Abstract
Increasing evidence indicates that survivin, an inhibitor of apoptosis protein (IAP), is expressed in human cancer cells but is absent from most normal adult tissues. Here, we examined the feasibility of using a survivin promoter (Sur-P) to direct therapeutic expression of a proapoptotic gene specifically in human tumor cells. First, we demonstrated that this promoter was highly active in human tumor cells but not in normal cells. Second, we found that Sur-P activity was upregulated by hypoxia in tumor cells. Third, to further enhance this promoter’s activity under hypoxia, we added a hypoxia-responsive element (HRE) from the vascular endothelial growth factor gene promoter in its 5′ region, and showed that this combination resulted in a further increase in the level of gene expression in hypoxic tumor cells. Finally, we demonstrated that expression of an autocatalytic reverse caspase-3 gene by this promoter specifically induced apoptotic cell death in human tumor cells but not in normal cells. These findings support the use of promoters Sur-P or chimeric HRE-Sur-P for generating novel vectors for cancer gene therapy.
Keywords: survivin promoter, reverse caspase-3 gene, hypoxia, hypoxia-responsive element, apoptosis, targeted cancer gene therapy, hypoxia-inducible factor1 (HIF-1)
Introduction
Development of therapeutic approaches specifically targeting cancer cells has been an intensive research focus for cancer therapy. A promising strategy is to deliver suicidal and/or cytotoxic genes into tumor cells through gene therapy vectors, which induces cancer cell death. Therefore, an important aspect of cancer gene therapy is to restrict the expression of the suicidal and cytotoxic genes to the tumor cells, thereby limiting damage to normal cells. Previous studies have demonstrated that tissue- or tumor-specific promoters, which are highly expressed in the liver, colon, melanoma, prostate or breast cancer, are capable of directing therapeutic gene expression in the tumor cells.1–5 However, this approach has limitations as many of these promoters are active only in specific tumor types and some of the tissue-specific promoters can lead to serious side effects due to expression of therapeutic genes in normal cells.1,6,7
Recently, a family of inhibitor of apoptosis (IAP) proteins has been characterized and their roles in blocking apoptotic pathways and heightening resistance to therapeutic reagents have been elucidated.8–10 Survivin, a novel member of the IAP family of proteins, is involved in apoptosis control as well as regulation of cell division.11 It is expressed in 60–70% of many common tumor types but not in normal, differentiated adult tissues.12–15 Its upregulation in human cancer cells has been related to the resistance of the cancer cells to chemo-and radiotherapy.16,17 Furthermore, the level of survivin expression in tumor tissues is correlated with the prognosis of the patients.15,18,19 These results have provided the rationale of targeting survivin for cancer therapy. For example, downregulation of survivin expression or function induces apoptotic cell death specifically in tumor cells and enhances the antitumor effects of several chemotherapy drugs.12,20–22 On the other hand, the unique expression of survivin in cancer cells but not in most normal adult tissues suggests that the survivin promoter (Sur-P) may be useful for cancer gene therapy. However, this research area has not yet been extensively explored.
In this study, we examined the activity of a number of Sur-P with different lengths in human breast and pancreatic cancer cell lines as well as in normal cell lines, and found that the 269-bp survivin core promoter has sufficient promoter activity and specificity for driving target gene expression in cancer but not normal cells. We also found that hypoxia increases Sur-P activity as well as its expression in human tumor cells. Moreover, a combination of the Sur-P with a hypoxia-responsive element (HRE) from the vascular endothelial growth factor (VEGF) gene further increased the activity of the Sur-P in tumor cells. Finally, we demonstrated the feasibility of using the Sur-P to control the expression of an apoptosis-inducing gene and to induce cell death specifically in human tumor cells.
Results
A 269-bp survivin core promoter is sufficient to drive specific expression of the luciferase gene in tumor cells
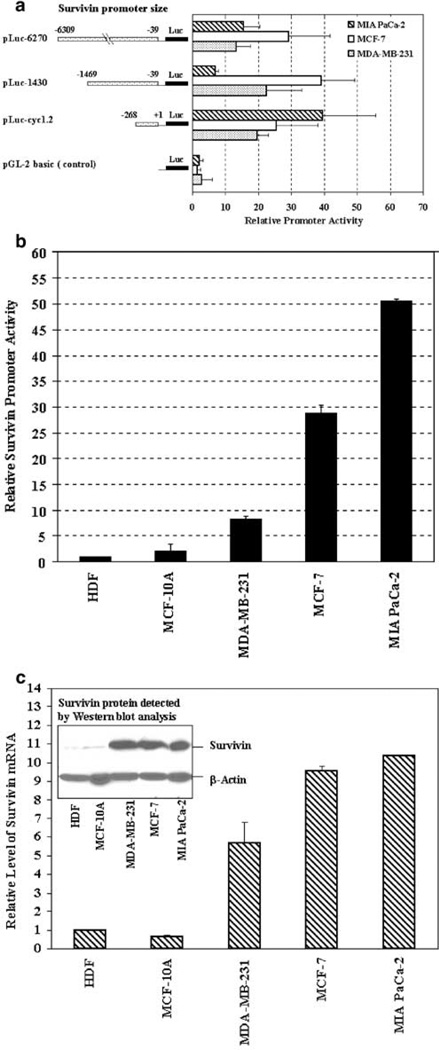
To determine the strength and specificity of different Sur-P fragments in controlling gene expression, we compared their ability to direct the expression of a luciferase reporter gene. pLuc-6270, pLuc-1430 and pLuc-cyc1.2 plasmids (see Materials and methods) were transiently transfected in human breast cancer MCF-7 and MDA-MB-231, and pancreatic cancer MIA PaCa-2 cell lines. Intermediate to high levels of promoter activities were detected in the tumor cell lines, transfected (Figure 1a). As compared to the luciferase activity measured in pGL-2-basic plasmid-transfected cells, relatively high levels of promoter activities (19- to 39-fold) were measured in all three tumor cell lines transfected with pLuc-cyc1.2. pLuc-1430 displayed high levels of promoter activity (22- to 39-fold) in MDA-MB-231 and MCF-7 cells and moderate activity (seven-fold) in MIA PaCa-2 cells. The relative activity of pLuc-6270 was intermediate (13-to 15-fold) in MDA-MB-231 and MIA PaCa-2 cells and high (29-fold) in MCF-7 cells (Figure 1a). Our results indicated that in all three human tumor cell lines, the activity of the 269-bp survivin core promoter (pLuc-cyc1.2) construct is comparable to or higher than the two other Sur-P containing 1430 (pLuc-1430) and 6270 (pLuc-6270) nt of 5′-flanking sequences.
Figure 1.
