Abstract
In land plants, xyloglucans (XyGs) tether cellulose microfibrils into a strong but extensible cell wall. The MUR2 and MUR3 genes of Arabidopsis encode XyG-specific fucosyl and galactosyl transferases, respectively. Mutations of these genes give precisely altered XyG structures missing one or both of these subtending sugar residues. Tensile strength measurements of etiolated hypocotyls revealed that galactosylation rather than fucosylation of the side chains is essential for maintenance of wall strength. Symptomatic of this loss of tensile strength is an abnormal swelling of the cells at the base of fully grown hypocotyls as well as bulging and marked increase in the diameter of the epidermal and underlying cortical cells. The presence of subtending galactosyl residues markedly enhance the activities of XyG endotransglucosylases and the accessibility of XyG to their action, indicating a role for this enzyme activity in XyG cleavage and religation in the wall during growth for maintenance of tensile strength. Although a shortening of XyGs that normally accompanies cell elongation appears to be slightly reduced, galactosylation of the XyGs is not strictly required for cell elongation, for lengthening the polymers that occurs in the wall upon secretion, or for binding of the XyGs to cellulose.
The plant cell wall is continually modified during cell growth and differentiation. The tensile strength of the wall is provided by a dense spool of cellulose microfibrils interlaced with cross-linking glycans (Carpita and Gibeaut, 1993). When plant cells grow, the wall is biochemically “loosened” to permit turgor-driven cell expansion (Cosgrove, 2000). One of the mysteries of cell growth in plants that researchers have pondered for decades is how these interlaced glycans loosen without compromising the tensile strength of the pliant wall.
In all dicots and certain monocots, xyloglucans (XyGs) are the principal cellulose tethering molecules, and the loosening of these tethers from the microfibrils provides a physical control point of cell expansion (Cosgrove, 2000). The unique ability of XyG endotransglucosylases (XETs) to cleave XyGs and rejoin the cut ends with new partners suggested a role for these enzyme activities in wall loosening during growth and in the restructuring of cell walls after extension (Nishitani and Tominaga, 1992). (A revised nomenclature has been adopted by consensus of researchers of the XyG endotransglucosylase/hydrolase gene/protein family [Rose et al., 2002]. The abbreviation XTH refers to any gene/protein of the family regardless of activity of the protein. However, when referring to endotransglucosylase activities, the abbreviation remains XET, and hydrolase activities are abbreviated XEH.) However, the only cell wall proteins proven to be capable of causing extension of isolated walls in vitro under mechanical stress are expansins, which increase wall extensibility under constant stress (McQueen-Mason et al., 1992), and yieldins, which lower the “yield threshold,” the minimum stress required that permits extension (Okamoto-Nakazato et al., 2000). Although XETs do not exhibit either of these activities in vitro (McQueen-Mason et al., 1993), a cooperativity between expansins and XET activity has been implicated in polymer lengthening during wall assembly (Thompson and Fry, 2001) and in the loosening and restructuring of the wall during cell growth and microfibril reorientation (Nishitani, 1998). The results of our studies with mutants with precisely altered XyG structures suggest a role for XET activity in the molecular grafting of XyGs—not for wall loosening during cell growth but principally to maintain tensile strength of the wall after growth.
Most XyGs consist of repeating heptasaccharide units of four β-d-glucosyl units linked (1→4), with three consecutive residues substituted with α-d-xylosyl units linked (1→6)- to the glucan backbone (Table I). About one-half of these units contain extensions of β-d-galactosyl-(1→2)- upon the first xylosyl (closest to the reducing end) of the glucan or to the middle Xyl or to both residues. An α-l-fucosyl-(1→2)- residue is then added to the Gal residue at the first position (Carpita and Gibeaut, 1993). Cleavage of XyG with a sequence-specific Trichoderma endoglucanase yields six kinds of oligomeric units that constitute a species-specific profile. In addition to the fundamental Xyl3Glc4 oligomer, called XXXG in a standardized nomenclature (Fry et al., 1993), the other oligomers are five possible permutations formed by addition of galactosyl residues at the first or middle Xyl residue to give XXLG or XLXG, and XLLG, and of subsequent addition of l-Fuc upon the first galactosyl unit, if present, to give XXFG and XLFG (Table I).
