Abstract
Pituitary adenylate cyclase-activating polypeptide (PACAP)-mediated activation of its G protein-coupled receptor PAC1 results in activation of the two G proteins Gs and Gq to alter second messenger generation and gene transcription in the nervous system, important for homeostatic responses to stress and injury. Heterologous expression of the three major splice variants of the rat PAC1 receptor, PAC1hop, null and hip, in neural NG108-15 cells conferred PACAP-mediated intracellular cAMP generation, while elevation of [Ca2+]i occurred only in PAC1hop-, and to a lesser extent in PAC1null-expressing cells. Induction of vasoactive intestinal polypeptide (VIP) and stanniocalcin 1 (STC1), two genes potentially involved in PACAP’s homeostatic responses, was examined as a function of the expressed PAC1 variant. VIP induction was greatest in PAC1hop-expressing cells, suggesting that a maximal transcriptional response requires combinatorial signaling through both cAMP and Ca2+. STC1 induction was similar for all three receptor splice variants and was mimicked by the adenylate cyclase activator forskolin, indicating that cAMP elevation is sufficient to induce STC1. The degree of activation of two different second messenger pathways appears to determine the transcriptional response, suggesting that cellular responses to stressors are fine-tuned through differential receptor isoform expression. Signaling to the VIP gene proceeded through cAMP and protein kinase A (PKA) in these cells, independently of the MAP kinase ERK1/2. STC1 gene induction by PACAP was dependent on cAMP and ERK1/2, independently of PKA. Differential gene induction via different cAMP dependent signaling pathways potentially provides further targets for the design of treatments for stress-associated disorders.
Keywords: PACAP, PAC1 receptor, cAMP, calcium, gene induction, nervous system
1. Introduction
Pituitary adenylate cyclase-activating polypeptide (PACAP) is a peptide that was isolated from ovine hypothalamus based on its ability to stimulate cyclic adenosine 3′5′-monophosphate (cAMP) in rat anterior pituitary cells [33]. The mature peptide PACAP occurs in two C-terminally α-amidated forms, PACAP-27 and PACAP-38, with PACAP-27 being identical to the first 27 amino acids of PACAP-38 [34]. PACAP-27 has 68% sequence homology with vasoactive intestinal polypeptide (VIP), identifying PACAP as a member of the VIP-secretin-growth hormone releasing hormone-glucagon superfamily. The first 27 amino acids of PACAP have almost been completely preserved through vertebrate evolution, from fish to mammals, and are responsible for its biological activity [37, 50]. PACAP is widely expressed in the nervous system, e.g., the hypothalamus, cerebral cortex, amygdala, nucleus accumbens, hippocampus and cerebellum of the central nervous system, and in sensory neurons, sympathetic preganglionic neurons and parasympathetic pre- and postganglionic neurons of the peripheral nervous system. PACAP-38 is the predominant form expressed [4, 18, 20, 56].
PACAP binds to three G protein-coupled receptors (GPCRs), named VPAC1, VPAC2 and PAC1, which are members of the class B family of GPCRs. VPAC1 and VPAC2 bind VIP and PACAP with similar affinity whereas PAC1 binds PACAP with high affinity and VIP with much lower affinity. PACAP receptors are abundantly expressed in the central nervous system, the anterior pituitary and adrenal gland [2, 23, 24, 28, 31, 45, 52, 53]. Several isoforms of the PACAP-preferring PAC1 receptor have been identified in vivo. These are generated through alternative splicing within two regions of the PAC1 gene: the N-terminus and the third intracellular loop (ic3). N-terminal variants result from deletions (21 or 57 amino acids) at the N-terminal extracellular domain affecting ligand binding and the relative potencies of the ligands in second messenger stimulation [9, 43]. Ic3 variants result from the presence or absence of different insertions at the C-terminal end of the loop, a domain thought to be crucial for interaction with G proteins. Each insertion, designated hip and hop, consists of an 84-bp cassette. The alternative use of two contiguous consensus splice acceptor sites at the 5′-end of the hop cassette generates hop1 and hop2. The hip cassette can be included together with the hop cassette to give rise to hiphop. The null form does not contain any insert. The hop cassette encodes a consensus motif for phosphorylation by protein kinase C (PKC) [54]. In the adrenal medulla the predominant PAC1 variant is PAC1hop, in the brain PAC1hop and null are abundantly expressed. PAC1 with a full-length N-terminus is the predominant form in the adult brain, whereas the embryonic brain expresses high levels of receptors containing a short N-terminus lacking 21 amino acids [15, 27, 38, 40, 46, 68].
All PACAP receptors regulate cAMP generation by coupling to adenylate cyclases (ACs) through Gs. Coupling to phospholipase Cβ (PLCβ), in contrast, varies among the different receptor sub-types. Regulation of inositol phosphate (IP) production by coupling to PLCβ through Gq is more efficacious in PAC1hop and null compared to hip receptors as assessed in non-neural heterologous cells [27, 46, 54]. The functional importance of Gs/AC- and Gq/PLCβ-mediated combinatorial signaling has been shown for sustained release of catecholamines (CAs) and neuropeptides from adrenomedullary chromaffin cells (CCs) [5, 16, 29, 49, 63], which predominantly express the PAC1hop receptor variant [38, 40]. In the adrenomedullary pheochromocytoma PC12 cell line [14] it has been shown that PAC1hop-activated sustained CA release proceeds through inositol-1,4,5-trisphosphate (IP3)-mediated Ca2+ release from intracellular stores and store-operated Ca2+ entry (SOCE) [39, 58]. Moreover, the activation of two second messenger pathways, cAMP and Ca2+, seems to be required for maximal transcriptional stimulation of the neuropeptide VIP in CCs [17]. Stanniocalcin 1 (STC1) is another PACAP-regulated gene in CCs [1] with potentially neurotrophic functions [65–67]; signaling pathways regulating its neural expression, however, remain unidentified.
Although the different ic3 splice variants of PAC1 were first discovered almost 20 years ago, an understanding of second messenger production and gene induction mediated by these different variants in neural cells is still lacking. Therefore, we investigated the induction of the second messengers cAMP and Ca2+ as well as the PACAP target genes VIP and STC1 by the rat PAC1hop1, null and hip receptor variants with a full-length N-terminus in neural NG108-15 cells. The NG108-15 cell line is a neuroblastoma x glioma hybrid, not responding to PACAP endogenously, therefore providing an appropriate model system to study the different PAC1 splice variants separately introduced into a neural cell line. We demonstrate here that combinatorial signaling through cAMP and Ca2+, mediated uniquely by PAC1hop, is required for a full transcriptional response of the VIP gene. Cyclic AMP generation by either PAC1hop, null or hip is sufficient for induction of the gene encoding the neuroprotective protein STC1. Furthermore, two separate cAMP-dependent signaling pathways activated by PACAP through PAC1 differentially regulate neural target genes. Our results provide evidence for the importance of differential expression of PAC1 splice variants and induction of second messenger pathways in shaping the PACAP-mediated transcriptional response in the nervous system.
2. Materials and Methods
2.1 Materials
PACAP-38 was purchased from Phoenix Pharmaceuticals (Mountain View, CA). Forskolin, H89, U0126 and 2′5′-dideoxyadenosine were obtained from Calbiochem (San Diego, CA). All cell culture media and supplements were obtained from Invitrogen (Carlsbad, CA) unless otherwise specified.
2.2 Culture of NG108-15 cells
NG108-15 cells (mouse neuroblastoma x rat glioma hybrid), obtained from the American Type Culture Collection (Manassas, VA) were cultured in high glucose Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum, penicillin-streptomycin (100 U/ml penicillin and 100 μg/ml streptomycin), 2 mM L-glutamine and 1X HAT (hypoxanthine, aminopterine, thymidine) at 37°C under 95% air and 5% CO2. For experiments, cells were plated at a density of 30,000 cells/0.95 cm2 growth area on poly-L-lysine-coated (0.1 mg/ml) multiple well culture plates and allowed to adhere overnight. Cells were used between passages seven and 25.
