Abstract
Introduction
Extract of Ginkgo biloba (EGb), a dietary supplement used for a number of conditions including dementia, has been suggested to increase cerebral blood flow (CBF). The purpose of this study was to determine if changes in CBF could be detected by dynamic susceptibility contrast-enhanced magnetic resonance imaging (DSC-MRI) in elderly human subjects taking EGb.
Methods
DSC-MRI was performed in nine healthy men (mean age 61±10 years) before and after 4 weeks of 60 mg EGb taken twice daily. One subject underwent six consecutive scans to evaluate intrasubject reproducibility. CBF values were computed before and after EGb, and analyzed at three different levels of spatial resolution, using voxel-based statistical parametric mapping (SPM), and regions of interest in different lobes, and all regions combined.
Results
Normalized intrasubject CBF (nCBF) measurements had a standard deviation of 7% and 4% in gray and white matter (WM) regions, respectively. SPM using an uncorrected, voxel-level threshold of P≤0.001 showed a small CBF increase in the left parietal–occipital region. CBF in individual lobar regions did not show any significant change post-EGb, but all regions combined showed a significant increase of non-normalized CBF after EGb (15% in white and 13% in gray matter, respectively, P≤0.0001).
Conclusion
nCBF measured by DSC-MRI has good intra-subject reproducibility. In this small cohort of normal elderly individuals, a mild increase in CBF is found in the left parietal–occipital WM after EGb, as well as a small but statistically significant increase in global CBF.
Keywords: Ginkgo, Cerebral blood flow, MR perfusion, Dementia
Introduction
Extract of Ginkgo biloba (EGb) has been extensively used as a herbal medicine to prevent cognitive decline as well as other disorders. It is estimated that more than $250 million is spent on EGb annually [1]. EGb is a standardized extract of G. biloba leaves and contains approximately 24% flavone glycosides (primarily quercetin, kaempferol, and isorhamnetin) and 6% terpene lactones (ginkgolides A, B, and C and bilobalide) [2, 3].
Several studies on animal models of subarachnoid hemorrhage and transient focal ischemia in mice showed that the administration of EGb resulted in increased cerebral blood flow (CBF) when compared to placebo [4–10]. It has been proposed that EGb, by antagonizing the overproduction of endothelin-1, partly reverses cerebral vasospasm, improves microcirculation, and thus relieves secondary ischemic brain injury after experimental subarachnoid hemorrhage [5]. Alternatively, in another study, it was proposed that EGb relieves cerebral vasospasm and cerebral ischemic damage by reversing the pathological alteration of nitric oxide [6]. EGb was shown to protect against transient and permanent focal cerebral ischemia and was effective after a prolonged reperfusion period, which the authors attributed to increase in cortical blood flow [7]. Additionally, the flavonoid constituents of EGb have been reported to have free radical scavenging properties; the terpenoid constituents inhibit platelet activating factor and decrease free radical release [2, 9, 10].
While EGb studies in animal models indicate therapeutic benefit, there are more variable results from clinical trials of EGb. One multicenter study found that EGb treatment significantly improved cognitive deficits in mildly to severely demented subjects with Alzheimer’s disease (AD) or multi-infarct dementia [11]. Another study showed a significant increase in CBF [using single-photon emission computed tomography (SPECT)], decrease in blood viscosity, and cognitive improvement after chronic EGb use in elder volunteers, suggesting a beneficial effect for demented patients [12]. However, results from the recently completed Ginkgo Evaluation of Memory (GEM) trial [1], a multicenter randomized controlled trial with 3069 participants older than 75 years, demonstrated that EGb did not lower the incidence of dementia or AD in elderly people with normal cognition or minimal cognition deficits. However, it was also found that the incidence of vascular dementia was infrequent in the EGb group compared to placebo (hazard ratio 1.56 [95% CI, 1.14–2.15], P=0.05) [3].
The current study was undertaken to determine if MR perfusion imaging (MRPI) can be used to detect effects of EGb on CBF in a small number of normal elderly individuals and to estimate sample sizes if CBF (as measured by MRPI) were to be used as an outcome biomarker in potential future clinical trials of EGb.
