Abstract
Objectives
Several studies have suggested the efflux transporter P-glycoprotein (P-gp) to play a role in the etiology of Alzheimer's disease through the clearance of amyloid beta (Aβ) from the brain. In this study, we aimed to investigate the possibility of P-gp as a potential therapeutic target for Alzheimer's disease by examining the impact of P-gp up-regulation on the clearance of Aβ, a neuropathological hallmark of Alzheimer's disease.
Methods
Uptake studies for 125I-radiolabelled Aβ1–40, and fluorescent immunostaining technique for P-gp and fluorescent imaging of Aβ1–40 were carried out in LS-180 cells following treatment with drugs known to induce P-gp expression.
Key findings
Approximately 10–35% decrease in 125I-Aβ1–40 intracellular accumulation was observed in cells treated with rifampicin, dexamethasone, caffeine, verapamil, hyperforin, β-estradiol and pentylenetetrazole compared with control. Also, fluorescent micrographs showed an inverse relationship between levels of P-gp expression and 5-carboxyfluorescein labelled Aβ (FAM-Aβ1–40) intracellular accumulation. Quantitative analysis of the micrographs revealed that the results were consistent with those of the uptake studies using 125I-Aβ1–40.
Conclusions
The investigated drugs were able to improve the efflux of Aβ1–40 from the cells via P-gp up-regulation compared with control. Our results elucidate the importance of targeting Aβ clearance via P-gp up-regulation, which will be effective in slowing or halting the progression of Alzheimer's disease.
Keywords: Alzheimer's disease, beta amyloid, clearance, P-glycoprotein, up-regulation
Introduction
Alzheimer's disease, a major cause of dementia, is a significant health care problem with about 5.3 million people currently affected in the USA. With the aging of the American population, projections suggest that by 2025, about 12 million Americans will suffer from this disease.[1] Aging is considered the major risk factor of Alzheimer's disease, which is characterized pathologically by the accumulation of tangles and senile plaques in the brain. Senile plaques are composed of beta amyloid peptides (Aβ).[2]
Significant efforts have been made to elucidate the mechanisms responsible for Aβ accumulation in the brain of Alzheimer's patients. Among such mechanisms is the increased level of Aβ as a result of its faulty clearance across the blood–brain barrier (BBB).[3] Continuous removal of Aβ from the CNS is important for preventing potentially neurotoxic Aβ accumulation. P-glycoprotein (P-gp) has been reported to play a role in Aβ clearance at the BBB.[4–6] Reduced expression of P-gp at the BBB has been observed during normal aging and in Alzheimer's patients.[5,7]
P-gp, the product of MDR1 gene, is perhaps the best-known and studied efflux transporter which, under normal physiological conditions, is widely expressed in barrier and excretory tissues.[8] P-gp is highly expressed on the luminal membrane of the endothelial cells at the BBB.[9] At the BBB, P-gp serves as a defence mechanism against a wide spectrum of non-polar therapeutic drugs and xenobiotics.[10] While its endogenous function is not fully understood, the expression pattern of P-gp has recently been suggested to play a key role in the etiology of Alzheimer's disease,[4] and has been shown to play substantial role in Aβ levels in the brain.[4–6] In-vitro binding studies showed that efflux of Aβ1–40 is mediated by P-gp as a result of direct interaction between Aβ and P-gp.[11] These results were confirmed in cell culture experiments using MDR1-transfected polarized kidney epithelial cells (LLC-PK1).[6] Moreover, when labelled Aβ1–40 protein was microinjected into the CNS of P-gp knockout mice, the clearance rate of Aβ1–40 across the BBB was reduced to half compared with the wild-type mice.[4]
With such a role of P-gp in the clearance of Aβ across the BBB, it is expected that a small decline in its expression or activity, by aging or Alzheimer's disease, will result in decreased clearance of Aβ from the brain, an increase in its level and consequent Aβ deposit. Thus, in the current studies we have hypothesized that up-regulation of the Aβ efflux transporter, P-gp, will enhance Aβ clearance and decelerate its intracellular accumulation.
In a previous study, we have demonstrated an in-vitro concentration-dependent increase in P-gp expression and activity by various drugs, including rifampicin, dexamethasone, caffeine, verapamil, hyperforin, β-estradiol and pentylenetetrazole, and ranked these drugs for their potencies to up-regulate P-gp (submitted). Subsequently, in the present study and to demonstrate the possibility of P-gp as potential therapeutic target for Alzheimer's disease, the impact of P-gp in-vitro up-regulation by the above drugs on Aβ cellular accumulation has been investigated in LS-180 cells as a model cell line for the BBB.