2.3 Production of gammaretroviral vectors and viral infection of NG108-15 cells
Retroviral vectors were produced by a three-plasmid co-transfection of 293T cells with the calcium phosphate method according to the manufacturer’s instructions (ProFection Mammalian Transfection System kit, Promega). 293T cells were plated at 2 × 106 cells per 10-cm plate (coated with 0.1 mg/ml poly-L-lysine) in high glucose DMEM supplemented with 10% fetal bovine serum, penicillin-streptomycin (100 U/ml penicillin and 100 μg/ml streptomycin) and 2 mM L-glutamine and incubated overnight at 37°C under 95% air and 5% CO2. Three hours before transfection the medium was replaced by 10 ml fresh medium. 10 μg of the bicistronic retroviral vector pPRIG (plasmid Polylinker Retroviral IRES GFP) [30] expressing the respective PAC1 receptor (rat PAC1null, hop1 and hip with a full-length N-terminus, which were subcloned from pRK8 vectors [54] into the EcoRI/SalI sites of pPRIG) and enhanced green fluorescent protein (eGFP) from a unique cytomegalovirus (CMV) promoter, together with a MuLV-based gag/pol plasmid, pIK6MuLVgp (2.5 μg) and a VSV-G envelope plasmid (5 μg) were co-transfected and incubated overnight. The medium was replaced with 5.5 ml fresh medium and retroviral vector-containing supernatant was harvested 48 h post-transfection. 5.5 ml fresh medium was added and the second supernatant was harvested 24 h later. The supernatant was filtered through a 0.45 μm syringe filter and used for infection of NG108-15 cells.
Before infection, NG108-15 cells were plated onto 12-well plates and incubated overnight. The medium was removed and 0.5 ml fresh medium and 0.5 ml virus preparation was added. After overnight incubation, the medium was changed. Transduction efficiency of PAC1 was determined by direct visualization of eGFP, expressed from its IRES-dependent cistron.
2.4 Analysis of PACAP receptor mRNAs by Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
RNA extraction of NG108-15 cells (6-well plate) was carried out with the RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. To remove genomic DNA, RNA samples were digested with RNase-free DNase I (Roche Applied Science, Mannheim, Germany). 1 μg RNA was reverse-transcribed with the SuperScript II Reverse Transcriptase (Invitrogen, Carlsbad, CA). Complementary DNA (cDNA) was PCR-amplified with gene-specific primers for PACAP receptors and GAPDH (see Table 1) and Ampli Taq Gold DNA Polymerase (Applied Biosystems, Carlsbad, CA) (5 min at 95°C, followed by 35 cycles at 95°C, 55°C and 72°C for 30 sec, respectively and a final incubation at 72°C for 7 min). Amplification products were electrophoresed on an ethidium bromide-stained 1.8% agarose/TAE gel.
Table 1.
Oligonucleotide primers used in the present study
| Primer | Sequence (5′→3′) | Amplicon [bp] | GenBank Accession |
|---|---|---|---|
| PAC1 F a | GGC CCC GTG GTT GGC TCT ATA ATG G | Z23279.1 and NM_001025372.1 | |
| PAC1 R a | GAG AGA AGG CGA ATA CTG TG | 187 or 271 | |
| PAC1hop R a | AGA GTA ATG GTG GAT AGT TCT GAC A | 200 | Z23274.1 and NM_007407.3 |
| PAC1hip R a | TGG GGA CTC TCA GTC TTA AA | 142 | Z23273.1 |
| VPAC1 F | GCA GCA ACA GAC CAA GTT CTA C | NM_012685.2 and NM_011703.4 | |
| VPAC1 R | TGA ACA GGC TCA AGA TAG CCA T | 107 | |
| VPAC2 F | AAG CAA AAA CTG CAC TAG TGA | NM_017238.1 and NM_009511.2 | |
| VPAC2 R | GCC CAA GGT ATA AAT GGC CTT CA | 133 | |
| GAPDH F | GTT ACC AGG GCT GCC TTC TC | NM_017008.3 and NM_008084.2 | |
| GAPDH R | GGG TTT CCC GTT GAT GAC C | 168 | |
| VIP F | GGA GTT TTC ACC AGC GAT TAC A | NM_053991.1 and NM_011702.2 | |
| VIP R | GCA CAG GAT CTT CCG AGA TGC | 112 | |
| STC1 F | CTA CTT TCC AGA GGA TGA TCG C | NM_031123.2 and NM_009285.3 | |
| STC1 R | ACT TCA GTG ATG GCT TCC GG | 100 |
Oligonucleotides were designed with Primer3 (http://biotools.umassmed.edu/bioapps/primer3_www.cgi) from both rat and mouse gene templates.
The PAC1 forward primer was used in combination with all PAC1 reverse primers. PAC1 F matches the beginning of transmembrane region five (TM 5) corresponding to bases 1018 to 1037 of the rat PAC1null sequence Z23279. PAC1 R matches the end of TM 6 corresponding to bases 1185 to 1204 of the rat PAC1null sequence Z23279. PAC1hop R matches the end of the hop cassette corresponding to bases 1193–1217 of the rat PAC1hop1 sequence Z23274. PAC1hip R matches the beginning of the hip cassette corresponding to bases 1140–1159 of the rat PAC1hip sequence Z23273. PAC1 F/PAC1 R generates a 187- or 271-bp fragment, depending on whether or not a hip- or hop-insert is present.
F, forward primer; R, reverse primer; PAC1, PACAP type 1 receptor; VPAC, VIP/PACAP receptor; VIP, vasoactive intestinal polypeptide; STC1, stanniocalcin 1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
2.5 Quantitative Real Time-Polymerase Chain Reaction (qRT-PCR)
qRT-PCR was performed on cDNA obtained from 1 μg DNase I-digested RNA of untreated and PACAP-treated samples with or without inhibitor pre-treatment, with 200 nM gene-specific primers for VIP, STC1 and GAPDH (see Table 1) and iQ SYBR Green Supermix on an iCycler iQ Real Time PCR System (Bio-Rad, Carlsbad, CA). cDNA levels of the gene of interest were normalized to GAPDH levels.
2.6 Measurement of intracellular cyclic AMP generation
Intracellular cAMP levels were measured with the cAMP Biotrak Enzymeimmunoassay (EIA) System (Amersham Biosciences) using the non-acetylation EIA procedure with the provided lysis reagent according to the manufacturer’s instructions. Cells were stimulated with 100 nM PACAP-38 or 25 μM forskolin in medium containing 500 μM of the non-selective phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX) for 20 min at 37°C (in 48-well plates).
2.7 Measurement of intracellular calcium concentration ([Ca2+]i) in single cells
[Ca2+]i was measured in cells plated on 1.5-cm-diameter glass cover slips (Assistent, Sondheim/Rhoen, Germany) coated with 0.5 mg/ml poly-L-lysine as previously described [38]. Briefly, cells were washed with Krebs-Ringer buffer (KRB: 20 mM HEPES, 125 mM NaCl, 5.5 mM glucose, 5 mM KCl, 1 mM Na2HPO4, 1 mM MgSO4 and 1 mM CaCl2; pH 7.3) and loaded with 4 μM fura-2 AM (Molecular Probes, Eugene, OR) for 22 min under gentle agitation. After loading, cells were washed with KRB and incubated for an additional 22 min in KRB. Cover slips were mounted onto a custom-built perfusion chamber and placed on an inverted Olympus IX70 microscope. Cells were perfused with KRB in the presence or absence of drugs at a flow rate of 800 μl/min. [Ca2+]i was measured using the 340/380 excitation ratio (R 340/380) and an emission wavelength of 510 nm. Images were captured every 2 sec and analyzed with the software MetaFluor (Molecular Devices). Cells stably expressing PAC1 receptors were identified by GFP co-expression.