Examination of Sur-P activity in human tumor and normal cell lines. (a) The promoter activity of different Sur-P–luciferase constructs in human tumor cell lines. The relative luciferase activity was a ratio of firefly and renilla luciferase activity for each sample. The relative promoter activity was calculated using the relative luciferase activity from MCF-7 cells transfected by pGL-2-basic construct as a reference of 1. Bars indicate mean values plus standard deviation (s.d.) of three (MDA-MB-231 and MIA PaCa-2) or four (MCF-7) independent experiments. (b) High levels of Sur-P activities were detected in human cancer lines, but the activity was very low in normal human cell lines. The relative Sur-P activity for each cell line was calculated using the relative luciferase activity of HDF cell line transfected by pLuc-cyc1.2 as a reference of 1. The numbers in the bar were the mean values plus s.d. of two to three repeat samples. (c) The levels of survivin expression in human tumor and normal cell lines detected by real-time RT-PCR and Western blot analyses. The relative level of survivin mRNA was a ratio of quantity of PCR products from the survivin gene and the quantity of β-actin products. Each bar in the figure represents the mean value and s.d. of two to three repeat samples. A high level of survivin protein located at 16.5 kDa was detected in human tumor cell lines MDA-MB-231, MCF-7 and MIA PaCa-2 by Western blot analysis. In contrast, a very low level of survivin protein was found in normal cell lines MCF-10A and HDF.
Next, we investigated the specificity of the 269-bp core Sur-P in driving gene expression in a number of normal and cancerous cell lines. High levels of pLuc-cyc1.2 Sur-P activities were observed in human tumor cell lines but not in normal cell lines (Figure 1b). Pancreatic cancer cell line MIA PaCa-2 displayed the strongest promoter activity among the three tumor cell lines with a relative luciferase activity 25- or 50-fold higher than that detected in normal human mammary epithelial cell line MCF-10A or primary human dermal fibroblast cell line HDF (Figure 1b). About 15- to 29-fold higher Sur-P activity was also detected in MCF-7 cells as compared with the two normal cell lines (Figure 1b). Of three tumor cell lines, MDA-MB-231 cells had a relatively low Sur-P activity, which was still about four- to eight-fold higher than that of the normal cell lines (Figure 1b). These data strongly suggest that the 269-bp survivin core promoter has sufficient strength and specificity for potential cancer gene therapy.
To determine whether the level of promoter activity in the reporter assay reflected the level of endogenous survivin expression, we examined survivin expression in human tumor and normal cell lines by real-time RT-PCR and Western blot analyses (Figure 1c). We found that the expression of survivin mRNA and protein is strikingly higher in human cancer cell lines than in normal cell lines, consistent with the results from Sur-P-luciferase reporter assay studies (Figure 1c).
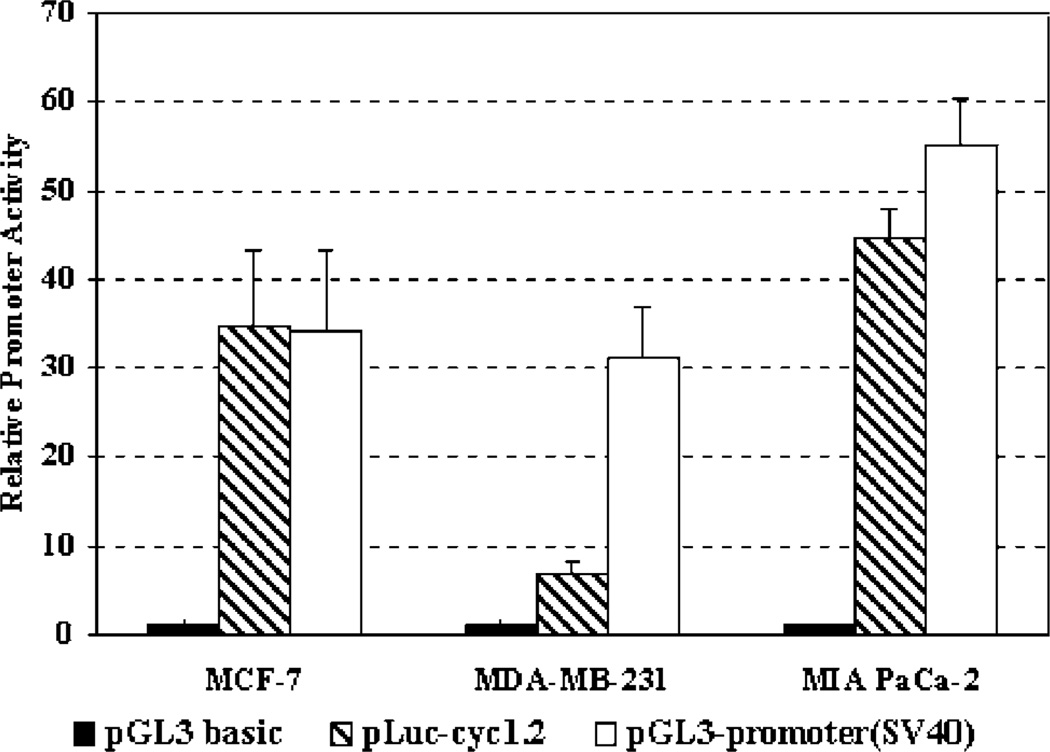
A common way to determine the strength of a tissue-or tumor-specific promoter is to compare the activity with viral promoters, such as CMV or SV-40 promoter. We compared the activity of the −269 nt Sur-P with the SV-40 viral promoter using a pGL-3 SV-40 promoter construct. Our results showed that the Sur-P activity in MCF-7 and MIA PaCa-2 cell lines was about the same as that obtained with the SV-40 promoter. While in MDA-MB-231 cells, it was about one-fourth (Figure 2). Therefore, the survivin core promoter is not only specifically activated in the tumor cells but also has high transcriptional activity, which makes it attractive for constructing gene therapy vectors that produce high levels of therapeutic gene expression specifically in human tumor cells.
Figure 2.
Comparison of the activity of the survivin core promoter with a viral promoter (SV 40). The tumor cell lines were transfected with pLuc-cyc1.2, pGL3 promoter (SV-40) or control pGL3 plasmid for 24 h. The firefly luciferase activities in the cell lysates were measured. The relative promoter activity was calculated based on the luciferase activity of pGL3-basic vector-transfected cells.
