Abstract
We report the discovery of a new hydroxylated abscisic acid (ABA) metabolite, found in the course of a mass spectrometric study of ABA metabolism in Brassica napus siliques. This metabolite reveals a previously unknown catabolic pathway for ABA in which the 9′-methyl group of ABA is oxidized. Analogs of (+)-ABA deuterated at the 8′-carbon atom and at both the 8′- and 9′-carbon atoms were fed to green siliques, and extracts containing the deuterated oxidized metabolites were analyzed to determine the position of ABA hydroxylation. The results indicated that hydroxylation of ABA had occurred at the 9′-methyl group, as well as at the 7′- and 8′-methyl groups. The chromatographic characteristics and mass spectral fragmentation patterns of the new ABA metabolite were compared with those of synthetic 9′-hydroxy ABA (9′-OH ABA), in both open and cyclized forms. The new compound isolated from plant extracts was identified as the cyclized form of 9′-OH ABA, which we have named neophaseic acid (neoPA). The proton nuclear magnetic resonance spectrum of pure neoPA isolated from immature seeds of B. napus was identical to that of the authentic synthetic compound. ABA and neoPA levels were high in young seeds and lower in older seeds. The open form (2Z,4E)-5-[(1R,6S)-1-Hydroxy-6-hydroxymethyl-2,6-dimethyl-4-oxo-cyclohex-2-enyl]-3-methyl-penta-2,4-dienoic acid, but not neoPA, exhibited ABA-like bioactivity in inhibiting Arabidopsis seed germination and in inducing gene expression in B. napus microspore-derived embryos. NeoPA was also detected in fruits of orange (Citrus sinensis) and tomato (Lycopersicon esculentum), in Arabidopsis, and in chickpea (Cicer arietinum), as well as in drought-stressed barley (Hordeum vulgare) and B. napus seedlings.
The plant hormone S-(+)-abscisic acid (ABA) regulates many aspects of plant growth and development including embryo maturation, seed dormancy, stress responses, and stomatal aperture (Zeevaart and Creelman, 1988). The mode of action of ABA is complex and is a very active subject of investigation. Although substantial progress has been made in understanding signaling processes in various plant systems, many questions regarding the pleiotropic responses to ABA remain to be answered (Hetherington, 2001; Ritchie et al., 2002; Xiong et al., 2002). The pathway and regulation of ABA biosynthesis has been much studied in the last few years and is relatively well understood, but there has been far less progress in understanding ABA catabolism (Cutler and Krochko, 1999; Zeevaart, 1999; Taylor et al., 2000; Milborrow, 2001; Schwartz et al., 2003).
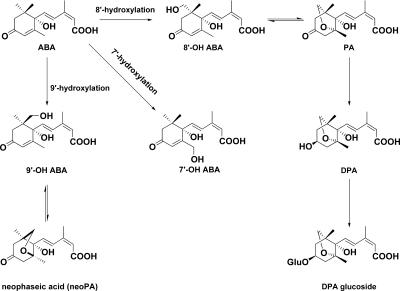
ABA can be metabolized by oxidation, reduction, or conjugation (Cutler and Krochko, 1999). The principal oxidation pathway is through hydroxylation of the 8′-carbon atom of ABA, affording 8′-hydroxy ABA (8′-OH ABA), mediated by a cytochrome P450 monooxygenase (Krochko et al., 1998). The oxidation product 8′-OH ABA exists in equilibrium with phaseic acid (PA;Balsevich et al., 1994; Zou et al., 1995). PA may be conjugated or further reduced to dihydrophaseic acid (DPA). DPA may also be metabolized to DPA conjugates (Zeevaart, 1999). A second, apparently minor oxidation pathway occurs through hydroxylation of the 7′-carbon atom of ABA, affording 7′-hydroxy ABA (7′-OH ABA; Lehmann et al., 1983; Lehmann and Schwenen, 1988). In cultured brome grass cells, the product of the minor pathway 7′-OH ABA does not accumulate and is further metabolized to unknown products (Hampson et al., 1992).
Although there are reports that PA and DPA are hormonally inactive (Cutler and Krochko, 1999), the hydroxylated metabolites 8′-OH ABA and 7′-OH ABA have exhibited significant hormonal activity in the few studies in which these labile compounds have been compared with ABA. In barley (Hordeum vulgare) half seeds, 7′-OH ABA inhibited GA3-stimulated α-amylase activity, although not as effectively as ABA (Hill et al., 1995). In microspore-derived embryos of Brassica napus, 8′-OH ABA was as effective as ABA in inducing expression of lipid modification and storage protein genes (Zou et al., 1995). Stable methyl ether analogs of 7′-OH ABA, 8′-OH ABA, and interestingly, 9′-hydroxy ABA (9′-OH ABA), are more potent than ABA in a number of assays (Todoroki et al., 1994; S.R. Abrams and M. Walker-Simmons, unpublished data). These results suggest the possibility that specific ABA catabolites could mediate some or all of the hormonal effects of ABA.
We are developing and employing analytical methods for targeted metabolite profiling of plant signaling molecules (Abrams et al., 2003; Chiwocha et al., 2003; Zhou et al., 2003). In the course of an investigation of ABA metabolism in silique development and seedpod shattering in B. napus, we detected a novel endogenous compound that appeared to be related to known ABA catabolites. Thus, we have evidence for a new ABA catabolic pathway in B. napus and here report the identification and preliminary assessment of the biological activity of two novel ABA metabolites, 9′-OH ABA and its cyclized product, which we have named neophaseic acid (neoPA).
