Abstract
Superoxide (O2•−) is implicated in inflammatory states including arteriosclerosis and ischemia-reperfusion injury. Cobalamin (Cbl) supplementation is beneficial for treating many inflammatory diseases and also provides protection in oxidative-stress-associated pathologies. Reduced Cbl reacts with O2•− at rates approaching that of superoxide dismutase (SOD), suggesting a plausible mechanism for its anti-inflammatory properties. Elevated homocysteine (Hcy) is an independent risk factor for vascular disease and endothelial dysfunction. Hcy increases O2•− levels in human aortic endothelial cells (HAEC). Here, we explore protective effects of Cbl in HAEC exposed to various O2•− sources, including increased Hcy levels. Hcy increased O2•− levels (1.6-fold) in HAEC, concomitant with a 20% reduction in cell viability and a 1.5-fold increase in apoptotic death. Pre-treatment of HAEC with physiologically relevant concentrations of cyanocobalamin (CNCbl) (10 – 50 nM) prevented Hcy-induced increases in O2•− and cell death. CNCbl inhibited both Hcy and rotenone- induced mitochondrial O2•− production. Similarly, HAEC challenged with paraquat showed a 1.5-fold increase in O2•− levels and a 30% decrease in cell viability, both of which were prevented with CNCbl (10 nM) pre-treatment. CNCbl also attenuated elevated O2•− levels following exposure of cells to a Cu/Zn-SOD inhibitor. Our data suggest that Cbl acts as an efficient intracellular O2•− scavenger.
Keywords: Superoxide, Homocysteine, Cobalamin, Apoptosis, Oxidative stress, Vascular Endothelium, Antioxidant
Introduction
Cobalamins (Cbls, vitamin B12 derivatives) are micronutrients essential for the synthesis of methylcobalamin (MeCbl) and adenosylcobalamin (AdoCbl), the respective cofactors for cytosolic methionine synthase (MS) and mitochondrial L-methylmalonyl-CoA mutase. Reduced Cbl cofactors in these catalytic cycles are sensitive to oxidation [1, 2]; hence, both MS and L-methylmalonyl-CoA mutase are inactivated by reactive oxygen species (ROS). MS plays a key role in the metabolism of homocysteine (L-Hcy, Hcy), a byproduct of methylation reactions which is metabolized to cysteine or methionine. In endothelial cells, Hcy metabolism depends exclusively on Cbl-dependent MS [3]. Impaired MS activity due to Cbl deficiency results in elevated Hcy, a risk factor for cardiovascular disease [4]. Hcy also elevates O2•− levels, leading to increased oxidative stress, which further hinders Cbl’s metabolism. Importantly, Cbl exhibits antioxidant effects in Hcy-independent systems [5, 6]. Birch et al reported that Cbl protects against hydrogen peroxide-induced oxidative stress [5], and our preliminary experiments demonstrate the potential of Cbl to attenuate elevated O2•− levels [6].
Cbl deficiency is a common and significant public health problem, particularly amongst the elderly [4]. Up to 40% of the elderly US population are B12-deficient [7]. Following folate fortification in food, Cbl deficiency became the primary modifiable cause of hyperhomocysteinemia [8]. Vitamin supplements containing cyanocobalamin (CNCbl, vitamin B12) decrease LDL oxidation in both healthy individuals and patients with coronary artery disease [9]. Cbl supplementation is also beneficial in treating many inflammatory diseases, and there is accumulating evidence that Cbl can protect against oxidative stress-associated pathologies [10-16]. Levels of the Cbl transport protein transcobalamin increase during inflammation [17, 18], concomitant with NF-κB activation induced by various stimuli, including ROS. Taken together, these studies suggest a potential role for Cbl in the regulation of inflammatory processes [15, 19].
Recently we demonstrated that the reduced form of Cbl, cob(II)alamin (Cbl(II)) reacts with O2•− with a second-order rate constant of 7 × 108 M−1.s−1, close to that observed for superoxide dismutase itself (Cu,Zn-SOD; k = 2 × 109 M−1.s−1) [6]. Given that Cbl(II) is a major intracellular Cbl form [2], we hypothesized that scavenging of O2•− by Cbl is an important mechanism by which Cbl can protect cells against oxidative stress.
O2•− is a free radical product of a 1 electron reduction of oxygen. It is produced by mitochondrial and reticular membrane electron transport systems, or enzymes including NADPH oxidase (NOX) and xanthine oxidase (XO), and uncoupled nitric oxide synthase (NOS)[20]. Exposing cells to Hcy also results in elevated levels of O2•− [21, 22]. O2•− is involved in both physiological and pathological processes [23], with O2•− overproduction implicated in a range of inflammatory states such as rheumatoid arthritis, osteoarthritis, arteriosclerosis, and ischemia-reperfusion [24]. The toxicity of increased O2•− levels is evident in homozygous SOD2 knockout mice, which die within the first three weeks of age [25]. O2•− can inactivate a range of enzymes in addition to causing direct molecular damage by initiating lipoperoxidation, leading to the destruction of neurotransmitters and hormones, and DNA single strand damage [20]. Moreover, O2•− can generate peroxynitrite via its reaction with nitric oxide and hydrogen peroxide via dismutation. From these species, stronger damaging oxidants can be generated such as the carbonate, hydroxyl and nitrogen dioxide radicals and oxoferryl complexes [26]. Therefore a tight control of O2•− levels is paramount to prevent the formation of secondary oxidants.
In this work we describe the protective effects of physiologically relevant concentrations of CNCbl against elevated intracellular O2•− levels induced by paraquat and L-Hcy and the associated cell injury in primary human aortic endothelial cells (HAEC). Both paraquat and L-Hcy induced elevated O2•− levels which paralleled cell death and which were prevented by pre-treating the cells with CNCbl prior to the insult. Elevated O2•− levels were also observed in cells treated with the Cu/Zn-SOD inhibitor diethyldithiocarbamate (DETC) [27], and were similarly attenuated by CNCbl pretreatment.
Materials and Methods
Synthesis of L- and D-Hcy
L- or D-Homocysteine thiolactone (20 mg, 130 μmol) was dissolved in NaOH (5 N, 200 μl) and incubated at 37 °C for 10 min. The solution was chilled and neutralized with HCl (5 N, 200 μl). PBS was added to a total volume of 1 ml and the solution bubbled with N2 for 10 min [28]. The yield was typically > 95%, determined by quantifying the reduced thiol groups by the Ellman’s assay [29].
Cell culture
Primary HAEC clones were a generous gift from Donald W. Jacobsen (Lerner Research Institute, The Cleveland Clinic). Each endothelial cell isolate was stored and passaged separately. HAEC were cultured in fibronectin coated flasks in M199 supplemented with LONZA bullet kit supplements for EBM-2 in a humidified 95% air, 5% CO2 incubator at 37 °C. For experiments cells were seeded onto 96- or 6-well plates at a density of 12000-20000 cells/cm2 and used up to passage 6.
Intracellular Cbl content and Cbl uptake
For Cbl uptake experiments, pre-confluent HAEC were incubated with 0.2 nM 57(Co)-CNCbl. Adherent cells were harvested at different time points up to 24 h and thoroughly washed with PBS. The intracellular Cbl uptake was determined by counting the cell-associated radioactivity. To measure total intracellular Cbl content, pre-confluent HAEC were incubated with or without varying concentrations of CNCbl for 24 h, and intracellular Cbl content was determined by the SimulTRAC radioassay.
