Abstract
Objective
Structural and functional brain reserves, thought to develop in childhood and adolescence, may be critical in determining the age at onset of cognitive impairment. Body height is affected by childhood conditions that promote growth. The authors examine the relationship of height in midlife and subsequent dementia, Alzheimer disease (AD), and vascular dementia.
Methods
Dementia was evaluated from 1999 to 2001 in 1,892 men age 76 to 95. Height had been measured when these men participated in the Israeli Ischemic Heart Disease project in 1963. Age, socioeconomic status (SES), and area of birth were also assessed in 1963.
Results
Older men and those with lower SES tended to be shorter. Relative to the shortest quartile, controlling for age, SES, and area of birth, the other quartiles had lesser respective odds ratios for dementia as a whole, AD, and vascular dementia.
Conclusion
Height was inversely associated with dementia, AD, and vascular dementia in a male sample. Since height is associated with childhood nutrition and may be associated with other risk factors for dementia, efforts to improve early life conditions that maximize body growth may diminish or delay the onset of dementia in later life.
There is growing evidence that structural and functional brain reserves, thought to develop in childhood and adolescence, may be critical in determining the age at onset of cognitive impairment.1 –4 If correct, these findings suggest that cognitive impairment and dementia in later life could be more frequent among elderly individuals who experienced nutritional, educational, and social deprivations in childhood. Body height and limb length as markers of childhood conditions that promote growth5 have been inversely associated with poor cognitive performance in late life.6,7 The purpose of this report is to examine height in midlife, in relation to dementia, Alzheimer disease (AD), and vascular dementia (VAD) in a large group of Jewish men participating in the Israeli Ischemic Heart Disease (IIHD) project.
The IIHD project was a longitudinal investigation of the incidence and risk factors for cardiovascular disease among about 10,000 male civil servants and municipal employees in Israel, chosen by stratified sampling of six geographical regions of birth. This cohort provided an extensive representation of the socioeconomic levels in the male working population of Israel at the time of inclusion; it was examined at three time-points, in 1963, 1965, and 1968.8 Survivors (mean age 82 at assessment) were examined for dementia between 1999 and 2001. The present report describes the relationship of height attained at adulthood and dementia.
METHODS
Subjects
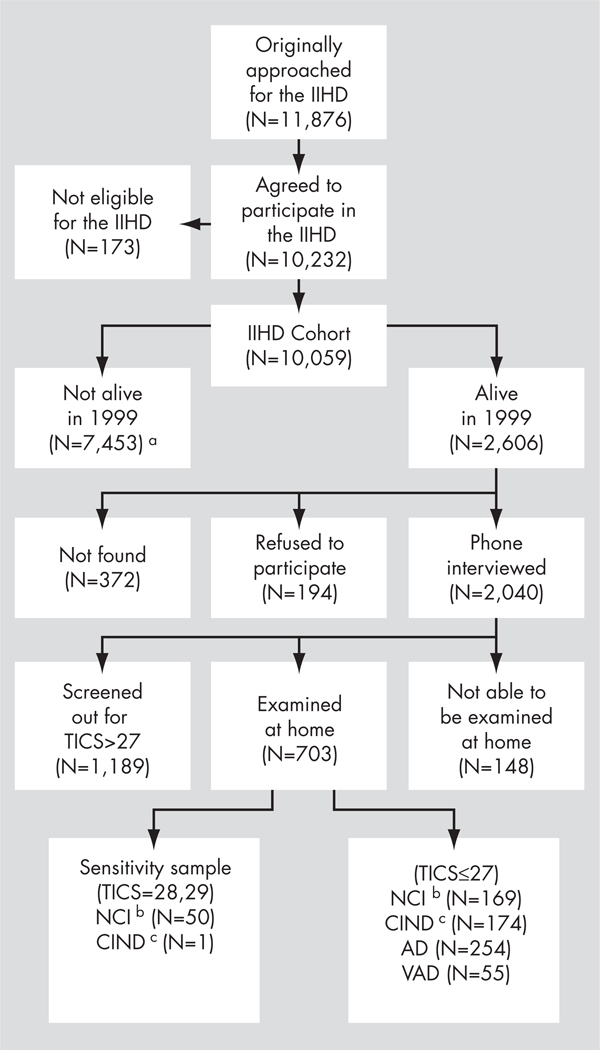
The sample of the IIHD Project (N = 11,876) was chosen by stratified sampling of civil servants and municipal employees in 1963 based on the following criteria: 1) they were men, age 40 to 65 years; 2) their place of work was limited to the three largest urban areas in Israel (Tel-Aviv, Jerusalem, and Haifa); and 3) the sampling fractions were aimed at obtaining numbers of study subjects from six geographical areas of birth (Central Europe, Eastern Europe, the Balkan countries, the Middle East, Northern Africa, and Israel) approximately proportional to the Israeli male population of this age; a group of 10,232 agreed to participate. Subsequent analyses excluded 173 men who were not born in the six pre-assigned areas of birth. Figure 1 presents the flow of