Abstract
Advances in transplantation techniques and supportive care strategies have resulted in a significant improvement in survival of those who have undergone treatment. However, hematopoietic stem cell transplantation (HSCT) survivors are at risk of developing long-term complications, such as endocrinopathies, musculoskeletal disorders, cardiopulmonary compromise and subsequent malignancies. These complications have a direct impact on the morbidity and mortality experienced by HSCT survivors. Two-thirds of HSCT survivors develop at least one chronic health condition; while a fifth develop severe or life-threatening conditions. HSCT patients who have survived for at least 5 years post-transplantation are at a fourfold to ninefold increased risk of late mortality for as long as 30 years from HSCT, producing an estimated 30% lower life expectancy compared with the general population. The high burden of morbidity experienced by HSCT survivors makes it critically important that there is standardized follow-up of HSCT survivors at high risk for post-HSCT complications. The Center for International Blood and Marrow Transplant Research/European Group for Blood and Marrow Transplantation/American Society for Blood and Marrow Transplantation and the Children’s Oncology Group long-term follow-up guidelines offer such standardized care. Future steps include wider dissemination and refinement of these guidelines.
Keywords: hematopoietic stem cell transplantation, late effects, long-term follow-up guidelines
Hematopoietic stem cell transplantation (HSCT) is an established curative option for a variety of hematological malignancies. Advances in transplantation techniques and supportive care strategies have resulted in a significant improvement in survival: 70–80% of those who survive the first 2 years following HSCT are expected to become long-term survivors [1–3]. However, cure or control of the underlying disease is not accompanied by full restoration of health. HSCT survivors are at risk of developing long-term complications, such as endocrinopathies, musculoskeletal disorders, cardiopulmonary compromise and subsequent malignancies (summarized in Table 1) [4–14]. These complications have a direct impact on the morbidity and mortality experienced by HSCT survivors. The high burden of morbidity experienced by HSCT survivors forms the basis for standardized follow-up of HSCT survivors at high risk for post-HSCT complications. This article summarizes the magnitude of risk of key long-term complications experienced by HSCT survivors, identifying those at increased risk for these complications owing to host characteristics and therapeutic exposures. The article also describes the current recommendations for follow-up, patterns of healthcare utilization by the HSCT survivors and adherence to these recommendations. Finally, the article identifies the need for future research efforts related to HSCT outcomes, identification of those at highest risk and refinement of the existing follow-up guidelines so that those at the highest risk are targeted.
Table 1.
Commonly occurring long-term complications in survivors of hematopoietic cell transplantation.
| Adverse outcome | Therapeutic exposures associated with increased risk |
Factors associated with highest risk |
|---|---|---|
| Cataracts | Cranial irradiation, TBI, steroids | Higher radiation dose, combination of steroids and radiation, single daily fraction |
| Cardiomyopathy/congestive heart failure |
Pre-HSCT anthracyclines | High cumulative doses, females, chest radiation, post-HSCT comorbidities (diabetes and hypertension) |
| Myocardial infarction | Pre-HSCT radiation to chest | Underlying risk factors for coronary artery disease (e.g., smoking, hypertension, hyperlipidemia, obesity) |
| Pulmonary fibrosis/interstitial pneumonitis |
Pre-HSCT bleomycin, radiation to chest, carmustine, TBI, chronic GvHD |
Younger age at treatment, bleomycin dose >400 U/m2 |
| Therapy-related myelodysplasia (after autologous HSCT) |
Pre-HSCT alkylating agents, topoisomerase II inhibitors, radiation, anthracyclines, platinum compound, use of topoisomerase II inhibitors for stem cell mobilization |
Increasing dose of chemotherapeutic agents, older age at therapeutic exposure |
| Thyroid cancer | Pre-HSCT radiation to the thyroid gland (e.g., neck, mantle), TBI |
Increasing dose up to 29 Gy, female sex, younger age at radiation |
| Skin cancer (basal cell, squamous cell, melanoma) |
Pre-HSCT radiation therapy, chronic GvHD | Additional excessive exposure to sun, tanning booths, males |
| Hypothyroidism | Pre-HSCT radiation to the thyroid gland (e.g., neck, mantle), TBI |
Increasing dose, female sex, older age at treatment |
| Hypogonadism | Pre-HSCT alkylating agents, craniospinal irradiation, abdomino–pelvic irradiation, gonadal irradiation, TBI |
Treatment during peripubertal or post-pubertal period in girls, higher cumulative doses of alkylators |
| Renal insufficiency | Pre-HSCT platinum-based therapy, ifosfamide, high-dose methotrexate, abdominal radiation, surgery, TBI, calcineurin inhibitors |
High-dose chemotherapy, younger age, abdominal radiation and chemotherapy |
| Short stature | Pre-HSCT cranial irradiation, corticosteroids, TBI | Younger age at treatment, cranial radiation dose >18 Gy, unfractionated (10 Gy) TBI |
| Osteopenia/osteoporosis | Pre-HSCT craniospinal irradiation, gonadal irradiation, TBI, corticosteroids |
Associated hypothyroidism, hypogonadism, growth hormone deficiency |
| Avascular necrosis | Corticosteroids, high-dose radiation to any bone, calcineurin inhibitors |
Dexamethasone, adolescence, female sex |
GvHD: Graft-versus-host disease; HSCT: Hematopoietic stem cell transplantation; TBI: Total body irradiation.
Cardiovascular risk factors & cardiac disease
Hematopoietic cell transplantation survivors are at risk for a variety of cardiac and cardiovascular complications owing to the combined effect of pre-HSCT therapeutic exposures, transplant-related conditioning and post-HSCT graft-versus-host disease (GvHD). Furthermore, HSCT recipients are at a 2.3- to 4.0-fold increased risk of death due to cardiac causes when compared with the general population [1,10].
Late cardiotoxicity includes cardiomyopathy, congestive heart failure (CHF), valvular dysfunction, arrhythmia and pericarditis. Anthracyclines are the main cause of cardiomyopathy. Mediastinal radiation can produce inflammation and fibrosis, resulting in restrictive cardiomyopathy and valvular defects. Fibrosis can also affect the electrical conduction pathways, causing arrhythmias.
Anthracycline-induced cardiotoxicity is dose dependent and the incidence approaches 26% in non-HSCT populations treated with doses exceeding 550 mg/m2 [15]. The modifying effects of female sex, younger age at exposure to anthracyclines and mediastinal radiation are now well established in survivors of childhood cancer [16,17]. However, less is known regarding risk factors for CHF in long-term survivors of HSCT. Potential risk factors unique to HSCT include conditioning with high-dose chemotherapy, total body irradiation (TBI) and presence of chronic GvHD following allogeneic HSCT. In addition, HSCT survivors are at increased risk of developing de novo cardiovascular risk factors such as diabetes and hypertension due, in part, to exposure to TBI, prolonged immunosuppressive therapy following allogeneic HSCT, or other conditions such as growth hormone deficiency or hypothyroidism [5,18]. A comprehensive approach to the independent influence of these cardiovascular risk factors on therapy-associated CHF was undertaken by Armenian et al. [4]. Using a case–control design, individuals with clinical CHF were identified from a cohort of 3820 individuals who had survived for 1 year or longer following transplantation between 1981 and 2006. Controls (without CHF) were matched by age and year of HSCT, type of HSCT (autologous or allogeneic) and length of follow-up. The mean ejection fraction was 36.7% (range: 13–53%). Multivariate analysis revealed the following factors to be independently associated with risk of CHF: female gender (odds ratio [OR]: 2.1; p < 0.01), high-dose (≥250 mg/m2) anthracycline alone (OR: 3.9; p < 0.01) or with chest radiation (OR: 6.7; p < 0.01) and the presence of multiple post-HSCT comorbidities (OR: 3.0; p < 0.01). Overall survival following CHF was 46.1% at 5 years. These data form the foundation for identifying high-risk individuals who should undergo targeted surveillance, as well as the basis for preventive strategies in the form of aggressive management of comorbidities.
Another serious complication is the development of therapy-related cardiovascular disease (CVD) [19,20]. Cardiovascular events including cerebrovascular disease (stroke, transient ischemic attack [TIA], carotid arterial occlusion or symptomatic lacunar infarcts) and coronary artery disease (myocardial infarction, atherosclerotic heart disease or angina pectoris) are prevalent and often occur earlier than would be expected in the general population [5,21,22]. The cumulative incidence of CVD approaches 23% at 25 years after HSCT in certain high-risk populations and the incidence appears to increase with time. The risk of CVD is reported to be greatest among allogeneic HSCT recipients. Factors such as increasing age, hypertension, diabetes, dyslipidemia, high BMI and male gender are well recognized as modifiers for CVD in the general population [23,24]. HSCT survivors are known to be at an increased risk of developing diabetes and hypertension, which potentially contributes to the risk of CVD in this population [5]. These reports indicate that CVD is a significant contributor to post-HSCT morbidity and mortality and that the risk of CVD could potentially be modified by the presence of comorbidities.
However, very few studies have evaluated the role of pre-HSCT therapeutic exposures or the contribution of comorbidities on the risk of late CVD. Armenian et al. addressed these gaps in the literature by evaluating the role of pre- and post-HSCT therapeutic exposures (chemotherapy and radiation), transplant-related conditioning and comorbidities, in the development of CVD in long-term HSCT survivors, using a nested case–control study design [25]. Cases were drawn from a cohort of HSCT recipients who had survived for 1 year or more after HSCT performed at City of Hope (CA, USA). Controls (without CVD) were selected using the following match criteria: age, year of HSCT, type of HSCT and length of follow-up. A total of 63 cases with late CVD were identified. Of these, 44 (69.8%) had coronary artery disease and 19 (30.2%) were diagnosed with cerebrovascular events. Using multivariate logistic regression techniques, the authors identified the presence of multiple cardiovascular risk factors (≥2 of the following: obesity, dyslipidemia, hypertension and diabetes) after HSCT to be associated with a 4.6-fold risk of late CVD (p < 0.01). Furthermore, exposure to chest radiation prior to HSCT was associated with a 9.3-fold risk of coronary artery disease (p = 0.04). Importantly, pre-HSCT chemotherapy and conditioning-related therapeutic exposures did not increase the risk.
Recommendations for screening
Patients exposed to anthracyclines prior to HSCT should undergo monitoring for late-onset cardiomyopathy using serial noninvasive testing (echocardiogram) and physical examination. The frequency of echocardiographic screening can range from annual to every 5 years, depending on cumulative anthracycline dose, age at exposure and treatment with mediastinal radiation. Pregnant women with prior exposure to anthracyclines should be monitored closely, since changes in blood volume during the third trimester could add significant stress to an already compromised myocardium. Survivors who received radiation involving the heart area should also be monitored for early-onset atherosclerosis. Lifestyles that promote good heart health should be recommended to all survivors, including a regular exercise program, dietary recommendations and screening for (and aggressive management of) dyslipidemia, hypertension and diabetes. Further specific recommendations for monitoring, based on age and therapeutic exposure, are delineated within the Children’s Oncology Group (COG) long-term follow-up guidelines [201].
Delayed pulmonary complications
Delayed pulmonary complications are frequent following HSCT and typically include noninfectious complications as well as infectious complications, related to the compromised immune system. Noninfectious pulmonary complications include the following: bronchiolitis obliterans (BO), bronchiolitis obliterans with organizing pneumonia (BOOP) and idiopathic pneumonia syndrome [26]. Noninfectious complications usually appear between 3 months and 2 years after HSCT and are closely related to chronic extensive GvHD. However, the functional consequences persist for many years. In a cohort of 438 patients surviving for 3 or more months, Patriarca et al. reported the cumulative incidence of late-onset pulmonary complications to be 10% at 2 years; the cumulative incidence was 15.6% among those diagnosed with chronic GvHD [27]. Furthermore, the overall survival at 5 years was significantly inferior among those with pulmonary complications when compared with those without. The magnitude of risk and severity of noninfectious pulmonary complications was worse after unrelated donor HSCT. A retrospective study of 69 allogeneic HSCT recipients demonstrated compromised pulmonary function (primarily restrictive in nature) from baseline in 45% of the patients. Compromised pulmonary functions prior to HSCT and presence of chronic GvHD were independently associated with later decline in function [28]. In children, the cumulative incidence of lung impairment evaluated in 162 HSCT recipients was 35% and was primarily restrictive in nature. Chronic GvHD was the major risk factor for reduced lung function [29].
