Abstract
It is unclear whether elevated spontaneous physical activity (SPA, very low-intensity physical activity) positively influences body composition long-term.
Objective
We determined whether SPA and caloric intake were differentially related to the growth curve trajectories of body weight, FM and FFM between obesity resistant and Sprague-Dawley rats at specific age intervals.
Design and Subjects
Body composition, SPA and caloric intake were measured in selectively-bred obesity resistant and out-bred Sprague-Dawley rats from 1-18 mo. Data from development throughout maturation were analyzed by longitudinal growth curve modeling to determine the rate and acceleration of body weight, fat mass (FM) and fat-free mass (FFM) gain.
Results
Obesity resistant rats had a lower rate of FM gain overall, a lower acceleration in body weight early in life, significantly greater SPA and lower cumulative caloric intake. Greater SPA in obesity resistant rats was significantly associated with a lower rate of FM gain overall and lower acceleration in body weight early in life. Obesity resistant rats lost less FFM compared to Sprague-Dawley rats despite that obesity resistant rats had a lower acceleration in FFM gain early in life. Obesity resistant rats gained less FM and more FFM per gram body weight and were less energy efficient than Sprague-Dawley rats. Caloric intake was significantly and positively related to body weight, FM and FFM gain in both groups. Circadian patterns of caloric intake were group and age-dependent. Our data demonstrate that elevated and sustained SPA during development and over the lifespan are related to the reduced the rate of FM gain and may preserve FFM.
Conclusion
These data support the idea that SPA level is a reproducible marker that reliably predicts propensity for obesity in rats, and that elevated levels of SPA maintained during the lifespan promote a lean phenotype.
Keywords: obesity, growth curve analysis
INTRODUCTION
The etiology of obesity has been reviewed and while it is clear that an imbalance between energy intake and expenditure promotes weight gain, it is unclear which factor has more profoundly driven the obesity epidemic [1-3]. Both factors contribute significantly and it is plausible that the magnitude of change in energy intake and expenditure that perpetuates obesity will be culturally, economically and regionally specific. Furthermore, the effect of increasing body weight on basal metabolism and inter-individual variability in the energetic cost of physical activity has made it difficult to understand whether low physical activity precedes obesity or whether body weight gain is an antecedent for low physical activity. Despite this, rising body weight was associated with reduced leisure time physical activity [4] and increased time spent viewing television [5, 6] while walking daily was associated with weight maintenance [7] and reductions in physical activity were associated with increased FM [8]. Longitudinal studies have tracked physical activity and fat gain in children and while some studies show that early and high physical activity levels predict lower adiposity [5, 9, 10] others report contradictory results [11, 12]. Overall, the majority of the data from longitudinal studies with objective measures of physical activity and adiposity suggest that early and sustained physical activity levels may contribute to reduced body weight gain during maturation.
Studies in rodents demonstrate that voluntary exercise attenuates increases in adiposity in adult [13] and post-weaned [14] diet-induced obese rats, melanocortin-4 receptor knockout mice [15] and rats lacking cholecystokinin-A receptors [16]. These data are consistent with clinical data; however, it is unclear whether early elevated and sustained physical activity levels dampen increases in adiposity long-term and thus whether early physical activity levels predict future adiposity. Based on our previous work showing that early differences in SPA were maintained at seven months between lean and obese rats [17, 18], we asked whether SPA was related to or predicted differences in growth curve trajectories of body composition over time. We hypothesized that SPA would be significantly and negatively associated with the rate and acceleration of adiposity over time. Therefore, energy intake, SPA and body composition were measured from development through maturation and aging (18 mo., which represents the age prior to senescence in most Sprague-Dawley rats) to capture the transition from the pre-obese to the obese state. Longitudinal multi-level growth curve modeling was used to determine whether trajectories of body weight, FM and FFM differed between lean and obese rats and then to determine whether SPA and caloric intake were related to the trajectories. Finally, because the SPA, adiposity and susceptibility to obesity may vary across developmental time points within the lifespan, we compared SPA, caloric intake and body composition at separate age intervals. Our data demonstrate that the maintenance of early and elevated SPA levels predicted a reduced rate of FM gain and may have contributed to a preservation of FFM through 18 mo.
MATERIALS AND METHODS
Animals
One-month old male Sprague-Dawley (n=12) and selectively-bred obesity resistant (n=24) rats (Charles River, Kingston, NY, USA) were housed individually in cages with a 12-h light/12-h dark photocycle (lights on at 0600-h in a temperature controlled room (21–22 °C)). Early mortality was noted in some obesity resistant rats in previous studies. Therefore, the sample size for obesity resistant rats was increased to ensure adequate power to detect group differences throughout maturation. Rats were fed a micro- and macronutrient specified and adequate low fat diet (Research Diets D12489B, New Brunswick, NJ, USA) ad libitum. The Institutional Animal Care and Use Committee at the Veterans Affairs Medical Center and the University of Minnesota approved the study.
SPA measurement
SPA was measured using racks of infrared (I/R) activity sensors (Med Associates, St. Albans, Vermont, USA) placed around a square acrylic cage as previously described [17, 19]. Briefly, two arrays in the ‘x-y’ direction and an elevated ‘x-y’ array measured ambulation and vertical movement. An activity unit was recorded and time stamped with each beam interruption and therefore movement was simultaneously detected in three axes. Food and water were available ad libitum as previously described [17]. Rats were acclimated to the SPA chambers for 24-h prior to a 24-h measurement period. From the SPA measurements time spent moving, the sum of time ambulatory (locomotor activity) and time vertical (rearing or standing) were reported for animals from 1-6 mo. Due to increased body length and an observed increase in hunched posture with age, the I/R array used to capture vertical movement (rearing) was raised one inch, the lowest possible increase. After the modification, vertical movement was 10 fold lower as compared to the measurement a month prior. It is plausible that the I/R array was too high after this modification, and we failed to detect all vertical movement. Thus as the vertical data from this point on was unreliable, time vertical was excluded and only time ambulatory was reported from 7-18 mo. Data are reported as time spent moving or time ambulatory in the cumulative 24-h measurement period.
Body composition
Total fat and FFM was measured using a quantitative magnetic resonance body composition analyzer (EchoMRI-900™, Houston, Texas) [20, 21]. Animals were weighed, placed in a small cylindrical plexiglass chamber within the body composition analyzer, and the measurement was taken over a 1-2 minute period.
Experimental design
One-month old rats were placed in hanging-wire bottom cages upon arrival to facilitate collecting spillage and obtaining accurate food intake measurements. Cumulative food intake was measured thrice weekly. Body weight and body composition were measured biweekly from 1-5.5 mo. and weekly thereafter. SPA was measured monthly. To determine if circadian caloric intake patterns differed between groups, food intake was measured once every four hours for 24-h at six, 12 and 18 mo. Ano-tail length was determined in isoflurane-anesthetized rats at 13.5 mo.
