the IRES element (Figure 2B). As shown in Figure 2 (compare A and B, left panels), RFP expression linked to the IRES-SVLT element is markedly enhanced in HEK293 cells transiently transfected with relatively small amounts of plasmid (40 ng/chamber slide well). Furthermore, RFP expression is readily detected in wells of a 96-well plate when transfected with only 1 and 10 ng of RFP-IRSV plasmid, while transfection of the standard RFP vector with these same amounts yields undetectable expression (Figure 2C). Using the standard FuGene6 transfection protocol, CV-1 cells are relatively resistant to transfection with rates at less than 10% (Figure 2A, right panel). However, using the RFP-IRSV plasmid, detectable RFP expression increases to ~50% of the cells (Figure 2B, right panel). These findings suggest that when an individual cell uptakes a small amount of plasmid, the presence of the SVLT/SV40 ori positive feedback loop induces replication of that plasmid within the cell and thus amplifies the expression of the RFP to detectable levels. Therefore, the use of this new vector system could enhance the use of transient transfection for analysis of gene function in cells that are otherwise resistant to this methodology, thus avoiding the costly and time-consuming production of stable cell lines. This same mechanism was put forth by de Chasseval and de Villartay (13), who reported that co-transfection of a protein on a plasmid bearing the SV40 ori with a second plasmid expressing the SVLT markedly enhances transfection rates in human lymphoid cell lines. The current vector system differs from this co-transfection methodology in that the SVLT is expressed on a vector bearing the SV40 ori, resulting in auto-amplification, and the IRES element assures that all cells receive both the gene of interest and the SVLT.
Figure 2.
Comparative RFP protein expression analysis in the absence and presence of the IRES-SVLT element. HEK293 cells (40 ng; left panels) or CV-1 cells (200 ng; right panels) were transiently tranfected in chamber slides using a standard pcDNA3.1 RFP vector (A) or the vector bicistronically linking RFP and SVLT expression via the IRES element (B). Confocal images of RFP expression using the same laser intensity and gain within each cell type are shown. Images are representative from at least three independent experiments. (C) HEK293 cells were transfected with either the standard RFP vector (RFP; green bars) or the IRES-SVLT containing vector (RFP-IRSV; red bars) with the indicated amounts of plasmid in black 96-well plates. Relative florescence was determined using a florescence plate reader (* p < 0.01; mean±s.d.; n=6). For each plasmid amount, RFP-IRSV was significantly greater than the RFP alone vector using the Student’s t-test (*p< 0.01). (D) 10 cm dishes of HEK293 cells were transfected (8 µg/dish) with either the RFP vector (green line) or the RFP-IRSV vector (red line). Cells were lifted from the plate and an emission scan for RFP expression was performed, as described in the Materials and Methods section. (E) Photographic image of cells from (F) HEK293 cells were transiently transfected with either 3.1-RFP or 3.1-RFP-IRSV and the number of colonies per µg of transfected DNA is shown (mean±s.d.; n=6) using the Hirt supernatant extraction method as described in the Materials and Methods section.
The IRES-SVLT element enhances RFP expression ~10-fold when HEK293 cells are transfected with optimal levels of plasmid (Figure 2D). Prior to analysis of the RFP emission scan, pelleting the cells from the 10 cm dish revealed visually detectable, colored cells in RFP-IRSV transfected preparations (Figure 2E), demonstrating an impressively high level of protein expression. Notably, under these high-level transfection conditions, the HEK293 were enlarged (compare A and B, left panels), but displayed no overt signs of stress (i.e. cell rounding) or death, as determined by exclusion of trypan blue. In order to confirm that this high-level of protein expression is due to plasmid replication, estimates of plasmid replication were determined for the pcDNA3.1-RFP and pcDNA3.1-RFP-IRSV plasmids in transiently transfected HEK293 cells using the Hirt supernatant extraction method. As shown in Figure 2F, the number of colonies per µg of originally transfected plasmid DNA is over two orders of magnitude greater for the SVLT containing plasmid when compared to the standard RFP vector, indicating that plasmid replication is likely to be the primary mechanism of enhanced expression. However, a report from Dean et al (14) demonstrated that the mere presence of the SV ori sequence enhanced nuclear uptake of the linked plasmid, thus facilitating expression of the gene of interest. Therefore, induced replication and enhanced nuclear uptake likely work in concert to obtain these pronounced expression levels. Many biophysical and biochemical methods require recombinant production. In order to generate large amounts of a particular protein of interest, bacterial expression is a common approach, however, often times mammalian proteins are poorly expressed in bacteria due to conflicts in codon usage and issues regarding proper protein folding and solubility. Furthermore, post-translational modifications are absent in bacterial expression systems. Recombinant protein production can also be achieved using the baculovirus/Sf9 cell system, but this requires the generation of viruses and the use of specialized cell culture techniques. As an alternative approach, the IRES-SVLT vector system could be used to generate large amounts of protein using the suspension culture-adapted 293s cell line, for example.
