Abstract
Juvenile dermatomyositis (JDM), a systemic vasculopathy, is characterized by inflammation of skin and muscle. Muscle biopsies from untreated JDM patients show upregulation of type I interferon (IFN)-inducible genes, including myxovirus resistance protein A (MxA). The present study examines whether MxA mRNA expression in peripheral blood mononuclear cells (PBMC) from JDM patients: (1) is elevated compared to healthy controls, (2) reflects disease activity, and (3) changes with the onset of clinically effective treatment. MxA mRNA expression in JDM PBMC obtained at the initial clinic visit was elevated compared to controls and was positively correlated with Disease Activity Score (DAS) for muscle, but not with DAS for skin, suggesting that damage to skin and muscle in JDM may each have a discrete pathophysiology. During the course of clinically effective treatment, decrease in muscle symptoms was associated with a decrease in PBMC MxA mRNA expression.
Keywords: Juvenile dermatomyositis, Interferon, MxA, PBMC, Muscle
Introduction
Although juvenile dermatomyositis (JDM) is a rare disease, it is the most common pediatric inflammatory myopathy, with an incidence of 3.2 cases/million children/year [1]. The mean age of disease onset (the time of the first symptom of JDM – rash or weakness) was 6.9 years and 25% of newly diagnosed children with JDM are younger than 4 years of age. Clinically, JDM is characterized by cutaneous manifestations, including the hallmark vasculitic rash, and progressive proximal muscle weakness. While the rash is requisite to meet the Bohan and Peter criteria for diagnosis [2,3], traditional assessments of disease activity have focused on muscle strength and do not quantify levels of cutaneous involvement. To address these issues, a validated Disease Activity Score (DAS), comprised of both patient-derived dermatological and musculoskeletal criteria [4], was developed to assess clinical observations of system-related disease activity individually (DAS skin and DAS muscle) or in total (DAS total). Such a categorization has permitted our group to observe associations between disease activity in the specific tissue compartments and laboratory and/or clinical measurements. For example, we have recently reported that abnormal nailfold capillary blood vessels are associated with DAS skin but not DAS muscle [5]. Conversely, a decrease in the absolute count of circulating natural killer (NK) cells is associated with DAS muscle but not DAS skin [6]. These observations suggest that there may be divergent disease mechanisms in the different tissue compartments (i.e., skin versus muscle).
Although JDM and adult-onset dermatomyositis may have distinct pathogeneses, gene expression profiling revealed increased expression of many type I interferon (IFN)-inducible genes in muscle biopsies from both untreated JDM and adult-onset dermatomyositis patients as compared to healthy age-matched controls [7-9]. Type I IFN, which includes IFN-α and -β, is difficult to measure in biological samples. Thus, several assays have been developed to detect type I IFN activity by assessing the expression of type I IFN-inducible genes in various cell types, including peripheral blood mononuclear cells (PBMC) [10-13]. Increased levels of type I IFN-inducible genes indicate that the cells have been exposed to type I IFN in vivo. Myxovirus resistance protein A (MxA), one of the genes found to be upregulated in JDM muscle biopsies, is unique in that its expression is specifically and tightly regulated by type I IFN and not other cytokines, including type II IFN (i.e., IFN-γ) [12,14]. In fact, MxA mRNA expression in PBMC has been successfully used as a marker of type I IFN activity in a variety of settings, including the assessment of the bioavailability of therapeutically administered IFN-α and -β in hepatitis C virus and multiple sclerosis patients, respectively [12,13,15].
It is not known whether the type I IFN activity observed in JDM patients parallels JDM disease activity or if evidence of type I IFN activity in children with JDM will be suppressed by clinically effective treatment. Given the near impossibility of collecting sequential muscle biopsies from children, we chose to assess the type I IFN response in JDM by examining MxA mRNA expression in PBMC. There are several clinical factors that may impact MxA mRNA expression in JDM, including age, previous therapy, and disease activity. Because many components of the immune response, including circulating lymphocytes subset distribution and cytokine production, are modified by the age of the child, age-matched, control data are essential in studies of children with JDM. Thus, the purpose of these studies was to determine whether: (1) MxA mRNA expression in PBMC from JDM patients is higher than in healthy age-matched controls, similar to the MxA mRNA expression in muscle biopsies; (2) MxA mRNA expression in PBMC is associated with disease severity at the initial visit; and (3) MxA mRNA expression decreases with the initiation of clinically effective treatment.
