Abstract
AIM: To assess feasibility, tolerability and efficacy of gemcitabine-based chemotherapy in patients ≥ 75 years old with advanced pancreatic cancer.
METHODS: All consecutive patients ≥ 75 years old with advanced pancreatic adenocarcinoma were included in this retrospective study. Necessary criteria to receive chemotherapy were: performance status 0-2, adequate biological parameters and no serious comorbidities. Other patients received best supportive care (BSC).
RESULTS: Thirty-eight patients (53% women, median age 78 years, range 75-84) with pancreatic cancer (metastatic: n = 20, locally advanced: n = 18) were studied. Among them, 30 (79%) were able to receive chemotherapy [median number: 9 infusions (1-45)]. Six patients (23%) had at least one episode of grade 3 neutropenia and one patient developed a grade 3 hemolytic-uremic syndrome. No toxic death occurred. Three patients (11%) had a partial tumor response, 13 (46%) had a stable disease and 12 (43%) had a tumor progression. Median survival was 9.1 mo (metastatic: 6.9 mo, locally advanced: 11.4 mo).
CONCLUSION: Tolerance and efficacy of gemcitabine-based chemotherapy is acceptable in elderly patients in good condition, with similar results to younger patients.
Keywords: Elderly, Pancreas, Cancer, Gemcitabine
INTRODUCTION
Although pancreatic cancer (PC) only accounts for 2% of all cancers, it is the fourth leading cause of cancer death in the United States (US)[1]. Prognosis is very poor, with an estimated incidence of 33 000 per year in the US and a similar death incidence rate[1]. Median survival in patients with advanced PC who receive the best supportive care (BSC) is only three to four months. Chemotherapy with gemcitabine has been considered the standard treatment of non-resectable PC since the study by Burris et al[2]; it slightly improves both survival and clinical response and is acceptably tolerated. Several drugs have been tested in combination with gemcitabine but with disappointing results. The only combination that showed a slight but significant increase in survival was erlotinib and gemcitabine in a study by Moore et al[3].
PC usually occurs in elderly patients. In the US, the incidence rate adjusted by age and for 100 000 is of 64.2 over 65 years old and of 3.7 under 65 years old[4]. In France 37.1% of PC cases occur in patients ≥ 75 years old[5]. Survival rates in this subgroup of patients seem to be shorter than in younger patients[4]. Physicians may hesitate to offer intravenous chemotherapy because of frequent comorbidities and short estimated survival; in addition, the motivation of elderly patients for this type of treatment should be carefully assessed. Nevertheless, it has clearly been shown that elderly patients are under-represented in cancer trials[6,7]. The efficacy and tolerance of chemotherapy in elderly patients with colorectal cancer has been shown in previous studies[8-12]. Most phase III studies of chemotherapy for PC include results of, but do not specifically analyze, the subset of patients ≥ 70-75 years old[2,13-15]. Results by Maréchal et al[16] in a pooled analysis of patients ≥ 70 years old who were included in seven prospective phase 2 or phase 3 studies testing various gemcitabine-based first line combinations, suggest that chemotherapy is feasible in the elderly as well as in younger patients with PC. Likewise, Locher et al[17] supported the use of gemcitabine in another study in elderly patients.
The aim of this retrospective monocentric study was to assess feasibility, tolerance and efficacy of gemcitabine-based palliative chemotherapy in patients ≥ 75 years old treated for PC.
MATERIALS AND METHODS
Selection of patients
All patients with digestive cancer in our hospital are discussed at the weekly multidisciplinary oncological committee meeting, even if they are only able to receive best supportive care on first intention. For the current study, all patients with pathologically-proven advanced adenocarcinoma of the exocrine pancreas who were ≥ 75 years old and listed in our database were considered. Patients with adenocarcinoma of the ampulla or the biliary tract were excluded. Overall, 40 patients were included for this retrospective analysis. Among them, 2 patients were excluded as they received gemcitabine in another institution (West Indies) and thus follow-up was not possible. Finally, 38 consecutive patients fulfilling these criteria and who were treated in our hospital between March 2000 and June 2006 were retrospectively studied. After clinical and imaging assessment, tumors were classified as locally advanced (stage III) or metastatic (stage IV) according to the UICC classification (UICC).
