Abstract
Liver cancer is the fifth and seventh most common cause of cancer in men and women, respectively. Wnt/β-catenin signalling has emerged as a critical player in both the development of normal liver as well as an oncogenic driver in hepatocellular carcinoma (HCC). Based on the current understanding, this article summarizes the possible mechanisms for the aberrant activation of this pathway with specific focus on HCC. Furthermore, we will discuss the role of dickkopfs (DKKs) in regulating Wnt/β-catenin signalling, which is poorly understood and understudied. DKKs are a family of secreted proteins that comprise at least four members, namely DKK1-DKK4, which act as inhibitors of Wnt/β-catenin signalling. Nevertheless, not all members antagonize Wnt/β-catenin signalling. Their functional significance in hepatocarcinogenesis remains to be further characterized for which these studies should provide new insights into the regulatory role of DKKs in Wnt/β-catenin signalling in hepatic carcinogenesis. Because of the important oncogenic roles, there are an increasing number of therapeutic molecules targeting β-catenin and the Wnt/β-catenin pathway for potential therapy of HCC.
Keywords: Dickkopf, Hepatocellular carcinoma, Tumourigenesis, Wnt/β-catenin signalling
HEPATOCELLULAR CARCINOMA AND THE UNMET MEDICAL NEEDS
Liver cancer ranks the fifth most common cancer in men and the second leading cause of cancer-related death. In women, it is the seventh most frequent cancer and sixth leading cause of cancer death[1]. Hepatocellular carcinoma (HCC) is the most common primary malignancy of liver. Men are three times more likely to develop HCC than women and the incidence increases with age[2]. HCC is prevalent in Asia and Africa, but recently it is on the rise in the Western world due to an increase in hepatitis C virus (HCV) infection[3]. Risk factors for HCC include chronic hepatitis B virus (HBV) and HCV infections, cirrhosis, chronic alcohol abuse, aflatoxin ingestion, non-alcoholic steatohepatitis and other metabolic liver diseases[4,5]. Much of HCC occurs in the background of cirrhosis. About 80%-90% of patients with cirrhosis go on to develop HCC eventually and the remaining 10%-20% of cases develop HCC without cirrhosis. Furthermore, HBV and HCV infections increase the risk of developing cirrhosis and later HCC. Among the HCC cases with cirrhosis, HCV infection has been identified in 27%-73% and HBV infection in 12%-55%[6,7].
HCC suffers from a high mortality rate due to lack of effective diagnostic methods for early detection as well as lack of treatment options especially for those with advanced disease conditions. Despite vigorous attempts to screen for early HCC by common surveillance techniques using serum α-fetoprotein (AFP) and ultrasound examination, early HCC is asymptomatic and most HCC cases are presented late when surgical treatments are not amenable[8]. Although surgical resection remains the treatment of choice for patients with well-preserved liver function, it is associated with a high risk of post-operative complications and tumour recurrence[7]. Liver transplantation is another treatment option for early HCC but this is limited by the shortage of suitable liver grafts[9]. Other surgical treatments for HCC include radio-frequency ablation (RFA), microwave ablation (MWA) and transcatheter arterial chemoembolization (TACE). RFA and MWA techniques utilise high frequency radio-waves and micro-waves, respectively, to kill tumour tissues by heat. Although several studies have reported better disease-free survival and a lower frequency of recurrence after surgical resection compared to RFA[10,11], others report better overall survival and disease-free survival for HCC patients with multi-nodular tumours following RFA[12]. Recently, Simo et al[13] reported no difference regarding the efficacies of MWA and RFA procedures. TACE is routinely performed on HCC patients who are not eligible for surgical resection or tumour ablation techniques. However, the survival benefits of TACE depend on careful patient selection. Patients with multi-nodular HCC, without vascular invasion and no extrahepatic metastases show a better 2-year survival (63%) after TACE than HCC patients with vascular invasion undergoing TACE (31%)[14,15]. However, tumour recurrence is an important limitation to any of the HCC treatments, and thus, understanding the molecular biology of HCC is crucial for the development of novel therapies.
In recent years, studies have shed light on the clinical implications of signalling pathways in HCC, including the Ras/Raf/MEK/ERK pathway[16], the PI3K/Akt/mTOR pathway[17], the JNK pathway[18] and the NF-κB pathway[19]. A promising approach would be to identify molecular pathways responsible for initiating and sustaining HCC as targets for HCC therapy. The canonical Wnt/β-catenin signalling pathway is another such oncogenic pathway, which is frequently activated in HCC and is reported to play a pivotal role in tumourigenesis[20]. This article reviews the canonical Wnt/β-catenin signalling pathway and its involvement in HCC development. In addition, antagonists of this pathway and their implications in HCC are described and discussed. From this prior knowledge, we hope to identify members of this pathway that could serve as potential targets for HCC therapy.
OVERVIEW OF WNT/β-CATENIN PATHWAY
The Wnt/β-catenin pathway is a well-conserved pathway that is important in embryonic development, cell proliferation, survival, regeneration and self-renewal[21-23]. Likewise, a great deal of understanding has been achieved by studying pathological specimens and using mouse models of liver diseases to understand the aberrations of this pathway in liver diseases ranging from hepatitis to HCC[24-26]. Based on these earlier studies, the Wnt/β-catenin pathway is a central player in maintaining liver health and is dysregulated in hepatic cancers, which makes it an attractive candidate for potential therapies of HCC.