subjects from 1963 through 2001 (the end of the present study). According to the Israel Mortality Registry, 7,136 subjects had died by the beginning of the study, in 1999; another 306 died before being approached for a phone interview, and 11 subjects were lost in the matching process. The remaining 2,606 subjects qualified for a telephone interview; 2,040 had such an interview. The telephone screening identified 800 potential dementia subjects for face-to-face home interview. Of these, 148 could not be examined at home. Of the remaining 1,240 subjects, 51 were examined at home for a sensitivity study of the phone interview instruments (50 were cognitively intact, and 1 cognitively impaired but not demented9), and the others were presumed to be cognitively intact. Thus, the follow-up study characterized the presence or absence of dementia in 1,892 subjects, of whom 309 had dementia (254 with AD and 55 with VAD), 175 had cognitive impairment not sufficient for the diagnosis of dementia, and 1,408 had no cognitive impairment.
FIGURE 1.
Flow of Subjects
Note: IIHD: Israeli Ischemic Heart Disease project; TICS: Telephone Interview for Cognitive Status; AD: Alzheimer disease; VAD: vascular dementia.
a This number consists of 7,136 who died before 1999, 306 who died before they could be interviewed in the 1999 follow-up, and 11 who were lost in the matching process.
b NCI: no cognitive impairment.
c CIND: cognitively impaired, no dementia.
Table 1 compares the 2,606 subjects who were alive by the time of the 1999 follow-up visit with the 7,453 who were not. The subjects who survived were younger, taller, had lower BMI, total cholesterol, systolic and diastolic blood pressure and glucose, were less likely to be diabetic, and less likely to have been smokers in 1963. Their socioeconomic status (SES) was slightly lower. More were born in Eastern Europe, and fewer were born in the Middle East. The groups had similar average weight and HDL cholesterol.
TABLE 1.
Comparisons Among Subjects Who Were Alive and Those Who Were Deceased in the 1999 Follow-Up
| Deceased (N = 7,453) | Alive (N = 2,606) | Statistics | |
|---|---|---|---|
| Age, years, 1963 | 50.9 (6.9) N = 7,453 | 44.8 (4.3) N = 2,606 | t = 42.3; p <0.005 |
| Height, cm, 1963 | 166.8 (6.6) N = 7,433 | 168.1 (6.5) N = 2,601 | t = 8.7; p <0.005 |
| Weight, kg, 1963 | 1.8 (10.9) N = 7,439 | 71.7 (10.3) N = 2,601 | t = 0.4; p = 0.68 |
| BMI (kg/m2), 1963 | 25.8 (3.4) N = 7,433 | 25.3 (3.1) N = 2,601 | t = 6.6; p <0.005 |
| Cholesterol, 1963/5/8) | 212.0 (40.0) N = 6,400 | 202.0 (38.0) N = 2,432 | t = 11.1; p <0.005 |
| HDL cholesterol (mg/dl), 1965 | 40.6 (9.5) N = 6,265 | 41.3 (9.4) N = 2,325 | t = 0.8; p = 0.45 |
| Systolic blood pressure, mm Hg, 1963/5/8 | 138.0 (22.0) N = 6,855 | 127.0 (15.0) N = 2,543 | t = 23.7; p <0.005 |
| Diastolic blood pressure, mm Hg, 1963/5/8 | 85.0 (12.0) N = 6,846 | 81.0 (9.0) N = 2,542 | t = 15.6; p <0.005 |
| % Diabetic, 1963/5/8 | 6.02 N = 7,254 | 1.15 N = 2,565 | χ2 = 101.1; p <0.005 |
| % Smokers, 1963 | 52.1 N = 7,453 | 45.8 N = 2,606 | χ2 = 30.5; p <0.005 |
| SES (1: low to 5: high), 1963 | 3.3 (1.2) N = 7,397 | 3.2 (1.2) N = 2,598 | t = 5.4; p <0.000 |
| Area of birth | N = 7,453 | N = 2,606 | χ2 = 57.2; p <0.005 |
| Israel | 13.6 | 14.5 | |
| Eastern Europe | 15.2 | 20.6 | |
| Central Europe | 15.0 | 13.2 | |
| Balkan countries | 16.5 | 17.5 | |
| Middle East | 26.9 | 22.3 | |
| North Africa | 12.8 | 11.9 |
Note: Values are mean (standard deviation), unless otherwise indicated.
