Abstract
AIM: To investigate the efficacy and safety of propofol sedation for endoscopic retrograde cholangiopancreatography (ERCP).
METHODS: Databases including PubMed, Embase, and the Cochrane Central Register of Controlled Trials updated as of October 2010 were searched. Main outcome measures were ERCP procedure duration, recovery time, incidence of hypotension and hypoxia.
RESULTS: Six trials with a total of 663 patients were included. The pooled mean difference in ERCP procedure duration between the propofol and traditional sedative agents was -8.05 (95% CI: -16.74 to 0.63), with no significant difference between the groups. The pooled mean difference in the recovery time was -18.69 (95% CI: -25.44 to -11.93), which showed a significant reduction with use of propofol sedation. Compared with traditional sedative agents, the pooled OR with propofol sedation for ERCP causing hypotension or hypoxia was 1.69 (95% CI: 0.82-3.50) and 0.90 (95% CI: 0.55-1.49), respectively, which indicated no significant difference between the groups.
CONCLUSION: Propofol sedation during ERCP leads to shorter recovery time without an increase of cardiopulmonary side effects. Propofol sedation can provide adequate sedation during ERCP.
Keywords: Endoscopic retrograde cholangiopancreatography, Propofol, Sedative agents, Meta-analysis, Outcomes
INTRODUCTION
Endoscopic retrograde cholangiopancreatography (ERCP), the most complex gastrointestinal procedure since its introduction in 1968[1], is a highly effective tool to diagnose or treat a variety of biliopancreatic diseases. It is generally recognized that ERCP is a lengthy and potentially uncomfortable procedure that should be performed under at least conscious sedation[2]. Over the past two decades, propofol, a short-acting agent with rapid metabolism in vivo has been used frequently worldwide as a sedative agent for standard endoscopic procedures[3]. However, propofol may lead to deep sedation or even dangerous adverse events that require cardiopulmonary support[4]. Previous studies and several meta-analyses[5,6] have demonstrated that, compared with the traditional sedative agents, propofol sedation is associated with a lower risk of complications in gastrointestinal endoscopy. To date, several studies have compared the effectiveness of propofol with conventional sedation during ERCP. However, the results of individual studies have been inconclusive. Thus, we propose that pooling all available studies together systematically may provide a better understanding of the procedure. Here, we performed a meta-analysis to assess the safety and efficacy of propofol sedation for ERCP, including all randomized controlled trials (RCTs).
MATERIALS AND METHODS
Searching strategy
Related articles in all languages were identified and selected by searching multiple electronic databases including PubMed, Embase, and the Cochrane Central Register of Controlled Trials updated to October 2010, and all bibliographies were identified in the reference lists to identify eligible studies. Due to the relatively small number of articles in this field, we did not use an automated RCT filter in the searching strategy. Key words including ERCP, propofol and diprivan, were used to identify as many articles as possible. Internet search engines, Google Scholar and Yahoo, were also searched with relevant keywords. Major proceedings of international meetings were hand-searched.
Inclusion and exclusion criteria
The primary objective of this meta-analysis was to determine the safety and efficacy of propofol sedation for ERCP by comparing with traditional sedative agents such as meperidine, midazolam, scopolamine, and/or pentazocine. Only RCTs in adult patients aged > 18 years who underwent ERCP, published as full articles or meeting abstracts in peer-reviewed journals were considered. Studies were included if they provided the sedation-related outcomes: patient monitoring and complications (i.e., hypoxia or hypotension), procedure-related outcomes (i.e., ERCP duration, sedation and recovery time). All the studies that used propofol plus other agents simultaneously in the same group were excluded. We also excluded studies that could not provide actual frequencies of the complications rather than percentages of complications or percentage decline in complications.
Data extraction and validity assessment
Two authors (Bo LL and Bai Y) selected the studies, extracted the data, and assessed study quality using a predesigned form. This process resulted in high inter-observer agreement (K = 0.86). Disagreement was resolved by consensus or discussion with the third author (Deng XM). Extracted information includes study design, interventions, outcomes, and adverse effects. When necessary, authors were contacted for data not reported or not fully clarified in the original article.
Included studies were assessed for methodological quality on a scale validated by Jadad et al[7] and scored from 0 to 5: randomization (0-2 points), blinding (0-2 points), and full accounting of all patients (0-1 point); a higher score indicating better quality. All the included studies had a score of at least 1 because randomization was a requirement for inclusion.