Materials and methods
Research design
Nine healthy male volunteers (mean age 61±10 years) were recruited through advertisement in local health clubs. The participants were screened for underlying medical conditions and had no history of psychiatric disorder, neurologic disease, major heart disease, diabetes, vascular disease, epilepsy, or head trauma. Furthermore, a neuroradiologist evaluated conventional MR images from each subject to determine that study subjects had no evidence of tumor, prior infarction, or any other imaging abnormality.
This study was approved by the local institutional review board, and written informed consent was obtained from all participants prior to evaluation. Each subject underwent a magnetic resonance imaging (MRI) study at baseline, and a second study after 4 weeks of 60 mg EGb capsules [DaVinci Laboratories, Discovery Company, Vermont, NY; 14.4 mg (24%) Ginkgo flavone glycosides per capsule] taken twice daily. In one of the subjects, six MR perfusion studies were conducted on different days over a 2-week period at baseline prior to Ginkgo administration to evaluate the reproducibility of perfusion measurements.
Image acquisition and processing
All studies were performed on a 1.5 T GE SIGNA scanner (GE Medical Systems, Milwaukee, WI) using a quadrature birdcage transmit–receive head coil. Conventional MRI included sagittal T1-weighted spin echo, axial fast fluid attenuated inversion recovery, and axial diffusion weighted scans. A gradient-echo, echo-planar perfusion-weighted imaging sequence (TR 2000 ms, TE 60 ms, flip angle 90°, 30 phases) was acquired dynamically during the bolus injection of 20 cc gadolinium-diethylenetriaminepentaacetic acid into the antecubital vein. Seventeen 5-mm-thick slices with a 2.5-mm gap were collected with a field of view of 24 cm and a matrix size of 128 mm×64 mm.
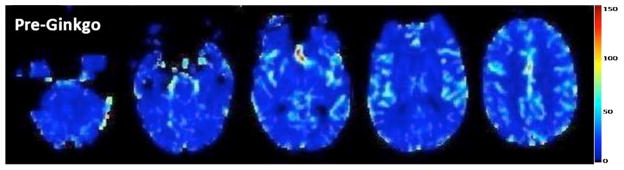
Processing of raw MRI perfusion images to generate maps of CBF was performed using the program “Penguin” [13], a MATLAB (Mathworks, Natick, MA)-based perfusion graphical user interface software (Center for Functionally Integrative Neuroscience, Aarhus University Hospital, Denmark). The arterial input function was selected automatically using a single slice at the level of the proximal circle of Willis in all patients, and a singular value decomposition was used to generate CBF maps (Fig. 1).
Fig. 1.
A sample CBF map (without normalization) generated using the Penguin software in a 69-year-old man
Data analysis
Data were analyzed at three levels of spatial resolution. Analysis was initially performed at the voxel level using the program statistical parametric mapping (SPM), then in segmented regions of interest (ROIs) in ten different lobar regions, and finally a “global” measurement of all regions combined. In all analyses, care was taken to avoid measurements within large vessels. The effects of EGb were analyzed by comparing both absolute CBF as determined by the Penguin program, as well as normalized CBF (nCBF), where each supratentorial brain region CBF is expressed relative to cerebellar white matter (WM) CBF values. Statistical significance was set at P= 0.05.
Voxel-level analysis
For the voxel-based analysis, SPM8 (Wellcome Trust Centre for Neuroimaging, London, England) implemented in MATLAB was used. The baseline and post-Ginkgo (non-normalized) CBF images of each subject were then registered based on the source perfusion images acquired pre-GdDTPA injection. The perfusion images were spatially normalized by 12-parameter affine warping and trilinear interpolation to a 2×2×2 mm3 Montreal Neurological Institute template. The resultant images were spatially smoothed using an 8-mm full-width at half-maximum Gaussian kernel. Within SPM, a voxel-based analysis consisting of paired t tests was used to compare CBF between the pre- and post-EGb. When comparisons using a family-wise error corrected P≤0.05 yielded no statistically significant clusters, more liberal voxel-level uncorrected thresholds of P≤0.001 and P≤0.005 were also used.
CBF (without and with normalization against cerebellar WM) from a single ROI corresponding to the cluster defined by SPM was then compared between the pre- and post-EGb by using a paired t test.