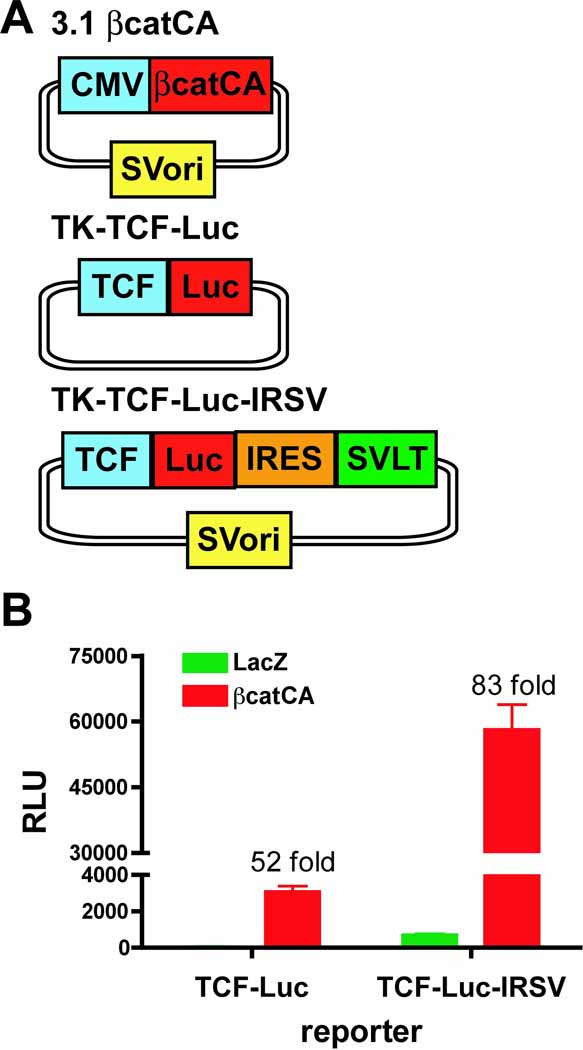
Up to this point, SVLT expression has been placed under the control of the constitutively active CMV promoter. Next, the effect of inducible SVLT expression in the context of regulated enhancer/promoter elements in conjunction with the luciferase reporter gene was examined. The well-known wnt-signaling pathway involving β-catenin-mediated activation of TCF response elements was employed as a test model. To circumvent the need for wnts, a constitutively active β-catenin (βcatCA), containing the S33Y mutation, was used (15). As shown in Figure 3A, three constructs were generated; a βcatCA expression vector containing the SV40 ori, a standard TCF-responsive luciferase reporter gene and a TCF-responsive luciferase linked to the SVLT via the IRES element in combination with the SV40 ori. Transient transfection of HEK293 cells with the standard TCF-responsive luciferase reporter gene demonstrates a robust, βcatCA-dependent activation process when compared to the LacZ control (52-fold increase) (Figure 3B). In contrast, βcatCA-dependent luciferase activation is substantially higher when the IRES-SVLT containing construct is co-transfected with luminescence ~150-fold greater than the standard reporter gene (Figure 3B). The basal activity of the luciferase-IRES-SVLT vector is somewhat higher, an effect that is likely due to leaking reporter expression and the linked SV40 ori on the plasmid inducing replication in the basal state. Despite this higher background, the fold-activation of the IRES-SVLT construct is almost twice (~83-fold) the fold-activation of the standard reporter gene. Luciferase is a common reporter gene used in high throughput screening methodologies. The markedly enhanced activation of this new SVLT-linked reporter gene system will certainly reduce the amount of reagents necessary for analysis and the number of cells required to detect the emitted luminescence, thus promoting well miniaturization, i.e. 384 to 1536, for example.