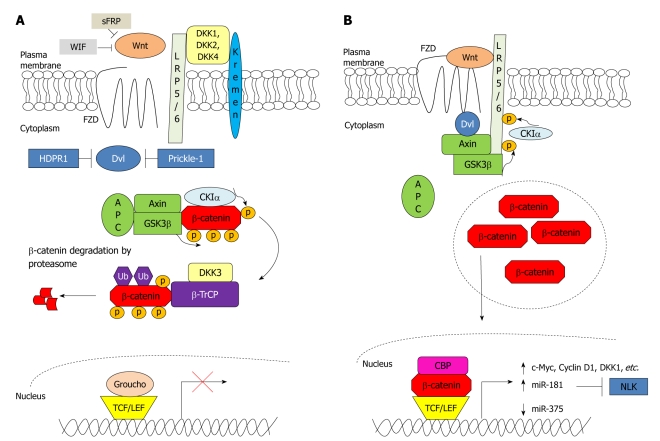
In an unstimulated cell, endogenous β-catenin is found at the adherens junctions, where it interacts with components of the cadherin-associated protein complexes to confer cell-cell adhesion functions[27,28]. On the other hand, surplus β-catenin in the cytoplasm is degraded by the action of a destruction complex which consists of glycogen synthase kinase 3β (GSK3β), Axin, adenomatous polyposis coli (APC) and casein kinase Iα (CKIα)[29]. β-Catenin is first phosphorylated at serine-45 (Ser45) by CKIα to further prime it for phosphorylation by GSK3β at Ser33, Ser37 and threonine-41 (Thr41). The phosphorylated β-catenin is then ubiquitinated by β-transducin repeat-containing protein (β-TrCP) and subsequently degraded by the proteasome[30] (Figure 1A). Maher et al[31] reported that β-catenin phosphorylated at Ser45 and not at Ser33/Ser37/Thr41 is predominantly located in the nucleus, whereas β-catenin phosphorylated at Ser33/Ser37/Thr41 is mostly localized to the cytoplasm. This spatial separation of β-catenin suggests that phosphorylation at Ser45 and at Ser33/Ser37/Thr41 is not necessarily coupled. It may also imply that phosphorylation at Ser45 by CKIα serves another function, yet to be delineated, other than priming β-catenin for further phosphorylation by GSK3β.
Figure 1.
Wnt/β-catenin signalling in the absence and presence of Wnt stimulus. A: Wnt/β-catenin signalling is regulated by several antagonists to prevent the formation of frizzled (FZD)-Wnt-low-density lipoprotein receptor-related protein 5/6 (LRP5/6) complex. Secreted frizzled-related protein (sFRP) and Wnt inhibitory factor (WIF) bind directly to Wnt, whereas dickkopfs (DKKs) bind to LRP5/6. Furthermore, human homologue of Dapper (HDPR1) and Prickle-1 inhibit the action of dishevelled (Dvl). In the absence of Wnt stimulus, β-catenin is first primed for phosphorylation by casein kinase Iα (CKIα) followed by phosphorylation by glycogen synthase kinase 3β (GSK3β) at three residues. The phosphorylated β-catenin is targeted for ubiquitination by β-transducin repeat-containing protein (β-TrCP) and is subsequently degraded by the proteasome. In the nucleus, T-cell factor (TCF)/lymphoid enhancer factor (LEF) represses transcription of the Wnt/β-catenin pathway target genes by interacting with co-repressor Groucho; B: Wnt binds to and activates FZD and LRP5/6 receptors. Dvl is recruited to the plasma membrane and binds to FZD. This results in the recruitment of Axin and GSK3β to LRP5/6. LRP5/6 is then phosphorylated by CKIα and GSK3β, resulting in an inactivation of the destruction complex and leading to β-catenin accumulation in the cytoplasm. β-catenin then subsequently translocates to the nucleus where it binds with TCF/LEF and other co-activators e.g. CREB binding protein (CBP) to mediate transcription of genes and microRNAs responsible for proliferation and growth. APC: Adenomatous polyposis coli; NLK: Nemo-like kinase; p: Phosphorylated; Ub: Ubiquitinated.
For diseased condition, the Wnt/β-catenin signalling pathway is activated upon binding of Wnt to one of the members of the frizzled (FZD) family and to low-density lipoprotein receptor-related protein 5 (LRP5) or LRP6. The FZD recruits dishevelled (Dvl) to the plasma membrane, which in turn recruits Axin and GSK3β to LRP5/LRP6[32]. The intercellular domain of LRP5/LRP6 contains five reiterated PPPSPxS motifs, which are dually phosphorylated by GSK3β and CKIα[33]. The phosphorylation of LRP5/LRP6 disrupts the formation of the destruction complex, thereby preventing GSK3β from phosphorylating β-catenin. Therefore, β-catenin is not degraded and accumulates in the cytoplasm from where it translocates to the nucleus. In the absence of Wnt, T-cell factor (TCF)/lymphoid enhancer factor (LEF) represses gene expression by interacting with co-repressor Groucho, which promotes histone deacetylation and chromatin modelling in the nucleus[34]. Nuclear accumulation of β-catenin displaces Groucho from TCF/LEF and recruits other transcriptional co-activators, e.g. CREB binding protein (CBP), for upregulation of target genes that are implicated in cell proliferation, anti-apoptosis, and angiogenesis, such as cyclin D1[35] (Figure 1B). Recent studies have supported receptor-mediated endocytosis for Wnt induced signalling[36,37]. Specifically, Wnt3a was shown to induce caveolin-dependent internalization of LRP6, which would in turn recruit Axin to LRP6 phosphorylated by GSK3β and CKIα, and thereby lead to β-catenin accumulation. Thus, caveolin plays a critical role in activating Wnt/β-catenin signalling[37].
Very recently, Chairoungdua et al[38] showed a novel mechanism of reducing cytoplasmic β-catenin levels independently of GSK3β phosphorylation or ubiquitination by exporting β-catenin out of the cell via exosomes. Exosomes are vesicles that form inside endosomes and the vesicles are then secreted when the endosomes fuse with the plasma membrane[39]. These exosomes are enriched in E-cadherin and tetraspanin proteins (CD9 and CD82). Expression of these tetraspanins was shown to decrease β-catenin protein levels, but further experiments showed that E-cadherin was also necessary for β-catenin secretion in exosomes. The molecular mechanism for the inclusion of CD9, CD82 and E-cadherin in exosomes warrants further investigation. Furthermore, how these tetraspanins induce exosome formation remains to be characterized. Although much remains to be investigated, this important and novel mechanism offers an alternative route for the regulation of Wnt/β-catenin activity, further highlighting the significance of keeping the Wnt/β-catenin pathway under check.