RESULTS
Detecting a Novel ABA Metabolite
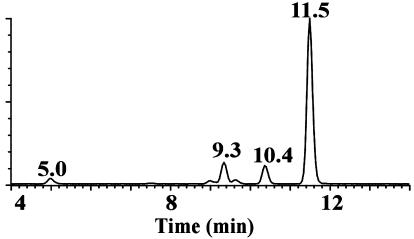
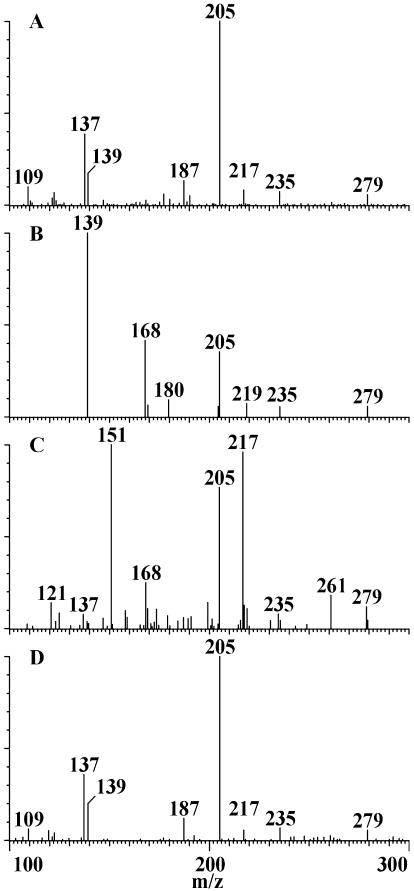
As part of a study of ABA metabolism in B. napus, liquid chromatography-electrospray-tandem mass spectrometry (LC-ESI-MS-MS) was used to detect ABA and its metabolites in extracts from both seeds and pericarps from siliques of B. napus and Brassica rapa. In the course of scanning for known oxidized ABA catabolites in extracts of immature seeds and pericarps, a new compound (HPLC retention time, 11.5 min) was detected with an apparent formula weight of 280, isomeric with PA (9.3 min) and 7′-OH ABA (10.4 min; Fig. 1). Under the conditions employed, the retention time of this compound was different from all known ABA metabolites including trans-PA and trans-7′-OH ABA. Daughter ion scans of m/z 279, the apparent [M-H]- of the unknown compound, showed major transitions to m/z 205 and to m/z 139, similar to those of PA and distinct from those of 7′-OH ABA (Fig. 2, A-C).
Figure 1.
HPLC chromatogram of a seed extract for oxidation products of ABA monitored by negative electrospray ionization MS-MS for the transition from m/z 279 to m/z 205. The retention times for PA, 7-OH ABA, and neoPA are 9.3, 10.4, and 11.5 min, respectively.
Figure 2.
Daughter ion mass spectra of natural neoPA (A), authentic PA (B), 7′-OH ABA (C), and neoPA (D). They were monitored by negative electrospray ionization MS-MS for m/z 279.
Feeding Studies with Deuterium-Labeled ABA Analogs
To determine whether this new compound was an ABA metabolite and, if so, which position of ABA was hydroxylated, feeding experiments were performed using (+)-ABA with deuterium labeling at specific carbon atoms. First, S-(+)-[8′,8′,8′-2H3]ABA (S-(+)-[8′-2H3]ABA) was fed to immature (green) B. napus siliques. Extracts from [8′-2H3]ABA-treated siliques (separated into pericarps and seeds) were analyzed by LC-MS-MS, scanning for daughter ions from ions with m/z 279, 281, and 282. Peaks with elution time of PA (9.3 min) were observed for endogenous PA (m/z 279) and [2H2]PA (m/z 281), and peaks (11.5 min) were observed for the unknown endogenous metabolite and its corresponding trideutero analog (m/z 282), indicating that the unknown was an ABA metabolite and the position of hydroxylation was not at the 8′-carbon atom. The position of oxidation in this putative hydroxylated ABA metabolite was determined by feeding S-(+)-[5,8′,8′,8′-2H4]ABA (S-(+)-[5,8′-2H4]ABA), and S-(+)- [8′,8′,8′,9′,9′,9′-2H6]ABA (S-(+)-[8′,9′-2H6]ABA) to green siliques on different B. napus plants either through surface application or by injection. Table I shows the results of LC-MS-MS analysis of metabolites derived from labeled ABA in the feeding study.
Table I.
Summary of LC-MS-MS analysis of oxidation products from labeled ABA supplied to B. napus siliques
| Supplied ABA | Retention Time | Deuterated Metabolites | Prominent Ions (m/z) (Abundance) |
|---|---|---|---|
| min | |||
| [5, 8′-2H4]ABA | 9.3 | [5, 8′, 8′-2H3]PA | 282 ([M-H]−, 5), 206 (53), 170 (26), 139 (100), 137 (7) |
| 10.4 | [5, 8′, 8′, 8′-2H4]7′-OH ABA | 283 ([M-H]−, 17), 221 (65), 209 (57), 154 (100) | |
| 11.5 | [5, 8′, 8′, 8′-2H4]neoPA | 283 ([M-H]−, 9), 209 (100), 142 (21), 140 (38) | |
| [8′, 9′-2H6]ABA | 9.3 | [8′, 8′, 9′, 9′, 9′-2H5]PA | 284 ([M-H]−, 8), 208 (38), 173 (21), 142 (100), 140 (6) |
| 10.4 | [8′, 8′, 8′, 9′, 9′, 9′-2H6]7′-OH ABA | 285 ([M-H]−, 8), 223 (60), 211 (38), 157 (100) | |
| 11.5 | [8′, 8′, 8′, 9′, 9′-2H5]neoPA | 284 ([M-H]−, 24), 208 (100), 142 (54), 140 (28) |
In the tissue extract from the [5,8′-2H4]ABA feeding experiment, [2H3]PA (m/z 282; 9.3 min) and [2H4]7′-OH ABA (10.4 min) were detected, as well as the unlabeled putative hydroxylated ABA and its corresponding tetradeutero analog (m/z 283) with emergence times of 11.5 min. From tissue obtained from the [8′,9′-2H6]ABA feeding experiment, we detected [2H5]PA (m/z 284, 9.3 min), [2H6]7′-OH ABA (10.4 min), the unlabeled putative hydroxylated ABA, and its corresponding pentadeutero analog (m/z 284, 11.5 min).
Structure Assignment of Unknown as Closed Form of 9′-OH ABA
These results suggested that hydroxylation had occurred at the 9′-carbon atom and that the unknown hydroxylated ABA could be either 9′-OH ABA or its ring closed form, which had been given the name epi-PA (Takahashi et al., 1989). To determine the structure of the metabolite in the plant extract, the (+)- and (-)-isomers of the open and ring-closed 9′-OH ABA were synthesized for comparison with the natural product. The methyl ester of neoPA was identical to that reported (Takahashi et al., 1989). Comparison by LC-MS-MS of the synthetic compounds with the unknown from the plant extract showed that the closed form of 9′-OH ABA (neoPA) and the unknown hydroxy ABA had the same elution times and fragmentation patterns (Fig. 2, A and D). The open form 9′-OH ABA eluted just slightly earlier than PA under the chromatographic conditions employed.