BMI: body mass index; SES: socioeconomic status.
Subjects were classified as having cognitive impairment but not dementia by the following criteria: memory complaint reported by both subject and informant, normal activities of daily living, normal general cognitive functioning, abnormal memory and no dementia. Thus, those subjects did not qualify for not cognitively impaired, AD, or VAD groups. Since their etiology is heterogeneous in that they may or may not have very early dementia, they were excluded from the evaluations of the associations between height and dementia. Thus, the following analysis included 1,706 subjects.
Dementia Diagnosis
The methods of this study have been described in detail elsewhere.9 Cases of dementia were identified by use of a two-phase screening and evaluation system. The first phase was locating the subjects and conducting a screening phone interview. Subjects were located by cross-linking the database of the original study8 with the current population registry of the Israel Ministry of Interior, using the ID number as linking identifier. All identified living participants in the original cohort were phoned, reminded of their participation in the original study, and asked for consent to participate in the new assessment. The subjects who agreed to participate were administered a 20-minute phone interview, including a sociodemographic questionnaire and the Hebrew version of The Modified Telephone Interview for Cognitive Status (TICS-m).10 The sociodemographic questionnaire included items that were not recorded during the original study in 1963, or might change with time, such as: marital status, retirement age, education, profession, leisure activities, degree of religiosity, smoking, and drinking habits.
The TICS-m is based on the Mini-Mental State Exam (MMSE).11 The interview includes questions regarding long- and short-term memory, orientation to time and place, attention, language, and abstraction. The scores of the TICS-m were highly correlated with the MMSE scores in clinical studies12 and in the Hebrew version in this cohort.9 At a level of 27/50, the sensitivity of this test was above 99%, and the specificity was 86%;10 the sensitivity at this level was 100% for the Hebrew version.9
Subjects with a TICS-m score of 27/50 or lower were classified as possibly cognitively impaired. The aim of the second phase of the study was to assess the diagnosis of dementia and its type. The patients were assessed at their residences by a neurologist or a psychiatrist with expertise in dementia. Physicians were blind to TICS-m scores. The clinical assessment included the Dementia Questionnaire (DQ),13 –15 Mini-Mental State Exam,11 Global Deterioration Scale (GDS),16 and the Hachinski Ischemic Scale (HIS).17 The entire interview lasted approximately 90 minutes.
For persons with suspected dementia, the DQ assesses, through informants, the symptoms, course, and features of the dementia, permitting a diagnostic classification of the presence and likely type of dementia. The DQ has previously demonstrated good inter-informant and inter-rater agreement in AD probands and normal elderly control populations for detecting the presence or absence of dementia and also, among identified cases of dementia, the type of dementia.13,14 More recently, several investigations have assessed the validity of this instrument. Compared with a clinical assessment of dementia, the DQ was found to have excellent sensitivity (100%) and very good specificity (90%).18,19 Also, in a series of autopsied elderly subjects, informant-based DQ scores were compared with independent neuropathological examinations; for AD, the DQ was found to be only slightly less sensitive than direct clinical assessment, and its specificity was virtually at the same level.15
A second physician, blind to the diagnosis assigned by the physician who did the face-to-face interview, examined the data gathered from each patient and assigned an independent diagnosis. In case of disagreement between the two physicians, the case was presented to a third physician for a consensus diagnosis. In a handful of cases, the patient was approached again.