ABERRANT WNT/β-CATENIN SIGNALLING IN HCC
Role of aberrant β-catenin activation in HCC
HCC is one of the cancers with a high rate of dysregulation in the Wnt/β-catenin pathway and although 40%-70%[40-43] of HCC patients have tumours with high levels of β-catenin accumulation, there is little agreement on the use of β-catenin in prognosis. Nuclear accumulation of β-catenin is strongly associated with β-catenin mutations[41]. A majority of β-catenin mutations in HCC are missense mutations occurring at exon 3. This region is responsible for phosphorylation and ubiquitination of β-catenin, and therefore, mutation in this region results in stable β-catenin that consequently accumulates in the nucleus. Mao et al[44] associated nuclear β-catenin accumulation to β-catenin mutation, non-invasive form of tumour and good prognosis. HCC tumours with mutant nuclear β-catenin resulted in a better 5-year survival than HCC tumours with wild-type nuclear β-catenin accumulation. This is suggestive of the fact that wild-type β-catenin accumulation and mutant β-catenin accumulation are not equivalent. Furthermore, in this study of 37 HCC tumours with nuclear mutant β-catenin accumulation, 19 mutations occurred at the sites of GSK3β phosphorylation (Ser45, Ser33, Ser37 and Thr41), 3 tumours had β-catenin deletions, and 15 mutations were reported at other sites. However, several studies have correlated nuclear β-catenin accumulation to tumour progression and poor prognosis[43,45,46]. Kondo et al[46] reported that β-catenin accumulation and β-catenin mutation do not occur early in hepatocarcinogenesis, but could be associated with malignant progression of HCC. Similar to these findings, Inagawa et al[45] observed poor prognosis in HCC patients with nuclear β-catenin accumulation in grade III HCC tumours and not in grade I or grade II HCC tumours. Furthermore, nuclear β-catenin accumulation in HCC has also been correlated to Ki67 (a marker for tumour cell proliferation), suggesting that β-catenin promotes tumour progression[43]. The discrepancy in β-catenin accumulation and HCC prognosis could be due to the type of β-catenin mutations. Functional studies on the role of different mutations on β-catenin stability may offer insights into mechanisms involved in β-catenin regulation. Other reasons for the discrepancy may include tumour histology and the size of the tumour. Additionally, the presence of β-catenin mutations demonstrates different phenotypical features in HCC. Cieply et al[47] reported that HCC tumours harbouring a missense mutation at exon 3 exhibit a more aggressive phenotype and may develop HCC without cirrhosis compared to HCC with non-mutated β-catenin. Thus, β-catenin mutations may serve as an independent risk factor for the development of HCC in the absence of cirrhosis. Greater tumour size has also been reported in HCC tumours with β-catenin mutations as compared to those without mutation in β-catenin[48]. Some studies have correlated cytoplasmic β-catenin (non-nuclear β-catenin) with poor cellular differentiation, large tumour size (> 5 cm in diameter) and short disease-free survival[41]. For reasons not yet elucidated, HCV-associated HCC has a greater frequency of β-catenin mutations than the HBV-associated type[42].
Several studies on transgenic animal models have shown that overexpression of mutant or stable forms of β-catenin on its own is not sufficient to induce tumours in liver[49-51]. However, deletion of APC in mice results in hepatomegaly, hepatocyte hyperplasia and rapid mortality[52]. Thus, β-catenin mutations or accumulation may cooperate with other genes or signalling pathways to result in hepatocarcinogenesis. However, it is also important to take into account the functional roles of APC that are independent of β-catenin, e.g. APC maintains epithelial integrity in non-transformed mouse mammary epithelial cells[53] and it regulates cell cycle progression through the S phase by inhibiting DNA replication via direct interaction with DNA in colon cancer cell lines[54]. New mouse models are required that mimic irregular Wnt/β-catenin pathway to understand the role of this pathway as well as its therapeutic implications.
Wnt/β-catenin pathway target genes in HCC
Several β-catenin target genes in association with liver carcinogenesis were identified by their high expression in chronic liver diseases and HCC. However, their specific role in hepatocarcinogenesis remains unknown. Frequent amplification and overexpression of c-Myc and cyclin D1 in HCC is associated with cytoplasmic and nuclear β-catenin accumulation along with poor prognosis[55-61]. However, there is little consensus on whether the overexpression of c-Myc and cyclin D1 is a result of mutations in β-catenin. Cadoret et al[49] did not report c-Myc or cyclin D1 induction in the liver of transgenic mice that express a mutant form of β-catenin, although such mice did exhibit hepatomegaly and marked hepatocellular proliferation. In contrast, de La Coste et al[62] reported activating somatic mutations in β-catenin in 50% of hepatic tumours in c-Myc transgenic mice. In addition to cyclin D1 and c-Myc, several other genes have been identified as downstream molecules of the Wnt/β-catenin pathway in HCC (Table 1). Expression of these genes was discovered in HCC transgenic mice or in HCC tissues exhibiting accumulation of wild type or mutated β-catenin. For example, glutamine synthetase and orphan G-protein-coupled receptor are frequently overexpressed in HCC with mutation in β-catenin[63,64]. Further studies are warranted to understand whether mutated or stable β-catenin results in transcription of different target genes, and if silencing these target genes affects aberrant Wnt/β-catenin pathway in a negative feedback manner. More importantly, can the expression of β-catenin target genes in HCC, e.g. glutamine synthetase or cyclin D1, be sufficient to identify β-catenin activation?
Table 1.
Downstream molecules of the Wnt/β-catenin pathway with overexpression in hepatocellular carcinoma
| Gene | Ref. |
| c-Myc | [159] |
| Cyclin D1 | [57] |
| Dickkopf 1 | [126] |
| Epidermal growth factor receptor | [51] |
| Glutamine synthetase | [63] |
| Glutamate transporter | [63] |
| Leukocyte cell-derived chemotaxin 2 | [160] |
| Ornithine aminotransferase | [63] |
| Orphan G-protein-coupled receptor | [64] |
| Regenerating islet-derived 1 α | [161] |
| Regenerating islet-derived 3 α | [161] |
Alterations of Wnt/β-catenin pathway components in HCC
Accumulation of β-catenin can also occur in the absence of β-catenin mutation or due to aberrant expression of other members of the Wnt/β-catenin pathway[65]. Table 2 summarizes the aberrant expression of Wnt/β-catenin signalling components in HCC.
Table 2.
Aberrant expression of Wnt/β-catenin pathway components in liver cancer
| Component | Expression in tumour tissues vs non-tumour/healthy tissues | Incidence (%) | Method | Ref. |
| β-catenin | High | 40-70 | IHC | [40-43] |
| Wnt1 | High | 41 | PCR | [69] |
| Wnt3 | High | 39-76 | PCR | [70,71] |
| Wnt4 | High | 20 | PCR | [71] |
| Wnt5A | High | 25 | PCR | [71] |
| FZD3 | High | 41 | PCR | [71] |
| FZD6 | High | 31 | PCR | [71] |
| FZD7 | High | 33-90 | PCR | [70-72] |
| phospho-GSK3β | High | 52 | IHC | [73] |
| Axin1 | Low | 67 | IHC | [76] |
| Dvl | High | 71 | Western blotting | [82] |
| Prickle-1 | Low | 55 | PCR | [82] |
| HDPR1 | Low | 58 | PCR | [83] |
| PIN1 | High | 53 | Western blotting | [65] |
| TCF-4 | High | 91 | PCR | [87] |
| LEF-1 | High | 52 | IHC | [88] |
| CDH17 | High | 72 | IHC | [90] |
CDH17: Cadherin-17; Dvl: Dishevelled; FZD: Frizzled; phospho-GSK3β: Phosphorylated glycogen synthase kinase 3β; HDPR1: Human homologue of Dapper; IHC: Immunohistochemistry; LEF: Lymphoid enhancer factor; PCR: Polymerase chain reaction; PIN1: Peptidyl-prolyl cis/trans isomerase; TCF: T-cell factor.
