Abstract
Accumulation of α-synuclein (Asyn) in neuronal perikarya and dystrophic neurites is characteristic of idiopathic and familial Parkinson’s disease. In this study, we investigated the relationship between α-synuclein expression and neurite outgrowth-maturation using MN9D dopaminergic cells and demonstrated key features of Asyn regulation in hippocampal neurons. Neurite elongation elicited by inhibition of Rho GTPase activity with C3 transferase or by db-cAMP treatment was associated with marked reduction of α-synuclein mRNA and protein expression. Rho inhibition resulted in reduction of transcription factor SRF in the nuclear fraction and retention of MKL-1 - the SRF co-transactivator of SRE - in cytosol, indicating that these effects of Rho inhibition may be mediated though reduction of SRF-SRE transcription. Inhibition of Rho GTPase activity led to decreased nuclear localization of GATA2, a key regulator of α-synuclein promoter activity. Rho inhibition-induced neurite extension was associated with increased VMAT2 and SNARE proteins synaptophysin and synapsin I. These results indicate that in the MN9D dopaminergic cell line, α-synuclein transcription and levels of synaptic vesicle associated proteins are inversely correlated with neurite growth. We confirm that in mature hippocampal neurons inhibition of RhoA and knock down of SRF by siRNA also leads to decrease GATA2 and Asyn. The results suggest that of RhoA signaling may be potential therapeutic target for the treatment of synucleinopathies.
Keywords: synuclein, Parkinson’s disease, Rho GTPase, C3 transferase
1. Introduction
Synucleinopathies are a group of degenerative diseases characterized by the accumulation of α-synuclein (Asyn) in dystrophic neurites and in neuronal perikarya that include idiopathic and familial Parkinson’s disease (PD) and dementia with Lewy bodies (DLB) (Marti et al., 2003). The discovery that missense mutations in the SCNA (α-synuclein) gene result in rare dominant forms of PD (Polymeropoulos et al., 1997) was followed by observations that duplication/triplication of the normal SCNA gene results also in autosomal dominant PD-DLB. Increased levels of α-synuclein correlate with disease onset and severity (Bandopadhyay and de Belleroche, 2009; Farrer et al., 2004; Singleton et al., 2003). The importance of α-synuclein levels in PD-DLB pathogenesis is supported further by observations that promoter polymorphisms causing increased transcription levels of α-synuclein are associated with the sporadic form of the disease (Maraganore et al., 2006), and observations from animal studies that overexpression of wild type or mutant Asyn in the substantia nigra of rodents causes dopaminergic denervation of the striatum and neurite pathology with accumulation of synaptic vesicles in varicosities (Lo Bianco et al., 2002). Asyn regulates activity of tyrosine hydroxylase (TH) (Lou et al., 2010), plays a key role in synaptic vesicle clustering and regulates neurotransmitter release (Nemani et al., 2010). In pathological states, Asyn accumulation in neurites is associated with dystrophic changes, neurite retraction and loss of synaptic connectivity. In PD, innervation of target fields is extensively lost early in the disease process (Albin et al., 2008; Bohnen et al., 2006), and it has been proposed that the vulnerability of select neuronal populations is in part related to the extent of axonal branching and the size of the synaptic fields supported by those neurons (Matsuda et al., 2009).
To explore the relationship between the development of dystrophic neurites and α-synuclein expression, we undertook a series of studies of neurite dynamics and α-synuclein expression. Rho GTPases function as molecular switches that translocate to the plasma membrane to transduce signals regulating growth cone morphology, and axon extension and branching. We used MN9D cells, a dopaminergic cell line that expresses the plasmalemmal dopamine transporter (DAT), TH, the vesicular monoamine transporter (VMAT), and release dopamine (DA); and we confirmed key findings in mature hippocampal neurons in vitro.
We found that inhibition of Rho-GTPase signaling by C3 transferase or by PKA activation resulted in neurite extension and was accompanied by a substantial reduction in expression of Asyn mRNA and protein. These effects were mediated through the reduction in nuclear transcription factors SRF and MKL-1, and in GATA-2, a transcriptional regulator of Asyn,
2. Materials and Methods
2.1. Cell culture
MN9D cells provided by by Alfred Heller and Lisa Won (University of Chicago, Chicago, USA) were grown in Dulbecco’s Modified Eagle’s Medium supplemented with 10% fetal clone III (Hyclone, Logan, UT). MN9D cells were treated with cell permeable C3 transferase (2.5 or 5μg/ml, Cytoskeleton, Denver, CO) for 24 or 48h or 2 mM dbCAMP (Sigma, St. Louis, MO) for 24h to 5d or 1μM forskolin (Sigma) for 48h. For primary neurons, hippocampi were dissected from P2 Sprague Dawley rats and digested with 0.05% trypsin/EDTA (Sigma) at 37° C for 15 min; cerebral cortex was dissected from E17 rat pups and dissociated by incubation with 0.25% trypsin/EDTA (Sigma) for 15 min at 37° C. Dissociated cells were plated in defined Neurobasal medium (Gibco) containing B27, Glutamax I, Albumax I, and penicillin/streptomycin. Hippocampal neurons maintained in vitro for 15–20 days (DIV15-20) and were treated with the cell permeable C3 transferase (5 μg/ml, Cytoskeleton) for 24 or 48h. For vector transfection, hippocampal neurons were infected for 2h at multiplicity of infection of 1 with a non-replicating Herpes simplex virus (HSV)-based vector vC3t expressing two copies of the C3 transferase transgene tagged with HA or vGFP expressing two copies of GFP (Mata et al., 2010). Transgene expression was confirmed by RT-PCR and Western blot to HA, and the Rho inhibitory activity of the transgene was determined by G-lisa (Cytoskeleton) of cell lysates. No sequence for release was attached and the transgene product remained intracellular (Mata et al., 2010).