Figure 3.
β-catenin-mediated activation of a TCF responsive luciferase reporter gene in the absence and presence of the IRES-SVLT-SV40 ori elements. (A) Schematic of constructs used in B. (B) HEK293 cells were co-transfected with either LacZ (green bars) or constitutively active β-catenin(S33Y) (red bars) with either a TCF-responsive luciferase reporter gene (TK-TCF-Luc) or a TCF-responsive reporter gene containing the IRES-SVLT element (TK-TCF-Luc-IRSV) in combination with the SV40 ori, as indicated, for 48 hours. Luciferase activities reported as relative luminescence units (RLU) are shown (mean±s.d.; n=4) and are representative of at least three independent experiments.
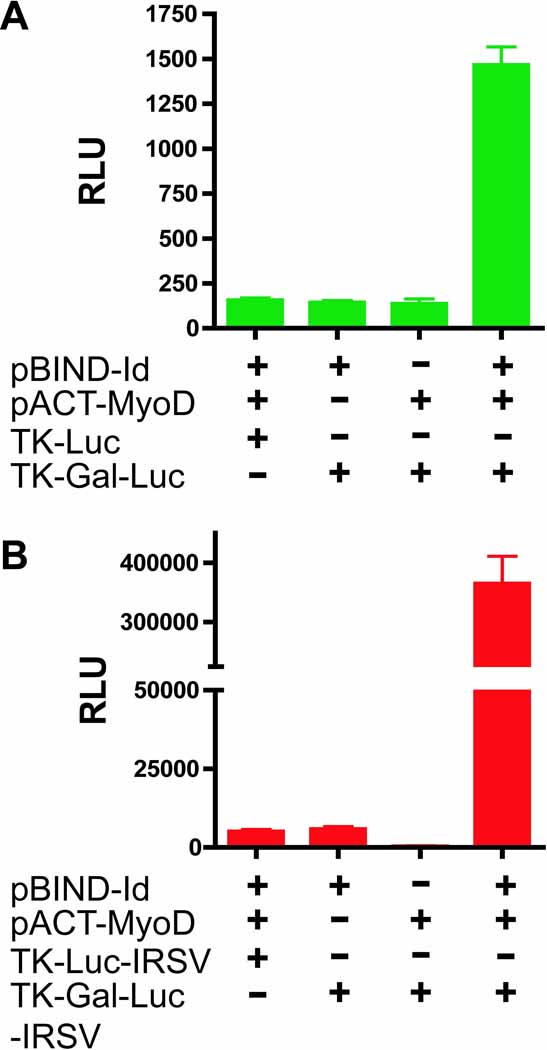
A second reporter gene-based application examined was the mammalian two-hybrid assay. Analogous to the yeast-based system, the mammalian two-hybrid assay assesses protein-protein interactions through the generation of two fusion constructs; the first interactor is fused the DNA-binding domain of gal4, a yeast transcription factor, and the second interactor is fused to the VP16 activation domain of the herpes simplex virus. Upon interaction of the two binding partners in a mammalian cell, the hybrid transcription factor (gal4-VP16) is capable of activating a gal4-responsive, luciferase reporter gene. Positive control vectors, purchased from Promega, analyze the interaction between MyoD and Id. Both of these expression vectors contain an SV40 ori. As shown in Figure 4A, co-transfection of the MyoD and Id fusion proteins readily activates the standard gal4-responsive luciferase reporter gene to levels that are 10-fold higher than basal levels. In contrast, co-transfection of the binding partners with the gal4-responsive luciferase-IRES-SVLT reporter generates a marked increase in the luminescent output to levels that are ~70-fold over basal (Figure 4B). Mechanistically, the initial interaction induces expression of the SVLT, which in turn enhances expression of the two binding partners within the cell and increases the formation of interaction-dependent hybrid transcriptional units, leading to further activation of the reporter gene and the generation of a strong, positive feedback loop. Thus, inclusion the IRES-SLVT element will likely allow for the analysis of weaker interactions within a mammalian cell. However, the SV40 ori on the reporter gene does increase the background level of interaction-independent luminescence, which could lead to masking of weak interactions or generation of false positives.