Patients and methods
Study population
The first study examined 14 children who attended the pediatric Immunology/Rheumatology clinic at Children’s Memorial Hospital (CMH; Chicago, Illinois) between August 1997 and March 2001 and, subsequently, had been followed at the CMH clinic for at least 36 months. Seven patients received some immunosuppressive therapy prior to their first visit to the CMH clinic, and seven were untreated. All 7 previously treated patients had been given oral prednisone, a subset had received methotrexate (n = 5) and hydroxychloroquine (n = 2), and one had received intravenous (IV) gamma-globulin. Irrespective of prior treatment status, all patients had had active disease and fulfilled the criteria of Bohan and Peter as having definite JDM [2,3]. All patients had a peripheral blood sample drawn during their first visit to the CMH clinic. Peripheral blood samples were also obtained from 24 healthy age-matched children attending outpatient clinics for well-child visits. The demographics of these groups are displayed in Table 1.
Table 1.
Demographic and clinical characteristics of patients with untreated JDM and healthy age-matched pediatric controls
| Healthy pediatric controls |
Active untreated JDM patients |
Active previously treated JDM patients |
Healthy adolescent control |
JDM patients 10 years post-diagnosis |
|
|---|---|---|---|---|---|
| n | 24 | 7 | 7 | 10 | 15 |
| # of females | 19 (79) | 6 (85) | 6 (85) | 6 (60) | 10 (67) |
| Age in years at diagnosis | N/A | 8.5 (4.3) | 10.4 (3.6) | N/A | 4.7 (2.2) |
| Age in years at blood draw | 12.6 (3.5) | 9.0 (3.9) | 11.3 (3.4) | 16.0 (2.3) | 14.7 (2.4) |
| DAS skin | N/A | 5.9 (1.4) | 6.4 (1.0) | N/A | 2.2 (2.5) |
| DAS muscle | N/A | 6.3 (3.5) | 3.9 (4.0) | N/A | 0.8 (2.1) |
Means (standard deviation) for continuous variables, n (%) for discrete variables.
In all 14 JDM patients described above, aggressive immunosuppressive therapy, which was tailored to the child’s clinical and laboratory findings, was initiated. In general, children with very active disease were given more than one dose of IV methylprednisolone (MP) at 30 mg/kg with 1 g maximum/day for varying periods of time. This was often accompanied by parenteral methotrexate, at a minimum of 15 mg/M2 and folic acid at 1–2 mg/day. Other components of the regimen included vitamin D (800 international units), appropriate exogenous calcium sources, and UVA, UVB PABA free sun blocks. Patients returned periodically to the clinic for at least 36 months. Eleven patients had at least 3 visits following the initiation of this aggressive treatment regimen where peripheral blood samples were drawn for follow-up analysis.
The second study examined a separate group of 15 JDM patients (mean age = 14.7; range 12.2–19.5). Ten years after the initial diagnosis, peripheral blood samples were drawn for comparison with samples obtained from 10 healthy age-matched adolescents attending outpatient clinics (mean age = 16.0; range 14.2–19.9). The demographics of these groups also are displayed in Table 1.
These studies were approved by the Institutional Review Board at the Children’s Memorial Research Center. Each participant or their guardian signed an informed consent.
Clinical and diagnostic laboratory data
Disease Activity Score (DAS)
One physician (LMP) determined the DAS for all patients. The DAS is a validated clinical estimate of disease activity for both musculoskeletal and dermatological criteria [4]. Nine points are given on the basis of clinical dermatological findings and 11 points based on musculoskeletal findings [4]. In this study, the dermatologic (DAS skin) and musculoskeletal (DAS muscle) components were analyzed for association with MxA mRNA expression in PBMC.
Serum muscle enzymes
The results of routine diagnostic serologic tests (creatine kinase [CK], lactic acid dehydrogenase [LDH], serum glutamic-oxaloacetic transaminase/aspartate aminotransferase [SGOT/AST], and aldolase) were standardized as previously described [16].