Lobar-segmented ROI analysis
Lobar ROI analysis was performed on CBF maps using the medical image processing, analysis, and visualization (MIPAV) software developed by the Biomedical Imaging Research Services Section at the National Institutes of Health (NIH, http://mipav.cit.nih.gov/) [14]. The “Draw levelset volume of interest” tool followed by manual refinement was used to semiautomatically extract the brain from non-brain tissue, including major vessels, on perfusion weighted images. Brains were segmented into WM, gray matter (GM), and cerebral spinal fluid (CSF) using tissue classification algorithms based on the fuzzy and noise-tolerant adaptive segmentation method [15]. Cortical GM was distinguished from basal ganglia by a semiautomatic delineation. Labeling of different lobes was done by transformation of perfusion images to the Talairach atlas by the Talairach transformation tool integrated in MIPAV [16, 17]. Average CBF of left and right frontal, occipital, parietal, and temporal lobes and limbic regions were calculated for each scan. The effects of EGb were analyzed by comparing absolute and nCBF for each region before and after treatment using paired t test with post hoc Bonferroni correction for multiple comparisons.
To test intrasubject variability, lobar-segmented CBF and nCBF values from six scans in one subject were compared using the same approach. Mean values and standard deviations of CBF and nCBF for each brain region over the six scans were calculated.
Global analysis
The mean lobar CBF (normalized and non-normalized) values from all cerebral regions were combined and compared between pre- and post-EGb treatment using a single two-tailed paired t test.
Results
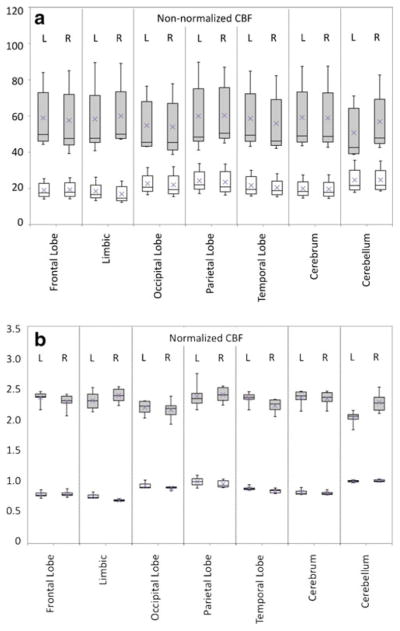
The CBF measurements from six MR perfusion sessions in the same subject on different days showed wide variance (Fig. 2a). The CBF values were comparable in various lobar regions, with an average CBF of the cortical GM being 57.67±18.68 (32%) and of the WM 21.24±5.95 (28%). With normalization against cerebellar WM, variance was decreased (Fig. 2b). Average nCBF of the GM was 2.54±0.18 (7%) and of the WM 0.94±0.04 (4%).
Fig. 2.
Box plots showing intrasubject variation of measurements of CBF in various lobar regions of each cerebral hemisphere after registration using the Talairach atlas, a without and b with normalization against mean CBF of the cerebellar WM. Each box encompasses the first to third quartiles, with error bars marking the full range of values, horizontal bar representing the median and x denoting the mean. GM is depicted in gray, and WM in white. From all regions, the absolute mean CBF of the cortical GM is 57.67±18.68 and of the WM 21.24±5.95. Average nCBF of the GM is 2.54±0.18 and of the WM 0.94±0.04
Voxel-based analysis using SPM8 demonstrated a cluster of mildly increased perfusion in the WM localized to the left parietal–occipital region (cluster size 14 voxels) after Ginkgo administration (P≤0.001). Further increasing the P value to 0.005 and setting the cluster-level threshold to 300 increased the cluster size (327 voxels; see Fig. 3 and Table 1). Reverse-paired t test analysis (assuming CBF pre-EGb>post-EGb) with the same P value failed to demonstrate any significant effect.
Fig. 3.