Hypoxia upregulates survivin expression and promoter activity in human tumor cell lines
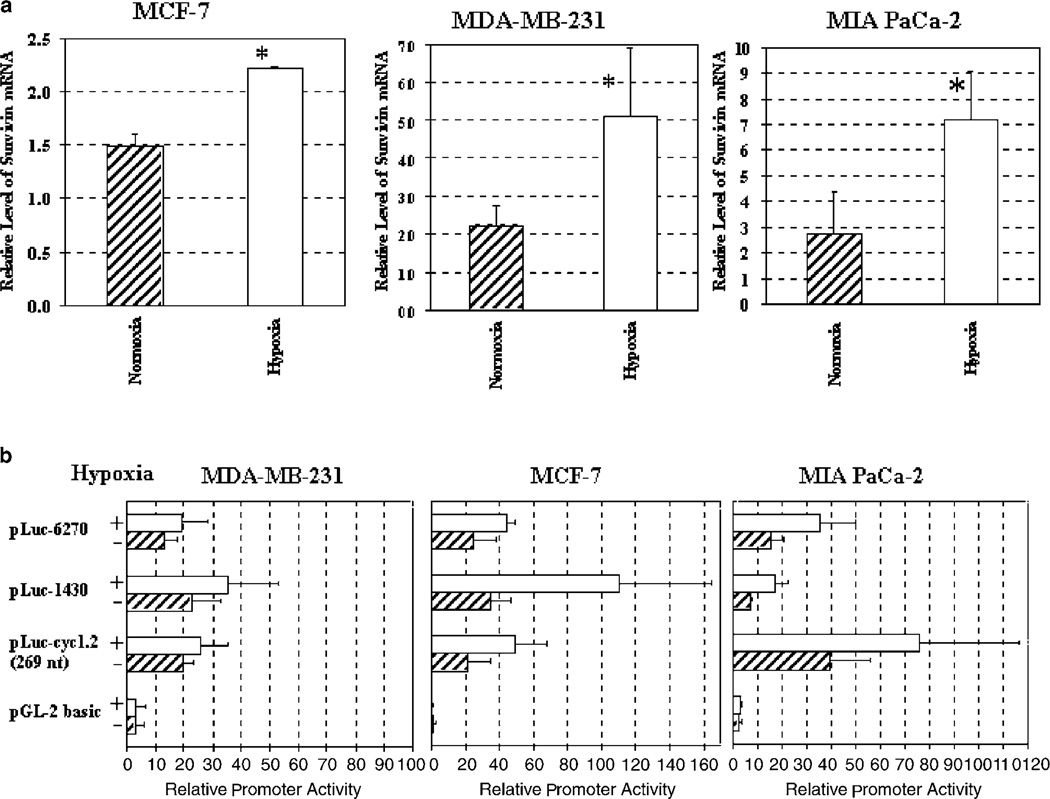
The presence of hypoxic regions in solid tumors is a common phenomenon and results in dramatic alterations in gene expression.23 We explored whether a hypoxic environment could affect survivin expression and promoter activity. Human breast cancer MDA-MB-231 and MCF-7, and pancreatic cancer MIA PaCa-2 cells were cultured in normoxic or hypoxic conditions (1% O2, 5% CO2 and 94% N2) and total RNAs were isolated. The level of survivin gene expression was examined by realtime RT-PCR analysis. As shown, survivin mRNA was significantly upregulated (1.5- to 2.6-fold) in all three cancer cell lines 8 h after hypoxia (Figure 3a, P < 0.05). The differences between the normoxic and hypoxic groups were statistically significant (MCF-7: P=0.0039; MDA-MB-231, P=0.05; and MIA PaCa-2: P=0.009, Student’s t-test, data obtained from two to three independent experiments).
Figure 3.
Upregulation of survivin gene expression and promoter activity in hypoxic tumor cells. (a) Hypoxia upregulated survivin gene expression in human tumor cell lines detected by real-time RT-PCR. The relative level of survivin mRNA was a ratio of quantity of survivin PCR products and the quantity of β-actin products. The bar in the figure represents the mean value and s.d. of two to three independent experiments. *Significant differences between the levels of survivin mRNA in normoxia and hypoxia groups were found in the tumor cell lines (Student’s t-test: P < 0.05 for all of the tumor cell lines). (b) The promoter activity of different sized Sur-P–luciferase constructs in human tumor cell lines cultured under normoxia or hypoxia. The relative Sur-P activity was calculated based on the relative luciferase activity of MCF-7 cell lysate transfected with a control pGL2-basic plasmid and cultured under normoxic conditions. The bars in the figure represent the mean values and s.d. of the relative Sur-P activity from four (MCF-7) or three (MDA-MB-231 and MIA PaCa-2) independent experiments.
Next, we examined the Sur-P activity in hypoxic tumor cells. Sur-P luciferase reporter constructs (pLuccyc1.2, pLuc-1430, pLuc-6270) were transfected into MCF-7, MDA-MB-231 and MIA PaCa-2 human tumor cell lines. After culture in the presence or absence of hypoxia, luciferase activities in the cell lysates were measured. Under hypoxia, promoter activities of all three Sur-P–luciferase constructs were increased in all tumor cell lines (Figure 3b). Interestingly, high levels of hypoxia-induced expression of the luciferase gene were observed in pLuc-cyc1.2-transfected MIA PaCa-2 and MCF-7 cells and in pLuc-1430-transfected MCF-7 cells. This observation once again suggested that the 269-nt survivin core promoter has strong activity in most tumor cell lines and its promoter activity can be enhanced in hypoxic tumor cells. Therefore, this survivin core promoter is a good choice for the construction of cancer gene therapy vectors, as it stays active in hypoxic tumor cells.
