Abstract
The “damage accumulation” phenomenon has not been quantitatively demonstrated in clinical cement mantles surrounding femoral hip stems. We stained transverse sections of 11 postmortem retrieved femoral hip components fixed with cement using fluorescent dye-penetrant and quantified cement damage, voids, and cement-bone interface gaps in epifluorescence and white light micrographs. Crack density (Cr.Dn), crack length-density (Cr.Ln.Dn), porosity, and cement-bone interface gap fraction (c/b-gap%) were calculated, normalized by mantle area. Multiple regression tests showed that cement damage (Cr.Ln.Dn. & Cr.Dn.) was significantly positively correlated (r2=0.98, p<0.001) with “duration of use” and body mass index (“BMI”) but not cement mantle “porosity”. There were significant interactions: “duration of use”*”BMI” was strongly predictive (p<0.005) of Cr.Dn.; and “duration of use”*”porosity” was predictive (p=0.04) of Cr.Ln.Dn. Stem related cracks accounted for approximately one fifth of Cr.Dn and one third of Cr.Ln.Dn. The mean c/b-gap% was 13.8% but it did not correlate (r2=0.01, p=0.8) with duration of use. We concluded that duration-dependent fatigue damage accumulation occurred during in vivo use. BMI strongly influenced cement crack length and the rate of new crack formation over time. Voids did not increase the rate of crack initiation but appeared to have promoted crack growth over time. Although not progressive, substantial bone resorption at the cement-bone interface appeared to be common.
Keywords: Hip, Arthroplasty, Bone cement, damage accumulation
Introduction
Several series of in vitro and in silico studies of cemented femoral stem function in total hip arthroplasty (THA) (Cristofolini et al., 2003; McCormack and Prendergast, 1999; Race et al., 2003; Stolk et al., 2004) have been predicated upon the assumption that fatigue damage accumulation occurs in clinical cement mantles. This assumption seemed reasonable since previous workers had documented the existence of fatigue cracks in revision-retrieved cement (Culleton et al., 1993; Topoleski et al., 1990) and in postmortem retrieved stem/cement/bone constructs (Jasty et al., 1991). However, the commonly described “damage accumulation” phenomenon has yet to be quantitatively demonstrated in clinical cement mantles. On the contrary, Bishop et al (Bishop et al., 2009), who examined an impressively large number of cemented total hip arthroplasty (THA) postmortem retrievals, reported that there was no sign of damage accumulation related to in vivo duration of use.
Along with the assumption of damage accumulation, it has long been thought that cement voids cause stress concentrations and initiate cracks in clinical mantles, leading to fatigue failure. A further factor that naturally fits within the damage accumulation scenario is patient obesity. It would seem likely that patients with a higher body mass index (BMI) would generate higher stresses in their cement mantles leading to greater fatigue damage rates.
The unproven status of the damage accumulation scenario, combined with the suspected roles of cement porosity and patient obesity, led us to our primary research question: (1) Does cement mantle damage correlate with in vivo duration of use and is it influenced by cement mantle porosity or patient body-mass-index (BMI)?
The function of the cement mantle is to support the stem within the bone. Therefore, in considering damage accumulation within the cement mantle, it is appropriate to consider also the mechanical competence of the stem/cement and cement/bone interfaces. Previous workers have suggested that cement damage originates at the stem-cement interface (Jasty et al., 1991), which prompted our second research question: (2) Does cement damage preferentially develop at the stem-cement interface?
Several previous studies of retrieved cemented components have suggested that the cement bone interface remains “intact” (Jasty et al., 1991; Maloney et al., 2002), but in vivo measurements have shown periprosthetic bone loss (Burchard et al., 2007; Damborg et al., 2008; Venesmaa et al., 2003). This prompted our final research question: (3) Do gaps at the cement-bone interface correlate with in vivo duration of use?
Methods
Specimen acquisition and dating
Eleven postmortem retrieved cemented femora were obtained en bloc from SUNY Upstate Medical University’s Anatomical Gift Program and from the University of Alabama – Birmingham retrieval program. Donor age, sex, height, BMI, type of implant, and duration of use were collected (Table 1). One undated implant (donor k) was also included in our dataset; since that particular stem type (Muller curved) had not been implanted since the 1980’s, it was assigned 20 years duration of use. Mean donor age was 82.5 years (SD 7.8; range 67–93 years), mean age at operation was 74.7 years (SD 12.3; range 53 – 88.5 years), and mean implant duration of use was 7.9 years (SD 6.7; range 0.2 – 20+ years).