Table I.
Distribution of oligomeric units in XyGs from leaves and hypocotyls of Arabidopsis wild-type, mur2, and mur3 seedlings and cells of mur2 and mur3 maintained in continuous liquid cell culture
Protocols for the preparation of cell walls, extraction and digestion of XyG, and the quantitation of the unit structures are described by Vanzin et al. (2002). n.d., not detected.
| Structure
|
Oligomer
|
Leaf
|
Hypocotyl
|
Cultured Cells
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| Wild Type | mur2 | mur3 | Wild Type | mur2 | mur3 | mur2 | mur3 | ||
| % of oligomers | |||||||||
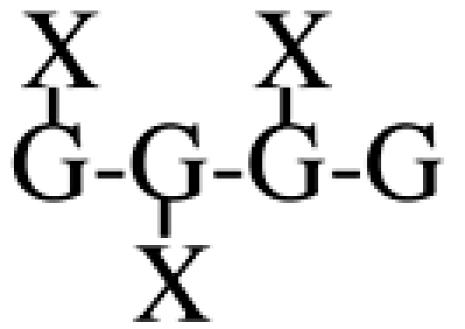 |
XXXG | 44.9 | 33.4 | 55.3 | 37.4 | 42.3 | 83.9 | 26.7 | 100.0 |
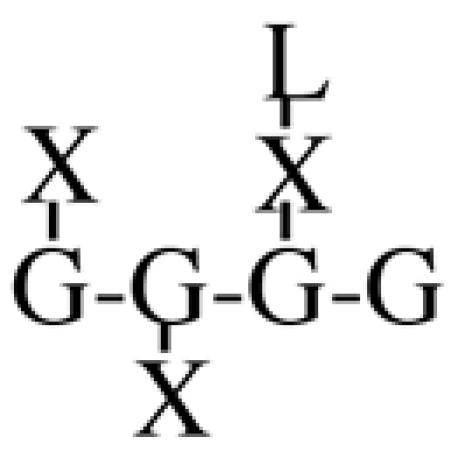 |
XXLG | 8.5 | 36.8 | n.d. | 14.7 | 49.7 | n.d. | 57.1 | n.d. |
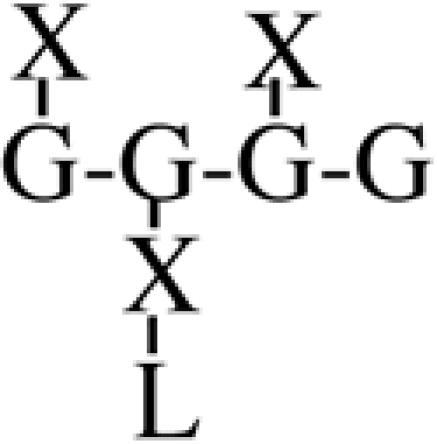 |
XLXG | 3.1 | 7.7 | 44.7 | 1.9 | 6.2 | 16.1 | 5.7 | n.d. |
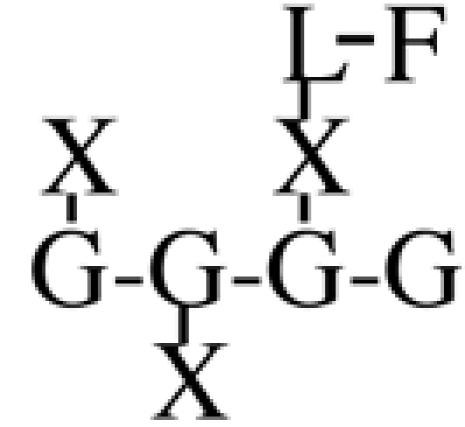 |
XXFG | 23.8 | n.d. | n.d. | 35.1 | n.d. | n.d. | n.d. | n.d. |
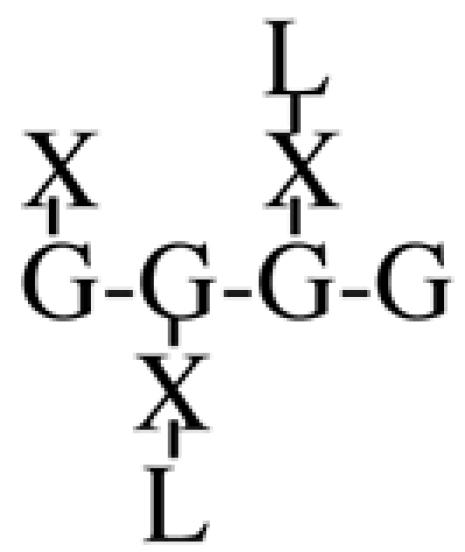 |
XLLG | 4.0 | 22.1 | n.d. | 0.8 | 1.8 | n.d. | 10.5 | n.d. |
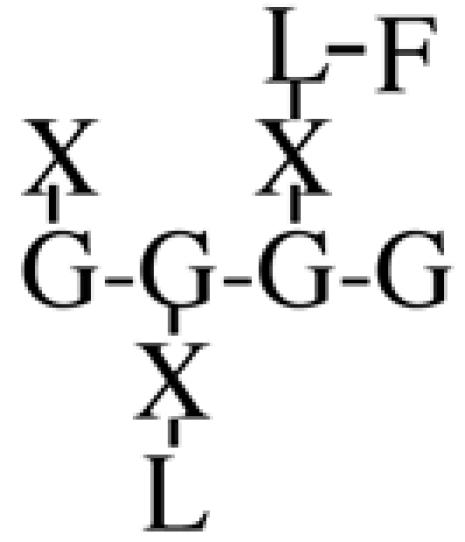 |
XLFG | 15.7 | n.d. | n.d. | 10.1 | n.d. | n.d. | n.d. | n.d. |
Two major hypotheses have been proposed for the function of the trisaccharide side group, α-l-Fuc-(1→2)-β-d-Gal-(1→2)-α-d-Xyl-, with particular importance placed on a requirement of the terminal fucosyl residue. First, computer-modeling studies of three-dimensional XyG structures suggest that the glucan backbone may assume either a twisted or straightened conformation, and trisaccharide side groups stabilize the straightened form to facilitate steric bonding with cellulose microfibrils (Levy et al., 1991). Second, the XyG oligomers containing the fucosylated trisaccharide side groups modulate auxin-induced growth in excised sections (Zablackis et al., 1996).
Two Arabidopsis mutants, called mur2 and mur3, were selected on the basis of an underrepresentation of Fuc in cell wall polymers (Reiter et al., 1997), and both of them affect XyG trisaccharide side-group structure specifically. MUR2 encodes a XyG-specific fucosyltransferase, which leads to the absence of XyG fucosylation in mur2 plants (Vanzin et al., 2002), and MUR3 encodes the galactosyltransferase specifically responsible for the first step in the formation of the α-l-Fuc-(1→2)-β-d-Gal-(1→2)- side group (Madson et al., 2003). The mur3 mutation eliminates the entire disaccharide extension from the first Xyl and results in marked enhancement of galactosylation at the middle Xyl in the instance of leaf-derived XyG. Remarkably, the growth habits of mur2 and mur3 plants are indistinguishable from those of wild type despite such radical alteration of their fundamental cross-linking polymers (Reiter et al., 1997). The tensile strengths of mur2 and mur3 floral stems were also comparable with wild type (Vanzin et al., 2002; Madson et al., 2003). Thus, if a fucosylated trisaccharide functions in facilitating normal growth or increasing the tensile strength of the wall during growth, then this functional property can be replaced by increased degrees of galactosylation.
Whereas growth form and physiology of both mur2 and mur3 mutants are indistinguishable from wild type in the shoot, we observed a strong phenotype in etiolated hypocotyls of mur3 seedlings. The tensile strengths of the hypocotyls was less than 40% those of wild-type or mur2 hypocotyls, and a visible swelling of the base of the hypocotyls occurred as a result of grossly enlarged epidermal and cortical cells. The phenotype is associated with a failure in hypocotyls of the enhancement of galactosylation of the middle Xyl in mur3 XyGs that occurs in mur3 shoot tissues. No other feature was affected by the altered XyG structure, because cell elongation, growth form, tenacity of cellulose binding, and polymer lengthening that occurs in muro were all indistinguishable from wild type. However, we found that XET activity is markedly enhanced by the presence of galactosyl residues, indicating that this enzyme plays a role in remodeling and religating XyGs during growth to maintain tensile strength.