2.2. siRNA Preparation and Transfection
Acell plus SMARTpool siRNA directed against SRF was used (Dharmacon, Chicago, IL). The siRNA sequences were ,1, 5′-GCACAGACCUCACGCAGAC-3′; sequence 2, 5′-UGAGACAGGCCAUGUGUAU-3′; sequence 3, 5′-AGACGGGCAUCAUGAAGAA-3′; sequence 4, 5′-ACAACAAGCUGCGGCGUUA-3′; Accell siCONTROL GAPDH-targeting pool siRNA (Dharmacon) was used as control (csiRNA). For siRNA transfection, 1μM siRNA solution was prepared in antibiotic-free cultured medium. Hippocampal neurons (DIV15) were incubated with the siRNA transfection solution for 72 h before cell lysis.
2.3. Isolation of nuclear proteins
MN9D cells were harvested at the indicated times and lysed in a solution containing 20 mM Tris-HCL, pH 7.8, 25mM KCl, 5mM MgCl2, 250 mM sucrose, 100 mM PMSF, 1 μg/ml pepstatin A, 2 μg/ml leupeptin, and 2 μg/ml aprotinin. After centrifugation (3,000 rpm, 10 min) the pellet was resuspended in the same buffer and was measured for protein by Bradford assay (BioRad, Hercules, CA).
2.4. Western blot
Cultured MN9D cells and hippocampal neurons were lysed in lysis buffer (50 mM Tris, 10 mM NaCl, 1% NP40, 0.02% sodium azide, and protease inhibitor cocktail (Sigma Aldrich) at pH 7.4) to get cytosolic extracts. Proteins from nuclear and cytosolic extracts were separated on 12% SDS-PAGE gels and then transferred onto a polyvinylidene diflouride membrane (Millipore, Medford, MA). Immunoblots were probed with primary antibody to anti-α synuclein (Abcam, Hartford, CT and BD Bioscience, San Jose, CA), anti-pTH (Millipore, Medford, MA), anti-TH (Millipore), anti-SRF (Santa Cruz Biotechnology), anti VMAT2 (Millipore), anti-synaptophysin (Millipore), anti-synapsin-1 (Millipore), anti-H3 (Cell Signaling, Danvers, MA), anti-GATA2 (Cell Signaling and Santa Cruz Biotechnology), anti-MKL (Genway, San Diego, CA), or anti-β-actin (Santa Cruz Biotechnology) then incubated with HRP-conjugated secondary antibody, followed by enhanced chemiluminescence detection (Amersham Biosciences, Arlington Heights, IL). The amount of chemiluminescent signal was quantitated using a chemiluminescence detector (ChemiDoc, BioRad).
2.5. Semiquantitative RT-PCR analysis
Total RNA was isolated from MN9D cells via TRIzol (Invitrogen, San Diego, CA). cDNA prepared from mRNA isolated from spinal cord neurons or rat spinal cord was amplified using following primer sets: β-actin-forward (5′-CAG TTC GCC ATG GAT GAC GAT ATC-3′) and β-actin-reverse (5′-CAC GCT CGG TCA GGA TCT TCA TG-3′) for β-actin, α-synuclein-forward (5′-AAA GGC CAA GGA GGG AGT TGT G-3′) and α-synuclein -reverse (5′-GGT GCA TAG TCT CAT GCT CAC-3′) for α-synuclein, VMAT2-forward (5′-CAA GCT GAT CCT GTT CAT CGT G-3′) and VMAT2-reverse (5′-ATC ATG GAG GAG TCC ACC ATC-3′) for VMAT2. All reactions involved initial denaturation at 94°C for 5 min followed by 30 cycles (94°C for 30 sec, 68°C for 3 min and 1 cycle 68°C for 8 min using a GeneAmp PCR 2700 (Applied Biosystems, Foster City, CA). The density of the bands was quantitated using a PC-based densitomtry system (ChemiDoc, BioRad).
2.6. Immunocytochemistry
Cells were fixed in 4% paraformaldehyde, washed with 0.2% Triton X-100 in PBS for 5 min, and the cells were placed in blocking serum (5% normal goat serum in PBS) for 30 min, after which they were exposed to anti-Tuj1 (Covance Research Products, Berkeley, CA), anti-VMAT2 (Millipore), anti-phosphorylated neurofilaments (SMI 31, Covance, Greenfield, IND), anti-MAP2 (Millipore), anti-synaptophysin (Millipore) and anti-α synuclein (BD Biosciences) followed by complementary antibody fluorescent tagged Alexa Fluor 488 and 594 (Molecular Probes). The nuclei were counterstained with Hoechst staining (Sigma). Morphometric analysis of neurite extension was carried out on images of individual or paired cells captured with a 20X lens on a Nikon E1000 microscope using Metmorph software (Nikon).
2.7. Data Analysis
Statistical significance of the difference between treatment and control groups was determined by two-tailed t-test SPSS 12.0 for Windows (SPSS, Chicago, IL). Each experiment was performed at least three independent times and the data expressed as mean ± standard error of mean (SEM), with P < 0.05 considered significant.