Wnt and FZD
The Wnt family is composed of nineteen secreted glycoproteins[66]. They bind to the extracellular domain of FZDs and activate the Wnt/β-catenin pathway[67]. Ten different FZD genes have been identified in mammals and all of them encode seven transmembrane receptors[68]. Wnt1 is upregulated in HCC tissues compared to adjacent non-tumour tissues and its expression has been associated with tumour recurrence[69]. Furthermore, three other Wnt genes (Wnt3, Wnt4 and Wnt5A), and three FZD genes (FZD3, FZD6 and FZD7) are also upregulated in HCC tissues and preneoplastic peritumoural tissues as compared with normal liver tissues, suggesting that their overexpression may be an early event in hepatocarcinogenesis. However, only the overexpression of FZD7 has been associated with nuclear and/or cytoplasmic accumulation of β-catenin in HCC[70-72].
GSK3β, CKIα, Axin and APC
There are no reports of mutations or aberrant expression of CKIα in HCC. A few studies have reported overexpression of phosphorylated GSK3β (phospho-GSK3β, inactive form of GSK3β) in HCC cells and tissues, but this has not been found to be associated with β-catenin accumulation[73,74].
There are two Axin genes. Axin1 is constitutively expressed, whereas Axin2 (also known as Axil or Conductin) is induced by Wnt/β-catenin signalling and takes part in a negative feedback loop[75]. Kim et al[76] recently observed reduced Axin1 expression only in HCC tissues and not in cirrhotic nodules, implying that its reduced expression is independent of cirrhosis. There are currently no reports of Axin2 expression in HCC. However, Axin1 and Axin2 mutations are rare in HCC occurring in only 10% and 3% of HCC cases, respectively[48,77,78]. A majority of these mutations are frameshift or nonsense mutations and more than half of HCC cases with Axin1 or Axin2 mutations have β-catenin accumulation in the cytoplasm or nucleus[77]. Interestingly, Zucman-Rossi et al[79] recently demonstrated that the loss of Axin1 function is not equivalent to gain-of-function of β-catenin, suggesting that Axin1 could also be participating in other pathways in HCC. APC mutations are very rare in HCC and promoter methylation has been reported to play an important role in its inactivation[80].
Dvl and Peptidyl-prolyl cis/trans isomerase
Dvl is a positive regulator of Wnt/β-catenin signalling and prevents GSK3β from phosphorylating β-catenin, leading to β-catenin stabilisation[81]. Its overexpression has been shown to be critical in Wnt/β-catenin signalling activation and β-catenin accumulation in various cancers including HCC[82]. Two inhibitors of Dvl have been identified including Prickle-1[82] and human homologue of Dapper[83] both of which are reduced in HCC and their reduced expression has significant association with β-catenin accumulation. Peptidyl-prolyl cis/trans isomerase (PIN1) is another positive regulator of the Wnt/β-catenin pathway and functions by inhibiting the interaction between β-catenin and APC[84]. It is also overexpressed in more than 50% of HCC cases and this has been correlated to increased β-catenin and cyclin D1 accumulation. Furthermore, β-catenin mutation and PIN1 overexpression are mutually exclusive events in HCC, suggesting that mechanisms other than β-catenin mutation also lead to β-catenin stabilisation and accumulation[65].
TCF/LEF
The human TCF/LEF family consists of four members, LEF-1, TCF-1, TCF-3 and TCF-4, and all members contain a conserved high mobility group box to bend DNA to allow binding of transcription factors, a β-catenin-binding domain to bind β-catenin and a transcription repression domain to recruit co-repressors like Groucho[85]. When β-catenin translocates to the nucleus, it binds to the β-catenin-binding domain of TCF/LEF and activates transcription of target genes. The TCF/LEF family has several spliced forms with unknown functional significance and it is suggested that they may activate preferential genes[86]. In HCC, mutations of TCF-4 are rare with Jiang et al[87] reporting mutations in 2 of 32 HCC cases, whereas Cui et al[25] did not report any mutation in 34 HCC cases. However, overexpression of TCF-4 in HCC tissues has been correlated to c-Myc overexpression, tumour capsule status and intrahepatic metastasis[87]. LEF-1 is also overexpressed in HCC and associated with cyclin D1 overexpression, but it is not found to be correlated with any clinicopathological parameters[88]. Therefore, it is not known whether the aberrant expression of TCF-4 and LEF-1 is a primary event in HCC or a result of aberrant Wnt/β-catenin signalling in HCC. Expression of other TCF/LEF family members in HCC has not been reported.
Cadherin-17
Cadherin-17 (CDH17) belongs to the cadherin superfamily comprising transmembrane glycoproteins that mediate calcium-dependent cell-cell adhesion[89]. Since β-catenin plays an essential role in cadherin-mediated cell-cell adhesion as well as in Wnt/β-catenin signalling, our laboratory identified CDH17 to affect Wnt/β-catenin signalling in HCC. CDH17 is overexpressed in HCC cells and in more than 70% of HCC cases[90]. In addition, overexpression of an alternative splice variant of CDH17 (lacking exon 7) in more than 50% of HCC is correlated to tumour recurrence, venous infiltration and reduced overall survival[91]. Interestingly, the CDH17 splice variant is found to be triggered by two single nucleotide polymorphisms that are identified as genetic factors contributing to the high development of HCC in Asia[92]. Furthermore, RNA interference (RNAi)-mediated knockdown of CDH17 in metastatic HCC cells has been shown to inhibit tumourigenesis in vivo and reduce Wnt/β-catenin signalling by decreasing phospho-GSK3β and cyclin D1. This was accompanied by re-localisation of β-catenin to the cytoplasm[93].