Large-Scale Purification of NeoPA from Seeds
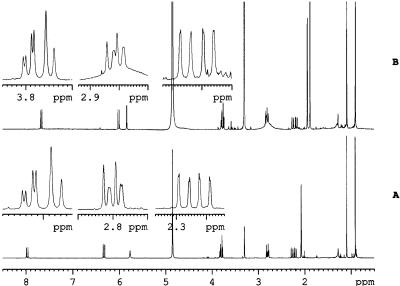
The natural product was isolated from a bulk sample of freeze-dried green (immature) seeds and shown to be identical with synthetic neoPA. The purified compound gave a single peak on HPLC on coinjection with synthetic neoPA. The increase in area was proportional to the amount of natural neoPA added (λmax = 265 nm). The 1H-NMR spectra of the natural and synthetic material were identical (Fig. 3). All of the chemical shifts were concentration dependent, and the olefinic signals showed the largest variation. The signals in the more abundant synthetic sample found initially at higher field than those of the natural compound. Dilution of the sample of the synthetic compound to 10-5 m afforded identical spectra.
Figure 3.
1H-NMR spectra of authentic neoPA (A) and natural neoPA (B).
Levels of ABA, PA, NeoPA, and 7′-OH ABA in B. napus Siliques
To determine whether neoPA levels changed during silique development, we analyzed the levels of ABA and the three initial products of its oxidation (note that 8′-OH ABA is converted to PA during sample processing) in siliques at 24 to 25 d after anthesis (seed bloom stage) and 46 d after anthesis (seed physiological maturity stage; Table II). In seeds at 24 to 25 d after anthesis, neoPA was the most abundant of the three oxidized catabolites (0.9 μg g-1 dry weight, corresponding to 3.2 nmol g-1 dry weight in Table II) although seed ABA levels were much higher at 6 μg g-1 dry weight (corresponding to 23 nmol g-1 dry weight as in Table II). Amounts of ABA, PA, neoPA, and 7′-OH ABA were at least 20-fold higher than those at 46 d after anthesis. Amounts of ABA, PA, and neoPA of seeds at 24 to 25 d after anthesis were also at least 50-fold higher than those in their corresponding pericarps. Although ABA levels in pericarps were similar at the two tested stages, at 24 to 25 d after anthesis, there was a higher content of PA and neoPA than at 46 d after anthesis.
Table II.
Levels of ABA, PA, neoPA, and 7′-OH ABA in B. napus siliques
Data shown are means ±SE of three siliques for samples from 46 d after anthesis (DAA) and four siliques for samples from 24 to 25 DAA.
| Samples | ABA | PA | neoPA | 7′-OH ABA |
|---|---|---|---|---|
| pmol g−1 dry weight | ||||
| Seeds at approximately 24 DAA | 23,394 ± 670 | 615.4 ± 58.6 | 3,208 ± 70 | 897.9 ± 44.6 |
| Pericarps at approximately 24 DAA | 327.3 ± 14.0 | 21.8 ± 11.4 | 97.9 ± 12.5 | 500.7 ± 63.2 |
| Seeds at 46 DAA | 241.7 ± 31.4 | 12.9 ± 7.1 | 48.2 ± 15.0 | 35.4 ± 20.4 |
| Pericarps at 46 DAA | 345.5 ± 26.1 | 2.8 ± 2.4 | —a | 878.2 ± 133.9 |
—, The analyte level is lower than the quantification limit.
Hormonal Activity of 9′-Oxygenated ABAs
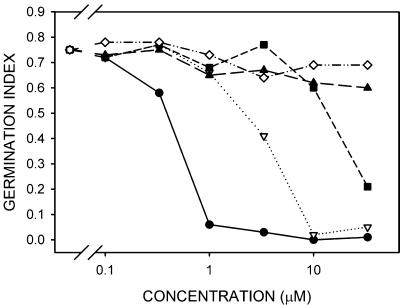
As an initial assessment of the hormonal activity of 9′-OH ABA, we compared the ability of the (+)- and (-)-isomers of 9′-OH ABA and neoPA to inhibit germination of Arabidopsis seeds. The (+)-isomer of 9′-OH ABA at a concentration of 10 μm inhibited germination completely; although the level of (+)ABA required to produce the same effect was 1 μm. The (-)-isomer of 9′-OH ABA was weakly active; significant inhibition of germination was apparent only at the highest concentration tested (33 μm; Fig. 4). Neither isomer of neoPA showed significant activity in the assay (Fig. 4).
Figure 4.
Inhibition of Arabidopsis seed germination by isomers of 9′-OH ABA. •, (+)-ABA; ▵, (+)-9′-OH ABA; ▪, (-)-9′-OH ABA; ⋄, (+)-neoPA; ▴, (-)-neoPA.
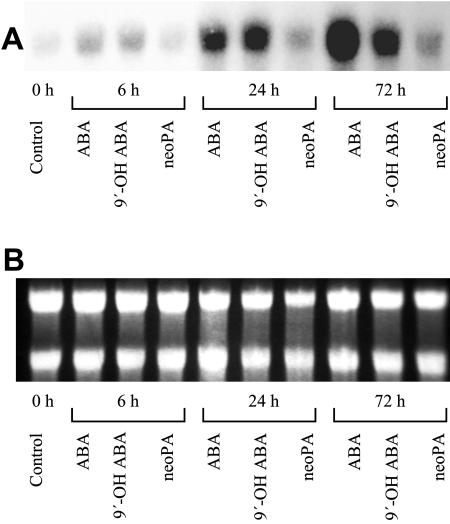
To test of the ability of 9′-OH ABA to induce gene expression, we compared the ability of ABA, (+) 9′-OH ABA, and neoPA as they affected 3-ketoacyl-CoA synthase (fatty acid elongase [FAE]) expression in microspore-derived embryos of B. napus. 9′-OH ABA (10 μm) was as strong as ABA in inducing FAE gene expression in a little as 6 h, whereas neoPA had a negligible effect, its signal similar to that observed in the untreated T0 control (Fig. 5). This trend continued through the 24-h treatment. After 72 h, the effect of ABA was slightly stronger than that observed in the 9′-OH ABA treatment, the latter staying at the same intensity as that observed after 24 h. As usual, the neoPA was much less active than either the ABA or the 9′-OH ABA analog.
Figure 5.
Effects of ABA, 9′-OH ABA, and neoPA on induction of elongase (FAE1) gene expression in torpedo stage microspore-derived embryos of B. napus cv Hero. Torpedo stage embryos were left untreated and harvested immediately (Control T0 time) or treated with 10 μm ABA, 9′-OH ABA, or neoPA, for 6, 24, and 72 h. After each time point, embryos were harvested and analyzed for 3-ketoacyl-CoA synthase (FAE1) gene induction as described in “Materials and Methods.” A, Relative expression of the 3-ketoacyl-CoA synthase (FAE1) gene transcript; B, relatively equal loading of total RNA on the gel as determined by comparative intensities of the two characteristic ribosomal RNA bands.