2.8 Immunoblotting of phosphorylated and total p44/42 MAPK
Immunoblotting was performed according to the protocol of Cell Signaling Technology (Beverly, MA) using the NuPAGE electrophoresis system (Invitrogen, Carlsbad, CA). Briefly, cells were treated with 100 nM PACAP-38 and various pharmacological inhibitors (in 12-well plates). After 2 min, cells were lysed in 100 μl of freshly prepared 1X lysis buffer (NuPAGE LDS sample buffer, NuPAGE reducing agent, Roche cocktail inhibitor tablet and Thermo Scientific Halt phosphatase inhibitor cocktail) and sonicated for 10–15 sec to reduce sample viscosity. Samples were heat-treated for 5 min at 95°C and micro-centrifuged. 20 μl of each sample was subjected to SDS-PAGE (120 V for 1.5 h) on 4–12% Novex Bis-Tris Gels followed by electrotransfer to a 0.45 μm nitrocellulose membrane (30 V for 1.5 h). After blocking, blots were incubated with a 1:1000 dilution of rabbit polyclonal antibody specific for phosphorylated p44/42 MAP Kinase and total p44/42 MAP Kinase (Cell Signaling Technology, Beverly, MA). Immunoreactive bands were detected with HRP-conjugated anti-rabbit secondary antibody (1:3000 dilution) and the Super Signal West Pico Chemiluminescence Substrate (Thermo Fisher Scientific, Rockford, IL). Bound phospho-p44/42 MAP Kinase antibody was removed with Restore Western Blot Stripping Buffer (Thermo Fisher Scientific, Rockford, IL) before incubating the same blot with total p44/42 MAP Kinase antibody.
2.9 Statistics
Statistical analysis was carried out in Prism 4 (GraphPad Software, La Jolla, CA) by unpaired t-test or one-way ANOVA with Dunnett’s or Tukey’s Multiple Comparison Test. Significance was set at p < 0.05.
3. Results
3.1 Generation of NG108-15 cells stably expressing the PAC1null, hop1 or hip receptor variant
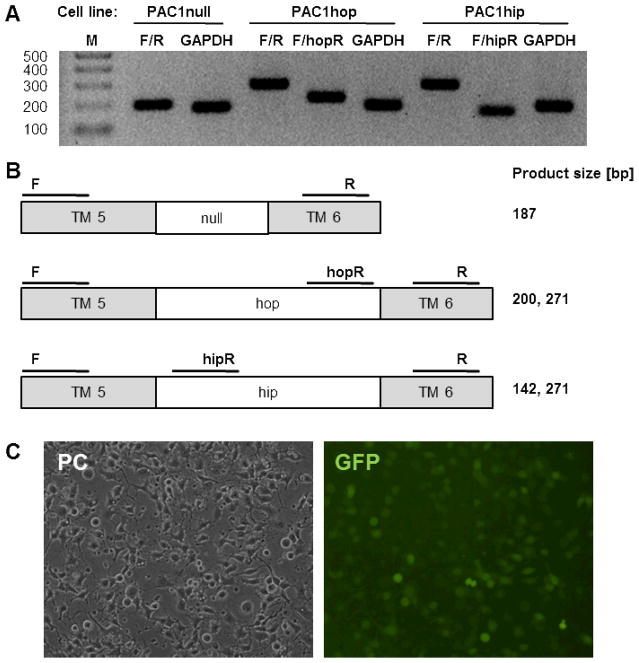
To characterize each individual PAC1 splice variant separately in neural cells, the neuroblastoma x glioma cell line NG108-15 was transduced with the rat PAC1null, hop1 or hip receptor variants. NG108-15 cells expressed negligible levels of the PACAP receptors VPAC2 and PAC1hop endogenously (not shown) and a PACAP response could only occur via activation of reconstituted but not endogenous receptors. Thus, although NG108-15 cells are capable of G protein signaling through activation of endogenous GPCRs for other neuropeptide ligands [12, 19, 22, 47], these cells possess no “background” PACAP response in the absence of exogenously expressed PACAP receptors. NG108-15 cells, stably expressing the rat PAC1 receptor splice variants hop1, null and hip, respectively, were generated through infection with gammaretroviral particles. Receptors were expressed from a CMV promoter generating stable cell lines with similar expression levels. Viral particles were made with the bicistronic retroviral vector pPRIG and the expression of the receptor was monitored by co-expression of enhanced green fluorescent protein (eGFP) from its IRES-dependent cistron [30]. The transfection efficiency was between 75 and 90%. Specifically, 89% of pPRIG-rPAC1hop, 81% of pPRIG-rPAC1null, 76% of pPRIG-rPAC1hip and 86% of pPRIG-control infected NG108-15 cells were GFP-positive. Fig. 1A and C show RT-PCR analysis of reverse-transcribed mRNAs from the NG108-15 cell lines PAC1null, PAC1hop and PAC1hip and representative micrographs of NG108-15-PAC1hop cells.
Figure 1. Expression of rat PAC1 receptor variants in NG108-15 cells after retroviral transduction.
(A) RT-PCR analysis of PAC1 receptors in NG108-15 cells, that were infected with viral particles made with the bicistronic retroviral vector pPRIG to generate stably expressing cell lines (PAC1null, PAC1hop and PAC1hip). Total RNA was reverse-transcribed and PCR-amplified (35 cycles) using different primers within the transmembrane region 5 and 6 (TM 5 and 6). M: 100-bp DNA ladder. (B) Schematic representation. The same forward primer (F) was used with different reverse primers (R): F/R generates a 187- or 271-bp fragment, depending on whether or not a hip- or hop-insert is present; the hop-specific primer pair F/hopR generates a 200-bp fragment; the hip-specific primer pair F/hipR generates a 142-bp fragment. GAPDH product size is 168-bp (see Table 1). (C) Photomicrographs of NG108-15 cells stably expressing rat PAC1hop-IRES-eGFP (NG108-15-rPAC1hop). PC: phase contrast, GFP: green fluorescent protein.
3.2 Expression of PAC1null, hop1 and hip confers PACAP-dependent intracellular cAMP generation
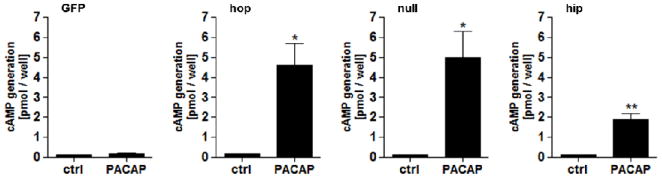
PAC1 receptors couple to Gs to potently stimulate adenylate cyclases (ACs) and increase intracellular cAMP levels. The expression of all three rat PAC1 receptor splice variants hop, null and hip in NG108-15 cells conferred an increase in intracellular cAMP generation upon treatment with 100 nM PACAP, indicating that coupling to ACs, presumably through Gs, was reconstituted in these cells. Cyclic AMP production was greater in PAC1hop- and null-compared to hip-expressing cells (~5 versus ~2 pmol/well). This suggests that the presence of the hip cassette in the third intracellular loop (ic3) of the receptor reduces coupling to ACs, in agreement with the first report published on the differential signaling properties of the different rat PAC1 splice variants in non-neural cells [54]. The expression of GFP alone was without effect (Fig. 2). The amount of cAMP generated upon stimulation with 25 μM of the AC activator forskolin was, as expected, similar in all cell lines (~15 pmol/well; not shown). Incubation of NG108-15-PAC1hop cells with 300 μM of the AC inhibitor 2′5′-dideoxyadenosine prior to PACAP treatment reduced cAMP generation approximately 75% (not shown).
Figure 2. Expression of the rat PAC1 receptor splice variants hop, null and hip in NG108-15 cells confers an intracellular cAMP production upon PACAP treatment. Cyclic AMP generation is greater in PAC1hop- and null- compared to PAC1hip-expressing cells.
Cells were treated with 100 nM PACAP-38 for 20 min. Values represent the grand mean +/− SEM of three independent experiments performed in triplicates and are expressed as pmol/well. Intracellular cAMP generation by stimulation with 25 μM forskolin was similar between all cell lines (~15 pmol/well; data not shown). ** P < 0.01, * P < 0.05 versus control, unpaired t-test.