Tetraspanins
Tetraspanins are transmembrane proteins known to affect a wide range of functions including cell-cell adhesion, cell growth and suppression of metastasis[94]. The recent involvement of tetraspanins CD9 and CD82 in a novel mechanism to antagonize Wnt/β-catenin signalling by exosomal release of β-catenin is an exciting avenue to explore in HCC. This exosomal release of β-catenin may be compromised in cancers with high Wnt/β-catenin signalling. CD9 and CD82 are suppressors of metastasis and their expression is reduced in HCC with portal vein invasion and/or intrahepatic metastasis[95]. Chairoungdua et al[38] demonstrated Wnt/β-catenin signalling inhibition in a metastatic cell line following restoration of CD82 expression. Thus, these tetraspanins may suppress metastasis by antagonizing Wnt/β-catenin signalling by targeting β-catenin for exosomal release. It will be important to investigate the correlation between CD9 and CD82 with β-catenin in HCC.
MicroRNAs
MicroRNAs (miRNAs) are small non-coding RNAs that regulate post-transcriptional gene expression[96]. They are aberrantly expressed in HCC compared to their non-tumour liver tissues[97-99] and contribute to liver tumourigenesis[100,101]. Several miRNAs have been identified to affect the Wnt/β-catenin pathway[102]. Using a global microarray-based miRNA profiling approach, Ji et al[103] identified miRNA-181 (miR-181) to be upregulated in HCC tumours that were positive for epithelial cell adhesion molecule (EpCAM) and AFP (EpCAM+AFP+). Such tumours demonstrated cancer stem cell properties and an activation of Wnt/β-catenin signalling. In vitro studies showed a correlation between overexpression of miR-181 and β-catenin in HCC cells and further demonstrated that miR-181 promoted the stemness of EpCAM+AFP+ HCC cells by targeting CDX2 (caudal type homeobox transcription factor 2), GATA6 (GATA binding protein 6, a hepatic transcriptional regulator of differentiation) and nemo-like kinase (NLK, an inhibitor of Wnt/β-catenin signalling). These findings provide evidence that miR-181 is transcriptionally activated by Wnt/β-catenin signalling and in turn inhibits its regulators. In addition, miR-375 is another miRNA involved in the Wnt/β-catenin pathway and it is downregulated by β-catenin in HCC[104]. However, the function of miR-375 and the mechanisms by which it is regulated by β-catenin are not clear. Further research is needed to investigate the involvement of miRNAs in Wnt/β-catenin signalling in HCC.
Yes-associated protein
The Hippo signalling pathway controls organ size by regulating cell proliferation and apoptosis. The signalling cascade of this pathway ultimately leads to the phosphorylation of yes-associated protein (YAP), a downstream effector of this pathway. YAP is a transcriptional co-activator and its phosphorylation causes it to remain in the cytoplasm and prevent the transcription of genes responsible for cell proliferation and inhibition of apoptosis[105]. Recently, a few studies have described the Hippo pathway as a negative regulator of Wnt/β-catenin signalling[106,107]. Varelas et al[106] reported phosphorylated Taz (component of the Hippo pathway) to inhibit the activation of Dvl, thereby preventing β-catenin stabilisation and activation. Heallen et al[107] recently showed nuclear interaction of unphosphorylated YAP and β-catenin in cardiac cells of mice with dysregulated Hippo signalling. These mice had enlarged hearts and overexpressed Wnt/β-catenin target genes. Additionally, dysregulation of the Hippo signalling pathway and inhibition of β-catenin resulted in restriction of cardiomyocyte overgrowth. This study offered insights into the direct interaction between the downstream effectors of these two important pathways. In HCC, nuclear overexpression of YAP (unphosphorylated YAP) has been reported in 62% of HCC and has been associated with short disease-free survival and overall survival[108]. Since β-catenin is also found to accumulate in the nucleus of HCC patients[43,45], further studies are warranted to understand the clinical implications of YAP and β-catenin overexpression in HCC.
WNT SIGNALLING ANTAGONISTS
As mentioned above, there are many factors affecting the Wnt/β-catenin pathway in HCC. However, the occurrence of these factors is not absolute in each clinical case of HCC. Therefore, the involvement of other factors in modulating the Wnt/β-catenin pathway is speculated. Factors upstream of GSK3β/Axin/APC protein complex, like those occurring at the extracellular level, may also be those involved. For this, secreted Wnt antagonists are distinguished examples. These antagonists can be divided broadly into two groups based on their different mechanisms in preventing the interaction of Wnt with LRP5/LRP6[109]. Group I consists of Wise, sclerostin and dickkopfs (DKKs) that bind directly to LRP5/LRP6. Group II comprises Wnt inhibitory factor and secreted frizzled-related protein that act by directly binding to Wnt proteins and prevent the formation of the Wnt receptor complex. This review will focus on the role of DKKs on the Wnt/β-catenin pathway in HCC.
Structure of DKKs
The DKK family consists of four members (DKK1 to DKK4). They are secreted glycoproteins of 225-350 amino acids with molecular weights between 25 and 29 kDa for DKK1, DKK2 and DKK4, and 38 kDa for DKK3. DKKs contain two conserved cysteine-rich domains, each of which is separated by a linker region of various lengths[110]. Each cysteine-rich domain contains ten cysteine residues. The amino-terminal cysteine-rich domain (Cys-1) is unique to each DKK, whereas the carboxyl-terminal cysteine-rich domain (Cys-2) is highly conserved among all members of the DKK family. The position of each cysteine residue in the Cys-2 domain closely resembles proteins in the colipase family[111]. Because of the role of colipases in lipid hydrolysis[112], the presence of this feature suggests the ability of DKKs to interact with lipids in regulating Wnt/β-catenin signalling[111]. Among all DKKs, DKK3 is the most divergent member[113]. It contains an extended amino-terminal domain preceding the Cys-1 region and an extended carboxyl-terminal domain following the Cys-2 domain. All DKKs possess several potential sites for proteolytic cleavage by furin-type proteases, indicating that they may be subjected to post-translational modifications[114]. Figure 2A and B illustrate the differences between different DKKs.
Figure 2.
Domain structure and phylogenetic tree of human dickkopf proteins. A: Each dickkopf (DKK) contains two cysteine-rich domains, each of which is separated by a linker region of various lengths. The amino-terminal cysteine-rich domain (Cys-1) domains are unique to each DKK, whereas the carboxyl-terminal cysteine-rich domain (Cys-2) domains are conserved among all members of the DKK family; B: Phylogenetic tree of the DKK proteins. Amino acid sequences were aligned by the Clustal W program, and the phylogenetic tree was constructed by the neighbour joining method. The scale bar indicates the estimated number of substitutions per 50 amino acids. SP: Signal peptide.