Figure 4.
Effect of the IRES-SVLT-SV40 ori elements on mammalian two-hybrid interaction analysis between Id and MyoD using gal4-responsive reporter genes. HEK293 cells were transiently co-transfected with indicated combinations of pBIND-Id and pACT-MyoD and either the standard control (TK-Luc) or gal4-responsive (TK-Gal-Luc) reporter genes (A) or the corresponding reporter genes containing the IRES-SVLT elements (TK-Luc-IRSV and TK-Gal-Luc-IRSV) (B). Luciferase activities reported as relative luminescence units (RLU) are shown (mean±s.d.; n=4) and are indicative of at least three independent experiments.
In conclusion, through the combination of two widely used viral elements, the IRES-SVLT-based vectors will likely enhance the utility of transient transfection for recombinant protein production, use of transfection-resistant cell lines and the sensitivity of luciferase-based reporter gene assays.
Acknowledgements
This work was supported by a grant from the National Institutes of Health (5P01DK011794-43) to Dr John Potts. This paper is subject to the NIH Public Access Policy. I would like to thank Dr. Marie Demay for the constitutively active β-catenin plasmid.
Footnotes
Competing Interests Statement
The author declares no competing interests.
References
- 1.Pipas JM. Common and unique features of T antigens encoded by the polyomavirus group. J Virol. 1992;66:3979–3985. doi: 10.1128/jvi.66.7.3979-3985.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahuja D, Saenz-Robles MT, Pipas JM. SV40 large T antigen targets multiple cellular pathways to elicit cellular transformation. Oncogene. 2005;24:7729–7745. doi: 10.1038/sj.onc.1209046. [DOI] [PubMed] [Google Scholar]
- 3.Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981;23:175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- 4.DuBridge RB, Tang P, Hsia HC, Leong PM, Miller JH, Calos MP. Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol Cell Biol. 1987;7:379–387. doi: 10.1128/mcb.7.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pear WS, Nolan GP, Scott ML, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci U S A. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jang SK, Pestova TV, Hellen CU, Witherell GW, Wimmer E. Cap-independent translation of picornavirus RNAs: structure and function of the internal ribosomal entry site. Enzyme. 1990;44:292–309. doi: 10.1159/000468766. [DOI] [PubMed] [Google Scholar]
- 7.Zhu J, Musco ML, Grace MJ. Three-color flow cytometry analysis of tricistronic expression of eBFP, eGFP, and eYFP using EMCV-IRES linkages. Cytometry. 1999;37:51–59. [PubMed] [Google Scholar]
- 8.Rees S, Coote J, Stables J, Goodson S, Harris S, Lee MG. Bicistronic vector for the creation of stable mammalian cell lines that predisposes all antibiotic-resistant cells to express recombinant protein. Biotechniques. 1996;20:102–104. doi: 10.2144/96201st05. 106, 108–110. [DOI] [PubMed] [Google Scholar]
- 9.Prost E, Moore DD. CAT vectors for analysis of eukaryotic promoters and enhancers. Gene. 1986;45:107–111. doi: 10.1016/0378-1119(86)90138-1. [DOI] [PubMed] [Google Scholar]
- 10.Baes M, Gulick T, Choi HS, Martinoli MG, Simha D, Moore DD. A new orphan member of the nuclear hormone receptor superfamily that interacts with a subset of retinoic acid response elements. Mol Cell Biol. 1994;14:1544–1552. doi: 10.1128/mcb.14.3.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967;26:365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- 12.Seed B, Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc. Natl. Acad. Sci. USA. 1987;84:3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Chasseval R, de Villartay JP. High level transient gene expression in human lymphoid cells by SV40 large T antigen boost. Nucleic Acids Res. 1992;20:245–250. doi: 10.1093/nar/20.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dean DA, Dean BS, Muller S, Smith LC. Sequence requirements for plasmid nuclear import. Exp Cell Res. 1999;253:713–722. doi: 10.1006/excr.1999.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ilyas M, Tomlinson IP, Rowan A, Pignatelli M, Bodmer WF. Beta-catenin mutations in cell lines established from human colorectal cancers. Proc Natl Acad Sci U S A. 1997;94:10330–10334. doi: 10.1073/pnas.94.19.10330. [DOI] [PMC free article] [PubMed] [Google Scholar]