3. Results
3.1. Link between neurite growth and α-synuclein expression uncovered by Rho inhibition
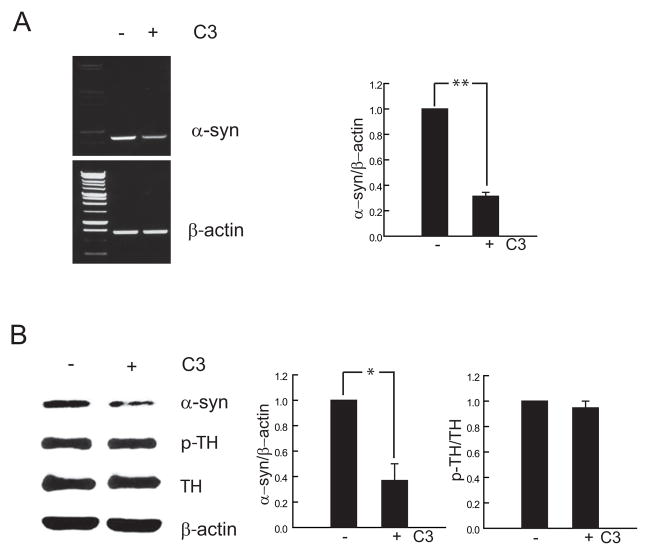
RhoA plays a central role in regulating growth cone collapse and for inhibiting neurite extension (Wang et al., 2002). C3 transferase is an enzyme produced by Clostridium botulinum that inhibits Rho activity through ADP-ribosylation of the Rho effector domain (Rubin et al., 1988). Targeted inhibition of RhoA by C3 transferase promotes neuronal regeneration and axonal extension after injury and during development (Dergham et al., 2002; Jin and Strittmatter, 1997; Niederost et al., 2002). To study the relationship between neurite growth and α-synuclein expression we used a cell permeable C3 transferase to induce neurite growth, C3 transferase treatment for 48 hours resulted in robust extension of neurites from what were otherwise rounded MN9D cells (Fig. 1A). Morphometric analysis of neurites stained with an antibody against β-III tubulin demonstrated an increased number of processes, increased length of processes and an increase in the number of branches per process (Fig. 1B). Neurite extension induced by Rho inhibition in these dopaminergic cells was associated with decreased Asyn mRNA and protein expression (Fig. 2A,B). There was no change in phosphorylated or total TH (Fig. 2B).
Figure 1.
A. MN9D cells treated with C3 transferase (2.5 μg/ml for 48h) show extension of neurites (top: phase contrast; bottom: immunostained with an antibody against β-III tubulin (Tuj-1). Bar = 40 μm. B. Quantitative analysis of the number of processes per cell, the mean length of processes per cell in μm, the maximum length of processes from individual cells in μm, and the number of branches per neuritic process. Mean ± SEM; * P < 0.05
Figure 2.
Treatment of MN9D cells with C3 transferase (2.5 μg/ml for 24h) resulted in a decrease in Asyn mRNA (A) and protein (B) but no change in pTH or TH (B). Quantitative analysis of RT-PCR and Western blots was determined for each sample as a ratio to β-actin or total TH and presented as percentage of control. Mean ± SEM; * P ≤ 0.05; ** P ≤ 0.01
3.2. Rho inhibition reduces nuclear SRF-MKL-1 and GATA-2
The effects of Rho-GTPases are mediated by downstream signaling through the serum response factor (SRF) transcription factor binding to the serum response element (SRE) in the promoter region of target genes (Liu et al., 2003). SRF acts as sensor of actin monomer (G-actin) levels in the cytoplasm. The G-actin level transducer of SRF is identified as the megakaryoblastic leukemia 1 (MKL-1) factor. MKL-1 is bound to G-actin in the cytoplasm; on activation of Rho and polymerization to F-actin, MKL-1 translocates to the nucleus to form a MKL-SRF complex that binds to SRE sites to coordinate the transcription of immediate early genes, including SRF (Knoll and Nordheim, 2009; Miralles et al., 2003; Sunavala-Dossabhoy et al., 2004). Treatment of MN9D cells with C3 transferase for 24 hrs resulted in a marked decrease in SRF in the nuclear fraction and retention of MKL-1 in the cytosolic fraction (Fig. 3A,B). SRF forms multiprotein complexes with the GATA family of transcription co-regulators. GATA-2 is abundantly expressed in dopaminergic neurons in brain regions affected by PD and binds to intron-1 of SCNA to regulate expression of endogenous neuronal Asyn (Scherzer et al., 2008). There was a marked decrease in GATA-2 protein in the nuclear fraction of C3 transferase treated compared to control MN9D cells suggesting that Rho signaling may alter Asyn transcription through regulation of GATA-2 (Fig. 3B).
Figure 3.
Treatment with C3 transferase for 24 h increased the amount of MKL1 in the cytosolic fraction (A) and decreases the amount of of MKL-1, SRF and GATA-2 in the nuclear fraction (B). Histone 3 (H3) is used as an internal control. Quantitative analysis was determined for each sample as a ratio to β-actin or H3 and presented as percentage of control. Mean ± SEM; * P ≤ 0.05; ** P ≤ 0.01.
3.3. cAMP-PKA activation results in neurite extension and a reduction in α synuclein mRNA and protein
Exposure of MN9D cells to dibutyryl cyclic AMP (db-cAMP) results in the formation of neurites similar to those seen in mature neurons. Treatment of MN9D cells with 2 mM db-cAMP resulted in a marked increase in the number and length of neuritic processes visualized by immunostaining with an antibody against β-III tubulin (Fig. 4A). The amount of phosphorylated and total TH was unchanged by the db-cAMP treatment, but the amount of α synuclein protein decreased as early as 24 hr after exposure to db-cAMP, and remained reduced through 4 days of treatment (Fig. 4B). The reduction in α-synuclein protein was probably a result of decreased expression, manifest by a reduction in Asyn mRNA (Fig. 4C).
Figure 4.
A. MN9D cells treated with 2 mM db-cAMP and immunostained with an antibody against β-III tubulin show extension of neurites characteristic of the differentiated state. Bar = 40 μm. B. Western blot of pTH, total TH, and α-synuclein in MN9D cells treated with db-cAMP for 48 to 96 h. β-actin was used as a loading control. C. α-synuclein mRNA from MN9D cells treated 48 or 96 h with 2 mM db-cAMP
PKA activation by cyclic AMP (cAMP) results in phosphorylation of Ser188 of RhoA leading to inactivation of RhoA (Lang et al., 1996). In order to understand the mechanism by which cAMP down-regulates α-synuclein expression during neurite outgrowth, we examined the effect of db-cAMP on Ser188 RhoA phosphorylation. We observed an increase in Ser188 phosphorylation of RhoA with unchanged levels of total RhoA (Fig. 5A), suggesting that PKA activation inhibits Rho activity through Ser188 phosphorylation. This was confirmed by the effect of 1 μM of the PKA activator forskolin, which reduced the amount of SRF in the nuclear fraction of treated MN9D cells (Fig. 5B). These results point to the convergence of cAMP-PKA and Rho-GTPase signaling pathways in promoting neurite growth and regulating Asyn transcription.