Criteria required to propose chemotherapy were an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2 and no serious comorbidities. Before starting chemotherapy, pain and biliary obstruction had to be controlled and adequate biological parameters (i.e., neutrophil count > 1500/mL, platelet count > 100 000/mL, serum creatinine < 1.5 × the upper limit of normal value (ULN), alkaline phosphatase < 5 × ULN, and bilirubin < 1.5 × ULN) were required. If one of these criteria was not fulfilled, BSC was decided.
Treatment
Chemotherapy included gemcitabine as a single agent according to the Burris regimen (gemcitabine 1000 mg/m² as a 30-min infusion weekly for 7 out of 8 wk and then for 3 out of 4 wk)[2] or combined with oxaliplatin according to the GemOx regimen (gemcitabine 1000 mg/m² as a 100-min infusion on day 1 and oxaliplatin 100 mg/m² as a 2-h infusion on day 2 every 2 wk)[18].
Patients who received at least one infusion of chemotherapy were placed in the “chemotherapy group”. All the other patients received BSC.
Chemotherapy was stopped if there was an unacceptable/life-threatening adverse event, if performance status worsened (i.e., ECOG ≥ 3) and/or if tumor progression occurred according to imaging results. The type of chemotherapy, the number of infusions and the reason why chemotherapy was not administered or was stopped were analysed.
Safety and efficacy evaluation
Baseline assessment included medical history, physical examination with an evaluation of clinical symptoms, and biological analyses (blood cell count, serum creatinine, bilirubin, ASAT, ALAT, alkaline phosphatase). During the treatment period, blood tests, toxicity evaluation and a physical examination were performed before each infusion.
Toxicity was graded according to the National Cancer Institute Common Toxicity Criteria (NCI-CTC) version 2. Chemotherapy was delayed if the grade of toxicity ≥2; the dose of gemcitabine was reduced by 20% if the toxicity grade was ≥ 3.
Tumor response was assessed by computed tomography scan at three month intervals according to RECIST (Response Evaluation Criteria In Solid Tumors)[19]. Evaluation procedures were performed ahead of schedule if the patient’s general condition worsened or severe toxicity occurred. Overall survival (OS) was calculated from the day of diagnosis of non-resectable PC to the date of death. This study was proposed after the agreement of our institution review board.
Statistical analysis
Qualitative data are expressed as numbers and percentages. Quantitative data are expressed as median (range). Survival was determined by the Kaplan-Meier method.
RESULTS
General characteristics
Twenty women and 18 men were studied. Median age was 78 years old (75-84). Tumors were metastatic in 20 patients (including tumor relapse after surgical resection in five patients) and locally advanced in 18 patients. Median follow up was 7 mo (1-44) (Table 1). Thirty of the 38 patients (79%) received gemcitabine-based chemotherapy (single agent: n = 28, combined with oxaliplatin: n = 2) with a median of 9 infusions (1-45). Twenty-four patients (83%) completed at least 2 mo of chemotherapy (i.e., 7 infusions). The relative dose-intensity of gemcitabine was 83%. Chemotherapy was stopped due to tumor progression (n = 21), toxicity (n = 1) or fatigue (n = 4); it was replaced by chemoradiotherapy (n = 2) in patients with controlled disease. The eight remaining patients did not receive chemotherapy due to exclusion criteria (n = 5) or a life-threatening medical event that occurred after the decision to treat but before the beginning of the treatment (n = 3) (Table 2).
Table 1.
Characteristics of the 38 patients and their pancreatic cancers
| Metastatic | Locally advanced | |
| Number of patients (%) | 20 (53) | 18 (47) |
| Median age (range) | 78 (75-84) | 78 (75-84) |
| Gender (M/F) | 8/12 | 10/8 |
| Site of metastases | ||
| -liver | 15 | 0 |
| -other1 | 10 | 0 |
lung, peritoneum, lymph nodes.
Table 2.
Characteristics of treatment in the 38 patients according to the stage of pancreatic cancer
| Metastatic | Locally advanced | |
| Number of patients treated by gemcitabine-based chemotherapy (%) | 15 (50%) | 15 (50%) |
| Number of patients with BSC on first intention | 5 | 3 |
| -staff decision ECO ≥ 2 | 3 | 2 |
| -others reasons | 2 (Septicaemia, pulmonary embolism) | 1 (Duodenal stenosis and deep venous thrombosis) |
| Median number of infusions (range)1 | n = 18 | n = 7 |
| (1-45) | (2-13) |
Data available for 28 patients.