Blood collection and sample preparation
Patients taking medication were instructed to forgo medication the day prior to attending the CMH clinic. Thus, patients did not take medication 24 h prior to blood collection. Blood was obtained in acid citrate dextrose (ACD; BD Vacutainer) tubes and subjected to Ficoll-Hypaque separation using Accuprep (Accurate Chemical and Scientific Corp.) according to the manufacturer’s instructions. Total RNA was isolated from PBMC using Trizol reagent (Invitrogen Corp.) according to the manufacturer’s instructions and subsequently DNase-treated using DNA-free™ Kits (Ambion) per manufacturer’s instructions. The concentration of total RNA was determined by spectrophotometry at 260 nm, and total RNA was diluted to 50 ng/μl.
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
First strand cDNA was synthesized by random-priming using 250 ng of total RNA and Superscript III (Invitrogen Corp.) according to the manufacturer’s instructions. Relative cDNA quantitations for MxA and an internal reference gene (β-actin) were done using a fluorescence-based real-time detection method (ABI PRISM 7700 Sequence Detection System; Perkin-Elmer Applied Biosystems). The PCR reaction was performed using the PCR Core Reagent Kit (Perkin-Elmer Applied Biosystems) and gene-specific primers and probes for MxA and β-actin. The primers and probe for MxA were: 5′-TTCAGCACCTGATGGCCTATC-3′ (forward primer), 5′-CCGTACGTCTGGAGCATGAAG-3′ (reverse primer), and 6FAM-CCAGGAGGCCAGCAAGCGCAT-QSY7 (probe). The primers and probe for β-actin were: 5′-TCACCCACACTGTGCCCATCTACGA-3′ (forward primer), 5′-CAGCGGAACCGCTCATTGCCAATGG-3′ (reverse primer), and 6FAM-ATGCCCTCCCCCATGCCATCCTGCGT-QSY7 (probe).
Statistics
In general, Student’s t tests were used to compare the mean MxA mRNA values between JDM patients and healthy age-matched controls. The Spearman Rank Correlation Test was used to assess correlations between MxA mRNA values and the DAS sub-scores and serum muscle enzyme levels.
Results
MxA expression PBMC at initial visit
Within the group of 14 JDM patients with clinical symptoms of active JDM (rash, weakness), 7 patients had been given immunosuppressive therapy prior to their initial visit to the CMH Immunology/Rheumatology Clinic. However, MxA mRNA expression in PBMC from previously treated children with active JDM did not significantly differ from MxA mRNA expression in PBMC from untreated children with active JDM (P = 0.503; Fig. 1). Consequently, the 7 JDM patients who were treated prior to their initial visit to the CMH clinic (but still had active disease) were combined with the 7 patients who were untreated prior to their initial visit in all subsequent analyses.
Figure 1.
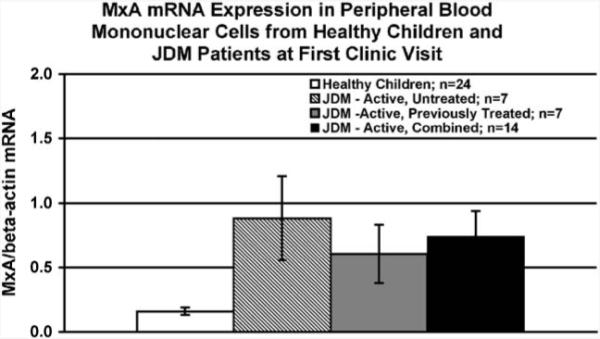
Peripheral blood mononuclear cell (PBMC) MxA mRNA expression as assessed by qRT-PCR is not different in previously treated children with active JDM as compared to untreated children with active JDM. PBMC MxA mRNA expression is higher in active JDM patients (irrespective of previous therapy) as compared to healthy age-matched pediatric controls (P = 0.004).
MxA mRNA levels in PBMC obtained at the first visit were significantly higher in children with active JDM (irrespective of previous therapy) as compared to healthy pediatric controls (P = 0.004; Fig. 1). There was no difference between the active JDM group and the healthy control group with respect to age. Furthermore, in JDM patients at the initial visit, MxA mRNA expression in PBMC was positively associated with DAS muscle (Spearman’s rho = 0.800; P = 0.001; Fig. 2A), but not DAS skin (Spearman’s rho = −0.208; P = 0.476; Fig. 2B). Levels of SGOT/AST, LDH, and aldolase were positively correlated with MXA expression, DAS weakness, and each other (data not shown). Levels of CK did not correlate with the other muscle enzymes or with DAS weakness and MXA expression (data not shown).