The Occurrence of 9′-OH ABA and NeoPA in Other Plants and Tissues
To determine whether ABA 9′-hydroxylation is a pathway that is widespread in plants, the occurrence of neoPA was investigated in a number of plant tissues. We detected neoPA in green Arabidopsis siliques, chickpea (Cicer arietinum) seed and pericarps, tomato (Lycopersicon esculentum) fruits, orange (Citrus sinensis) fruits, drought-stressed barley seedlings, and drought-stressed B. napus seedlings (Table III).
Table III.
Survey of plant tissues for presence of neoPA
The tissues were extracted and purified as described in `Materials and Methods.' The purified extracts were loaded to LC-MS-MS for multiple reaction monitoring and compared with the authentic standard. +, neoPA was detected with the same retention time on LC and with the right transition; +/−, a trace of neoPA was detected; and −, no neoPA was detected.
| Plant Tissue | Detection of neoPA |
|---|---|
| Green Arabidopsis siliques | + |
| Green mature Arabidopsis leaves | − |
| Green chick pea seed | + |
| Green chick pea capsule | + |
| Green young tomato fruits | + |
| Orange peel | +/− |
| Orange fruits | +/− |
| Green rice leaves | − |
| Barley seedling | − |
| Barley seedling (drought) | + |
| B. napus seedling | − |
| B. napus seedling (drought) | + |
DISCUSSION
ABA metabolites were first identified and studied either by large-scale isolation (MacMillan and Pryce, 1968) or using tracer experiments with radioactive ABA (Milborrow, 1968, 1969, 1970; Tinelli et al., 1973; Lehmann et al., 1983; Boyer and Zeevaart, 1986; Balsevich et al., 1994; Wang et al., 2002). Such studies revealed the existence of the ABA 8′-hydroxylation and 7′-hydroxylation pathways. Using LC-MS-MS we have now discovered a novel ABA 9′-hydroxylation pathway and deduced the structure of the metabolite. The discovery of this novel catabolic pathway was made possible by recent developments in MS, particularly in HPLC-MS-MS. HPLC can be employed to separate components of a complex mixture, which can be directly analyzed with the use of the tandem mass spectrometers by selecting ions with specific mass to charge ratios and by observing fragmentation of the selected ions. The technique of multiple reaction monitoring (MRM) in which the transition of specific parent to fragment ions can be measured allows the analysis of compounds that are not chromatographically resolved. The new metabolite neoPA was first observed in HPLC-MS-MS experiments selecting for the negative ion with m/z 279, corresponding to the molecular weights of the anions of PA and 7′-OH ABA. Such experiments also reveal structural information, especially, as in the present case, when combined with feeding studies with deuterated hormone analogs.
It is interesting to speculate about why neoPA had not been observed before. Levels of hormone catabolites in plant tissues are very low, making identification difficult. Radiolabeled ABA has been used to study known catabolites in many plant tissues and processes. Detection of labeled catabolites has been accomplished by thin-layer chromatography (TLC) or by HPLC, using standards to identify known catabolites. It is likely that neoPA and 9′-OH ABA were not previously identified because they can coelute with known metabolites. For example, in a typical HPLC chromatographic system employed for quantitation of ABA catabolites (on a 33 × 4.6 mm C18 column using isocratic acetonitrile-water-acetic acid [15:85:0.07, v/v] solvent system, the retention times of 9′-OH ABA, ABA glucose ester (ABA-GE), PA, neoPA, and trans-ABA are 2.05 min, 2.08 min, 2.16 min, 4.01 min, and 4.03 min), PA, 9′-OH ABA, and ABA-GE elute very close together as do neoPA and trans-ABA. Using typical TLC conditions, the pairs trans-PA and neoPA as well as trans-7′-OH ABA and 9′-OH ABA have similar chromatographic properties. Likely TLC bands or HPLC peaks with low levels of activity not corresponding to known metabolites were attributed to photoisomerization products, metabolism products from radiolabeled unnatural (-)-ABA, or degradation products. As well as these technical problems, previous studies on ABA catabolism may have been performed on tissues in which the levels of neoPA were not high. In this study, we found that levels were higher early in seed development and decreased as the seed matured.
For earlier studies on the importance of ABA turnover in plant physiological processes regulated by ABA, we had synthesized and tested ABA analogs with deuterium atoms on the 8′- and 9′-carbon atoms (Lamb et al., 1996; Rose et al., 1997; Schmitz et al., 2000). These molecules have proven to be useful tools for determining the position of hydroxylation of the unknown ABA catabolite in B. napus. On the basis of the observation of a trideutero hydroxylated ABA formed from [8′-2H3]ABA feeding, we predicted that this putative hydroxylated ABA was an ABA metabolite distinct from 8′-OH ABA or PA because all three deuterium atoms were retained in the product. In contrast, the [8′,9′-2H6]ABA feeding results indicated that there was a loss of one deuterium in the unknown hydroxylated ABA, suggesting that the unknown was 9′-OH ABA. Comparison with authentic synthetic 9′-OH ABA and its cyclized form confirmed that the isolated compound was cyclized 9′-OH ABA. The methyl ester of this compound had previously been synthesized and fully characterized as a byproduct in a synthesis of PA and called epi-PA (Takahashi et al., 1989). We have renamed the ring-closed form of 9′-OH ABA neoPA to distinguish this new metabolite from PA, the cyclized form of 8′-OH ABA. This is the first report of a metabolite derived from 9′-hydroxylation of ABA. Metabolites arising from 7′-, 8′-, and 9′-hydroxylation can be observed simultaneously in Brassica sp. seeds and pericarps. We found that neoPA disappeared at late stages of seed and pericarp development, implying that it is further metabolized. Later products in the 9′-hydroxylation pathway remain to be identified.