Figure 5.
A. MN9D cells treated with 2 mM db-cAMP for 24h show increased pRhoA (Ser188) as compared to levels of total Rho protein by Western blot. B. The amount of SRF in the nuclear fraction was decreased after treatment with 1 μM forskolin for 48h. H 3 is used as an internal control. Quantitative analysis was determined for each sample as a ratio to β-actin or H3 and presented as percentage of control. Mean ± SEM; * P ≤ 0.05.
3.4. Upregulation of VMAT2 and synaptic vesicle protein expression by Rho inhibition
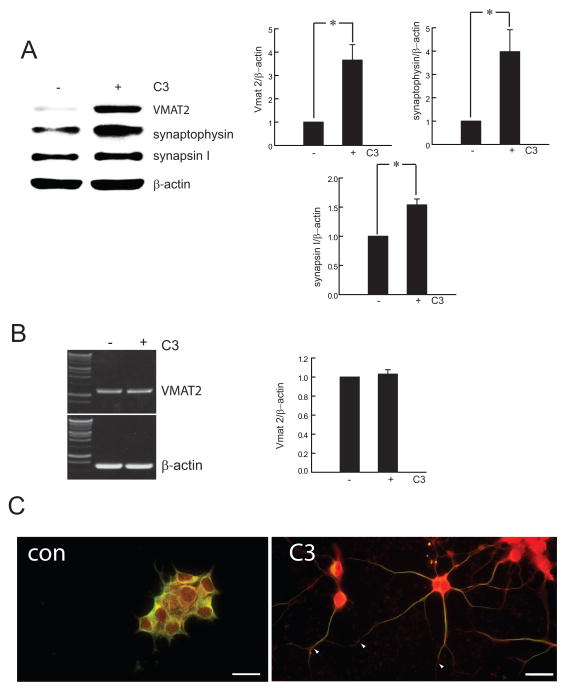
The vesicular monoamine transporter VMAT2, responsible for vesicle loading of neurotransmitter amines, is important in protecting neurons against MPTP toxicity (Takahashi et al., 1997; Uhl et al., 2000). We found that treatment with C3 transferase to inhibit Rho signaling resulted in a marked increase in VMAT2 protein levels as well as increased synaptic vesicle proteins synaptophysin and synapsin I (Fig 6A), suggesting a coordinated relationship between Asyn expression and synaptic vesicle protein levels during neurite growth induced Rho-GTPase inhibition. VMAT2 mRNA level was unchanged between treated and control cells (Fig 6B) indicating that the increase in VMAT2 must occur through a posttranscriptional mechanism. VMAT2 immunoreactivity colocalized with β-III tubulin along neuritic processes and extended into growth cones in MN9D cells treated with C3 transferase (Fig 6C).
Figure 6.
Treatment of MN9D cells with 2.5 μg/ml C3 transferase for 48h resulted in an increase in VMAT2, synaptophysin and synapsin I (A). VMAT2 mRNA levels were unchanged (B). Quantitative analysis of RT-PCR and Western blots was determined for each sample as a ratio to β-actin and presented as percentage of control. Mean ± SEM; * P ≤ 0.05. VMAT2 (red) and β-III tubulin (green) co-localized in neuritic processes, with VMAT2 particularly prominent in growth cones (arrowheads) after C3 transferase treatment (C). Bar = 30 μm.
3.5. Asyn regulation in primary hippocampal neurons
In order to investigate whether the regulation of Asyn observed in MN9D cells also occurs in primary neurons known to express Asyn, we examined P2 hippocampal neurons grown in vitro for 15–20 days, a time at which these neurons have fully differentiated dendrites and axons, and form extensive synaptic contacts (Fig. 7A). Asyn immunoreactivity was found in a punctuate pattern on MAP2-immunoreactive dendrites suggesting a localization at synaptic contacts (Fig.7A). Treatment of hippocampal neurons (DIV15) with C3 transferase to inhibit Rho-GTPase or transfection of the cells with a non-replicated HSV-based vector expressing C3 transferase transgene (vC3t) (Mata et al., 2010) resulted in a significant reduction in Asyn levels (Fig. 7B,C). These results demonstrate that in primary non-dopaminergic neurons regulation Asyn expression is also downstream of Rho signaling.
Figure 7.
P2 hippocampal neurons grown in vitro for 18 days display a mature phenotype with clear differentiation of axons labeled with SMI 31 and dendrites labeled with MAP2 (A, top panel) and formation of extensive synaptic contacts labeled with synaptophysin on MAP2 dendrites (A, middle panel) and Asyn immunostaining distributed along MAP2 positive and negative neurites (A, bottom panel). Figure enlargement shows Asyn-positive (green) in a punctuate pattern on dendrites. Scale bar = 20 μm. Hippocampal neurons (DIV 16) treated with C3 transferase (5 μg/ml) for 48h causes a reduction in Asyn protein in cell lysates as compared to control (B). Transfection with HSV-vector vC3t expressing C3 transferase but not with control vector vGFP (MOI 1) for 48h also causes reduction of Asyn protein (C). Treatment with SRF siRNA 1μM for 72h caused a marked reduction in SRF protein that was accompanied by a decreased in GATA2 transcription factor and Asyn levels. These changes were not seen in neurons treated with csiRNA for 72h. Results are representative of at least three independent experiments. Each sample was calculated as a ratio to β-actin and presented as percentage of control. Mean ± SEM; *p< 0.05, **p<0.01, ***p<0.005
3.6. Is SRF an upstream regulator of GATA2 and Asyn in primary neurons?
In order to test if SRF transcription regulation is upstream of GATA2, we knocked down SRF levels using an siRNA. P2 hippocampal neurons (DIV 15) were treated with either SRF siRNA or control siRNA for 72h. We observed a marked reduction in SRF protein that was accompanied by a significant decreased in GATA2 levels in cells treated with SRF siRNA but not with csiRNA. These changes in GATA2 occurred coincident with a decrease in Asyn protein, suggesting that SRF regulates expression of GATA2 and Asyn, and thus establishing a possible link between Rho GTPase and Asyn (Fig 7D).