Statistical analysis
All statistical analysis was performed using Review Manager (RevMan version 5.0), the Cochrane Collaboration’s software for preparing and maintaining Cochrane systematic reviews. Meta-analysis was performed using fixed-effect or random-effect methods, depending on the absence or presence of significant heterogeneity. We used the χ2 test to assess heterogeneity between trials and the I2 statistic to assess the extent of inconsistency. P < 0.10 was defined as significant heterogeneity. Results were expressed as OR or mean difference with 95% CI. P < 0.05 was considered statistically significant. Potential publication bias was examined by funnel plot.
RESULTS
Selected RCTs
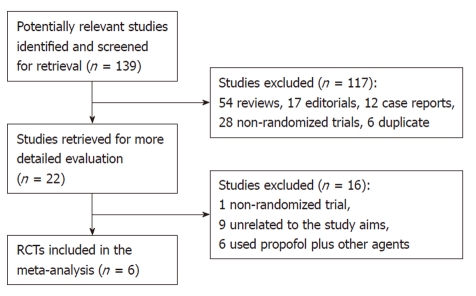
Figure 1 shows the process of study selection. Our initial searching strategy yielded 139 citations in Embase, PubMed, and Cochrane library (updated to October 12, 2010), of which 117 were excluded on the basis of the title or abstract. Of the remaining 22 articles, we excluded one study that was not randomized, nine unrelated to the study aims, and six having used some other agents plus propofol in the same group or in the control.
Figure 1.
Flow diagram of included and excluded trials.
Finally, six RCTs[8-13], with a total of 663 subjects, 331 who received propofol, and 332 who received traditional agents for sedation, fulfilled our inclusion criteria. Among them, three trials were reported from Germany[8-10] (Riphaus, 2005 #30), one from China[11], one from Israel[12], and one from Thailand[13]. All eligible articles were reported in the form of full-text articles.
Characteristics of the selected studies
The characteristics of the six included studies are summarized in Table 1. The median number of enrolled patients was 107 (range, 32-197). The indication for ERCP in these trials was generally biliary diseases. All of them were randomized controlled single-center trials. Four of them[8,10,12,13] reported the method of randomization with a Jadad score of ≥ 3, which suggested a good study design or high quality of report.
Table 1.
Characteristics of studies included in the meta-analysis
| Included studies | Country | Administrator | Procedure | Sedation | Sample size | Hypoxia (SaO2 < 90%) | Hypotension (SBP < 90 mmHg) | Procedure duration (min) | Recovery time (min) | Jadad score |
| Chen et al[11], 2005 | China | ICU physician | ERCP | Propofol | 35 | 2 | 7 | 49.22 ± 24.51 | 5.20 ± 1.94 | 2 |
| Meperidine + scopolamine | 35 | 3 | 0 | 69.59 ± 25.16 | 63.94 ± 78.02 | |||||
| Jung et al[10], 2000 | Germany | Anesthesiologist | ERCP | Propofol | 40 | 1 | 2 | |||
| Midazolam | 40 | 0 | ||||||||
| Kongkam et al[13], 2008 | Thailand | ACLS trained gastroenterologist | ERCP | Propofol | 67 | 15 | 6 | 39.79 ± 32.49 | 17.24 ± 5.99 | 5 |
| Meperidine + midazolam | 67 | 21 | 6 | 41.82 ± 21.85 | 34.25 ± 16.06 | |||||
| Krugliak et al[12], 2000 | Israel | Anesthesiologist | ERCP | Propofol | 15 | 13.1 ± 5.8 | 5 | |||
| Midazolam | 17 | 58.4 ± 29.4 | ||||||||
| Riphaus et al[8], 2005 | Germany | ICU physician | ERCP | Propofol | 75 | 8 | 6 | 22 ± 7 | 5 | |
| Meperidine + midazolam | 75 | 7 | 4 | 31 ± 8 | ||||||
| Wehrmann et al[9], 1999 | Germany | Physician unspecified | ERCP | Propofol | 99 | 11 | 7 | 27 ± 16 | 19 ± 8 | 4 |
| Midazolam + pentazocine | 98 | 8 | 2 | 32 ± 14 | 29 ± 8 |
SBP: Systolic blood pressure; ERCP: Endoscopic retrograde cholangiopancreatography.