RESULTS AND DISCUSSION
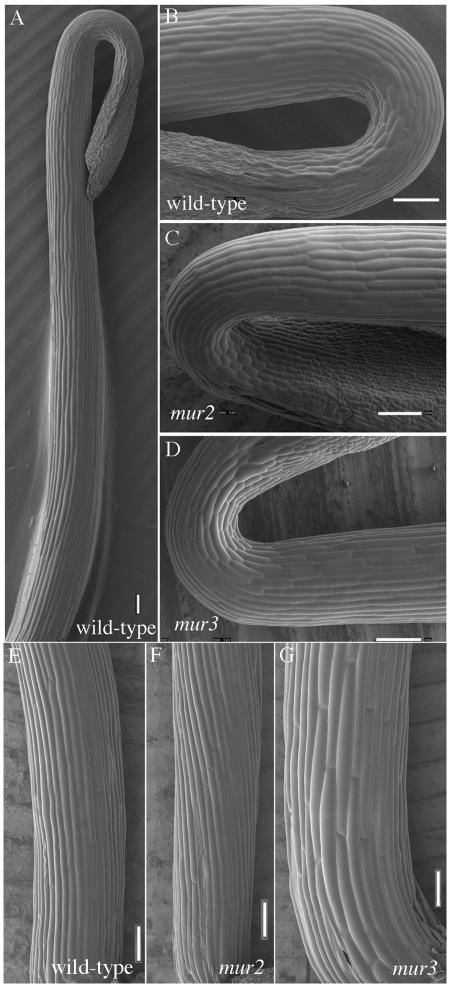
The hypocotyl is a tapered cylinder of tissue that elongates in darkness to about 2 cm (Fig. 1A). The shape of the hypocotyl “hook” and the initiation of cell elongation are indistinguishable among wild-type and mur mutants (Fig. 1, B-D). During maximal growth, the wild-type hypocotyls is about 260 μm at the base and tapers to 190 μm just below the hook. After elongation, the base of the hypocotyls continues to enlarge to about 300 μm. However, the average diameters of the base of the mur3 hypocotyl swell to nearly 400 μm, and the epidermal cells exhibit abnormal swelling or bulging that accompanies the onset of elongation but disappears in wild-type and mur2 hypocotyls after growth has ceased (Fig. 1, E-G). Although the epidermal cells display obvious bulging, the increases in hypocotyl diameters are due primarily to increases in diameters of the cortical cells comprising two layers within the epidermis. The diameters of the inner layer of eight cortical cells of wild type are about 45 and 48 μm in periclinal and radial directions, respectively, whereas mur2 inner cells are somewhat smaller at 40 to 42 μm. However, the mur3 inner cortical cells swell to more than 70 μm radially and about 65 μm periclinally (Table II). The cortical cells underlying the epidermis were also larger in mur3, which have radial diameters of about 65 μm and periclinal diameters of 59 μm. The comparable cortical cells of wild type and mur2 were more similar to the inner cells and varied slightly from 43 to 50 μm in both radial and periclinal directions. In mur3, the cortical cells underlying the epidermis are smaller than those of the inner cells underlying them. Whereas the number of inner cells is an invariant eight in wild type and mutants, the underlying cells of mur3 increase one or two cells compared with wild type and mur2, from about 14 to 16, and this factor resulted in slightly lower diameters compared with the inner cells (Table II). Epidermal cells are much smaller than cortical cells. Wild-type and mur2 epidermal cells are nearly isodiametric, with radial diameters of about 22 μm, and 23 to 24 μm in the periclinal direction. By comparison, the epidermal cells of mur3 are larger than either wild type or mur2, particularly in the periclinal direction, where the average diameters are 32 μm (Table II).
Figure 1.
SEM images of elongating Arabidopsis etiolated wild-type, mur2, and mur3 hypocotyls. A, Low magnification SEM image of 3.5-d-old wild-type hypocotyl. Elongation begins just below the hypocotyls hook. B through D, Wild-type, mur2, and mur3 hypocotyl “hook” regions, where cell elongation begins. Bars = 50 μm. E through G, The SEM images of the basal cells of 4.5- to 5.2-d-old hypocotyls demonstrate the pronounced swelling of the mur3 epidermal cells after growth compared with that of mur2 and wild-type cells. The diameters of bases of the hypocotyls are wild type (297 ± 5 μm), mur2 (274 ± 15 μm), and mur3 (395 ± 5 μm; minimum sample size = 20). Bars = 100 μm.
Table II.
Diameters of the epidermal cells, the cortical cells underlying the epidermal layer, and the inner cortical cells of wild-type, mur2, and mur3 etiolated hypocotyls determined at the culmination of growth
Diameters were determined in both radial and periclinal directions from automated analysis of digital images of basal cross sections. Values are the mean ± sd of at least four hypocotyls each.
| Line
|
Epidermal
|
Underlying Cortical
|
Inner Cortical
|
|||
|---|---|---|---|---|---|---|
| Radial | Periclinal | Radial | Periclinal | Radial | Periclinal | |
| μm | ||||||
| Wild type | 22.4 ± 1.1 | 23.0 ± 0.3 | 49.9 ± 3.7 | 48.1 ± 3.9 | 47.6 ± 2.4 | 44.9 ± 1.8 |
| mur2 | 21.6 ± 1.5 | 23.5 ± 2.9 | 43.9 ± 2.0 | 43.1 ± 2.6 | 40.6 ± 7.0 | 41.6 ± 3.5 |
| mur3 | 26.1 ± 3.1 | 32.3 ± 0.3 | 65.4 ± 4.4 | 59.3 ± 2.1 | 70.8 ± 4.8 | 64.6 ± 3.5 |
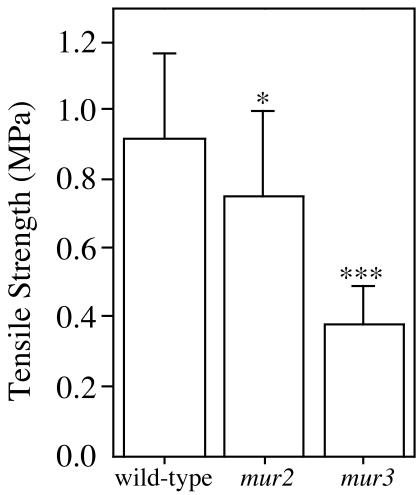
During maximal rates of growth, the tensile strength of the mur3 hypocotyls is less than one-half that of wild type, whereas the strength of mur2 hypocotyls is only slightly lowered (Fig. 2). The XyGs from wild-type, mur2, and mur3 etiolated hypocotyls all exhibit much reduced galactosylation of the middle Xyl of leaf XyGs (Table I). More than 80% of mur3 XyG is composed of XXXG units. The swollen nature of the basal cells and loss of tensile strength in mur3 hypocotyls correlate with the presence of a poorly galactosylated XyG (Table I).
Figure 2.
Tensile strengths of the basal 3 mm of 4-d-old wild-type, mur2, and mur3 hypocotyls. Etiolated hypocotyls were grown and tested submerged with a TA-XT2i texture analyzer (Stable Microsystems, Godalming Surrey, UK), with a load cell sensitive to 1 mn (Ryden et al., 2003). Tensile strength values and error bars are the means ± sd of 20 samples. Errors in strength were proportional to absolute strength, so the statistical comparisons used the loge transformation, comparing the strength of each mutant with that of the wild type (*, P < 0.05; ***, P < 0.001).