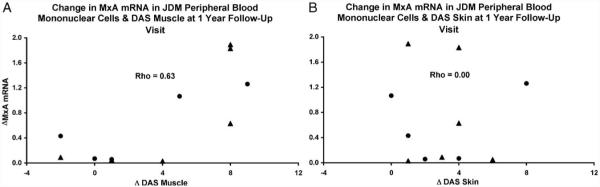
Figure 2.
At the initial clinic visit, MxA mRNA expression in peripheral blood mononuclear cells from JDM patients is positively associated with Disease Activity Score (DAS) muscle (A), but not DAS skin (B) for both previously treated children with active JDM and untreated children with active JDM. Triangles (▲) represent patients who were untreated at the initial clinic visit and circles (●) represent partially treated patients who had received ineffective therapy prior to the initial clinic visit.
MxA expression during treatment
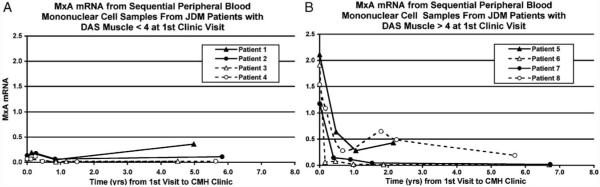
Following the initiation of aggressive immunosuppressive therapy, PBMC samples were obtained from at least 3 follow-up visits for 11 of the 14 JDM patients. Difference scores were obtained by subtracting the DAS muscle, DAS skin, and MxA mRNA levels obtained at the one-year follow-up visit from the appropriate values obtained at the initial visit. At the one-year follow-up visit, the change in MxA mRNA expression in PBMC samples was positively correlated with the change in DAS muscle (Spearman’s rho = 0.63, P = 0.04; Fig. 3A), but not with the change in DAS skin (Spearman’s rho = 0.00, P > 0.99; Fig. 3B). Figs. 4A and B show MxA mRNA expression from sequential PBMC for selected individuals whose DAS muscle at the initial visit was >4 (mean DAS muscle = 8.25) or ≤4 (mean DAS muscle = 1.75), respectively. It should be noted that both subsets of patients had substantial skin involvement at the initial visit. The mean DAS skin for the group of patients with DAS muscle >4 at the initial visit was 5.00, while the mean DAS skin for the group of patients with DAS muscle ≤4 was 6.75.
Figure 3.
A difference score was calculated for Disease Activity Score (DAS) muscle, DAS skin, and peripheral blood mononuclear cell MxA mRNA expression by subtracting the values obtained at the one-year follow-up visit from the corresponding values obtained at the initial visit. The change in MxA mRNA expression in peripheral blood mononuclear cells is positively correlated with the change in DAS muscle (A), but not with the change DAS skin (B). Triangles (▲) represent patients who were untreated at the initial clinic visit and circles (●) represent partially treated patients who had received ineffective therapy prior to the initial clinic visit.
Figure 4.
Sequential peripheral blood mononuclear cell MxA mRNA expression as assessed by qRT-PCR from 4 patients who originally presented with a Disease Activity Score (DAS) muscle <4 [A; mean DAS muscle = 1.75; mean DAS skin = 6.75] and 4 patients who originally presented with a DAS muscle >4 [B; mean DAS muscle = 8.25; mean DAS skin = 5.00]. Triangles (open (△) and closed (▲) represent patients who were untreated at the initial clinic visit and circles (open (○) and closed (●) represent partially treated patients who had received ineffective therapy prior to the initial clinic visit. Patients 1, 3, 5, and 6 were untreated prior to initial visit. Patients 2, 4, 7, and 8 were treated prior to initial visit. Prior to the initial visit, patient 2 received oral prednisone; patient 4 received oral prednisone, methotrexate, and hydroxychloroquine; patient 7 received oral prednisone and hydroxychloroquine; and patient 8 received oral prednisone, methotrexate, and intravenous gamma-globulin. Following the initial clinic visit, patients 1, 4, 6, and 7, and 8 received intravenous (IV) methylprednisolone, oral prednisone, methotrexate, and hydroxychloroquine; patients 2, 5, and 8 subsequently received IV methylprednisolone, oral prednisone, and methotrexate; patient 3 received oral prednisone and hydroxychloroquine.