Functions of DKKs on the Wnt/β-catenin pathway
Members of the DKK family differ not only in their structures but also in their mRNA expression in HCC and in their ability to modulate Wnt/β-catenin signalling. Table 3 summarizes these differences between members of the DKK family.
Table 3.
Differences between the DKK family members
| Property | DKK1 | DKK2 | DKK3 | DKK4 | Ref. |
| Variants | Unknown | Unknown | 2 | Unknown | [130] |
| Location of Cys-1 | 85-138 amino acids | 78-127 amino acids | 147-195 amino acids | 41-90 amino acids | [110] |
| Location of Cys-2 | 189-263 amino acids | 183-256 amino acids | 208-284 amino acids | 145-218 amino acids | [110] |
| Length of linker region (number of amino acids) | 50 | 56 | 12 | 54 | [110] |
| Receptor | LRP5/LRP6 | LRP5/LRP6 | Not identified | LRP5/LRP6 | [113] |
| Activity in Wnt/β-catenin signalling in HCC | Antagonist | Not reported | Not reported | Not reported | [126] |
| Expression in HCC tissues | Overexpressed | Reduced | Reduced | Not reported | [126,143] |
| Phenotype of knockout mice | Embryonic lethality | Viable, fertile, blindness, osteopaenia | Viable, fertile, hyperactive, increased IgM, natural killer cells and haematocrit levels | Not reported | [116,162-164] |
Cys-1: Amino-terminal cysteine-rich domain; Cys-2: Carboxyl-terminal cysteine-rich domain; HCC: Hepatocellular carcinoma; LRP: Lipoprotein receptor-related protein; DKK: Dickkopf.
DKK1 is the most studied member of the DKK family. It was originally identified as a head inducer when its mRNA was injected into Xenopus embryos[115]. Experiments inactivating Xenopus DKK1 with anti-DKK1 antibodies[115] and involving DKK1 knockout mice[116] show a lack of anterior head structures, highlighting its importance in head formation. DKK1 inhibits Wnt-induced stabilisation of β-catenin and two models have been proposed. The first model proposes that DKK1 binds to the extracellular domain of LRP5/LRP6 and prevents the formation of the FZD-Wnt-LRP5/LRP6 complex in response to Wnt, thereby attenuating Wnt activity[117]. The second model proposes that DKK1 inhibits Wnt signalling by inducing clathrin-dependent internalisation of LRP6[37]. However, contrary to the results of Yamamoto
et al[37], Blitzer et al[118] used mouse fibroblast cells to show that clathrin-dependent internalisation of LRP6 is required to propagate Wnt/β-catenin signalling, and disturbing this clathrin-mediated endocytosis blocks Wnt activity. These recent findings suggest that mechanisms of DKK1 antagonistic activity may vary in different cell types.
In addition to binding to LRP5/LRP6, DKK1 also binds to Kremen 1 (Krm1) and Krm2, which belong to another class of transmembrane receptors[119,120]. DKK1 binds to Krm1 and Krm2 with high affinity and this inhibits Wnt/β-catenin signalling[121]. Despite that, gene knockout studies in mice have shown that Krm1 and Krm2 are not universally required for DKK1-associated function[122]. It was demonstrated that DKK1 mutants are able to antagonize Wnt activity without binding to Krm1 or Krm2[123]. Therefore, Krms may not be essential for DKK1 function and further studies are needed to understand their involvement in Wnt/β-catenin signalling.
Among all DKKs, DKK4 demonstrates similar antagonistic activity towards the Wnt/β-catenin pathway as DKK1 by binding to LRP5/LRP6 and Krms[120]. While functioning upstream of the Wnt/β-catenin pathway, DKK1 and DKK4 are also downstream targets of the Wnt/β-catenin pathway, creating a negative feedback loop to regulate Wnt/β-catenin signalling[124,125]. However, this feedback mechanism is often abrogated in cancers including HCC[125,126].
Like DKK1 and DKK4, DKK2 also binds to LRP5/LRP6 and Krms. However, DKK2 may serve as an antagonist or an agonist to the Wnt/β-catenin pathway depending on cellular context. For instance, overexpression of DKK2 in 293 fibroblast cells results in Wnt/β-catenin pathway activation, whereas co-transfection of DKK2 and Krm2 in the same cell type inhibits this pathway[127]. To explain this phenomenon, Chen et al[128] identified YWTD β-propeller domains of LRP5/LRP6 as the docking sites for Cys-2 of mouse DKK2, while this Cys-2 domain also contains binding site for Krm1 and Krm2. Therefore, the expression of Krms serves as a switch for the dual role of DKK2 on the Wnt/β-catenin pathway.
Unlike other DKKs having a role in Wnt/β-catenin signalling by binding to LRP5/LRP6 and Krms, the receptor for DKK3 has not been identified and its effect on this pathway remains unclear. Earlier studies in Xenopus suggested that DKK3 is not involved in Wnt/β-catenin signalling[113,115], but recent studies have demonstrated that DKK3 is associated with a reduction in cytoplasmic and nuclear accumulation of β-catenin in Saos-2 osteosarcoma cells, lung cancer cells and cervical cancer cells[129-131]. Interestingly, Lee et al[130] identified β-TrCP as a binding partner to DKK3, and possibly DKK3 may reduce β-catenin levels via ubiquitination. Therefore, the potential role of DKK3 in Wnt/β-catenin pathway remains to be determined.
Since different DKKs exhibit different effects on Wnt/β-catenin activity, it will be important to study the mechanisms by which each member of the DKK family exerts its effect on the Wnt/β-catenin pathway. Furthermore, as reported by Yamamoto et al[37] and Blitzer et al[118], DKKs may have different mechanisms of action in different cell types. Additionally, sharing the same receptor may not imply exhibiting the same effect as in the case of DKK1 and DKK2. For instance, they both bind to LRP5/LRP6 and Krms, but DKK1 serves as an antagonist, whereas DKK2 serves as both an agonist and an antagonist to the Wnt/β-catenin pathway.
DKKs in HCC
As mentioned above, the Wnt/β-catenin pathway plays a critical role in HCC and not surprisingly, Wnt inhibitors, including DKKs, are involved. More research studies have been dedicated to studying the role of DKKs in HCC over the past few years.