The results obtained from this study should furthermore establish the most promising modes of intervention and contribute to drug discovery efforts aimed at selective up-regulation of P-gp as a therapeutic approach for treatment or prevention of Alzheimer's disease.
Materials and Methods
Materials
LS-180 cell line and all cell culture reagents were obtained from American Type Culture Collection (Manassas, USA). Rifampicin, dexamethasone, hyperforin, pentylenetetrazole, caffeine, β-estradiol and verapamil were purchased from Sigma-Aldrich (St Louis, USA). The reagents and supplements required for Western blotting were purchased from Bio-Rad (Hercules, USA). The mouse monoclonal P-gp antibody (C-219) was obtained from Covance Research Products (Dedham, USA). Anti-β-actin (C-11) and horseradish peroxidase (HRP)-labelled secondary antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, USA). Normal donkey serum, normal goat serum and Rhodamine Red-X-conjugated AffiniPure donkey anti-mouse IgG (H + L) secondary antibody against P-gp were purchased from Jackson ImmunoResearch Laboratories Inc. (West Grove, USA). Synthetic 125I-Aβ1–40 (human) was purchased from PerkinElmer Inc. (Waltham, USA). FAM-labelled Aβ1–40 was purchased from AnaSpec, Inc. (Fremont, USA). All other reagents and supplies were purchased from VWR (West Chester, USA).
Cell culture
LS-180 cells were cultured in RPMI-1640 growth medium supplemented with 10% fetal bovine serum (FBS), 50 IU/ml penicillin and 50 μg/ml streptomycin. The cells were grown to confluence in 75-cm2 cell culture flasks for 3–6 days at 37°C/5% CO2 and used between passage numbers 5–20.
Drug treatment of LS-180 for in-vitro induction study
Induction of P-gp was carried out as follows. Cells were seeded in 10-mm cell culture dishes at a density of 5 × 106 cells/dish and allowed to attach and grow up to 50–60% confluence. Stock solutions of rifampicin, pentylenetetrazole, β-estradiol, dexamethasone, hyperforin, verapamil and caffeine were diluted to a final concentration of 50 μm (hyperforin, 100 nm) in growth medium before use. Forty-eight hours after cell seeding, control and drug-containing media were added to the respective treatment cells. The cells were then incubated for 48 h at 37°C/5% CO2. The media were not renewed for the duration of the experiment.
Western blot analysis of P-gp
Cellular protein (16 μg) was resolved on 7.5% SDS-polyacrylamide gels and transferred onto nitrocellulose membrane. The monoclonal antibody for P-gp (C-219) and β-actin (C-11) at dilutions 1 : 200 and 1 : 3000, respectively, were used. For protein detection, the membrane was incubated with HRP-labelled secondary IgG antibody for P-gp (anti-mouse) and β-actin (anti-goat) at dilution 1 : 5000. The bands were visualized using Pierce chemiluminescence detection kit (Thermo Scientific; Rockford, USA). Quantitative analysis of the immunoreactive bands was performed using Syngene luminescent image analyzer (Scientific Resources Southwest, Inc., Stafford, USA). Three independent Western blotting experiments were carried out for each drug.
In-vitro uptake of 125I-Aβ1–40 in control and treated LS-180 cells
125I-Aβ1–40 was used after aqueous reconstitution according the manufacturer recommendation to avoid the formation of Aβ1–40 oligomer aggregates. [12] 125I-Aβ1–40 uptake in control and treated LS-180 cells was investigated as follows. LS-180 cells were cultured onto 48-well plates at 5 × 104 cells/well and treated with 50 μm of rifampicin, dexamethasone, caffeine, verapamil, β-estradiol, pentylenetetrazole or 100 nm of hyperforin, as described above. The medium was aspirated and the cells were washed by incubation in fresh growth medium for 4 h. The cells were washed, and then pre-incubated for 30 min with or without 100 μm verapamil as a P-gp inhibitor in transport buffer (in mm: 141 NaCl, 4 KCl, 2.8 CaCl2, 1 MgSO4, 10 d-glucose and 10 HEPES). The activity experiments were started by the addition of 250 nCi/ml of 125I-Aβ1–40 in transport buffer with or without 100 μm verapamil for 1.0 h at 37°C/5% CO2. The activity experiment was terminated by washing the cells with ice-cold phosphate-buffered saline (PBS). Cells then were lysed for 1.0 h at 37°C. Intracellular radioactivity of 125I-Aβ1–40 was measured using Wallac 1470 Wizard Gamma Counter (PerkinElmer Inc.), and the data were normalized for the protein content. Intracellular accumulation of 125I-Aβ1–40 was expressed as percent of the means and standard deviation for the amount of Aβ1–40 (nCi/mg protein) detected in treated cells compared with control cells, both in the presence and absence of verapamil. For each drug three independent experiments were carried out with four replicates in each experiment.