MxA expression 10 years after diagnosis
A separate group of patients was used to examine MxA mRNA expression 10 years after diagnosis. While some patients had active disease 10 years after diagnosis (mean DAS skin: 2.3; mean DAS muscle: 0.8), the majority was free of any clinical symptoms. MxA mRNA expression in PBMC obtained from patients diagnosed with JDM 10 years earlier was not different from that of healthy age-matched controls (P = 0.319; Fig. 5).
Figure 5.
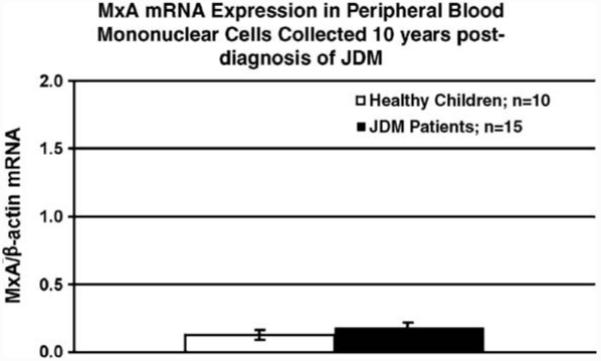
Peripheral blood mononuclear cell MxA mRNA expression as assessed by qRT-PCR is not different in JDM patients 10 years after diagnosis as compared to age-matched healthy adolescent controls.
Discussion
These results confirm that JDM, like other rheumatic diseases, is associated with activation of the type I interferon system [17-20]. Furthermore, these results show that the type I interferon response (as assessed by MxA mRNA expression) observed in JDM is not restricted to muscle and may be monitored in PBMC. However, the type I IFN response in JDM appears to be associated with muscle symptoms, rather than with skin symptoms, suggesting that damage to skin and muscle in JDM may each have a distinct pathophysiology. In addition, at the initial visit, the type I IFN response in JDM is associated with serum levels of SGOT/AST, LDH, and aldolase. Finally, clinically effective treatment, which at the CMH clinic consists of high dose IV MP, methotrexate, and on occasion cyclosporine, reduces MxA mRNA expression in PBMC obtained from patients initially presenting with severe muscle symptoms.
The pathophysiology of JDM is not known, but an autoimmune mechanism has been proposed. Studies of circulating lymphocyte subsets in the peripheral blood of children with JDM demonstrated a profound lymphopenia and a marked decrease in CD8+ memory T cells and NK cells in those children compared with age-matched controls [6,21]. Removal of these cells from the circulation may be a reflection of trafficking to the sites of inflammation and, furthermore, participation in the pathophysiology of the disease [6,21]. Indeed, JDM muscle biopsies contain macrophages, CD8+ T cells, and, to a lesser extent, NK cells [22-24]. Of note, oligoclonal CD8+ T cells with a memory/effector phenotype have been shown to be prevalent, particularly around vessels [23].
Moreover, in JDM, as well as in other autoimmune diseases, responses to microbial antigens have been proposed as either direct or accessory etiopathogenic mechanisms [25]. A case–control study showed that antecedent illness in the 3 months prior to the first symptom of JDM was more prevalent in children with JDM compared to controls [26]. Furthermore, the infectious process involved the respiratory system in over half of cases and gastrointestinal tract in a third of the group [26]. Finally, transcription of type I IFN-inducible genes is considered a hallmark of the host defense mechanism against infection by a range of microorganisms.
Type I IFN establishes an anti-microbial state in target cells and is predominantly produced in response to microbial infection. In addition to direct anti-microbial activity, type I IFN exerts a number of different effects on the immune system, including positively regulating the survival and proliferation of memory CD8+ T cells [27], the activation of NK cell mediated lysis [28,29], and the expression of major histocompatibility complex (MHC) class I molecules [30]. Each of these aspects of the immune response has been implicated in the pathogenesis of JDM [6,21,24].