Safety evaluation
Tolerance data were available in 26 of 30 patients. Six patients (23%) had at least one episode of grade 3 hematological toxicity (neutropenia). One patient developed grade 3 hemolytic-uremic syndrome, so gemcitabine was discontinued. No grade 4 toxicity or toxic deaths occurred.
Tumor response rate
Response rate was available in 28 of 30 patients. During the first assessment (at 3 mo), 3 patients (11%) had partial tumor response (PR), disease was stable in 13 (46%) (SD) and 12 (43%) had tumor progression (PD). For 14/15 patients with metastatic PC, 1 PR (7%), 4 SD (29%) and 9 PD (64%) were observed.
Second line treatment included chemotherapy in four patients with progressive disease (GemOx after gemcitabine alone: n = 3, and Folfiri after GemOx: n = 1), and chemoradiotherapy was proposed in two 75-year old patients with locally advanced tumors who were in very good condition with controlled tumors after 3 mo of chemotherapy. The latter treatment involved irradiation of 50.4 Gy with a continuous infusion (200 mg/m²) of 5-fluorouracile as a radiosensitizer based on the results of a previous study[20].
Overall survival
Median survival of all patients (n = 38) was 8.9 mo and the one-year survival rate was 33.2%. Median survival of the 8 patients who received BSC was 2.95 mo. In patients receiving chemotherapy, median survival was 9.1 mo; this was 6.9 mo in patients with metastatic cancer and 11.4 mo in patients with locally advanced cancer; the 1-year survival rate was 40.6% and 44%, respectively.
Overall survival in patients treated with gemcitabine-based chemotherapy according to disease stage is presented in Figure 1.
Figure 1.
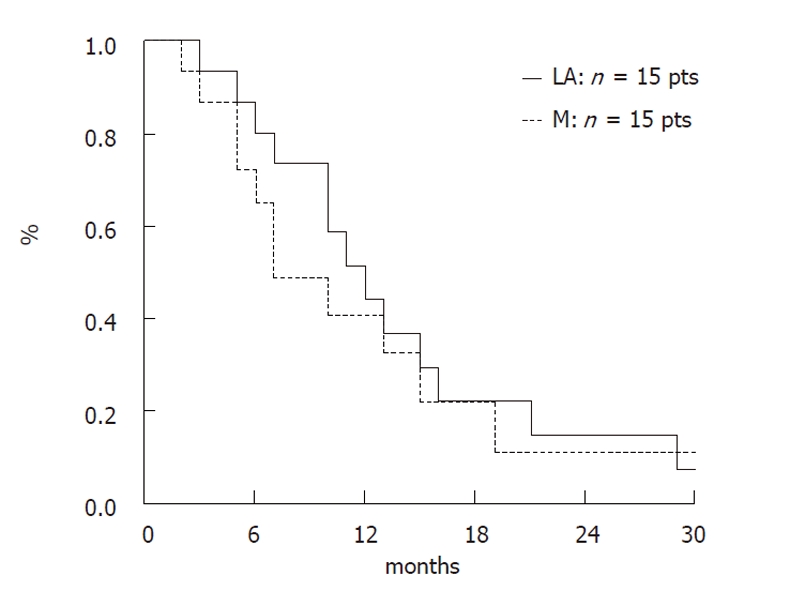
Overall survival of treated patients according to disease stage.
DISCUSSION
Although a direct comparison was not performed, this monocentric retrospective study suggests that the safety and efficacy of gemcitabine-based chemotherapy in elderly patients is similar to that in younger patients. Most eligible patients (79%) received a median of 9 infusions of chemotherapy. Safety was acceptable with grade 3 neutropenia in 23% of patients (with no grade 4), and one case of grade 3 hemolytic-uremic syndrome requiring treatment discontinuation. There were no toxic deaths. These safety results are similar to those in randomised studies including younger patients which report neutropenia as the most frequent type of toxicity with gemcitabine (grade 3-4 toxicities from 9% to 27.6%)[2,3,13-15,18].
In our study, disease control was obtained in 57% of patients (PR: 11% and SD: 46%) who received chemotherapy, which compares favourably to other published randomised studies (41.2% to 52.8%)[2,3,13,21]. The objective response rates in these studies, which include patients with both locally advanced and metastatic cancers, was 7.1% to 17.3%[3,14,15,18]. The survival rate in our study was 9.1 mo in patients who received chemotherapy; this was 6.9 mo in patients in the metastatic subgroup and 11.4 mo in the locally advanced subgroup. The 1-year survival rate of patients with metastatic and locally advanced disease who received chemotherapy was 40.6% and 44%, respectively. In the randomised series with younger patients, median survival rates in the gemcitabine arm were 5.6 and 7.2 mo, respectively[2,3,13-15,18,21].