Immunofluorescence staining of P-gp and fluorescence imaging of FAM-Aβ1–40
To confirm the effect of drug treatment on P-gp and the consequent intracellular accumulation levels of Aβ, both protein and peptide expressions were visualized using confocal microscopy following treatment with selected drugs, including rifampicin, caffeine, verapamil and β-estradiol. LS-180 cells (5 × 104) were seeded on 35-mm poly-d-lysine-coated glass bottom plates (MatTek Corporation, Ashland, USA) and treated with 50 μm of rifampicin, caffeine, verapamil or β-estradiol as described above. After 48 h, the cells were washed with PBS and incubated with 500 nm of FAM-labelled Aβ1–40 in serum-free medium for 1.0 h. The cells were then washed with PBS, fixed with 4% formaldehyde and blocked with 10% of normal donkey and goat sera. This step was followed by overnight incubation at 4°C with a 1 : 50 dilution of primary antibody against P-gp in solution composed of 1% normal donkey and goat sera in PBS. The cells were washed with PBS and incubated for 30 min with Rhodamine Red goat anti-mouse secondary antibody at 1 : 250 dilution. Images for P-gp and Aβ were captured using Leica TCS-SP5 inverted confocal microscope equipped with 488 nm line of Argon Laser (Leica Microsystems Inc., Bannockburn, USA). Negative controls for each treatment that were processed without primary antibody showed negligible background fluorescence (data not shown). P-gp and Aβ1–40 membrane fluorescence for each sample were quantified using ImageJ version 1.44 software (Research Services Branch, NIMH/NIH, Bethesda, USA). This experiment was repeated three times.
Statistical analysis
Wherever possible, the experimental results were statistically analysed for significant difference using two-tailed Student's t-test to evaluate differences between controls and treated groups. P < 0.05 was considered to be statistically significant.
Results
In-vitro induction of P-gp expression
The ability of the investigated drugs to induce P-gp expression was assessed by Western blotting using the C-219 antibody. Figure 1a–1e illustrates the fold increase in P-gp expression obtained following Western blotting in LS-180 cells treated with 50 μm of rifampicin, dexamethasone, caffeine, verapamil or 100 nm hyperforin, and quantified by densitometry of the immunoblots. In addition, and as a representative drug, Figure 1f illustrates a dose-dependent increase in P-gp expression and activity as a result of verapamil treatment. All investigated drugs resulted in an increase in P-gp expression following 48 h treatment to different extents. Under the in-vitro cell culture conditions, a 2.7-fold increase in the expression of P-gp protein was observed post-incubation with verapamil while rifampicin increased P-gp expression by 5.6 fold compared with control. Hyperforin showed up to 2.6-fold increase at 100 nm. Other investigated drugs, including dexamethasone and caffeine, resulted in up-regulation of P-gp expression by 70% and 30%, respectively, when compared with control, while pentylenetetrazole and β-estradiol increased P-gp expression only by 10%.
Figure 1.
Representative Western blot and densitometry analysis for the ratio of P-gp to β-actin expression levels in LS-180 cells treated with (a) 50 μm caffeine (CAF) (b) 50 μm dexamethasone (DEX) (c) 100 nm hyperforin (HYP) (d) 50 μm rifampicin (RIF) and (e) 50 μm verapamil (VER) compared with control (CTRL). (f) A representative figure for dose dependent increase in P-gp expression (column bars) and activity (line) following treatment with verapamil. The data are expressed as the mean + SD, n = 3 independent experiments. *P ≤ 0.05 vs control.