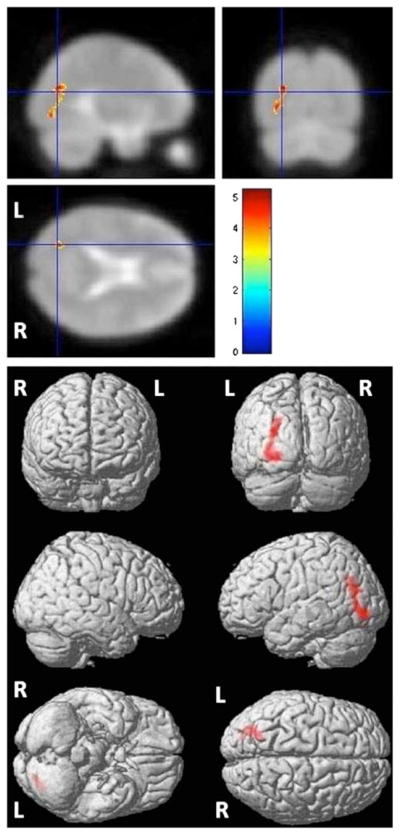
Statistical parametric map of regional increased perfusion after Ginkgo administration (uncorrected P≤0.005). The red region representing hyperperfusion is localized to the left parietal–occipital WM. This result was demonstrated only in the “pre≤post” design matrix based on paired t test. The intensities of color-coding in the 3D rendering are weighted sum of t values, where the weights are proportional to the depth within the brain; that is, deeper regions will be shown with less intensities
Table 1.
Regions with increased CBF after 4 weeks Gingko consumption
| Value | Z score | P value (uncorrected) | MNIa coordinates (x, y, z) | ||
|---|---|---|---|---|---|
| 5.24 | 3.36 | 0.000 | −26 | −70 | 24 |
| 4.72 | 3.17 | 0.001 | −26 | −82 | −6 |
| 4.69 | 3.16 | 0.001 | −22 | −68 | 32 |
Table shows three local maxima more than 8.0 mm apart. Height threshold: T=3.36, P=0.005 (1.000 corrected). Extent threshold: k=300 voxels, P=0.004 (0.291 corrected)
MNI Montreal Neurological Institute
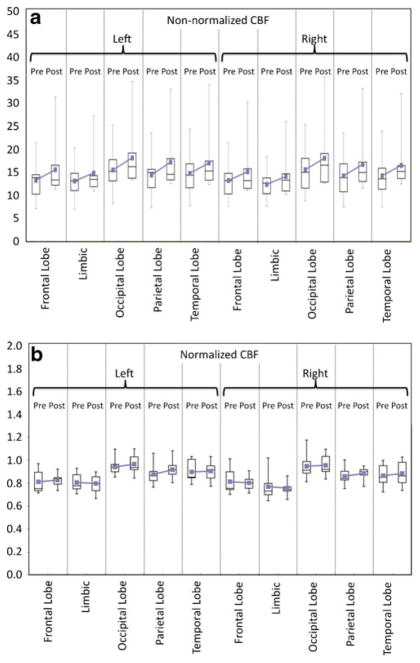
The voxel-based SPM findings in the left parietal–occipital WM were corroborated by a single-paired t test after brain segmentation guided by the SPM cluster. Based on the a priori knowledge, the paired t test on the mean nCBF confirmed a mild increase in the same left parietal–occipital WM region (mean pre-EGb nCBF 0.904±0.075 and mean post-EGb nCBF 0.939±0.068, P=0.022). Without normalization, the mean CBF is also significantly higher after EGb (mean pre-EGb CBF 15.11±4.68 compared to mean post-EGb CBF 17.81±6.34, P=0.0047). No other lobar regions, either in the GM or in the WM, showed any significant difference after correction for multiple comparisons. However, a general trend of higher mean and median CBF values was observed in all lobar regions (including cerebellum) without normalization (Fig. 4a) and in more than 50% of the regions after normalization against cerebellar WM (Fig. 4b). When combining all ROIs of the cerebrum, CBF was greater in post-EGb than pre-EGb (16.47±1.37 vs. 14.21±1.07, P≤0.0001 in the WM; 42.37± 1.82 vs. 37.20±1.77, P≤0.0001 in the GM) using a single two-tailed paired t test. Similarly, nCBF was slightly greater in post-EGb than pre-EGb, but the statistical significance was much lower or lost (0.85±0.05 vs. 0.84±0.05, P=0.03 in the WM; 2.22±0.08 vs. 2.20±0.10, P=0.08 in the GM).