Assessing ROS production
Cells with or without CNCbl pre-treatment (500 pM - 10 μM) were incubated with L-Hcy (100 or 150 μM), H2O2 (50 - 200 μM), paraquat (1.5 mM), rotenone (5 μM), or with culture medium alone. To assess general ROS production, cells were incubated with the oxidation sensitive fluorescent probe dichlorofluorescein acetate (DCFA, 3 μM) for the duration of the L-Hcy or H2O2 treatment. For the assessment of O2•− production, cells were incubated with the oxidative fluorescent probes, dihydroethidium DHE (5 μM), or MitoSOX (5 μM) for 1 h subsequent to the L-Hcy, paraquat, or rotenone treatment. Fluorescence was quantified in a microplate reader (DCF: λex/em = 420/520; 2-hydroxyethidium: λex/em = 510/605 nm; MitoSOX: λex/em = 510/580 nm).
Cell viability
Cell viability was assessed with trypan blue staining or with the MTT assay. For the MTT assay cells were incubated with thiazolyl blue tetrazolium (0.4 mg/ml) in M199 for 3 h at 37°C. Mitochondrial-dependent tetrazolium reduction to formazan was measured by reading optical density at 540 nm.
Cbl protection against O2•−-generating insults- L-Hcy, paraquat or rotenone
Pre-confluent HAEC were incubated for 24 h in the absence or presence of CNCbl (500 pM – 10 μM) prior to adding L-Hcy (100 or 150 μM), paraquat (1.5 mM), or rotenone (5 μM). For some experiments SOD (3 μM) was added at the time of paraquat addition or apocynin (0.1 mM) was added 30 min before adding L-Hcy or paraquat. Cells were incubated for 24 h with L-Hcy, H2O2, or paraquat, or for 1 h with rotenone. ROS and cell viability were assessed as described above.
Diethyldithiocarbamate (DETC)
Pre-confluent HAEC were incubated for 24 h in the absence or presence of CNCbl, then subjected to 10 mM DETC for 2 hr. This concentration has been previously shown to inhibit Cu/ZnSOD activity by up to 50% and to increase vascular O2•− [30].
DNA and Cbl quantification
Confluent cells were harvested in lysis buffer (50 mM Tris pH 7.4, 0.5 % Triton X-100) and DNA quantified using the CyQuant cell proliferation kit (Roche). Cbl was quantified using the SimulTRAC Radioassay for vitamin B12 and folate by MP Biomedicals (Orangeburg, NY) according to the manufacturer’s specifications.
Apoptosis measurement
To detect apoptotic cell death, cells were seeded onto 6-well plates and pre-treated with or without CNCbl (10 – 100 nM) for 24 h. Cells were washed then incubated in the absence or presence of L-Hcy for 18 h. Apoptosis was assessed using the Cell Death Detection ELISA (Roche) according to the manufacturer’s specifications.
General solution preparation
Thiol solutions were prepared immediately before use and the concentrations were determined by the Ellman’s method [29]. A fresh solution of H2O2 was prepared before experiments and the concentration determined spectrophotometrically (ε240nm = 43.6 M−1cm−1 [31]). The concentration of the stock solution of CNCbl was determined by the dicyanocobalamin test (ε368nm=30.4 mM−1 cm−1 [32]).
Statistics
All experiments were carried out using at least three separate cell clones. Results are expressed as mean ± SEM. Statistical comparisons were carried out using ANOVA with the Bonferroni post hoc test.
Online Supplemental Material
Figures S1-S4 are included as supplemental figures.
Results
Cbl protects against elevated O2•− levels induced by Hcy exposure
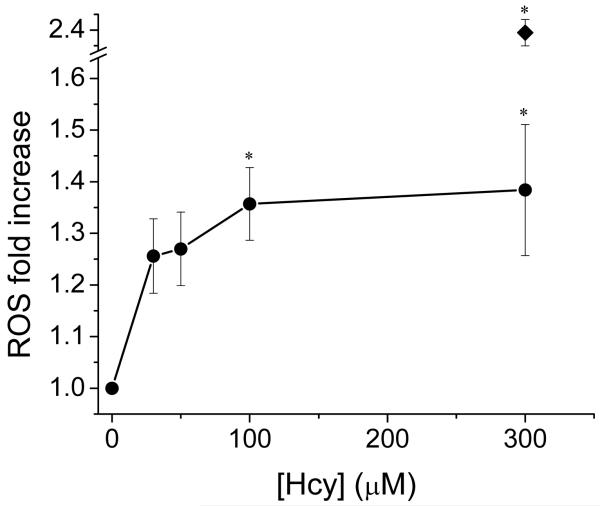
Treatment of HAEC with varying concentrations of L-Hcy induced a concentration-dependent increase in ROS detected by increasing dichlorofluorescein (DCF) fluorescence, a general probe for ROS. L-Hcy (100 μM) elicited a significant increase in ROS compared to control (Figure 1). To verify that the ROS increase is a L-Hcy-specific effect, HAEC were incubated for 48 h with 0.1 mM of a range of thiols (glutathione, L-cysteine, D-Hcy, L-Hcy, and β-mercaptoethanol (βME)). Only L-Hcy elicited a significant increase in DCF fluorescence (Figure S1A), concomitant with a significant decrease in cell viability as measured using the MTT assay (Figure S1B).
Figure 1. L-Hcy-induced ROS increase.
Pre-confluent HAEC were incubated with increasing concentrations of L-Hcy in the presence of 3 μM DCFA. After 48 h ROS production was assessed fluorometrically. *p < 0.05 compared to control cells not exposed to L-Hcy. Positive control exposed to 200 μM H2O2 (◆). Data are expressed as mean ± SEM; N = 3.
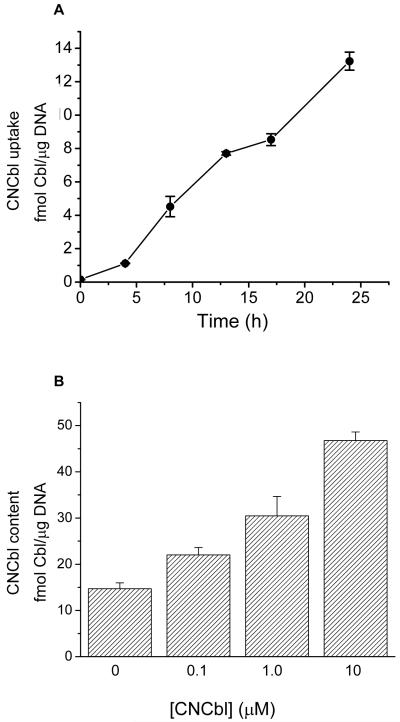
Prior to determining the effect of vitamin B12 (CNCbl) on L-Hcy-dependent ROS levels, the concentration- and time-dependent uptake of CNCbl by HAEC was assessed. Incubating HAEC with 57Co-labeled CNCbl (0.2 nM) induced a time dependent increase in intracellular CNCbl (Figure 2A). Increasing the CNCbl in the medium (0.1 – 10 μM non-labeled Cbl) lead to higher intracellular Cbl levels after 24 h (Figure 2B). A 24 h incubation time with CNCbl was selected as an appropriate time for all subsequent experiments.
Figure 2. Effect of exogenous CNCbl on intracellular Cbl content.
A. Pre-confluent HAEC were incubated with 0.2 nM 57(Co)-CNCbl for up to 24 h, then harvested at different time points. The intracellular Cbl uptake was determined by counting the cell-associated radioactivity. Data represents mean ± SEM; N = 3. B. Pre-confluent HAEC were incubated with or without varying concentrations of CNCbl for 24 h, and intracellular Cbl content was determined by the SimulTRAC radioassay. Data represents mean ± SEM; N = 3.