Meta-analysis results
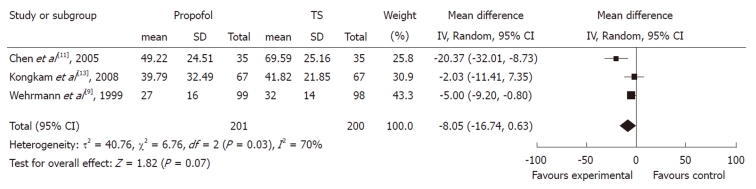
Procedure time: The duration of ERCP procedure between propofol and control groups was measured in three studies. Although all of them showed a trend towards duration reduction in the propofol group, the pooled mean difference between the propofol and control groups was -8.05 (95% CI: -16.74 to 0.63), which suggested a statistically non-significant difference between the two groups. The χ2 and I2 were 6.76 (P < 0.10) and 70%, which indicated heterogeneity among the studies (Figure 2).
Figure 2.
Forest plot of meta-analysis of propofol vs traditional sedative agents in endoscopic retrograde cholangiopancreatography procedure duration. IV: Inverse variance; TS: Traditional sedation.
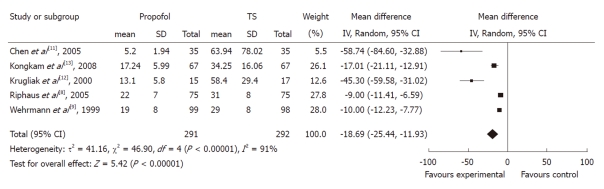
Recovery time: Five studies with 583 patients reported recovery time. All of them found a shorter mean recovery time using propofol with pooled weighted mean difference (WMD) of -18.69 (95% CI: -25.44 to -11.93), which indicated a statistically significant difference between the two groups. The χ2 and I2 were 46.9 (P < 0.10) and 91%, which suggested heterogeneity among the studies. Sensitivity analysis omitting two studies[11,12] with a high risk of bias did not alter the findings, pooled WMD -11.61 (95% CI: -15.45 to -7.78) (Figure 3).
Figure 3.
Forest plot of meta-analysis of propofol vs traditional sedative agents in endoscopic retrograde cholangiopancreatography recovery time. IV: Inverse variance; TS: Traditional sedation.
Complications: The complications of hypotension and hypoxia were recorded in most of the studies. However, amnesia was recorded in only three studies. Systolic blood pressure < 75% of baseline and heart rate < 75% of baseline were recorded in only two studies. Due to the limited number of studies, and different criteria for amnesia recognition, only hypotension and hypoxia were eligible for inclusion in the present meta-analysis.
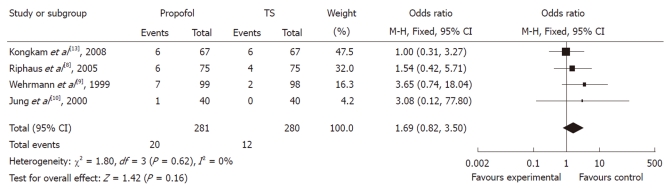
Of the four studies, the OR of hypotension in three studies was in favor of traditional agents, with one showing no difference. The meta-analysis demonstrated that hypotension occurred in 4.29% of controls (12/280) vs 7.12% (20/281) of the propofol group. Compared with traditional agents for sedation, the pooled OR of developing hypotension using propofol was 1.69 (95% CI: 0.82-3.50), which indicated no statistically significant difference between the two groups (Figure 4).
Figure 4.
Forest plot of meta-analysis of propofol vs traditional sedative agents in occurrence of hypotension during endoscopic retrograde cholangiopancreatography. M-H: Mantel-Haenszel; TS: Traditional sedation.
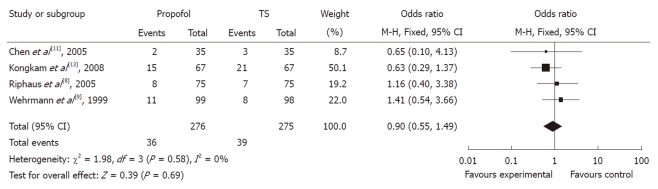
In evaluating the OR between propofol and traditional sedation agents causing hypoxia, two studies favored propofol, whereas two studies favored traditional sedation agents. The meta-analysis demonstrated that hypoxia occurred in 14.19% of controls (39/275) vs 13.04% (36/276) of the propofol group. Overall, the pooled OR of developing hypoxia using propofol was 0.90 (95% CI: 0.55-1.49), which suggested no statistically significant difference between the two groups (Figure 5).