Fig. 4.
Box plots showing CBF values in various segmented lobar WM regions before and after Ginkgo administration: (a) non-normalized CBF values, (b) CBF values normalized to cerebellar WM CBF (nCBF). In all lobar regions (including cerebellum), there is a general trend of higher mean and median absolute CBF values after Ginkgo administration; however, this is not statistically significant when corrections for multiple comparisons are performed. This trend is not consistently observed after normalization, but still holds in more than 50% of the regions. If correction for multiple comparisons is not performed, a single ROI of the left occipital and parietal WM region shows significantly increased CBF and nCBF post-Ginkgo (see text)
Discussion
In this study of nine healthy elderly subjects, SPM analysis showed a mild increase in nCBF in the left parietal–occipital WM after EGb administration, although only when an uncorrected P≤0.001 was used. As the parieto-occipital region has been implicated in visual memory and cognition, this is an interesting finding in regard to a possible beneficial effect of EGb on these functions [18–20]. However, the relationship between nCBF and neurocognitive function requires further investigation, and overall, this CBF change was slight and was undetected at the P≤0.05 level, if correction for multiple comparisons was performed. Only when combining results from all ROIs were significant results found; the SPM findings should be considered of borderline significance at best, since when correction for multiple comparisons was performed, no significant clusters were found. That only very subtle CBF changes are found may be consistent with the results of the GEM study, which failed to show any significant clinical benefit of EGb in preventing the onset of dementia in a similar subject group [1]. Nevertheless, EGb is likely to have some physiological effects as demonstrated in previous studies in vitro and in animal models [2, 4–10], and it is possible that it may have a greater effect on CBF in subjects with cognitive deficits or other brain pathology.
The current results did not duplicate the findings of the previous SPECT study [12], which reported significantly increased CBF in many various brain regions including the left frontal, frontoparietal, and parietal and right frontal and parietal lobes. This could be due to a number of methodological differences, including the dose and duration of EGb administration, lobar segmentation and ROI selections, subject inclusion criteria, and imaging methods.
Quantitative cerebral perfusion measurements are traditionally made by nuclear medicine techniques such as 99 m technetium-hexamethylpropyleneamine oxime (99mTc-HMPAO-SPECT) or oxygen-15 positron emission tomography (15O-PET). MRPI has the advantage of avoiding ionizing radiation, a much higher spatial resolution, and superior anatomic depiction afforded by conventional MR images obtained in the same study. Through the use of arterial input function estimation and singular value decomposition, it has been shown that CBF can be determined with reasonable reliability, for instance, in studies of acute ischemic stroke [21, 22]. In the current study, good nCBF reproducibility was demonstrated within the same individual studied multiple times, with low standard deviations in the normalized values of the baseline studies (4% in WM and 7% in GM). As expected, the absolute CBF values showed much greater variance, with approximately 30% standard deviation in both WM and GM. Scan-to-scan variations in CBF can occur for many technical reasons [23], but with perhaps the greatest influence arising from partial volume effects in the arterial input function. However, normalization can potentially introduce errors; as EGb appears to have a general effect, albeit not statistically significant, on CBF in all brain regions (including the cerebellum), this normalization procedure would obscure such a change. Nevertheless, this is unlikely to account for the differences between the current results and previously published nuclear medicine studies, since normalization against the cerebellum is also commonly used in PET and SPECT studies. In the future, other normalization approaches, for instance, using quantitative measures of cerebral blood volume [23], could be considered to estimate absolute CBF without requiring the assumption of unaltered cerebellar flow.
The current study is limited by the small sample size, and our preliminary data showing mild increase in CBF in the left parietal–occipital WM after EGb, while significant, lack statistical power (0.2). The data nevertheless allow planning of future studies; using the current quantitative MR perfusion technique without the use of normalization, to obtain a power of 0.8 at an α value of 0.05, we estimate that a sample size of 41 is required.