Bronchiolitis obliterans is characterized by a nonspecific inflammatory injury affecting the small airways. Among 2512 allogeneic HSCT recipients reported in nine studies, the incidence of BO was 8%. BO is strongly associated with chronic GvHD. Additional factors shown to be associated with increased risk included the use of peripheral blood stem cells, exposure to methotrexate for GvHD prophylaxis, older age of the recipient and/or donor at HSCT, exposure to busulfan for conditioning, prior history of respiratory infection and low serum immunoglobulin levels [30]. The onset of BO is protracted and typically occurs approximately 1 year after HSCT. Manifesting as an obstructive defect in the initial stages, it progresses to peribronchiolar fibrosis, with emergence of restrictive changes [31]. Patients present with a dry cough, progressive dyspnea and wheezing. High-resolution CT images of the chest in inspiration and expiration are considered to be the radiologic procedure of choice to measure the structural alterations in the lung. A characteristic finding in patients with BO is a mosaic image. This technique carries a sensitivity of 74–91% and specificity of 69–94% [32]. Criteria used to make a clinical diagnosis of BO include:
Forced expiratory volume in 1 s/forced vital capacity <0.7 and forced expiratory volume in 1 s <75% of predicted value;
Evidence of air trapping or small airway thickening or bronchiectasis on high-resolution computed tomography;
Absence of respiratory infection [33].
Bronchiolitis obliterans organizing pneumonia is a clinicopathologic syndrome involving bronchioles, alveolar ducts and alveoli. The syndrome has a cumulative incidence of less than 2%. It typically presents as an interstitial pneumonia and usually occurs within the first 12 months following HSCT. The clinical presentation in patients is acute, with the sudden onset of a dry cough, as well as dyspnea and fever. Pulmonary function tests suggest a restrictive pattern and observations from chest x-rays show peripheral patchy consolidation, ground glass attenuation and nodular opacities [34]. Definitive diagnosis necessitates histologic confirmation. Patients with BOOP are more likely to have acute or chronic GvHD.
Idiopathic pneumonia syndrome usually occurs within the first 4 months after HSCT. Risk factors include exposure to TBI and pre-HSCT chemotherapy, presence of GvHD and increasing age at HSCT [35]. Late-onset interstitial pneumonitis occurs several years after HSCT, typically in patients with severe chronic GvHD of the sclerodermatous cutaneous variety. A restrictive defect is found on pulmonary function tests [36].
Recommendations for screening
Monitoring for pulmonary dysfunction in HSCT survivors should include the assessment of symptoms such as chronic cough or dyspnea. Patients should be warned of the risks of smoking and exposure to secondhand smoke. It is recommended that pulmonary function tests and chest x-ray are carried out upon entry into long-term follow-up for at-risk patients and should be repeated as clinically indicated in symptomatic patients and in those with subclinical abnormalities identified at screening. Influenza and pneumococcal vaccines are recommended in survivors at risk for pulmonary compromise.
Endocrine complications
Endocrine complications are among the most common chronic health conditions encountered following HSCT and include thyroid dysfunction, osteoporosis, metabolic syndrome, growth impairment and gonadal dysfunction. The risk of these complications is influenced by pre-HSCT therapeutic exposures, transplantation-related conditioning and post-transplantation management of GvHD.
Thyroid abnormalities primarily include subclinical and overt hypothyroidism. Subclinical or compensated hypothyroidism is defined as elevated thyroid-stimulating hormone (TSH) accompanied by normal T4 levels. On the other hand, overt hypothyroidism is characterized by low T4 levels accompanied by elevated TSH. The incidence of compensated hypothyroidism ranges from 25 to 30% with a median latency of 2 years. On the other hand, the cumulative incidence of overt hypothyroidism ranges from 3.4 to 9% with a latency of 2.7 years. Hypothyroidism is directly related to radiation of the thyroid gland (either as part of neck/mediastinal radiation or TBI). Younger age increases the risk of hypothyroidism [37,38].
Hematopoietic cell transplantation recipients are at risk for osteopenia and osteoporosis. The decreased bone mineral density (BMD) is due to the use of steroids in the treatment of GvHD [39], the known association of HSCT with growth hormone deficiency [40] and hypogonadism [41,42], physical inactivity and a diet low in calcium [43]. The incidence of osteopenia in adults is reported to approach 50% at 4–6 years after HSCT, while the incidence of osteoporosis approaches 20% at 2 years [44,45]. In a recent retrospective analysis conducted at a median time of 5 years after HSCT, quantitative computed tomography demonstrated that 26% of children had osteopenia and 21% had osteoporosis [46,47]. Mattano found that the average loss in BMD was 6.2–10% in the femoral neck region and 2–5.7% in the lumbar region and that the most significant loss in BMD occurred during the first 6 months after HSCT [48]. In their 2004 long-term follow-up study, Schulte and Beelen demonstrated significant bone loss with nadir BMD for the spine at month 6 and at month 24 for total body and femoral neck BMD. Spine and total body BMD returned to baseline at month 48 [42]. Bone mineral loss increases the risk of fractures in the HSCT population, as it does in the general population. Nontraumatic fractures were observed in 10.6% of the patients within 3 years of HSCT [44].
Metabolic syndrome represents a collection of symptoms including central obesity, hyperglycemia, dyslipidemia and hypertension and conveys an increased risk of cardiovascular disease and Type 2 diabetes mellitus. Disturbances of the hypothalamic–pituitary axis resulting in growth hormone deficiency and hypogonadism play a role in the development of metabolic syndrome. Chemotherapy and radiation can have direct impact on the vascular endothelium. Dyslipidemia, glucose intolerance and arterial hypertension can arise as a consequence of prolonged immunosuppression with cyclosporine, sirolimus, mycophenolate, tacrolimus and corticosteroids. Data on the prevalence of metabolic syndrome indicate that 34–49% of adult survivors of HSCT have one or more component of metabolic syndrome [18,49]. Adjusting for age, sex, race and BMI, survivors of allogeneic HSCT are 3.7-times more likely to report diabetes mellitus and 2.1-times more likely to report hypertension than their siblings [5]. Oudin et al. evaluated the prevalence of and risk factors for metabolic syndrome in young adults surviving childhood leukemia, treated with and without HSCT [50]. Among the HSCT recipients, metabolic syndrome was diagnosed in 5.9% of those who did not receive TBI and 18.6% of those who did. HSCT recipients exposed to TBI were at a 3.9-fold increased risk of developing metabolic syndrome when compared with those treated with conventional therapy without radiation. Exposure to TBI was associated with hypertriglyceridemia, low levels of high-density lipoprotein cholesterol and elevated fasting glucose.
Growth impairment is a frequent complication observed in children after HSCT [51]. While central damage to the hypothalamic–pituitary axis due to cranial radiation or TBI is the primary mediator, several modifying factors may be present, including genetic, nutritional, hormonal (gonadal failure, hypothyroidism) and psychological factors, as well as exposure to corticosteroids for the management of GvHD and peripheral lesions of the bones, cartilage and epiphyseal growth plate [52]. Thus, short stature after exposure to TBI could be due to the direct impairment of growth, by damaging epiphyseal growth plates, or could be indirect, by decreasing growth hormone secretion or by causing hypogonadism or hypothyroidism. The final height of the patient conditioned with TBI is significantly lower than that of the patient conditioned without TBI [53]. The loss of height is more severe in boys compared with girls. Patient age at exposure to TBI is a significant factor in predicting adult height. Children younger than 10 years of age at HSCT are at greatest risk for short stature [54]. Patients conditioned with chemotherapy alone attain final height values within predicted ranges. Chemaitilly et al. have demonstrated that final height in patients treated with hyperfractionated TBI is similar to that in patients receiving standard fractionation [52]; however, the reduction in height in this group is due to a reduction in sitting height, suggesting impairment of spinal column growth [52].
While growth hormone impairment is the leading cause of growth impairment, the prevalence varies widely after HSCT, ranging from 20 to 85%. Sanders et al. have demonstrated a maximum benefit of GH replacement for patients transplanted before 10 years of age and with documented GH deficiency [55]. Although GH replacement therapy is not associated with an increased risk of relapse of original disease, preliminary evidence suggests an increased risk of second malignancies [56]. However, these findings need to be confirmed in additional cohorts.
Gonadal failure is a common endocrine complication observed after HSCT. Pubertal disturbances after HSCT are caused by radiation -related perturbations of the hypothalamic–pituitary axis and/or by chemoradiotherapy-related damage to the gonads. The testicular germinal epithelium (Sertoli cells) is responsible for spermatogenesis and is more sensitive to radiation and chemotherapy than the testicular Leydig cell component, which is involved in testosterone secretion. The probability of developing gonadal failure increases with cumulative doses of gonadotoxic therapies. Recovery of spermatogenesis seems to occur more frequently in patients receiving lower doses of cyclophosphamide (120 mg/kg) than in those treated with higher doses (200 mg/kg). Rovo et al. reported that males who are long-term survivors have a considerable chance of recovering some degree of sperm production even when treated with TBI, provided that they are under 25 years of age when undergoing HSCT and that they do not experience chronic GvHD [57,58].
Compared with the testes, the ovaries are more vulnerable to irradiation and chemotherapy. It has been shown that while approximately 50% of prepubertal girls who underwent fractionated TBI enter puberty spontaneously and achieve menarche at a normal age, almost all female patients who are above 12 years of age when undergoing HSCT show ovarian failure, probably as a result of a decreased reserve of primordial follicles [59]. It has also been demonstrated that high-dose busulfan is a significant cause of ovarian failure, even when it is administered in the prepubertal period [60]. Irreversibility of ovarian function after HSCT in most patients highlights the necessity of timely hormonal replacement therapy to prevent osteoporosis and other complications resulting from lack of hormones.
Recommendations for screening
Growth and growth velocity should be measured every 6 months during childhood. Any deviation beyond the standard deviation score of age- and sex-expected means should prompt a consultation with the endocrinologist.
Screening for thyroid dysfunction relies on a good medical history and physical examination, as well as annual thyroid function tests (free thyroxine, THS). Survivors with abnormalities on screening evaluation should be referred to an endocrinologist for hormone replacement therapy.
Screening for hypogonadism in males should include establishing an age-appropriate history and Tanner staging, with particular attention to issues related to libido, impotence or fertility. Measurement of serum luteinizing hormone, follicle-stimulating hormone and testosterone levels at 11 years of age has been recommended as a baseline in boys whose puberty appears to be delayed. Abnormalities in testicular function should prompt a consultation with the endocrinologist. Screening for ovarian dysfunction in females involves establishing a history (primary or secondary amenorrhea, menstrual irregularity, and pregnancies or difficulties in becoming pregnant), Tanner staging and serum gonadotropin and estradiol levels. For patients who show no clinical evidence of puberty, baseline hormone levels should be obtained at the expected time of onset of puberty in order to assess the need for hormone therapy to induce puberty.
Fertility
Permanent gonadal damage and infertility are known toxicities of preparative regimens used for patients undergoing HSCT [13,61–63]. Using a retrospective study design, Carter et al. described the extent of compromise in reproductive function as well as pregnancy outcomes in 619 women and partners of men treated with autologous (n = 241) or allogeneic (n = 378) HSCT between 21 and 45 years of age who had survived for 2 years or longer [64]. Older age at transplantation (≥30 years; OR: 4.8), female sex (OR: 3.0) and exposure to TBI (OR: 3.3) were associated with an increased risk for infertility. While HSCT survivors were 36-times more likely to not report a conception when compared with age- and sex-matched siblings, they did not differ from siblings with respect to risk of miscarriage or stillbirth (OR: 0.7).
Infertility should be regarded as a late effect of significant concern as it has the potential to influence various medical and quality of life (QOL) aspects of survivorship. In fact, some survivors have reported that dealing with their loss of fertility was as painful as confronting cancer [65,66]. Although HSCT survivors are at an extremely high risk for infertility secondary to gonadotoxic myeloablative therapy, survivor fertility status cannot be definitely known without testing. Hammond et al. described the fertility status and the prevalence of risk factors for elevated infertility concern in 10-year adult cancer survivors who had been treated with myeloablative HSCT [67]. The majority of survivors under the age of 40 years (54%) were shown to express elevated infertility concern. Survivors without children before HSCT had greater risk for elevated concern after 10 years (OR: 3.4; p = 0.05).