Table 1.
donor and implant data. Superscripts in the “Retrieval” column indicate a common donor
| Retrieval | Chart Symbol | Age (years) | Sex | BMI (Kg/m2) | Duration of use (years) | Cause of death | Implant | Implant type | Radiographic status |
|---|---|---|---|---|---|---|---|---|---|
| a | ■ | 88 | M | 20.7 | 0.2 | Cardiac arrest | Perfecta PDA calcar replacement (Wright Medical Technology) | Hemi | fixed |
| b |
|
87 | F | 27.1 | 0.9 | Cardiac arrest | Perfecta (Wright Medical Technology) | Bipolar | fixed |
| c1 |
|
76 | F | 26.6 | 2 | Breast cancer | Modular calcar replacement (Zimmer) | Bipolar | fixed |
| d | ▲ | 93 | F | 17.8 | 4.5 | Cardiac arrest | VerSys cemented (Zimmer) | Bipolar | fixed |
| e1 |
|
76 | F | 26.6 | 5 | Breast cancer | Precision long stem (Howmedica) | Bipolar | fixed |
| f |
|
92 | F | 23.8 | 6 | Renal insufficiency | Endurance (Depuy Orthopaedics) | Total | fixed |
| g2 | ● | 85 | F | 29.6 | 8 | Bacterial endocarditis | VerSys cemented (Zimmer) | Total | fixed |
| h2 |
|
85 | F | 29.6 | 8 | Bacterial endocarditis | VerSys cemented (Zimmer) | Total | loose |
| i |
|
67 | F | 20.8 | 14 | Alzheimer’s disease | Harris precoat (Zimmer) | Total | fixed |
| j | ◆ | 79 | F | 38.5 | 18 | Cardiac arrest | Exeter matte (Howmedica) | Total | fixed |
| k |
|
80 | F | 26 | 20 | Cardiac arrest | Müller curved (JRI) | Total | fixed |
The study sample was random in that it represented the continuous set of those implants that came into our possession for which we could obtain, or in one case deduce, the date of implantation. The sample was “selected” in that we waited until the accumulated set included several longer-term specimens in order to investigate our primary research question regarding duration of use.
Assessment of cement damage and mantle morphology
All constructs were radiographed and then assessed by an experienced total joint surgeon (THI) for radiographic signs of loosening (Table 1). Subsequently, constructs were transversely sectioned using a water irrigated silicone carbide abrasive blade (Isomet 2000, Buehler, Lake Bluff, IL, USA). Quantification of cement damage was based on the analysis of four transverse sections per donor bone, the locations of which were defined by the length of each stem: a proximal section was made at the level of the lesser trochanter; a distal section was made 10 mm proximal the tip of the implant; and two further sections were made evenly spaced between the proximal and distal ones. The resulting sections were stained using a fluorescent dye penetrant (Aqua Check WB200, Sherwin Inc., South Gate, CA, USA) in order to show cement microcracks with maximum contrast (Figure 1). The proximal transverse surfaces were soaked in the dye penetrant for 10 minutes and then rinsed with water for 1 minute to remove excess dye. Whole section bright-field and epifluorescence images were then generated at a resolution of 5.7microns/pixel for each section. These composite images were automatically captured and tiled using a digital camera (Spot RT Monochrome, Diagnostic Instruments Inc., SterlingHeights, MI) and epifluorescence microscope (Nikon SMZ800, Nikon Instruments Inc., Melville,NY) with ring illuminator, mounted to a computer-controlled milling machine (Benchman 2000, Light Machines, Manchester, NH).
Figure 1.
Epifluorescence images (with zoomed sections below) showing lesser trochanter level sections: (left) a short-term lower BMI donor (“b” in Table 1) showing minimal cracking; and (right) from a long-term, higher BMI donor (“j” in Table 1) showing extensive cracking. Both of these specimens were radiographically well fixed.
Cement mantle cracks were manually identified on the epifluorescence images and digitally traced for each section. Crack lengths were then determined using ImagePro (Media Cybernetics, Silver Spring, MD, USA).