Conclusion
Dynamic susceptibility contrast-enhanced MRI can be useful for evaluating hemodynamic changes following pharmaceutical intervention. In this pilot study consisting of a small cohort of normal elderly individuals, a mild increase in CBF was found in the left parietal–occipital WM after EGb administration. A future study including a larger sample of at least 41 patients is required to confirm this finding with a statistical power of 0.8. Whether EGb has a similar or perhaps larger effect on regional CBF in elderly subjects with cognitive impairment, and the relationship between CBF and neurocognitive function, requires further investigation.
Acknowledgments
We thank Rena Geckle for the research support and patient recruitment. This study was supported in part by NIH grant P41RR015241.
Footnotes
Conflict of interest statement We declare that we have no conflict of interest.
References
- 1.DeKosky ST, Williamson JD, Kronmal FAL, RAIDG, Saxton JA, Lopez OL, Burke G, Carlson MC, Fried LP, Kuller LH, Robbins JA, Tracy RP, Woolard NF, Dunn L, Snitz BE, Nahin RL, Furberg CD. Ginkgo biloba for prevention of dementia: a randomized controlled trial. JAMA. 2008;300:2253–2262. doi: 10.1001/jama.2008.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahlemeyer B, Krieglstein J. Neuroprotective effects of Ginkgo biloba extract. Cell Mol Life Sci. 2003;60:1779–1792. doi: 10.1007/s00018-003-3080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schneider LS. Ginkgo biloba extract and preventing Alzheimer disease. JAMA. 2008;300:2306–2308. doi: 10.1001/jama.2008.675. [DOI] [PubMed] [Google Scholar]
- 4.Sun BL, Yuan H, Xia YMF, ZLZSM, Wang LX. Effects of extract of Ginkgo biloba on intracranial pressure, cerebral perfusion pressure, and cerebral blood flow in a rat model of subarachnoid haemorrhage. Int J Neurosci. 2007;117:655–665. doi: 10.1080/00207450600773871. [DOI] [PubMed] [Google Scholar]
- 5.Sun BL, Zhang J, Xia WXC, ZLYMF, Zhang SM, Ye WJ, Yuan H. Effects of extract of Ginkgo biloba on spasms of the basilar artery and cerebral microcirculatory perfusion in rats with subarachnoid hemorrhage. Clin Hemorheol Microcirc. 2003;29:231–238. [PubMed] [Google Scholar]
- 6.Sun BL, Xia ZL, Yang MF, Qiu PM. Effects of Ginkgo biloba extract on somatosensory evoked potential, nitric oxide levels in serum and brain tissue in rats with cerebral vasospasm after subarachnoid hemorrhage. Clin Hemorheol Microcirc. 2000;23:139–144. [PubMed] [Google Scholar]
- 7.Lee EJ, Chen HY, Wu TS, Chen TY, Ayoub IA, Maynard KI. Acute administration of Ginkgo biloba extract (EGb 761) affords neuroprotection against permanent and transient focal cerebral ischemia in Sprague–Dawley rats. J Neurosci Res. 2002;68:636–645. doi: 10.1002/jnr.10251. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, Fu S, Liu S, Mao T, Xiu R. The therapeutic effect of Ginkgo biloba extract in SHR rats and its possible mechanisms based on cerebral microvascular flow and vasomotion. Clin Hemorheol Microcirc. 2000;23:133–138. [PubMed] [Google Scholar]
- 9.Clark WM, Rinker LG, Lessov NS, Lowery SL, Cipolla MJ. Efficacy of antioxidant therapies in transient focal ischemia in mice. Stroke. 2001;32:1000–1004. doi: 10.1161/01.str.32.4.1000. [DOI] [PubMed] [Google Scholar]
- 10.Pietri S, Maurelli E, Drieu K, Culcasi M. Cardioprotective and anti-oxidant effects of the terpenoid constituents of Ginkgo biloba extract (EGb 761) J Mol Cell Cardiol. 1997;29:733–742. doi: 10.1006/jmcc.1996.0316. [DOI] [PubMed] [Google Scholar]