Statistical analyses
Data are expressed as mean ± SEM and an alpha level of .05 was used for all statistical tests.
Rationale for using mixed-multilevel regression models
The assumptions for repeated measures ANOVA are frequently violated in longitudinal designs and thus a repeated measures analysis would not be appropriate for this longitudinal study. Mixed-level regression modeling [22] permits the estimation of the effect of fixed and time-invariant (e.g. group) and time-varying (e.g. SPA and caloric intake) variables on longitudinal change in an outcome. Mixed models [23-26] have been used to demonstrate whether physical activity [5] or television viewing [27] were related to adiposity. The modeling approach is advantageous as it allows for missing data, subjects measured at different time intervals, the inclusion of both fixed and time-varying covariates, and the estimation of individual rates of change. Auto-correlated errors are incorporated into the model, thereby reducing error variance and increasing statistical power. Our dataset fit best with this modeling approach given that we include group and body composition as covariates, subject’s data prior to death remain in the dataset after the subject died, and the measurement interval varied for body composition measurements.
Longitudinal growth curve analysis
Mixed-multilevel regression models with unstructured error covariance matrices [22, 28-30] were fitted using data obtained from the same rats for 1-18 mo. to determine whether growth curves of body weight, FM and FFM differed between groups (obesity resistant and Sprague-Dawley), (SPSS version 17.1, Chicago, IL, USA). A series of models were considered, including unadjusted growth models and growth models adjusted for SPA and food intake. Unadjusted models assessed the effect of group on a given outcome over time (body weight, FM and FFM). Adjusted models assessed the effect of covariates and group on a given outcome over time. Separate models were run for each outcome for the following age intervals: 1-6, 6-12, 12-18 and 1-18 mo.
We first determined whether the trajectories of each outcome were significantly different between groups for each age interval (Unadjusted growth model – Model 1). The 1-6 and 1-18 mo. trajectories for body weight and FFM were curvilinear while FM at each age interval and body weight and FFM at the 6-12 and 12-18 mo. intervals were linear. Therefore, the quadratic models for body weight and FFM at 1-6 and 1-18 mo. include parameters for slope (rate of increase over time) and acceleration (change in the rate of increase over time) while linear models include only slope. Thus intercept, time, group, group × time were included in linear models and time2 and group × time2 were added to the curvilinear models. A separate unadjusted model was run for each outcome at each age interval. The slope and acceleration were considered to be significantly different between groups if the group × time and group × time2 interaction effects, respectively, were significant.
Next, we assessed whether SPA and food intake as time-varying predictor variables were significantly related to the trajectory of each outcome and determined whether the slope or acceleration differed between groups at each age interval (Covariate adjusted growth model – Model 2). To do so, all two-way and three-way interaction terms were included in the models. Three-way interaction terms were added to determine if group interacted with the predictor variable (e.g. SPA or food intake) and the slope or acceleration of each outcome over time. Therefore, a significant three-way interaction would indicate that either the slope or acceleration of the predictor variable and outcome was significantly different between groups at a specific age interval. Two-way interaction terms were added to determine if the predictor variable significantly interacted with either the slope or acceleration of each outcome over time. A significant two-way interaction and non-significant three-way interaction would therefore indicate that the predictor variable was related to the growth curves but not differentially across the groups.
Separate models were run with each of the following predictor variables: SPA, estimated monthly SPA, estimated cumulative SPA, cumulative monthly caloric intake and cumulative caloric intake. Estimated monthly SPA (SPA during the 24-h measurement period for a specific month multiplied by the number of days in that month) and estimated cumulative SPA (sum of estimated monthly SPA for a given age interval) were chosen as an approximation of accumulated SPA given that caloric intake was measured and therefore cumulative caloric intake was an actual measure. Last, since body length may be related to adiposity, body length was included as a covariate for each adjusted group comparison model for the 1-18 mo. interval.
Covariate adjusted growth model with additional baseline outcome adjustment (Model 3)
Model 1 indicated a significant variance in intercept for each outcome and age interval. Therefore, baseline body weight, FM or FFM was added as an additional covariate to each adjusted growth model to control for baseline differences in each outcome and models were re-analyzed.
Models excluding outcome data from the last month of life
We noted that weight loss preceded death for several rats. Therefore, we removed the last month of outcome data prior to death and analyzed Model 1 again to determine whether the trajectories were different between groups for each age interval with this “limited” dataset. These results were compared to Model 1, which included the whole dataset. Models 2 and 3 were re-analyzed with the limited dataset. Finally, we collapsed the outcome data across group for models where the group variable was not a significant predictor for an outcome and re-analyzed Models 1 and 3.
SPA, caloric intake, body composition, ano-tail length, energy efficiency and circadian-dependent caloric intake patterns
Data were also analyzed with Prism version 5.0b (GraphPad Software Inc., San Diego, CA, USA). Group differences were determined with t-tests for the following endpoints: monthly SPA; percent body fat and FFM at 1, 6, 12 and 18 mo.; change in FM and FFM; loss of FFM; ano-tail length; cumulative caloric intake and energy efficiency (ratios of body weight gain/caloric intake, FM gain/caloric intake, FFM gain/caloric intake and FM gain/body weight gain and FFM gain/body weight gain). Loss of FFM was defined as the absolute loss of FFM at 18 mo. from peak FFM and as a percentage of peak FFM (e.g. FFM at 18 mo. minus peak FFM / peak FFM). T-tests were performed on food intake during the following time periods: 24-h, light/dark cycle and 4-h at six, 12 and 18 mo.
Multiple linear regression
The effect of three predictors (fat mass at baseline, total caloric intake and estimated SPA) on the outcome fat mass gain over three age intervals (1-6, 1-12 and 1-18 mo.) was determined (SPSS version 17.1, Chicago, IL, USA). A model for each age interval was run separately and adjusted for baseline fat mass.
RESULTS
Mixed-multilevel regression models
Model 1 (Figure 1 and Table 1)
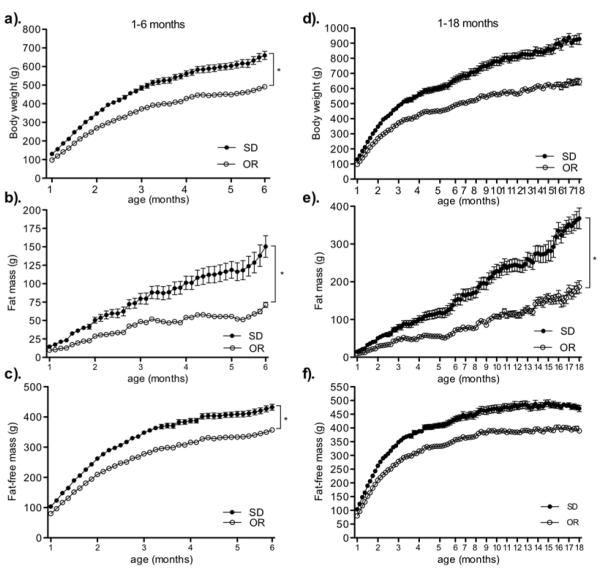
Figure 1.