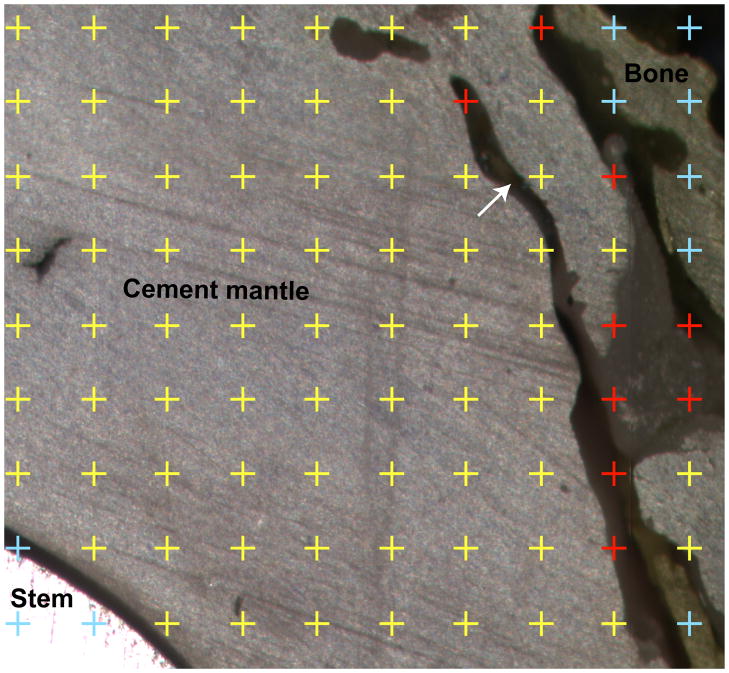
The areas of the cement mantle, mid-mantle porosity, and cement-bone interface gaps were quantified in each section using a stereological technique. A randomly positioned regular point grid was digitally superimposed upon the bright-field images using ImagePro (Media Cybernetics, Silver Spring, MD, USA). Areas were then calculated from point counts for cement, porosity, and cement-bone interface gap (Figure 2). Note that the cement-bone interface gap was that between the cement mantle and adjacent bone (trabecular or cortical) rather than that between the mantle and the cortex.
Figure 2.
White light image of a partial transverse section illustrating the stereological method (randomly placed regular point grid) used to estimate the areas of the cement mantle, cement bone gaps and porosity. Note the trabecular shaped defect in the cement mantle (arrow), which was strongly suggestive of bone resorption.
Data analysis
In order to answer our first research question, regarding damage accumulation, total cement damage for the four sections of each specimen was normalized by total cement mantle area, giving estimates for crack number density (Cr.Dn) and crack length density (Cr.Ln.Dn). Cr.Dn was defined as the number of measured cracks divided by the sum of the measured mantle areas. Cr.Ln.Dn was defined as the sum of measured crack lengths divided by the sum of the measured mantle areas. Similarly, “porosity” was defined as the sum of measured void areas divided by sum of the measured mantle areas. These data were then used to generate least-squares-fit multiple regression models with “duration of use”, “BMI”, “porosity”, and the mean-centered interaction terms “duration of use”*”BMI” and “duration of use”*”porosity” as a predictors. The simple predictors tested whether there was a proportional increase in damage associated with an increase in each predictor. The interaction terms tested for the possibility that the rate of proportional increase in damage for one interacting predictor was changed by the value of the other (for example, a significant positive interaction between porosity and duration of use would show that the rate of damage accumulation over time increased with increasing porosity).
Our second research question concerned the distribution of cement damage. We divided the cement cracks into two categories, those that involved the stem-cement interface and those that did not. Stem versus non-stem crack densities were compared using paired t-tests.
Our third research question, regarding the integrity of the cement-bone interface over time, was answered by testing the correlation between “cement-bone interface gap” (normalized by cement mantle area) and “duration of use”.