From subjects with a diagnosis of dementia, we collected previously obtained MRI or CT scan results whenever available. When there was no previous procedure, we recommended that a CT or MRI be conducted and shared with our project. Dementia was diagnosed by DSM-IV criteria,20 and the NINCDS-ADRDA criteria were used for the diagnosis of AD.21 AD was defined to include all persons whose AD diagnosis was “probable” or “possible.” Diagnosis of VAD used NINDS-AIREN criteria.22 VAD diagnosis included “probable” and “possible” cases. When the diagnosis was mixed (meeting the criteria of both AD and VAD), the diagnostician assigned a diagnosis of AD or VAD according to the primary cause of dementia. The study was reviewed and approved by the Helsinki Committee of the Sheba Medical Center, Ramat Gan, Israel, and all participants provided informed consent.
Height Measurement
Body height was measured in 1963 to the nearest centimeter, without shoes. Height was divided into four quartiles on the basis of the 1999 follow-up sample: 164 cm or shorter, 165–168 cm, 169–172 cm, and 173 cm or taller.
Socioeconomic Status (SES) Index
This 5-point scale of socioeconomic status (SES) was based on formal education and type of employment. Formal education included nine levels, ranging from no formal schooling to completed university education. Employment was the current one at the time of the examination (1963) and included five levels, ranging from “laborer” to “professional.” The SES scale ranged from 1: very low SES (three lowest education and the lowest employment level) to 5: very high SES (three highest education and two highest employment levels).23
Statistical Analysis
The sociodemographic characteristics of the height quartiles were compared by ANOVA and Pearson’s chi-square. Logistic-regression analysis was performed to compare the dementia prevalence rates in the four height quartiles, controlling for age, SES, and area of birth. Age and SES were treated as continuous variables, and geographical area of birth as categorical. Parallel logistic-regression analyses were performed for AD and VAD. For the analyses of AD, subjects with AD were compared with those with no cognitive impairment, excluding those with VAD. Similarly, VAD cases were compared with cases with no cognitive impairment, excluding those with AD.
RESULTS
Table 2 presents the distribution of age, SES, and area of birth by height quartiles. The four groups did not differ in age (F[1, 1,877] = 1.84; p = 0.14). The tallest group had significantly higher SES levels than each of the other three height groups. The Asia and Eastern Europe birth areas had relatively large numbers of short subjects, whereas Israel had a relatively high number of tall subjects.
TABLE 2.
Age, SES, and Area of Birth by Body Height in Men Screened for Dementia
| H1 (≤164 cm.) (N = 515) | H2 (165–168 cm.) (N = 460) | H3 (169–172 cm.) (N = 424) | H4 (≥173) (N = 482) | p | |
|---|---|---|---|---|---|
| Age in 1963, years | 44.8 (4.1) | 44.4 (4.0) | 44.4 (4.1) | 44.2 (4.0) | 0.14a |
| SES (%) | |||||
| 1: very low | 23.5 | 15.9 | 15.6 | 10.6 | <0.0005 b |
| 2: low | 23.7 | 26.5 | 27.4 | 19.7 | H1 < H4 |
| 3: moderate | 32.8 | 35.7 | 36.3 | 43.4 | H2 < H4 |
| 4: high | 9.7 | 9.3 | 9.9 | 14.7 | H3 < H4 |
| 5: very high | 10.3 | 12.6 | 10.8 | 11.6 | |
| Mean (SD) | 2.6 (1.2) | 2.8 (1.2) | 2.77 (1.17) | 3.0 (1.1) | |
| Area of birth (%) | 0.02 c | ||||
| Israel | 10.7 | 13.3 | 13.9 | 16.8 | |
| Eastern Europe | 19.2 | 17.4 | 15.6 | 11.8 | |
| Central Europe | 16.1 | 15.4 | 13.2 | 18.3 | |
| Balkan countries | 14.6 | 15.7 | 17.5 | 18.5 | |
| Middle East | 27.6 | 26.7 | 26.6 | 21.3 | |
| North Africa | 11.8 | 11.5 | 13.2 | 13.3 |
Note: Values are mean (standard deviation [SD]), unless otherwise indicated. SES: socioeconomic status.