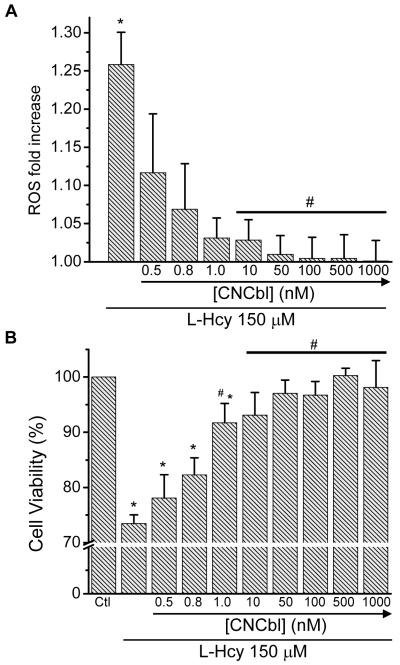
Exposing HAEC to L-Hcy (150 μM) over 48 h induced a 1.25-fold increase in DCF fluorescence (Figure 3A) that correlated with a ~ 25% decrease in cell viability (Figure 3B).To assess the effects of CNCbl on the L-Hcy-dependent ROS production and the L-Hcy-dependent decrease in cell viability, HAEC were pre-incubated with increasing concentrations of CNCbl for 24 h prior to treating the cells with L-Hcy (150 μM). To ensure that extracellular Cbl was not responsible for Cbl effects on ROS, cells were washed after Cbl treatment and medium replaced prior to further treatments. Pre-incubation of HAEC with CNCbl prevented the L-Hcy-dependent increase in ROS and decrease in cell viability in a concentration-dependent fashion (Figure 3). 10 nM CNCbl completely inhibited both the L-Hcy-dependent ROS increase (p < 0.05) and the L-Hcy-dependent decrease in cell viability ( p < 0.05). L-Hcy (150 μM) treatment of cells over a 24 h period resulted in a ~20% decrease in cell viability; hence, subsequent experiments were conducted using a 24 h L-Hcy treatment protocol, unless otherwise stated.
Figure 3. Cbl protection against L-Hcy-induced oxidative stress.
Pre-confluent HAEC were incubated with increasing concentrations of CNCbl for 24 h prior to the addition of 150 μM L-Hcy. ROS was measured by detecting oxidation of 3 μM DCFA. A. ROS fold-increase after 48 h. B. Cell viability after 48 h as measured with MTT assay. Data are expressed as mean ± SEM; N = 7; *p<0.05 with respect to control cells not exposed to L-Hcy; #p<0.05 with respect to L-Hcy-treated cells not exposed to CNCbl
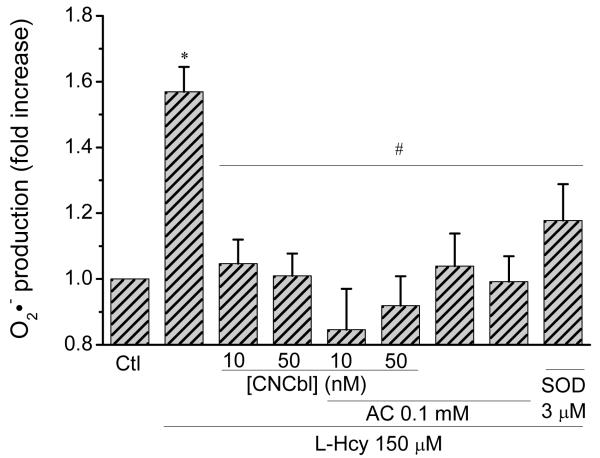
Exposing cells to L-Hcy is reported to increase intracellular O2•−levels [21, 22]. To determine if O2•− is indeed generated in our system, O2•− levels following exposure to L-Hcy were assessed using the O2•− specific probe hydroethidine (DHE), which upon reacting with O2•− yields the fluorescent product 2-hydroxyethidium. Incubation of HAEC with L-Hcy (150 μM) for 24 h induced a 1.6-fold increase in hydroxyethidium fluorescence (Figures 4 and S2). This increase was completely inhibited by pre-incubation of the cells with 10 nM CNCbl (p < 0.05), the antioxidant apocynin (0.1 mM), or SOD itself (3 μM) (Figures 4, S2 and S3).
Figure 4. Cbl protection against L-Hcy induced superoxide production.
Pre-confluent HAEC were incubated in the absence or in the presence of 10 or 50 nM CNCbl for 24 h. Cells were washed with PBS, prior to incubation with 150 μM L-Hcy with or without 0.1 mM apocynin or 3 μM SOD. Hydroxyethidium fluorescence was measured with a fluorescent plate reader after 24 hr. * p < 0.05 with respect to untreated HAEC; # p < 0.05 with respect to L-Hcy-treated cells. Data are expressed as mean ± SEM; N = 6 except for the SOD experiment where N = 3.
Cbl protects against elevated mitochondrial O2•− levels
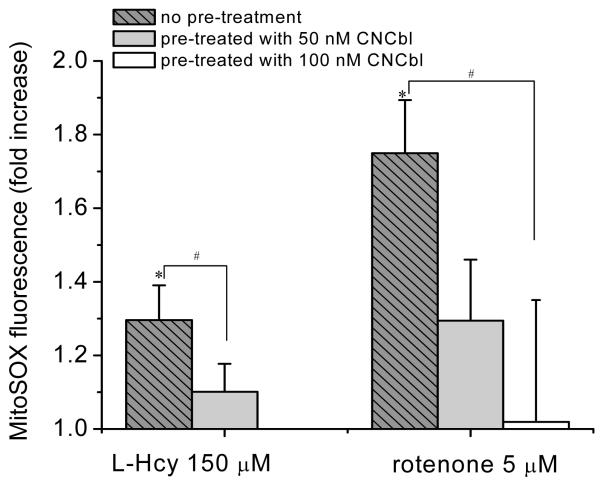
To investigate the subcellular localization of the L-Hcy-induced increase in O2•−, cells were assayed with mitoSOX, a mitochondrial specific O2•− probe. Incubation of HAEC with L-Hcy (150 μM) for 24 h elicited a moderate but significant increase in mitoSOX fluorescence, which was completely inhibited by preincubation of the cells with CNCbl (50 nM) (Figure 5). Moreover, treatment of HAEC with the mitochondrial electron transport chain inhibitor rotenone (5 μM) induced an increase in mitochondrial O2•− which was also significantly inhibited by pre-incubation with CNCbl (100 nM) (Figures 5 and S4).
Figure 5. Subcellular localization of Hcy-induced oxidative stress.
HAEC were incubated in the absence or in the presence of CNCbl (50 nM or 100 nM) for 24 h. HAEC were washed and exposed to L-Hcy (150 μM, 24 h) or rotenone (5 μM; 1 h). Mitochondrial O2•− was detected by quantifying mitoSOX fluorescence in a microplate reader (λex/em = 510/580 nm). Data are expressed as mean ± SEM; N ≥ 4; * p < 0.05 compared to control, # p < 0.05.
Cbl protects against elevated O2•− levels induced by paraquat
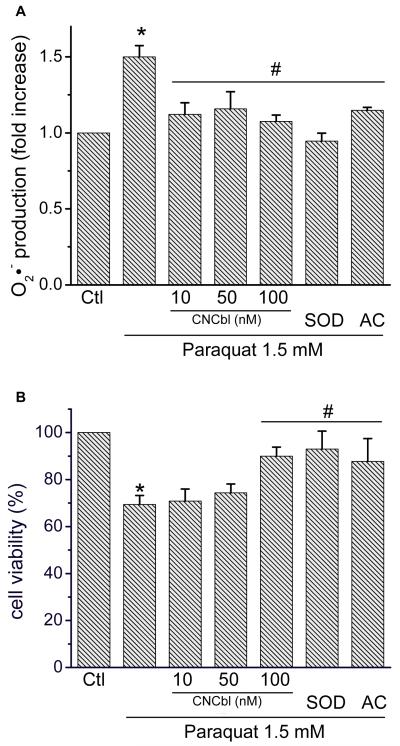
To assess the ability of CNCbl to protect against a direct source of O2•−, HAEC were exposed to paraquat (1.5 mM, 24 h), a well established O2•− source [33]. Paraquat induced a 1.5-fold increase in O2•− production measured by hydroxyethidium fluorescence, which was prevented by CNCbl pre-treatment (10 nM), apocynin (0.1 mM), and SOD (3 μM) (Figure 6A). The paraquat-induced increase in oxidative stress correlated with a 30% decrease in cell viability (Figure 6B). Pre-incubation with CNCbl for 24 h, treatment with apocynin (0.1 mM), or SOD (3 μM) protected HAEC against the O2•−-dependent decrease in cell viability (Figure 6B).