The high prevalence of infertility and the high rate of infertility- related concerns among cancer survivors increases the need for transplanters to discuss fertility-preserving options prior to HSCT with prospective HSCT recipients and for healthcare providers taking care of long-term HSCT survivors to provide family-building options and psychosocial support to those at risk.
Musculoskeletal complications
Musculoskeletal symptoms are prevalent and persist among HSCT survivors. Syrjala et al. reported that 35% of the those treated with HSCT who had survived for 10 years or longer had one or more musculoskeletal symptoms, compared with 17% of the matched controls [68]. Survivors of childhood HSCT were more likely to report muscle weakness (5.5 vs 1.6% of the controls) and pain (21 vs 10%) [69]. Muscle weakness was shown to be strongly associated with a positive history of chronic GvHD.
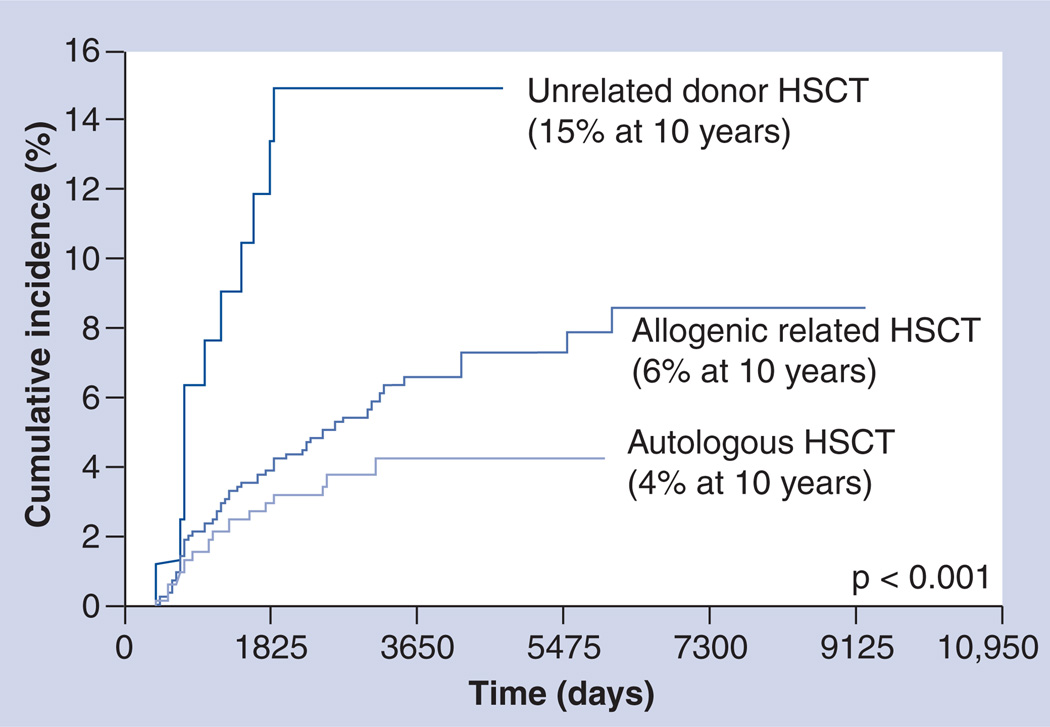
Avascular necrosis (AVN) of the bone is a debilitating and painful condition that develops when the blood supply to the bone is disturbed, usually in areas with terminal circulation. AVN has been reported to be a complication of HSCT, which can cause significant morbidity and often requires surgery. Campbell et al. followed 1346 HSCT survivors to assess the development of AVN [70]. The cumulative incidence of AVN was shown to be almost 2.9% at 10 years following autologous HSCT, 5.4% after allogeneic matched-related donor HSCT and 15% after unrelated donor HSCT (Figure 1). Among recipients of allogeneic HSCT, males (relative risk [RR]: 2.1), those with chronic GvHD (RR: 2.2) and those exposed to cyclosporine, FK506, prednisone and mycophenolate mofetil (MMF) were at increased risk.
Figure 1. Cumulative incidence of avascular necrosis by type of hematopoietic stem cell transplantation.
HSCT: Hematopoietic stem cell transplantation.
Adapted with permission from [70].
Recommendations for screening
Clinical anticipation in at-risk survivors, in the form of building up a careful history and a thorough physical examination are the cornerstone of screening. Success with interventions (including the use of calcium supplementation, calcitonin, bisphosphonates and hormone replacement in patients with gonadal failure) suggests a need for baseline dual-emission x-ray absorptiometry or quantitative CT scans with repeat studies as clinically indicated.
Chronic kidney disease
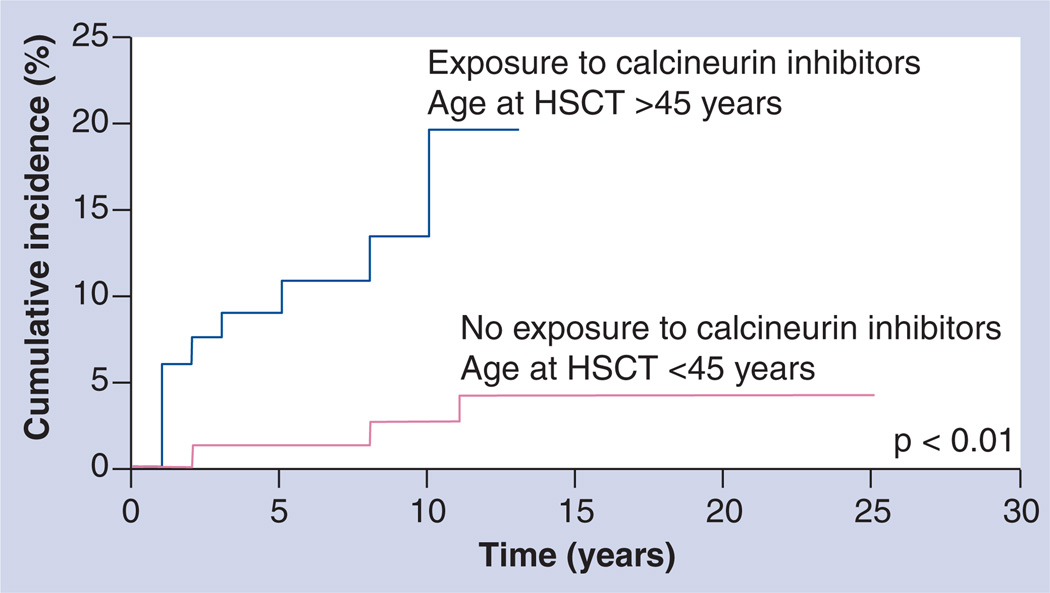
Renal dysfunction has long been recognized as a complication of HSCT [71–77]. Previous studies have been limited by small sample sizes and relatively short follow-up periods following HSCT [78], thus restricting their ability to describe the magnitude of risk and to adequately evaluate the contribution of various nephrotoxic exposures, in addition to host and demographic factors, to the development of delayed chronic kidney disease. Choi et al. addressed these limitations by evaluating the risk of delayed chronic kidney disease in 1190 HSCT survivors followed for a median of 7 years [7]. Presence of chronic kidney disease was defined as a persistent elevation of serum creatinine indicating a glomerular filtration rate of less than 60 ml per minute per 1.73 m2 for at least 3 months. Using this definition, a total of 60 patients with chronic kidney disease were identified, conferring a cumulative incidence of 4.4% at 5 years for the entire cohort; the cumulative incidence was 3.8% among autologous HSCT recipients, 4.5% for matched sibling HSCT recipients and 10% for unrelated donor HSCT recipients. For every 5-year increment in age at HSCT the risk of chronic kidney disease increased by 33%. In addition, exposure to cyclosporine without tacrolimus (RR: 1.9) or with tacrolimus (RR: 4.6) and a primary diagnosis of multiple myeloma (RR: 2.5) were associated with an increased risk of delayed chronic kidney disease. Older patients exposed to calcineurin inhibitors were at the highest risk (Figure 2).
Figure 2. Cumulative incidence of chronic kidney disease by age at exposure to calcineurin inhibitors among allogeneic hematopoietic stem cell transplantation recipients.
HSCT: Hematopoietic stem cell transplantation.
Adapted with permission from [7].
Recommendations for screening
Patients at risk for chronic kidney disease should be monitored with evaluation of serum creatinine, blood urea nitrogen and serum chemistries at entry into long-term follow-up; this should be repeated as clinically indicated. Urinalysis and measurement of blood pressure should be performed at baseline and annually thereafter. Patients at risk for chronic kidney disease should be cautioned to avoid nephrotoxic agents (e.g., ibuprofen, aminoglycosides) and to seek early intervention for urinary tract infections.
Visual impairments
Cataracts are a well-described complication in HSCT survivors. Among survivors of HSCT performed in childhood, the cumulative incidence of cataracts was shown to be 36% at 15 years post-HSCT [69]. Cataracts were confined to patients who had received TBI for conditioning or cranial irradiation prior to HSCT. Survivors of allogeneic HSCT were more likely to report a cataract (cumulative incidence: 40% at 15 years), when compared with autologous HSCT recipients (cumulative incidence: 21%). Furthermore, among allogeneic HSCT recipients, those with chronic GvHD were more likely to report a cataract (cumulative incidence: 46% at 15 years). When the analysis was limited to the patients who had received TBI, no difference in cataract incidence was observed by transplant type or by history of chronic GvHD.
The association between cataract formation and exposure to ionizing radiation has been recognized since the late 19th Century. Following HSCT, it is very well known that TBI-based conditioning regimens are more cataractogenic [79–81]. Besides TBI-related parameters such as total dose, fractionation or dose rate [79,82–85], other risk factors include age, sex, steroid administration and pre-HSCT cranial irradiation [80,83].
Recommendations for screening
Patients at risk for cataracts should undergo annual fundoscopic examination and assessment for visual acuity. Surgical interventions (cataract extraction or photocoagulation) may be indicated, with preservation of the sight being the overall goal.
Subsequent malignant neoplasms
An important and potentially devastating complication of HSCT is the occurrence of SMNs. The magnitude of risk of subsequent malignant neoplasms (SMNs) after HSCT ranges from four- to 11-fold of the general population. Several host and clinical factors are associated with an increased risk of SMNs after HSCT. These include age at HSCT, pre-HSCT exposure to chemotherapy and radiation, exposure to TBI as part of conditioning, infection with oncogenic viruses (Epstein–Barr virus [EBV] and hepatitis B and C viruses), prolonged immunosuppression after HSCT, autologous versus allogeneic HSCT and original cancer [86,87].
Inherent heterogeneity among the SMNs due to varying clinicopathologic characteristics prevents measurement of risk factors in aggregate. It is for this reason that SMNs are conventionally classified into three groups [87]:
Therapy-related myelodysplasia (t-MDS) and therapy-related acute myeloid leukemia (t-AML)
Lymphoma
Solid nonhematopoietic tumors
t-MDS/AML
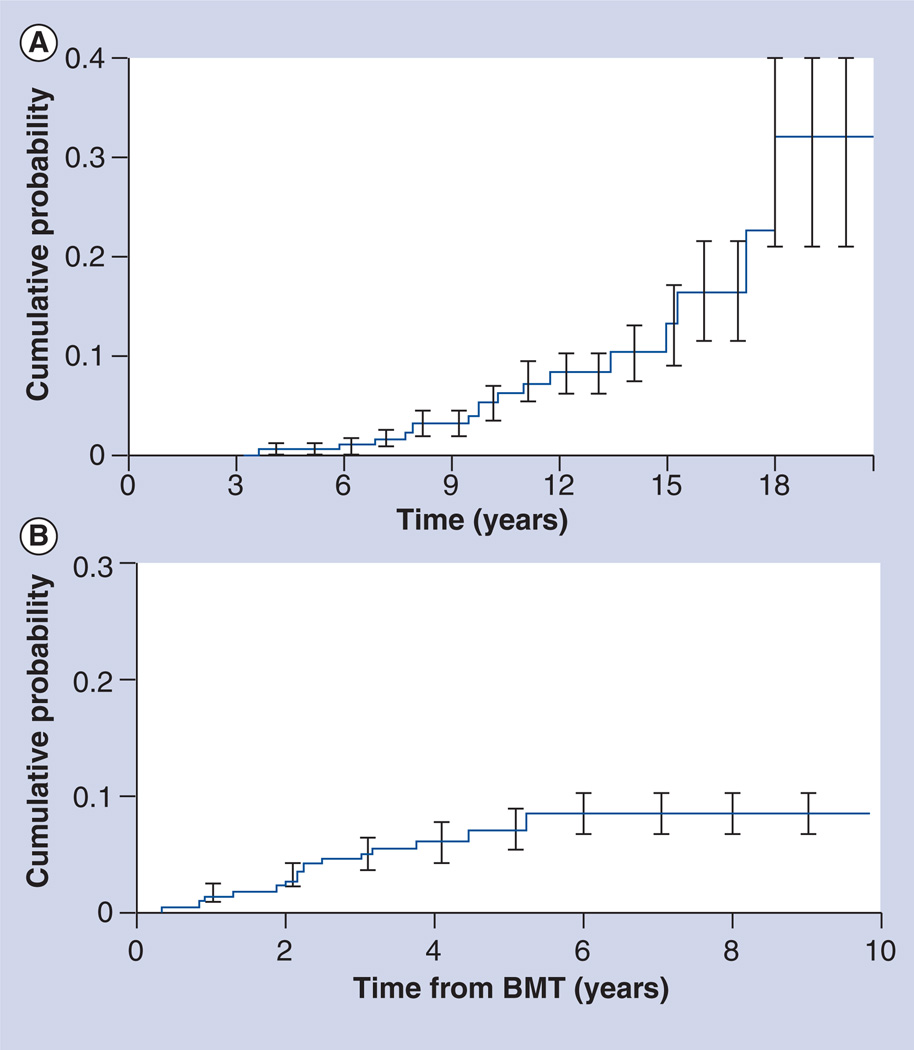
Typically, t-MDS/AML and lymphoma develop soon after HSCT; secondary solid tumors have a longer latency (Figure 3).