Effect of P-gp up-regulation on 125I-Aβ1–40 cellular uptake in vitro
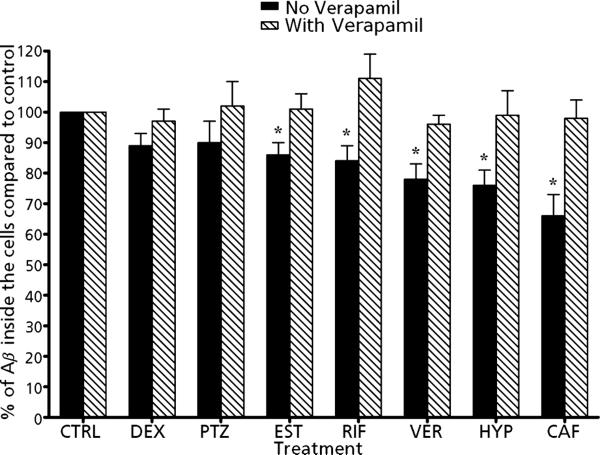
To determine the consequence of P-gp up-regulation on the accumulation of Aβ1–40, the cellular uptake of radiolabelled 125I-Aβ1–40 was evaluated following drug treatment in the presence and absence of 100 μm verapamil, a specific P-gp inhibitor. Figure 2 shows the percentage change in the intracellular accumulation of Aβ1–40 with and without verapamil in treated LS-180 cells compared with control. As a result of P-gp up-regulation by the drugs, the cellular accumulation of Aβ1–40 has decreased, and the inhibition of P-gp by verapamil enhanced Aβ1–40 accumulation confirming the specific role of P-gp on Aβ1–40 cellular uptake. This difference in Aβ1–40 was more apparent and statistically significant (P < 0.05) in cells treated with caffeine, rifampicin, verapamil, β-estradiol and 100 nm hyperforin, which resulted in 15–35% decrease in Aβ1–40 cellular uptake (Figure 2). When LS-180 cells treated with these five drugs were co-incubated with verapamil and 125I-Aβ1–40, a significant increase in 125I-Aβ1–40 uptake by 16–32% was observed (P < 0.05). On the other hand, cells treated with dexamethasone and pentylenetetrazole showed moderate decrease in 125I-Aβ1–40 cellular uptake by approximately 10%, and their co-incubation with verapamil increased its intracellular uptake by 8–12%. The above results confirm the role of P-gp in determining Aβ1–40 intracellular levels. The induction of P-gp lowered Aβ1–40 cellular uptake by enhancing its clearance, while the inhibition of P-gp increased Aβ1–40 uptake.
Figure 2.
Effect of treatment of LS-180 cells with rifampicin (RIF), verapamil (VER), dexamethasone (DEX), b-estradiol (EST), pentylenetetrazole (PTZ), hyperforin (HYP) and caffeine (CAF), on the intracellular accumulation of radiolabelled 125I-Aβ1–40. Percent change in the accumulation of Aβ1–40 was evaluated in the presence (dashed bars) and absence (solid bars) of verapamil, a specific P-gp inhibitor. The data are expressed as mean ± SD, n = 3 independent experiments, each drug 4 replicate/experiment. *P < 0.05.
Effect of P-gp up-regulation on the intracellular fluorescence intensity of FAM-Aβ1–40
Figure 3a–3c show representative fluorescent micrograph images of P-gp expression of untreated (control, Figure 3a), rifampicin-treated (Figure 3b) and caffeine-treated (Figure 3c) cells captured by TCS-SP5 Leica confocal microscope. Fluorescence of secondary antibody against P-gp was located mainly on the cell membrane, while the fluorescence associated with FAM-Aβ1–40 was taken up into the cells (Figure 3d–3f). The conjugation of Aβ peptide with FAM fluorophore did not affect the structure and function of the peptide, and the in-vitro uptake of labelled and unlabelled Aβ by cells was similar.[13]
Figure 3.
Representative fluorescent micrographs of P-gp (a, b and c) and FAM-Aβ1–40 (d, e and f) for control (a and d) and treated LS-180 cells with rifampicin (b and e) and caffeine (c and f). Quantitative folds change in the P-gp expression and FAM-Aβ1–40 intracellular accumulation were measured using ImageJ version 1.44. The data are expressed as mean ± SD, n = 3. *P < 0.05. Scale bar = 25 μm.
Quantitative analysis of P-gp expressed in LS-180 cells showed significant increase in membrane P-gp expression of drug-treated LS-180 compared with control. For example, rifampicin and caffeine resulted in 6.2-fold and 1.5-fold increase, respectively, in P-gp expression compared with control (P < 0.05; Figure 3, top bar graph). These results are in agreement with those obtained from Western blotting data (Figure 1). At the same time, this increase in P-gp expression caused by both drugs was accompanied by 43–67% decrease in the intracellular accumulation of FAM-Aβ1–40 when compared with control (P < 0.05; Figures 3d–3f, bottom bar graph). In addition, treatment with β-estradiol and verapamil decreased the intracellular accumulation of FAM-Aβ1–40 by 28% and 59%, respectively.