Figure 6. Effect of Cbl on paraquat-induced O2•− levels and cell death.
HAEC were incubated in the absence or in the presence of varying concentrations of CNCbl for 24 h prior to subjection to paraquat (1.5 mM). DHE (5 μM) was added for the final 1 h of treatment. Also shown are the effects of SOD (3 μM) and apocynin (AC, 0.1 mM) on the paraquat-induced increase in O2•− and cell death. A. O2•− measured as hydroxyethidium fluorescence (λex/em = 520/605 nm) compared to untreated cells. B. Cell viability was assessed with the MTT assay. Data expressed as ± SEM; N = 3; * p < 0.05 compared to control, # p < 0.05 compared to paraquat alone.
Cbl attenuates elevated O2•− levels resulting from Cu/Zn-SOD inhibition
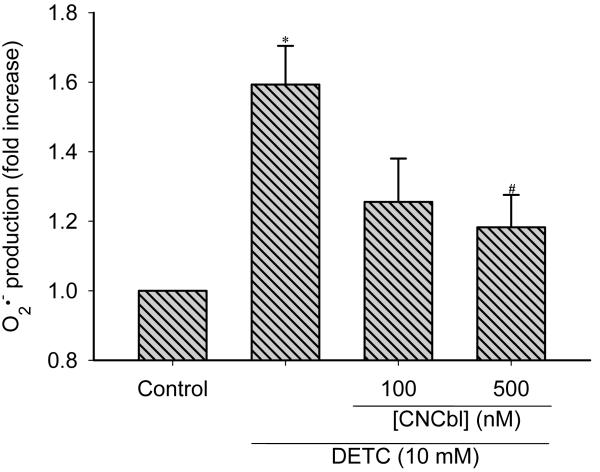
To explore the possibility that Cbl can act as a second line of defense when O2•− production overwhelms the SOD capacity, we inhibited Cu/Zn-SOD by treating HAEC with diethyldithiocarbamate (DETC, 10 mM) for 2 h with or without CNCbl pre-treatment. Incubation with DETC elicited an increase in DHE fluorescence indicative of higher intracellular O2•− levels. Pre-treatment with CNCbl (100 nM) significantly reduced the DETC-induced DHE fluorescence (Figure 7), providing support for CNCbl’s ability to scavenge O2•− in SOD-compromised cells.
Figure 7. Cbl attenuates the DETC-induced increase in superoxide levels.
Pre-confluent HAEC were incubated in the absence or in the presence of 100 or 500 nM CNCbl for 24 h. Cells were washed with PBS, prior to incubation with 10 mM DETC. After 2 h, cells were incubated with 5 μM DHE for 1 h. Hydroxyethidium fluorescence was measured with a fluorescent plate reader. * p < 0.05 with respect to untreated HAEC; # p < 0.05 with respect to DETC alone. Data are expressed as mean ± SEM; N = 5.
Cbl protects against Hcy-dependent increase in apoptotic cell death
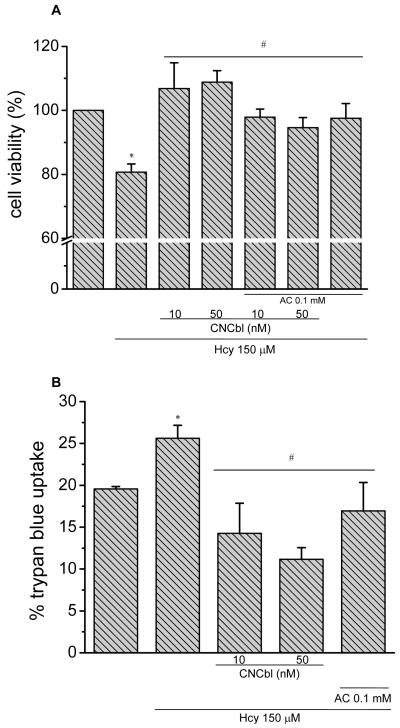
Exposing HAEC to L-Hcy (150 μM) for 24 h caused a significant decrease in cell viability as measured by the MTT assay (Figure 8A). Since the MTT assay is a measure of mitochondrial function and metabolic activity, cell death was directly assessed by trypan blue staining, which corresponded with the MTT results (Figure 8B). Finally, to characterize the L-Hcy induced cell death, apoptosis was assessed by measuring cytosolic fragmented DNA with an ELISA-based cell death assay (Roche). HAEC showed a significant increase in apoptotic cell death in response to L-Hcy (150 μM) for 18 h (Figure 9). Since both CNCbl and apocynin prevented the L-Hcy-induced decrease in cell viability as measured by the MTT assay (Figure 8A), and the increase in cell death, as measured by trypan blue staining (Figure 8B) or ELISA (Figure 9), this is indicative of CNCbl providing protection against apoptosis.
Figure 8. Cbl protection against L-Hcy-induced cell death.
Pre-confluent HAEC were incubated in the presence or absence of 10 or 50 nM CNCbl for 24 h prior to adding 150 μM L-Hcy for 24 h in the presence or absence of apocynin (0.1 mM). *p < 0.05 with respect to control cells not exposed to L-Hcy; #p < 0.05 with respect to L-Hcy-treated HAEC. A. Cell viability by the MTT assay. Data are expressed as mean ± SEM; N = 4. B. Trypan blue uptake. Data are expressed as mean ± SEM; N = 3.
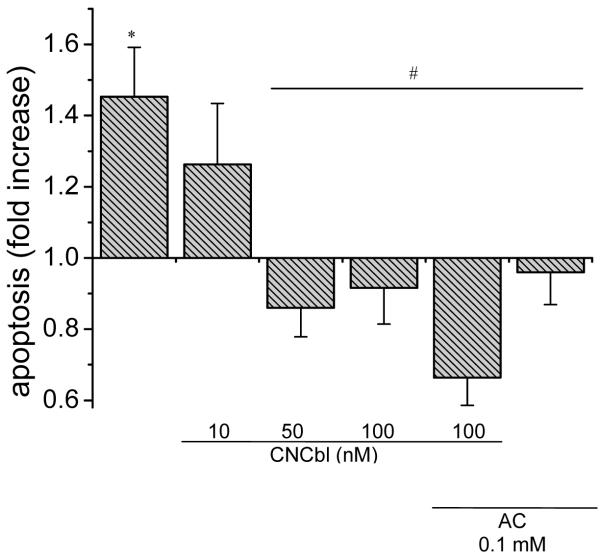
Figure 9. Cbl protection against L-Hcy-induced apoptosis.
Pre-confluent HAEC were incubated in the presence of absence of varying concentrations of CNCbl for 24 h prior to exposing the cells to 150 μM L-Hcy in the presence or absence of apocynin (0.1 mM). Shown also are corresponding data for apocynin and apocynin + CNCbl. Apoptotic DNA fragmentation was measured by ELISA. Data are expressed as mean ± SEM; N = 4; *p < 0.05 with respect to control; #p < 0.05 with respect to L-Hcy-treated HAEC.