ANOVA; F[3, 1,877] = 1.84
ANOVA; F[3, 1,877] = 8.5. Tukey HSD (honestly significant difference) post-hoc analysis was undertaken to specify between which groups and in what direction the difference was found at the 0.05 significance level.
χ2[15] = 28.04; p = 0.02.
Controlling for age, SES, and geographical area of birth in a logistic regression, the four height categories differed significantly in their dementia rates (χ2[3] = 21.7; p <0.0005). Relative to the shortest quartile, the other quartiles had respective odds ratios (ORs) of 0.78 (95% confidence interval [CI]: 0.56–1.10), 0.47 (95% CI: 0.32–0.68), and 0.51 (95% CI: 0.35–0.74); thus rates declined for taller quartiles. Results were similar when age, SES, and geographical area of birth were not controlled (χ2[3] = 32.1; p <0.0005). Age-adjusted rates of dementia were 0.25, 0.20, 0.13 and 0.13 for respective quartiles.
As expected, a substantial majority (82%) of the dementia cases had a clinical diagnosis of AD. The pattern of results was similar for these; after adjusting for age, SES, and area of birth, the four height categories differed significantly in their AD rates (χ2[3] = 16.9; p = 0.001). Relative to the shortest-height quartile, the odds ratios were 0.81 (95% CI: 0.56–1.2), 0.46 (95% CI: 0.31–0.70), and 0.57(95% CI: 0.38–0.85). Results were similar when age, SES, and geographical area of birth were not controlled (χ2[3] = 22.6; p <0.0005). Age-adjusted rates of AD were 0.20, 0.16, 0.11, and 0.12, for the respective quartiles.
Since the rates of VAD were so much smaller than for dementia or AD, the power for this comparison was substantially reduced. Adjusting for age, SES, and area of birth, the four height categories differed significantly in their VAD rates (χ2[3] = 9.9; p = 0.02). The ORs relative to the shortest quartile were 0.73 (95% CI: 0.37–01.5), 0.43 (95% CI: 0.20–0.94), and 0.29 (95% CI: 0.11–0.74). Again, results were similar when age, SES, and area of birth were not controlled (χ2[3] = 14.6; p = 0.002). Age-adjusted rates for VAD were 0.05, 0.04, 0.02, and 0.01, for the respective quartiles.
DISCUSSION
The present study results indicate associations between body height and dementia for both AD and VAD. These associations remain when not controlling for age, SES, and area of birth. Similar results were found in the Honolulu–Asia Aging Study.6 In that study, height was associated with poorer cognitive functioning; the relationship with dementia was less clear, possibly because of lower rates of dementia. Our dementia rates were higher because the subjects were older. Height was also associated with stroke in a follow-up mortality study24 analyzing the IIHD cohort.
Malnutrition may be the most important modifiable determinant both of height and cognitive development.25 –27 The Barker hypothesis proposes that intrauterine malnutrition, marked by low birth weight (and with short stature), predisposes individuals to Type 2 diabetes, hypertension, dyslipidemia, cardiovascular disease, and renal disease in adult life.28 –32 It is possible that cognitive impairment and dementia are other dimensions that could be added to the Barker hypothesis. Prenatal and early-life exposures have been proposed as influential in the clinical expression of AD, particularly in those who are more vulnerable.2,3,33,34 Low linguistic ability in early life, which may reflect suboptimal neurological and cognitive development, has a strong association with dementia in late life.2 Also, low SES and barren cognitive milieu in childhood have some influence on cognitive functioning in old age.35
An alternative and/or complementary explanation for our results is the association of growth hormone (GH) levels to both height and cognition. Cognitive impairment was related to GH deficiency in a study examining men suffering from isolated growth hormone deficiency.30,36. A study evaluating the effects of 2 years of GH therapy on cognitive performance in adults with childhood-onset growth hormone deficiency concluded that GH replacement therapy improved cognition in those subjects.37 A study evaluating attention before and after 2 years of GH treatment in intrauterine growth-retarded children found that attention is related to stature and that GH treatment seems to have beneficial effects on attention capacity.38 Finally, binding sites for GH are found in various areas of the brain, and their distribution suggests that GH contributes to the function of the hippocampus, a brain structure specifically involved in the development of dementia.39
Short stature may also be a consequence of socioeconomic deprivation, interfering with growth in childhood and adolescence. Better socioeconomic conditions in childhood have been shown to be associated with relatively more intact later-life cognitive ability.40 Our assessment of SES, based on number of years of formal schooling and type of employment, was done at ages 40 and above. Thus, it included both direct and indirect effects of education, which may be associated with childhood socioeconomic environment. Although baseline (1963) socioeconomic status was associated with dementia, controlling it did not alter the height–dementia association. Other unrecorded socioeconomic characteristics may also have been associated both with height attained and dementia. A majority of the sample were immigrants, from Europe and the Middle East and North African Arab countries, in which many Jewish communities were disrupted.