Results
Radiographically, ten of the eleven implants were judged to be well fixed and one was obviously loose (donor implant h, Table 1). Cement damage was observed in all sections, but varied considerably in degree; Figure 1 shows epifluorescence images of two proximal sections representing the extrema of cement damage. The smallest micro-cracks that could be reliably observed were 28 microns in length (5 pixels in the digital images). The largest cracks were over 1mm in length. The average of the per-donor-bone mean crack lengths was 0.163mm (0.028 SD, range 0.097–0.194). Again considering per-donor-bone means, cracks which involved the stem (average 0.302mm, 0.180 SD, range 0.109–0.731), were significantly (p=0.014) longer than those that did not (average 0.142mm, 0.021 SD, range 0.096–0.165). Stem related cracks that extended to the cement-bone interface (through-cracks) were found in all of the retrieved mantles bar one (implant h, Table 1), mostly in the proximal section. The average of the per-donor-bone mean through-crack lengths was 0.53mm (0.40 SD, range 0.076–1.2). Mean through-crack length was not always greater than the mean length of the remainder of the stem related cracks (7 of 10 cases) and there was no significant difference (p = 0.066).
Damage accumulation and the effect of BMI and cement porosity
The mean normalized cement damage measures were: Cr.Dn. 0.56/mm2 (0.34 SD, range 0.28 – 1.47); Cr.Ln.Dn. 0.093mm/mm2 (0.058SD, range 0.037 – 0.228). Mean porosity was 3.0% (3.0 SD, range 0.1 – 8.6).
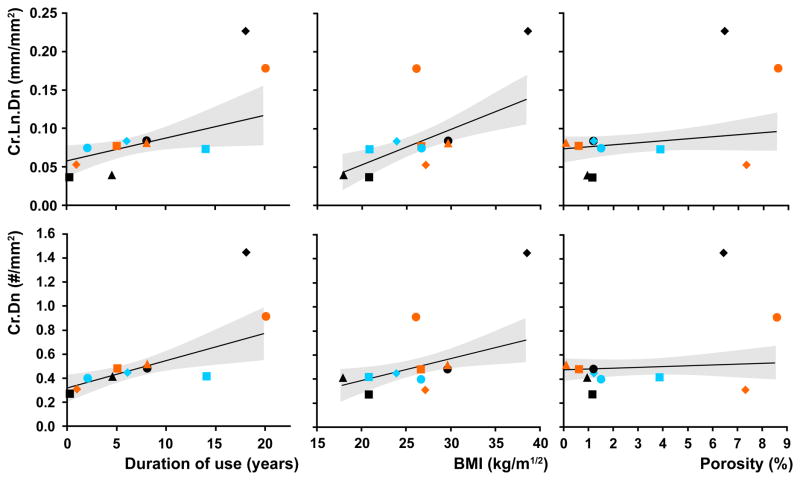
The multiple regression models were strongly predictive of cement damage (r2=0.98, p=0.0003 for both Cr.Dn. and Cr.Ln.Dn.). Cement damage was significantly positively correlated with “duration of use” and “BMI” but not “porosity” (Figure 3, Table 2). The regression models included significant interaction terms (Table 2): “duration of use”*”BMI” was strongly predictive of Cr.Dn.; and “duration of use”*”porosity” was predictive of Cr.Ln.Dn.
Figure 3.
Bivariate plots of cement damage (top Cr.Dn, bottom Cr.Ln.Dn) versus duration of use, BMI and porosity fraction. The plots are overlaid with the profiles of the regression models (solid lines) along with 95% confidence intervals (dashed lines). Results of the multiple regression analyses are shown in Table 2.
Table 2.
Statistical results of the multiple regression tests for crack density (Cr.Dn) and crack length density (Cr.Ln.Dn). Note that data for the interaction terms was mean-centered. P-values are for the leverage of each predictor, significant predictors are denoted by an asterisk.
| Predictor | Cr.Dn (R2 = 0.98, p = 0.0003)
|
Cr.Ln.Dn (R2 = 0.98, p = 0.0003)
|
||||
|---|---|---|---|---|---|---|
| Estimate | Std error | p-value | Estimate | Std error | p-value | |
| Duration-of-use | 0.023 | 0.006 | 0.013* | 0.0029 | 0.0010 | 0.038* |
| BMI | 0.018 | 0.005 | 0.020* | 0.0045 | 0.0010 | 0.0051* |
| Porosity | 0.68 | 0.87 | 0.47 | 0.26 | 0.15 | 0.15 |
| (Duration of use – 7.87)*(BMI - 26.1) | 0.0033 | 0.0007 | 0.0050* | 0.00019 | 0.00012 | 0.18 |
| (Duration of use – 7.87)*(Porosity – 2.97) | 0.17 | 0.15 | 0.30 | 0.072 | 0.0003 | 0.041* |
| Intercept | −0.18 | 0.12 | 0.22 | −0.066 | 0.022 | 0.03* |
Cement cracks at the stem-cement interface
The majority of cement damage did not involve the stem (Table 3). Stem related cracks accounted for approximately one fifth of Cr.Dn and one third of Cr.Ln.Dn.