Discussion
Vitamin B12 is an essential micronutrient required for one-carbon metabolism and branched amino acid catabolism. Recent studies in our laboratories showed that Cbl(II) can directly scavenge O2•− to form aquacobalamin (and hydrogen peroxide) extremely rapidly, at a rate approaching that of SOD-catalyzed dismutation (7 × 108 vs 2 × 109 M−1.s−1) [6]. Substantial free (non-protein bound) intracellular Cbl can be achieved with supplementation [36-39]. Upon entering cells the cobalt(III) center of Cbl is reduced to cobalt(II) (= Cbl(II)) prior to binding to the B12-dependent enzymes [40]. The ability of cells to re-reduce free aquacobalamin to Cbl(II) (“aquacobalamin reductase activity”) is well established [41-46]; therefore, providing the theoretical basis for Cbl-mediated catalytic O2•− scavenging. This led us to speculate that Cbl might protect cells from oxidative stress by efficient O2•− scavenging.
In our studies, intracellular O2•− was generated by treating HAEC with paraquat , and the ability of CNCbl to protect cells against damage was assessed. CNCbl at nanomolar concentrations could prevent the increase inO2•− levels and the associated reduction in cell viability (Figure 6). The inhibitory effects of CNCbl were comparable to those observed with apocynin or SOD itself (Figure 6).
The inhibition of Cu/Zn-SOD with DETC treatment also elevated cytosolic O2•− levels. DETC-treated cells were round and loosely attached compared to spindle-shaped control cells, demonstrating the dramatic effect of inhibiting Cu/Zn-SOD in HAEC. Pre-treating HAEC with CNCbl (100 nM) attenuated the DETC-induced increase in O2•− and also partially reverted the altered cell morphology, which further supports the direct effect of Cbl on intracellular O2•−.
Additionally, CNCbl could blunt the generation of O2•− by Hcy. Hcy has been shown to induce an increase in intracellular O2•− levels [21, 22]. Moreover, elevated Hcy is associated with vascular oxidative stress. Normally, plasma Hcy levels are maintained below 12 μM; however, in severe clinical hyperhomocysteinemia, Hcy levels can exceed 100 μM in severe instances [47]. To ensure a universal response among our individual HAEC clones, our experiments used a high but still pathophysiological range of Hcy concentrations (100 – 150 μM), which increased O2•− levels and resulted in associated loss of cell viability. As with paraquat, these effects were completely inhibited by pre-treating HAEC with CNCbl (10 nM) or by treating the cells with apocynin or SOD itself.
The increase in L-Hcy-induced cell death correlated with increased apoptotic cell death. L-Hcy induces apoptosis in human bone marrow stromal cells [52], human umbilical vein endothelial cells [51, 53], and endothelial progenitor cells [54]. It also inhibits growth [55] and reduces cell viability in HAEC [56]. Our results are consistent with previous studies in other endothelial cell lines; however to our knowledge, we are the first to show L-Hcy-induced apoptotic cell death in primary cultures of HAEC. L-Hcy-induced apoptosis was prevented by apocynin or by pretreating HAEC with CNCbl (50 nM).
The protective effects of Cbl against L-Hcy-induced oxidative stress are perhaps not suprising, given the cofactor role of Cbl in Hcy metabolism. However, our experiments indicate that Cbl shows effects against O2•− generated in response to a variety of insults apart from Hcy (ie paraquat, rotenone, DETC). Such results indicate that Cbl protection against Hcy-mediated oxidative stress may not be due to increasing Hcy metabolism alone, and that Cbl can act to protect against oxidative stress in a general manner. These data, combined with our previous in vitro studies showing a direct and fast reaction between Cbl(II) and O2•− strongly implicate Cbl as an intracellular O2•− scavenger. However, in the case of Hcy, we cannot rule out the involvement of other mechanisms independent of O2•− scavenging in the CNCbl-mediated protective effects.
An important finding from our studies is the effectiveness of CNCbl against mitochondrial oxidative stress. Oxidative stress-associated mitochondrial dysfunction is a common feature in cardiovascular pathologies [57, 58] and there is considerable interest in developing mitochondrial-specific antioxidants [58, 59]. Hcy increases mitochondrial oxidative stress in brain [60] and cardiac myocytes [61]. Cbl is present in the mitochondria (Cbl-dependent L-methylmalonyl-CoA mutase is a mitochondrial enzyme) and ~80% of Cbl is in its Cbl(II) form [2]. In endothelial cells the mitochondrial AdoCbl concentration is 4-fold higher than cytosolic MeCbl concentration [62] and a substantial fraction of mitochondrial Cbl is not protein-bound [63]. In our studies, CNCbl treatment effectively inhibited the generation of mitochondrial O2•− by Hcy or rotenone treatment (Figure 5).
DHE remains one of the most widely used probes for detection of O2•− in live cells, although 2-hydroxyethidium, the specific reaction product between DHE and O2•−, is not the only DHE fluorescent oxidation product. Ethidium, a 1 e− oxidation product of DHE has an emission spectrum with a 45% overlap with that of 2-hydroxyethidium [64]. The same applies for the mitochondria-targeted ethidium derivative, mitoSOX and its oxidation products [64]. Therefore, the specificity of DHE for detecting O2•−levels has been questioned. However, our conclusions are not solely based on the effect of Cbl on DHE or mitoSOX oxidation. The oxidative-stress-inducing insults used in our studies have been shown to increase O2•− levels in previous studies using lucigenin chemiluminescence or EPR [21, 22, 30, 65, 66], and these data correlate well with an increase in DHE-derived fluorescence. Thus, because O2•− is the predominant ROS produced in these systems, the Cbl effect was mimicked by SOD, and our previous studies show that Cbl reacts very rapidly with O2•−, we conclude that the Cbl-dependent decrease in DHE fluorescence is due to a direct Cbl-mediated O2•−-scavenging mechanism.
It is likely that Cbl has biological roles beyond its ability to act as a cofactor for the two mammalian B12-dependent enzyme reactions (reviewed by Solomon) [19]. Cbl supplementation can be beneficial in treating a range of inflammatory and viral based diseases associated with oxidative stress [10-16] and also modulates the immune response [68, 69] . Moreover, high doses of Cbl have been used to treat pernicious anaemia for decades with no apparent toxicity [67]. Cbl therapy normalizes levels of TNF-α and epidermal growth factor in Cbl deficient patients [68], by mechanism(s) which are currently unclear. Thus, there are intriguing clinical implications for our observed association between Cbl and intracellular O2•− levels. Our results support the hypothesis that Cbl can act as a second line of defense when O2•− production overwhelms the SOD protection system. This perhaps accounts for significantly increased oxidative damage markers in patients with inherited disorders of intracellular Cbl metabolism [70].
Our data show that physiologically relevant concentrations (up to 10−7 M are achievable in plasma [34, 35]) of CNCbl (the common form of Cbl in vitamin supplements) effectively protect against increased intracellular levels of O2•−, both in the cytosol and in the mitochondria, resulting in a concomitant reduction in cell death. Importantly, these effects were found to be independent of Hcy metabolism. These results combined with the in vitro kinetic data demonstrating that Cbl(II) efficiently scavenges O2•− [6], suggest that direct scavenging of O2•− by Cbl is an important mechanism by which Cbl protects against intracellular oxidative stress. Our results have important implications both in regard to the high percentage of the elderly who are B12-deficient and in the treatment of chronic inflammatory diseases associated with oxidative stress. These results encourage further studies with animal models to test the efficacy of Cbl as a O2•− scavenger in vivo.
Supplementary Material
Acknowledgements
The authors thank Dr. Donald Jacobsen, The Cleveland Clinic, for providing HAEC and Dr. William Chilian, Northeastern Ohio Universities Colleges of Medicine and Pharmacy, and Dr. Andrew McCaddon, Cardiff School of Medicine, for insightful input.