The variables such as education and area of birth, that were controlled for in the data analysis, were assumed to affect childhood development to a considerable extent. Other variables that were not available, such as birth weight, nutrition in the first years of life, parents’ education, occupation, and income; and physical environment during the participants’ childhood, which might be better predictors of dementia and might reduce the apparent association with height, are not available in this group, many of whom were refugees and/or pioneers. Parental height would be a particularly useful genetic factor to control, but many of these subjects were separated from their parents at very young ages, so this information would have been unreliable. More careful assessments would involve a lifetime of follow-up of large cohorts of individuals that bridge the gap between studies of cognition in childhood with those that recruit elderly subjects.
A limitation of this study is the lack of dementia characterization of the 7,453 subjects in the original IIHD study who died before the follow-up study was conducted. Those who survived to the time of this study were younger and healthier. Moreover, the 1,892 survivors who participated in the study similarly differed in their profile of sociodemographic and health characteristics from the 714 survivors who were not found or refused to participate (data not shown). Thus, the conclusions of this study are applicable to a population of those who survive to a relatively old age and who are amenable to participation in research. Height was measured in middle age, rather than young adulthood; although intervening factors (e.g., degenerative arthritis, osteoporosis) might have occurred, this is very unlikely, since these diseases tend to occur at later ages, and the cohort was actively employed.
We have chosen to compare subjects with definite dementia to unequivocally nondemented subjects, excluding those with cognitive impairment but not dementia, who are a heterogeneous group, with some subjects early in the course of cognitive decline. When they were included with the dementia-subjects group as cognitively impaired, the results of the analysis—more impairment in the shorter quartiles—remained essentially unchanged.
Our results are consistent with the hypothesis that early-childhood development, as reflected by body height, influences the probability of suffering from dementia later in life. Height reflects childhood nutrition, especially in this study, which included many subjects who lived in disturbed childhood environments. Height could also be associated with other environmental conditions in childhood and adolescence. Specifically, height is associated with GH, which is also associated with cognitive functioning. More studies examining those relationships are warranted.
Acknowledgments
This research was supported, in part, by the Israel Science Foundation, founded by the Israel Academy of Sciences and Humanities (Grant no. 67/99).
References
- 1.Stern Y, Gurland B, Tatemichi T. Influence of education and occupation on the incidence of Alzheimer’s disease. Neurology. 1993;43:839–844. [PubMed] [Google Scholar]
- 2.Snowdon DA, Greiner LH, Markesbery WR. Linguistic ability in early life and the neuropathology of Alzheimer’us disease and cerebrovascular disease. Findings from The Nun Study. Ann N Y Acad Sci. 2000;903:34–38. doi: 10.1111/j.1749-6632.2000.tb06347.x. [DOI] [PubMed] [Google Scholar]
- 3.Graves AB, Mortimer JA, Larson EB, et al. Head circumference as a measure of cognitive reserve: association with severity of impairment in Alzheimer’s disease. Br J Psychiatry. 1996;169:86–92. doi: 10.1192/bjp.169.1.86. [DOI] [PubMed] [Google Scholar]