Type I IFN has long been believed to be involved in the induction of tolerance and, conversely, autoimmune disease. It has been proposed that prolonged exposure of the immune system to type I IFN can break tolerance and eventually lead to autoimmune disease [20,31,32]. Consistent with this hypothesis, a case report has identified a case of dermatomyositis in association with IFN-α treatment [32]. Not surprisingly, numerous autoimmune rheumatic diseases have been associated with activation of the type I interferon system [17-20]. For example, gene expression analysis of PBMC collected from patients with systemic lupus erythematosus (SLE) reveals evidence of a type I IFN response [18]. Moreover, high expression of 3 different IFN-inducible genes in PBMC from SLE patients was associated with increased disease activity as assessed by a variety of measures, including the SLE Disease Activity Index 2000 [33]. Plasma levels of type I interferon from SLE patients were associated with cutaneous manifestations of SLE and renal involvement, but not neurological involvement [33]. Similarly, in this study, we observed that evidence of type I IFN activation in JDM is associated with muscle involvement, but not skin involvement. More research will be needed to ascertain the similarities and differences between the type I IFN response in SLE and JDM and the association of this response with involvement of different physiological systems (e.g., muscle vs. skin) in the disease process.
The present study reveals that immunosuppressive therapy that is sufficient to reduce muscle symptoms is associated with decreased expression of MxA mRNA in PBMC. However, in vitro, glucocorticoids do not reduce IFN-α2-induced MxA mRNA expression in PBMC (unpublished observations). Therefore, the suppression of MxA mRNA expression in JDM patients may be attributable to the suppression of type I interferon activity or production. Indeed, glucocorticoids reduce both the amount of IFN-α produced by PBMC in vitro and the number of circulating plasmacytoid dendritic cells (pDC) [34], a primary cell type involved in production of type I IFN [35] that have been identified in muscle biopsies from adult-onset dermatomyositis patients [9]. pDC have also been identified in cutaneous SLE lesions [36]. The role of pDC in JDM pathogenesis and the effect of corticosteroid therapy on pDC will require further examination.
In summary, the type I IFN response observed in JDM may play an important role in the disease pathogenesis, as it does in SLE. This study documents that the type I IFN response (as assessed by MxA mRNA levels) may be measured in PBMC, allowing for a less invasive mode of monitoring. Moreover, the type I IFN response is highly associated with muscle, but not skin, symptoms, supporting the conjecture that the pathophysiology of the inflammatory response in skin and muscle in JDM may each have a discrete pathway, as in SLE. Finally, results of this study suggest that treatment that effectively reduces muscle symptoms in JDM, as measured by clinical parameters, also may suppress the type I IFN activity that may be central to muscle involvement.
Acknowledgments
The authors wish to acknowledge the contribution of Violet Kula, Susan Spiropoulos, and Joyce Sundberg, who performed the invaluable task of collecting and processing blood samples. Supported in part by grants from: The Myositis Association, NIAMS: RO1-AR48289-02, and The Arthritis Foundation.
References
- [1].Mendez EP, Lipton R, Ramsey-Goldman R, Roettcher P, et al. US incidence of juvenile dermatomyositis, 1995–1998: results from the National Institute of Arthritis and Musculo-skeletal and Skin Diseases Registry. Arthritis Rheum. 2003;49(3):300–305. doi: 10.1002/art.11122. [DOI] [PubMed] [Google Scholar]
- [2].Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts) N. Engl. J. Med. 1975;292(7):344–347. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- [3].Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts) N. Engl. J. Med. 1975;292(8):403–407. doi: 10.1056/NEJM197502202920807. [DOI] [PubMed] [Google Scholar]