DKK1
Several studies have reported overexpression of DKK1 in HCC cell lines and tissues[126,132,133], while Yu et al[126] was the first to demonstrate a correlation between DKK1 overexpression and cytoplasmic/nuclear β-catenin accumulation in HCC. It was demonstrated through in vitro assays that DKK1 failed to inhibit TCF-mediated transcriptional activity in HCC cells with cytoplasmic or nuclear β-catenin accumulation, suggesting an abrogation of the negative feedback loop of DKK1 in HCC. Survival analysis correlated overexpression of DKK1 with poor prognosis of HCC patients, and DKK1 was identified as an independent prognostic marker for overall survival and disease-free survival. The 5-year overall survival and disease-free survival rates for HCC patients overexpressing DKK1 (43.4% and 34.2%, respectively) were significantly lower than HCC patients with reduced DKK1 expression (59.3% and 55.2%, respectively). Although elevated levels of AFP remain the gold standard for screening HCC, there are, however, a subgroup of patients who have HCC and normal levels of AFP. When patients were stratified according to AFP levels, DKK1 overexpression demonstrated worse prognosis for AFP-normal HCC patients, suggesting that DKK1 may serve as a prognostic marker for this group of patients. Furthermore, HCC patients with high DKK1 and β-catenin expression also showed poor prognosis. The 5-year overall survival and disease-free survival rates were 66.0% and 59.8%, respectively, for HCC patients without DKK1 and β-catenin expression, and 46.0% and 18.0% for HCC patients with high DKK1 and high β-catenin expression[126]. This study highlights the important role of DKK1 in Wnt/β-catenin signalling in HCC.
Other than total AFP levels, lens culinaris agglutinin–reactive AFP (AFP-L3) and prothrombin induced by vitamin K absence-II (PIVKA-II) have been reported as tumour markers for HCC. High serum AFP-L3 levels have recently been reported as a prognostic marker even in HCC patients with low AFP levels[134]. Additionally, high serum levels of PIVKA-II have been associated with advanced HCC with portal vein invasion[135].
Because of its high expression in HCC tissues and its secretory nature, DKK1 is hypothesized to be present at high levels in the serum of HCC patients. However, there are no studies evaluating high DKK1 serum levels on HCC progression or prognosis and it would be important to conduct such a study to understand the clinical significance of DKK1 in HCC. On the other hand, high levels of DKK1 in patients’ serum are associated with poor prognosis in various cancers including oesophageal squamous cell carcinoma[136], lung cancer[137], breast cancer[138] and cervical cancer[138], suggesting that the serum level of DKK1 may also reflect the prognosis of HCC patients. In multiple myeloma, high DKK1 serum levels are associated with osteolytic bone lesions[139] and patients responding to anti-myeloma treatment show a decrease in DKK1 serum levels[140], suggesting the involvement of DKK1 in this aspect. Recently, Fulciniti et al[141] evaluated the effect of anti-DKK1 monoclonal antibody (BHQ880) in a multiple myeloma mouse model and found that it induced bone formation and inhibited tumour-induced osteolytic bone lesions. Like multiple myeloma, HCC is also osteolytic in nature with 20% of HCC patients having bone metastasis[142], making it important to assess the role of DKK1 in bone metastasis in HCC.
DKK2
In HCC, a higher level of DKK2 methylation was detected in HCC tissues compared to corresponding non-cancerous cirrhotic tissues[143], suggesting its role in hepatocarcinogenesis. Epigenetic silencing of DKK2 has also been reported in gastric cancer[144], oesophageal squamous cell carcinoma[145] and renal cell carcinoma[146]. In renal cell carcinoma, no significant relationship was found between DKK2 methylation and β-catenin expression[146]. Although the effect of DKK2 on Wnt/β-catenin signalling depends on the expression of LRP5/LRP6 and Krms, DKK2 expression has not been studied in the context of these molecules. Furthermore, there are currently no reports on the effect of DKK2 on Wnt/β-catenin signalling in HCC.
DKK3
There are few reports on the clinical significance of DKK3 in HCC[143,147]. Reduction in DKK3 expression is associated with increased frequency of methylation in HCC tissues compared to corresponding non-cancerous cirrhotic tissues, implying that DKK3 methylation may not be an early event in HCC, but may function in the progression of HCC. Furthermore, DKK3 methylation has been significantly associated with short progression-free survival in HCC patients[143]. The effect of DKK3 on Wnt/β-catenin signalling has not been reported in HCC, but reduced DKK3 expression has been shown to correlate with β-catenin accumulation in lung cancer[131]. Although there are no reports of DKK3 level in HCC serum, reduced DKK3 serum levels in ovarian cancer are associated with the presence of lymph node metastasis, and high DKK3 serum levels have been associated with large tumours in cervical cancer[148]. Interestingly, multigene methylation status of a combination of Wnt antagonist genes, including DKK3 and others, in the serum have been proposed as markers for diagnosis, staging and prognosis of renal cell carcinoma[149]. Although different Wnt antagonists may function differently, their cumulative silencing may serve as a molecular marker for cancer detection.
DKK4
DKK4 is least studied in cancer. It is located on chromosome 8p11.2-p11.1 and this chromosomal region experiences frequent loss of heterozygosity in HCC[150]. This may explain reduced expression of DKK4 in HCC cell lines without detection of DKK4 methylation[144]. Currently there are no reports on DKK4 expression in HCC tissues or its effect on Wnt/β-catenin signalling in HCC. Recently, Hirata et al[151] reported a reduction of Wnt target genes after ectopic expression of DKK4 in renal carcinoma-derived Caki cells. However, overexpression of DKK4 also resulted in activation of the JNK pathway and enhanced tumour growth in vivo. This suggests that DKK4 may be involved in pathways other than Wnt/β-catenin signalling.
POTENTIAL THERAPEUTIC TARGETS FOR WNT/β-CATENIN SIGNALLING IN HCC
Mounting evidence suggests the role of β-catenin stabilisation in promoting tumour proliferation in HCC patients[43,152]. Such observations make Wnt/β-catenin signalling an attractive target for cancer therapy. In line with this hypothesis, small molecule inhibitors have been developed to hinder Wnt/β-catenin signalling by disrupting protein-protein interactions of components of this pathway. These include the fungal derivatives, PKF115-584 and CGP049090, that function to impede the interaction between β-catenin and the TCF complex[153]. Emami et al[154] reported another molecule called ICG-001, which disrupts the interaction between β-catenin and CBP. More recently, pyrvinium pamoate, an anthelmintic drug, was shown to inhibit the Wnt/β-catenin pathway by allosteric activation of CKIα and subsequently increased levels of β-catenin destruction complex secondary to Axin stabilisation[155]. Therapeutic antibodies against Wnt1 and Wnt2 have also demonstrated Wnt/β-catenin signalling inhibition and suppression of tumour growth in vivo[156,157]. β-catenin suppression through chemoprophylaxis may offer another alternative therapy. R-Etodolac (enantiomer of the non-steroidal anti-inflammatory drug Etodolac) reduced proliferation and survival of two HCC cell lines (HepG2 and Hep3B) by decreasing the total and active forms (dephosphorylated at Ser33/Ser37/Thr41) of β-catenin[158]. Further studies will be important to access clinical implications of these potential targets in HCC.