Figure 3. Cumulative incidence of subsequent malignant neoplasms after hematopoietic stem cell transplantation.
(A) Solid tumors. (B) Therapy-related myelodysplasia/acute myeloid leukemia.
BMT: Bone marrow transplantation.
(A) Adapted with permission from [99].
(B) Adapted with permission from [88].
Therapy-related myelodysplasia/acute myeloid leukemia are now the major cause of nonrelapse mortality in patients undergoing autologous HSCT for patients with a primary diagnosis of Hodgkin’s lymphoma (HL) or non-Hodgkin’s lymphoma (NHL) [2,88]. The cumulative probability of t-MDS/t-AML ranges from 1.1% at 20 months to 24.3% at 43 months after autologous HSCT, with a median latency of 12–24 months after HSCT (range: 4 months–6 years). Using the WHO classification, two types of t-MDS/t-AML are recognized, closely related to the therapeutic exposure: alkylating agent/radiation and topoisomerase II inhibitor [89]. The alkylating agent-related t-MDS/t-AML typically develops 4–7 years after exposure. Cytopenias are common. Approximately 65% of the patients present with MDS; the remaining present with AML but carry myelodysplastic features. Abnormalities involving chromosomes 5 (−5/del[5q]) and 7 (−7/del[7q]) are frequently observed. AML secondary to topoisomerase II inhibitors presents as overt leukemia, without a preceding myelodysplastic phase. The latency is brief, ranging from 6 months to 5 years and is associated with balanced translocations involving chromosome bands 11q23 or 21q22.
The common and nonspecific nature of cytopenias after autologous HSCT has resulted in the development of criteria for diagnosing t-MDS/t-AML after HSCT. These include myelodysplasia in two or more cell lines, cytopenias with no medical explanation and blasts in the bone marrow using the French–American–British classification [90]. Often, patients may not present with blasts in the bone marrow and so evidence for clonal cytogenetic abnormality in addition to dysplasia may help in making the diagnosis.
Several studies have described an increased risk of t-MDS/t-AML with older age at HSCT [86], pretransplantation therapy with alkylating agents, topoisomerase II inhibitors and radiation therapy [88], use of peripheral blood hematopoietic cells, stem cell mobilization with etoposide, difficult stem cell harvests, conditioning with TBI, number of CD34+ cells infused and a history of multiple transplants [86,88].The diagnosis of t-MDS/t-AML after autologous HSCT confers a uniformly poor prognosis, with a median survival of 6 months in patients treated with conventional chemotherapy. Allogeneic HSCT has been attempted with actuarial survival ranging from between 0 and 24% at 3 years [91]. Male sex, increased age (over 37 years), positive recipient cytomegalovirus serology, absence of complete response at HSCT and intensive conditioning schedules were independently associated with poor outcome [92]. Among t-MDS/t-AML patients with balanced aberrations, 11q23 translocations are an independent adverse risk factor [93]. Treatment-related mortality (TRM) and relapse were reported to be 41 and 27% at 1 year and 48 and 31% at 5 years, respectively, in a large cohort of patients undergoing allogeneic HSCT [94]. Disease-free survival (DFS) and overall survival (OS) were 32 and 37% at 1 year and 21 and 22% at 5 years, respectively. It was demonstrated that age above 35 years, poor-risk cytogenetics, t-AML not in remission or advanced t-MDS, and a donor who was not an HLA-identical sibling or a partially or well-matched unrelated donor had adverse impacts on DFS and OS. It was demonstarted that 5-year survival for subjects with none, one, two, three or four of these risk factors was 50, 26, 21, 10 and 4%, respectively. These studies show that despite the significant TRM, the DFS was improved when transplantation was performed earlier in the progress of disease because it resulted in a lower relapse rate. These data also help identify subjects more likely to benefit from allogeneic transplantation. It is important to closely monitor patients at risk for the development of t-MDS/t-AML in order to identify the disease as early as possible. Prompt transplantation should be considered after diagnosis of t-MDS/t-AML. Innovative transplantation strategies are needed to reduce the high risk of relapse and nonrelapse mortality. Owing to the fact that the poor outcomes of allogeneic transplant for t-MDS/t-AML are associated with the high risk of TRM, it is important to consider the role of reduced-intensity conditioning approaches in this setting.
Lymphoma
Post-transplantation lymphoproliferative disorder (PTLD) is the most common SMN in the first year following allogeneic T-cell-depleted HSCT and is associated with a compromised immune status and EBV infection [95]. The risk of PTLD is strongly associated with T-cell depletion of the donor marrow, anti-thymocyte globulin use, unrelated or HLA-mismatched grafts, presence of acute or chronic GvHD, older age at HSCT and multiple transplants. The cumulative incidence is low (0.2%) among patients with no major risk factors, but increased to 1.1, 3.6 and 8.1% with one, two and more than three major risk factors, respectively. The large majority of the PTLDs have a B-cell origin. The more recent use of nonmyeloablative therapy coupled with highly immunosuppressive therapy needs close observation to monitor for the development of PTLD. Therapeutic options available for these patients include B-cell-specific monoclonal antibodies and cellular therapy. Anti-CD20 monoclonal antibody (rituximab) has been used with success in patients with PTLD, especially in the early stage of disease, based on increasing EBV load.
Late-occurring lymphoma is clinically distinct from PTLD [96], and is associated with extensive chronic GvHD. HL has also been described after HSCT [97]. The most common subtype is mixed cellularity and most of the cases contain the EBV genome. Late-occurring lymphoma is not associated with risk factors typically associated with EBV-PTLD; it is characterized by a later onset (>2.5 years) and a relatively good prognosis.
The magnitude of risk of solid tumors exceeds that of an age- and sex-matched general population by twofold [11,98,99]. Rizzo et al. reported the risk of solid tumors in a multi-institutional cohort of 28,874 allogeneic HSCT recipients [11]. While the overall risk of solid malignancies is twofold that of the general population, the risk reaches threefold among patients followed for 15 or more years after HSCT [11]. Solid tumors are unequivocally related to radiation therapy used to treat the primary cancer and typically have a long latency. The risk is high among those exposed to irradiation at a young age. Rizzo et al. demonstrated that the risk of developing a nonsquamous cell carcinoma following conditioning radiation is dependent on age at exposure to radiation [11]. Thus, among patients exposed to radiation at less than 30 years of age, the risk is ninefold that of the general population, while for those older than 30 years of age when exposed, it approaches that of the general population.
Immunologic alterations predispose patients to squamous cell carcinoma (SCC) of the buccal cavity, hence the association with chronic GvHD [100]. In patients with prolonged immunosuppression, oncogenic viruses such as human papillomavirus contribute to SCC of the skin and buccal mucosa [100]. Solid tumors commonly observed after HSCT include melanoma, and cancers of the oral cavity and salivary glands, brain, liver, cervix, thyroid, breast, bone and connective tissue [11,98].
Nonhematopoietic tumors
Skin cancer
Among allogeneic HSCT recipients, the incidence of basal cell carcinoma (BCC) is 6.5% at 20 years, while that for SCC is 3.4% [100]. TBI increases the risk of BCC, especially in younger patients. SCC risk is increased among patients with acute GvHD, while chronic GvHD is associated with both BCC and SCC [101].
Breast cancer
The 25-year cumulative incidence of breast cancer is reported to be 11% after allogeneic HSCT [102]. Allogeneic HSCT survivors are at a 2.2-fold increased risk of developing breast cancer when compared with the age- and sex-matched general population. The median latency from HSCT to diagnosis of breast cancer is 12.5 years. The incidence is higher among those exposed to TBI (17%) than among those who did not receive TBI (3%). The risk is increased among those exposed to TBI at a younger age.
Thyroid cancer
Hematopoietic cell transplantation recipients are at a 3.3-fold increased risk of thyroid cancer, when compared with the age-and sex-matched general population [103]. An age of younger than 10 years at HSCT, neck radiation, female sex and chronic GvHD are associated with an increased risk of thyroid cancer. Thyroid cancer develops after a latency of 8.5 years and is associated with an excellent outcome.
Recommendations for screening
Close monitoring is important for those at risk. The period at risk for therapy-related leukemia usually extends to 10 years after HSCT. Recommendations include monitoring with annual complete blood count for 10 years after autologous HSCT. Screening recommendations for radiation-related second cancers include careful physical examination of the skin and underlying tissues in the radiation field. Since outcome after treatment for breast cancer is closely linked to stage at diagnosis, close surveillance in order to promote early diagnosis should confer a survival advantage. The COG recommendations for those at risk (i.e., radiation doses of 20 Gy or higher to the mantle, mediastinal, whole lung and axillary fields) include: monthly self-examination of the breast beginning at puberty; annual clinical breast examinations beginning at puberty until the age of 25 years; and clinical breast examinations every 6 months, with annual mammograms and MRIs beginning 8 years after radiation or at age 25 (whichever occurs later). Screening of those at risk for early onset colorectal cancer (i.e., radiation doses of 30 Gy or higher to the abdomen, pelvis or spine) should include colonoscopy every 5 years beginning at 35 years of age or 10 years following radiation (whichever occurs later).