- 4.Schofield PW, Mosesson RE, Stern Y, et al. The age at onset of Alzheimer’s disease and an intracranial area measurement. Arch Neurol. 1995;52:95–98. doi: 10.1001/archneur.1995.00540250103019. [DOI] [PubMed] [Google Scholar]
- 5.Barker JD. The malnourished baby and infant. Br Med Bull. 2001;60:69–88. doi: 10.1093/bmb/60.1.69. [DOI] [PubMed] [Google Scholar]
- 6.Abbott RD, White LR, Ross GW, et al. Height as a marker of childhood development and late-life cognitive function: The Honolulu-Asia Aging Study. Pediatrics. 1998;102:602–609. doi: 10.1542/peds.102.3.602. [DOI] [PubMed] [Google Scholar]
- 7.Kim JM, Stewart R, Shin IS, et al. Limb length and dementia in an older Korean population. J Neurol Neurosurg Psychiatry. 2003;74:427–432. doi: 10.1136/jnnp.74.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groen JJ, Medalie JH, Neufeld HN, et al. An epidemiologic investigation of hypertension and ischemic heart disease within a defined segment of the adult male population of Israel. Isr J Med Sci. 1968;4:177–194. [PubMed] [Google Scholar]
- 9.Beeri MS, Werner P, Davidson M, et al. Validation of the modified telephone interview for cognitive status (TICS-m) in Hebrew. Int J Geriatr Psychiatry. 2003;18:381–386. doi: 10.1002/gps.840. [DOI] [PubMed] [Google Scholar]
- 10.Gallo JJ, Breitner JC. Alzheimer’ s disease in the NAS-NRC Registry of aging twin veterans, IV: performance characteristics of a two-stage telephone screening procedure for Alzheimer’ s dementia. Psychol Med. 1995;25:1211–1219. doi: 10.1017/s0033291700033183. [DOI] [PubMed] [Google Scholar]
- 11.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 12.Brandt J, Spencer M, Folstein M. The Telephone Instrument for Cognitive Status. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1:11–17. [Google Scholar]
- 13.Silverman JM, Breitner JCS, Mohs RC, et al. Reliability of the family-history method in genetic studies of Alzheimer’ s disease and related dementias. Am J Psychiatry. 1986;143:1279–1282. doi: 10.1176/ajp.143.10.1279. [DOI] [PubMed] [Google Scholar]
- 14.Silverman JM, Keefe RS, Mohs RC, et al. A study of the reliability of the family-history method in genetic studies of Alzheimer disease. Alzheimer Dis Assoc Disord. 1989;4:218–223. [PubMed] [Google Scholar]
- 15.Li G, Aryan M, Silverman JM, et al. A validity study of family-history assessment of primary progressive dementia. Arch Neurol. 1997;54:634–640. doi: 10.1001/archneur.1997.00550170104021. [DOI] [PubMed] [Google Scholar]
- 16.Reisberg B, Ferris SH, de Leon MJ, et al. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139:1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 17.Moroney JT, Bagiella E, Hachinski VC, et al. Misclassification of dementia subtype using the Hachinski Ischemic Score: results of a meta-analysis of patients with pathologically verified dementias. Ann N Y Acad Sci. 1997;2(826):490–492. doi: 10.1111/j.1749-6632.1997.tb48510.x. [DOI] [PubMed] [Google Scholar]
- 18.Ellis RJ, Jan K, Kawas C, et al. Diagnostic validity of the dementia questionnaire for Alzheimer disease. Arch Neurol. 1998;55:360–365. doi: 10.1001/archneur.55.3.360. [DOI] [PubMed] [Google Scholar]
- 19.Kawas C, Segal J, Stewart WF, et al. A validation study of the Dementia Questionnaire. Arch Neurol. 1994;51:901–906. doi: 10.1001/archneur.1994.00540210073015. [DOI] [PubMed] [Google Scholar]
- 20.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 21.McKhann G, Drachman DA, Folstein T. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group Under the Auspices of the Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 22.Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies: report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 23.Eaton CB, Medalie JH, Flocke SA, et al. Self-reported physical activity predicts long-term coronary heart disease and all-cause mortalities: 21-year follow-up of the Israeli Ischemic Heart Disease Study. Arch Fam Med. 1995;4:323–329. doi: 10.1001/archfami.4.4.323. [DOI] [PubMed] [Google Scholar]