Growth curves of body weight (a, d), fat mass (b, e), and fat-free mass (c, f) from 1-6 and 1-18 mo. among obesity resistant, (OR, open circles), and Sprague-Dawley (SD, filled circles) rats. * P < .0001 as compared to the slope for fat mass and the acceleration for body weight and fat-free mass of the growth curve for Sprague-Dawley rats. Data represent mean ± SE. N = 9-24 (OR) and 8-12 (SD). Please note different y-axes.
Table 1.
Model 1: Unadjusted growth model of body weight, fat mass, and fat-free mass trajectories in obesity resistant and Sprague-Dawley rats from 1 to 18 mo.
| Intercept | Group | Time | Time2 | Time * Group | Time2 * Group | |
|---|---|---|---|---|---|---|
| Body weight 1-18 mo. p-values | <.0001 | <.0001 | <.0001 | <.0001 | 0.174 | 0.335 |
| Coefficient | 178.65 | 76.05 | 19.47 | −0.24 | 2.68 | 0.04 |
| SE | 8.23 | 14.13 | 1.12 | 0.02 | 1.92 | 0.04 |
| t-ratio | 21.71 | 5.38 | 17.33 | −9.46 | 1.4 | 0.98 |
| Fat mass 1-18 mo. p-values | <.0001 | <.0001 | <.0001 | <.0001 | ||
| Coefficient | 18.9 | 21.18 | 2.26 | 2.13 | ||
| SE | 3.15 | 5.42 | 0.18 | 0.32 | ||
| t-ratio | 6 | 3.9 | 12.2 | 6.7 | ||
| Fat-free mass 1-18 mo. p-values | <.0001 | <.0001 | <.0001 | <.0001 | 0.76 | 0.17 |
| Coefficient | 150.3 | 59.74 | 12.83 | −0.17 | −0.37 | 0.03 |
| SE | 5.86 | 10.06 | 0.7 | 0.01 | 1.2 | 0.02 |
| t-ratio | 25.66 | 5.93 | 18.22 | −11.33 | −0.31 | 1.41 |
| Body weight 1-6 mo. p-values | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 |
| Coefficient | 87.87 | 28.58 | 37.62 | −0.88 | 10.84 | −0.2 |
| SE | 3.52 | 6.09 | 0.87 | 0.02 | 1.51 | 0.04 |
| t-ratio | 24.98 | 4.69 | 42.12 | −34.29 | 7.18 | −4.46 |
| Fat mass 1-6 mo. p-values | <.0001 | 0.156 | <.0001 | <.0001 | ||
| Coefficient | 14.27 | 2.53 | 2.64 | 3.5 | ||
| SE | 1 | 1.74 | 0.29 | 0.52 | ||
| t-ratio | 14.12 | 1.45 | 14.19 | 6.8 | ||
| Fat-free mass 1-6 mo. p-values | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 |
| Coefficient | 77.67 | 23.86 | 27.01 | −0.65 | 6.08 | −0.17 |
| SE | 2.76 | 4.77 | 0.54 | 0.17 | 0.93 | 0.3 |
| t-ratio | 28.17 | 5 | 50.1 | −38.35 | 6.52 | −5.64 |
| Body weight 6-12 mo. p-values | <.0001 | 0.001 | <.0001 | <.0001 | ||
| Coefficient | 450.29 | 115.94 | 2079 | 2.69 | ||
| SE | 17.91 | 29.76 | 0.38 | 0.61 | ||
| t-ratio | 25.14 | 3.9 | 7.4 | 4.38 | ||
| Fat mass 6-12 mo. p-values | 0.004 | 0.168 | 0.001 | 0.002 | ||
| Coefficient | 43.91 | 33.49 | 1.51 | 2.21 | ||
| SE | 14.23 | 23.65 | 0.39 | 0.64 | ||
| t-ratio | 3.08 | 1.42 | 3.89 | 3.46 | ||
| Fat-free mass 6-12 mo. p-values | <.0001 | <.0001 | <.0001 | 0.038 | ||
| Coefficient | 351.02 | 64 | 0.83 | 0.57 | ||
| SE | 9.08 | 15.16 | 0.16 | 0.26 | ||
| t-ratio | 38.64 | 4.22 | 5.14 | 2.91 | ||
| Body weight 12-18 mo. p-values | <.0001 | 0.108 | 0.628 | 0.626 | ||
| Coefficient | 650.26 | 190.99 | −0.68 | 1.02 | ||
| SE | 76.87 | 113.83 | 1.39 | 2.06 | ||
| t-ratio | 8.46 | 1.67 | −0.49 | 0.49 | ||
| Fat mass 12-18 mo. p-values | <.0001 | 0.002 | 0.72 | 0.138 | ||
| Coefficient | 402.87 | 148.59 | −0.16 | −1.01 | ||
| SE | 28.76 | 43.14 | 0.44 | 0.66 | ||
| t-ratio | 14.01 | 3.44 | −0.62 | −1.53 | ||
| Fat-free mass 12-18 mo. p-values | <.0001 | 0.002 | 0.72 | 0.138 | ||
| Coefficient | 402.87 | 148.59 | −0.16 | −1.01 | ||
| SE | 28.76 | 43.14 | 0.44 | 0.66 | ||
| t-ratio | 14.01 | 3.44 | −0.62 | −1.53 |
During the 1-18 mo. interval, the rate of FM gain was significantly less in obesity resistant rats compared to Sprague-Dawley rats. Body weight and FFM increased over time in both groups as indicated by the significant time and group effects; however, the acceleration of the body weight and FFM trajectories were not significantly different between groups. During the 1-6 and 6-12 mo. intervals, obesity resistant rats had a lower rate of FM gain and lower acceleration of body weight and FFM gain. In contrast, the slope and acceleration of each outcome during the 12-18 mo. interval were not significantly different between groups. The intercept was significant in each model at each time interval indicating body weight, FM and FFM was significantly different between groups at 1, 6, and 12 mo.
Model 2 and 3 (Figure 1, table 1 and supplementary table 1)
Based on the results from Model 1 showing group differences for trajectories, we determined whether predictor variables had a differential effect on trajectories between groups for each age interval. We report the results from Model 3 since the results from Model 2 were unchanged after controlling for baseline differences in each outcome (Model 3).