Combination of the basic Sur-P with a HRE enhances its activity in hypoxic tumor cells while retaining its specificity
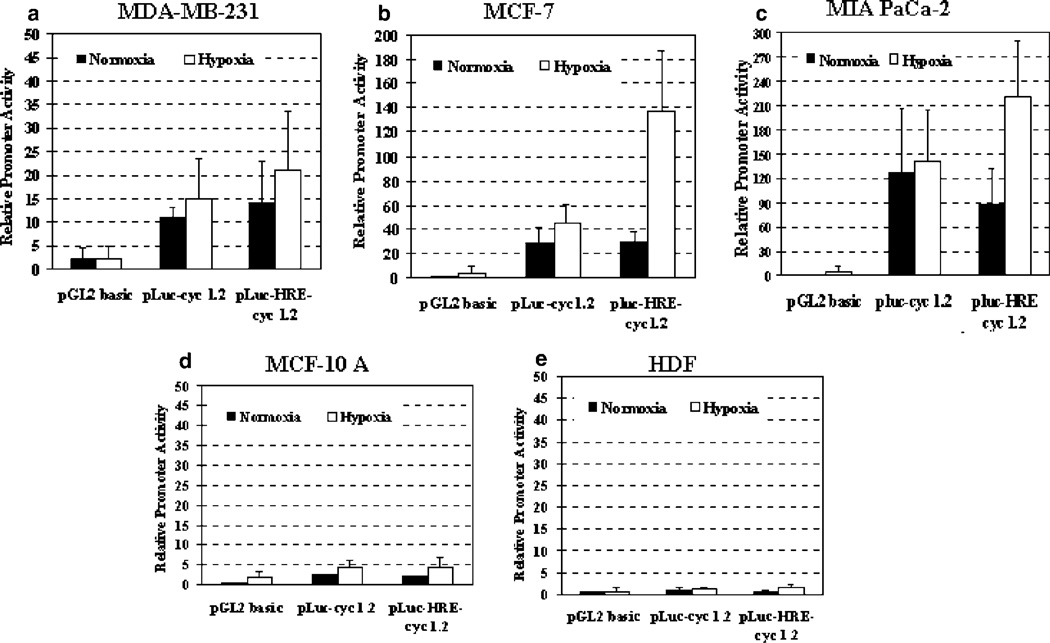
To further augment the levels of tumor-specific expression of the Sur-P, we decided to combine it with an exogenous HRE. It has been shown that hypoxia activates the expression of a number of genes through binding of two basic helix–loop–helix PAS proteins (HIF-1α and HIF-1β) to an HRE consensus DNA sequence.24 Several studies have demonstrated that HREs regulating the human VEGF and the erythropoietin (EPO) genes induce specific gene expression in hypoxic cells, and those constructs have great potential for cancer gene therapy.25–27 In this study, an HRE fragment composed of six tandem copies of VEGF-HRE (V6R)28 was cloned upstream of the 269-bp survivin core promoter in pLuc-cyc1.2. The resultant pLuc-HRE-cyc1.2 vector was transfected into human tumor and normal cell lines. After the cells were cultured with or without hypoxia for 24 h, luciferase activities were examined (Figure 4). As expected, a higher promoter activity was found in pLuc-HRE-cyc1.2-transfected tumor cells under hypoxic conditions (Figure 4a–c). pLuc-HRE-cyc1.2 showed 1.6- (MIA PaCa-2), 2-(MDA-MB-231) and 4.7- (MCF-7) fold stronger promoter activity than that of pLuc-cyc1.2 in the transfected cells after culture in hypoxic conditions (Figure 4; MCF-7 cells: P = 0.001).
Figure 4.
A combination of the survivin core promoter with VEGF-HRE increased the level of gene expression in tumor but not normal cells. Human breast cancer cell lines MDA-MB-231 (a) and MCF-7 (b), pancreatic cancer MIA PaCa-2 (c) and normal cell lines MCF-10A (d) and HDF (e) were transfected with Sur-P–luciferase construct (pLuc-cyc1.2) or chimeric VEGF-HRE–survivin promoter construct (pLuc-HRE-cyc1.2) and control pRL-SV-40 plasmid. The relative Sur-P activity was calculated based on the relative luciferase activity of MCF-7 cell lysate transfected with a control pGL2-basic plasmid under normoxic conditions. The bars in the figure represent the mean values plus s.d. from two (MDA-MB-231), four (MCF-10A and MIA PaCa-2), five (HDF) or six (MCF-7) independent experiments. There was a significant difference in the luciferase activity between pLuc-cyc1.2-transfected and pluc-HRE-cyc1.2-transfected MCF-7 cells under hypoxic conditions (P < 0.0013, Student’s t-test). Although the level of luciferase activity was higher in pLuc-HRE-cyc1.2-transfected MDA-MB-231 or MIA Paca-2 cells than that in pLuc-cyc1.2 transfected cells after hypoxia treatment, the difference was not statistically significant (MDA-MB-231: P < 0.6, MIA PaCa-2: P < 0.14, Student’s t-test).
Most importantly, we found that the VEGF-HRE did not alter the tumor specificity of the Sur-P, since low levels of promoter activity were detected in normal human cell lines MCF-10A (Figure 4d) and HDF (Figure 4e), transfected with pLuc-cyc1.2 or pLuc-HRE-cyc1.2 and then cultured with or without hypoxia. Thus, our results strongly suggest that a combination of the 269-bp survivin core promoter with the VEGF-HRE fragment V6R should direct cancer cell-specific transcription in normoxic and hypoxic tumor cells.
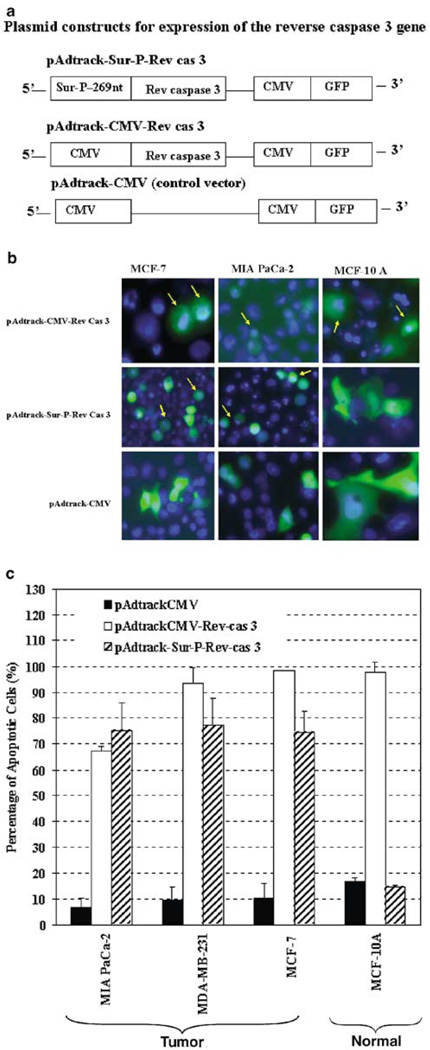
Expression of an autocatalytic reverse caspase-3 (Rev-cas-3) gene driven by the 269-nt survivin core promoter selectively induced cell death in human tumor but not normal cells
Next, we examined the feasibility of gene therapy using the 269-nt survivin core promoter to control therapeutic gene expression in tumor cells. We employed an autocatalytic Rev-cas-3 gene, which was derived from the procaspase-3 gene by switching the small subunit gene to the front of the large subunit gene.29 The resultant Rev-cas-3 gene products were able to process themselves autocatalytically into active forms of caspase-3 without the requirement of an active upstream apoptotic signaling. Our previous studies indicated that the constitutive expression of the Rev-cas-3 gene from a CMV promoter induced a high level of apoptosis in both tumor and normal cell lines.20 Here, we replaced the CMV promoter in a pAdtrackCMV-Rev-cas-3 vector with the survivin core promoter to obtain pAdtrack-Sur-P-Rev-cas-3 (Figure 5a). The Rev-cas-3 gene-expressing vectors controlled either by a CMV or by the survivin core promoter were transfected into human tumor and normal cell lines and the percentages of apoptotic cells, in the transfected cells (GFP positive) were determined 48 h later by Hoechst 33342 staining. As shown, expression of the Rev-cas-3 gene driven by the CMV promoter induced apoptosis in both tumor and normal cell lines (Figure 5b and c). Over 90% of breast cancer (MCF-7 and MDA-MB-231) and normal (MCF-10A) cells, as well as 68% of pancreatic cancer (MIA PaCa-2) cells, were undergoing apoptosis after transfection with pAdtrackCMV-Rev-cas-3 (Figure 5c). In contrast, expression of the Rev-casp-3 gene driven by the survivin core promoter specifically induced a high percentage (~75%) of apoptotic cell death in tumor cell lines but not in MCF-10A normal cell line (Figure 5b and c). The level of apoptosis in pAdtrack-Sur-P-Rev-cas–3-transfected MCF-10A normal cells was 15%, which was comparable with the control pAdtrackCMV vector-transfected group (Figure 5c). Thus, our results for the first time demonstrate that the 269-nt survivin core promoter is a good candidate to direct cancer cell-specific expression of therapeutic or cytotoxic genes, which exclusively results in apoptosis of tumor but not normal cells.