These results should be cautiously interpreted since methodological biases are inevitable in such a retrospective study. In addition, it was conducted in a tertiary care institution, thus our population should not entirely reflect the “true life” practice for elderly patients with PC. Likewise, our study does not allow distinguishing of the potential influence of performance status (i.e., 0-1 vs 2) on both treatment safety and efficacy.
One retrospective phase II trial analysed the impact of age (< or ≥ 65 years) on the efficacy and tolerance to gemcitabine in advanced non-small cell lung cancers. Hematological, non-hematological toxicities and dose reductions, or the mean number of cycles were similar in both age groups[22].
A recent study by Locher et al[17] reported 39 patients ≥ 70 years old with PC treated by a fixed-dose rate of gemcitabine[23]. The authors showed a good efficacy of this treatment with a clinical benefit observed in 20%, a tumor response rate in 10% and a stabilization of the disease in 33% of patients. The median survival was 10 mo and the time to progression was 7 mo. Grade 3-4 neutropenia and alopecia occurred in respectively 38% and 18%. These side-effects were higher than in others trials probably due to the fixed dose rate of gemcitabine[2,3,13-15,18-23]. Maréchal et al[16] analyzed 42 patients > 70 years old pooled from seven prospective studies evaluating gemcitabine-based chemotherapy and compared them to 57 younger patients. Two thirds of the elderly patients received gemcitabine alone and one third received gemcitabine-based combinations (mainly gemcitabine-oxaliplatin). The median overall survival (220 d vs 240 d), time to progression (104 d vs 119 d), response rate (4.8% vs 8.9%) and clinical benefit (57.1% vs 59.6%) were similar in elderly and non-elderly patients. Tolerance to chemotherapy was acceptable in the elderly group despite a dose reduction or delay in therapy in 62%, a higher figure than that observed in our study. As in our study, neutropenia was the most common cause of grade 3-4 toxicity. Grade 3-4 neutropenia, anaemia and peripheral neuropathy occurred more often in the elderly group than in younger patients (30.9% vs 8.8%, 14.3% vs 8.8% and 4.8% vs 0% respectively). Age was not an independent prognostic factor in multivariate analysis of the whole population. Multivariate analysis identified ASAT and Karnofsky index as independent prognostic factors in the elderly group[16].
A Japanese study specifically reported results in 25 patients ≥ 70 years old receiving gemcitabine 800-1000 mg/m² compared to 43 patients receiving BSC. Patients receiving chemotherapy had a more favourable prognosis and acceptable tolerance[24]. Another retrospective study by Nakachi et al[25], presented in abstract form at the ASCO GI meeting in 2007, suggested that gemcitabine was effective and well tolerated in selected elderly patients. Thirty-seven patients ≥ 75 years old were compared to 137 younger patients. Grade 3-4 neutropenia (18.9% vs 19 %) and tumor response rates (8.1% vs 4.3%) were similar. In contrast, median overall survival was better in the elderly group (8 mo vs 5.6 mo, P = 0.009).
Recently, the promising schema FOLFIRINOX (5-fluorouracil, irinotecan and oxaliplatin) was shown to be superior to gemcitabine in terms of tumor response and overall survival[26]. However, patients treated in this study were less than 75 years-old and in very good condition (PS 0-1). Moreover, significant toxicity was seen [45.7% of patients experienced a significant (grade 3-4) hematological toxicity with 5.4% of febrile neutropenia] that could be problematic in elderly patients[26]. Further studies are warranted in latter patients using such drugs.
In conclusion, gemcitabine chemotherapy seems to be effective and safe in elderly patients with PC in good condition. The risk/benefit ratio of this treatment should be discussed in a multidisciplinary context and these patients should actively participate in therapeutic decisions. Prospective studies of this specific subgroup of patients with PC are needed.
ACKNOWLEDGMENTS
The authors thank Pr. Christophe Louvet for his critical review of this manuscript.