Sources of Funding
Funding for this work was provided by an American Heart Association predoctoral fellowship (E.S.M.), Kent State University (GSS research grant, E.S.M.), the Ohio Board of Regents (J.Y. and N.E.B.), and the NIH (HL52234 and HL71907 to Donald W. Jacobsen; Department of Cell Biology, Lerner Research Institute, Cleveland Clinic, Cleveland, OH 44106).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Present address of E.S.M.: Division of Vascular Surgery, Department of Surgery, Northwestern University, Chicago, IL.
References
- [1].Olteanu H, Banerjee R. Redundancy in the pathway for redox regulation of mammalian methionine synthase: reductive activation by the dual flavoprotein, novel reductase 1. J Biol Chem. 2003;278(40):38310–4. doi: 10.1074/jbc.M306282200. [DOI] [PubMed] [Google Scholar]
- [2].Padovani D, Banerjee R. Assembly and protection of the radical enzyme, methylmalonyl-CoA mutase, by its chaperone. Biochemistry. 2006;45(30):9300–6. doi: 10.1021/bi0604532. [DOI] [PubMed] [Google Scholar]
- [3].Chen P, Poddar R, Tipa EV, Dibello PM, Moravec CD, Robinson K, Green R, Kruger WD, Garrow TA, Jacobsen DW. Homocysteine metabolism in cardiovascular cells and tissues: implications for hyperhomocysteinemia and cardiovascular disease. Adv Enzyme Regul. 1999;39:93–109. doi: 10.1016/s0065-2571(98)00029-6. [DOI] [PubMed] [Google Scholar]
- [4].Carmel R, Green R, Rosenblatt DS, Watkins D. Update on cobalamin, folate, and homocysteine. Hematology (Am Soc Hematol Educ Program) 2003:62–81. doi: 10.1182/asheducation-2003.1.62. [DOI] [PubMed] [Google Scholar]
- [5].Birch CS, Brasch NE, McCaddon A, Williams JH. A novel role for vitamin B(12): Cobalamins are intracellular antioxidants in vitro. Free Radic Biol Med. 2009;47(2):184–8. doi: 10.1016/j.freeradbiomed.2009.04.023. [DOI] [PubMed] [Google Scholar]
- [6].Suarez-Moreira E, Yun J, Birch CS, Williams JH, McCaddon A, Brasch NE. Vitamin B(12) and Redox Homeostasis: Cob(II)alamin Reacts with Superoxide at Rates Approaching Superoxide Dismutase (SOD) J Am Chem Soc. 2009 doi: 10.1021/ja904670x. [DOI] [PubMed] [Google Scholar]
- [7].Wolters M, Strohle A, Hahn A. Cobalamin: a critical vitamin in the elderly. Prev Med. 2004;39(6):1256–66. doi: 10.1016/j.ypmed.2004.04.047. [DOI] [PubMed] [Google Scholar]
- [8].Green R, Miller JW. Vitamin B12 deficiency is the dominant nutritional cause of hyperhomocysteinemia in a folic acid-fortified population. Clin Chem Lab Med. 2005;43(10):1048–51. doi: 10.1515/CCLM.2005.183. [DOI] [PubMed] [Google Scholar]
- [9].Earnest CP, Wood KA, Church TS. Complex multivitamin supplementation improves homocysteine and resistance to LDL-C oxidation. J Am Coll Nutr. 2003;22(5):400–7. doi: 10.1080/07315724.2003.10719323. [DOI] [PubMed] [Google Scholar]
- [10].Flynn MA, Irvin W, Krause G. The effect of folate and cobalamin on osteoarthritic hands. J Am Coll Nutr. 1994;13(4):351–6. doi: 10.1080/07315724.1994.10718421. [DOI] [PubMed] [Google Scholar]
- [11].Greenberg SS, Xie J, Zatarain JM, Kapusta DR, Miller MJ. Hydroxocobalamin (vitamin B12a) prevents and reverses endotoxin-induced hypotension and mortality in rodents: role of nitric oxide. J Pharmacol Exp Ther. 1995;273(1):257–65. [PubMed] [Google Scholar]
- [12].Crocket JA. Cyanocobalamin in asthma. Acta Allergol. 1957;11(4):261–8. doi: 10.1111/j.1398-9995.1957.tb03037.x. [DOI] [PubMed] [Google Scholar]
- [13].Miller JW. Vitamin B12 deficiency, tumor necrosis factor-alpha, and epidermal growth factor: a novel function for vitamin B12? Nutr Rev. 2002;60(5 Pt 1):142–4. doi: 10.1301/00296640260093805. [DOI] [PubMed] [Google Scholar]
- [14].Volkov I, Rudoy I, Press Y. Successful treatment of chronic erythema nodosum with vitamin B12. J Am Board Fam Pract. 2005;18(6):567–9. doi: 10.3122/jabfm.18.6.567. [DOI] [PubMed] [Google Scholar]
- [15].Wheatley C. A scarlet pimpernel for the resolution of inflammation? The role of supra-therapeutic doses of cobalamin, in the treatment of systemic inflammatory response syndrome (SIRS), sepsis, severe sepsis, and septic or traumatic shock. Med Hypotheses. 2006;67(1):124–42. doi: 10.1016/j.mehy.2006.01.036. [DOI] [PubMed] [Google Scholar]
- [16].Yamashiki M, Nishimura A, Kosaka Y. Effects of methylcobalamin (vitamin B12) on in vitro cytokine production of peripheral blood mononuclear cells. J Clin Lab Immunol. 1992;37(4):173–82. [PubMed] [Google Scholar]
- [17].Molad Y, Rachmilewitz B, Sidi Y, Pinkhas J, Weinberger A. Serum cobalamin and transcobalamin levels in systemic lupus erythematosus. Am J Med. 1990;88(2):141–4. doi: 10.1016/0002-9343(90)90463-n. [DOI] [PubMed] [Google Scholar]
- [18].Remacha AF, Montagud M, Cadafalch J, Riera A, Martino R, Gimferrer E. Vitamin B12 transport proteins in patients with HIV-1 infection and AIDS. Haematologica. 1993;78(2):84–8. [PubMed] [Google Scholar]
- [19].Solomon LR. Disorders of cobalamin (vitamin B12) metabolism: emerging concepts in pathophysiology, diagnosis and treatment. Blood Rev. 2007;21(3):113–30. doi: 10.1016/j.blre.2006.05.001. [DOI] [PubMed] [Google Scholar]
- [20].McCord JM, Omar BA. Sources of free radicals. Toxicol Ind Health. 1993;9(1-2):23–37. doi: 10.1177/0748233793009001-204. [DOI] [PubMed] [Google Scholar]
- [21].Lang D, Kredan MB, Moat SJ, Hussain SA, Powell CA, Bellamy MF, Powers HJ, Lewis MJ. Homocysteine-induced inhibition of endothelium-dependent relaxation in rabbit aorta: role for superoxide anions. Arterioscler Thromb Vasc Biol. 2000;20(2):422–7. doi: 10.1161/01.atv.20.2.422. [DOI] [PubMed] [Google Scholar]
- [22].Ungvari Z, Csiszar A, Edwards JG, Kaminski PM, Wolin MS, Kaley G, Koller A. Increased superoxide production in coronary arteries in hyperhomocysteinemia: role of tumor necrosis factor-alpha, NAD(P)H oxidase, and inducible nitric oxide synthase. Arterioscler Thromb Vasc Biol. 2003;23(3):418–24. doi: 10.1161/01.ATV.0000061735.85377.40. [DOI] [PubMed] [Google Scholar]
- [23].Buetler TM, Krauskopf A, Ruegg UT. Role of superoxide as a signaling molecule. News Physiol Sci. 2004;19:120–3. doi: 10.1152/nips.01514.2003. [DOI] [PubMed] [Google Scholar]