Figure 5.
Selective induction of apoptotic cell death in human tumor cell lines by expressing an autocatalytic Rev-cas-3 gene from the survivin core promoter. (a) Schematic illustration of the Rev-cas-3 gene expressing constructs. (b and c) Survivin core promoter-mediated expression of the Rev-cas-3 gene-induced apoptotic cell death in human tumor cells but not normal cells. Human cancer and normal cell lines were transfected with the plasmid constructs as shown in (a). (b) At 48 h after transfection, the cells were stained with Hoechst 33342 and examined under fluorescence microscope for the percentage of GFP-positive cells (Green) that displayed apoptotic nuclei (Blue, b). The representative morphology of GFP-positive cells undergoing apoptotic cell death was indicated by arrows in, some late-stage apoptotic cells were found with very weak or lost GFP fluorescence (c) Quantitative analysis of the percentage of the apoptotic cells in the Rev-cas-3 gene expressing plasmid-transfected cell population. The bars are the mean values plus s.d. of three to 12 fields under a × 20 microscope lens, which includes about 50–150 GFP-positive cells. There were significant differences in the percentage of apoptotic cells in pAdtrack-Sur-P-Rev-cas-3 vector-transfected MCF-10A cells in comparison with the pAdtrack-Surp-Rev-cas-3 vector-transfected MCF-7 (P < 4 × 10−11), MDA-MB-231 (P < 5.9 × 10−12) and MIA PaCa-2 cells (P < 1.2 × 10−4). Student’s t-test was used for all statistical analyses.
Discussion
Recent studies have shown that a novel member of the IAP protein family, survivin, is expressed at a high level in 60–100% of the most common human tumor types, including the colon, pancreas, breast, lung, liver, brain, lymphoma, melanoma and prostate cancers.12–15 Survivin is highly expressed in fetal tissues but is not found in most normal adult tissues.12 Therefore, the Sur-P should be an excellent candidate for controlling specific expression of therapeutic genes in human tumor cells. Since expression of survivin is upregulated in most common tumors, construction of cancer gene therapy vectors with the Sur-P has an advantage over many other tumorspecific promoters, which are only active in one or several types of human tumors.1
A previous study showed that a 1092-bp fragment of the human Sur-P could direct the expression of an alkaline phosphatase marker gene in human ovarian, breast, colon, lung and uterus cancer cell lines. Alkaline phosphatase activity was also detected in plasma samples of mice implanted with ovarian tumor cell lines stably transfected with this construct.30 Based on the observation of a low Sur-P activity in normal mouse fibroblast (NIH3T3) and mouse ovarian surface epithelial cell lines, it was concluded that Sur-P activity is specifically activated in tumor cells.30 However, it remained unclear what activity level the Sur-P would have in normal human cell lines and whether the 1092-bp promoter is optimal for the design of cancer gene therapy vectors. While a previous study had shown the activity of different sized Sur-Ps in Hela cells, it had not been determined which construct produced high activity while retaining tumor specificity.31
To answer some of these remaining questions, we examined human Sur-P activities in human breast and pancreatic cancer cells as well as in primary human normal cells. First, we examined a number of Sur-P-luciferase constructs containing 269, 1430 or 6270 nt of survivin gene. 5′-flanking sequences, and found that the promoter activity of the survivin core promoter (+1 to −268 bp of 5′-flanking region of the survivin gene) is comparable or higher than that of the other constructs. We further demonstrated that the 269-nt survivin core promoter directs a high level of luciferase activity in the three human tumor cell lines examined but not in two normal human cell lines. Consistent with the result of a previous study using the 1092 promoter fragment,30 we found that the −269 nt survivin core promoter has the same activity as the SV-40 promoter in two of three human tumor cell lines. Therefore, our results demonstrate, for the first time that the 269-nt survivin core promoter has strong promoter activity and is sufficient to direct specific gene expression in human tumor cells while maintaining minimal promoter activity in primary human fibroblast and normal immortalized human mammary epithelial cells. The identification of a small sized tumor-specific promoter has an important advantage for constructing virus-based vectors, since most of the viral vectors have a size limitation on inserting exogenous sequences.32
It is well known that human tumors contain regions that are deficient in oxygen due to a rapid growth rate of the tumor cells and the presence of an abnormal vasculature.23,24 Studies have shown that there are significant associations between intratumoral hypoxia and tumor metastases, response to chemotherapy or radiotherapy and prognosis of cancer patients.33–37 It has been shown that hypoxia modulates the expression of different sets of genes in cancer cells.36 To generate gene therapy vectors that are effective for hypoxic tumor cells, it is crucial to use transcriptional control elements that are active in these cells. The effect of hypoxia on the expression of the survivin gene was unknown. We found that hypoxia moderately upregulated the endogenous survivin steady state mRNA levels and the expression of Sur-P–luciferase reporter constructs. The promoter activities of all three promoter–luciferase constructs including the 5′-flanking region from −269 to −6270 nt were upregulated, suggesting the presence of hypoxia regulatory sequences within +1 to −268 nt of the Sur-P. Consistent with this notion, inspection of the 269-nt Sur-P revealed the presence of a putative core HIF-1α binding site (5′-CGTG-3′)38 in the −82 to −85 bp 5′-flanking region of the survivin gene. However, the role of the HIF-1α-like binding site in regulation of survivin gene expression in hypoxic condition requires further investigation.