4. Discussion
4.1. Neurite extension is regulated by Rho GTPases
Extension and retraction of neurites is under control of the Rho family of GTPases. Rac/cdc42 activation promotes neurite outgrowth with RhoA activation leading to growth cone collapse and neurite retraction through modulation of the assembly and stabilization of actin filaments and microtubule networks (Luo, 2000; Peng et al., 2010). In some populations of neurons, neurite extension is also under the regulatory control of adenylyl cyclases-PKA controlled by growth factor receptors (Cai et al., 1999; Zhou et al., 2009). PKA activation of CREB transcription and up-regulation of polyamines play important roles as downstream effectors of axonal regeneration (Gao et al., 2004; Hannila and Filbin, 2008; Zhou et al., 2009). PKA may also inhibit RhoA function via phosphorylation-dependent binding of RhoA to RhoGDI to result in retention of the complex in the cytosolic compartment, leading to a decrease in SRE transcriptional activity (Neumann et al., 2002; Qiao et al., 2008). Our studies address the role of RhoA and PKA in the differentiation and neurite formation and suggest that the convergence of these two pathways in neurite growth may result in part from direct phosphorylation of RhoA Ser 188 by PKA.
4.2. Regulation of Asyn expression in a model of dopaminergic neurons is negatively correlated with neurite extension
The vulnerability of neuronal populations affected in PD correlates with the extent of axonal branching and synaptic terminal fields (Matsuda et al., 2009). By the time clinical symptoms of PD become apparent projections to the receptor fields are already substantially reduced (Albin et al., 2008; Bohnen et al., 2006), indicating that a slow chronic process of neurite retraction and denervation precedes the clinical phenotype. In rodents, over-expression of wild type Asyn in dopaminergic neurons of the substantia nigra causes neurite pathology with accumulation of synaptic vesicles in varicosities (Lo Bianco et al., 2002). In neurons, RhoA activation following injury is one mechanism responsible for the failure of central axonal regeneration, while targeted inhibition of RhoA enhances extension of injured axons and enhances synaptic plasticity (Dergham et al., 2002; Jin and Strittmatter, 1997; Niederost et al., 2002). Our studies demonstrate that extension of neurites by Rho inhibition is correlated with repression of α-synuclein expression during differentiation of MN9D cells.
The complex morphological changes regulated by Rho-GTPase are mediated by downstream signaling that modify the levels SRF-MKL-1 in the nucleus and binding to SRE in the promoter region of many immediate early genes to coordinate their transcription. In addition, SRF forms multiprotein complexes with other transcription factors and chromatin remodeling factors to produced disparate programs of gene expression (Miano, 2003). SRF forms complexes with the GATA family of transcription co-regulators and GATA-1 and -2 directly regulates the expression of Asyn (Scherzer et al., 2008). Inhibition of RhoA and suppression of SRE transcription causes marked decrease in nuclear SRF and GATA-2, suggesting that these two factors may be transcriptional regulators of Asyn expression in MN9D cells and in hippocampal neurons.
4.3. Accumulation of Asyn is characteristic of PD and other synucleinopathies
In disease states Asyn accumulates in dystrophic neuritis and neuronal perikarya where it self-associates leading to formation of oligomers and fibrils. Similar accumulations have also been described in peripheral axon fibers in animal models of aging and in disease states (Phillips et al., 2009; Shishido et al., 2010). Many potential contributors to protein aggregation have been described including: free radical formation (Ostrerova-Golts et al., 2000), mitochondrial toxicity (Betarbet et al., 2000), and nitration (Giasson et al., 2000) or phosphorylation of the protein (Fujiwara et al., 2002), and alterations in proteasomal processing (Bence et al., 2001; Lindersson et al., 2004) may occur independently or as a consequence of the aggregates. Transgenic overexpression of wild-type Asyn in mouse models produces only subtle behavioral phenotypes (Chandra et al., 2005; Masliah et al., 2000; Sharon et al., 2003). Despite the lack of obvious pathology in these transgenic mouse models, the inherited forms of PD resulting from Asyn gene duplication/triplication (Singleton et al., 2003), and the sporadic cases of PD related to promoter polymorphisms leading to increased Asyn (Maraganore et al., 2006) serve to support the notion that the amount of Asyn by itself may be a critical variable, independent of post-translational modification or defects in processing of the protein.
4.4. Asyn and synaptic vesicles
Wild type Asyn, in its native state, binds to acidic phospholipids (Uversky, 2007) including vesicles and the presynaptic membrane (Kahle et al., 2000). Even modest overexpression of Asyn inhibits neurotransmitter release by impairing synaptic vesicle exocytosis (Nemani et al.). In vitro overexpression of Asyn reduces the readily releasable pool of synaptic vesicles without affecting the kinetics of fusion. In transgenic mice overexpressing normal Asyn, there is a failure of synaptic transmission and reduced synaptic vesicle clustering and reduced vesicle density adjacent to the active zone by electron microscopy (Nemani et al.). Over-expression of Asyn also causes inactivation of tyrosine hydroxylase (Lou et al., 2010). Reducing Asyn levels by gene knock down in mice or by siRNA interference in human derived dopaminergic neurons in vitro prevents MPTP and L-DOPA induced toxicity (Dauer et al., 2002; Fountaine et al., 2008; Mosharov et al., 2009). Taken together these studies suggest that while Asyn is essential for synaptic vesicle function and neurotransmitter release, high levels of Asyn are deleterious and may contribute to the sensitivity of dopaminergic neurons to exogenous toxins. In this context maintaining low levels of α-synuclein by Rho inhibition may contribute to efficient synaptic function.