Discussion
There is compelling evidence that Aβ protein aggregation is an essential early event in Alzheimer's disease pathogenesis.[2] In fact, upon aging, human brain inevitably starts to accumulate Aβ suggesting that the Alzheimer's disease cascade is initiated as a normal consequence of the aging process. However, as compared with healthy aged brains, the accumulation of neurotoxic Aβ in the brains of Alzheimer's patients, although starting slowly, begins to accelerate exponentially after a certain point.[14] This implies that a slight increase in the steady state of Aβ above normal in the brain of Alzheimer's patients may initiate one or multiple vicious processes causing dramatic increase in Aβ levels.[14]
LS-180, a human colon adenocarcinoma cell line,[15] is a well characterized in-vitro model utilized in P-gp efflux studies. LS-180 cell line endogenously expresses P-gp and pregnane-X-receptor (PXR), which controls expression of P-gp.[16,17] As a result, this cell line is considered a useful tool for the mechanistic investigation of P-gp substrates and is frequently utilized to investigate the effect of P-gp modulation by inhibition or up-regulation on the uptake or clearance of substrate drugs.[18] As most drugs investigated, including rifampicin, dexamethasone, verapamil and hyperforin, have been reported to up-regulate P-gp by activating PXR,[19–22] in the current study LS-180 cell line was chosen as a model for the BBB to evaluate the effect of induced expression of P-gp caused by these drugs on the increased efflux, or clearance, of Aβ, a P-gp substrate.
Available experimental data strongly suggest impaired clearance of Aβ across the BBB might largely contribute to the formation of Aβ brain deposits and Alzheimer's disease progression. Also, it has been demonstrated that the efflux transporter P-gp plays a substantial role in the elimination of Aβ1–40 and Aβ1–42 from the brain across the BBB.[4–6,11] Accordingly, in this study we report the impact of P-gp up-regulation on the intracellular accumulation of Aβ1–40 in vitro by rifampicin, dexamethasone, verapamil, caffeine, pentylenetetrazole and β-estradiol at 50 μm, and hyperforin at 100 nm concentrations. As both Aβ peptide species have been implicated in the pathogenesis of Alzheimer's disease,[23] in this study Aβ1–40 was used to investigate our hypothesis.
The examined drugs were particularly investigated as they demonstrated potency to up-regulate P-gp expression and activity in a concentration-dependent manner (Figure 1f), and these same drugs have been reported to have positive impact on Alzheimer's disease. For example, in humans, a 3-month course of rifampicin (300 mg p.o.) and doxycycline attenuated the rate of cognitive decline in patients with mild-to-moderate Alzheimer's disease. The authors concluded that such efficacy was unrelated to the antibiotic activity of either drug.[24] In addition, several studies showed caffeine intake as a protective factor for Alzheimer's disease. In a case-controlled study conducted on 54 patients with probable Alzheimer's disease,[25] results showed that patients with Alzheimer's disease had an average daily caffeine intake of 73.9 ± 97.9 mg during the 20 years that preceded diagnosis of Alzheimer's disease, whereas the controls had an average daily caffeine intake of 198.7 ± 135.7 mg during the corresponding 20 years of their lifetimes. Further, in-vivo studies on mice models of Alzheimer's disease confirmed caffeine's protective role against cognitive impairment and showed that it reduced brain and plasma levels of Aβ1–40.[26,27] Similar positive effects have been reported with hyperforin, which reduced amyloid deposit formation in rats injected with amyloid fibrils in the hippocampus, prevented Aβ-induced neurotoxicity and improved spatial learning acquisition.[28]
Pentylenetetrazole,[29] β-estradiol,[30] verapamil[31] and dexamethasone[32] have also shown anti-Alzheimer's effects in human or animal Alzheimer's disease models. For example, pentylenetetrazole, an old drug once used to treat epilepsy, has been shown to up-regulate P-gp at the BBB of rats treated with 30 mg/kg for 24 days, where P-gp levels in the brain increased by 1.7-fold.[33] When pentylenetetrazole (3 mg/kg, a non-epileptic dose) was administered to a mouse model with Down's syndrome symptoms, Ts65Dn, for 17 days, pentylenetetrazole substantially improved learning and memory in these mice.[29] Ts65Dn mice demonstrate increased levels of Aβ in the hippocampus and astrocytic hypertrophy.[29]