We previously observed a primary deuterium isotope effect in the oxidation of (+)-ABA to 8′-OH ABA in vivo using suspension cultured maize (Zea mays) cells (Lamb et al., 1996) and from in vitro enzyme assays (Cutler et al., 2000). The greater strength of the C-2H bond versus the C-H bond resulted in a 2-fold reduction in the rate of oxidation. Similar reduction of the rate of oxidation would be expected to occur in the present case. Thus, at this stage, it is premature to comment on the flux through the pathways to different hydroxylation products and our results are qualitative in nature. It appears that there are three different oxidation sites for catabolism in ABA, each involving one of the three methyl groups of the ring (Fig. 6). Although in seeds at 24 to 25 d after anthesis, neoPA levels at about 1 μg g-1 dry weight were much higher than the levels of PA and 7′-OH ABA, we also detected DPA levels at about 7 μg g-1 dry weight. Therefore, the relative importance of the 9′-hydroxylation pathway in relation to 8′-hydroxylation is not clear. Also, it is not known whether each of these oxidations requires a separate cytochrome P450 monooxygenase; this will be an objective for future investigation. In this context, it is worth noting that 9′-derivatives of ABA were equally or more effective inhibitors of ABA 8′-hydroxylation than 8′-derivatives of ABA when assayed in vitro (Cutler et al., 2000). This suggests the possibility that 8′-hydroxylases also oxidize the 9′-carbon atom of ABA, perhaps as a side reaction.
Figure 6.
ABA oxidation pathways in plants, including 9′-hydroxylation pathway.
By analogy with 8′-hydroxylation, it is expected that the first product of 9′-oxidation would be (2Z,4E)-5-[(1R,6S)-1-Hydroxy-6-hydroxymethyl-2,6-dimethyl-4-oxo-cyclohex-2-enyl]-3-methyl-penta-2,4-dienoic acid ((+)-9′-OH ABA), which can cyclize to, and exist in equilibrium with, the closed form neoPA (Fig. 6). Our first indications from studies with the synthetic material are that the open form has greater persistence at physiological pH than 8′-OH ABA. Under the experimental conditions used to isolate the hydroxylated ABA compounds, we found that the open form 9′-OH ABA closed to neoPA. Isolation of the closed form after extensive manipulation does not necessarily indicate that this is the predominant form in plant cells.
Subsequent to our initial identification of neoPA in B. napus siliques, we showed that neoPA occurs in a variety of other plants and tissues (Table III). The widespread occurrence of neoPA in different plants implies that ABA 9′-hydroxylation pathway is significant and general. In the germination assay with Arabidopsis seeds, we observed that the open form of the natural (+)-isomer of 9′-OH ABA is hormonally active (although less so than (+)-ABA), whereas the closed form (neoPA) is inactive (Fig. 4). In the B. napus microspore-derived embryo model system, we were able to demonstrate that (+)-9′-OH ABA is hormonally active on a level equivalent to natural ABA with respect to induction of the 3-ketoacyl-CoA synthase gene, the latter encoding a condensing enzyme essential to the production of erucic and eicosenoic acids in the developing embryo at the (early) torpedo stage. NeoPA (the closed form of (+)-9′-OH ABA) was comparatively far less active (Fig. 5). These findings are analogous to the observations that 8′-OH ABA and the stable analog 8′ methylene ABA (Qi et al., 1998), like ABA, induce expression of specific genes in mid-cotyledonary microspore-derived embryos of B. napus, whereas PA (the closed form of 8′-OH-ABA) does not (Zou et al., 1995; Qi et al., 1998). The reduction of activity of 9′-OH ABA in the induction of 3-ketoacyl-CoA synthase gene from 24 to 72 h could be due to cyclization of the 9′-OH ABA to neoPA, similar to the observation in 8′-OH ABA-induced gene expression (Zou et al., 1995). Thus, 9′-OH-ABA plays a critical role in lipid-related gene induction in developing embryos.
This study and others in which hydroxylated ABAs exhibit significant hormonal activity (Todoroki et al., 1994; Hill et al., 1995) suggest the possibility that these compounds may play a role in ABA signaling before cyclization or further degradation. It is noteworthy that neoPA was found in drought-stressed seedlings of barley and B. napus but not well-watered seedlings. In future studies, we will compare the gene expression changes induced by each of the hydroxylated ABA catabolites with those induced by ABA, and we will identify metabolites of neoPA and 9′-OH ABA.
In this paper, we have described the discovery of two novel ABA metabolites, 9′-OH ABA and neoPA, arising from a new pathway of ABA metabolism in plants, and we demonstrate that 9′-OH ABA has significant activity in two ABA bioassays. These findings enrich our knowledge of ABA metabolism by revealing hitherto unappreciated complexity, and open new avenues of research in hormone signaling.
MATERIALS AND METHODS
ABA, ABA Catabolites, and Deuterium-Labeled Analogs
The following chemicals were prepared as described previously: (+)ABA (Balsevich et al., 1994); [8′,9′-2H6]ABA (Lamb et al., 1996); [5,8′-2H4]ABA (Abrams et al., 2003); [8′-2H3]ABA (Rose et al., 1997); S-(+)-7′-OH ABA (Nelson et al., 1991); and (-)-PA (Balsevich et al., 1994). (+)-9′-OH ABA, plus its enantiomer, and (2Z,4E)-5-[(1S,5S,8S)-8-hydroxy-1,5-dimethyl-3-oxo-6-oxa-bicyclo[3.2.1]oct-8-yl]-3-methyl-penta-2,4-dienoic acid ((+)-neoPA), plus its enantiomer, were synthesized. The methyl ester of (+)-neoPA was identical to that reported (Takahashi et al., 1989). The 1H-NMR of synthetic (-)-neoPA is shown in Figure 3.
Plant Materials
Brassica napus cv Quantum plants were grown in a greenhouse under natural light supplemented with artificial light and heating to provide a 16-h light (25°C)/8-h night (18°C) cycle. Drip irrigation (Netafim Irrigation Inc., East Hamilton, Fresno, CA) of Plant-Prod 24-10-20 (Plant Products Co. Ltd., Brampton, ON, Canada) at 165 mg nitrogen L-1 was used for each young plant/seedling once a day for 2 min (rate of application 80-100 mL min-1); increased to 2 min twice daily for mature plants; and later increased to 4 to 6 min twice daily (a total of 320-600 mL d-1). The humidity was maintained at 45% to 65%. Only the primary and two secondary inflorescences were retained on each plant. Siliques were separated into seeds and pericarps, weighed, and frozen in liquid nitrogen before lyophilization.
For large-scale purification and identification of ABA metabolites, B. napus cv AC EXCEL plants grown at the experimental farm of the Saskatoon Research Centre, Agriculture and Agri-Food Canada were collected between September 12 and 17, 2002. The branches with green siliques were shipped on dry ice and stored at -20°C. Green seeds were isolated from pericarps and frozen immediately in liquid nitrogen. The lyophilized seeds were mixed and stored for extraction.