The FM growth curve was significantly different between groups from 1-18 mo. Model 3 indicated that after controlling for baseline differences in FM, greater SPA and estimated monthly SPA were associated with a significantly lower rate of FM gain in obesity resistant rats only. This suggests that greater SPA in obesity resistant rats dampened the rate of FM gain from 1-18 mo. The three-way interaction effect was not significant for cumulative monthly or total caloric intake; however, the two-way interaction effects were significant. This indicates that lower cumulative monthly and total caloric intake were associated with a lower rate of FM gain over time in both groups over the study duration.
During the 1-6 mo. interval, greater SPA was associated with a significantly lower rate of FM gain and a slower acceleration of body weight and FFM over time in obesity resistant rats. Estimated monthly SPA was also associated with a significantly slower acceleration of body weight and FFM gain in obesity resistant rats only and associated with a lower rate of FM gain in both groups during this age interval. Lower cumulative monthly caloric intake was associated with a significantly slower acceleration of body weight and FFM gain and a lower rate of FM gain in both groups during this age interval.
Despite group differences in the trajectories for each outcome during the 6-12 mo. interval, the two- and three-way interaction effects were not significant for body weight or FFM. In contrast, greater SPA in obesity resistant rats and lower cumulative caloric intake in both groups was associated with a significantly lower rate of FM gain during this time period. Body length was not associated with a lower rate of FM gain between groups from 1-18 mo. despite that ano-tail length was significantly less in obesity resistant rats (Table 2). Finally, we included body length as a covariate for each adjusted group comparison model at the 1-18 mo. interval. Controlling for body length did not change results in model 2 or 3 (data not shown).
Table 2.
Body composition, energy efficiency, caloric intake and ano-tail length.
| Obesity Resistant | Sprague-Dawley | |
|---|---|---|
| Fat mass(FM, %) | ||
| 1 mo. | 9.9 ± 0.2** | 11.18 ± 0.3 |
| 6 mo. | 14.5 ± 0.6δ | 22.7 ± 1.5 |
| 12 mo. | 19.51 ± 1.1δ | 29.90 ± 1.3 |
| 18 mo. | 28.55 ± 1.6 *** | 39.22 ± 1.6 |
| Fat-free mass (FFM, %) | ||
| 1 mo. | 82.15 ± 0.3δ | 79.67 ± 0.4 |
| 6 mo. | 72.70 ± 0.5δ | 65.94 ± 1.4 |
| 12 mo. | 68.54 ± 1.0δ | 59.39 ± 1.1 |
| 18 mo. | 67.78 ± 1.3 *** | 51.11 ± 1.4 |
| Loss of FFM (g) | −22.7 ± 4.5*** | −68.6 ± 15.5 |
| Loss of FFM (%) | −5.6 ± 1.2* | −13.0 ± 2.7 |
| Energy Efficiency (1-18 mo.) | ||
| FM gain / kcal intake | 0.0041 ± 0.0003δ | 0.0075 ± 0.0004 |
| FM gain / kcal intake | 0.0070 ± 0.0002 | 0.0076 ± 0.0003 |
| BW gain / kcal intake | 0.0125 ± 0.0004δ | 0.0167 ± 0.0006 |
| Caloric intake | ||
| 1-6 mo. | 13 003 ± 145δ | 15 170 ± 404 |
| 6-12 mo. | 14 483 ± 864* | 17 308 ± 429 |
| 12-18 mo. | 14 272 ± 548* | 16 287 ± 565 |
| 1-18 mo. | 42 970 ± 665*** | 48 817 ± 1,316 |
| Ano-tail lengthb (cm) | 27.1 ± 0.2*** | 28.4 ± 0.2 |
BW = body weight; kcal = caloric intake. Data represent mean ± SEM.
p < .05,
p < .005,
p < .0005, and
p < .0001 as compared to Sprague-Dawley rats.
N (obesity resistant and Sprague-Dawley) = 1 and 6 mo.: 24 and 12; 12 mo. 18 and 12; 18 mo.: 10 and 8; length: 16 and 12.
Models excluding outcome data from the last month of life
After the outcome data were limited, we determined whether growth curves differed between groups and compared these to Model 1, which included the entire dataset. Significant differences in trajectories shown in Model 1 remained significant with one exception. The group difference in the rate of FFM gain during the 6-12 mo. interval failed to reach statistical significance (p = .079) with the limited dataset. Next, Models 2 and 3 were re-analyzed with the limited dataset. The results with the limited dataset were similar with and without controlling for baseline differences in each outcome. Therefore we report results from Model 3 with the limited data set and compared those to Model 3 with the whole dataset. Results were similar with three exceptions. Use of the limited dataset resulted in a non-significant three-way interaction between estimated monthly SPA and the rate of FM gain during the 1-18 and 6-12 mo. intervals and a significant three-way interaction between estimated cumulative monthly caloric intake and the rate of FM gain during the 1-18 mo. interval in obesity resistant rats. Last, controlling for body length had no effect on the results with the limited dataset during the 1-18 mo. interval (data not shown).
Collapsing across groups
Model 1 indicated that the trajectories for each outcome during the 12-18 mo. interval were not different between groups. Therefore, we removed the “group” variable to test whether the outcome variables increased over time. Fat mass increased; however, body weight and FFM did not increase significantly during this time interval. We re-analyzed Model 3 to determine whether SPA and food intake were associated with the rate of FM gain during this time interval. Greater estimated monthly SPA, lower cumulative monthly and lower total caloric intake were associated with a significantly lower rate of FM gain.
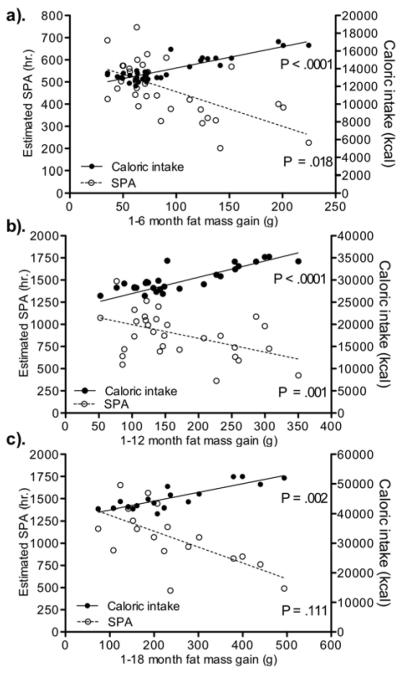
Multiple linear regression (Figure 2)
Figure 2.
Multiple linear regression to determine the effect of fat mass at baseline, estimated spontaneous physical activity (SPA) and caloric intake as predictors on fat mass gain from 1-6 (a), 1-12 (b) and 1-18 (c) months of age in obesity resistant and Sprague-Dawley rats. Data represent mean ± SE. N = 9-24 (obesity resistant), and 8-12 (Sprague-Dawley). Please note different y-axes.