VMAT2 is the brain form of the vesicular monoamine transporter responsible for packaging biogenic amines, including dopamine, into synaptic vesicles (Uhl et al., 2000). In heterozygous knockout mice decreased expression of VMAT2 results in reduced neurotransmitter content in synaptic vesicles, decreased magnitude of quantal release and modifying behavior measures (Fon et al., 1997; Pothos et al., 2000; Takahashi et al., 1997). VMAT2 protects against MPTP induced parkinsonism by sequestering MPP+ into synaptic vesicles and VMAT2 heterozygous knockout mice have increased sensitivity to MPTP toxicity (Guillot and Miller, 2009; Mooslehner et al., 2001; Uhl et al., 2000). In humans, gain of function haplotypes in the VMAT2 promoter display significant increased transcriptional activity have shown to be protective and have a reduced association with PD (Glatt et al., 2006). The transcriptional control of VMAT2 is complex and a number of endogenous and exogenous molecules participate in regulating the promoter activity of VMAT2 in cell lines. Our studies show that transcriptional repression of Asyn observed during neurite growth is associated with a substantial increase in VMAT2 levels, as well as synaptophysin and synapsin I; a state that would serve to promote loading of neurotransmitter in synaptic vesicles for synaptic release. This agrees with previous studies demonstrating that lowering Asyn levels by siRNA increases total VMAT2 particle density and number of VMAT2 particles per synaptic vesicle.
5. Conclusions
Inhibition of Rho-GTPase signaling by C3 transferase or by PKA activation resulted in neurite extension in the MN9D cells and was accompanied by a substantial reduction in expression of Asyn mRNA and protein. These effects were mediated through the reduction in nuclear transcription factors SRF and MKL-1, known to complex and bind to SRE elements on promoters of immediate early genes, and with GATA-2, a transcriptional regulator of Asyn (Fig. 8). Increased neurite outgrowth in MN9D cells was accompanied by an increase in VMAT2 and other synaptic vesicle associated proteins characteristic of synaptic vesicle maturation. In hippocampal neurons inhibition of Rho-GTPase may also regulate Asyn levels by a similar SRF-GATA2 pathway. These results suggest that C3 transferase-like molecules applied directly or by vector delivery may have potential as therapeutic agents for the treatment of synucleinopathy.
Figure 8.
Schematic representation of the pathways involved in regulation of α-synuclein expression.
Acknowledgments
We acknowledge the technical support of Aaron Ellis. The MN9D cells were provided by Drs. Alfred Heller and Lisa Won (University of Chicago). The work was supported by grants to MM and DJF from the National Institutes of Health and the Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Zhigang Zhou, Email: zhouzhigang8@gmail.com.
Jeeyong Kim, Email: jyokimc@umich.edu.
Ryan Insolera, Email: ryi2002@med.cornell.edu.
Xiangmin Peng, Email: flosarah@gmail.com.
David J. Fink, Email: djfink@umich.edu.
References
- Albin RL, Koeppe RA, Bohnen NI, Wernette K, Kilbourn MA, Frey KA. Spared caudal brainstem SERT binding in early Parkinson’s disease. J Cereb Blood Flow Metab. 2008;28:441–444. doi: 10.1038/sj.jcbfm.9600599. [DOI] [PubMed] [Google Scholar]
- Bandopadhyay R, de Belleroche J. Pathogenesis of Parkinson’s disease: emerging role of molecular chaperones. Trends Mol Med. 2009;16:27–36. doi: 10.1016/j.molmed.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Bence NF, Sampat RM, Kopito RR. Impairment of the ubiquitin-proteasome system by protein aggregation. Science. 2001;292:1552–1555. doi: 10.1126/science.292.5521.1552. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Albin RL, Koeppe RA, Wernette KA, Kilbourn MR, Minoshima S, Frey KA. Positron emission tomography of monoaminergic vesicular binding in aging and Parkinson disease. J Cereb Blood Flow Metab. 2006;26:1198–1212. doi: 10.1038/sj.jcbfm.9600276. [DOI] [PubMed] [Google Scholar]
- Cai D, Shen Y, De Bellard M, Tang S, Filbin MT. Prior exposure to neurotrophins blocks inhibition of axonal regeneration by MAG and myelin via a cAMP-dependent mechanism. Neuron. 1999;22:89–101. doi: 10.1016/s0896-6273(00)80681-9. [DOI] [PubMed] [Google Scholar]
- Chandra S, Gallardo G, Fernandez-Chacon R, Schluter OM, Sudhof TC. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123:383–396. doi: 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Dauer W, Kholodilov N, Vila M, Trillat AC, Goodchild R, Larsen KE, Staal R, Tieu K, Schmitz Y, Yuan CA, Rocha M, Jackson-Lewis V, Hersch S, Sulzer D, Przedborski S, Burke R, Hen R. Resistance of alpha -synuclein null mice to the parkinsonian neurotoxin MPTP. Proc Natl Acad Sci U S A. 2002;99:14524–14529. doi: 10.1073/pnas.172514599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dergham P, Ellezam B, Essagian C, Avedissian H, Lubell WD, McKerracher L. Rho signaling pathway targeted to promote spinal cord repair. J Neurosci. 2002;22:6570–6577. doi: 10.1523/JNEUROSCI.22-15-06570.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer M, Kachergus J, Forno L, Lincoln S, Wang DS, Hulihan M, Maraganore D, Gwinn-Hardy K, Wszolek Z, Dickson D, Langston JW. Comparison of kindreds with parkinsonism and alpha-synuclein genomic multiplications. Ann Neurol. 2004;55:174–179. doi: 10.1002/ana.10846. [DOI] [PubMed] [Google Scholar]
- Fon EA, Pothos EN, Sun BC, Killeen N, Sulzer D, Edwards RH. Vesicular transport regulates monoamine storage and release but is not essential for amphetamine action. Neuron. 1997;19:1271–1283. doi: 10.1016/s0896-6273(00)80418-3. [DOI] [PubMed] [Google Scholar]