Chronic disease burden after HSCT
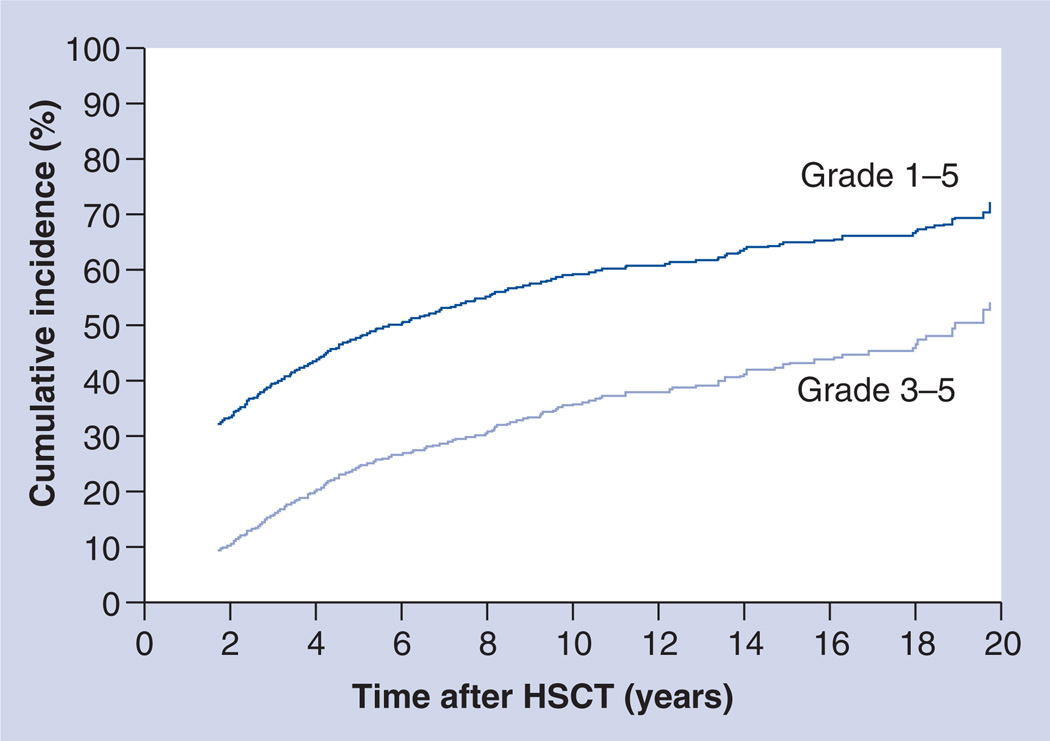
As described previously, HSCT survivors are at risk for developing long-term complications such as endocrinopathies, musculoskeletal disorders, cardiopulmonary compromise and SMNs. Previous reports had conventionally focused on specific complications, providing insight into their etiology and pathogenesis and identifying individuals at increased risk, so that targeted surveillance and interventions can be instituted to reduce morbidity and mortality. The burden of morbidity resulting from the cumulative impact of these individual complications was examined recently. Sun et al. described the risk of chronic health conditions among 1022 HSCT survivors and 309 siblings [104]. The Common Terminology Criteria for Adverse Events, (Version 3.0) was used to assign severity score (grades 1 [mild] to 4 [life-threatening]) to each health condition. Two-thirds of the HSCT survivors had at least one chronic health condition; a fifth developed severe/life-threatening conditions. By comparison, 39% of the siblings developed at least one chronic health condition and 8% developed life-threatening conditions (p < 0.001). The cumulative incidence of a chronic health condition was 59% at 10 years after HSCT. For severe/life-threatening conditions, the 10-year cumulative incidence approached 35% (Figure 4). HSCT survivors were twice as likely as siblings to develop a chronic condition and 3.5-times as likely to develop severe/life-threatening conditions. HSCT survivors with chronic GvHD were 4.7-times as likely to develop severe/life-threatening conditions. HSCT survivors were more likely to report gastrointestinal, musculoskeletal and cardiovascular problems compared with an age-, gender- and race-adjusted sibling group.
Figure 4. Cumulative incidence of any chronic health conditions (grades 1–5) and severe or life-threatening chronic health conditions (grade 3 or 4) or death due to a chronic health condition (grade 5) after hematopoietic stem cell transplantation.
HSCT: Hematopoietic stem cell transplantation.
Adapted with permission from [104].
Syrjala et al. described the health problems experienced by 10-year survivors of HSCT [68]. Survivors reported twice as many medical problems when compared with controls (p < 0.001). Survivors were more likely to report more musculoskeletal symptoms, cataract surgery, liver problems, sexual problems, memory and attention concerns (p = 0.003), urinary symptoms and use of psychotropic medication.
Understanding the burden of morbidity after HSCT is important for a variety of reasons. It is important to the healthcare providers and policy makers in identifying and procuring resources for the long-term care of individuals with a high burden of morbidity; to the researchers in identifying common etiologic pathways that increase the risk of overall morbidity; and to the HSCT survivors in making informed decisions regarding the long-term QOL concerns after HSCT.
Late mortality
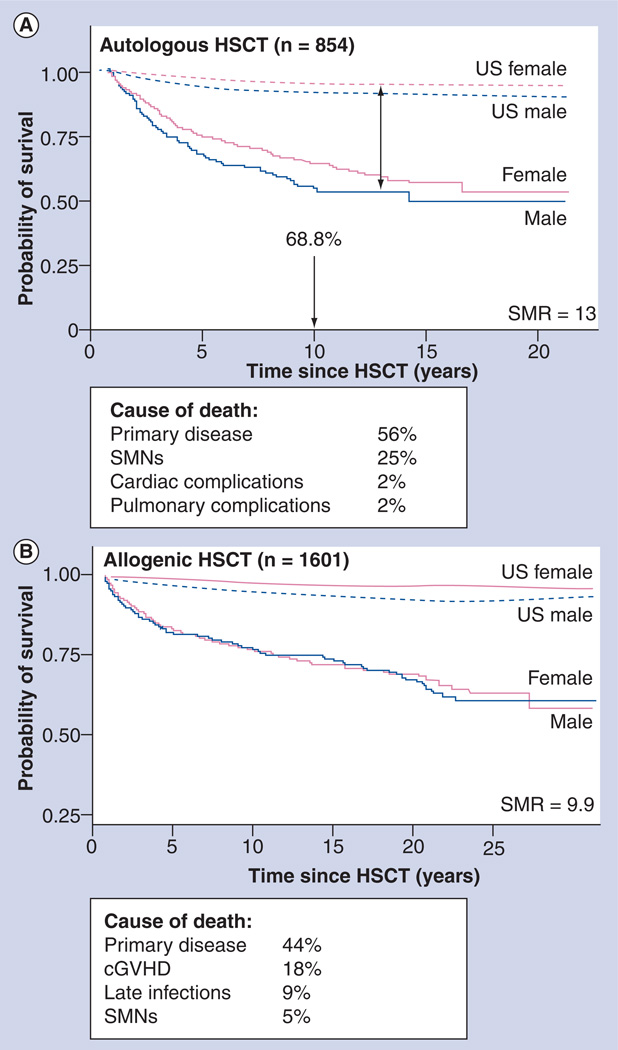
The high burden of morbidity carried by HSCT recipients can result in premature death. Several studies have examined late mortality in patients undergoing allogeneic or autologous HSCT and are summarized here. Late mortality was evaluated in a cohort of 854 individuals who had survived for 2 or more years after autologous HSCT for hematologic malignancies [2]. The OS was 68.8% at 10 years (Figure 5A) and the cohort was at a 13-fold increased risk for premature death compared with the general population. Relapse of primary disease (56%) and SMNs (25%) were leading causes of premature death. Compared with patients with AML, relapse-related mortality was increased among patients with HL (RR: 3.6), NHL (RR: 2.1) and ALL (RR: 6.5). TBI provided a protective effect (RR: 0.6). Nonrelapse-related mortality was increased after carmustine treatment (RR: 2.3) and with the use of peripheral blood stem cells (RR: 2.4). Subsequent malignancies accounted for a 12-fold higher mortality rate than that expected in the general population; pulmonary dysfunction accounted for a 5.6-fold increased risk; and cardiac compromise a 4.4-fold increased risk.
Figure 5. Late mortality experience of patients undergoing hematopoietic stem cell transplantation.
(A) Late mortality after autologous HSCT. (B) Late mortality after allogeneic HSCT.
cGVHD: Chronic graft-versus-host disease; HSCT: Hematopoietic stem cell transplantation; SMN: Subsequent malignant neoplasm; SMR: Standardized mortality ratio.
(A) Adapted with permission from [2].
(B) Adapted with permission from [1].
Late mortality experience was also evaluated in 1479 individuals who had survived for 2 years or longer after allogeneic HSCT [1]. It was demonstrated that the conditional survival probability at 15 years after HSCT was 80.2% for those individuals who were disease free at entry into the cohort (Figure 5B), with a relative mortality of 9.9. Relapse of primary disease (29%) and chronic GvHD (22%) were the main causes of premature death. Nonrelapse-related mortality was greater among patients who were over 18 years of age when undergoing HSCT, as well as among those with chronic GvHD (RR: 2.7) and was reduced among individuals who received methotrexate for GvHD prophylaxis (RR: 0.5). Compared with the general population, allogeneic HSCT recipients were 3.6-times more likely to have died of a SNM, 15.1-times more likely to have died of pulmonary dysfunction and 3.6-times more likely to have died of a cardiac compromise.
Wingard et al. followed a cohort of 10,632 allogeneic HSCT recipients who survived 2 or more years after HSCT [105]. With a median follow-up of 9 years, the probability of being alive 10 years after HSCT approached 85%. Relapse was the major cause of death. Major causes for nonrelapse-related mortality included older age and GvHD. In a recent cohort study of 2574 patients who had survived for at least 5 years after allogeneic or autologous HSCT without recurrence of the original disease, Martin et al. evaluated mortality, projected life expectancy and cause of death [106]. The estimated survival of the cohort at 20 years follow-up after HSCT was 80.4%. Mortality rates were four- to ninefold higher than expected in the population for at least 30 years following HSCT, producing an estimated lower life expectancy of 30% compared with the general population, regardless of current age. Leading causes of the higher mortality rate were SMNs and recurrent diseases, followed by infections, chronic GvHD, respiratory disease and cardiovascular disease.
In summary, patients who have survived for 2–5 years after HSCT without recurrence of original disease have a high probability of surviving for an additional 15 years. However, life expectancy is not fully restored to the level seen in the normal population.
Health behaviors of long-term survivors of HSCT
The high burden of morbidity carried by HSCT survivors necessitates that the survivors engage in health promotion as well as prevention and screening behaviors after HSCT. Bishop et al. evaluated the health and screening patterns of HSCT survivors and demonstrated that HSCT survivors had health and screening behaviors simlar to matched controls [107]. Armenian et al. described health behaviors and cancer screening practices in a cohort consisting of 1040 HSCT survivors [108]. High-risk health behaviors included current smoker status and ‘risky’ alcohol intake (≥four drinks/day in males and ≥three drinks/day in females). American Cancer Society recommendations were utilized to assess cancer screening practices. Survivors were more likely to have received a mammogram (OR: 2.8; p = 0.05) than their siblings. Furthermore, HSCT survivors were less likely to indulge in high-risk behavior (OR: 0.5; p < 0.01) than their siblings. In the ana lysis restricted to HSCT survivors, patients younger than 35 years of age at study participation (OR: 4.7; p < 0.01) and those with lower educational status (educated to less than college level: OR: 2.1; p < 0.01) were more likely to demonstrate high-risk behavior.
In summary, HSCT survivors had comparable cervical and testicular cancer screening practices, were more likely to have had breast cancer screening by mammography and were less likely to be engaged in high-risk behaviors when compared with healthy sibling controls. However, despite potential long-term risks, certain subsets of survivors continue to engage in high-risk behaviors such as smoking and excessive alcohol intake, indicating the need for targeted interventions for these high-risk populations. Continued vigilance in encouraging appropriate cancer screening and healthy behaviors for HSCT survivors is critical.
Healthcare utilization by HSCT survivors
Exposure to high-intensity therapy combined with prolonged immune suppression after HSCT increase the risk of adverse sequelae and hence the healthcare needs of survivors. Shankar et al. described healthcare utilization reported by 845 long-term survivors and examined subpopulations at increased risk for lack of utilization [109]. In this cohort, 98% of allogeneic HSCT recipients and 94% of autologous HSCT recipients reported medical contact a decade after HSCT. While cancer-related visits decreased with time from HSCT, the prevalence of general physical examinations increased. This study demonstrates that primary physicians assume increasing responsibility for providing healthcare for long-term HSCT survivors, thus making it critical that there are standardized guidelines for follow-up of these survivors.
Long-term follow-up guidelines
The Center for International Blood and Marrow Transplantation Research (CIBMTR), European Group for Blood and Marrow Transplantation (EBMT) and American Society for Bone Marrow Transplantation (ASBMT) have developed recommendations to offer care providers suggested screening and prevention practices for autologous and allogeneic HSCT survivors [110]. These recommendations concentrate on risks faced by patients beyond 6 months after transplantation. Most of these recommendations are derived from studies that have identified specific complications in long-term survivors and the risk factors associated with them. The recommendations are organized (and driven) by tissue or organ system, with details regarding potential late effects, the known risk factors and recommendations for monitoring tests and preventive measures. The recommendations are also organized by time after HSCT – detailing suggested recommendations at 6 months, 1 year and then annually.