COMMENTS
Background
Pancreatic cancer is a severe disease that is often treated using systemic chemotherapy as it is non-resectable in up to 80% of patients at the time of diagnosis. Significant rates of patients with this disease are older than 75 years. However, most phase III studies of chemotherapy for pancreatic cancer include elderly patients, but they do not provide a specific analysis of patients ≥ 70-75 years old. Thus, this specific population is strongly underrepresented in therapeutic trials for digestive cancers and thus guidelines for clinical practice are lacking. In this paper, the results suggest that elderly patients with pancreatic cancer, when they are in acceptable condition, could receive gemcitabine-based chemotherapy which is safe and seems to be as efficient as in younger patients.
Research frontiers
Tumor response rates, toxicity and duration of tumor control were specifically analyzed in a homogeneous population of 38 elderly patients with pancreatic cancer treated in one center.
Innovations and breakthroughs
This is a homogeneous study of consecutive patients treated by an experienced team in digestive cancers, particularly pancreatic cancer. The authors have shown that toxicity of gemcitabine was manageable, and tumor control and overall survival were encouraging, as they appear to be similar to that of younger patients. The authors hope it will encourage physicians to evaluate and consider chemotherapy in such patients.
Applications
It is time to pave the way of chemotherapy in elderly patients with pancreatic cancer knowing that a significant subset of them may benefit of these treatments. In the future, patients should be better selected for the treatments using molecular markers (i.e., hENT-1 expression and gemcitabine).
Terminology
A locally advanced pancreatic cancer is a tumor involving the arterial axis (celiac trunk, mesenteric artery) and thus is non-resectable despite there being no detectable metastases. This form of cancer should be distinguished from metastatic tumors as the prognosis is different (slightly better, and some patients can return to surgical treatment in cases of good tumor response after chemotherapy), and thus separate analyses are needed.
Peer review
This is an article describing gemcitabine in elderly patients with advanced pancreatic cancer.
Footnotes
Peer reviewer: Hiroyuki Uehara, MD, PhD, Chief, Division of Pancreatology, Department of Gastroenterology, Osaka Medical Center for Cancer and Cardiovascular Diseases, 1-3-3 Nakamichi, Higashinari, 537-8511 Osaka, Japan
S- Editor Sun H L- Editor Rutherford A E- Editor Zhang DN
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 3.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 4.SEER Cancer Statistics Review, 1975-2005 In: Ries LAG, Melbert D, Krapcho M, Stinchcomb DG, Howlader N, Horner MJ, Mariotto A, Miller BA, Feuer EJ, Altekruse SF, Lewis DR, Clegg L, Eisner MP, Reichman M, Edwards BK, editors. National Cancer Institute. Bethesda, MD. Available from: http://seer.cancer.gov/csr/1975_2005/
- 5.Belot A, Grosclaude P, Bossard N, Jougla E, Benhamou E, Delafosse P, Guizard AV, Molinié F, Danzon A, Bara S, et al. Cancer incidence and mortality in France over the period 1980-2005. Rev Epidemiol Sante Publique. 2008;56:159–175. doi: 10.1016/j.respe.2008.03.117. [DOI] [PubMed] [Google Scholar]
- 6.Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–2067. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 7.Yee KW, Pater JL, Pho L, Zee B, Siu LL. Enrollment of older patients in cancer treatment trials in Canada: why is age a barrier? J Clin Oncol. 2003;21:1618–1623. doi: 10.1200/JCO.2003.12.044. [DOI] [PubMed] [Google Scholar]
- 8.Aparicio T, Desramé J, Lecomte T, Mitry E, Belloc J, Etienney I, Montembault S, Vayre L, Locher C, Ezenfis J, et al. Oxaliplatin- or irinotecan-based chemotherapy for metastatic colorectal cancer in the elderly. Br J Cancer. 2003;89:1439–1444. doi: 10.1038/sj.bjc.6601310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aparicio T, Mitry E, Sa Cunha A, Girard L. [Management of colorectal cancer of elderly patients] Gastroenterol Clin Biol. 2005;29:1014–1023. doi: 10.1016/s0399-8320(05)88176-x. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg RM, Tabah-Fisch I, Bleiberg H, de Gramont A, Tournigand C, Andre T, Rothenberg ML, Green E, Sargent DJ. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol. 2006;24:4085–4091. doi: 10.1200/JCO.2006.06.9039. [DOI] [PubMed] [Google Scholar]