Figure 5.
Forest plot of meta-analysis of propofol vs traditional sedative agents in occurrence of hypoxia during endoscopic retrograde cholangiopancreatography. M-H: Mantel-Haenszel; TS: Traditional sedation.
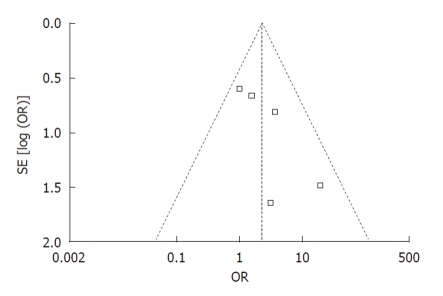
Publication bias: Funnel plot analysis was conducted using the occurrence of hypotension as the index. The graphical funnel plot of the five studies appeared to be asymmetrical (Figure 6).
Figure 6.
Funnel plot of trials of propofol sedation during endoscopic retrograde cholangiopancreatography. OR: Odds ratio.
DISCUSSION
By summarizing the current best evidence, this meta-analysis conclusively revealed that there are clear benefits of propofol sedation during ERCP regarding the recovery time, without an increase in hypotension and hypoxia occurrence.
Propofol is widely used to induce and maintain anesthesia. It is also used to induce moderate to deep sedation for other procedures, and its advantages include rapid onset, rapid recovery time, and absence of nausea or vomiting[14]. During the past decade, with the growing interest in sedation for gastrointestinal endoscopy worldwide, the use of propofol sedation during endoscopy has been increased[15]. In a previous meta-analysis[6], propofol sedation for colonoscopy was associated with significantly fewer adverse effects. Our present meta-analysis of propofol in ERCP indicated that propofol was not inferior to traditional sedation agents.
Our present meta-analysis showed that the recovery time with propofol sedation was significantly reduced when compared with that with traditional sedation. We also confirmed that the incidence of hypotension and hypoxia during ERCP with propofol sedation was comparable to traditional sedation. It has been reported that propofol for sedation during colonoscopy for generally healthy individuals can lead to a faster recovery time without an increase in side effects[6]. Our results in ERCP also found a significant reduction in recovery time. Qadeer et al[6] also concluded that propofol is not inferior to other agents when used for ERCP/endoscopic ultrasound sedation (EUS) in terms of complications of hypoxia and hypotension. However, their meta-analysis of propofol sedation in ERCP included only three studies; since then, three new RCTs have been published[8,11,13]. Estimation based on the three trials, involving only 304 patients, was underpowered to detect the risk of hypoxia or hypotension. Several differences should also be highly noted. First, our present meta-analysis focused specifically on ERCP, whereas the previous meta-analysis focused on colonoscopy. Second, the procedure duration and recovery time were compared in the present meta-analysis, whereas the previous analysis only estimated the risk of hypoxia and hypotension caused by propofol sedation during ERCP/EUS.
Guidelines and a position statement[16] published jointly by four American gastroenterology and hepatology societies regarding non-anesthesiologist administration of propofol for gastrointestinal endoscopy state that, non-anesthesiologist administration of propofol is more cost-effective than standard sedation with benzodiazepines and opioids. Propofol has the potential to induce general anesthesia, and there is no pharmacological antagonist to reverse its effect. Although propofol sedation appears to be a promising strategy during ERCP, its side effects should never be underestimated. With respect to its potential side effects, the administrator should be aware of the risk of hypotension and respiratory depression[4]. Further studies with standardized end-points are also needed to compare propofol administration by anesthesiologists to that by non-anesthesiologists.
The objectives of a meta-analysis include increasing power to detect an overall therapeutic effect by estimating the degree of benefit associated with a particular study treatment[17]. In the case of propofol sedation during ERCP, the current meta-analysis pooled all available data from published RCTs, which substantially reduced the type II error. However, the present meta-analysis also has several limitations that need to be taken into account in interpreting the results.