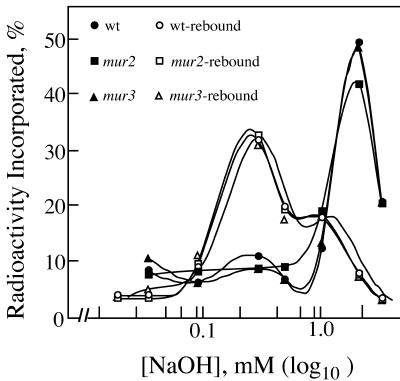
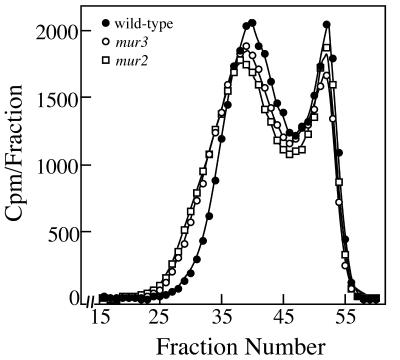
The mur2 and mur3 hypocotyls contain more cellulose per unit length than those of wild type (about 54.2 ± 6.1 and 54.9 ± 7.3 ng mm-1 in mur2 and mur3, respectively, compared with 41.2 ± 3.6 ng mm-1 in wild type) and proportionally higher amounts of XyG and pectins. Thus, the swollen phenotype and loss of tensile strength cannot be attributed to lower wall mass or changes in composition of other matrix polysaccharides besides XyG. The mur3 hypocotyl XyGs are assembled upon cellulose and exhibit the same tenacity of binding as the galactosylated XyGs of wild-type and mur2 hypocotyls (Fig. 3). Whereas 4 m NaOH is required to extract hypocotyl XyGs from walls of wild-type and mur mutants, these extracted XyGs rebind equally well to purified crystalline cellulose but require only about 0.6 m NaOH to dislodge them (Fig. 3). In contrast to the predictions of Levy et al. (1997), the tenacity of binding in vivo and in vitro show that the absence of the Gal residues has no influence on the physical interaction of XyG and cellulose during wall assembly in the living hypocotyl. The greater tenacity of binding in the nascent wall implicates an assembly mechanism that is lost in vitro, but this mechanism does not rely on Gal residues. On the basis of gel permeation chromatography, the molecular size distribution of the XyGs from mur2 and mur3 hypocotyls is shifted to slightly larger masses than those of wild type, indicating no apparent loss of the ability for the extracellular assembly of long XyGs from the secreted precursor polymers (Fig. 4). On the contrary, wild-type XyGs are generally shorter than either mur2 or mur3 XyGs. A shift to shorter XyGs accompanies auxin-induced cell elongation of excised pea (Pisum sativum) epicotyls (Talbott and Ray, 1992), and an anti-sensed member of the XyG endotransgluosylase/hydrolase (XTH) gene family blocks the growth-dependent decrease in the molecular size distribution of tobacco (Nicotiana tabacum) XyGs (Herbers et al., 2001). Together, these data indicate that these precisely altered XyG structures have little impact on their extended polymerization in muro or on their assembly around cellulose into a functional wall. What appears to be affected specifically is the remodeling of the wall structure to maintain tensile strength. The discrepancy of molecular sizes between wild-type versus mur2 and mur3 XyGs prompted us to examine the XET activity against these altered polymers.
Figure 3.
Tenacity of binding of wild-type, mur2, and mur3 XyGs to cellulose in vivo and in vitro. Seeds of each were germinated in [14C]d-Glc, and the cell walls of the hypocotyls were purified and depectinated. Values are the proportions of labeled XyGs extracted from the walls at each step of increasing alkali concentration.
Figure 4.
Molecular mass distributions of wild-type, mur2, and mur3 XyGs. Radiolabeled hypocotyls XyGs were purified from the 4 m NaOH extract of purified depectinated walls pre-extracted with 1 m NaOH. The XyGs in 1 m NaOH were loaded onto a 60- × 2.5-cm column of Sepharose 4B-CL (Pharmacia) equilibrated in 1 m NaOH. Fractions (3.5 mL) were collected into 2 mL of 2 m acetic acid; 2 mL was assayed for radioactivity by liquid scintillation spectroscopy.
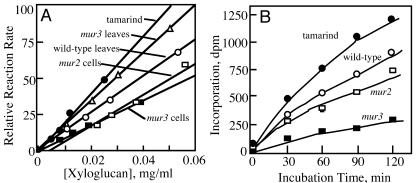
The activities of Arabidopsis hypocotyl XETs in vitro are markedly enhanced by the side-group extensions of XyGs. When the concentration-dependent activities of XETs are tested on purified, buffer-soluble XyGs, highly galactosylated tamarind seed storage XyGs are the best substrate (Fig. 5A). XyGs from mur3 and wild-type leaves, which contain predominately XLXG and XXFG oligomers, respectively (Table I), are also good substrates for the enzyme. The mur2 XyG extracted from late-stationary phase cells in liquid culture, which contain predominately XXXG and XXLG, are poorer substrates, but XyGs from mur3 late-stationary-cultured cells, which contain only XXXG, are the poorest substrates (Fig. 5A). In addition to the galactosyl residues, XET activity is also somewhat dependent on molecular size of the XyGs (Nishitani and Tominaga, 1992). Total XyGs extracted from plant tissues provide a rich array of sizes (Fig. 4). Although slight differences in the size distribution were observed between wild-type and mur2 and mur3 XyGs, given the size-dependent differences in activity predicted by the data of Nishitani and Tominaga (1992), it is unlikely that these small differences can account for the gross differences in XET activity (Fig. 5A). The XyGs extracted from late-stationary culture cells and tamarind XyGs behave chromatographically like those of the hypocotyls XyGs shown in Figure 4 (data not shown).
Figure 5.
Activity of XET with altered XyGs as substrates. Total XTHs were purified from Arabidopsis hypocotyls and cells. The enzyme mixture was assayed by a paper-binding assay (Fry et al., 1992), measured as attachment of the various XyG substrates to a common [3H]XLLGol acceptor or mixed-oligomer preparation of mostly XLLGol, which was synthesized by chemical reduction of high-performance anion exchange chromatography-purified XLLG by NaB3H4 (Steele and Fry, 1999). A, Concentration-dependent hypocotyl XET activity with soluble XyGs. Sources of the XyG enriched in specific oligomers are: mur3 cells in liquid culture (only XXXG), mur2 cells in liquid culture (predominately XXXG and XXLG), mur3 leaves (predominately XXXG and XLXG), wild-type leaves (predominately XXXG, XXFG, and XLFG), and tamarind seed flour XyG (predominately XXXG, XXLG, and XLLG). The XyG oligomeric distributions for each substrate are shown in Table I. B, Activity of Arabidopsis cultured cell XET with purified XyGs from tamarind seed flours or from hypocotyls of wild type, mur2, and mur3. The XyGs were suspended in boiling water at 3 mg mL-1 and cooled, and insoluble material was removed from the supernatant before assay of the substrate remaining soluble.