- [4].Bode RK, Klein-Gitelman MS, Miller ML, Lechman TS, et al. Disease activity score for children with juvenile dermatomyositis: reliability and validity evidence. Arthritis Rheum. 2003;49(1):7–15. doi: 10.1002/art.10924. [DOI] [PubMed] [Google Scholar]
- [5].Smith RL, Sundberg J, Shamiyah E, Dyer A, et al. Skin involvement in juvenile dermatomyositis is associated with loss of end row nailfold capillary loops. J. Rheumatol. 2004;31(8):1644–1649. [PubMed] [Google Scholar]
- [6].O’Gorman MR, Bianchi L, Zaas D, Corrochano V, et al. Decreased levels of CD54 (ICAM-1)-positive lymphocytes in the peripheral blood in untreated patients with active juvenile dermatomyositis. Clin. Diagn. Lab. Immunol. 2000;7(4):693–697. doi: 10.1128/cdli.7.4.693-697.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tezak Z, Hoffman EP, Lutz JL, Fedczyna TO, et al. Gene expression profiling in DQA1*0501+ children with untreated dermatomyositis: a novel model of pathogenesis. J. Immunol. 2002;168(8):4154–4163. doi: 10.4049/jimmunol.168.8.4154. [DOI] [PubMed] [Google Scholar]
- [8].Zhou X, Dimachkie MM, Xiong M, Tan FK, et al. cDNA microarrays reveal distinct gene expression clusters in idiopathic inflammatory myopathies. Med. Sci. Monit. 2004;10(7):BR191–BR197. [PubMed] [Google Scholar]
- [9].Greenberg SA, Pinkus JL, Pinkus GS, Burleson T, et al. Interferon-alpha/beta-mediated innate immune mechanisms in dermatomyositis. Ann. Neurol. 2005;57(5):664–678. doi: 10.1002/ana.20464. [DOI] [PubMed] [Google Scholar]
- [10].Files JG, Gray JL, Do LT, Foley WP, et al. A novel sensitive and selective bioassay for human type I interferons. J. Interferon Cytokine Res. 1998;18(12):1019–1024. doi: 10.1089/jir.1998.18.1019. [DOI] [PubMed] [Google Scholar]
- [11].Pungor E, Jr., Files JG, Gabe JD, Do LT, et al. A novel bioassay for the determination of neutralizing antibodies to IFN-beta1b. J. Interferon Cytokine Res. 1998;18(12):1025–1030. doi: 10.1089/jir.1998.18.1025. [DOI] [PubMed] [Google Scholar]
- [12].Roers A, Hochkeppel HK, Horisberger MA, Hovanessian A, et al. MxA gene expression after live virus vaccination: a sensitive marker for endogenous type I interferon. J. Infect. Dis. 1994;169(4):807–813. doi: 10.1093/infdis/169.4.807. [DOI] [PubMed] [Google Scholar]
- [13].Bertolotto A, Gilli F, Sala A, Audano L, et al. Evaluation of bioavailability of three types of IFNbeta in multiple sclerosis patients by a new quantitative-competitive-PCR method for MxA quantification. J. Immunol. Methods. 2001;256(1–2):141–152. doi: 10.1016/s0022-1759(01)00434-3. [DOI] [PubMed] [Google Scholar]
- [14].von Wussow P, Jakschies D, Hochkeppel HK, Fibich C, et al. The human intracellular Mx-homologous protein is specifically induced by type I interferons. Eur. J. Immunol. 1990;20(9):2015–2019. doi: 10.1002/eji.1830200920. [DOI] [PubMed] [Google Scholar]
- [15].Antonelli G, Simeoni E, Turriziani O, Tesoro R, et al. Correlation of interferon-induced expression of MxA mRNA in peripheral blood mononuclear cells with the response of patients with chronic active hepatitis C to IFN-alpha therapy. J. Interferon Cytokine Res. 1999;19(3):243–251. doi: 10.1089/107999099314171. [DOI] [PubMed] [Google Scholar]
- [16].Pachman LM, Abbott K, Sincacore JM, Amoruso L, et al. Duration of illness is an important variable for untreated children with juvenile dermatomyositis. J. Pediatr. 2006;148(2):247–253. doi: 10.1016/j.jpeds.2005.10.032. [DOI] [PubMed] [Google Scholar]
- [17].Bave U, Nordmark G, Lovgren T, Ronnelid J, et al. Activation of the type I interferon system in primary Sjogren’s syndrome: a possible etiopathogenic mechanism. Arthritis Rheum. 2005;52(4):1185–1195. doi: 10.1002/art.20998. [DOI] [PubMed] [Google Scholar]
- [18].Crow MK, Kirou KA, Wohlgemuth J. Microarray analysis of interferon-regulated genes in SLE. Autoimmunity. 2003;36(8):481–490. doi: 10.1080/08916930310001625952. [DOI] [PubMed] [Google Scholar]
- [19].Hjelmervik TO, Petersen K, Jonassen I, Jonsson R, et al. Gene expression profiling of minor salivary glands clearly distinguishes primary Sjogren’s syndrome patients from healthy control subjects. Arthritis Rheum. 2005;52(5):1534–1544. doi: 10.1002/art.21006. [DOI] [PubMed] [Google Scholar]