3.3 Expression of PAC1null and hop1 confers PACAP-dependent [Ca2+]i elevation
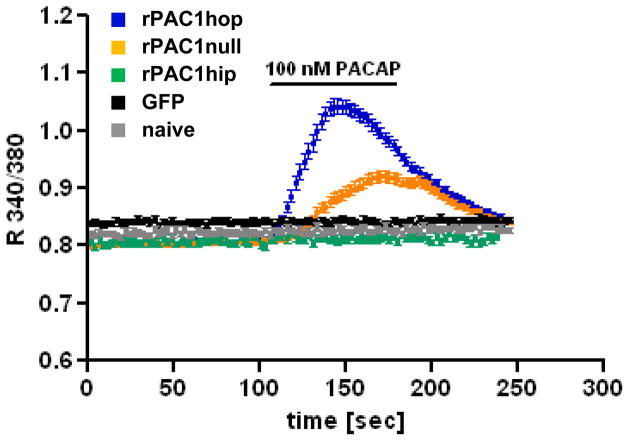
PAC1 receptor activation can stimulate combinatorial signaling through cAMP and Ca2+. Expression of the ratPAC1hop and null receptors in NG108-15 cells conferred a rise in intracellular Ca2+ concentrations ([Ca2+]i) upon treatment with 100 nM PACAP, whereas expression of the PAC1hip receptor was without effect. In both cell lines the response consisted of a rapid and transient rise of [Ca2+]i reminiscent of IP3 receptor-mediated Ca2+ release from intracellular stores. [Ca2+]i elevation was greater in PAC1hop- than in PAC1null-expressing cells, suggesting that the efficacy of coupling to Ca2+ is increased by expression of the hop cassette in the third intracellular loop of the receptor, whereas coupling to Ca2+ is abolished by expression of the hip cassette. Expression of GFP alone was without effect (Fig. 3). Depolarization-induced Ca2+ influx was similar in the five cell lines as determined by stimulation with 55 mM KCl (R 340/360: ~1; not shown). Stimulation of NG108-15-PAC1hop and -PAC1null cells with 10 nM PACAP also elicited an intracellular Ca2+ response, which was somewhat lower than the response observed upon stimulation with 100 nM. 1 nM PACAP failed to increase [Ca2+]i in either cell line (not shown).
Figure 3. Expression of the rat PAC1hop and null but not the hip receptor in NG108-15 cells confers an increase in intracellular Ca2+ upon PACAP treatment. The Ca2+ response is greater in PAC1hop- than in PAC1null-expressing cells.
Cells were loaded with 4 μM fura-2 AM and stimulated with 100 nM PACAP-38 for 75 sec in KRB. Intracellular Ca2+ concentrations were measured in single cells using the 340/380 excitation ratio (R 340/380) and an emission wavelength of 510 nm. Images were captured every 2 sec. Plot represents the average +/− SEM of five (naive), six (rPAC1hip and GFP) or 19 (rPAC1hop and null) independent experiments. Each experiment represents 10–25 cells (hop: n=376, null: n=302, hip: n=85, GFP: n=94, naive: n=94).
3.4 Differential gene induction by PAC1 splice variants
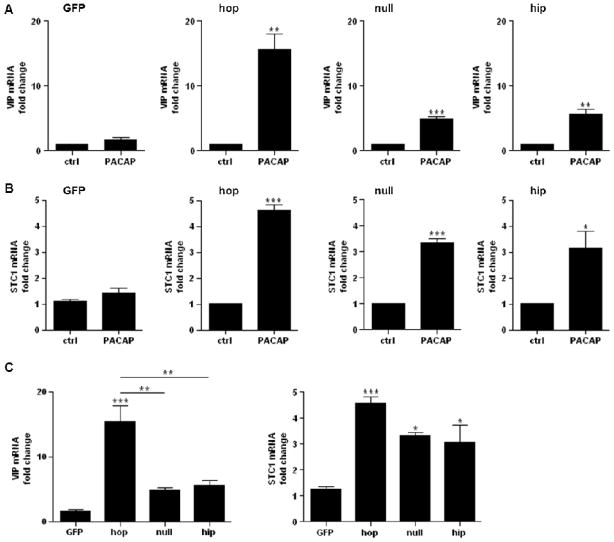
The different PAC1 splice variants were examined for a role in the induction of PACAP target genes as measured by qRT-PCR. Maximal induction of the VIP gene has been shown to depend on combinatorial signaling of cAMP and Ca2+ in primary bovine chromaffin cells (BCCs) [17]. Here we show that the PAC1null and hip receptor induced VIP gene expression about five-fold after 6 h of PACAP treatment, whereas the PAC1hop receptor lead to an 11-fold induction (Fig. 4A and C), suggesting that cAMP leads to a low-level gene expression, which is significantly enhanced by sufficiently high levels of Ca2+. The small Ca2+ response elicited by PAC1null was not high enough to enhance cAMP-induced VIP mRNA.
Figure 4. Expression of the rat PAC1hop, null and hip receptors confers induction of VIP (A) and STC1 (B) mRNA upon PACAP treatment. No induction is seen in GFP only-expressing control cells.
Cells were stimulated with 100 nM PACAP-38 and lysed after 6 h. Total RNA was extracted and reverse-transcribed. Transcript levels were measured by qRT-PCR. Values represent the grand mean +/− SEM of three independent experiments performed in triplicates and are expressed as fold change of VIP or STC1 versus GAPDH mRNA. In C values from A and B are shown as fold of untreated control. *** P < 0.001; ** P < 0.01, * P < 0.05; A and B: versus control, unpaired t-test; C: versus GFP only- and between PAC1 variant-expressing cell lines, one-way ANOVA, with Tukey’s Multiple Comparison Test.
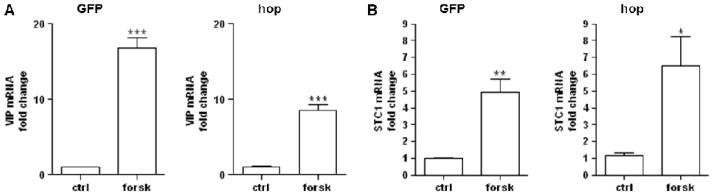
In a microarray analysis of BCCs and PC12-G cells expressing physiological levels of the PAC1hop receptor, we identified stanniocalcin 1 (STC1) as a PACAP-regulated gene [1]. Here we show that STC1 was upregulated about three- to four-fold in PAC1hop-, null- and hip-expressing NG108-15 cell lines (Fig. 4B and C), suggesting that STC1 gene induction proceeds through cAMP, and is not further enhanced by Ca2+. The AC activator forskolin induced VIP and STC1 gene induction in both GFP only- and PAC1hop-expressing cells (Fig. 5), demonstrating that AC activation and cAMP generation can occur independently of PAC1 receptor activation in NG108-15 cells.
Figure 5. Forskolin induces VIP (A) and STC1 (B) mRNA in GFP only- and PAC1hop-expressing NG108-15 cells.
Cells were stimulated with 25 μM forskolin and lysed after 6 h. Total RNA was extracted and reverse-transcribed. Transcript levels were measured by qRT-PCR. Values represent the grand mean +/− SEM of three independent experiments performed in duplicates or triplicates. Values are expressed as fold change of VIP or STC1 versus GAPDH mRNA. *** P < 0.001; ** P < 0.01, * P < 0.05; versus control, unpaired t-test.