Comprehensive guidelines for follow-up of pediatric cancer survivors (including patients who have been treated with HSCT) have been developed by the COG and can be found at [201]. The overall goal is early identification of treatment-related complications, potentially allowing for early intervention with resultant reduction in morbidity and mortality and attendant reduction in healthcare costs. The guidelines are evidence based (utilizing established associations between therapeutic exposures and late effects to identify high-risk categories) and consensus based (matching the magnitude of risk of complications with intensity of screening). Since treatment exposures vary by primary diagnosis, HSCT type, patient age and treatment era, a therapy-based approach was chosen. These guidelines have been developed as a resource for clinicians who provide ongoing healthcare to HSCT survivors. The COG guidelines are intended for use beginning 2 or more years after completion of therapy and provide a framework for monitoring of late effects in survivors. The recommendations for periodic screening evaluations provided in these guidelines are appropriate for asymptomatic survivors who present for routine medical follow-up. Box 1 gives an overview of the key areas covered in the guidelines. The major difference between the COG guidelines and those developed by EBMT/CIBMTR/ASBMT is the fact that the former utilize the cumulative therapeutic exposures to generate a tailored list of potential late effects and the recommended screening for early detection of those late effects. The intensity of screening is determined by whether the patient is at standard risk or increased risk owing to cumulative therapeutic exposures and/or host/demographic characteristics. This provides a greater level of granularity and specificity to the screening that the patient undergoes and therefore eliminates the possibility of over- or under-screening.
Box 1. Key components of guidelines for long-term follow-up of survivors.
Therapeutic agent: all major therapeutic agents used in the treatment including pre-HSCT chemotherapy, radiation, surgery and transfusions as well as HSCT-related preparative regimens
Potential late effects: sequelae observed after treatment potentially resulting from pre-HSCT therapy, preparative regimens used for HSCT or as a result of post-HSCT events (psychosocial, dermatologic, ocular, audiologic, oral, craniofacial, cardiovascular, pulmonary, hepatic, gastrointestinal, renal, musculoskeletal, neurological, endocrine, reproductive, immune reconstitution and risk of infection, as well as secondary malignant neoplasms)
-
Potential risk factors
-
–
Host factors (sex, age at therapeutic exposure, genetic predisposition, ethnicity) Medical conditions (premorbid/comorbid conditions)
-
–
Therapeutic exposures (cumulative dose)
-
–
Health behaviors (diet, smoking, exercise)
-
–
Highest risk categories: host factors, treatment factors, medical conditions, genetic predisposition
Periodic evaluations: health history, clinical examinations, laboratory ana lysis, diagnostic imaging, psychosocial assessment
Minimum recommended frequency: baseline and periodic testing and recommendations based on risk factors and magnitude of risk (supported by literature and clinical experience)
Health protective counseling: to prevent/reduce risk of complications and promote early detection
General healthcare: linked to recommendations as per US Preventive Services Task Force [202]
Cancer screening guidelines: based on American Cancer Society recommendations for standard risk. For increased risk of subsequent malignant neoplasm (therapeutic exposures, family history orpremorbid/comorbid conditions), modified guidelines tailored to the magnitude of risk will be available
HSCT: Hematopoietic stem cell transplantation.
Expert commentary
The high burden of morbidity suffered by HSCT survivors necessitates that strategies be developed for the prevention or early detection of these complications. However, many survivors are no longer under the care of transplant centers and community healthcare providers may be unfamiliar with the unique needs of HSCT survivors. Despite the differences in approach used by the organizations described above, having a set of consensus -based guidelines represents a huge first step in standardizing the long-term follow-up of HSCT survivors. The next important step is to ensure that these guidelines are disseminated and accepted by the healthcare providers, as well as by the agencies that reimburse the costs of screening tests. Adherence to these guidelines, the yield from benefit of recommendations and cost–effectiveness of standardized follow-up of the HSCT survivors will provide evidence for refinement of these guidelines.
Five-year view
It is abundantly clear that HSCT survivors are at an increased risk for chronic morbidity many years after HSCT. It is important to follow these survivors in the longterm in order to determine the lifetime risk of long-term complications. It is also important to understand how pretransplant therapeutic exposures interact with HSCT-related exposures to increase the risk of these complications. Over the next few years, it will also become important to develop a better understanding of the pathogenesis of the adverse outcomes, so that targeted interventions can be developed.
Learning objectives.
Upon completion of this activity, participants will be able to:
Evaluate cardiovascular diagnoses associated with HSCT.
Analyze pulmonary, endocrine, musculoskeletal and renal effects of HSCT.
Perform appropriate screening for chronic illness among survivors of HSCT.
Distinguish the most significant cause of non-relapse mortality among patients who received HSCT for lymphoma.
Key issues.
This article describes the long-term risk of chronic morbidity and premature mortality among hematopoietic stem cell transplantation (HSCT) patients.
We need to further our understanding of the contribution of pre-HSCT exposures, HSCT-related exposures and comorbidities in the development of adverse events.
It is necessary to identify those at highest risk of complications.
There is a need to develop intervention strategies.
Long-term follow-up guidelines need to be disseminated and refined.
Footnotes
Financial & competing interests disclosure
Editor
Elisa Manzotti
Editorial Director, Future Science Group, London, UK.
Disclosure: Elisa Manzotti has disclosed no relevant financial relationships.
CME Author
Charles P Vega, MD
Associate Professor, Residency Director, Department of Family Medicine, University of California, Irvine, CA 92697, USA.
Disclosure: Charles P Vega, MD, has disclosed no relevant financial relationships.
Author and Credentials
Smita Bhatia, MD, MPH
Department of Population Sciences, City of Hope, 1500 E. Duarte Road, Duarte, CA 91010, USA.
Disclosure: Smita Bhatia has disclosed no relevant financial relationships.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Bhatia S, Francisco L, Carter A, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110(10):3784–3792. doi: 10.1182/blood-2007-03-082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bhatia S, Robison LL, Francisco L, et al. Late mortality in survivors of autologous hematopoietic-cell transplantation: report from the Bone Marrow Transplant Survivor Study. Blood. 2005;105(11):4215–4222. doi: 10.1182/blood-2005-01-0035.. • Utilizes a comprehensive approach (National Death Index, medical records) to describe late mortality and functional status among survivors of 2 or more years after autologous hematopoietic stem cell transplantation (HSCT).
- 3.Socie G, Stone JV, Wingard JR, et al. Long-term survival and late deaths after allogeneic bone marrow transplantation. Late Effects Working Committee of the International Bone Marrow Transplant Registry. N. Engl. J. Med. 1999;341(1):14–21. doi: 10.1056/NEJM199907013410103. [DOI] [PubMed] [Google Scholar]
- 4. Armenian SH, Sun CL, Francisco L, et al. Late congestive heart failure after hematopoietic cell transplantation. J. Clin. Oncol. 2008;26(34):5537–5543. doi: 10.1200/JCO.2008.17.7428.. • Examines the independent and simultaneous impact of pre-HSCT therapeutic exposures, HSCT conditioning, and post-HSCT comorbidities in the development of post-HSCT congestive heart failure.
- 5. Baker KS, Ness KK, Steinberger J, et al. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: a report from the bone marrow transplantation survivor study. Blood. 2007;109(4):1765–1772. doi: 10.1182/blood-2006-05-022335.. • Describes the risk of diabetes and hypertension after HSCT, as well as the role of total body irradiation in the development of these complications.
- 6.Brennan BM, Shalet SM. Endocrine late effects after bone marrow transplant. Br. J. Haematol. 2002;118(1):58–66. doi: 10.1046/j.1365-2141.2002.03527.x. [DOI] [PubMed] [Google Scholar]
- 7.Choi M, Sun CL, Kurian S, et al. Incidence and predictors of delayed chronic kidney disease in long-term survivors of hematopoietic cell transplantation. Cancer. 2008;113(7):1580–1587. doi: 10.1002/cncr.23773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fraser CJ, Bhatia S, Ness K, et al. Impact of chronic graft-versus-host disease on the health status of hematopoietic cell transplantation survivors: a report from the Bone Marrow Transplant Survivor Study. Blood. 2006;108(8):2867–2873. doi: 10.1182/blood-2006-02-003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leiper AD. Non-endocrine late complications of bone marrow transplantation in childhood: part II. Br. J. Haematol. 2002;118(1):23–43. doi: 10.1046/j.1365-2141.2002.03471.x. [DOI] [PubMed] [Google Scholar]
- 10.Majhail NS, Ness KK, Burns LJ, et al. Late effects in survivors of Hodgkin and non-Hodgkin lymphoma treated with autologous hematopoietic cell transplantation: a report from the bone marrow transplant survivor study. Biol. Blood Marrow Transplant. 2007;13(10):1153–1159. doi: 10.1016/j.bbmt.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rizzo JD, Curtis RE, Socie G, et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood. 2009;113(5):1175–1183. doi: 10.1182/blood-2008-05-158782.. • Describes the magnitude of risk and associated risk factors for the development of solid malignancies after allogeneic HSCT.
- 12.Sanders JE. Chronic graft-versus-host disease and late effects after hematopoietic stem cell transplantation. Int. J. Hematol. 2002;76 Suppl. 2:15–28. doi: 10.1007/BF03165081. [DOI] [PubMed] [Google Scholar]
- 13.Socie G, Salooja N, Cohen A, et al. Nonmalignant late effects after allogeneic stem cell transplantation. Blood. 2003;101(9):3373–3385. doi: 10.1182/blood-2002-07-2231. [DOI] [PubMed] [Google Scholar]
- 14.Wingard JR, Vogelsang GB, Deeg HJ. Stem cell transplantation: supportive care and long-term complications. Hematology (Am. Soc. Hematol. Educ. Program) 2002:422–444. doi: 10.1182/asheducation-2002.1.422. [DOI] [PubMed] [Google Scholar]
- 15.Yahalom J, Portlock CS. Long-term cardiac and pulmonary complications of cancer therapy. Hematol. Oncol. Clin. North Am. 2008;22(2):305–318. doi: 10.1016/j.hoc.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Grenier MA, Lipshultz SE. Epidemiology of anthracycline cardiotoxicity in children and adults. Semin. Oncol. 1998;25(4 Suppl. 10):72–85. [PubMed] [Google Scholar]
- 17.Barry E, Alvarez JA, Scully RE, Miller TL, Lipshultz SE. Anthracycline-induced cardiotoxicity: course, pathophysiology, prevention and management. Expert Opin Pharmacother. 2007;8(8):1039–1058. doi: 10.1517/14656566.8.8.1039. [DOI] [PubMed] [Google Scholar]
- 18.Majhail NS, Flowers ME, Ness KK, et al. High prevalence of metabolic syndrome after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2009;43(1):49–54. doi: 10.1038/bmt.2008.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armenian SH, Bhatia S. Cardiovascular disease after hematopoietic cell transplantation - lessons learned. Haematologica. 2008;93(8):1132–1136. doi: 10.3324/haematol.13514. [DOI] [PubMed] [Google Scholar]