First, this meta-analysis is a study-level but not an individual patient-level meta-analysis. It is known that study-level analysis can lead to biased assessments, and use of aggregated summary values has some limitations for explaining the heterogeneity[18]. Second, the administrator of propofol sedation was not the same in all the included studies: two studies by anesthesiologists[10,12], two by ICU physicians[8,11], one by ACLS trained gastroenterologists[13], and one by an unspecified physician[9]. This may be considered as a source of heterogeneity. However, due to the limited number of included studies and differently recorded data, subgroup analysis was not carried out. Third, we originally intended to analyze other complications (e.g., arrhythmias, antegrade amnesia, and apnea), assessment of the procedure by the patients (i.e., satisfaction, pain or discomfort), and assessment of the procedure by physicians (i.e., satisfaction with sedation and patient cooperation). However, due to the limited number of studies that reported relevant outcomes, and the different methods in reporting outcomes, it was not appropriate to combine them together for the present meta-analysis. It should be emphasized that future studies should take into a comprehensive consideration of uniform outcome reporting.
A high incidence of hypotension was noticed among all original studies except one[13]. Although the present meta-analysis found no significant statistical difference between two sedative agents, there was a trend toward a higher incidence of hypotension with propofol sedation. This result may have been caused by the relatively small numbers included in each study, leading to a high possibility of type II error, which could weaken the conclusions. Further studies with a large number of patients are warranted to clarify the safety of propofol sedation during ERCP.
In conclusion, propofol sedation during ERCP can lead to a shorter recovery time without an increase of cardiopulmonary side effects. Propofol sedation seems to be an effective method for providing adequate sedation during ERCP.
ACKNOWLEDGMENTS
The authors thank all the authors of the primary studies, without which this study would not have been possible.
COMMENTS
Background
Endoscopic retrograde cholangiopancreatography (ERCP), a highly effective tool to diagnose or treat a variety of biliopancreatic diseases, is considered as the most complex gastrointestinal procedure. It is generally recognized that ERCP is a lengthy and potentially uncomfortable procedure that should be performed under at least conscious sedation.
Research frontiers
Propofol, a short-acting agent with rapid metabolism in vivo, has been used frequently worldwide as a sedative agent for standard endoscopic procedures. However, propofol may lead to deep sedation or even dangerous adverse events that require cardiopulmonary support.
Innovations and breakthroughs
The current meta-analysis summarized all available studies to support the propofol sedation for ERCP. The authors found from this meta-analysis that propofol can lead to a shorter recovery time without an increase of cardiopulmonary side effects. Propofol sedation can provide adequate sedation during ERCP.
Applications
This study generated the best evidence to support the clinical use of propofol for ERCP sedation.
Terminology
Propofol: a short-acting, intravenously administered hypnotic agent. It is used in the induction and maintenance of general anesthesia, sedation for mechanically ventilated adults, and procedural sedation. ERCP: a technique that combines the use of endoscopy and fluoroscopy to diagnose and treat certain problems of the biliary or pancreatic ductal systems.
Peer review
The authors made a meta-analysis comparing the effect and adverse effects of propofol for ERCP sedation. Nowadays, propofol is more frequently used especially to sedate patients undergoing ERCP. Clearly, there is a need to update on the propofol effect and safety. The great effort provided by the authors is appreciated.
Footnotes
Supported by The grants from the Department of Anesthesiology and Intensive Care of Changhai Hospital, Shanghai, China
Peer reviewer: Ibrahim A Al Mofleh, Professor, Department of Medicine, College of Medicine, King Saud University, PO Box 2925, 11461 Riyadh, Saudi Arabia
S- Editor Sun H L- Editor Ma JY E- Editor Zheng XM