Other plant materials were: a green chickpea (Cicer arietinum L. cv Kabula Yuma) capsule from a greenhouse-grown plant with the seed separated from the remainder of the fruit; a portion of the peel and fruit of a Navel orange (Citrus sinensis L. Osbeck); green siliques and green leaves of Arabidopsis ecotype Columbia; garden-grown green cherry tomatoes (Lycopersicon esculentum Mill cv Favorita); mature rice (Oryza sativa L. cv Nipponbare) leaves; 10-d-old B. napus cv Quantum seedlings, which were air-dried to lose 10% to 15% of fresh weight and then placed in a sealed plastic bag for 5 h; 10-d-old barley (Hordeum vulgare cv Himalaya) seedlings, which were air-dried to lose 10% to 15% of fresh weight and then placed in a sealed plastic bag for 5 h.
Feeding Studies with Labeled ABA
Deuterium-labeled ABA S-(+)-[8′-2H3]ABA (1 and 10 μg silique-1), S-(+)-[5,8′-2H4]ABA (33 μg silique-1), and S-(+)-[8′,9′-2H6]ABA (33 μg silique-1) were dissolved in methanol at 100 μg μL-1 and then diluted with 1% (v/v) detergent MERGE to the desired concentrations. The ABA solutions were applied to immature green siliques either through surface application or injection at 20 μL silique-1. Tissues were harvested after 48 h and immediately frozen in liquid nitrogen before lyophilization.
Sample Extraction and Purification
Freeze-dried tissues were extracted and prepared by the same procedures as described previously (Zhou et al., 2003).
Analysis of ABA and Catabolites by MS
LC-ESI-MS-MS
To track and analyze neoPA during large-scale isolations, 50-μL portions (1/1,000) of the sample were collected at each step, combined with 50 ng of [2H4]ABA, dried under vacuum, and reconstituted in 200 μL of acetonitrile: water:acetic acid (15:85:0.07, v/v) before LC-MS-MS.
Analyses for these experiments were performed on two LC-ESI-MS-MS systems. The HP1100 series binary HPLC system (Agilent, Palo Alto, CA) with a Quattro LC mass spectrometer (Micromass, Manchester, UK) has been described previously (Zhou et al., 2003). A similar system consisting of an Alliance 2695 quaternary HPLC system (Waters, Milford, MA) with a Quattro Ultima mass spectrometer (Micromass) was also used to analyze ABA and related metabolites. Chromatographic separations were performed using a 100- × 2.1-mm, 4-μm Genesis C18 HPLC column (Jones Chromatography Ltd., Hengoed, UK) and a solvent system comprising 0.07% (v/v) acetic acid in aqueous acetonitrile, with a linear gradient of 15% to 45% (v/v) acetonitrile over 10 min. Electrospray source conditions for the Quattro Ultima were: electro-spray (ES) capillary, 2.25 kV; cone, 25 V; aperture, 0.2 V; source temperature, 120°C; desolvation (N2) gas flow, 590 L h-1 at a temperature 350°C; and cone gas flow, 107 L h-1. MRM of target compounds was performed by MS-MS using a collision cell pressure of 4.5 × 10-3 mbar (Ar) and a collision cell potential (ELAB) of 8 to 18 eV, depending on the analyte. The following precursor [M-H]- to product ion transitions were used for MRM: ABA, m/z 263 → 153; [8′-2H3]ABA, m/z 266 → 156; [5,8′-2H4]ABA, m/z 267 → 156; PA, m/z 279 → 139 and 205; neoPA, m/z 279 → 205; 7′-OH ABA, m/z 279 → 151 and 205; and DPA, m/z 281 → 171. These compounds were quantified using calibration curves based on the ratio of the MRM peak area for each analyte to that for the appropriate internal standard ([2H3]ABA for plant tissues and [2H4]ABA for neoPA isolation) using known amounts of analyte and internal standard (Zhou et al., 2003).
Electron Impact MS
Methyl neoPA and methyl PA were analyzed on a Trio 2000 mass spectrometer (Fisons Instruments, VG Biotech, Altrincham, UK) by direct probe positive ion electron impact MS using a probe temperature of 200°C, a source temperature of 200°C, and an electron energy of 70 eV. The m/z ratios of the most prominent ions are as follows (percentage relative abundance in parentheses):
Methyl neoPA (M•+): 294 (7), 276 (8), 262 (4), 139 (39), 135 (62), 125 (100), 121 (79), 111 (19), 94 (71), 91 (56).
Methyl PA (M•+): 294 (2), 262 (20), 139 (58), 135 (64), 125 (53), 121 (90), 111 (74), 94 (85), 91 (100).