- [20].Pascual V, Banchereau J, Palucka AK. The central role of dendritic cells and interferon-alpha in SLE. Curr. Opin. Rheumatol. 2003;15(5):548–556. doi: 10.1097/00002281-200309000-00005. [DOI] [PubMed] [Google Scholar]
- [21].Uzel G, Conboy CM, Giczewski D, Abbott K, et al. Decreased ICAM-1+ memory suppressor T cells in untreated juvenile dermatomyositis peripheral blood. Clin. Invest. Med. 2004;27(Suppl.) [Google Scholar]
- [22].McDouall RM, Dunn MJ, Dubowitz V. Nature of the mononuclear infiltrate and the mechanism of muscle damage in juvenile dermatomyositis and Duchenne muscular dystrophy. J. Neurol. Sci. 1990;99(2–3):199–217. doi: 10.1016/0022-510x(90)90156-h. [DOI] [PubMed] [Google Scholar]
- [23].Mizuno K, Yachie A, Nagaoki S, Wada H, et al. Oligoclonal expansion of circulating and tissue-infiltrating CD8+ T cells with killer/effector phenotypes in juvenile dermatomyositis syndrome. Clin. Exp. Immunol. 2004;137(1):187–194. doi: 10.1111/j.1365-2249.2004.02500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Li CK, Varsani H, Holton JL, Gao B, et al. MHC Class I overexpression on muscles in early juvenile dermatomyositis. J. Rheumatol. 2004;31(3):605–609. [PubMed] [Google Scholar]
- [25].Massa M, Costouros N, Mazzoli F, De Benedetti F, et al. Self epitopes shared between human skeletal myosin and Streptococcus pyogenes M5 protein are targets of immune responses in active juvenile dermatomyositis. Arthritis Rheum. 2002;46(11):3015–3025. doi: 10.1002/art.10566. [DOI] [PubMed] [Google Scholar]
- [26].Pachman LM, Lipton R, Ramsey-Goldman R, Shamiyeh E, et al. History of infection before the onset of juvenile dermatomyositis: results from the National Institute of Arthritis and Musculoskeletal and Skin Diseases Research Registry. Arthritis Rheum. 2005;53(2):166–172. doi: 10.1002/art.21068. [DOI] [PubMed] [Google Scholar]
- [27].Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272(5270):1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- [28].Biron CA. Role of early cytokines, including alpha and beta interferons (IFN-alpha/beta), in innate and adaptive immune responses to viral infections. Semin. Immunol. 1998;10(5):383–390. doi: 10.1006/smim.1998.0138. [DOI] [PubMed] [Google Scholar]
- [29].Reiter Z. Interferon-a major regulator of natural killer cell-mediated cytotoxicity. J. Interf. Res. 1993;13(4):247–257. doi: 10.1089/jir.1993.13.247. [DOI] [PubMed] [Google Scholar]
- [30].Keir ME, Stoddart CA, Linquist-Stepps V, Moreno ME, et al. IFN-alpha secretion by type 2 predendritic cells up-regulates MHC class I in the HIV-1-infected thymus. J. Immunol. 2002;168(1):325–331. doi: 10.4049/jimmunol.168.1.325. [DOI] [PubMed] [Google Scholar]
- [31].Ronnblom L, Eloranta ML, Alm GV. Role of natural interferon-alpha producing cells (plasmacytoid dendritic cells) in autoimmunity. Autoimmunity. 2003;36(8):463–472. doi: 10.1080/08916930310001602128. [DOI] [PubMed] [Google Scholar]
- [32].Dietrich LL, Bridges AJ, Albertini MR. Dermatomyositis after interferon alpha treatment. Med. Oncol. 2000;17(1):64–69. doi: 10.1007/BF02826219. [DOI] [PubMed] [Google Scholar]
- [33].Kirou KA, Lee C, George S, Louca K, et al. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 2005;52(5):1491–1503. doi: 10.1002/art.21031. [DOI] [PubMed] [Google Scholar]
- [34].Shodell M, Shah K, Siegal FP. Circulating human plasmacytoid dendritic cells are highly sensitive to corticosteroid administration. Lupus. 2003;12(3):222–230. doi: 10.1191/0961203303lu362xx. [DOI] [PubMed] [Google Scholar]
- [35].Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284(5421):1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- [36].Farkas L, Beiske K, Lund-Johansen F, Brandtzaeg P, et al. Plasmacytoid dendritic cells (natural interferon-alpha/beta-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am. J. Pathol. 2001;159(1):237–243. doi: 10.1016/s0002-9440(10)61689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]