Baseline fat mass was not a significant predictor of fat mass gain in the Models (1-6 months: p = .927, 1-12 months: p = .086 and 1-18 months: p = .745). Total caloric intake and estimated total SPA were significant predictors of fat mass gain during the 1-6 and 1-12 month age intervals. Total caloric intake but not estimated SPA was a significant predictor of fat mass gain during the 1-18 month age interval.
Spontaneous physical activity (Figure 3)
Figure 3.
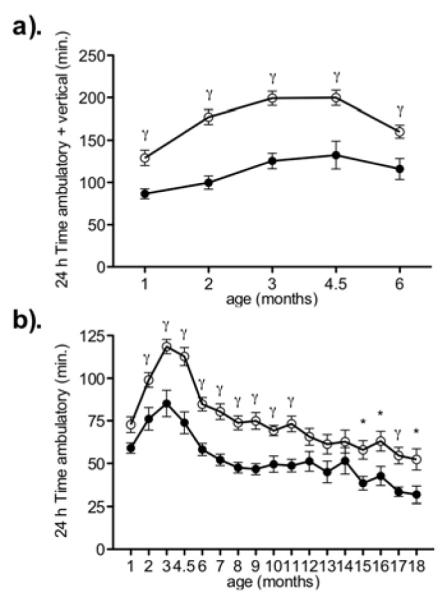
Time spent moving (sum of time ambulatory and time vertical) from 1-6 mo. (a) and time ambulatory from 1-18 mo. (b) in obesity resistant (open circles) and Sprague-Dawley (filled circles) rats. γ P < .05 and * P < .005 as compared to age-matched Sprague-Dawley rats. Data represent mean ± SE. N = 9-24 (obesity resistant), and 8-12 (Sprague-Dawley). Please note different y-axes.
Obesity resistant rats moved significantly more than Sprague-Dawley rats from 1-6 mo. (p < .004 for all comparisons excluding 1 month: p = .0572). Over the study duration, obesity resistant rats spent significantly more time ambulating than Sprague-Dawley rats at most measurements (2-11 mo. and 15-18mo.) excluding month 1, 12, 13, 14 and 18.
Body composition, loss of FFM, energy efficiency, cumulative caloric intake (table 2)
Obesity resistant rats had significantly less FM and more FFM at the beginning of each age interval and gained significantly less body mass (p = .0002; 516.2±21.6g and 758.0±50.6g), FM and FFM during the study (Figure 4). Adjusting for body mass revealed that obesity resistant rats gained more FFM and less FM for their body mass (p = .001 and p = .003, respectively). Obesity resistant rats lost significantly less FFM from the peak FFM, which suggests potentially greater sarcopenia in Sprague-Dawley rats compared to obesity resistant rats. Obesity resistant rats consumed significantly fewer calories during each age interval, were less energy efficient over the study duration and gained significantly less FM per calorie of intake.
Figure 4.
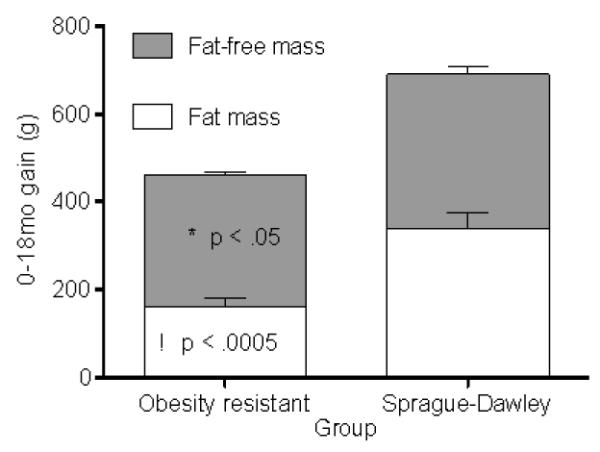
Fat mass (a) and fat-free mass gain (b) in obesity resistant and Sprague-Dawley rats over the 1-18 mo. interval. * P < .05 as compared to fat-free mass and γ P < .0005 as compared fat mass in Sprague-Dawley rats. Data represent mean ± SEM. N = 10 (obesity resistant) and 8 (Sprague-Dawley) rats.
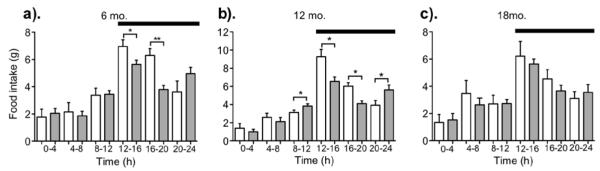
Circadian caloric intake patterns
Food intake was measured at four-hour periods over 24-h at six, 12 and 18 mo. (Figure 5). Circadian differences in caloric intake over four-hour intervals across the 24-h time period resulted in less 24-h food intake in obesity resistant rats at six but not 12 or 18 mo. Six-month old obesity resistant rats ate significantly less during the first and second four-hour periods after the onset of, and during the dark cycle but not during the light cycle. Twelve-month old obesity resistant rats had significantly greater food intake during the four-hour period before light and dark onset, while Sprague-Dawley rats had significantly greater food intake during the first and second four-hour period after dark onset. Cumulative food intake during the light and dark cycle were not significantly different between 12 mo. old rats or between 18 mo. old rats during the four-hour time periods, light/dark or 24-h time period.
Figure 5.
Food intake measured at four-hour intervals across the 24-h time period in obesity resistant (shaded bars) and Sprague-Dawley (open bars) rats at six (a), 12 (b), and 18 (c) mo. * P < .05 and ** P < .005 as compared to Sprague-Dawley rats. Black bar = dark cycle. Data represent mean ± SEM. N = 9-24 (obesity resistant) and 8-12 (Sprague-Dawley). Please note different y-axes.
DISCUSSION
We measured SPA, food intake and body composition from 1-18 mo., in rats with different propensities for obesity, to determine whether SPA levels were related to changes in body composition, and whether SPA early in life predicted and influenced adiposity gain later in life. The data are novel and show that elevated SPA was associated with a lower rate of FM gain during growth and maturation in obesity resistant rats and during aging in both groups. Second, SPA significantly predicts fat mass gain. Third, results were unchanged after controlling for body length or baseline differences in body weight, FM and FFM. Fourth, the correlation between SPA and the rate of FM gain was most influential during the 1-6 and 6-12 mo. intervals. Fifth, obesity resistant rats had greater SPA and gained significantly less fat and more FFM for their body mass over the study period. Finally, circadian patterns of caloric intake were significantly different between groups and patterns were age-dependent.
Obesity resistant rats had consistently greater SPA and a lower rate of FM gain despite that acceleration in body weight and FFM gains were not significantly different relative to Sprague-Dawley rats throughout the study duration. These data are consistent with and extend our earlier work showing that elevations in SPA at two months in obesity resistant rats are maintained through seven months of age [17]. The correlation between SPA and adiposity levels shown here is consistent with other rodent studies with mice [31] and rats [32] bred for high or low wheel running, mice lacking the melanocortin 4 receptor [15] and rats trained to voluntary wheel run [13, 14, 16].