Isolation of 9′-OH ABA/neoPA from B. napus Seeds
For large-scale extraction, 158 g of lyophilized seeds was extracted once with acetone:water:acetic acid (80:19:1, v/v; 1.5 L). The filtrate volume was reduced to about 300 mL under reduced pressure using a rotary evaporator and was partitioned against hexane (3 × 300 mL). The aqueous phase was freeze-dried. Then the dry extract was dissolved in 5% (w/v) NaHCO3 (450 mL) and washed with ethyl acetate (3 × 450 mL). The aqueous phase was adjusted to pH 2.6 with 3 M HCl and extracted with ethyl acetate (5 × 450 mL). The combined organic fractions were washed with aqueous NaCl (180 g of NaCl, 1 L of water), dried over anhydrous Na2SO4, filtered, and evaporated under reduced pressure. The filtrate (440 mg) was dissolved in methanol (5 mL) and diluted to 50 mL with 1% (v/v) acetic acid. The solution was passed through a 50-mL filter (0.22-μm cellulose acetate Tube Top Filter, Corning Incorporated, Corning, NY). A 50-μL aliquot was removed for LC-MS-MS. The remainder of the clarified extract was loaded on a 35 cc Oasis HLB cartridge (6 g of sorbent; Waters) preconditioned with methanol (50 mL) followed by 1% (v/v) acetic acid (100 mL). After loading the sample, the cartridge was washed with 1% (v/v) acetic acid (50 mL) and eluted with 50-mL portions of solvent with increasing proportions of methanol. A 50-μL aliquot of each fraction was removed for LC-MS-MS. NeoPA was found in the fraction of methanol:water:acetic acid (60:39:1, v/v). This fraction was dried under reduced pressure using a rotary evaporator and reconstituted in 1 mL of acetonitrile:water:acetic acid (15:84:1, v/v). Subsequently, this fraction was loaded on two normal-phase silica gel plates (20 × 20 cm, 250-μm layer thickness; Silica gel 60 F254, EM Science, Gibbstown, NJ) and developed with ethyl acetate:chloroform:acetic acid (100:50:7.5, v/v). The relative retention (RF value) for each compound was ABA = 0.68; neoPA = 0.60; PA = 0.52; 9′-OH-ABA = 0.43; 7′-OH-ABA = 0.36; DPA = 0.21; ABA-GE = 0.04. The band corresponding to neoPA, identified by LC-MS-MS, was excised, and the silica was extracted with ethyl acetate: methanol (100:5, v/v; 10 mL). The neoPA fraction was dried and then reconstituted in acetonitrile:water:acetic acid (20:79:1, v/v) and clarified with a 0.2-μm nylon filter (Costar Spin-X HPLC Micro Centrifuge filter, Corning). The sample was further purified by semipreparative HPLC (25 cm×10 mm, 5 μm; Supelcosil LC-18 HPLC column, Supelco, Belletone, PA). The mobile phase was acetonitrile:water:acetic acid (20:80:0.07, v/v) that ran at a flow rate of 5 mL min-1. The separation was carried out on a 1100 series HPLC (Hewlett-Packard, Palo Alto, CA) equipped with a guard column (5 cm×4.6 mm; Pelliguard LC-18, Supelco) and a HP 1040 photodiode array detector (Hewlett-Packard) at 262 nm. NeoPA had a retention time of 15 min with λmax = 265 nm. Approximately 30 μg of neoPA was isolated. 1H-NMR (C2H3O2H): δ 7.67 (d, J = 16.0 Hz, 1H, C-4), 6.01 (d, J = 16.0 Hz, 1H, C-5), 5.84 (s, 1H, C-2), 3.80 (dd, J = 2.5, 9.0 Hz 1H, C9′), 3.76 (d, J = 9.0 Hz, 1H, C-9′), 2.83 (d, J = 17.5 Hz, 1H, C-3′), 2.81 (d, J = 2.5, 17.5 Hz, 1H, C-5′), 2.26 (dd, J = 1.5, 17.5 Hz, 1H, C-3′), 2.19 (dd, J = 1.5, 17.5 Hz, 1H, C-5′), 1.95 (s, 3H, C-6), 1.10 (s, 3H, C-7′), 0.91 (s, 3H, C-8′). The NMR spectrum was obtained on a BrukerAvance DRX 500 MHz NMR (Bruker Biospin, Rheinstetten, Germany) equipped with a CryoProbe accessory (Bruker Biospin AG, Fällanden, Switzerland).
Germination Assay
For each replicate assay, 50 seeds of Arabidopsis (ecotype Columbia) were placed on two sheets of filter paper (70 mm; No. 1, Whatman, Clifton, NJ) in a petri plate (100 × 15 mm) wetted with 2 mL of sterile water containing the additives indicated. Plates were sealed with Parafilm and incubated at 24°C, with a 16-h/8-h light/dark cycle for the duration of the experiment (7 d). The number of seeds germinated in each plate was recorded twice each day. ABA and ABA analogs were added from ethanolic stock solutions, and control plates contained ethanol at the highest amount in the test plates. The results were quantified by a weighted germination index (Walker-Simmons, 1988).
Study of 3-Ketoacyl-CoA Gene Induction by 9′-OH ABA: Embryo Treatments and Northern Analysis
B. napus cv Hero plants were grown in controlled environment growth chambers and microspores were isolated and cultured according to the methods described previously for B. napus cv Reston (Zou et al., 1995; Qi et al., 1998). The microspore-derived embryos were enriched in the torpedo stage. Embryos in liquid culture were supplemented with 10 μm ABA, 9′-OH ABA, or neoPA. An initial T0 control was untreated. Embryos were maintained in the dark at 25°C on a rotary shaker 50 rpm. After 0, 6, 24, and 72 h of hormone treatment, individual plates of embryos for each treatment were harvested and rinsed with sterile distilled water, and the medium was stored at -20°C. Total RNA was isolated from harvested embryos of each treatment and fractionated on 1.2% (w/v) formaldehyde agarose gels. The RNA was subsequently transferred to Hybond N+ membrane and hybridized at high stringency (65°C) overnight with a 32P-labeled Arabidopsis 3-ketoacyl-CoA synthase1 (FAE1) DNA probe, prepared using the Random Primers DNA labeling kit as described previously (Qi et al., 1998).
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor.
Acknowledgments
We thank Dr. G.F.W. Rakow and Mr. Don Rode for permission to collect B. napus siliques from Agiculture and Agri-Food Canada field plots, Jenny Yang for help with large-scale seed isolation, and Drs. Irina Zaharia and Garth Abrams for helpful discussions.
This work was supported by Dow AgroSciences and Natural Sciences and Engineering Research Council of Canada (strategic grant no. 234754-2000 to S.R.A.). This paper is National Research Council of Canada number 45,271.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.030734.