Hypoxia induces upregulation of HIF-1α, a transcription factor, that mediates transcriptional responses in cells through binding to an HRE existing in hypoxia-inducible genes.35 The potential of using an HRE to control the expression of therapeutic genes in hypoxic cells has been examined by several laboratories. For example, combination of the VEGF-HRE or Epo-HRE with minimal promoters of either CMV or SV-40 greatly enhanced gene expression in hypoxic tumor cells or ischemic myocardium.28,39–41 An HRE from the VEGF gene was also engineered in the adenoviral vector to control the expression of the E1 gene, resulting in a hypoxia-activated replication-competent adenoviral vector that specifically replicates and induces cell death in hypoxic tumor cells.27
We also examined the feasibility of enhancing the level of specific gene expression in hypoxic tumor cells through the combination of six copies of VEGF-HRE with the survivin core promoter. The results from our study indicated that the presence of VEGF-HRE fragments in the 5′-flanking region of the Sur-P significantly increased the level of luciferase reporter gene expression in hypoxic cancer cells. Most importantly, we found that this chimeric enhancer/promoter construct retained the ability of controlling specific gene expression in human tumor cells while only exhibiting a very basal activity in normal cells. Thus, the results of this study demonstrate that a combination of Sur-P with VEGF-HRE further enhances the level of specific gene expression in hypoxic tumor cells. This chimeric construct should provide a powerful transcriptional control element for generating cancer gene therapy vectors that are able to target tumor cells with or without hypoxia. This tumor-specific promoter should also have advantages over other promoters since its activity is upregulated in hypoxic tumor cells, which are a cell population resistant to chemo- and/or radiotherapy.
Finally, as a proof-of-principle we wanted to demonstrate that the Sur-P’s ability to direct tumor-specific gene expression to tumor cells could be used to mediate specifically therapeutic tumor cell death. We constructed a vector with the expression of an apoptosis-inducing gene (Rev-cas-3) directed by the survivin core promoter (269 nt). Expression of this vector induced apoptotic cell death specifically in human tumor cells. Therefore, the Sur-P is an excellent candidate for generating novel gene therapy vectors that control the expression of apoptotic or other cytotoxic genes specifically to tumor cells.
Materials and methods
Human tumor and normal cell lines
Breast cancer cell lines MDA-MB-231 and MCF-7, pancreatic cancer cell line MIA PaCa-2 and normal immortalized human mammary epithelial cell line MCF-10A were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Primary normal human dermal fibroblast cell line HDF was purchased from Emory University Skin Disease Center (Atlanta, GA, USA). MIA PaCa-2 cells were cultured in DMEM medium (Mediatech, Herndon, VA, USA). Human breast cancer cell lines were maintained in DMEM/F-12 medium (50:50, Mediatech). All the above media were supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT, USA), 2 mm l-glutamine, 100 IU/ml penicillin and 100 µg/ml streptomycin (Mediatech). HDF was maintained in DMEM medium with 20% FBS. MCF-10A cells were cultured in DMEM/F12 medium supplemented with 20 ng/ml epidermal growth factor, 500 ng/ml hydrocortisome, 100 ng/ml of cholera toxin, 10 µg/ml insulin, 2 mm l-glutamine and 5% FBS.
Real-time RT-PCR
Total RNAs were isolated using RNA Bee kit (Tel-test, Friendswood, TX, USA). RNA samples (2 µg) were amplified with Omniscript RT kit using an oligo-dT primer (QIAGEN Inc., Valencia, CA, USA) to generate 20 µl of cDNAs. cDNA (1–2 µl) was then quantified by real-time PCR with primer pairs for survivin or β-actin using SYBR Green PCR Master mix. The real-time PCR was performed on ABI PRISM 7000 sequence detection system (Applied Biosystems, Foster City, CA, USA) or Bio-Rad iCycler (Bio-Rad laboratories, Hercules, CA, USA). The primer pairs for detection of the expression of survivin gene were: survivin forward, 5′-TCCACTGCCCCACTGAGAAC-3′; and survivin reverse, 5′-TGGCTCCCAGCCTTCCA-3′, which amplify a 76 nt PCR product located from 130 to 206 nt of survivin mRNA. Amplification of β-actin gene was used as an internal control for real-time RT-PCR. The primer pair for β-actin gene were: β-actin forward, 5′-AAAGACCTGTACGCCAACACAGTGCTGTCTGG-3′; and β-actin reverse, 5′-CG TCATACTCCTGCTTGCTGATCCACATCTGC-3′, which generate a 219 nt PCR product from 870 to 1089 nt of the β-actin mRNA sequence. The start quantity of PCR products from the amplification of the survivin gene was standardized with the start quantity of β-actin products for each sample to obtain a relative level of gene expression.
Western blot analyses
Cells were lysed in 50 mm HEPES, 50 mm NaCl, 5 mm EDTA, 10 mm Na2P2O7 ·10 H2O, 50 mm NaF, 1 mm NaVO4, 1% Triton X-100 and protease inhibitor cocktail tablets (Complete mini, Roche Molecular Biochemical, Indianapolis, IN, USA). Protein concentrations of the resulting lysates were determined using Bio-Rad protein assay kit. Protein (50 µg) was resolved on 12–15% polyacrylamide-SDS gels and then transferred to PVDF membranes (Bio-Rad laboratories). The membranes were blocked with 5% of nonfat milk in Tris-buffered saline for 1 h, and then incubated for 2 h with goat anti-human survivin (1:600) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) or mouse monoclonal anti-β-actin antibody (1:2000) (Sigma Chemical Co., St Louis, MO, USA). After three washings, the membranes were incubated with horseradish-peroxidase conjugated with anti-goat or mouse secondary antibody (Santa Cruz Biotechnology) for 1 h. The levels of specific proteins in each lysate were detected by Enhanced Chemiluminescence using ECL plus (Amersham International, Buckingham, UK) followed by autoradiography.
Sur-P–luciferase reporter constructs
Human Sur-P–firefly luciferase report constructs include: (1) pLuc-cyc 1.2, which contains a 269 nt 5′-flanking region from +1 to −268 bp of the survivin gene; (2) pLuc-1430 that contains 1430 nt of 5′-flanking region from −39 to −1469 bp of the survivin gene; and (3) pLuc-6270 containing 6270 nt of Sur-P sequence from −39 to −6309 bp of the survivin gene (see Figure 1a). Generation of these plasmids were described previously.31
To determine whether the HRE from the VEGF (VEGF-HRE) enhances the Sur-P activity, we engineered a construct with VEGF-HRE placed in the 5′ region of the 269 nt Sur-P. Six tandem copies of the VEGF-HRE (V6R) were cut from pBI-HRE vector using Xho1 and Stu1.28 Plasmid pLuc-HRE-cyc 1.2 was generated by cloning the blunt VEGF-HRE V6R fragment into pLuc-cyc 1.2 plasmid at a blunt BamH1 site, located 5′ region to the Sur-P. pGL2- or pGL3-basic plasmids (Promega Corp., Madison, WI, USA), which are promoter-less vectors and contain a firefly luciferase gene, were used as a control plasmid. The pGL-3 promoter plasmid (Promega) has an SV-40 promoter driving expression of the firefly luciferase gene.