When boiled XyG-enriched preparations from hypocotyls were tested at relatively high concentrations of substrate, enhanced XET activity against Arabidopsis XyGs is observed when they are enriched in galactosylated oligomers, but mur3 hypocotyl XyGs are exceptionally poor substrates (Fig. 5B). Upon centrifugation of the reaction mixture, the mur3 preparation is significantly depleted of XyG in the supernatant, suggesting that the relatively ungalactosylated XyGs were inaccessible to enzyme action. Thus, the poor activity against XyGs with little or no side-group substitution is a consequence of two factors. First, XET activities with water-soluble XyGs enriched in XLXG or XXFG oligomers directly enhance enzyme recognition of the substrate (Fig. 5A). Second, loss of galactosyl residues from XyG changes the physical state and lowers solubility (Shirakawa et al., 1998; Yamanaka et al., 1999), and this feature renders the substrate inaccessible to XET action. XyGs exist in at least two domains in the wall, one that is accessible to enzymes that modify its structure and one that is not (Pauly et al., 1999), and there is a shift of polymers from the former to the latter during cell growth (Pauly et al., 2001). The mur3 hypocotyl XyGs may more rapidly enter the enzyme-inaccessible domain. As Levy et al. (1997) described, the backbone can exist in twisted and straightened conformations, and XLXG and XXFG are particularly effective in maintenance of the backbone in the straightened conformation. Thus, if the computer simulations of Levy et al. (1997) are correct, then the chain straightening function may enhance the binding of any member of the XTH family to XyG (Fig. 5A). However, the behavior of polymeric substrates is to a certain extent consistent with the activity of oligomeric substrates, where XLLG is significantly better acceptor than either XXXG or XXFG (Purugganan et al., 1997), indicating a strong preference toward substrate and acceptor with Gal residues on the middle Xyl.
A role of XyGs in tensile strength runs contrary to biophysical data suggesting that the addition of unfucosylated XyG to cellulose composites decreases strength (Chanliaud et al., 2002). However, these biophysical parameters are valid for unilamellate artificial structures devoid of enzymatic activities that may drastically alter the biochemical interactions among its molecular components. The formation of the hook and initiation of cell elongation proceed indistinguishably between wild type and mutant (Fig. 1, B-D), and elongation rates of the mur3 hypocotyl are only lowered marginally, demonstrating that the predicted lowering of XET activity does not impair cell growth. This is a surprising finding given evidence implicating XET activity directly in growth (Takeda et al., 2002). However, the abnormal swelling and bulging of the cortical and epidermal cells of mur3 hypocotyls occur well after growth (Fig. 1, E-G; Table II). This phenomenon may result from failure to religate XyGs after wall expansion and, thus, be symptomatic of the loss of tensile strength (Fig. 2).
Although the galactosylation of XyGs has a marked effect only on tensile strength and activity of XET, the roles of these enzymes in other events of wall dynamics remain to be confirmed by direct experiments. In Arabidopsis, these enzymes are encoded by a XTH gene family (Rose et al., 2002) comprising nearly three dozen members in at least three subclasses (Campbell and Braam, 1999; Yokoyama and Nishitani, 2001; Rose et al., 2002). The numerous XTH isoforms suggest a multiplicity of roles in cell development (Yokoyama and Nishitani, 2001). Thus, if members of some classes of XTH function in polymer elongation, as has been suggested (Thompson and Fry, 2001), then Gal residues are not essential for these particular reactions. Likewise, mur3 and wild-type hypocotyl XyGs bind equally well to cellulose, and the enhanced tenacity of native XyG binding compared with rebinding in vitro suggest that proteins other than XTHs facilitate assembly in vivo.
Mutants and alteration of expression of XTHs have given mixed results in demonstration of an association between XET or XEH activities and growth and wall remodeling (Campbell and Braam, 1999). No less than 10 XTHs are highly expressed in Arabidopsis roots (Yokoyama and Nishitani, 2001), although the number expressed specifically in hypocotyls is unknown. Redundancy of expression of several XTHs with similar function in any one cell may explain the difficulty using this approach to characterize gene function (Campbell and Braam, 1999; Yokoyama and Nishitani, 2001). In the experiments described here, the alteration of a common substrate has been more informative than underexpression of a single XTH gene. The total complement of XTHs from cell and hypocotyls were extracted to determine all nascent activities to be expected. All of the members of the XTH family, regardless of binding to XyG during affinity purification or not, exhibit poor activity toward ungalactosylated XyGs. For the first time, a role for XET activity in remodeling the wall through religation of XyGs to tighten the wall after wall extension finally has empirical data to support the concept.
Although the collective data in this study implicate XET activity in wall restructuring to maintain tensile strength, the data are still indirect, and the function of XTHs in this role could be bolstered by measurements of activity in muro. XyGs are integral to growth because of their dynamic interaction with cellulose microfibrils, and alterations of structure could impact other enzymes and proteins for which XyG is a common substrate. To our knowledge, the activity of expansins in in vitro extension assays with mur2 and mur3 hypocotyls has not been examined. Also, hydrolysis of XyG by α-xylosidases and β-glucosidases is modulated by Gal residues at the middle Xyl (Edwards et al., 1988; de Alcântara et al., 1999), and this feature may impact the failure of mur2 and mur3 hypocotyl XyGs to undergo shortening during growth (Fig. 4). A β-galactosidase is active only against XyG oligomers (or the non-reducing terminal oligomer of XyG polymers) and removes solely the middle galactosyl residue. This β-galactosidase activity generates an oligomer that becomes accessible to α-xylosidases (Fanutti et al., 1991) and β-glucosidases (Crombie et al., 1998) that act cooperatively to digest the xylosyl and backbone glucosyl units. However, these activities cannot occur in the absence of XET or XEH activity, and in contrast to the observed results (Fig. 4), the mur2 and mur3 should be better substrates for degradation if XET or XEH activities were optimal.
MATERIALS AND METHODS
Growth of Plants
Seeds of Arabidopsis (ecotype Columbia) were from wild-type or mur2-1 and mur3-1 lines that had been back-crossed at least four times (Reiter et al., 1997). Surface-sterilized seeds were chilled in water for 3 d to synchronize the germination response and then plated on one-half-strength Murashige and Skoog salts (Sigma-Aldrich, St. Louis), pH 5.7, in 0.8% (w/v) plant agar (Sigma-Aldrich). Etiolated hypocotyls were harvested after germination and grown for up to 5 d in darkness, whereas plantlets were obtained after 14 d of growth on agar at 23°C under continuous light of approximately 60 μmol m-2 s-1 from high-output fluorescent lamps. Leaves of plants, grown as described (Vanzin et al., 2002), were harvested at 32 d. Callus cultures were initiated from wild-type seedlings grown on an agar medium of Gamborg salts (Sigma-Aldrich), pH 5.7, supplemented with 5 μm 2,4-dichlorophenoxyacetic acid and 2% (w/v) Suc, and the callus was used to initiate liquid cell suspension cultures, with sub-culture every 2 to 3 weeks.