Table 3.
Crack density (Cr.Dn) and crack length density (Cr.Ln.Dn) with paired comparisons between cracks that involved the stem and those that did not.
| All cracks | Stem | Not Stem | p (stem=not stem) | |
|---|---|---|---|---|
| Cr.Dn (#/mm2) | 0.56 ± 0.34 | 0.11 ± 0.14 | 0.45 ± 0.21 | < 0.0001 |
| Cr.Ln.Dn (mm/mm2) | 0.093 ± 0.058 | 0.030 ± 0.034 | 0.063 ± 0.029 | 0.0005 |
Gap fraction at the cement-bone interface with duration of use
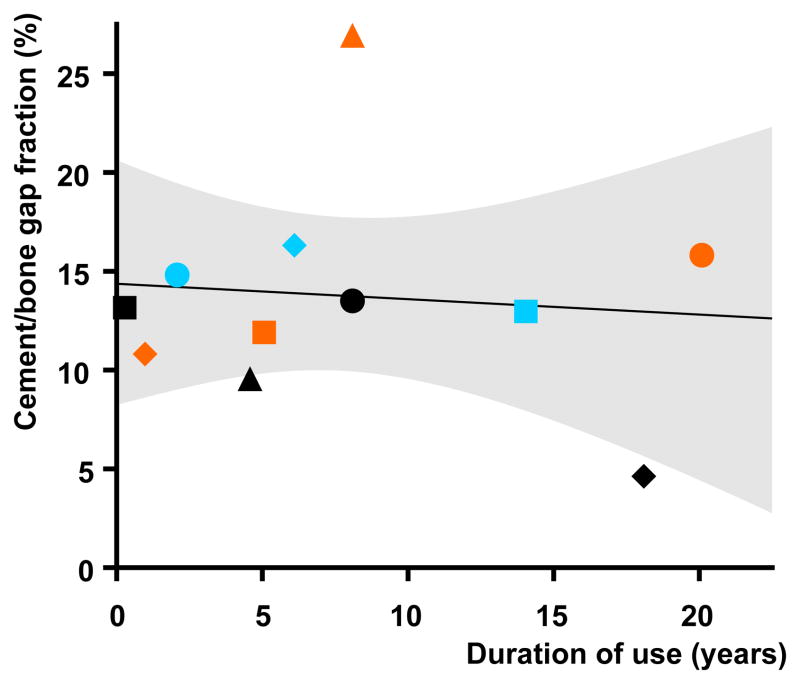
Cement-bone interface gap area did not correlate (r2=0.01, p=0.77) with duration of use (Figure 4). The mean gap area, normalized by cement mantle area, was 13.8% (5.5 SD, range 4.8 – 27.1). This was significantly (p = 0.0010) greater than the 4.4% maximum areal gap that could be theoretically expected without bone resorption (based on a typical volumetric curing shrinkage of PMMA cement of about 6.7% (Gilbert et al., 2000) and assuming adequate lavage).
Figure 4.
Chart showing area of gap at the cement-bone interface (normalized by cement mantle area to give “gap fractions”) versus duration of use: there was no significant correlation. Solid line shows linear fit, dashed line 95% CI on fit (R2 = 0.010, p = 0.77).
Discussion
Our data quantitatively demonstrated that duration-dependent fatigue damage accumulation occurred within the cement mantles surrounding femoral stems during in vivo use. Furthermore, BMI strongly influenced crack extension and the rate of new crack formation over time, indicated by increasing Cr.Ln.Dn. and Cr.Dn. respectively.
Limitations
Our study was based on a fairly small, somewhat older, group of donors from the United States of America. We did not know the surgical technique used or what comorbidities there may have been. As a result, prudent caution should be exercised in extending our conclusions to the wider population of cemented THA recipients.