- [24].Muscoli C, Cuzzocrea S, Riley DP, Zweier JL, Thiemermann C, Wang ZQ, Salvemini D. On the selectivity of superoxide dismutase mimetics and its importance in pharmacological studies. Br J Pharmacol. 2003;140(3):445–60. doi: 10.1038/sj.bjp.0705430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Huang TT, Carlson EJ, Raineri I, Gillespie AM, Kozy H, Epstein CJ. The use of transgenic and mutant mice to study oxygen free radical metabolism. Ann N Y Acad Sci. 1999;893:95–112. doi: 10.1111/j.1749-6632.1999.tb07820.x. [DOI] [PubMed] [Google Scholar]
- [26].Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82(1):47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- [27].Iqbal J, Whitney P. Use of cyanide and diethyldithiocarbamate in the assay of superoxide dismutases. Free Radic Biol Med. 1991;10(1):69–77. doi: 10.1016/0891-5849(91)90023-v. [DOI] [PubMed] [Google Scholar]
- [28].Barbato JC, Catanescu O, Murray K, Dibello PM, Jacobsen DW. Targeting of Metallothionein by L-Homocysteine. A Novel Mechanism for Disruption of Zinc and Redox Homeostasis. Arterioscler Thromb Vasc Biol. 2007;27(1):49–54. doi: 10.1161/01.ATV.0000251536.49581.8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ellman GL. A colorimetric method for determining low concentrations of mercaptans. Arch Biochem Biophys. 1958;74(2):443–50. doi: 10.1016/0003-9861(58)90014-6. [DOI] [PubMed] [Google Scholar]
- [30].Munzel T, Hink U, Yigit H, Macharzina R, Harrison DG, Mulsch A. Role of superoxide dismutase in in vivo and in vitro nitrate tolerance. Br J Pharmacol. 1999;127(5):1224–30. doi: 10.1038/sj.bjp.0702622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hildebrandt AG, Roots I, Tjoe M, Heinemeyer G. Hydrogen peroxide in hepatic microsomes. Methods Enzymol. 1978;52:342–50. doi: 10.1016/s0076-6879(78)52037-5. [DOI] [PubMed] [Google Scholar]
- [32].Barker HA, Smyth RD, Weissbach H, Munch-Petersen A, Toohey JI, Ladd JN, Volcani BE, Wilson RM. Assay, purification, and properties of the adenylocobamide coenzyme. J Biol Chem. 1960;235:181–90. [PubMed] [Google Scholar]
- [33].Day BJ, Patel M, Calavetta L, Chang LY, Stamler JS. A mechanism of paraquat toxicity involving nitric oxide synthase. Proc Natl Acad Sci U S A. 1999;96(22):12760–5. doi: 10.1073/pnas.96.22.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Honma K, Kohsaka M, Fukuda N, Morita N, Honma S. Effects of vitamin B12 on plasma melatonin rhythm in humans: increased light sensitivity phase-advances the circadian clock? Experientia. 1992;48(8):716–20. doi: 10.1007/BF02124286. [DOI] [PubMed] [Google Scholar]
- [35].Sombolos K, Fragia T, Natse T, Bartholomatos G, Karagianni A, Katsaris G, Christidou F, Bamichas G, Stangou M, Papagalanis N. The effect of long-term intravenous high dose B-complex vitamins with or without folic acid on serum homocysteine in hemodialysis patients. J Nephrol. 2002;15(6):671–5. [PubMed] [Google Scholar]
- [36].Quadros EV, Jacobsen DW. The dynamics of cobalamin utilization in L-1210 mouse leukemia cells: a model of cellular cobalamin metabolism. Biochim Biophys Acta. 1995;1244(2-3):395–403. doi: 10.1016/0304-4165(95)00037-c. [DOI] [PubMed] [Google Scholar]
- [37].Beedholm-Ebsen R, van de Wetering K, Hardlei T, Nexo E, Borst P, Moestrup SK. Identification of multidrug resistance protein 1 (MRP1/ABCC1) as a molecular gate for cellular export of cobalamin. Blood. 115(8):1632–9. doi: 10.1182/blood-2009-07-232587. [DOI] [PubMed] [Google Scholar]
- [38].Dan N, Cutler DF. Transcytosis and processing of intrinsic factor-cobalamin in Caco-2 cells. J Biol Chem. 1994;269(29):18849–55. [PubMed] [Google Scholar]
- [39].Seetharam B, Presti M, Frank B, Tiruppathi C, Alpers DH. Intestinal uptake and release of cobalamin complexed with rat intrinsic factor. Am J Physiol. 1985;248(3 Pt 1):G326–31. doi: 10.1152/ajpgi.1985.248.3.G326. [DOI] [PubMed] [Google Scholar]
- [40].Banerjee R, Gherasim C, Padovani D. The tinker, tailor, soldier in intracellular B12 trafficking. Curr Opin Chem Biol. 2009;13(4):484–91. doi: 10.1016/j.cbpa.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Fiskerstrand T, Riedel B, Ueland PM, Seetharam B, Pezacka EH, Gulati S, Bose S, Banerjee R, Berge RK, Refsum H. Disruption of a regulatory system involving cobalamin distribution and function in a methionine-dependent human glioma cell line. J. Biol. Chem. 1998;273(32):20180–84. doi: 10.1074/jbc.273.32.20180. [DOI] [PubMed] [Google Scholar]
- [42].Watanabe F, Saido H, Yamaji R, Miyatake K, Isegawa Y, Ito A, Yubisui T, Rosenblatt DS, Nakano Y. Mitochondrial NADH- or NADPH-linked aquacobalamin reductase activity is low in human skin fibroblasts with defects in synthesis of cobalamin coenzymes. J. Nutr. 1996;126(12):2947–51. doi: 10.1093/jn/126.12.2947. [DOI] [PubMed] [Google Scholar]
- [43].Watanabe F, Nakano Y, Tachikake N, Kitaoka S, Tamura Y, Yamanaka H, Haga S, Imai S, Saido H. Occurrence and subcellular location of NADH- and NADPH-linked aquacobalamin reductases in human liver. Int. J. Biochem. 1991;23(5-6):531–3. [PubMed] [Google Scholar]
- [44].Watanabe F, Nakano Y, Maruno S, Tachikake N, Tamura Y, Kitaoka S. NADH- and NADPH-linked aquacobalamin reductases occur in both mitochondrial and microsomal membranes of rat liver. Biochem. Biophys. Res. Commun. 1989;165(2):675–9. doi: 10.1016/s0006-291x(89)80018-x. [DOI] [PubMed] [Google Scholar]
- [45].Pezacka EH. Identification and characterization of two enzymes involved in the intracellular metabolism of cobalamin. Cyanocobalamin beta -ligand transferase and microsomal cob(III)alamin reductase. Biochim. Biophys. Acta, Gen. Subj. 1993;1157(2):167–77. doi: 10.1016/0304-4165(93)90061-c. [DOI] [PubMed] [Google Scholar]
- [46].Yamada K, Gravel RA, Toraya T, Matthews RG. Human methionine synthase reductase is a molecular chaperone for human methionine synthase. Proc Natl Acad Sci U S A. 2006;103(25):9476–81. doi: 10.1073/pnas.0603694103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Welch GN, Loscalzo J. Homocysteine and atherothrombosis. N Engl J Med. 1998;338(15):1042–50. doi: 10.1056/NEJM199804093381507. [DOI] [PubMed] [Google Scholar]
- [48].Handy DE, Zhang Y, Loscalzo J. Homocysteine down-regulates cellular glutathione peroxidase (GPx1) by decreasing translation. J Biol Chem. 2005;280(16):15518–25. doi: 10.1074/jbc.M501452200. [DOI] [PubMed] [Google Scholar]