Luciferase reporter assays
Cells were plated in 24-well tissue culture plates at 80– 90% confluence for 24 h. The cells were then cotransfected with 1 µg of various Sur-P–luciferase gene constructs and 10 ng of pRL-SV-40 internal control plasmid that expresses a renilla luciferase gene (Promega.) using LipofectAMINE 2000 (Invitrogen, Carlsbad, CA, USA). The transfected cells were cultured in 5% CO2 tissue culture incubator at 371C for 24 h. For experimental groups receiving hypoxia treatment, the plates were placed in a hypoxia chamber with 1% O2, 5% CO2 and 94% N2 (Specialty Gases, GA, USA) and incubated at 371C for 24 h. The luciferase activity in the cell lysates was measured with a luminometer (Lumistar galaxy, BMG, Winooski, VM, USA) using Dual Luciferase Assay System (Promega). The relative luciferase activity for each sample was calculated as a ratio of firefly luciferase activity divided by renilla luciferase activity. The level of renilla lucifease activity, which was expressed from the cotransfected pRL-SV-40 plasmid, was used as an internal control for differences in transfection efficiencies among the cell lines.
Construction of the Rev-cas-3 gene-expressing vectors
An autocatalytic Rev-cas-3 gene, which was engineered by switching the position of the small subunit to the front of the large subunit, was kindly provided by Dr Emad S Alnemri at Thomas Jefferson University in Philadelphia, PA, USA. The Rev-cas-3 gene29 was cloned into the Xho1 and EcoRV site of pAdtrackCMV vector42 (Dr Bert Vogelstein, Johns Hopkins University, MD, USA), which generated pAdtrackCMV-Rev-cas-3 plasmid. To engineer a plasmid with the Rev-cas-3 gene expressed from the Sur-P, we first transferred the BamH1–HindIII Sur-P fragment from pLuc-cyc1.2 plasmid to BgII and HindIII site of p-shuttle plasmid,42 resulting in a p-shuttle-Sur-P plasmid. Then, the CMV promoter in pAdtrackCMV-Rev-cas-3 vector was replaced by the Sur-P through cloning the Kpn1–Bstπ1 fragment from the p-shuttle-Sur-P vector into Kpn1–Bstχ1 site in pAdtrackCMV-Rev-cas-3 plasmid, generating a pAdtrack-Sur-P-Rev-cas-3 vector with the Rev-cas-3 expressed from the survivin core promoter.
Transfection and analysis of apoptotic cells
Plasmids (1 µg) were transfected into cultured tumor or normal cell lines in 24-well tissue culture plates using LipofectAMINE 2000 (Invitrogen). At 48 h after transfection, the cells were stained with 10 µg/ml of Hoechst 33342 (Molecular Probes, Inc., Eugene, OR, USA) for 10 min and examined under an inverted fluorescence microscope (Nikon Eclipse E800, Nikon Instrument Inc., Melville, NY, USA) within 1 h. Since the cloning vector pAdtrackCMV has an expressing cassette for the green fluorescence protein (GFP) gene, the percentages of apoptotic cells in transfected cell populations were determined by counting the number of GFP-positive cells with apoptotic nuclear morphology within all GFP-positive cells in each field. Fluorescent images were taken using Optronics Magnafire digital imaging system (Meyer Instrument, Houston, TX, USA).
Acknowledgements
We express our sincere thanks to Dr Bert Vogelstein at Johns Hopkins University for pAdtrackCMV and p-shuttle vectors, and Dr Emad S Alnemri at Thomas Jefferson University for providing us with the reverse caspase-3 gene. This research project is supported in part by the Breast Cancer Research Program of Avon Foundation (to LY), NIH Grants # CA95643 (to LY), CA80017 (to LY), CA87830 NS41403. CA86335 (to EGVM) and The Brain Tumor Society (to EGVM and DEP).
References
- 1.Nettelbeck DM, Jerome V, Muller R. Gene therapy: designer promoters for tumour targeting. Trends Genet. 2000;16:174–181. doi: 10.1016/s0168-9525(99)01950-2. [DOI] [PubMed] [Google Scholar]
- 2.Robson T, Hirst DG. Transcriptional targeting in cancer gene therapy. J Biomed Biotechnol. 2003;2003:110–137. doi: 10.1155/S1110724303209074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brand K, et al. Tumor cell-specific transgene expression prevents liver toxicity of the adeno-HSVtk/GCV approach. Gene Therapy. 1998;5:1363–1371. doi: 10.1038/sj.gt.3300728. [DOI] [PubMed] [Google Scholar]
- 4.Hernandez-Alcoceba R, Pihalja M, Qian D, Clarke MF. New oncolytic adenoviruses with hypoxia- and estrogen receptor-regulated replication. Hum Gene Ther. 2002;13:1737–1750. doi: 10.1089/104303402760293574. [DOI] [PubMed] [Google Scholar]
- 5.Lee SJ, et al. Novel prostate-specific promoter derived from PSA and PSMA enhancers. Mol Ther. 2002;6:415–421. doi: 10.1006/mthe.2002.0682. [DOI] [PubMed] [Google Scholar]
- 6.Bui LA, et al. In vivo therapy of hepatocellular carcinoma with a tumor-specific adenoviral vector expressing interleukin-2. Hum Gene Ther. 1997;8:2173–2182. doi: 10.1089/hum.1997.8.18-2173. [DOI] [PubMed] [Google Scholar]
- 7.Kitazono M, Chuman Y, Aikou T, Fojo T. Adenovirus HSV-TK construct with thyroid-specific promoter: enhancement of activity and specificity with histone deacetylase inhibitors and agents modulating the camp pathway. Int J Cancer. 2002;99:453–459. doi: 10.1002/ijc.10307. [DOI] [PubMed] [Google Scholar]
- 8.Altieri DC. Survivin and apoptosis control. Adv Cancer Res. 2003;88:31–52. doi: 10.1016/s0065-230x(03)88303-3. [DOI] [PubMed] [Google Scholar]
- 9.Reed JC. The Survivin saga goes in vivo. J Clin Invest. 2001;108:965–969. doi: 10.1172/JCI14123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holcik M, Gibson H, Korneluk RG. XIAP: apoptotic brake and promising therapeutic target. Apoptosis. 2001;6:253–261. doi: 10.1023/a:1011379307472. [DOI] [PubMed] [Google Scholar]
- 11.Li F, et al. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature. 1998;396:580–584. doi: 10.1038/25141. [DOI] [PubMed] [Google Scholar]
- 12.Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003;3:46–54. doi: 10.1038/nrc968. [DOI] [PubMed] [Google Scholar]