Collectively, the above summarized data highlight the possible link between the induction of P-gp by the investigated drugs and their associated decreasing risk or protection against Alzheimer's disease. Consequently, in this study we have investigated the hypothesis that P-gp up-regulation enhances Aβ clearance and decelerates Aβ deposition, thus providing effective therapeutic strategy in slowing or halting the progression of Alzheimer's disease. We have shown that exposure of LS-180 cells to rifampicin, verapamil, hyperforin, caffeine, β-estradiol and, to a lesser extent, pentylenetetrazole and dexamethasone resulted in an increase in P-gp expression ranging from 10 to 460%, and a subsequent decrease in Aβ1–40 cellular uptake ranging from 10 to 35%. In addition, P-gp inhibition by verapamil following its up-regulation by the investigated drugs enhanced Aβ1–40 cellular uptake by 8–32%. Verapamil has been reported to be a specific competitive inhibitor for P-gp and is commonly used to evaluate the functionality of the P-gp efflux transporter.[34,35]
The above results confirm that decreased intracellular accumulation of Aβ1–40 is associated with P-gp up-regulation caused by the drugs. The investigated drugs demonstrated different potencies in up-regulating P-gp expression (Figure 1). Most of the drugs evaluated in this study up-regulate P-gp via PXR activation,[19–22] where, following drug binding, PXR forms a heterodimer with the retinoid X receptor (RXR) and subsequently binds to PXR response elements in the 5′-flanking region of the MDR1 gene resulting in transcriptional activation.[36] PXR activation is drug dependent,[36] which may explain the variability in drugs potencies to up-regulate P-gp. Nonetheless, these results support the role of such drugs in enhancing Aβ clearance as a result of P-gp up-regulation at the BBB, reducing its brain levels and thus improving cognitive function and preventing Aβ-induced neurotoxicity. Furthermore, these findings support our hypothesis that targeting P-gp at the BBB as a therapeutic strategy will be beneficial for patients with Alzheimer's disease.
In addition, the results from expression studies of P-gp by confocal microscopy, while somewhat higher, were consistent with those of the uptake studies using radiolabelled 125I-Aβ1–40, confirming the inverse relationship between P-gp expression and Aβ1–40 intracellular accumulation. Images of LS-180 cells demonstrated that rifampicin and caffeine treatments increased P-gp expression by 6.2 and 1.5 fold, respectively. Such increase in P-gp expression was associated with a decrease in FAM-Aβ1–40 by both drugs when compared with control. However, the decrease in 125I-Aβ1–40 and FAM-Aβ1–40 accumulation in LS-180 cells treated with caffeine (35 and 67%, respectively) was higher than in those treated with rifampicin (16 and 43%, respectively) despite the ~ 6.0-fold increase in P-gp expression caused by rifampicin compared with the 1.5-fold increase by caffeine. Possible explanations for this observation could be that the increase in P-gp expression caused by rifampicin is not equivalently functional, or the presence of an additional mechanism of Aβ1–40 transport across the membrane could be modulated by either treatment. This information is currently under investigation in our laboratory. Similarly, treatment of cells with β-estradiol and verapamil resulted in a significant decrease in the cellular uptake of 125I-Aβ1–40 (15 and 22%, respectively) and FAM-Aβ1–40 (28 and 59%, respectively), while pentelenetetrazole and dexamethasone had a moderate effect on both P-gp expression and 125I-Aβ cellular uptake. Overall, these results confirm the effect of P-gp up-regulation in enhanced efflux, hence clearance, of Aβ from the cells. Further studies are in progress to evaluate the above drugs in vivo and verify the consequences of such induction on P-gp activity at the BBB and Aβ clearance.
Conclusions
Our results showed that in-vitro treatment of LS-180 cells with rifampicin, caffeine, verapamil, hyperforin and β-estradiol, and to a lesser extent pentylenetetrazole and dexamethasone, resulted in significant decrease in the intracellular accumulation of Aβ1–40 compared with control as a result of P-gp up-regulation. In addition, the results support the link between P-gp up-regulation caused by these drugs and cognitive improvement in patients with Alzheimer's disease. Thus, targeting P-gp could be an effective strategy in slowing or halting the progression of Alzheimer's disease.