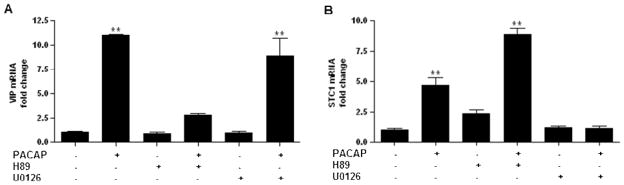
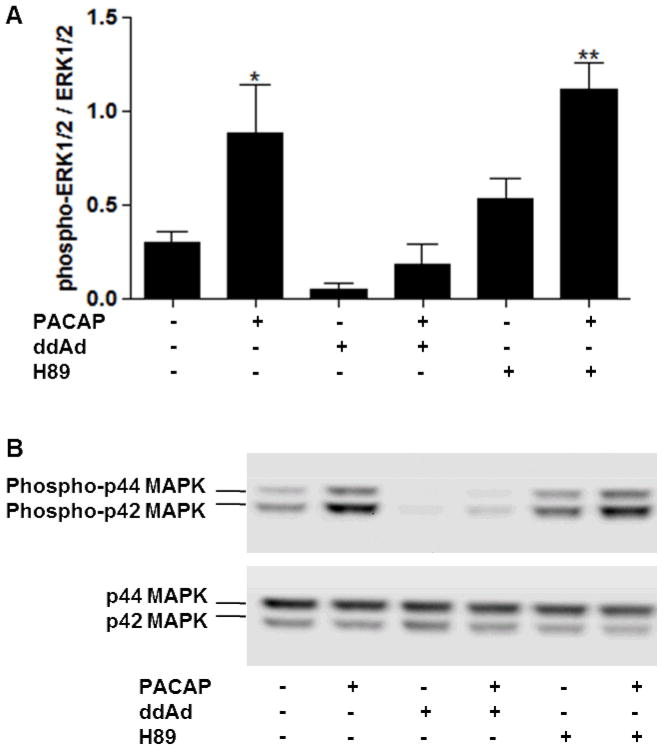
To characterize PACAP-induced gene induction pharmacologically, we examined the effect of the PKA inhibitor H89 and the MEK1/2 inhibitor U0126 (10 μM each) in NG108-15 cells expressing the PAC1hop receptor, the predominant variant in the nervous system. We show that induction of VIP by 100 nM PACAP was blocked by H89 but not by U0126, suggesting a PKA dependent but ERK1/2 independent signaling pathway. STC1 induction, in contrast, was not blocked by H89 but was blocked by U0126, suggesting cAMP and ERK1/2 dependent but PKA independent signaling to the STC1 gene (Fig. 6). Consistent with the presence of such a pathway, the activation of the mitogen-activated protein kinase (MAPK) extracellular signal-regulated kinase (ERK) was blocked by the AC inhibitor 2′5′-dideoxyadenosine (ddAd, 300 μM) but not by the PKA inhibitor H89 as determined by phosphorylation of the p44/42 MAPK (ERK1/2) (Fig. 7). As expected, U0126 completely blocked ERK1/2 phosphorylation (not shown). In NG108-15-GFP control cells, PACAP failed to activate ERK1/2 (not shown).
Figure 6. Induction of VIP (A) by PACAP is blocked by the PKA inhibitor H89 but not blocked by the MEK1/2 inhibitor U0126, whereas STC1 induction (B) is not blocked by H89 but blocked by U0126 in NG108-15-PAC1hop cells.
Cells were stimulated with 100 nM PACAP-38 in the presence or absence of the PKA inhibitor H89 (10 μM) or the MEK1/2 inhibitor U0126 (10 μM) (30 min pre-treatment with inhibitors). Cells were lysed after 6 h, total RNA was extracted and reverse-transcribed. Transcript levels were measured by qRT-PCR. Values represent the mean +/− SEM of triplicates and experiments were repeated once with similar results. Values are expressed as fold change of VIP or STC1 versus GAPDH mRNA. ** P < 0.01 versus control; one-way ANOVA, with Dunnett’s Multiple Comparison Test.
Figure 7. Activation of the p44/42 MAPK (ERK1/2) by PACAP is blocked by the AC inhibitor 2′5′-dideoxyadenosine but not blocked by the PKA inhibitor H89 in NG108-15-PAC1hop cells.
Cells were stimulated with 100 nM PACAP-38 in the presence or absence of the AC inhibitor 2′5′-dideoxyadenosine (ddAd, 300 μM) or the PKA inhibitor H89 (10 μM) (30 min pre-treatment with inhibitors). Whole cell extracts were harvested after 2 min. (A) Plot represents the mean +/− SEM of three independent experiments performed in singlicates. Values are expressed as the ratio of phospho-ERK divided by total ERK. ** P < 0.01, * P < 0.05 versus control; one-way ANOVA, with Dunnett’s Multiple Comparison Test. (B) Representative Western Blot.
4. Discussion
PACAP is an important neuropeptide slow transmitter acting as a neurotrophic factor during brain development, a neuroprotective factor after brain injury and a regulator of the adrenal gland during prolonged stress [3, 37, 42, 51]. Its effects on prolonged secretion of epinephrine from the adrenal medulla and corticosterone from the adrenal cortex are accompanied by transcriptional changes that occur at the level of the adrenal gland but also the hypothalamus, reflecting the importance of PACAP in central and peripheral control of stress responses. Moreover, potentially neuroprotective genes are up-regulated after middle cerebral artery occlusion (MCAO) in a PACAP-dependent manner [8, 18, 41, 55]. PACAP engages multiple signal transduction pathways in the nervous system through activation of its cognate receptor PAC1, regulating gene transcription [10, 13, 17, 21, 25, 44] and cellular outputs such as proliferation [32], differentiation [26, 48, 61] and cell survival [6, 57, 60, 62]. PAC1 occurs in different splice variants resulting in variable coupling to second messenger production, but the importance of specific PAC1 receptor splice variants in engaging combinatorial transduction pathways to induce gene induction in neural cells has not been previously delineated.
We transduced NG108-15 cells, which endogenously do not respond to PACAP, with the three major PAC1 splice variants occurring in the adult nervous system: the PAC1null, hop1 and hip variant with a full-length N-terminus and measured the PACAP-induced cAMP, Ca2+ and transcriptional response. We found that PACAP triggered intracellular cAMP generation in all three cell lines. Maximal cAMP generation by 100 nM PACAP-38 was greater in PAC1hop- and null- compared to hip-expressing cells, which supports results generated from rat and human PAC1 splice variants expressed in non-neural cell lines [27, 54]. The PACAP-induced Ca2+ response consisted of a rapid and transient rise in [Ca2+]i indicative of Ca2+ release from intracellular stores, which was not followed by a prolonged Ca2+ influx plateau phase that can be observed in bovine chromaffin cells (BCCs) and cortical neurons [15, 17]. The intracellular Ca2+ response in NG108-15 cells expressing the bovine PAC1hop receptor has been shown to be mainly mediated through IP3 receptor-activated Ca2+ mobilization [38], which is likely to also apply for the PAC1-mediated Ca2+ response measured here. This suggests that PAC1 receptor expression in NG108-15 cells reconstitutes cAMP and Ca2+ signaling, presumably through coupling to Gs and Gq, respectively. A Ca2+ response was induced by PAC1hop and null but not hip and the increase in [Ca2+]i was greater in PAC1hop- than PAC1null-expressing cells. VIP mRNA induction was also greatest in PAC1hop-expressing cells, whereas induction was lower in PAC1null- and hip-expressing cells, where the level of induction did not differ. These results suggest that the Ca2+ response induced by PAC1null did not reach the threshold required for the induction of a full transcriptional response.
Our laboratory has previously shown that VIP mRNA is maximally induced through combinatorial signaling of cAMP and Ca2+ influx in BCCs, two signal transduction pathways induced by PACAP in this cell type [17]. Results from the present study indicate that a sufficiently high transient Ca2+ response, presumably Ca2+ mobilization, is sufficient to increase cAMP-mediated gene induction, suggesting that combinatorial signaling occurs whether the rise in [Ca2+]i results from mobilization or influx. This induction can only be supported by the PAC1hop receptor variant, which is efficiently coupled to both cAMP and Ca2+ elevation in NG108-15 cells. In contrast, STC1, a gene up-regulated under hypoxic/ischemic conditions in vivo [64–66] was induced by the PAC1hop, null and hip receptor to a similar extent, suggesting that cAMP elevation alone is sufficient to induce STC1. The AC activator forskolin, which acts downstream of receptor activation and induces a supra-maximal cAMP response, lead to a similar increase in STC1 mRNA, further supporting the conclusion that cAMP signaling is both required and sufficient to induce STC1. Taken together, these results suggest that the presence of the hop cassette in the third intracellular loop (ic3) of the receptor is required for the induction of a full Ca2+ response and therefore a maximal transcriptional response requiring combinatorial signaling through cAMP and Ca2+.
Two separate cAMP dependent pathways mediated signaling for gene induction in NG108-15 cells upon PAC1hop expression. PACAP signaling to the VIP gene proceeded through cAMP/PKA, but not ERK1/2, whereas cAMP and ERK1/2, but not PKA, were required for STC1 induction. Moreover, ERK1/2 activation was itself dependent on the cAMP dependent and PKA independent signaling pathway. The importance of the latter pathway in PACAP-mediated signaling has been shown before for neuronal differentiation of PC12 [48] and neuroblastoma cells [35]. It is noteworthy that VIP gene induction is accessed via the cAMP/ERK pathway in BCCs [17], but via the cAMP/PKA pathway in PC12 [59] and NG108-15 cells (this study). These results imply a complex signaling code for PACAP-regulated genes that differs among different cell types, perhaps as a function of their state of development and differentiation [7]. PACAP’s protective action on fully differentiated neurons has been shown in an in vivo mouse stroke model [8] and in cell culture during glutamate-induced excitotoxicity [11], mimicked by intracellular elevation of cAMP levels [36]. It will be of particular interest to investigate whether the induction of STC1 by PACAP in differentiated neurons of the central nervous system proceeds through the cAMP and ERK1/2 dependent but PKA independent signaling pathway, and is a mediator of PACAP’s neuroprotective effects after brain injury.