We chose BMI rather than simply weight because we thought it likely to be a better predictor of the severity of mechanical stresses (load per unit area): BMI normalizes weight (which is proportional to load on the implant) by the square of height (which is roughly proportional to bone cross-sectional area). Clinical evidence of the effect of BMI on revision rates is mixed. Some clinical studies have shown that BMI correlated with revision risk (Münger et al., 2006; Röder et al., 2008) others that it did not (Lübbeke et al., 2010). Ideally, we would have included donor activity level as an additional predictor but the data were not available. The finding that cement damage appeared to be predicted by duration of use and BMI without reference to activity level was intriguing. It is possible that our study sample all had similar activity levels since, in the USA, cemented hips are generally selected for older and less active patients.
It is possible that some through cracks originated at the cement-bone interface, so our tally of stem-initiated cracks may be overestimated.
In considering the physical significance of porosity related damage, it was difficult to determine whether a given void was causally or incidentally involved in a crack that traversed it. Thus, testing the correlation between porosity and void-related crack measures could not have helped in determining whether those voids caused the cracks to initiate. We dealt with this issue by including porosity as a predictor in the regression models for cement damage.
Cement areas and gap/pore areas were stereologically estimated rather than absolutely measured. The repeatability of the randomly placed regular point grid was investigated during a previous study (Messick et al., 2007). Ten measurements were made of a transverse section of a cemented stem with 6.6% porosity (which was of similar magnitude to the porosity (3%) and interface gaps (14%) quantified in the current study). The coefficient of variation (COV = SD/mean) for porosity was 0.07 and that for total cement area was 0.006.
Relation to previous studies
Previously published quantitative studies of cement damage following in vivo service have used white light microscopy/digital-imaging of whole transverse sections (Bin and Noble, 2009; Bishop et al., 2009; Kawate et al., 1998). However, these techniques were only suitable to observe substantially opened cracks and were thus incapable of observing the early stages of fatigue damage development. Scanning electron microscopy (SEM) can resolve cracks at a much earlier stage of development, and has been used in qualitative studies of in vivo cement damage (Jasty et al., 1991; Maloney et al., 2002). However, SEM is cumbersome to utilize across whole sections and the required dehydration can introduce artifacts, making quantitative studies challenging. The present study is the first to systematically quantify cement damage in en bloc retrieved mantles using the dye-penetrant techniques that are normally used by engineers to highlight early fatigue damage.
Clinical relevance
The formation of cracks in the cement mantle has two potential consequences relevant to implant failure: debris generation (previously demonstrated by crack surface polishing in clinical samples (Culleton et al., 1993)) and fluid/debris transport. The latter effect is particularly relevant if cyclic stem motions cause fluid to be pumped from the joint space to the cement-bone interface via a through crack. The finding that the majority of cracks were related not to the stem-cement interface but to the cement-bone interface indicated that the cement-bone interface can be directly compromised by local cement failure.
Our data suggest that reducing a patient’s BMI could reduce the initiation and growth of cracks and thus positively impact the longevity of their hip arthroplasty.
The historical observation of fatigue cracks in clinical cement mantles led to the development of methods to improve the fatigue resistance of cement, most commonly through porosity reduction. Pores have been shown to initiate cement cracks in damage accumulation studies of isolated cement specimens (Murphy and Prendergast, 2002) and simplified laboratory models (McCormack and Prendergast, 1999) and, unsurprisingly, porosity reduction has been shown to improve static strength and fatigue life (Lidgren et al., 1984; Lidgren et al., 1987). However, a lack of demonstrable clinical benefit led R. S. Ling to describe porosity reduction as “clinically irrelevant” (Ling, 1998). Subsequent reports from the Swedish Hip Arthroplasty Register (Malchau et al., 2000) showed that vacuum mixing increased the relative risk of revision in the first 5 post-operative years and reduced it subsequently. Our data suggest that porosity reduction may not reduce fatigue crack initiation but might be of long-term benefit by reducing crack growth rates. We suggest that improvements in the fatigue resistance of the bone cement itself are likely to produce more substantial clinical benefits than did porosity reduction.
From the measures of cement-bone interface gaps, we concluded that, although not progressive, substantial bone resorption at the cement-bone interface is a common occurrence after cemented THA. This conclusion was supported by the frequent observation of trabecular shaped concavities within the cement at the mantle periphery (Figure 2). A substantially smaller cement-bone interface gap was observed in one long-term specimen (donor bone j, Table 1; Figure 1) that displayed clear signs that the bone had remodeled into extensive apposition to the mantle. This indicated that post-implantation bone loss, whilst common, is not universal. The fact that widespread bone resorption from the interface appeared to occur rapidly post-op, but not progress, suggested that some bone resorption may be initiated by the operative procedure itself rather than as a response to fatigue loading or stress-shielding. If the mechanism for this postoperative resorption could be identified and mitigated, it may improve cemented THA longevity.