- 20.Tichelli A, Bhatia S, Socie G. Cardiac and cardiovascular consequences after haematopoietic stem cell transplantation. Br. J. Haematol. 2008;142(1):11–26. doi: 10.1111/j.1365-2141.2008.07165.x. [DOI] [PubMed] [Google Scholar]
- 21.Tichelli A, Passweg J, Wojcik D, et al. Late cardiovascular events after allogeneic hematopoietic stem cell transplantation: a retrospective multicenter study of the Late Effects Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2008;93(8):1203–1210. doi: 10.3324/haematol.12949. [DOI] [PubMed] [Google Scholar]
- 22.Tichelli A, Bucher C, Rovo A, et al. Premature cardiovascular disease after allogeneic hematopoietic stem-cell transplantation. Blood. 2007;110(9):3463–3471. doi: 10.1182/blood-2006-10-054080. [DOI] [PubMed] [Google Scholar]
- 23.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 24.Grundy SM, Pasternak R, Greenland P, Smith S, Jr, Fuster V. AHA/ACC scientific statement: assessment of cardiovascular risk by use of multiple-risk-factor assessment equations: a statement for healthcare professionals from the American Heart Association and the American College of Cardiology. J. Am. Coll. Cardiol. 1999;34(4):1348–1359. doi: 10.1016/s0735-1097(99)00387-3. [DOI] [PubMed] [Google Scholar]
- 25.Armenian SH, Sun CL, Mills G, et al. Predictors of late cardiovascular complications in survivors of hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 2010;16(8):1138–1144. doi: 10.1016/j.bbmt.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshihara S, Yanik G, Cooke KR, Mineishi S. Bronchiolitis obliterans syndrome (BOS), bronchiolitis obliterans organizing pneumonia (BOOP), and other late-onset noninfectious pulmonary complications following allogeneic hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 2007;13(7):749–759. doi: 10.1016/j.bbmt.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Patriarca F, Skert C, Bonifazi F, et al. Effect on survival of the development of late-onset non-infectious pulmonary complications after stem cell transplantation. Haematologica. 2006;91(9):1268–1272. [PubMed] [Google Scholar]
- 28.Savani BN, Montero A, Srinivasan R, et al. Chronic GVHD and pretransplantation abnormalities in pulmonary function are the main determinants predicting worsening pulmonary function in long-term survivors after stem cell transplantation. Biol. Blood Marrow Transplant. 2006;12(12):1261–1269. doi: 10.1016/j.bbmt.2006.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uderzo C, Pillon M, Corti P, et al. Impact of cumulative anthracycline dose, preparative regimen and chronic graft-versus-host disease on pulmonary and cardiac function in children 5 years after allogeneic hematopoietic stem cell transplantation: a prospective evaluation on behalf of the EBMT Pediatric Diseases and Late Effects Working Parties. Bone Marrow Transplant. 2007;39(11):667–675. doi: 10.1038/sj.bmt.1705652. [DOI] [PubMed] [Google Scholar]
- 30.Santo Tomas LH, Loberiza FR, Jr, Klein JP, et al. Risk factors for bronchiolitis obliterans in allogeneic hematopoietic stem-cell transplantation for leukemia. Chest. 2005;128(1):153–161. doi: 10.1378/chest.128.1.153. [DOI] [PubMed] [Google Scholar]
- 31.Pandya CM, Soubani AO. Bronchiolitis obliterans following hematopoietic stem cell transplantation: a clinical update. Clin. Transplant. 2010;24(3):291–306. doi: 10.1111/j.1399-0012.2009.01122.x. [DOI] [PubMed] [Google Scholar]
- 32.Bankier AA, Van Muylem A, Knoop C, Estenne M, Gevenois PA. Bronchiolitis obliterans syndrome in heart-lung transplant recipients: diagnosis with expiratory CT. Radiology. 2001;218(2):533–539. doi: 10.1148/radiology.218.2.r01fe09533. [DOI] [PubMed] [Google Scholar]
- 33.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol. Blood Marrow Transplant. 2005;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Tichelli A, Rovo A, Passweg J, et al. Late complications after hematopoietic stem cell transplantation. Expert Rev. Hematol. 2009;2(5):583–601. doi: 10.1586/ehm.09.48. [DOI] [PubMed] [Google Scholar]
- 35.Fukuda T, Hackman RC, Guthrie KA, et al. Risks and outcomes of idiopathic pneumonia syndrome after nonmyeloablative and conventional conditioning regimens for allogeneic hematopoietic stem cell transplantation. Blood. 2003;102(8):2777–2785. doi: 10.1182/blood-2003-05-1597. [DOI] [PubMed] [Google Scholar]
- 36.Wolff D, Reichenberger F, Steiner B, et al. Progressive interstitial fibrosis of the lung in sclerodermoid chronic graft-versus-host disease. Bone Marrow Transplant. 2002;29(4):357–360. doi: 10.1038/sj.bmt.1703386. [DOI] [PubMed] [Google Scholar]
- 37.Berger C, Le-Gallo B, Donadieu J, et al. Late thyroid toxicity in 153 long-term survivors of allogeneic bone marrow transplantation for acute lymphoblastic leukaemia. Bone Marrow Transplant. 2005;35(10):991–995. doi: 10.1038/sj.bmt.1704945. [DOI] [PubMed] [Google Scholar]
- 38.Ishiguro H, Yasuda Y, Tomita Y, et al. Long-term follow-up of thyroid function in patients who received bone marrow transplantation during childhood and adolescence. J. Clin. Endocrinol. Metab. 2004;89(12):5981–5986. doi: 10.1210/jc.2004-0836. [DOI] [PubMed] [Google Scholar]
- 39.Ebeling PR, Thomas DM, Erbas B, Hopper JL, Szer J, Grigg AP. Mechanisms of bone loss following allogeneic and autologous hematopoietic stem cell transplantation. J. Bone Miner. Res. 1999;14:341–350. doi: 10.1359/jbmr.1999.14.3.342. [DOI] [PubMed] [Google Scholar]
- 40.Aisenberg J, Hsieh K, Kalaitzoglou G, et al. Bone mineral density in young adult survivors of childhood cancer. J. Pediatr. Hematol. Oncol. 1998;20(3):241–245. doi: 10.1097/00043426-199805000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Atkinson SA, Halton JM, Bradley C, Wu B, Barr RD. Bone and mineral abnormalities in childhood acute lymphoblastic leukemia: influence of disease, drugs and nutrition. Int. J. Cancer Suppl. 1998;11:35–39. [PubMed] [Google Scholar]
- 42.Schulte CM, Beelen DW. Bone loss following hematopoietic stem cell transplantation: a long-term follow-up. Blood. 2004;103(10):3635–3643. doi: 10.1182/blood-2003-09-3081. [DOI] [PubMed] [Google Scholar]
- 43.Schimmer AD, Minden MD, Keating A. Osteoporosis after blood and marrow transplantation: clinical aspects. Biol. Blood Marrow Transplant. 2000;6(2A):175–181. doi: 10.1016/s1083-8791(00)70040-1. [DOI] [PubMed] [Google Scholar]
- 44.Stern JM, Sullivan KM, Ott SM, et al. Bone density loss after allogeneic hematopoietic stem cell transplantation: a prospective study. Biol. Blood Marrow Transplant. 2001;7(5):257–264. doi: 10.1053/bbmt.2001.v7.pm11400947. [DOI] [PubMed] [Google Scholar]
- 45.Schimmer AD, Mah K, Bordeleau L, et al. Decreased bone mineral density is common after autologous blood or marrow transplantation. Bone Marrow Transplant. 2001;28(4):387–391. doi: 10.1038/sj.bmt.1703149. [DOI] [PubMed] [Google Scholar]
- 46.Kaste SC, Shidler TJ, Tong X, et al. Bone mineral density and osteonecrosis in survivors of childhood allogeneic bone marrow transplantation. Bone Marrow Transplant. 2004;33(4):435–441. doi: 10.1038/sj.bmt.1704360. [DOI] [PubMed] [Google Scholar]
- 47.Petryk A, Bergemann TL, Polga KM, et al. Prospective study of changes in bone mineral density and turnover in children after hematopoietic cell transplantation. J. Clin. Endocrinol. Metab. 2006;91(3):899–905. doi: 10.1210/jc.2005-1927. [DOI] [PubMed] [Google Scholar]
- 48.Mattano LA., Jr Strategic approaches to osteoporosis in transplantation. Pediatr. Transplant. 2004;8 Suppl. 5:51–55. doi: 10.1111/j.1398-2265.2004.00184.x. [DOI] [PubMed] [Google Scholar]
- 49.Annaloro C, Usardi P, Airaghi L, et al. Prevalence of metabolic syndrome in long-term survivors of hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;41(9):797–804. doi: 10.1038/sj.bmt.1705972. [DOI] [PubMed] [Google Scholar]
- 50.Oudin C, Simeoni MC, Sirvent N, et al. Prevalence and risk factors of the metabolic syndrome in adult survivors of childhood leukemia. Blood. 2011;117(17):4442–4448. doi: 10.1182/blood-2010-09-304899. [DOI] [PubMed] [Google Scholar]
- 51. Cohen A, Bekassy AN, Gaiero A, et al. Endocrinological late complications after hematopoietic SCT in children. Bone Marrow Transplant. 2008;41 Suppl. 2:S43–S48. doi: 10.1038/bmt.2008.54.. • Provides a comprehensive overview of endocrine complications following HSCT.
- 52.Chemaitilly W, Sklar CA. Endocrine complications of hematopoietic stem cell transplantation. Endocrinol. Metab. Clin. North Am. 2007;36(4):983–998. doi: 10.1016/j.ecl.2007.07.002. ix. [DOI] [PubMed] [Google Scholar]
- 53.Jung MH, Cho KS, Lee JW, et al. Endocrine complications after hematopoietic stem cell transplantation during childhood and adolescence. J. Korean Med. Sci. 2009;24(6):1071–1077. doi: 10.3346/jkms.2009.24.6.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sanders JE. Growth and development after hematopoietic cell transplant in children. Bone Marrow Transplant. 2008;41(2):223–227. doi: 10.1038/sj.bmt.1705875. [DOI] [PubMed] [Google Scholar]
- 55.Sanders JE, Guthrie KA, Hoffmeister PA, Woolfrey AE, Carpenter PA, Appelbaum FR. Final adult height of patients who received hematopoietic cell transplantation in childhood. Blood. 2005;105(3):1348–1354. doi: 10.1182/blood-2004-07-2528. [DOI] [PubMed] [Google Scholar]
- 56.Sklar CA, Mertens AC, Mitby P, et al. Risk of disease recurrence and second neoplasms in survivors of childhood cancer treated with growth hormone: a report from the Childhood Cancer Survivor Study. J. Clin. Endocrinol. Metab. 2002;87(7):3136–3141. doi: 10.1210/jcem.87.7.8606. [DOI] [PubMed] [Google Scholar]
- 57.Rovo A, Tichelli A, Passweg JR, et al. Spermatogenesis in long-term survivors after allogeneic hematopoietic stem cell transplantation is associated with age, time interval since transplantation, and apparently absence of chronic GvHD. Blood. 2006;108(3):1100–1105. doi: 10.1182/blood-2006-01-0176. [DOI] [PubMed] [Google Scholar]
- 58.Savani BN, Kozanas E, Shenoy A, Barrett AJ. Recovery of spermatogenesis after total-body irradiation. Blood. 2006;108(13):4292–4294. doi: 10.1182/blood-2006-08-044289. [DOI] [PubMed] [Google Scholar]
- 59.Sanders JE. The impact of marrow transplant preparative regimens on subsequent growth and development. The Seattle Marrow Transplant Team. Semin. Hematol. 1991;28(3):244–249. [PubMed] [Google Scholar]
- 60.Teinturier C, Hartmann O, Valteau-Couanet D, Benhamou E, Bougneres PF. Ovarian function after autologous bone marrow transplantation in childhood: high-dose busulfan is a major cause of ovarian failure. Bone Marrow Transplant. 1998;22(10):989–994. doi: 10.1038/sj.bmt.1701483. [DOI] [PubMed] [Google Scholar]
- 61.Gulati SC, Van Poznak C. Pregnancy after bone marrow transplantation. J. Clin. Oncol. 1998;16(5):1978–1985. doi: 10.1200/JCO.1998.16.5.1978. [DOI] [PubMed] [Google Scholar]
- 62.Sanders JE, Buckner CD, Amos D, et al. Ovarian function following marrow transplantation for aplastic anemia or leukemia. J. Clin. Oncol. 1988;6(5):813–818. doi: 10.1200/JCO.1988.6.5.813. [DOI] [PubMed] [Google Scholar]
- 63.Schechter T, Finkelstein Y, Doyle J, Koren G. Pregnancy after stem cell transplantation. Can. Fam. Physician. 2005;51:817–818. [PMC free article] [PubMed] [Google Scholar]
- 64.Carter A, Robison LL, Francisco L, et al. Prevalence of conception and pregnancy outcomes after hematopoietic cell transplantation: report from the Bone Marrow Transplant Survivor Study. Bone Marrow Transplant. 2006;37(11):1023–1029. doi: 10.1038/sj.bmt.1705364. [DOI] [PubMed] [Google Scholar]
- 65.Schover LR. Motivation for parenthood after cancer: a review. J. Natl Cancer Inst. Monogr. 2005;(34):2–5. doi: 10.1093/jncimonographs/lgi010. [DOI] [PubMed] [Google Scholar]