References
- 1.McCune WS, Shorb PE, and Moscovitz H. Endoscopic cannulation of the ampulla of Vater: a preliminary report. Gastrointest Endosc. 1988;34:278–280. [PubMed] [Google Scholar]
- 2.Wang P, Li ZS, Liu F, Ren X, Lu NH, Fan ZN, Huang Q, Zhang X, He LP, Sun WS, et al. Risk factors for ERCP-related complications: a prospective multicenter study. Am J Gastroenterol. 2009;104:31–40. doi: 10.1038/ajg.2008.5. [DOI] [PubMed] [Google Scholar]
- 3.Bryson EO, Sejpal D. Anesthesia in remote locations: radiology and beyond, international anesthesiology clinics: gastroenterology: endoscopy, colonoscopy, and ERCP. Int Anesthesiol Clin. 2009;47:69–80. doi: 10.1097/AIA.0b013e3181939b20. [DOI] [PubMed] [Google Scholar]
- 4.Coté GA, Hovis RM, Ansstas MA, Waldbaum L, Azar RR, Early DS, Edmundowicz SA, Mullady DK, Jonnalagadda SS. Incidence of sedation-related complications with propofol use during advanced endoscopic procedures. Clin Gastroenterol Hepatol. 2010;8:137–142. doi: 10.1016/j.cgh.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Singh H, Poluha W, Cheung M, Choptain N, Baron KI, Taback SP. Propofol for sedation during colonoscopy. Cochrane Database Syst Rev. 2008:CD006268. doi: 10.1002/14651858.CD006268.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qadeer MA, Vargo JJ, Khandwala F, Lopez R, Zuccaro G. Propofol versus traditional sedative agents for gastrointestinal endoscopy: a meta-analysis. Clin Gastroenterol Hepatol. 2005;3:1049–1056. doi: 10.1016/s1542-3565(05)00742-1. [DOI] [PubMed] [Google Scholar]
- 7.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 8.Riphaus A, Stergiou N, Wehrmann T. Sedation with propofol for routine ERCP in high-risk octogenarians: a randomized, controlled study. Am J Gastroenterol. 2005;100:1957–1963. doi: 10.1111/j.1572-0241.2005.41672.x. [DOI] [PubMed] [Google Scholar]
- 9.Wehrmann T, Kokabpick S, Lembcke B, Caspary WF, Seifert H. Efficacy and safety of intravenous propofol sedation during routine ERCP: a prospective, controlled study. Gastrointest Endosc. 1999;49:677–683. doi: 10.1016/s0016-5107(99)70281-6. [DOI] [PubMed] [Google Scholar]
- 10.Jung M, Hofmann C, Kiesslich R, Brackertz A. Improved sedation in diagnostic and therapeutic ERCP: propofol is an alternative to midazolam. Endoscopy. 2000;32:233–238. doi: 10.1055/s-2000-96. [DOI] [PubMed] [Google Scholar]
- 11.Chen WX, Lin HJ, Zhang WF, Gu Q, Zhong XQ, Yu CH, Li YM, Gu ZY. Sedation and safety of propofol for therapeutic endoscopic retrograde cholangiopancreatography. Hepatobiliary Pancreat Dis Int. 2005;4:437–440. [PubMed] [Google Scholar]
- 12.Krugliak P, Ziff B, Rusabrov Y, Rosenthal A, Fich A, Gurman GM. Propofol versus midazolam for conscious sedation guided by processed EEG during endoscopic retrograde cholangiopancreatography: a prospective, randomized, double-blind study. Endoscopy. 2000;32:677–682. doi: 10.1055/s-2000-9021. [DOI] [PubMed] [Google Scholar]
- 13.Kongkam P, Rerknimitr R, Punyathavorn S, Sitthi-Amorn C, Ponauthai Y, Prempracha N, Kullavanijaya P. Propofol infusion versus intermittent meperidine and midazolam injection for conscious sedation in ERCP. J Gastrointestin Liver Dis. 2008;17:291–297. [PubMed] [Google Scholar]
- 14.Trapani G, Altomare C, Liso G, Sanna E, Biggio G. Propofol in anesthesia. Mechanism of action, structure-activity relationships, and drug delivery. Curr Med Chem. 2000;7:249–271. doi: 10.2174/0929867003375335. [DOI] [PubMed] [Google Scholar]
- 15.Wehrmann T, Triantafyllou K. Propofol sedation in gastrointestinal endoscopy: a gastroenterologist’s perspective. Digestion. 2010;82:106–109. doi: 10.1159/000285554. [DOI] [PubMed] [Google Scholar]
- 16.Vargo JJ, Cohen LB, Rex DK, Kwo PY. Position statement: Nonanesthesiologist administration of propofol for GI endoscopy. Am J Gastroenterol. 2009;104:2886–2892. doi: 10.1038/ajg.2009.607. [DOI] [PubMed] [Google Scholar]
- 17.Normand SL. Meta-analysis: formulating, evaluating, combining, and reporting. Stat Med. 1999;18:321–359. doi: 10.1002/(sici)1097-0258(19990215)18:3<321::aid-sim28>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 18.Berlin JA, Santanna J, Schmid CH, Szczech LA, Feldman HI. Individual patient- versus group-level data meta-regressions for the investigation of treatment effect modifiers: ecological bias rears its ugly head. Stat Med. 2002;21:371–387. doi: 10.1002/sim.1023. [DOI] [PubMed] [Google Scholar]