Purification of XyGs
Plant materials were frozen in liquid nitrogen and homogenized in a glass-glass grinder (Duall, Kontes Glass, Vineland, NJ) in 50 mm Tris[HCl], pH 7.2, supplemented with 1% (w/v) SDS. The homogenate was heated to 65°C for 15 min, and the walls were collected on a nylon mesh (47-μm square pores; Nitex, Briarcliff, NJ). The walls were washed extensively with water, ethanol, acetone, and finally suspended in water. Pectins were removed from the walls by extraction with excess 2 m imidazole[HCl], pH 7, at ambient temperature, followed by extraction with up to 0.045 m NaOH (supplemented with 3 mg mL-1 sodium borohydride to prevent end elimination). De-pectinated walls were then subjected to 1 m NaOH (with borohydride) to extract other material and additional pectin, and XyGs were extracted with excess 4 m NaOH (with borohydride) overnight with constant stirring under an argon atmosphere. The 4 m extract was passed through a glass-fiber filter mat to remove unsedimented wall remnants, and the eluant was chilled to ice temperature and acidified to pH 5 with glacial acetic acid. The XyGs were dialyzed extensively against running deionized water, and either used directly or freeze-dried. In some experiments, freeze-dried XyGs were suspended in water and boiled for 10 min to dissolve the polymers, and then Sephadex A-25 anion exchange resin was added to remove a small amount of contaminating uronic-acid rich polymers.
Determination of the Distribution of the Oligomers
The percentage of each oligomeric unit is based on separation of Trichoderma endo-β-glucanase (Megazyme, Bray, County Wicklow, Ireland) digests of XyG oligomers from leaves, hypocotyls, and cultured cells that were separated by high-performance anion exchange chromatography on a CarboPac PA-1 (Dionex, Sunnyvale, CA) anion exchange column and detected by pulsed amperometric detection (Vanzin et al., 2002). Electrospray mass spectrometry (MS) of the total digest and MS/MS of m/z 1247 were used to determine the proportions of XXLG and XLXG.
Microscopy
Hypocotyls harvested at several times during rapid elongation phase (3.5- to 5.5-d-old) were rapidly frozen by plunging them into nitrogen slush and then sputter-coating them with gold for 4 s at -165°C. Samples were then imaged at -140°C in a scanning electron microscope (JSM-840, JEOL, Tokyo) using 5 kV of accelerating voltage. For determinations of cell diameters, etiolated hypocotyls were fixed in a mixture of 2.5% (w/v) glutaraldehyde and 2% (w/v) paraformaldehyde in 0.05 m sodium phosphate, pH 6.8, washed with the same buffer, and post-fixed in 1% (w/v) osmium tetroxide in the same buffer. After dehydration in ethanol, the hypocotyls were embedded in Spurr's resin. Semithin sections were cut from the base of a minimum of four hypocotyls and observed by bright-field microscopy in a microscope (AH-2, Olympus, Tokyo), and the images were recorded digitally by a SPOT RT camera accessory (Diagnostic Instruments, Sterling Heights, MI).
Determination of Tensile Strength
Etiolated 4-d-old hypocotyls were grown and tested submerged as described (Ryden et al., 2003) using a TA-XT2i texture analyzer (Stable Microsystems, Godalming Surrey, UK) with a load cell sensitive to 1 mn. In brief, tensile tests were made on about 4-mm lengths of hypocotyls starting from just above the root junction. The hypocotyls were fixed with cyanoacrylate (Resist H20, Holdtite, Gateshead, UK) across two aluminum tabs about 3 mm apart, and the adhesive was cured rapidly with an activator (Cyanolit Plus, Eurobond, Sittingbourne UK). Taking specimens to be cylindrical, the tensile strength was derived (Cleland, 1967). The average hypocotyl diameters were: wild type, 260 ± 16 μm; mur2, 248 ± 23 μm; and mur3, 287 ± 11 μm. Tensile strength values and error bars are the means ± sd of 20 samples, and Dunnett's test was performed as described in the Minitab Release 10 Reference Manual (1994).
Determination of Tenacity of XyG Binding to Cellulose in Vivo and in Vitro
Arabidopsis wild-type, mur2, and mur3 hypocotyls were labeled by addition of [U-14C]d-Glc to the agar growth medium. Walls were purified and depectinated as described previously. The cell walls were then extracted sequentially with increasing concentrations of NaOH (each with 3 mg mL-1 sodium borohydride), and after neutralization, the amount of radioactivity was determined by liquid scintillation counting of a small sample of each supernatant. The 4 m NaOH extracted the bulk of the wall material, which was about 80 mole % XyG based on monosaccharide analysis. In a separate experiment, XyGs from cell walls, depectinated as above and pre-extracted with up to 1 m NaOH, were exhaustively extracted with 4 m NaOH, and the supernatant was filtered over glass-fiber mats, mixed with a 10-fold excess of pre-alkali extracted microcrystalline cellulose (Cellex N-1, Bio-Rad Laboratories, Hercules, CA), and gradually neutralized with acetic acid and vigorous stirring over a 2-h period at ambient temperature. The cellulose with bound labeled XyGs were then pelleted by centrifugation, washed several times with water and 0.02 m NaOH, and subjected to a gradient extraction with NaOH as before.
Activities of Arabidopsis Hypocotyls XET with Precisely Altered XyGs
XTHs were isolated from the medium, and 0.2 m CaCl2 extracts of cell walls were purified from Arabidopsis cells and hypocotyls and precipitated by increasing saturation with ammonium sulfate as described (Steele and Fry, 1999). A preparation containing most of the XET activity precipitated between 20% and 70% saturation. The XTHs were also purified by affinity binding to XyG essentially as described (Steele and Fry, 1999). In brief, XTHs dissolved in 100 mm succinate[NaOH], pH 5.5, and 10 mm CaCl2, and 0.2% (w/v) tamarind XyG was added. In the absence of oligomers, the bound XTHs void a column of Bio-Gel A-0.5m (Bio-Rad Laboratories) equilibrated in the suspension buffer, and upon addition of excess tamarind oligomers, generated by Trichoderma endoglucanase digestion, XTHs elute near the included volume. After concentration, the enzymes were separated osmotically from the oligomers by passing them over a Sephadex G-25 de-salting column (Pharmacia, Uppsala).