Acknowledgments
This work was financially supported by NIH AR42017 (KAM). Two donor bones were provided from the University of Alabama at Birmingham via NIH EB001715 (Alan W Eberhardt). The authors also acknowledge Preston Beck and Dan Jaeger for help with specimen procurement and Caroline Mann for performing stereological area measurements. This study would not have been possible without the generous assistance of the donors’ next of kin, for which we are most grateful.
Footnotes
Conflict of interest statement: The authors have no conflict of interests to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
A. Race, SUNY Upstate Medical University.
M. A. Miller, SUNY Upstate Medical University.
T. H. Izant, Crouse Hospital, Syracuse NY.
K. A. Mann, SUNY Upstate Medical University.
References
- Bin D, Noble PC. Role of mantle cracks in the loosening and micromotion of cemented femoral stems: Analysis of autopsy material of 18 cemented femoral stems. Journal of Clinical Rehabilitative Tissue Engineering Research. 2009;13:9583–9586. [Google Scholar]
- Bishop NE, Schönwald MA, Schultz P, Püschel K, Morlock MM. The condition of the cement mantle in femoral hip prosthesis implantations - A post mortem retrieval study. HIP International. 2009;19:87–95. doi: 10.1177/112070000901900202. [DOI] [PubMed] [Google Scholar]
- Burchard R, Leppek R, Schmitt J, Lengsfeld M. Volumetric measurement of periprosthetic bone remodeling: Prospective 5 years follow-up after cemented total hip arthroplasty. Archives of Orthopaedic and Trauma Surgery. 2007;127:361–368. doi: 10.1007/s00402-007-0293-z. [DOI] [PubMed] [Google Scholar]
- Cristofolini L, Teutonico AS, Monti L, Cappello A, Toni A. Comparative in vitro study on the long term performance of cemented hip stems: validation of a protocol to discriminate between “good” and “bad” designs. Journal of Biomechanics. 2003;36:1603–15. doi: 10.1016/s0021-9290(03)00191-x. [DOI] [PubMed] [Google Scholar]
- Culleton P, Prendergast PJ, Taylor D. Fatigue failure in the cement mantle of an artificial hip joint. Clinical Materials. 1993;12:95–102. doi: 10.1016/0267-6605(93)90056-d. [DOI] [PubMed] [Google Scholar]
- Damborg F, Nissen N, Jørgensen HRI, Abrahamsen B, Brixen K. Changes in bone mineral density (BMD) around the cemented Exeter stem: A prospective study in 18 women with 5 years follow-up. Acta Orthopaedica. 2008;79:494–498. doi: 10.1080/17453670710015481. [DOI] [PubMed] [Google Scholar]
- Gilbert JL, Hasenwinkel JM, Wixson RL, Lautenschlager EP. A theoretical and experimental analysis of polymerization shrinkage of bone cement: A potential major source of porosity. Journal of Biomedical Materials Research. 2000;52:210–218. doi: 10.1002/1097-4636(200010)52:1<210::aid-jbm27>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Jasty M, Maloney WJ, Bragdon CR, O’Connor DO, Haire T, Harris WH. The initiation of failure in cemented femoral components of hip arthroplasties. Journal of Bone & Joint Surgery - British Volume. 1991;73:551–8. doi: 10.1302/0301-620X.73B4.2071634. [DOI] [PubMed] [Google Scholar]
- Kawate K, Maloney WJ, Bragdon CR, Biggs SA, Jasty M, Harris WH. Importance of a thin cement mantle. Autopsy studies of eight hips. Clinical Orthopaedics & Related Research. 1998;355:70–6. doi: 10.1097/00003086-199810000-00008. [DOI] [PubMed] [Google Scholar]
- Lidgren L, Bodelind B, Moller J. Bone cement improved by vacuum mixing and chilling. Acta Orthopaedica Scandinavica. 1987;58:27–32. doi: 10.3109/17453678709146338. [DOI] [PubMed] [Google Scholar]