Acknowledgements
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. In addition, this work utilized the facilities of the Cell Biology and Bioimaging Core that are supported in part by COBRE (NIH P20-RR021945) and NORC (NIH 1P30-DK072476) center grants from the National Institutes of Health.
Funding The project described was supported by Grant Number P20RR016456 from the National Center For Research Resources.
Footnotes
Conflict of interest The Author(s) declare(s) that they have no conflicts of interest to disclose.
References
- 1.Maslow K. 2010 Alzheimer's disease facts and figures. Alzheimers Dement. 2010;6:158–194. doi: 10.1016/j.jalz.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 3.Zlokovic BV, Frangione B. Transport-clearance hypothesis for Alzheimer's disease and potential therapeutic implications. In: Saido TC, editor. Aβ Metabolism in Alzheimer's Disease. Landes Bioscience; Georgetown, TX: 2003. pp. 114–122. [Google Scholar]
- 4.Cirrito JR, et al. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J Clin Invest. 2005;115:3285–3290. doi: 10.1172/JCI25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogelgesang S, et al. Deposition of Alzheimer's beta-amyloid is inversely correlated with P-glycoprotein expression in the brains of elderly non-demented humans. Pharmacogenetics. 2002;12:535–541. doi: 10.1097/00008571-200210000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Kuhnke D, et al. MDR1-P-Glycoprotein (ABCB1) mediates transport of Alzheimer's amyloid-beta peptides – implications for the mechanisms of Abeta clearance at the blood-brain barrier. Brain Pathol. 2007;17:347–353. doi: 10.1111/j.1750-3639.2007.00075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toornvliet R, et al. Effect of age on functional P-glycoprotein in the blood-brain barrier measured by use of (R)-[(11)C]verapamil and positron emission tomography. Clin Pharmacol Ther. 2006;79:540–548. doi: 10.1016/j.clpt.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Thiebaut F, et al. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci USA. 1987;84:7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beaulieu E, et al. P-glycoprotein is strongly expressed in the luminal membranes of the endothelium of blood vessels in the brain. Biochem J. 1997;326(Pt 2):539–544. doi: 10.1042/bj3260539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller DS, et al. Modulation of P-glycoprotein at the blood-brain barrier: opportunities to improve central nervous system pharmacotherapy. Pharmacol Rev. 2008;60:196–209. doi: 10.1124/pr.107.07109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lam FC, et al. beta-Amyloid efflux mediated by p-glycoprotein. J Neurochem. 2001;76:1121–1128. doi: 10.1046/j.1471-4159.2001.00113.x. [DOI] [PubMed] [Google Scholar]
- 12.LaRue B, et al. Method for measurement of the blood-brain barrier permeability in the perfused mouse brain: application to amyloid-beta peptide in wild type and Alzheimer's Tg2576 mice. J Neurosci Methods. 2004;138:233–242. doi: 10.1016/j.jneumeth.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 13.Jungbauer LM, et al. Preparation of fluorescently-labeled amyloid-beta peptide assemblies: the effect of fluorophore conjugation on structure and function. J Mol Recognit. 2009;22:403–413. doi: 10.1002/jmr.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saido TC. Overview-Abeta metabolism: from Alzheimer research to brain aging control. In: Saido TC, editor. Aβ Metabolism in Alzheimer's Disease. Landes Bioscience; Georgetown, TX: 2003. pp. 1–16. [Google Scholar]
- 15.Tom BH, et al. Human colonic adenocarcinoma cells. I. Establishment and description of a new line. In Vitro. 1976;12:180–191. doi: 10.1007/BF02796440. [DOI] [PubMed] [Google Scholar]
- 16.Ott M, et al. Pregnane X receptor (PXR) regulates P-glycoprotein at the blood-brain barrier: functional similarities between pig and human PXR. J Pharmacol Exp Ther. 2009;329:141–149. doi: 10.1124/jpet.108.149690. [DOI] [PubMed] [Google Scholar]
- 17.Bauer B, et al. Pregnane X receptor up-regulation of P-glycoprotein expression and transport function at the blood-brain barrier. Mol Pharmacol. 2004;66:413–419. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- 18.Perloff MD, et al. Rapid assessment of P-glycoprotein inhibition and induction in vitro. Pharm Res. 2003;20:1177–1183. doi: 10.1023/a:1025092829696. [DOI] [PubMed] [Google Scholar]