- 66.Dow KH. Having children after breast cancer. Cancer Pract. 1994;2(6):407–413. [PubMed] [Google Scholar]
- 67.Hammond C, Abrams JR, Syrjala KL. Fertility and risk factors for elevated infertility concern in 10-year hematopoietic cell transplant survivors and case-matched controls. J. Clin. Oncol. 2007;25(23):3511–3517. doi: 10.1200/JCO.2007.10.8993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Syrjala KL, Langer SL, Abrams JR, Storer BE, Martin PJ. Late effects of hematopoietic cell transplantation among 10-year adult survivors compared with case-matched controls. J. Clin. Oncol. 2005;23(27):6596–6606. doi: 10.1200/JCO.2005.12.674. [DOI] [PubMed] [Google Scholar]
- 69.Gurney JG, Ness KK, Rosenthal J, Forman SJ, Bhatia S, Baker KS. Visual, auditory, sensory, and motor impairments in long-term survivors of hematopoietic stem cell transplantation performed in childhood: results from the Bone Marrow Transplant Survivor study. Cancer. 2006;106(6):1402–1408. doi: 10.1002/cncr.21752. [DOI] [PubMed] [Google Scholar]
- 70.Campbell S, Sun CL, Kurian S, et al. Predictors of avascular necrosis of bone in long-term survivors of hematopoietic cell transplantation. Cancer. 2009;115(18):4127–4135. doi: 10.1002/cncr.24474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Antignac C, Gubler MC, Leverger G, Broyer M, Habib R. Delayed renal failure with extensive mesangiolysis following bone marrow transplantation. Kidney Int. 1989;35(6):1336–1344. doi: 10.1038/ki.1989.132. [DOI] [PubMed] [Google Scholar]
- 72.Atkinson K, Biggs JC, Hayes J, et al. Cyclosporin A associated nephrotoxicity in the first 100 days after allogeneic bone marrow transplantation: three distinct syndromes. Br. J. Haematol. 1983;54(1):59–67. doi: 10.1111/j.1365-2141.1983.tb02067.x. [DOI] [PubMed] [Google Scholar]
- 73.Cruz DN, Perazella MA, Mahnensmith RL. Bone marrow transplant nephropathy: a case report and review of the literature. J. Am. Soc. Nephrol. 1997;8(1):166–173. doi: 10.1681/ASN.V81166. [DOI] [PubMed] [Google Scholar]
- 74.Glynne P, Powles R, Steele J, et al. Renal dysfunction following autologous bone marrow transplantation in adult patients with acute leukemia. Acta Oncol. 1996;35(6):709–712. doi: 10.3109/02841869609084003. [DOI] [PubMed] [Google Scholar]
- 75.Kist-van Holthe JE, Bresters D, Ahmed-Ousenkova YM, et al. Long-term renal function after hemopoietic stem cell transplantation in children. Bone Marrow Transplant. 2005;36(7):605–610. doi: 10.1038/sj.bmt.1705110. [DOI] [PubMed] [Google Scholar]
- 76.Parikh CR, McSweeney PA, Korular D, et al. Renal dysfunction in allogeneic hematopoietic cell transplantation. Kidney Int. 2002;62(2):566–573. doi: 10.1046/j.1523-1755.2002.00455.x. [DOI] [PubMed] [Google Scholar]
- 77.Kersting S, Hene RJ, Koomans HA, Verdonck LF. Chronic kidney disease after myeloablative allogeneic hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 2007;13(10):1169–1175. doi: 10.1016/j.bbmt.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 78.Stevens LA, Levey AS. Measurement of kidney function. Med. Clin. North Am. 2005;89(3):457–473. doi: 10.1016/j.mcna.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 79.Bray LC, Carey PJ, Proctor SJ, Evans RG, Hamilton PJ. Ocular complications of bone marrow transplantation. Br. J. Ophthalmol. 1991;75(10):611–614. doi: 10.1136/bjo.75.10.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Calissendorff BM, Bolme P. Cataract development and outcome of surgery in bone marrow transplanted children. Br. J. Ophthalmol. 1993;77(1):36–38. doi: 10.1136/bjo.77.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Giri N, Davis EA, Vowels MR. Long-term complications following bone marrow transplantation in children. J. Paediatr. Child Health. 1993;29(3):201–205. doi: 10.1111/j.1440-1754.1993.tb00487.x. [DOI] [PubMed] [Google Scholar]
- 82.Deeg HJ, Flournoy N, Sullivan KM, et al. Cataracts after total body irradiation and marrow transplantation: a sparing effect of dose fractionation. Int. J. Radiat. Oncol. Biol. Phys. 1984;10(7):957–964. doi: 10.1016/0360-3016(84)90163-9. [DOI] [PubMed] [Google Scholar]
- 83.Fife K, Milan S, Westbrook K, Powles R, Tait D. Risk factors for requiring cataract surgery following total body irradiation. Radiother. Oncol. 1994;33(2):93–98. doi: 10.1016/0167-8140(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 84.Livesey SJ, Holmes JA, Whittaker JA. Ocular complications of bone marrow transplantation. Eye. 1989;3(3):271–276. doi: 10.1038/eye.1989.37. [DOI] [PubMed] [Google Scholar]
- 85.Ozsahin M, Belkacemi Y, Pene F, et al. Total-body irradiation and cataract incidence: a randomized comparison of two instantaneous dose rates. Int. J. Radiat. Oncol. Biol. Phys. 1994;28(2):343–347. doi: 10.1016/0360-3016(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 86.Bhatia S, Ramsay NK, Steinbuch M, et al. Malignant neoplasms following bone marrow transplantation. Blood. 1996;87(9):3633–3639. [PubMed] [Google Scholar]
- 87.Witherspoon RP, Fisher LD, Schoch G, et al. Secondary cancers after bone marrow transplantation for leukemia or aplastic anemia. N. Engl. J. Med. 1989;321(12):784–789. doi: 10.1056/NEJM198909213211203. [DOI] [PubMed] [Google Scholar]
- 88.Krishnan A, Bhatia S, Slovak ML, et al. Predictors of therapy-related leukemia and myelodysplasia following autologous transplantation for lymphoma: an assessment of risk factors. Blood. 2000;95(5):1588–1593. [PubMed] [Google Scholar]
- 89.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 90.Gilliland DG, Gribben JG. Evaluation of the risk of therapy-related MDS/AML after autologous stem cell transplantation. Biol. Blood Marrow Transplant. 2002;8(1):9–16. doi: 10.1053/bbmt.2002.v8.pm11846355. [DOI] [PubMed] [Google Scholar]
- 91.Witherspoon RP, Deeg HJ. Allogeneic bone marrow transplantation for secondary leukemia or myelodysplasia. Haematologica. 1999;84(12):1085–1087. [PubMed] [Google Scholar]
- 92.Yakoub-Agha I, de La Salmoniere P, Ribaud P, et al. Allogeneic bone marrow transplantation for therapy-related myelodysplastic syndrome and acute myeloid leukemia: a long-term study of 70 patients-report of the French society of bone marrow transplantation. J. Clin. Oncol. 2000;18(5):963–971. doi: 10.1200/JCO.2000.18.5.963. [DOI] [PubMed] [Google Scholar]
- 93.Bloomfield CD, Archer KJ, Mrozek K, et al. 11q23 balanced chromosome aberrations in treatment-related myelodysplastic syndromes and acute leukemia: report from an international workshop. Genes Chromosomes Cancer. 2002;33(4):362–378. doi: 10.1002/gcc.10046. [DOI] [PubMed] [Google Scholar]
- 94.Litzow MR, Tarima S, Perez WS, et al. Allogeneic transplantation for therapy-related myelodysplastic syndrome and acute myeloid leukemia. Blood. 2010;115(9):1850–1857. doi: 10.1182/blood-2009-10-249128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Landgren O, Gilbert ES, Rizzo JD, et al. Risk factors for lymphoproliferative disorders after allogeneic hematopoietic cell transplantation. Blood. 2009;113(20):4992–5001. doi: 10.1182/blood-2008-09-178046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schouten HC, Hopman AH, Haesevoets AM, Arends JW. Large-cell anaplastic non-Hodgkin’s lymphoma originating in donor cells after allogenic bone marrow transplantation. Br. J. Haematol. 1995;91(1):162–166. doi: 10.1111/j.1365-2141.1995.tb05262.x. [DOI] [PubMed] [Google Scholar]
- 97.Rowlings PA, Curtis RE, Passweg JR, et al. Increased incidence of Hodgkin’s disease after allogeneic bone marrow transplantation. J. Clin. Oncol. 1999;17(10):3122–3127. doi: 10.1200/JCO.1999.17.10.3122. [DOI] [PubMed] [Google Scholar]
- 98.Curtis RE, Rowlings PA, Deeg HJ, et al. Solid cancers after bone marrow transplantation. N. Engl. J. Med. 1997;336(13):897–904. doi: 10.1056/NEJM199703273361301. [DOI] [PubMed] [Google Scholar]
- 99.Bhatia S, Louie AD, Bhatia R, et al. Solid cancers after bone marrow transplantation. J. Clin. Oncol. 2001;19(2):464–471. doi: 10.1200/JCO.2001.19.2.464. [DOI] [PubMed] [Google Scholar]
- 100.Leisenring W, Friedman DL, Flowers ME, Schwartz JL, Deeg HJ. Nonmelanoma skin and mucosal cancers after hematopoietic cell transplantation. J. Clin. Oncol. 2006;24(7):1119–1126. doi: 10.1200/JCO.2005.02.7052. [DOI] [PubMed] [Google Scholar]
- 101.Schwartz JL, Kopecky KJ, Mathes RW, Leisenring WM, Friedman DL, Deeg HJ. Basal cell skin cancer after total-body irradiation and hematopoietic cell transplantation. Radiat. Res. 2009;171(2):155–163. doi: 10.1667/RR1469.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Friedman DL, Rovo A, Leisenring W, et al. Increased risk of breast cancer among survivors of allogeneic hematopoietic cell transplantation: a report from the FHCRC and the EBMT-Late Effect Working Party. Blood. 2008;111(2):939–944. doi: 10.1182/blood-2007-07-099283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cohen A, Rovelli A, Merlo DF, et al. Risk for secondary thyroid carcinoma after hematopoietic stem-cell transplantation: an EBMT Late Effects Working Party Study. J. Clin. Oncol. 2007;25(17):2449–2454. doi: 10.1200/JCO.2006.08.9276. [DOI] [PubMed] [Google Scholar]
- 104. Sun CL, Francisco L, Kawashima T, et al. Prevalence and predictors of chronic health conditions after hematopoietic cell transplantation: a report from the Bone Marrow Transplant Survivor Study. Blood. 2010;116(17):3129–3139. doi: 10.1182/blood-2009-06-229369. quiz 3377.. •• Describes the cumulative burden of morbidity experienced by long-term survivors of HSCT.
- 105. Wingard JR, Majhail NS, Brazauskas R, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J. Clin. Oncol. 2011;29(16):2230–2239. doi: 10.1200/JCO.2010.33.7212.. • Describes the late mortality in a large cohort of patients following allogeneic HSCT.
- 106. Martin PJ, Counts GW, Jr, Appelbaum FR, et al. Life expectancy in patients surviving more than 5 years after hematopoietic cell transplantation. J. Clin. Oncol. 2010;28(6):1011–1016. doi: 10.1200/JCO.2009.25.6693.. •• Utilizes state-of-the-art methodology to describe the life expectancy in patients who have survived for at least 5 years after HSCT.
- 107.Bishop MM, Lee SJ, Beaumont JL, et al. The preventive health behaviors of long-term survivors of cancer and hematopoietic stem cell transplantation compared with matched controls. Biol Blood Marrow Transplant. 2010;16(2):207–214. doi: 10.1016/j.bbmt.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Armenian SH, Sun C-L, Kawashima T, et al. Long-term health-related outcomes in survivors of childhood cancer treated with hematopoietic cell transplantation (HSCT) versus conventional therapy: a report from the Bone Marrow Transplant Survivor Study (BMTSS) and Childhood Cancer Survivor Study (CCSS) Blood. 2010 doi: 10.1182/blood-2011-01-331835. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shankar SM, Carter A, Sun CL, et al. Health care utilization by adult long-term survivors of hematopoietic cell transplant: report from the Bone Marrow Transplant Survivor Study. Cancer Epidemiol Biomarkers Prev. 2007;16(4):834–839. doi: 10.1158/1055-9965.EPI-06-0714. [DOI] [PubMed] [Google Scholar]
- 110. Rizzo JD, Wingard JR, Tichelli A, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation: joint recommendations of the European Group for Blood and Marrow Transplantation, the Center for International Blood and Marrow Transplant Research, and the American Society of Blood and Marrow Transplantation. Biol. Blood Marrow Transplant. 2006;12(2):138–151. doi: 10.1016/j.bbmt.2005.09.012.. •• Describes consensus-based recommendations for patients after undergoing HSCT.
Websites
- 201.Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. www.survivorshipguidelines.org.
- 202.Agency for Healthcare Research and Quality prevention and care management. doi: 10.1080/15360280802537332. www.ahrq.gov/clinic/prevenix.htm. [DOI] [PubMed]