The association between SPA and the reduced rate of FM gain shown here is consistent with data in children suggesting that high levels of physical activity dampen gains in BMI and adipose tissue [5] and that fewer hours of television viewing slows acceleration in BMI [27]. While our data are consistent with other longitudinal [9, 10, 33] and cross-sectional studies [34-39], which report a negative association between physical activity and various measures of adiposity, others report no association between physical activity and adiposity [40] or only an association between physical activity or aerobic fitness [11] and adiposity in normal weight children [12]. Inconsistent results appear to be due to methodological differences related to study design; objectivity of physical activity and adiposity measurements; age of study participants; intensity of physical activity and location of FM measurements.
We reasoned that greater SPA levels long-term would promote lower fat and body mass gain and greater FFM gain based on previous work [5, 10, 17, 18, 27] and studies showing that physical activity levels promotes maintenance of FFM [1, 2, 32]. We compared the ratio of change in FFM and body weight between groups to account for the proportional increase in FFM due to the physical demand of increasing body mass and loss of FFM in attempt to account for sarcopenia during aging. Obesity resistant rats gained less FM and more FFM for their body mass and were lost less FFM then Sprague-Dawley rats. Together these data suggest that elevated and sustained SPA may promote greater FFM gains during development and maturation and dampen loss of FFM during aging. Given that age-related reductions in FFM negatively impact functional mobility and health, our data underscores public health efforts to increase physical activity specifically among older persons.
We determined whether the effect of SPA on FM was age-dependent since physical activity declines with age in humans. Data suggests that the beneficial effect of increased physical activity among aged persons may be partial to improvements in functional capacity (skeletal muscle strength and increased mobility) versus reductions in body weight [41]. Elevated SPA was associated with a lower rate of FM gain during the 1-6, 6-12 and 1-18 mo. intervals in obesity resistant rats but there was no significant difference in the rate of FM gain between groups during the final interval (12-18 mo.). The ‘group’ variable was not significant during 12-18 mo. and so the outcome data were collapsed across groups to determine whether SPA was associated with FM gain over time among all rats. Greater monthly SPA was associated with a lower rate of FM gain during the 12-18 mo. interval. Multiple regression analysis indicated that greater SPA predicted lower fat mass gain from 1-12 but not from 1-18 mo. These data imply that elevated SPA early in life (1-12 mo.) most profoundly influences the rate of FM gain observed over the lifespan and absolute fat mass gain during the 1-12 mo interval. That elevated SPA was associated with a lower rate of FM gain but didn’t predict absolute fat mass gain over 1-18 mo is likely due to the decline in SPA during the 12-18 mo intervals and the high variability in 1-18 mo. estimated SPA. Obesity early in life increase risk for obesity later in life in humans [5, 27, 42] and obesity risk varies across developmental periods [43]. Therefore, the current data may have implications for public health efforts to increase physical activity across the lifespan and specifically early in life, to reduce risk for developing obesity during adulthood.
Our data show that the direct association between caloric intake and the rate of FM gain was similar between groups and that caloric intake predicts absolute fat mass gain despite different adiposity profiles. Circadian caloric intake patterns and meal structure differ between lean and obese rodents and age-related decline in food intake parallels increasing body weight in humans. Therefore, we determined whether circadian caloric intake patterns differed across age and between groups. Twenty-four hour food intake increased during development, peaked during maturation and declined during late adulthood (Figure 5); however, 24-h food intake was statistically different between groups at six months only. This is consistent with data in humans showing age-related reductions in caloric intake [44-46]. That food intake was similar between groups yet SPA was greater in obesity resistant rats at 18mo. further suggests that elevated SPA levels are beneficial to adiposity profiles during aging. Despite that 67% of the 24-h food intake was consumed during the dark cycle across groups and ages, which is consistent with previous reports [47], food intake during the four-hour time intervals was not consistently greater in Sprague-Dawley. Food intake during the first two intervals early in the dark cycle was greater at six and 12 mo. only, but obesity resistant rats had significantly greater food intake during the intervals before light and dark onset. This indicates that circadian caloric intake patterns were inconsistent across age and were group-dependent. It is unclear whether these age-dependent circadian caloric intake patterns contribute to the differential adiposity profiles observed. Previous studies show greater body weight in mice with abnormal circadian caloric intake patterns [48], large infrequent meals in obese rodents [49] and shifts in meal size and frequency after gastric bypass surgery [50] which is similar to caloric intake behaviors in humans [51-56]. Therefore, an analysis of meal microstructure including meal size and frequency with parallel adiposity profiles across age among obese and lean rats may be more informative.
We acknowledge limitations of our study. First, additional factors aside from those measured likely contributed to the lower rate of FM gain in obesity resistant rats. Factors in addition to SPA contribute to energy expenditure including resting metabolic rate, brown adipose tissue thermogenesis and the thermic effect of food. However, based on thermodynamics, an increase in physical activity requires an increase in energy expenditure. Second, the type of analysis used does not define a cause-effect relationship between elevated SPA and lower FM; instead, the results show a significant association between elevated SPA and a reduced FM gain, which supports studies demonstrating that physical activity significantly impacts body composition. Third, we acknowledge that some mixed model analyses, especially in small samples such as ours, can give inflated type I error rates and confidence intervals that are too narrow [57]. However, our data are consistent with other physical activity intervention-type studies and longitudinal studies in humans. Finally, we acknowledge that intervention studies are needed to validate our conclusions and demonstration of similar differences between obesity resistant and obesity prone rats fed on an obesogenic diet would lend further support to the conclusions.
In conclusion these studies demonstrate for the first time that group differences in SPA were associated with a differential rate of FM gain between obesity resistant and Sprague-Dawley rats and were not due to baseline differences in FM or body length. These data suggest that early and sustained elevations in SPA are associated with lower rate of FM gain and contributed to less FM gain over the study duration. The data also show that elevated SPA may preserve FFM through aging. Intervention studies are needed; however, to show a definitive causal relationship between sustained and elevated SPA and reduced FM gain across the lifespan. These findings have implications for human obesity and public health efforts to increase physical activity levels among young children and maintain such levels throughout maturation and aging to prevent obesity in adulthood.
Supplementary Material
ACKNOWLEDGEMENTS
Funding for this research was provided by the Department of Veterans Affairs, the National Institute of Health (NIDDK R01DK078985 to CMK) and the Minnesota Partnership for Biotechnology and Genomics.
Footnotes
CONFLICT OF INTEREST: The authors declare no conflict of interest.