- 24.Goldbourt U, Tanne D. Body height is associated with decreased long-term stroke but not coronary heart disease mortality? Stroke. 2002;33:743–748. doi: 10.1161/hs0302.103814. [DOI] [PubMed] [Google Scholar]
- 25.Lifshitz F, Tarim O. Nutritional dwarfing. Curr Probl Pediatr. 1993;23:322–336. doi: 10.1016/0045-9380(93)90023-6. [DOI] [PubMed] [Google Scholar]
- 26.Hack M, Breslau N, Weissman B. Effect of very low birth weight and subnormal head size on cognitive abilities at school age. N Engl J Med. 1991;325:231–237. doi: 10.1056/NEJM199107253250403. [DOI] [PubMed] [Google Scholar]
- 27.Lynn R. A nutrition theory of the secular increases in intelligence: positive correlations between height, head size, and IQ. Br J Educ Psychol. 1989;59:372–377. [Google Scholar]
- 28.Barker DJP, Hales CN, Fall CHD, et al. Type 2 diabetes mellitus, hypertension and hyperlipidemia (Syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36:62–67. doi: 10.1007/BF00399095. [DOI] [PubMed] [Google Scholar]
- 29.Barker DJP, Martyn CN. The maternal and infant origins of cardiovascular disease. J Epidemiol Community Health. 1992;46:8–11. doi: 10.1136/jech.46.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams S, St. George IM, Silva PA. Intrauterine growth retardation and blood pressure at age 7 and 18. J Clin Epidemiol. 1992;45:1257–1263. doi: 10.1016/0895-4356(92)90167-l. [DOI] [PubMed] [Google Scholar]
- 31.Klebanoff MA, Secher NJ, Mednick BR, et al. Maternal size at birth and the development of hypertension during pregnancy: a test of the Barker hypothesis. Arch Intern Med. 1999;159:1607–1612. doi: 10.1001/archinte.159.14.1607. [DOI] [PubMed] [Google Scholar]
- 32.Hoy WE, Rees M, Kile E, et al. A new dimension to the Barker hypothesis: low birthweight and susceptibility to renal disease. Kidney Int. 1999;56:1072–1077. doi: 10.1046/j.1523-1755.1999.00633.x. [DOI] [PubMed] [Google Scholar]
- 33.Mortimer JA, Graves AB. Education and other socioeconomic determinants of dementia and Alzheimer’s disease. Neurology. 1993;43:839–844. [Google Scholar]
- 34.Snowdon DA, Kemper SJ, Mortimer JA, et al. Linguistic ability in early life and cognitive function and Alzheimer’ s disease in late life: findings from The Nun Study. JAMA. 1996;275:528–532. [PubMed] [Google Scholar]
- 35.Everson-Rose SA, Mendes de Leon CF, Bienias JL, et al. Early life conditions and cognitive functioning in later life. Am J Epidemiol. 2003;158:1083–1089. doi: 10.1093/aje/kwg263. [DOI] [PubMed] [Google Scholar]
- 36.Deijen JB, de Boer H, Block GJ, et al. Cognitive impairments and mood disturbances in growth hormone-deficient men. Psycho-neuroendocrinology. 1996;21:313–322. doi: 10.1016/0306-4530(95)00050-x. [DOI] [PubMed] [Google Scholar]
- 37.Deijen JB, de Boer H, van der Veen EA. Cognitive changes during growth hormone replacement in adult men. Psychoneuroendocrinology. 1998;23:45–55. doi: 10.1016/s0306-4530(97)00092-9. [DOI] [PubMed] [Google Scholar]
- 38.Van der Reijden-Lakeman IE, de Sonneville LM, Swaab-Barneveld HJ, et al. Evaluation of attention before and after 2 years of growth hormone treatment in intrauterine-growth-retarded children. J Clin Exp Neuropsychol. 1997;19:101–118. doi: 10.1080/01688639708403840. [DOI] [PubMed] [Google Scholar]
- 39.Van Dam PS, Aleman A, de Vries WR, et al. Growth hormone, insulin-like growth factor I, and cognitive function in adults. Growth Horm IGF Res. 2000;10:S69–S73. doi: 10.1016/s1096-6374(00)80013-1. [DOI] [PubMed] [Google Scholar]
- 40.Kaplan GA, Turrell G, Lynch JW, et al. Childhood socioeconomic position and cognitive function in adulthood. Int J Epidemiol. 2001;30:256–263. doi: 10.1093/ije/30.2.256. [DOI] [PubMed] [Google Scholar]