- Fountaine TM, Venda LL, Warrick N, Christian HC, Brundin P, Channon KM, Wade-Martins R. The effect of alpha-synuclein knockdown on MPP+ toxicity in models of human neurons. Eur J Neurosci. 2008;28:2459–2473. doi: 10.1111/j.1460-9568.2008.06527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- Gao Y, Deng K, Hou J, Bryson JB, Barco A, Nikulina E, Spencer T, Mellado W, Kandel ER, Filbin MT. Activated CREB is sufficient to overcome inhibitors in myelin and promote spinal axon regeneration in vivo. Neuron. 2004;44:609–621. doi: 10.1016/j.neuron.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- Glatt CE, Wahner AD, White DJ, Ruiz-Linares A, Ritz B. Gain-of-function haplotypes in the vesicular monoamine transporter promoter are protective for Parkinson disease in women. Hum Mol Genet. 2006;15:299–305. doi: 10.1093/hmg/ddi445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot TS, Miller GW. Protective actions of the vesicular monoamine transporter 2 (VMAT2) in monoaminergic neurons. Mol Neurobiol. 2009;39:149–170. doi: 10.1007/s12035-009-8059-y. [DOI] [PubMed] [Google Scholar]
- Hannila SS, Filbin MT. The role of cyclic AMP signaling in promoting axonal regeneration after spinal cord injury. Exp Neurol. 2008;209:321–332. doi: 10.1016/j.expneurol.2007.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z, Strittmatter SM. Rac1 mediates collapsin-1-induced growth cone collapse. J Neurosci. 1997;17:6256–6263. doi: 10.1523/JNEUROSCI.17-16-06256.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle PJ, Neumann M, Ozmen L, Muller V, Jacobsen H, Schindzielorz A, Okochi M, Leimer U, van Der Putten H, Probst A, Kremmer E, Kretzschmar HA, Haass C. Subcellular localization of wild-type and Parkinson’s disease-associated mutant alpha -synuclein in human and transgenic mouse brain. J Neurosci. 2000;20:6365–6373. doi: 10.1523/JNEUROSCI.20-17-06365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll B, Nordheim A. Functional versatility of transcription factors in the nervous system: the SRF paradigm. Trends Neurosci. 2009;32:432–442. doi: 10.1016/j.tins.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Lang P, Gesbert F, Delespine-Carmagnat M, Stancou R, Pouchelet M, Bertoglio J. Protein kinase A phosphorylation of RhoA mediates the morphological and functional effects of cyclic AMP in cytotoxic lymphocytes. EMBO J. 1996;15:510–519. [PMC free article] [PubMed] [Google Scholar]
- Lindersson E, Beedholm R, Hojrup P, Moos T, Gai W, Hendil KB, Jensen PH. Proteasomal inhibition by alpha-synuclein filaments and oligomers. J Biol Chem. 2004;279:12924–12934. doi: 10.1074/jbc.M306390200. [DOI] [PubMed] [Google Scholar]
- Liu HW, Halayko AJ, Fernandes DJ, Harmon GS, McCauley JA, Kocieniewski P, McConville J, Fu Y, Forsythe SM, Kogut P, Bellam S, Dowell M, Churchill J, Lesso H, Kassiri K, Mitchell RW, Hershenson MB, Camoretti-Mercado B, Solway J. The RhoA/Rho kinase pathway regulates nuclear localization of serum response factor. Am J Respir Cell Mol Biol. 2003;29:39–47. doi: 10.1165/rcmb.2002-0206OC. [DOI] [PubMed] [Google Scholar]
- Lo Bianco C, Ridet JL, Schneider BL, Deglon N, Aebischer P. alpha -Synucleinopathy and selective dopaminergic neuron loss in a rat lentiviral-based model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2002;99:10813–10818. doi: 10.1073/pnas.152339799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou H, Montoya SE, Alerte TN, Wang J, Wu J, Peng XM, Hong CS, Friedrich EE, Mader SA, Pedersen CJ, Marcus BS, McCormack AL, Di Monte DA, Daubner SC, Perez RG. Serine 129 phosphorylation reduces {alpha}-synuclein’s ability to regulate tyrosine hydroxylase and protein phosphatase 2A in vitro and in vivo. J Biol Chem. 2010 doi: 10.1074/jbc.M110.100867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L. Rho GTPases in neuronal morphogenesis. Nat Rev Neurosci. 2000;1:173–180. doi: 10.1038/35044547. [DOI] [PubMed] [Google Scholar]
- Maraganore DM, de Andrade M, Elbaz A, Farrer MJ, Ioannidis JP, Kruger R, Rocca WA, Schneider NK, Lesnick TG, Lincoln SJ, Hulihan MM, Aasly JO, Ashizawa T, Chartier-Harlin MC, Checkoway H, Ferrarese C, Hadjigeorgiou G, Hattori N, Kawakami H, Lambert JC, Lynch T, Mellick GD, Papapetropoulos S, Parsian A, Quattrone A, Riess O, Tan EK, Van Broeckhoven C. Collaborative analysis of alpha-synuclein gene promoter variability and Parkinson disease. JAMA. 2006;296:661–670. doi: 10.1001/jama.296.6.661. [DOI] [PubMed] [Google Scholar]
- Marti MJ, Tolosa E, Campdelacreu J. Clinical overview of the synucleinopathies. Mov Disord. 2003;18(Suppl 6):S21–27. doi: 10.1002/mds.10559. [DOI] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, Mucke L. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287:1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- Mata M, Zhou Z, Peng X, Chiang P, Kim J, Fink DJ. HSV-mediated gene transfer of C3 transferase to inhibit Rho promotes CNS axonal regeneration. Neuroscience Meeting Planner; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda W, Furuta T, Nakamura KC, Hioki H, Fujiyama F, Arai R, Kaneko T. Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. J Neurosci. 2009;29:444–453. doi: 10.1523/JNEUROSCI.4029-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miano JM. Serum response factor: toggling between disparate programs of gene expression. J Mol Cell Cardiol. 2003;35:577–593. doi: 10.1016/s0022-2828(03)00110-x. [DOI] [PubMed] [Google Scholar]