- 11.Magné N, François E, Broisin L, Guardiola E, Ramaïoli A, Ferrero JM, Namer M. Palliative 5-fluorouracil-based chemotherapy for advanced colorectal cancer in the elderly: results of a 10-year experience. Am J Clin Oncol. 2002;25:126–130. doi: 10.1097/00000421-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Popescu RA, Norman A, Ross PJ, Parikh B, Cunningham D. Adjuvant or palliative chemotherapy for colorectal cancer in patients 70 years or older. J Clin Oncol. 1999;17:2412–2418. doi: 10.1200/JCO.1999.17.8.2412. [DOI] [PubMed] [Google Scholar]
- 13.Colucci G, Labianca R, Di Costanzo F, Gebbia V, Cartenì G, Massidda B, Dapretto E, Manzione L, Piazza E, Sannicolò M, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with single-agent gemcitabine as first-line treatment of patients with advanced pancreatic cancer: the GIP-1 study. J Clin Oncol. 2010;28:1645–1651. doi: 10.1200/JCO.2009.25.4433. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham D, Chau I, Stocken DD, Valle JW, Smith D, Steward W, Harper PG, Dunn J, Tudur-Smith C, West J, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2009;27:5513–5518. doi: 10.1200/JCO.2009.24.2446. [DOI] [PubMed] [Google Scholar]
- 15.Herrmann R, Bodoky G, Ruhstaller T, Glimelius B, Bajetta E, Schüller J, Saletti P, Bauer J, Figer A, Pestalozzi B, et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol. 2007;25:2212–2217. doi: 10.1200/JCO.2006.09.0886. [DOI] [PubMed] [Google Scholar]
- 16.Maréchal R, Demols A, Gay F, de Maertelaer V, Arvanitaki M, Hendlisz A, Van Laethem JL. Tolerance and efficacy of gemcitabine and gemcitabine-based regimens in elderly patients with advanced pancreatic cancer. Pancreas. 2008;36:e16–e21. doi: 10.1097/MPA.0b013e31815f3920. [DOI] [PubMed] [Google Scholar]
- 17.Locher C, Fabre-Guillevin E, Brunetti F, Auroux J, Delchier JC, Piedbois P, Zelek L. Fixed-dose rate gemcitabine in elderly patients with advanced pancreatic cancer: an observational study. Crit Rev Oncol Hematol. 2008;68:178–182. doi: 10.1016/j.critrevonc.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Louvet C, Labianca R, Hammel P, Lledo G, Zampino MG, André T, Zaniboni A, Ducreux M, Aitini E, Taïeb J, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23:3509–3516. doi: 10.1200/JCO.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 19.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 20.Huguet F, André T, Hammel P, Artru P, Balosso J, Selle F, Deniaud-Alexandre E, Ruszniewski P, Touboul E, Labianca R, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol. 2007;25:326–331. doi: 10.1200/JCO.2006.07.5663. [DOI] [PubMed] [Google Scholar]
- 21.Heinemann V, Quietzsch D, Gieseler F, Gonnermann M, Schönekäs H, Rost A, Neuhaus H, Haag C, Clemens M, Heinrich B, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol. 2006;24:3946–3952. doi: 10.1200/JCO.2005.05.1490. [DOI] [PubMed] [Google Scholar]
- 22.Shepherd FA, Abratt RP, Anderson H, Gatzemeier U, Anglin G, Iglesias J. Gemcitabine in the treatment of elderly patients with advanced non-small cell lung cancer. Semin Oncol. 1997;24(Suppl 7):50–55. [PubMed] [Google Scholar]
- 23.Tempero M, Plunkett W, Ruiz Van Haperen V, Hainsworth J, Hochster H, Lenzi R, Abbruzzese J. Randomized phase II comparison of dose-intense gemcitabine: thirty-minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinoma. J Clin Oncol. 2003;21:3402–3408. doi: 10.1200/JCO.2003.09.140. [DOI] [PubMed] [Google Scholar]
- 24.Hanada K, Hino F, Amano H, Fukuda T, Kuroda Y. Current treatment strategies for pancreatic cancer in the elderly. Drugs Aging. 2006;23:403–410. doi: 10.2165/00002512-200623050-00004. [DOI] [PubMed] [Google Scholar]
- 25.Nakachi K, Furuse J, Ishii H, Suzuki E, Shimizu S, Yoshino M. Tolerability and efficacy of standard chemotherapy with gemcitabine for elderly patients with advanced pancreatic cancer. Gastrointest Cancer Res. 2007;1:73. [Google Scholar]
- 26.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]