In conclusion we provide evidence that the maximal induction of some PACAP target genes requires combinatorial signaling through cAMP and Ca2+, which is mediated uniquely by PAC1hop. PACAP dependent induction of other genes can proceed through a single cAMP-mediated pathway, which is supported by any of the three PAC1 receptor splice variants hop, hip or null. Cell-specific expression of PAC1 splice variants, providing variable coupling to second messengers and differential gene induction upon exposure to PACAP, might be an important regulatory mechanism implicated in the pleiotropic actions of PACAP in ischemic injury in the brain, and stress-responding throughout the neuraxis. The engagement of two different cAMP dependent signaling pathways inducing differential gene induction implies even more diversity in PACAP-mediated signaling. A more complete understanding of the complex signaling pathways induced by PACAP for the induction of target genes in a variety of cell types will be crucial for the development of therapeutic strategies for the treatment of stroke and other neurodegenerative and stress-associated diseases, based on PACAP’s pharmacological and physiological effects in vivo.
Acknowledgments
We thank Dr. Laurent Journot for sharing their pRK8-PAC1 vectors, James Walsh for assistance in the subcloning of the PAC1 receptor variants, James Nagle and Debbie Kauffman (DNA Sequencing Facility, NINDS, National Institutes of Health) for carrying out sequencing of all DNA samples, Dr. Maribeth V. Eiden and members of her lab (NIMH, National Institutes of Health) for assistance in producing viral particles and Prof. E. Weihe (Philipps-University, Marburg, Germany) for advice and consultation in preparation of this report. This work was supported by NIMH Intramural Research Program Project Z01 MH002386-22.
Abbreviations
- PACAP
pituitary adenylate cyclase-activating polypeptide
- PAC1
PACAP type 1 receptor
- ic3
third intracellular loop
- VIP
vasoactive intestinal polypeptide
- STC1
stanniocalcin 1
- GPCR
G protein-coupled receptor
- AC
adenylate cyclase
- PKA
protein kinase A
- ERK
extracellular signal-regulated kinase
- NG108-15 cells
mouse neuroblastoma x rat glioma hybrid
Footnotes
Disclosure statement: The authors have nothing to disclose.
References
- 1.Ait-Ali D, Samal B, Mustafa T, Eiden LE. Neuropeptides, Growth Factors, and Cytokines: A Cohort of Informational Molecules Whose Expression Is Up-Regulated by the Stress-Associated Slow Transmitter PACAP in Chromaffin Cells. Cell Mol Neurobiol. 2010 doi: 10.1007/s10571-010-9620-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arimura A. Receptors for pituitary adenylate cyclase-activating polypeptide: comparison with vasoactive intestinal peptide receptors. Trends Endocrinol Metab. 1992;3:288–94. doi: 10.1016/1043-2760(92)90139-r. [DOI] [PubMed] [Google Scholar]
- 3.Arimura A. Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems. Jpn J Physiol. 1998;48:301–31. doi: 10.2170/jjphysiol.48.301. [DOI] [PubMed] [Google Scholar]
- 4.Arimura A, Somogyvari-Vigh A, Miyata A, Mizuno K, Coy DH, Kitada C. Tissue distribution of PACAP as determined by RIA: highly abundant in the rat brain and testes. Endocrinology. 1991;129:2787–9. doi: 10.1210/endo-129-5-2787. [DOI] [PubMed] [Google Scholar]
- 5.Bobrovskaya L, Gelain DP, Gilligan C, Dickson PW, Dunkley PR. PACAP stimulates the sustained phosphorylation of tyrosine hydroxylase at serine 40. Cell Signal. 2007;19:1141–9. doi: 10.1016/j.cellsig.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Cavallaro S, Copani A, D’Agata V, Musco S, Petralia S, Ventra C, et al. Pituitary adenylate cyclase activating polypeptide prevents apoptosis in cultured cerebellar granule neurons. Mol Pharmacol. 1996;50:60–6. [PubMed] [Google Scholar]
- 7.Cavallaro S, D’Agata V, Guardabasso V, Travali S, Stivala F, Canonico PL. Differentiation induces pituitary adenylate cyclase-activating polypeptide receptor expression in PC-12 cells. Mol Pharmacol. 1995;48:56–62. [PubMed] [Google Scholar]
- 8.Chen Y, Samal B, Hamelink CR, Xiang CC, Chen Y, Chen M, et al. Neuroprotection by endogenous and exogenous PACAP following stroke. Regul Pept. 2006;137:4–19. doi: 10.1016/j.regpep.2006.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dautzenberg FM, Mevenkamp G, Wille S, Hauger RL. N-terminal splice variants of the type I PACAP receptor: isolation, characterization and ligand binding/selectivity determinants. J Neuroendocrinol. 1999;11:941–9. doi: 10.1046/j.1365-2826.1999.00411.x. [DOI] [PubMed] [Google Scholar]
- 10.Eiden LE, Samal B, Gerdin MJ, Mustafa T, Vaudry D, Stroth N. Discovery of pituitary adenylate cyclase-activating polypeptide-regulated genes through microarray analyses in cell culture and in vivo. Ann N Y Acad Sci. 2008;1144:6–20. doi: 10.1196/annals.1418.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frechilla D, Garcia-Osta A, Palacios S, Cenarruzabeitia E, Del Rio J. BDNF mediates the neuroprotective effect of PACAP-38 on rat cortical neurons. Neuroreport. 2001;12:919–23. doi: 10.1097/00001756-200104170-00011. [DOI] [PubMed] [Google Scholar]
- 12.Ghadessy RS, Kelly E. Second messenger-dependent protein kinases and protein synthesis regulate endogenous secretin receptor responsiveness. Br J Pharmacol. 2002;135:2020–8. doi: 10.1038/sj.bjp.0704655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Girard BM, Keller ET, Schutz KC, May V, Braas KM. Pituitary adenylate cyclase activating polypeptide and PAC1 receptor signaling increase Homer 1a expression in central and peripheral neurons. Regul Pept. 2004;123:107–16. doi: 10.1016/j.regpep.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 14.Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976;73:2424–8. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grimaldi M, Cavallaro S. Functional and molecular diversity of PACAP/VIP receptors in cortical neurons and type I astrocytes. Eur J Neurosci. 1999;11:2767–72. doi: 10.1046/j.1460-9568.1999.00693.x. [DOI] [PubMed] [Google Scholar]
- 16.Hahm SH, Hsu CM, Eiden LE. PACAP activates calcium influx-dependent and -independent pathways to couple met-enkephalin secretion and biosynthesis in chromaffin cells. J Mol Neurosci. 1998;11:43–56. doi: 10.1385/JMN:11:1:43. [DOI] [PubMed] [Google Scholar]
- 17.Hamelink C, Lee HW, Chen Y, Grimaldi M, Eiden LE. Coincident elevation of cAMP and calcium influx by PACAP-27 synergistically regulates vasoactive intestinal polypeptide gene transcription through a novel PKA-independent signaling pathway. J Neurosci. 2002;22:5310–20. doi: 10.1523/JNEUROSCI.22-13-05310.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamelink C, Tjurmina O, Damadzic R, Young WS, Weihe E, Lee HW, et al. Pituitary adenylate cyclase-activating polypeptide is a sympathoadrenal neurotransmitter involved in catecholamine regulation and glucohomeostasis. Proc Natl Acad Sci U S A. 2002;99:461–6. doi: 10.1073/pnas.012608999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamprecht B, Glaser T, Reiser G, Bayer E, Propst F. Culture and characteristics of hormone-responsive neuroblastoma X glioma hybrid cells. Methods Enzymol. 1985;109:316–41. doi: 10.1016/0076-6879(85)09096-6. [DOI] [PubMed] [Google Scholar]