- [49].Sawle P, Foresti R, Green CJ, Motterlini R. Homocysteine attenuates endothelial haem oxygenase-1 induction by nitric oxide (NO) and hypoxia. FEBS Lett. 2001;508(3):403–6. doi: 10.1016/s0014-5793(01)03117-9. [DOI] [PubMed] [Google Scholar]
- [50].Topal G, Brunet A, Millanvoye E, Boucher JL, Rendu F, Devynck MA, David-Dufilho M. Homocysteine induces oxidative stress by uncoupling of NO synthase activity through reduction of tetrahydrobiopterin. Free Radic Biol Med. 2004;36(12):1532–41. doi: 10.1016/j.freeradbiomed.2004.03.019. [DOI] [PubMed] [Google Scholar]
- [51].Dong F, Zhang X, Li SY, Zhang Z, Ren Q, Culver B, Ren J. Possible involvement of NADPH oxidase and JNK in homocysteine-induced oxidative stress and apoptosis in human umbilical vein endothelial cells. Cardiovasc Toxicol. 2005;5(1):9–20. doi: 10.1385/ct:5:1:009. [DOI] [PubMed] [Google Scholar]
- [52].Kim DJ, Koh JM, Lee O, Kim NJ, Lee YS, Kim YS, Park JY, Lee KU, Kim GS. Homocysteine enhances apoptosis in human bone marrow stromal cells. Bone. 2006;39(3):582–90. doi: 10.1016/j.bone.2006.03.004. [DOI] [PubMed] [Google Scholar]
- [53].Zhang C, Cai Y, Adachi MT, Oshiro S, Aso T, Kaufman RJ, Kitajima S. Homocysteine induces programmed cell death in human vascular endothelial cells through activation of the unfolded protein response. J Biol Chem. 2001;276(38):35867–74. doi: 10.1074/jbc.M100747200. [DOI] [PubMed] [Google Scholar]
- [54].Alam MM, Mohammad AA, Shuaib U, Wang C, Ghani U, Schwindt B, Todd KG, Shuaib A. Homocysteine reduces endothelial progenitor cells in stroke patients through apoptosis. J Cereb Blood Flow Metab. 2009;29(1):157–65. doi: 10.1038/jcbfm.2008.99. [DOI] [PubMed] [Google Scholar]
- [55].Wang H, Yoshizumi M, Lai K, Tsai JC, Perrella MA, Haber E, Lee ME. Inhibition of growth and p21ras methylation in vascular endothelial cells by homocysteine but not cysteine. J Biol Chem. 1997;272(40):25380–5. doi: 10.1074/jbc.272.40.25380. [DOI] [PubMed] [Google Scholar]
- [56].Lin CP, Chen YH, Chen JW, Leu HB, Liu TZ, Liu PL, Huang SL. Cholestin (Monascus purpureus rice) inhibits homocysteine-induced reactive oxygen species generation, nuclear factor-kappaB activation, and vascular cell adhesion molecule-1 expression in human aortic endothelial cells. J Biomed Sci. 2008;15(2):183–96. doi: 10.1007/s11373-007-9212-0. [DOI] [PubMed] [Google Scholar]
- [57].Ballinger SW. Mitochondrial dysfunction in cardiovascular disease. Free Radic Biol Med. 2005;38(10):1278–95. doi: 10.1016/j.freeradbiomed.2005.02.014. [DOI] [PubMed] [Google Scholar]
- [58].Victor VM, Apostolova N, Herance R, Hernandez-Mijares A, Rocha M. Oxidative stress and mitochondrial dysfunction in atherosclerosis: mitochondria-targeted antioxidants as potential therapy. Curr Med Chem. 2009;16(35):4654–67. doi: 10.2174/092986709789878265. [DOI] [PubMed] [Google Scholar]
- [59].Cocheme HM, Kelso GF, James AM, Ross MF, Trnka J, Mahendiran T, Asin-Cayuela J, Blaikie FH, Manas AR, Porteous CM, Adlam VJ, Smith RA, Murphy MP. Mitochondrial targeting of quinones: therapeutic implications. Mitochondrion. 2007;7(Suppl):S94–102. doi: 10.1016/j.mito.2007.02.007. [DOI] [PubMed] [Google Scholar]
- [60].Baydas G, Koz ST, Tuzcu M, Etem E, Nedzvetsky VS. Melatonin inhibits oxidative stress and apoptosis in fetal brains of hyperhomocysteinemic rat dams. J Pineal Res. 2007;43(3):225–31. doi: 10.1111/j.1600-079X.2007.00465.x. [DOI] [PubMed] [Google Scholar]
- [61].Moshal KS, Tipparaju SM, Vacek TP, Kumar M, Singh M, Frank IE, Patibandla PK, Tyagi N, Rai J, Metreveli N, Rodriguez WE, Tseng MT, Tyagi SC. Mitochondrial matrix metalloproteinase activation decreases myocyte contractility in hyperhomocysteinemia. Am J Physiol Heart Circ Physiol. 2008;295(2):H890–7. doi: 10.1152/ajpheart.00099.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Hannibal L, Axhemi A, Glushchenko AV, Suarez-Moreira E, Brasch NE, Jacobsen DW. Accurate assessment and identification of naturally occurring cellular cobalamins. Clin Chem Lab Med. 2008 doi: 10.1515/CCLM.2008.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Fenton WA, Ambani LM, Rosenberg LE. Uptake of hydroxocobalamin by rat liver mitochondria. Binding to a mitochondrial protein. J Biol Chem. 1976;251(21):6616–23. [PubMed] [Google Scholar]
- [64].Robinson KM, Janes MS, Pehar M, Monette JS, Ross MF, Hagen TM, Murphy MP, Beckman JS. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc Natl Acad Sci U S A. 2006;103(41):15038–43. doi: 10.1073/pnas.0601945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Guzik TJ, Olszanecki R, Sadowski J, Kapelak B, Rudzinski P, Jopek A, Kawczynska A, Ryszawa N, Loster J, Jawien J, Czesnikiewicz-Guzik M, Channon KM, Korbut R. Superoxide dismutase activity and expression in human venous and arterial bypass graft vessels. J Physiol Pharmacol. 2005;56(2):313–23. [PubMed] [Google Scholar]
- [66].Dikalov SI, Kirilyuk IA, Voinov M, Grigor’ev IA. EPR detection of cellular and mitochondrial superoxide using cyclic hydroxylamines. Free Radic Res. doi: 10.3109/10715762.2010.540242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Mangiarotti G, Canavese C, Salomone M, Thea A, Pacitti A, Gaido M, Calitri V, Pelizza D, Canavero W, Vercellone A. Hypervitaminosis B12 in maintenance hemodialysis patients receiving massive supplementation of vitamin B12. Int J Artif Organs. 1986;9(6):417–20. [PubMed] [Google Scholar]
- [68].Scalabrino G, Veber D, Mutti E. Experimental and clinical evidence of the role of cytokines and growth factors in the pathogenesis of acquired cobalamin-deficient leukoneuropathy. Brain Res Rev. 2008;59(1):42–54. doi: 10.1016/j.brainresrev.2008.05.001. [DOI] [PubMed] [Google Scholar]
- [69].Tamura J, Kubota K, Murakami H, Sawamura M, Matsushima T, Tamura T, Saitoh T, Kurabayshi H, Naruse T. Immunomodulation by vitamin B12: augmentation of CD8+ T lymphocytes and natural killer (NK) cell activity in vitamin B12-deficient patients by methyl-B12 treatment. Clin Exp Immunol. 1999;116(1):28–32. doi: 10.1046/j.1365-2249.1999.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Mc Guire PJ, Parikh A, Diaz GA. Profiling of oxidative stress in patients with inborn errors of metabolism. Mol Genet Metab. 2009;98(1-2):173–80. doi: 10.1016/j.ymgme.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.