The various XyGs were mixed at up to 3 mg mL-1 in 100 mm sodium succinate, pH 5.5, and boiled to maximize solubility, and after cooling to ambient temperature, the insoluble residues were pelleted by centrifugation at 14,000 rpm in a microfuge. The XyG concentration remaining in the supernatant was estimated by phenol-sulfuric sugar assay (DuBois et al., 1956). In experiments designed to determine XyG concentration-dependent activity, affinity-purified Arabidopsis cell XTHs were used, and XET activities were tested against the supernatant liquids and were determined by the attachment of the XyG fragment to the non-reducing end of [3H]XyG oligomer acceptor in a filter paper-binding assay (Fry et al., 1992). As stated in the figure legend, either purified [3H]XLLGol or [3H]tamarind XyG mixed oligomers were used as acceptors. Aliquots of the reaction were stopped by addition of 50% (v/v) formic acid at various times and air-dried on strips of filter paper (No. 1, Whatman, Clifton, NJ). The strips were then washed extensively with water to remove unreacted [3H]oligomers, and the radioactivity bound to the filter paper was assayed by liquid scintillation spectroscopy. In experiments involving depleted XyG preparations, hypocotyl XTHs between 20% and 70% (w/w) ammonium sulfate saturation were used without affinity purification.
Cellulose Binding Assays
Shoot XyGs were radiolabeled by incubation of 30-d-old wild-type, mur2, and mur3 plants with 14CO2 in a sealed fumigation chamber at 25°C under 60 μmol m-2 s-1 light from fluorescent lamps for 2 d. Leaves were harvested into liquid nitrogen, and cell walls were prepared as described earlier. XyGs, solubilized by 4 m NaOH from 1 m NaOH-pre-extracted walls, were collected, neutralized, dialyzed against water, and freeze-dried. The dry XyGs were suspended in 5 mm succinate [NaOH], pH 5.8, boiled to maximize solubility, and cooled to ambient temperature. A small amount of insoluble material was removed by centrifugation at 14,000g in a microfuge, and equimolar amounts of soluble XyG (independent of radiolabel) were mixed with a 10-fold excess of microcrystalline cellulose (Cellex N-1, Bio-Rad Laboratories) that was pre-washed with 4 m NaOH (supplemented with 3 mg mL-1 of sodium borohydride) and washed extensively with water and finally suspended in 5 mm succinate [NaOH], pH 5.8, to start the reactions. Binding at 25°C was determined by centrifugation of a portion of the reaction mixture, and assay of the depletion of the labeled XyG from soluble fraction by liquid scintillation spectroscopy. Residual label incapable of binding to cellulose was subtracted from the total when binding rates were calculated.
Molecular Mass Distribution of XyGs
The molecular mass distributions of radiolabeled hypocotyls XyGs used in the cellulose binding assays were purified from the 4 m NaOH-extract of purified depectinated walls pre-extracted with 1 m NaOH. The 4 m NaOH extract was filtered of small remnants of unsedimented cell walls, chilled and neutralized with glacial acetic acid, dialyzed against running deionized water, and freeze-dried. The materials were dissolved 1 m NaOH, and a small amount of insoluble material was removed by centrifugation. An equivalent amount of radioactivity in the 1 m NaOH solution was applied to a 60- × 2.5-cm column of Sepharose 4B-CL (Pharmacia) equilibrated in 1 m NaOH. Fractions (3.5 mL) were collected into 2 mL of 2 m acetic acid; 2 mL was assayed for radioactivity by liquid scintillation spectroscopy, and the remainders were pooled into high- and low-mass fractions, dialyzed against deionized water, and lyophilized for monosaccharide analysis. The XyGs were judged to comprise the vast majority of each fraction based on the amounts and ratios of Xyl and Glc.
Acknowledgments
We thank Debbie Sherman and Chia-Ping Huang in the Purdue University Microscopy Center for their help with the SEM and fixation of materials for bright-field microscopy. We are also grateful to Drs. Wolf-Dieter Reiter and Maureen McCann for helpful discussions and critical reviews of the manuscript.
This work was supported by the U.S. Department of Agriculture-National Research Initiative Competitive Grants Program, Plant Growth and Development (grant to N.C.C.), and by the Biotechnology and Biological Science Research Council (competitive strategic grant to P.R. and A.C.S.). This is journal paper no. 17,217 of the Purdue University Agricultural Experiment Station.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.027508.
References
- Campbell P, Braam J (1999) Xyloglucan endotransglycosylases: diversity of genes, enzymes and potential wall-modifying functions. Trends Plant Sci 4: 361-366 [DOI] [PubMed] [Google Scholar]
- Carpita NC, Gibeaut DM (1993) Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J 3: 1-30 [DOI] [PubMed] [Google Scholar]
- Chanliaud E, Burrows KM, Jeronimidis G, Gidley MJ (2002) Mechanical properties of primary plant cell wall analogues. Planta 215: 989-996 [DOI] [PubMed] [Google Scholar]
- Cleland RE (1967) Extensibility of isolated cell walls: measurement and changes during cell elongation. Planta 74: 197-209 [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ (2000) Loosening of plant cell walls by expansins. Nature 407: 321-326 [DOI] [PubMed] [Google Scholar]
- Crombie HJ, Chengappa S, Hellyer A, Reid JSG (1998) A xyloglucan oligosaccharide-active, transglycosylating β-d-glucosidase from the cotyledons of nasturtium (Tropaeolum majus L) seedlings: purification, properties and characterization of a cDNA clone. Plant J 15: 27-38 [DOI] [PubMed] [Google Scholar]
- de Alcântara PHN, Dietrich SMC, Buckeridge MS (1999) Xyloglucan mobilization and purification of a (XLLG/XLXG) specific β-galactosidase from cotyledons of Copaifera langsdorfii. Plant Physiol Biochem 37: 653-663 [Google Scholar]
- DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28: 350-356 [Google Scholar]
- Edwards M, Bowman YJL, Dea ICM, Reid JSG (1988) A β-d-galactosidase from nasturtium (Tropaeolum majus L.) cotyledons: purification, properties, and demonstration that xyloglucan is the natural substrate. J Biol Chem 263: 4333-4337 [PubMed] [Google Scholar]