- 20.Hannibal J. Pituitary adenylate cyclase-activating peptide in the rat central nervous system: an immunohistochemical and in situ hybridization study. J Comp Neurol. 2002;453:389–417. doi: 10.1002/cne.10418. [DOI] [PubMed] [Google Scholar]
- 21.Hashimoto H, Hagihara N, Koga K, Yamamoto K, Shintani N, Tomimoto S, et al. Synergistic induction of pituitary adenylate cyclase-activating polypeptide (PACAP) gene expression by nerve growth factor and PACAP in PC12 cells. J Neurochem. 2000;74:501–7. doi: 10.1046/j.1471-4159.2000.740501.x. [DOI] [PubMed] [Google Scholar]
- 22.Ishihara T, Nakamura S, Kaziro Y, Takahashi T, Takahashi K, Nagata S. Molecular cloning and expression of a cDNA encoding the secretin receptor. EMBO J. 1991;10:1635–41. doi: 10.1002/j.1460-2075.1991.tb07686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishihara T, Shigemoto R, Mori K, Takahashi K, Nagata S. Functional expression and tissue distribution of a novel receptor for vasoactive intestinal polypeptide. Neuron. 1992;8:811–9. doi: 10.1016/0896-6273(92)90101-i. [DOI] [PubMed] [Google Scholar]
- 24.Joo KM, Chung YH, Kim MK, Nam RH, Lee BL, Lee KH, et al. Distribution of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide receptors (VPAC1, VPAC2, and PAC1 receptor) in the rat brain. J Comp Neurol. 2004;476:388–413. doi: 10.1002/cne.20231. [DOI] [PubMed] [Google Scholar]
- 25.Just L, Morl F, Barmann C, Olenik C, Meyer DK. Evidence for cell specific regulation by PACAP38 of the proenkephalin gene expression in neocortical cells. Glia. 2000;30:242–52. doi: 10.1002/(sici)1098-1136(200005)30:3<242::aid-glia4>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 26.Lazarovici P, Jiang H, Fink D., Jr The 38-amino-acid form of pituitary adenylate cyclase-activating polypeptide induces neurite outgrowth in PC12 cells that is dependent on protein kinase C and extracellular signal-regulated kinase but not on protein kinase A, nerve growth factor receptor tyrosine kinase, p21(ras) G protein, and pp60(c-src) cytoplasmic tyrosine kinase. Mol Pharmacol. 1998;54:547–58. doi: 10.1124/mol.54.3.547. [DOI] [PubMed] [Google Scholar]
- 27.Lutz EM, Ronaldson E, Shaw P, Johnson MS, Holland PJ, Mitchell R. Characterization of novel splice variants of the PAC1 receptor in human neuroblastoma cells: consequences for signaling by VIP and PACAP. Mol Cell Neurosci. 2006;31:193–209. doi: 10.1016/j.mcn.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Lutz EM, Sheward WJ, West KM, Morrow JA, Fink G, Harmar AJ. The VIP2 receptor: molecular characterisation of a cDNA encoding a novel receptor for vasoactive intestinal peptide. FEBS Lett. 1993;334:3–8. doi: 10.1016/0014-5793(93)81668-p. [DOI] [PubMed] [Google Scholar]
- 29.Marley PD, Cheung CY, Thomson KA, Murphy R. Activation of tyrosine hydroxylase by pituitary adenylate cyclase-activating polypeptide (PACAP-27) in bovine adrenal chromaffin cells. J Auton Nerv Syst. 1996;60:141–6. doi: 10.1016/0165-1838(96)00044-6. [DOI] [PubMed] [Google Scholar]
- 30.Martin P, Albagli O, Poggi MC, Boulukos KE, Pognonec P. Development of a new bicistronic retroviral vector with strong IRES activity. BMC Biotechnol. 2006;6:4. doi: 10.1186/1472-6750-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mazzocchi G, Malendowicz LK, Neri G, Andreis PG, Ziolkowska A, Gottardo L, et al. Pituitary adenylate cyclase-activating polypeptide and PACAP receptor expression and function in the rat adrenal gland. Int J Mol Med. 2002;9:233–43. [PubMed] [Google Scholar]
- 32.Meyer DK. The effects of PACAP on neural cell proliferation. Regul Pept. 2006;137:50–7. doi: 10.1016/j.regpep.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 33.Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang L, et al. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun. 1989;164:567–74. doi: 10.1016/0006-291x(89)91757-9. [DOI] [PubMed] [Google Scholar]
- 34.Miyata A, Jiang L, Dahl RD, Kitada C, Kubo K, Fujino M, et al. Isolation of a neuropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP38) Biochem Biophys Res Commun. 1990;170:643–8. doi: 10.1016/0006-291x(90)92140-u. [DOI] [PubMed] [Google Scholar]
- 35.Monaghan TK, Mackenzie CJ, Plevin R, Lutz EM. PACAP-38 induces neuronal differentiation of human SH-SY5Y neuroblastoma cells via cAMP-mediated activation of ERK and p38 MAP kinases. J Neurochem. 2008;104:74–88. doi: 10.1111/j.1471-4159.2007.05018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morio H, Tatsuno I, Hirai A, Tamura Y, Saito Y. Pituitary adenylate cyclase-activating polypeptide protects rat-cultured cortical neurons from glutamate-induced cytotoxicity. Brain Res. 1996;741:82–8. doi: 10.1016/s0006-8993(96)00920-1. [DOI] [PubMed] [Google Scholar]
- 37.Mustafa T, Eiden LE. The Secretin Superfamily: PACAP, VIP, and Related Neuropeptides. In: Lim R, editor. Handbook of Neurochemistry and Molecular Neurobiology: XIII Neuroactive Peptides and Proteins. XIII. Springer; Heidelberg: 2006. pp. 1–36. [Google Scholar]
- 38.Mustafa T, Grimaldi M, Eiden LE. The hop cassette of the PAC1 receptor confers coupling to Ca2+ elevation required for pituitary adenylate cyclase-activating polypeptide-evoked neurosecretion. J Biol Chem. 2007;282:8079–91. doi: 10.1074/jbc.M609638200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mustafa T, Walsh J, Grimaldi M, Eiden LE. PAC1hop receptor activation facilitates catecholamine secretion selectively through 2-APB-sensitive Ca(2+) channels in PC12 cells. Cell Signal. 2010;22:1420–6. doi: 10.1016/j.cellsig.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nogi H, Hashimoto H, Fujita T, Hagihara N, Matsuda T, Baba A. Pituitary adenylate cyclase-activating polypeptide (PACAP) receptor mRNA in the rat adrenal gland: localization by in situ hybridization and identification of splice variants. Jpn J Pharmacol. 1997;75:203–7. doi: 10.1254/jjp.75.203. [DOI] [PubMed] [Google Scholar]
- 41.Ohtaki H, Nakamachi T, Dohi K, Aizawa Y, Takaki A, Hodoyama K, et al. Pituitary adenylate cyclase-activating polypeptide (PACAP) decreases ischemic neuronal cell death in association with IL-6. Proc Natl Acad Sci U S A. 2006;103:7488–93. doi: 10.1073/pnas.0600375103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohtaki H, Nakamachi T, Dohi K, Shioda S. Role of PACAP in ischemic neural death. J Mol Neurosci. 2008;36:16–25. doi: 10.1007/s12031-008-9077-3. [DOI] [PubMed] [Google Scholar]
- 43.Pantaloni C, Brabet P, Bilanges B, Dumuis A, Houssami S, Spengler D, et al. Alternative splicing in the N-terminal extracellular domain of the pituitary adenylate cyclase-activating polypeptide (PACAP) receptor modulates receptor selectivity and relative potencies of PACAP-27 and PACAP-38 in phospholipase C activation. J Biol Chem. 1996;271:22146–51. doi: 10.1074/jbc.271.36.22146. [DOI] [PubMed] [Google Scholar]