- Lidgren L, Drar H, Moller J. Strength of polymethylmethacrylate increased by vacuum mixing. Acta Orthopaedica Scandinavica. 1984;55:536–41. doi: 10.3109/17453678408992954. [DOI] [PubMed] [Google Scholar]
- Ling RS. Porosity reduction in acrylic cement is clinically irrelevant. Clinical Orthopaedics & Related Research. 1998;355:249–53. doi: 10.1097/00003086-199810000-00026. [DOI] [PubMed] [Google Scholar]
- Lübbeke A, Garavaglia G, Barea C, Roussos C, Stern R, Hoffmeyer P. Influence of obesity on femoral osteolysis five and ten years following total hip arthroplasty. Journal of Bone and Joint Surgery - Series A. 2010;92:1964–1972. doi: 10.2106/JBJS.I.00749. [DOI] [PubMed] [Google Scholar]
- Malchau H, Herberts P, Soderman P, Oden A. Prognosis of Total Hip Replacement: Update and validation of results from the Swedish National Hip Arthroplasty Register 1979–1998. Scientific Exhibition presented at the 67th Annual Meeting of the American Academy of Orthopaedic Surgeons; March 15–19, 2000; Orlando, USA. 2000. https://www.jru.orthop.gu.se/ [Google Scholar]
- Maloney WJ, Schmalzried T, Harris WH. Analysis of long-term cemented total hip arthroplasty retrievals. Clinical Orthopaedics & Related Research. 2002:70–8. doi: 10.1097/00003086-200212000-00009. [DOI] [PubMed] [Google Scholar]
- Messick KJ, Miller MA, Damron LA, Race A, Clarke MT, Mann KA. Vacuum-mixing cement does not decrease overall porosity in cemented femoral stems: an in vitro laboratory investigation. The Journal of Bone and Joint Surgery British Volume. 2007;89:1115–1121. doi: 10.1302/0301-620X.89B8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack BA, Prendergast PJ. Microdamage accumulation in the cement layer of hip replacements under flexural loading. Journal of Biomechanics. 1999;32:467–75. doi: 10.1016/s0021-9290(99)00018-4. [DOI] [PubMed] [Google Scholar]
- Münger P, Röder C, Ackermann-Liebrich U, Busato A. Patient-related risk factors leading to aseptic stem loosening in total hip arthroplasty: A case-control study of 5,035 patients. Acta Orthopaedica. 2006;77:567–574. doi: 10.1080/17453670610012629. [DOI] [PubMed] [Google Scholar]
- Murphy BP, Prendergast PJ. The relationship between stress, porosity, and nonlinear damage accumulation in acrylic bone cement. Journal of Biomedical Materials Research. 2002;59:646–54. doi: 10.1002/jbm.10028. [DOI] [PubMed] [Google Scholar]
- Race A, Miller MA, Ayers DC, Mann KA. Early cement damage around a femoral stem is concentrated at the cement/bone interface. Journal of Biomechanics. 2003;36:489–496. doi: 10.1016/s0021-9290(02)00460-8. [DOI] [PubMed] [Google Scholar]
- Röder C, Eggli S, Münger P, Melloh M, Busato A. Patient characteristics differently affect early cup and stem loosening in THA: A case-control study on 7,535 patients. International Orthopaedics. 2008;32:33–38. doi: 10.1007/s00264-006-0291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolk J, Verdonschot N, Murphy BP, Prendergast PJ, Huiskes R. Finite element simulation of anisotropic damage accumulation and creep in acrylic bone cement. Engineering Fracture Mechanics. 2004;71:513–528. [Google Scholar]
- Topoleski LD, Ducheyne P, Cuckler JM. A fractographic analysis of in vivo poly(methyl methacrylate) bone cement failure mechanisms. Journal of Biomedical Materials Research. 1990;24:135–54. doi: 10.1002/jbm.820240202. [DOI] [PubMed] [Google Scholar]
- Venesmaa PK, Kröger HPJ, Jurvelin JS, Miettinen HJA, Suomalainen OT, Alhava EM. Periprosthetic bone loss after cemented total hip arthroplasty: A prospective 5-year dual energy radiographic absorptiometry study of 15 patients. Acta Orthopaedica Scandinavica. 2003;74:31–36. doi: 10.1080/00016470310013617. [DOI] [PubMed] [Google Scholar]