- 19.Haslam IS, et al. Rifampin and digoxin induction of MDR1 expression and function in human intestinal (T84) epithelial cells. Br J Pharmacol. 2008;154:246–255. doi: 10.1038/bjp.2008.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Durr D, et al. St John's Wort induces intestinal P-glycoprotein/ MDR1 and intestinal and hepatic CYP3A4. Clin Pharmacol Ther. 2000;68:598–604. doi: 10.1067/mcp.2000.112240. [DOI] [PubMed] [Google Scholar]
- 21.Tannergren C, et al. St John's wort decreases the bioavailability of R- and S-verapamil through induction of the first-pass metabolism. Clin Pharmacol Ther. 2004;75:298–309. doi: 10.1016/j.clpt.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 22.Scheer N, et al. In vivo responses of the human and murine pregnane X receptor to dexamethasone in mice. Drug Metab Dispos. 2010;38:1046–1053. doi: 10.1124/dmd.109.031872. [DOI] [PubMed] [Google Scholar]
- 23.Itkin A, et al. Calcium ions promote formation of amyloid beta-Peptide (1–40) oligomers causally implicated in neuronal toxicity of Alzheimer's disease. PLoS ONE. 2011;6:e18250. doi: 10.1371/journal.pone.0018250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loeb MB, et al. A randomized, controlled trial of doxycycline and rifampin for patients with Alzheimer's disease. J Am Geriatr Soc. 2004;52:381–387. doi: 10.1111/j.1532-5415.2004.52109.x. [DOI] [PubMed] [Google Scholar]
- 25.Maia L, de Mendonca A. Does caffeine intake protect from Alzheimer's disease? Eur J Neurol. 2002;9:377–382. doi: 10.1046/j.1468-1331.2002.00421.x. [DOI] [PubMed] [Google Scholar]
- 26.Cao C, et al. Caffeine suppresses amyloid-beta levels in plasma and brain of Alzheimer's disease transgenic mice. J Alzheimers Dis. 2009;17:681–697. doi: 10.3233/JAD-2009-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arendash GW, et al. Caffeine reverses cognitive impairment and decreases brain amyloid-beta levels in aged Alzheimer's disease mice. J Alzheimers Dis. 2009;17:661–680. doi: 10.3233/JAD-2009-1087. [DOI] [PubMed] [Google Scholar]
- 28.Dinamarca MC, et al. Hyperforin prevents beta-amyloid neurotoxicity and spatial memory impairments by disaggregation of Alzheimer's amyloid-beta-deposits. Mol Psychiatry. 2006;11:1032–1048. doi: 10.1038/sj.mp.4001866. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez F, et al. Pharmacotherapy for cognitive impairment in a mouse model of Down syndrome. Nat Neurosci. 2007;10:411–413. doi: 10.1038/nn1860. [DOI] [PubMed] [Google Scholar]
- 30.Pike CJ, et al. Protective actions of sex steroid hormones in Alzheimer's disease. Front Neuroendocrinol. 2009;30:239–258. doi: 10.1016/j.yfrne.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Popovic M, et al. Neuroprotective effect of chronic verapamil treatment on cognitive and noncognitive deficits in an experimental Alzheimer's disease in rats. Int J Neurosci. 1997;92:79–93. doi: 10.3109/00207459708986392. [DOI] [PubMed] [Google Scholar]
- 32.Alisky JM. Intrathecal corticosteroids might slow Alzheimer's disease progression. Neuropsychiatr Dis Treat. 2008;4:831–833. doi: 10.2147/ndt.s3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu X, et al. Increased P-glycoprotein expression and decreased phenobarbital distribution in the brain of pentylenetetrazole-kindled rats. Neuropharmacology. 2007;53:657–663. doi: 10.1016/j.neuropharm.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 34.Pham YT, et al. Interactions of racemic mefloquine and its enantiomers with P-glycoprotein in an immortalised rat brain capillary endothelial cell line, GPNT. Biochim Biophys Acta. 2000;1524:212–219. doi: 10.1016/s0304-4165(00)00160-4. [DOI] [PubMed] [Google Scholar]
- 35.Barraud de Lagerie S, et al. Cerebral uptake of mefloquine enantiomers with and without the P-gp inhibitor elacridar (GF1210918) in mice. Br J Pharmacol. 2004;141:1214–1222. doi: 10.1038/sj.bjp.0705721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma X, et al. The pregnane X receptor: from bench to bedside. Expert Opin Drug Metab Toxicol. 2008;4:895–908. doi: 10.1517/17425255.4.7.895. [DOI] [PMC free article] [PubMed] [Google Scholar]