- 44.Pisegna JR, Moody TW, Wank SA. Differential signaling and immediate-early gene activation by four splice variants of the human pituitary adenylate cyclase-activating polypeptide receptor (hPACAP-R) Ann N Y Acad Sci. 1996;805:54–64. doi: 10.1111/j.1749-6632.1996.tb17473.x. discussion -6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pisegna JR, Wank SA. Molecular cloning and functional expression of the pituitary adenylate cyclase-activating polypeptide type I receptor. Proc Natl Acad Sci U S A. 1993;90:6345–9. doi: 10.1073/pnas.90.13.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pisegna JR, Wank SA. Cloning and characterization of the signal transduction of four splice variants of the human pituitary adenylate cyclase activating polypeptide receptor. Evidence for dual coupling to adenylate cyclase and phospholipase C. J Biol Chem. 1996;271:17267–74. doi: 10.1074/jbc.271.29.17267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Propst F, Moroder L, Wunsch E, Hamprecht B. The influence of secretin, glucagon and other peptides, of amino acids, prostaglandin endoperoxide analogues and diazepam on the level of adenosine 3′,5′-cyclic monophosphate in neuroblastoma x glioma hybrid cells. J Neurochem. 1979;32:1495–500. doi: 10.1111/j.1471-4159.1979.tb11090.x. [DOI] [PubMed] [Google Scholar]
- 48.Ravni A, Vaudry D, Gerdin MJ, Eiden MV, Falluel-Morel A, Gonzalez BJ, et al. A cAMP-dependent, protein kinase A-independent signaling pathway mediating neuritogenesis through Egr1 in PC12 cells. Mol Pharmacol. 2008;73:1688–708. doi: 10.1124/mol.107.044792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rius RA, Guidotti A, Costa E. Pituitary adenylate cyclase activating polypeptide (PACAP) potently enhances tyrosine hydroxylase (TH) expression in adrenal chromaffin cells. Life Sci. 1994;54:1735–43. doi: 10.1016/0024-3205(94)00614-8. [DOI] [PubMed] [Google Scholar]
- 50.Sherwood NM, Krueckl SL, McRory JE. The origin and function of the pituitary adenylate cyclase-activating polypeptide (PACAP)/glucagon superfamily. Endocr Rev. 2000;21:619–70. doi: 10.1210/edrv.21.6.0414. [DOI] [PubMed] [Google Scholar]
- 51.Shioda S, Ohtaki H, Nakamachi T, Dohi K, Watanabe J, Nakajo S, et al. Pleiotropic functions of PACAP in the CNS: neuroprotection and neurodevelopment. Ann N Y Acad Sci. 2006;1070:550–60. doi: 10.1196/annals.1317.080. [DOI] [PubMed] [Google Scholar]
- 52.Shioda S, Shimoda Y, Hori T, Mizushima H, Ajiri T, Funahashi H, et al. Localization of the pituitary adenylate cyclase-activating polypeptide receptor and its mRNA in the rat adrenal medulla. Neurosci Lett. 2000;295:81–4. doi: 10.1016/s0304-3940(00)01595-0. [DOI] [PubMed] [Google Scholar]
- 53.Shioda S, Shuto Y, Somogyvari-Vigh A, Legradi G, Onda H, Coy DH, et al. Localization and gene expression of the receptor for pituitary adenylate cyclase-activating polypeptide in the rat brain. Neurosci Res. 1997;28:345–54. doi: 10.1016/s0168-0102(97)00065-5. [DOI] [PubMed] [Google Scholar]
- 54.Spengler D, Waeber C, Pantaloni C, Holsboer F, Bockaert J, Seeburg PH, et al. Differential signal transduction by five splice variants of the PACAP receptor. Nature. 1993;365:170–5. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- 55.Stroth N, Eiden LE. Stress hormone synthesis in mouse hypothalamus and adrenal gland triggered by restraint is dependent on pituitary adenylate cyclase-activating polypeptide signaling. Neuroscience. 2010;165:1025–30. doi: 10.1016/j.neuroscience.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sundler F, Ekblad E, Hannibal J, Moller K, Zhang YZ, Mulder H, et al. Pituitary adenylate cyclase-activating peptide in sensory and autonomic ganglia: localization and regulation. Ann N Y Acad Sci. 1996;805:410–26. doi: 10.1111/j.1749-6632.1996.tb17501.x. discussion 27–8. [DOI] [PubMed] [Google Scholar]
- 57.Tanaka J, Koshimura K, Murakami Y, Sohmiya M, Yanaihara N, Kato Y. Neuronal protection from apoptosis by pituitary adenylate cyclase-activating polypeptide. Regul Pept. 1997;72:1–8. doi: 10.1016/s0167-0115(97)01038-0. [DOI] [PubMed] [Google Scholar]
- 58.Taupenot L, Mahata M, Mahata SK, O’Connor DT. Time-dependent effects of the neuropeptide PACAP on catecholamine secretion: stimulation and desensitization. Hypertension. 1999;34:1152–62. doi: 10.1161/01.hyp.34.5.1152. [DOI] [PubMed] [Google Scholar]
- 59.Vaudry D, Chen Y, Ravni A, Hamelink C, Elkahloun AG, Eiden LE. Analysis of the PC12 cell transcriptome after differentiation with pituitary adenylate cyclase-activating polypeptide (PACAP) J Neurochem. 2002;83:1272–84. doi: 10.1046/j.1471-4159.2002.01242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vaudry D, Gonzalez BJ, Basille M, Pamantung TF, Fontaine M, Fournier A, et al. The neuroprotective effect of pituitary adenylate cyclase-activating polypeptide on cerebellar granule cells is mediated through inhibition of the CED3-related cysteine protease caspase-3/CPP32. Proc Natl Acad Sci U S A. 2000;97:13390–5. doi: 10.1073/pnas.97.24.13390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vaudry D, Stork PJ, Lazarovici P, Eiden LE. Signaling pathways for PC12 cell differentiation: making the right connections. Science. 2002;296:1648–9. doi: 10.1126/science.1071552. [DOI] [PubMed] [Google Scholar]
- 62.Villalba M, Bockaert J, Journot L. Pituitary adenylate cyclase-activating polypeptide (PACAP-38) protects cerebellar granule neurons from apoptosis by activating the mitogen-activated protein kinase (MAP kinase) pathway. J Neurosci. 1997;17:83–90. doi: 10.1523/JNEUROSCI.17-01-00083.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Watanabe T, Masuo Y, Matsumoto H, Suzuki N, Ohtaki T, Masuda Y, et al. Pituitary adenylate cyclase activating polypeptide provokes cultured rat chromaffin cells to secrete adrenaline. Biochem Biophys Res Commun. 1992;182:403–11. doi: 10.1016/s0006-291x(05)80159-7. [DOI] [PubMed] [Google Scholar]
- 64.Westberg JA, Serlachius M, Lankila P, Andersson LC. Hypoxic preconditioning induces elevated expression of stanniocalcin-1 in the heart. Am J Physiol Heart Circ Physiol. 2007;293:H1766–71. doi: 10.1152/ajpheart.00017.2007. [DOI] [PubMed] [Google Scholar]
- 65.Westberg JA, Serlachius M, Lankila P, Penkowa M, Hidalgo J, Andersson LC. Hypoxic preconditioning induces neuroprotective stanniocalcin-1 in brain via IL-6 signaling. Stroke. 2007;38:1025–30. doi: 10.1161/01.STR.0000258113.67252.fa. [DOI] [PubMed] [Google Scholar]
- 66.Zhang K, Lindsberg PJ, Tatlisumak T, Kaste M, Olsen HS, Andersson LC. Stanniocalcin: A molecular guard of neurons during cerebral ischemia. Proc Natl Acad Sci U S A. 2000;97:3637–42. doi: 10.1073/pnas.070045897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang KZ, Westberg JA, Paetau A, von Boguslawsky K, Lindsberg P, Erlander M, et al. High expression of stanniocalcin in differentiated brain neurons. Am J Pathol. 1998;153:439–45. doi: 10.1016/S0002-9440(10)65587-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou CJ, Kikuyama S, Shibanuma M, Hirabayashi T, Nakajo S, Arimura A, et al. Cellular distribution of the splice variants of the receptor for pituitary adenylate cyclase-activating polypeptide (PAC(1)-R) in the rat brain by in situ RT-PCR. Brain Res Mol Brain Res. 2000;75:150–8. doi: 10.1016/s0169-328x(99)00300-9. [DOI] [PubMed] [Google Scholar]