REFERENCES
- 1.Westerterp KR, Speakman JR. Physical activity energy expenditure has not declined since the 1980s and matches energy expenditures of wild mammals. Int J Obes (Lond) 2008;32(8):1256–63. doi: 10.1038/ijo.2008.74. [DOI] [PubMed] [Google Scholar]
- 2.Monasta L, et al. Early-life determinants of overweight and obesity: a review of systematic reviews. Obes Rev. 2010 doi: 10.1111/j.1467-789X.2010.00735.x. [DOI] [PubMed] [Google Scholar]
- 3.Bleich SN, Ku R, Wang YC. Relative contribution of energy intake and energy expenditure to childhood obesity: a review of the literature and directions for future research. Int J Obes (Lond) 2010 doi: 10.1038/ijo.2010.252. [DOI] [PubMed] [Google Scholar]
- 4.Seo DC, Li K. Leisure-time physical activity dose-response effects on obesity among US adults: results from the 1999-2006 National Health and Nutrition Examination Survey. J Epidemiol Community Health. 2010;64(5):426–31. doi: 10.1136/jech.2009.089680. [DOI] [PubMed] [Google Scholar]
- 5.Moore LL, et al. Does early physical activity predict body fat change throughout childhood? Prev Med. 2003;37(1):10–7. doi: 10.1016/s0091-7435(03)00048-3. [DOI] [PubMed] [Google Scholar]
- 6.Proctor MH, et al. Television viewing and change in body fat from preschool to early adolescence: The Framingham Children’s Study. Int J Obes Relat Metab Disord. 2003;27(7):827–33. doi: 10.1038/sj.ijo.0802294. [DOI] [PubMed] [Google Scholar]
- 7.Gordon-Larsen P, et al. Fifteen-year longitudinal trends in walking patterns and their impact on weight change. Am J Clin Nutr. 2009;89(1):19–26. doi: 10.3945/ajcn.2008.26147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Westerterp KR, Plasqui G. Physically active lifestyle does not decrease the risk of fattening. PLoS ONE. 2009;4(3):e4745. doi: 10.1371/journal.pone.0004745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janz KF, Burns TL, Levy SM. Tracking of activity and sedentary behaviors in childhood: the Iowa Bone Development Study. Am J Prev Med. 2005;29(3):171–8. doi: 10.1016/j.amepre.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Janz KF, et al. Sustained effect of early physical activity on body fat mass in older children. Am J Prev Med. 2009;37(1):35–40. doi: 10.1016/j.amepre.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson MS, et al. Aerobic fitness, not energy expenditure, influences subsequent increase in adiposity in black and white children. Pediatrics. 2000;106(4):E50. doi: 10.1542/peds.106.4.e50. [DOI] [PubMed] [Google Scholar]
- 12.Stevens J, et al. Physical activity as a predictor of body composition in American Indian children. Obes Res. 2004;12(12):1974–80. doi: 10.1038/oby.2004.248. [DOI] [PubMed] [Google Scholar]
- 13.Levin BE, Dunn-Meynell AA. Chronic exercise lowers the defended body weight gain and adiposity in diet-induced obese rats. Am J Physiol Regul Integr Comp Physiol. 2004;286(4):R771–8. doi: 10.1152/ajpregu.00650.2003. [DOI] [PubMed] [Google Scholar]
- 14.Patterson CM, Dunn-Meynell AA, Levin BE. Three weeks of early-onset exercise prolongs obesity resistance in DIO rats after exercise cessation. Am J Physiol Regul Integr Comp Physiol. 2008;294(2):R290–301. doi: 10.1152/ajpregu.00661.2007. [DOI] [PubMed] [Google Scholar]
- 15.Haskell-Luevano C, et al. Voluntary exercise prevents the obese and diabetic metabolic syndrome of the melanocortin-4 receptor knockout mouse. Faseb J. 2009;23(2):642–55. doi: 10.1096/fj.08-109686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bi S, et al. Running wheel activity prevents hyperphagia and obesity in Otsuka long-evans Tokushima Fatty rats: role of hypothalamic signaling. Endocrinology. 2005;146(4):1676–85. doi: 10.1210/en.2004-1441. [DOI] [PubMed] [Google Scholar]
- 17.Teske JA, et al. Elevated hypothalamic orexin signaling, sensitivity to orexin A, and spontaneous physical activity in obesity-resistant rats. Am J Physiol Regul Integr Comp Physiol. 2006;291(4):R889–99. doi: 10.1152/ajpregu.00536.2005. [DOI] [PubMed] [Google Scholar]
- 18.Kotz CM, Teske JA, Billington CJ. Neuroregulation of nonexercise activity thermogenesis and obesity resistance. Am J Physiol Regul Integr Comp Physiol. 2008;294(3):R699–710. doi: 10.1152/ajpregu.00095.2007. [DOI] [PubMed] [Google Scholar]
- 19.Thorpe AJ, Kotz CM. Orexin A in the nucleus accumbens stimulates feeding and locomotor activity. Brain Res. 2005;1050(1-2):156–62. doi: 10.1016/j.brainres.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 20.Taicher GZ, et al. Quantitative magnetic resonance (QMR) method for bone and whole-body-composition analysis. Anal Bioanal Chem. 2003;377(6):990–1002. doi: 10.1007/s00216-003-2224-3. [DOI] [PubMed] [Google Scholar]
- 21.Nixon JP, et al. Evaluation of a Quantitative Magnetic Resonance Imaging System for Whole Body Composition Analysis in Rodents. Obesity (Silver Spring) 2010 doi: 10.1038/oby.2009.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singer J, Willett J. Applied longitudinal data analysis: modeling change and event occurrence. Oxford Press; New York: 2003. [Google Scholar]
- 23.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963–74. [PubMed] [Google Scholar]
- 24.Suppes T, et al. Clinical outcome in a randomized 1-year trial of clozapine versus treatment as usual for patients with treatment-resistant illness and a history of mania. Am J Psychiatry. 1999;156(8):1164–9. doi: 10.1176/ajp.156.8.1164. [DOI] [PubMed] [Google Scholar]
- 25.Bryk AS, Raudenbush SW. Toward a more appropriate conceptualiatio of research on school effects: a three-level hierarchical lineral model. Am J Educ. 1987;97(1):65–108. [Google Scholar]
- 26.DeLucia C, Pitts SC. Applications of individual growth curve modeling for pediatric psychology research. J Pediatr Psychol. 2006;31(10):1002–23. doi: 10.1093/jpepsy/jsj074. [DOI] [PubMed] [Google Scholar]
- 27.Danner FW. A national longitudinal study of the association between hours of TV viewing and the trajectory of BMI growth among US children. J Pediatr Psychol. 2008;33(10):1100–7. doi: 10.1093/jpepsy/jsn034. [DOI] [PubMed] [Google Scholar]
- 28.Littell RC, et al. SAS system for mixed models. SAS Institute; 1996. [Google Scholar]
- 29.Kincaid C. Guidelines for selecting the covariance structure in mixed model analysis. Proceedings of the Thirtieth Annual SAS Users Group International Conference; Cary, NC: SAS Institute Inc.; 2005. [Google Scholar]
- 30.Keselman HJ, et al. A comparison of two approaches for selecting covariance structures in the analysis of repeated measurements. 1998 Available from: http://home.cc.umanitoba.ca/~kesel/cis1998.pdf.