- 11.Le Bars PL, Katz MM, Berman N, Itil TM, Freedman AM, Schatzberg AF. A placebo-controlled, double-blind, randomized trial of an extract of Ginkgo biloba for dementia. North American EGb Study Group. JAMA. 1997;278:1327–1332. doi: 10.1001/jama.278.16.1327. [DOI] [PubMed] [Google Scholar]
- 12.Santos RF, Galduróz JC, Barbieri A, Castiglioni ML, Ytaya LY, Bueno OF. Cognitive performance, SPECT, and blood viscosity in elderly non-demented people using Ginkgo biloba. Pharmacopsychiatry. 2003;36:127–133. doi: 10.1055/s-2003-41197. [DOI] [PubMed] [Google Scholar]
- 13.Østergaard L. Principles of cerebral perfusion imaging by bolus tracking. J Magn Reson Imaging. 2005;22:710–717. doi: 10.1002/jmri.20460. [DOI] [PubMed] [Google Scholar]
- 14.McAuliffe M, Lalonde E, McGarry D, Gandler W, Csaky K, Trus B. Medical image processing, analysis and visualization in clinical research. Proceedings of the 14th IEEE Symposium on Computer-Based Medical Systems; Piscataway, NJ. 2001. pp. 381–386. [Google Scholar]
- 15.Pham DL. Robust fuzzy segmentation of magnetic resonance images. Proceedings of the 14th IEEE Symposium on Computer-Based Medical Systems; Bethesda, MD. 2001. pp. 127–131. [Google Scholar]
- 16.Bazin PL, Cuzzocreo JL, Yassa MA, Gandler W, McAuliffe MJ, Bassett SS, Pham DL. Volumetric neuroimage analysis extensions for the MIPAV software package. J Neurosci Methods. 2007;165:111–121. doi: 10.1016/j.jneumeth.2007.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pham DL, Xu C, Prince JL. Current methods in medical image segmentation. Annu Rev Biomed Eng. 2000;2:315–237. doi: 10.1146/annurev.bioeng.2.1.315. [DOI] [PubMed] [Google Scholar]
- 18.Stenset V, Hofoss D, Berstad AE, Negaard A, Gjerstad L, Fladby T. White matter lesion subtypes and cognitive deficits in patients with memory impairment. Dement Geriatr Cogn Disord. 2008;26:424–431. doi: 10.1159/000165355. [DOI] [PubMed] [Google Scholar]
- 19.Ota M, Nemoto K, Sato N, Yamashita F, Asada T. Relationship between white matter changes and cognition in healthy elders. Int J Geriatr Psychiatry. 2009;24:1463–1469. doi: 10.1002/gps.2289. [DOI] [PubMed] [Google Scholar]
- 20.Sepulcre J, Masdeu JC, Sastre-Garriga J, Goñi J, Vélez-de-Mendizábal N, Duque B, Pastor MA, Bejarano B, Villoslada P. Mapping the brain pathways of declarative verbal memory: evidence from white matter lesions in the living human brain. Neuroimage. 2008;42:1237–1243. doi: 10.1016/j.neuroimage.2008.05.038. [DOI] [PubMed] [Google Scholar]
- 21.Wu O, Østergaard L, Weisskoff RM, Benner T, Rosen BR, Sorensen AG. Tracer arrival timing-insensitive technique for estimating flow in MR perfusion-weighted imaging using singular value decomposition with a block-circulant deconvolution matrix. Magn Reson Med. 2003;50:164–174. doi: 10.1002/mrm.10522. [DOI] [PubMed] [Google Scholar]
- 22.Christensen S, Mouridsen K, Wu O, Hjort N, Karstoft H, Thomalla G, Röther J, Fiehler J, Kucinski T, Østergaard L. Comparison of 10 perfusion MRI parameters in 97 sub-6-hour stroke patients using voxel-based receiver operating characteristics analysis. Stroke. 2009;40:2055–2061. doi: 10.1161/STROKEAHA.108.546069. [DOI] [PubMed] [Google Scholar]
- 23.Shin W, Horowitz S, Ragin A, Chen Y, Walker M, Carroll TJ. Quantitative cerebral perfusion using dynamic susceptibility contrast MRI: evaluation of reproducibility and age- and gender-dependence with fully automatic image postprocessing algorithm. Magn Reson Med. 2007;58(6):1232–1241. doi: 10.1002/mrm.21420. [DOI] [PubMed] [Google Scholar]