- Fanutti C, Gidley MJ, Reid JSG (1991) A xyloglucan oligosaccharide-specific α-d-xylosidase or exo-oligoxyloglucan-α-xylohydrolase from germinated nasturtium (Tropaeolum majus L.) seeds. Planta 184: 137-147 [DOI] [PubMed] [Google Scholar]
- Fry SC, Smith RC, Renwick KF, Martin DJ, Hodge SK, Matthews KJ (1992) Xyloglucan endotransglycosylase, a new wall-loosening enzyme activity from plants. Biochem J 282: 821-828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry SC, York WS, Albersheim P, Darvill A, Hayashi T, Joseleau J-P, Kato Y, Lorences EP, MacLachlan GA, McNeil M et al. (1993) An unambiguous nomenclature for xyloglucan-derived oligosaccharides. Physiol Plant 89: 1-3 [Google Scholar]
- Herbers K, Lorences EP, Barrachina C, Sonnewald U (2001) Functional characterisation of Nicotiana tabacum xyloglucan endotransglycosylase (NtXET-1): generation of transgenic tobacco plants and changes in cell wall xyloglucan. Planta 212: 279-287 [DOI] [PubMed] [Google Scholar]
- Levy S, Maclachlan G, Staehelin LA (1997) Xyloglucan sidechains modulate binding to cellulose during in vitro binding assays as predicted by conformational dynamics simulations. Plant J 11: 373-386 [DOI] [PubMed] [Google Scholar]
- Levy S, York WS, Stuikeprill R, Meyer B, Staehelin LA (1991) Simulations of the static and dynamic molecular conformations of xyloglucan: the role of the fucosylated side-chain in surface-specific side-chain folding. Plant J 1: 195-215 [PubMed] [Google Scholar]
- Madson M, Dunand C, Li X, Verma R, Vanzin GF, Caplan J, Shoue DA, Carpita NC, Reiter W-D (2003) The MUR3 gene of Arabidopsis thaliana encodes a xyloglucan galactosyltransferase that is evolutionarily related to animal exostosins. Plant Cell 15: 1662-1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen-Mason S, Durachko DM, Cosgrove DJ (1992) Two endogenous proteins that induce cell wall extension in plants. Plant Cell 4: 1425-1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen-Mason SJ, Fry SC, Durachko DM, Cosgrove DJ (1993) The relationship between xyloglucan endotransglycosylase and in vitro cell wall extension in cucumber hypocotyls. Planta 190: 327-331 [DOI] [PubMed] [Google Scholar]
- Minitab Release 10 Reference Manual (1994) Minitab Release 10 Reference Manual. Minitab, State College, PA
- Nishitani K (1998) Construction and restructuring of the cellulose-xyloglucan framework in the apoplast as mediated by the xyloglucan related protein family: a hypothetical scheme. J Plant Res 111: 159-166 [Google Scholar]
- Nishitani K, Tominaga R (1992) Endoxyloglucan transferase, a novel class of glycosyltransferase that catalyzes transfer of a segment of xyloglucan molecule to another xyloglucan molecule. J Biol Chem 267: 21058-21064 [PubMed] [Google Scholar]
- Okamoto-Nakazato A, Nakamura T, Okamoto H (2000) The isolation of wall-bound proteins regulating yield threshold tension in glycerinated hollow cylinders of cowpea hypocotyls. Plant Cell Environ 23: 145-154 [Google Scholar]
- Pauly M, Albersheim P, Darvill A, York W (1999) Molecular domains of the cellulose/xyloglucan network in the cell walls of higher plants. Plant J 20: 629-639 [DOI] [PubMed] [Google Scholar]
- Pauly M, Qin Q, Greene H, Albersheim P, Darvill A, York WS (2001) Changes in the structure of xyloglucan during cell elongation. Planta 212: 842-850 [DOI] [PubMed] [Google Scholar]
- Purugganan MM, Braam J, Fry SC (1997) The Arabidopsis TCH4 xyloglucan endotransglycosylase: substrate specificity, pH optimum, and cold tolerance. Plant Physiol 115: 181-190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter W-D, Chapple C, Somerville CR (1997) Mutants of Arabidopsis thaliana with altered cell wall polysaccharide composition. Plant J 12: 335-345 [DOI] [PubMed] [Google Scholar]
- Rose JKC, Braam J, Fry SC, Nishitani K (2002) The XTH family of enzymes involved in xyloglucan endotransglucosylation and endohydrolysis: current perspectives and a new unifying nomenclature. Plant Cell Physiol 43: 1421-1435 [DOI] [PubMed] [Google Scholar]
- Ryden P, Sugimoto-Shirasu K, Smith AC, Findlay K, Reiter W-D, McCann MC (2003) Tensile properties of Arabidopsis cell walls depend on both a xyloglucan cross-linked network and rhamnogalacturonan II-borate complexes. Plant Physiol 132: 1033-1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa M, Yamatoya K, Nishinari K (1998) Tailoring of xyloglucan properties using an enzyme. Food Hydrocoll 12: 25-28 [Google Scholar]
- Steele NM, Fry SC (1999) Purification of xyloglucan endotransglycosylases (XETs): a generally applicable and simple method based on reversible formation of an enzyme-substrate complex. Biochem J 340: 207-211 [PMC free article] [PubMed] [Google Scholar]
- Takeda T, Furuta Y, Awano T, Mizuno K, Mitsuishi Y, Hayashi T (2002) Suppression and acceleration of cell elongation by integration of xyloglucans in pea stem segments. Proc Natl Acad Sci USA 99: 9055-9060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott LD, Ray PM (1992) Changes in molecular size of previously deposited and newly synthesized pea cell wall matrix polysaccharides: effects of auxin and turgor. Plant Physiol 98: 369-379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JE, Fry SC (2001) Restructuring of wall-bound xyloglucan by transglycosylation in living plant cells. Plant J 26: 23-34 [DOI] [PubMed] [Google Scholar]
- Vanzin GF, Madson M, Carpita NC, Raikhel NV, Keegstra K, Reiter W-D (2002) The mur2 mutant of Arabidopsis thaliana lacks fucosylated xyloglucan because of a lesion in fucosyltransferase AtFUT1. Proc Natl Acad Sci USA 99: 3340-3345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka S, Yaguchi Y, Urakawa H, Kajiwara K, Shirakawa M, Yamatoya K (1999) Gelation of enzymatically degraded xyloglucan extracted from tamarind seed. Sen Gakk 55: 528-532 [Google Scholar]
- Yokoyama R, Nishitani K (2001) A comprehensive expression analysis of all members of a gene family encoding cell-wall enzymes allowed us to predict cis-regulatory regions involved in cell-wall construction in specific organs of Arabidopsis. Plant Cell Physiol 42: 1025-1033 [DOI] [PubMed] [Google Scholar]
- Zablackis E, York WS, Pauly M, Hantus S, Reiter W-D, Chapple CCS, Albersheim P, Darvill A (1996) Substitution of l-fucose by l-galactose in cell walls of Arabidopsis mur1. Science 272: 1808-1810 [DOI] [PubMed] [Google Scholar]