- 31.Nehrenberg DL, et al. Voluntary exercise and its effects on body composition depend on genetic selection history. Obesity (Silver Spring) 2009;17(7):1402–9. doi: 10.1038/oby.2009.51. [DOI] [PubMed] [Google Scholar]
- 32.Swallow JG, et al. Phenotypic and evolutionary plasticity of body composition in rats selectively bred for high endurance capacity. J Appl Physiol. 2010;109(3):778–85. doi: 10.1152/japplphysiol.01026.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riddoch CJ, et al. Prospective associations between objective measures of physical activity and fat mass in 12-14 year old children: the Avon Longitudinal Study of Parents and Children (ALSPAC) BMJ. 2009;339:b4544. doi: 10.1136/bmj.b4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roemmich JN, et al. Pubertal alterations in growth and body composition. V. Energy expenditure, adiposity, and fat distribution. Am J Physiol Endocrinol Metab. 2000;279(6):E1426–36. doi: 10.1152/ajpendo.2000.279.6.E1426. [DOI] [PubMed] [Google Scholar]
- 35.Abbott RA, Davies PS. Habitual physical activity and physical activity intensity: their relation to body composition in 5.0-10.5-y-old children. Eur J Clin Nutr. 2004;58(2):285–91. doi: 10.1038/sj.ejcn.1601780. [DOI] [PubMed] [Google Scholar]
- 36.Dencker M, et al. Daily physical activity related to body fat in children aged 8-11 years. J Pediatr. 2006;149(1):38–42. doi: 10.1016/j.jpeds.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Ness AR, et al. Objectively measured physical activity and fat mass in a large cohort of children. PLoS Med. 2007;4(3):e97. doi: 10.1371/journal.pmed.0040097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ortega FB, Ruiz JR, Sjostrom M. Physical activity, overweight and central adiposity in Swedish children and adolescents: the European Youth Heart Study. Int J Behav Nutr Phys Act. 2007;4:61. doi: 10.1186/1479-5868-4-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saelens BE, et al. Visceral abdominal fat is correlated with whole-body fat and physical activity among 8-y-old children at risk of obesity. Am J Clin Nutr. 2007;85(1):46–53. doi: 10.1093/ajcn/85.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berentzen T, et al. Physical activity in leisure-time is not associated with 10-year changes in waist circumference. Scand J Med Sci Sports. 2008;18(6):719–27. doi: 10.1111/j.1600-0838.2007.00761.x. [DOI] [PubMed] [Google Scholar]
- 41.Westerterp KR. Physical activity as determinant of daily energy expenditure. Physiol Behav. 2008;93(4-5):1039–43. doi: 10.1016/j.physbeh.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 42.Freedman DS, et al. The relation of childhood BMI to adult adiposity: the Bogalusa Heart Study. Pediatrics. 2005;115(1):22–7. doi: 10.1542/peds.2004-0220. [DOI] [PubMed] [Google Scholar]
- 43.Dietz WH. Periods of risk in childhood for the development of adult obesity--what do we need to learn? J Nutr. 1997;127(9):1884S–1886S. doi: 10.1093/jn/127.9.1884S. [DOI] [PubMed] [Google Scholar]
- 44.Morley JE. Anorexia in older persons: epidemiology and optimal treatment. Drugs Aging. 1996;8(2):134–55. doi: 10.2165/00002512-199608020-00007. [DOI] [PubMed] [Google Scholar]
- 45.Blanton CA, et al. Meal patterns associated with the age-related decline in food intake in the Fischer 344 rat. Am J Physiol. 1998;275(5 Pt 2):R1494–502. doi: 10.1152/ajpregu.1998.275.5.R1494. [DOI] [PubMed] [Google Scholar]
- 46.Horwitz BA, Blanton CA, McDonald RB. Physiologic determinants of the anorexia of aging: insights from animal studies. Annu Rev Nutr. 2002;22:417–38. doi: 10.1146/annurev.nutr.22.120301.071049. [DOI] [PubMed] [Google Scholar]
- 47.Rosenwasser AM, Boulos Z, Terman M. Circadian organization of food intake and meal patterns in the rat. Physiol Behav. 1981;27(1):33–9. doi: 10.1016/0031-9384(81)90296-1. [DOI] [PubMed] [Google Scholar]
- 48.Fonken LK, et al. Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci U S A. 2010;107(43):18664–9. doi: 10.1073/pnas.1008734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marco A, Schroeder M, Weller A. Microstructural pattern of palatable food intake from weaning to adulthood in male and female OLETF rats. Behav Neurosci. 2009;123(6):1251–60. doi: 10.1037/a0017740. [DOI] [PubMed] [Google Scholar]
- 50.Zheng H, et al. Meal patterns, satiety, and food choice in a rat model of Roux-en-Y gastric bypass surgery. Am J Physiol Regul Integr Comp Physiol. 2009;297(5):R1273–82. doi: 10.1152/ajpregu.00343.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Larson NI, Neumark-Sztainer D, Story M. Weight control behaviors and dietary intake among adolescents and young adults: longitudinal findings from Project EAT. J Am Diet Assoc. 2009;109(11):1869–77. doi: 10.1016/j.jada.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 52.Koletzko B, Toschke AM. Meal patterns and frequencies: do they affect body weight in children and adolescents? Crit Rev Food Sci Nutr. 2010;50(2):100–5. doi: 10.1080/10408390903467431. [DOI] [PubMed] [Google Scholar]
- 53.Flegal KM, et al. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298(17):2028–37. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 54.Finkelstein EA, et al. Individual and aggregate years-of-life-lost associated with overweight and obesity. Obesity (Silver Spring) 2010;18(2):333–9. doi: 10.1038/oby.2009.253. [DOI] [PubMed] [Google Scholar]
- 55.Ludwig DS. Childhood obesity--the shape of things to come. N Engl J Med. 2007;357(23):2325–7. doi: 10.1056/NEJMp0706538. [DOI] [PubMed] [Google Scholar]
- 56.van Dam RM, et al. The relationship between overweight in adolescence and premature death in women. Ann Intern Med. 2006;145(2):91–7. doi: 10.7326/0003-4819-145-2-200607180-00006. [DOI] [PubMed] [Google Scholar]
- 57.Cheng J, et al. Real longitudinal data analysis for real people: building a good enough mixed model. Stat Med. 2010;29(4):504–20. doi: 10.1002/sim.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.