- 13.Li F. Survivin study: what is the next wave? J Cell Physiol. 2003;197:8–29. doi: 10.1002/jcp.10327. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka K, et al. Expression of survivin and its relationship to loss of apoptosis in breast carcinomas. Clin Cancer Res. 2000;6:127–134. [PubMed] [Google Scholar]
- 15.Satoh K, et al. Expression of survivin is correlated with cancer cell apoptosis and is involved in the development of human pancreatic duct cell tumors. Cancer. 2001;92:271–278. doi: 10.1002/1097-0142(20010715)92:2<271::aid-cncr1319>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 16.Asanuma K, et al. A role for survivin in radioresistance of pancreatic cancer cells. Jpn J Cancer Res. 2002;93:1057–1062. doi: 10.1111/j.1349-7006.2002.tb02483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaffaroni N, et al. Expression of the anti-apoptotic gene survivin correlates with taxol resistance in human ovarian cancer. Cell Mol Life Sci. 2002;59:1406–1412. doi: 10.1007/s00018-002-8518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kennedy SM, et al. Prognostic importance of survivin in breast cancer. Br J Cancer. 2003;88:1077–1083. doi: 10.1038/sj.bjc.6600776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller M, Smith D, Windsor A, Kessling A. Survivin gene expression and prognosis in recurrent colorectal cancer. Gut. 2001;48:137–138. doi: 10.1136/gut.48.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang L, Cao Z, Yan H, Wood WC. Co-existence of high levels of apoptotic signaling and inhibitor of apoptosis proteins in human tumor cells: implication for cancer specific therapy. Cancer Res. 2003;63:6815–6824. [PubMed] [Google Scholar]
- 21.Mesri M, et al. Cancer gene therapy using a survivin mutant adenovirus. J Clin Invest. 2001;108:981–990. doi: 10.1172/JCI12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olie RA, et al. A novel antisense oligonucleotide targeting survivin expression induces apoptosis and sensitizes lung cancer cells to chemotherapy. Cancer Res. 2000;60:2805–2809. [PubMed] [Google Scholar]
- 23.Hockel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93:266–276. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 24.Semenza GL. Hypoxia, clonal selection, and the role of HIF-1 in tumor progression. Crit Rev Biochem Mol Biol. 2000;35:71–103. doi: 10.1080/10409230091169186. [DOI] [PubMed] [Google Scholar]
- 25.Shibata T, Giaccia AJ, Brown JM. Hypoxia-inducible regulation of a prodrug-activating enzyme for tumor-specific gene therapy. Neoplasia. 2002;4:40–48. doi: 10.1038/sj.neo.7900189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruan H, et al. A hypoxia-regulated adeno-associated virus vector for cancer-specific gene therapy. Neoplasia. 2001;3:255–263. doi: 10.1038/sj.neo.7900157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Post DE, Van Meir EG. A novel hypoxia-inducible factor (HIF) activated oncolytic adenovirus for cancer therapy. Oncogene. 2003;22:2065–2072. doi: 10.1038/sj.onc.1206464. [DOI] [PubMed] [Google Scholar]
- 28.Post DE, Van Meir EG. Generation of bidirectional hypoxia/HIF-responsive expression vectors to target gene expression to hypoxic cells. Gene Therapy. 2001;8:1801–1807. doi: 10.1038/sj.gt.3301605. [DOI] [PubMed] [Google Scholar]
- 29.Srinivasula SM, et al. Generation of constitutively active recombinant caspases-3 and -6 by rearrangement of their subunits. J Biol Chem. 1998;273:10107–10111. doi: 10.1074/jbc.273.17.10107. [DOI] [PubMed] [Google Scholar]
- 30.Bao R, et al. Activation of cancer-specific gene expression by the survivin promoter. J Natl Cancer Inst. 2002;94:522–528. doi: 10.1093/jnci/94.7.522. [DOI] [PubMed] [Google Scholar]
- 31.Li F, Altieri DC. Transcriptional analysis of human survivin gene expression. Biochem J. 1999;344(Pt 2):305–311. doi: 10.1042/0264-6021:3440305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roth JA, Cristiano RJ. Gene therapy for cancer: what have we done and where are we going? J Natl Cancer Inst. 1997;89:21–39. doi: 10.1093/jnci/89.1.21. [DOI] [PubMed] [Google Scholar]
- 33.Bos R, et al. Levels of hypoxia-inducible factor-1alpha independently predict prognosis in patients with lymph node negative breast carcinoma. Cancer. 2003;97:1573–1581. doi: 10.1002/cncr.11246. [DOI] [PubMed] [Google Scholar]
- 34.Zhong H, et al. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830–5835. [PubMed] [Google Scholar]
- 35.Semenza GL. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med. 2002;8:S62–S67. doi: 10.1016/s1471-4914(02)02317-1. [DOI] [PubMed] [Google Scholar]
- 36.Ghafar MA, et al. Acute hypoxia increases the aggressive characteristics and survival properties of prostate cancer cells. Prostate. 2003;54:58–67. doi: 10.1002/pros.10162. [DOI] [PubMed] [Google Scholar]
- 37.Dachs GU, Tozer GM. Hypoxia modulated gene expression: angiogenesis, metastasis and therapeutic exploitation. Eur J Cancer. 2000;36:1649–1660. doi: 10.1016/s0959-8049(00)00159-3. [DOI] [PubMed] [Google Scholar]
- 38.Semenza GL, et al. Hypoxia-inducible factor 1: from molecular biology to cardiopulmonary physiology. Chest. 1998;114:40S–45S. doi: 10.1378/chest.114.1_supplement.40s. [DOI] [PubMed] [Google Scholar]
- 39.Shibata T, Giaccia AJ, Brown JM. Development of a hypoxia-responsive vector for tumor-specific gene therapy. Gene Therapy. 2000;7:493–498. doi: 10.1038/sj.gt.3301124. [DOI] [PubMed] [Google Scholar]
- 40.Greco O, et al. Novel chimeric gene promoters responsive to hypoxia and ionizing radiation. Gene Therapy. 2002;9:1403–1411. doi: 10.1038/sj.gt.3301823. [DOI] [PubMed] [Google Scholar]
- 41.Su H, Arakawa-Hoyt J, Kan YW. Adeno-associated viral vector-mediated hypoxia response element-regulated gene expression in mouse ischemic heart model. Proc Natl Acad Sci USA. 2002;99:9480–9485. doi: 10.1073/pnas.132275299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He TC, et al. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]