- Miralles F, Posern G, Zaromytidou AI, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell. 2003;113:329–342. doi: 10.1016/s0092-8674(03)00278-2. [DOI] [PubMed] [Google Scholar]
- Mooslehner KA, Chan PM, Xu W, Liu L, Smadja C, Humby T, Allen ND, Wilkinson LS, Emson PC. Mice with very low expression of the vesicular monoamine transporter 2 gene survive into adulthood: potential mouse model for parkinsonism. Mol Cell Biol. 2001;21:5321–5331. doi: 10.1128/MCB.21.16.5321-5331.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosharov EV, Larsen KE, Kanter E, Phillips KA, Wilson K, Schmitz Y, Krantz DE, Kobayashi K, Edwards RH, Sulzer D. Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron. 2009;62:218–229. doi: 10.1016/j.neuron.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemani VM, Lu W, Berge V, Nakamura K, Onoa B, Lee MK, Chaudhry FA, Nicoll RA, Edwards RH. Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron. 2010;65:66–79. doi: 10.1016/j.neuron.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann S, Bradke F, Tessier-Lavigne M, Basbaum AI. Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron. 2002;34:885–893. doi: 10.1016/s0896-6273(02)00702-x. [DOI] [PubMed] [Google Scholar]
- Niederost B, Oertle T, Fritsche J, McKinney RA, Bandtlow CE. Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. J Neurosci. 2002;22:10368–10376. doi: 10.1523/JNEUROSCI.22-23-10368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrerova-Golts N, Petrucelli L, Hardy J, Lee JM, Farer M, Wolozin B. The A53T alpha-synuclein mutation increases iron-dependent aggregation and toxicity. J Neurosci. 2000;20:6048–6054. doi: 10.1523/JNEUROSCI.20-16-06048.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Zhou Z, Hu J, Fink DJ, Mata M. Soluble Nogo receptor down-regulates expression of neuronal Nogo-A to enhance axonal regeneration. J Biol Chem. 2010;285:2783–2795. doi: 10.1074/jbc.M109.046425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RJ, Walter GC, Ringer BE, Higgs KM, Powley TL. Alpha-synuclein immunopositive aggregates in the myenteric plexus of the aging Fischer 344 rat. Exp Neurol. 2009;220:109–119. doi: 10.1016/j.expneurol.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Pothos EN, Larsen KE, Krantz DE, Liu Y, Haycock JW, Setlik W, Gershon MD, Edwards RH, Sulzer D. Synaptic vesicle transporter expression regulates vesicle phenotype and quantal size. J Neurosci. 2000;20:7297–7306. doi: 10.1523/JNEUROSCI.20-19-07297.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J, Holian O, Lee BS, Huang F, Zhang J, Lum H. Phosphorylation of GTP dissociation inhibitor by PKA negatively regulates RhoA. Am J Physiol Cell Physiol. 2008;295:C1161–1168. doi: 10.1152/ajpcell.00139.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin EJ, Gill DM, Boquet P, Popoff MR. Functional modification of a 21-kilodalton G protein when ADP-ribosylated by exoenzyme C3 of Clostridium botulinum. Mol Cell Biol. 1988;8:418–426. doi: 10.1128/mcb.8.1.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzer CR, Grass JA, Liao Z, Pepivani I, Zheng B, Eklund AC, Ney PA, Ng J, McGoldrick M, Mollenhauer B, Bresnick EH, Schlossmacher MG. GATA transcription factors directly regulate the Parkinson’s disease-linked gene alpha-synuclein. Proc Natl Acad Sci U S A. 2008;105:10907–10912. doi: 10.1073/pnas.0802437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon R, Bar-Joseph I, Frosch MP, Walsh DM, Hamilton JA, Selkoe DJ. The formation of highly soluble oligomers of alpha-synuclein is regulated by fatty acids and enhanced in Parkinson’s disease. Neuron. 2003;37:583–595. doi: 10.1016/s0896-6273(03)00024-2. [DOI] [PubMed] [Google Scholar]
- Shishido T, Ikemura M, Obi T, Yamazaki K, Terada T, Sugiura A, Saito Y, Murayama S, Mizoguchi K. alpha-synuclein accumulation in skin nerve fibers revealed by skin biopsy in pure autonomic failure. Neurology. 2010;74:608–610. doi: 10.1212/WNL.0b013e3181cff6d5. [DOI] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Sunavala-Dossabhoy G, Fowler M, De Benedetti A. Translation of the radioresistance kinase TLK1B is induced by gamma-irradiation through activation of mTOR and phosphorylation of 4E-BP1. BMC Mol Biol. 2004;5:1. doi: 10.1186/1471-2199-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Miner LL, Sora I, Ujike H, Revay RS, Kostic V, Jackson-Lewis V, Przedborski S, Uhl GR. VMAT2 knockout mice: heterozygotes display reduced amphetamine-conditioned reward, enhanced amphetamine locomotion, and enhanced MPTP toxicity. Proc Natl Acad Sci U S A. 1997;94:9938–9943. doi: 10.1073/pnas.94.18.9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl GR, Li S, Takahashi N, Itokawa K, Lin Z, Hazama M, Sora I. The VMAT2 gene in mice and humans: amphetamine responses, locomotion, cardiac arrhythmias, aging, and vulnerability to dopaminergic toxins. FASEB J. 2000;14:2459–2465. doi: 10.1096/fj.00-0205rev. [DOI] [PubMed] [Google Scholar]
- Uversky VN. Neuropathology, biochemistry, and biophysics of alpha-synuclein aggregation. J Neurochem. 2007;103:17–37. doi: 10.1111/j.1471-4159.2007.04764.x. [DOI] [PubMed] [Google Scholar]
- Wang KC, Kim JA, Sivasankaran R, Segal R, He Z. P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature. 2002;420:74–78. doi: 10.1038/nature01176. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Peng X, Fink DJ, Mata M. HSV-mediated transfer of artemin overcomes myelin inhibition to improve outcome after spinal cord injury. Mol Ther. 2009;17:1173–1179. doi: 10.1038/mt.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]