References
- Abrams SR, Nelson K, Ambrose SJ (2003) Deuterated abscisic acid analogs for mass spectrometry and metabolism studies. J Label Compd Radiopharm 46: 273-283 [Google Scholar]
- Balsevich JJ, Cutler AJ, Lamb N, Friesen LJ, Kurz EU, Perras MR, Abrams SR (1994) Response of cultured maize cells to (+)-abscisic acid, (-)abscisic acid, and their metabolites. Plant Physiol 106: 135-142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer GL, Zeevaart JAD (1986) 7′-Hydroxy (-)-R-abscisic acid: a metabolite of feeding (-)-R-abscisic acid to Xanthium strumarium. Phytochemistry 25: 1103-1105 [Google Scholar]
- Chiwocha SDS, Abrams SR, Ambrose SJ, Cutler AJ, Loewen M, Ross ARS, Kermode AR (2003) A method for profiling classes of plant hormones and their metabolites using liquid chromatography-electrospray ionization tandem mass spectrometry: an analysis of hormone regulation of thermodormancy of lettuce (Lactuca sativa L.) seeds. Plant J 35: 405-417 [DOI] [PubMed] [Google Scholar]
- Cutler AJ, Krochko JE (1999) Formation and breakdown of ABA. Trends Plant Sci 4: 472-478 [DOI] [PubMed] [Google Scholar]
- Cutler AJ, Rose PA, Squires TM, Loewen MK, Shaw AC, Quail JW, Krochko JE, Abrams SR (2000) Inhibitors of abscisic acid 8′-hydroxylase. Biochemistry 39: 13614-13624 [DOI] [PubMed] [Google Scholar]
- Hampson CR, Reaney MJT, Abrams GD, Abrams SR, Gusta LV (1992) Metabolism of (+)-abscisic acid to (+)-7′-hydroxyabscisic acid by bromegrass cell cultures. Phytochemistry 31: 2645-2648 [Google Scholar]
- Hetherington AM (2001) Guard cell signaling. Cell 107: 711-714 [DOI] [PubMed] [Google Scholar]
- Hill RD, Liu JH, Durnin D, Lamb N, Shaw A, Abrams SR (1995) Abscisic acid structure-activity relationships in barley aleurone layers and protoplasts. Plant Physiol 108: 573-579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krochko JE, Abrams GD, Loewen MK, Abrams SR, Cutler AJ (1998) (+)-Abscisic acid 8′-hydroxylase is a cytochrome P450 monooxygenase. Plant Physiol 118: 849-860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb N, Wahab N, Rose PA, Shaw AC, Abrams SR, Cutler AJ, Smith PJ, Gusta LV, Evan B (1996) Synthesis, metabolism and biological activity of a deuterated analogue of the plant hormone S-(+)-abscisic acid. Phytochemistry 41: 23-28 [Google Scholar]
- Lehmann H, Preiss A, Schmidt J (1983) A novel abscisic acid metabolite from cell suspension cultures of Nigellas damascena. Phytochemistry 22: 1277-1278 [Google Scholar]
- Lehmann H, Schwenen L (1988) Nigellic acid: an endogenous abscisic acid metabolite from Vicia faba leaves. Phytochemistry 27: 677-678 [Google Scholar]
- MacMillan J, Pryce RJ (1968) Phaseic acid, a putative relative of abscisic acid, from seed of Phaseolus multiflorus. Chem Commun 124-126 [DOI] [PubMed]
- Milborrow BV (1968) Identification and measurement of (+)-abscisic acid in plants. In F Wightman, G Setterfield, eds, Biochemistry and Physiology of Plant Growth Substances. The Runge Press Ltd., Ottawa, pp 1531-1545
- Milborrow BV (1969) Identification of “Metabolite C” from abscisic acid and a new structure for phaseic acid. Chem Commun 966-967
- Milborrow BV (1970) The metabolism of abscisic acid. J Exp Bot 21: 17-29 [Google Scholar]
- Milborrow BV (2001) The pathway of biosynthesis of abscisic acid in vascular plants: a review of the present state of knowledge of ABA biosynthesis. J Exp Bot 52: 1145-1164 [PubMed] [Google Scholar]
- Nelson LAK, Shaw AC, Abrams SR (1991) Synthesis of (+)-, (-)-, and (+/-)-7′-hydroxy abscisic acid. Tetrahedron 47: 3259-3270 [Google Scholar]
- Qi Q, Rose PA, Abrams GD, Taylor DC, Abrams SR, Cutler AJ (1998) (+)-Abscisic acid metabolism, 3-ketoacyl-coenzyme A synthase gene expression, and very long-chain monounstaurated fatty acid biosynthesis in Brassica napus embryos. Plant Physiol 117: 979-987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie SM, Swanson SJ, Gilroy S (2002) From common signaling components to cell specific responses: insights from the cereal aleurone. Physiol Plant 115: 342-351 [DOI] [PubMed] [Google Scholar]
- Rose PA, Cutler AJ, Irvine NM, Shaw AC, Squires TM, Loewen MK, Abrams SR (1997) 8′-Acetylene ABA: an irreversible inhibitor of ABA 8′-hydroxylase. Bioorg Med Chem Lett 7: 2543-2546 [Google Scholar]
- Schmitz N, Abrams SR, Kermode AR (2000) Changes in abscisic acid content and embryo sensitivity to (+)-abscisic acid during the termination of dormancy of yellow cedar seeds. J Exp Bot 51: 1159-1162 [DOI] [PubMed] [Google Scholar]
- Schwartz SH, Qin X, Zeevaart JAD (2003) Elucidation of the indirect pathway of abscisic acid biosynthesis by mutants, genes, and enzymes. Plant Physiol 131: 1591-1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Oritani T, Yamashita K (1989) Synthesis of (+)-methyl phaseate and its isomer from (-)-β-pinene. Agric Biol Chem 53: 2711-2718 [Google Scholar]
- Taylor IB, Burbidge A, Thompson AJ (2000) Control of abscisic acid synthesis. J Exp Bot 51: 1563-157511006307 [Google Scholar]
- Tinelli ET, Sondheimer E, Walton DC (1973) Metabolites of 2-14C-abscisic acid. Tetrahedron Lett 2: 139-140 [Google Scholar]
- Todoroki Y, Hirai N, Koshimizu K (1994) 8′- and 9′-Methoxyabscisic acids as antimetabolic analogs of abscisic acid. Biosci Biotech Biochem 58: 707-715 [Google Scholar]
- Walker-Simmons M (1988) Enhancement of ABA responsiveness in wheat embryos by high temperature. Plant Cell Environ 11: 769-775 [Google Scholar]
- Wang Z, Mambelli S, Setter TL (2002) Abscisic acid catabolism in maize kernels in response to water deficit at early endosperm development. Ann Bot 90: 623-630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L, Schumaker KS, Zhu JK (2002) Cell signaling during cold, drought, and salt stress. Plant Cell Suppl 14: S165-S183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart JAD (1999) Abscisic acid metabolism and its regulation. In PJJ Hooykaas, MAK Hall, R Libbenga, eds, Biochemistry and Molecular Biology of Plant Hormones. Elsevier Science. Amsterdam, pp 189-207
- Zeevaart JAD, Creelman RA (1988) Metabolism and physiology of abscisic acid. Annu Rev Plant Physiol Plant Mol Biol 39: 439-473 [Google Scholar]
- Zhou R, Squires TM, Ambrose SJ, Abrams SR, Ross ARS, Cutler AJ (2003) Rapid extraction of ABA and its metabolites for liquid chromatography-tandem mass spectrometry analysis. J Chromatogr A 1010: 75-85 [DOI] [PubMed] [Google Scholar]
- Zou J, Abrams GD, Barton DL, Taylor DC, Pemeroy MK, Abrams SR (1995) Induction of lipid and oleosin biosynthesis by (+)-abscisic acid and its metabolites in microspore-derived embryos of Brassica napus L. cv Reston. Plant Physiol 108: 563-571 [DOI] [PMC free article] [PubMed] [Google Scholar]