PERSPECTIVES
Aberrant activation of Wnt/β-catenin signalling is an important event in HCC that leads to transcription of various target genes involved in carcinogenesis. It will be clinically relevant to target the Wnt/β-catenin pathway and regulate its activity. However, since the pathway can be aberrantly activated by the dysfunction of several genes, it is important to ensure that when one member is targeted the other functions of the same target, if any, are not disrupted. For instance, β-catenin is involved in the Wnt/β-catenin pathway and plays a critical role in cell-cell adhesion. Therefore, care should be taken in designing reagents for disruption of molecules with dual functions. For tumours with mutated β-catenin, one approach could be to utilize RNAi targeting mutant β-catenin for therapy, as this will target selectively for tumour cells, but not healthy cells. However, mutant β-catenin-negative tumours with aberrant Wnt/β-catenin signalling offer the greatest challenge as there could be a multitude of reasons for aberrant Wnt/β-catenin activation. Therefore, altering Wnt/β-catenin signalling may be advantageous for therapeutic means but there are caveats and limitations involved.
In line with these investigations, DKKs have been discovered over the past 12 years for their abilities to interfere in the Wnt/β-catenin pathway. Since then, much effort has been dedicated to exploring their functions and implications in liver development and disease. Different DKKs exhibit different expressions and functions in various cancers. Some important questions remain to be answered. For example, where are DKKs localised in HCC cells? Is their expression associated with cytoplasmic/nuclear β-catenin accumulation in HCC cells? Do DKKs have prognostic value in HCC patients? Do they exert their effects on both wild type and mutant β-catenin in HCC cells? Do DKKs degrade β-catenin via a ubiquitination-dependent mechanism? Are different DKKs involved in different stages of HCC development? What is the receptor for DKK3? Are DKKs redundant in their functions? Furthermore, the negative feedback mechanism of DKK1 also warrants further research. Although DKK1 inhibits Wnt/β-catenin signalling, its overexpression has no effect on HCC cells with cytoplasmic/nuclear β-catenin accumulation[126]. This may be suggestive of an abrogated negative feedback loop or it may also imply that the inhibitory effect of DKK1 is only functional until reaching a point of saturation beyond which it cannot exert its inhibitory effect. In summary, compared to the overwhelming body of evidence showing the functional role of Wnt/β-catenin signalling in HCC, the biological and physiological roles of DKKs in relation to this pathway remain to be discovered. DKKs provide a new avenue to explore and they add another level of complexity to the already complex Wnt/β-catenin signalling in HCC.
Footnotes
Peer reviewers: Steven A Rosenzweig, PhD, Professor, Department of Cell and Molecular Pharmacology and Experimental Therapeutics, Medical University of South Carolina, 173 Ashley Avenue, Charleston, SC 29425, United States; Eiji Miyoshi, MD, PhD, Professor, Department of Molecular Biochemistry and Clinical Investigation, Professor and Chairman, Osaka University Graduate School of Medicine, 565-0871 1-7 Yamada-oka Suita, Japan
S- Editor Tian L L- Editor Webster JR E- Editor Zheng XM
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Wands J. Hepatocellular carcinoma and sex. N Engl J Med. 2007;357:1974–1976. doi: 10.1056/NEJMcibr075652. [DOI] [PubMed] [Google Scholar]
- 3.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 5.Severi T, van Malenstein H, Verslype C, van Pelt JF. Tumor initiation and progression in hepatocellular carcinoma: risk factors, classification, and therapeutic targets. Acta Pharmacol Sin. 2010;31:1409–1420. doi: 10.1038/aps.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanwal F, Hoang T, Kramer JR, Asch SM, Goetz MB, Zeringue A, Richardson P, El-Serag HB. Increasing prevalence of HCC and cirrhosis in patients with chronic hepatitis C virus infection. Gastroenterology. 2011;140:1182–1188.e1. doi: 10.1053/j.gastro.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen VT, Law MG, Dore GJ. Hepatitis B-related hepatocellular carcinoma: epidemiological characteristics and disease burden. J Viral Hepat. 2009;16:453–463. doi: 10.1111/j.1365-2893.2009.01117.x. [DOI] [PubMed] [Google Scholar]
- 8.Kudo M, Han KH, Kokudo N, Cheng AL, Choi BI, Furuse J, Izumi N, Park JW, Poon RT, Sakamoto M. Liver Cancer Working Group report. Jpn J Clin Oncol. 2010;40 Suppl 1:i19–i27. doi: 10.1093/jjco/hyq123. [DOI] [PubMed] [Google Scholar]
- 9.Mazzaferro V, Chun YS, Poon RT, Schwartz ME, Yao FY, Marsh JW, Bhoori S, Lee SG. Liver transplantation for hepatocellular carcinoma. Ann Surg Oncol. 2008;15:1001–1007. doi: 10.1245/s10434-007-9559-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim H, Rhim H, Choi D, Lim HK, Kim YS, Lee WJ, Joh JW. Recurrence and treatment pattern in long-term survivors with hepatocellular carcinoma: a comparison between radiofrequency ablation and surgery as a first-line treatment. World J Surg. 2010;34:1881–1886. doi: 10.1007/s00268-010-0533-1. [DOI] [PubMed] [Google Scholar]
- 11.Vivarelli M, Guglielmi A, Ruzzenente A, Cucchetti A, Bellusci R, Cordiano C, Cavallari A. Surgical resection versus percutaneous radiofrequency ablation in the treatment of hepatocellular carcinoma on cirrhotic liver. Ann Surg. 2004;240:102–107. doi: 10.1097/01.sla.0000129672.51886.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ueno S, Sakoda M, Kubo F, Hiwatashi K, Tateno T, Baba Y, Hasegawa S, Tsubouchi H. Surgical resection versus radiofrequency ablation for small hepatocellular carcinomas within the Milan criteria. J Hepatobiliary Pancreat Surg. 2009;16:359–366. doi: 10.1007/s00534-009-0069-7. [DOI] [PubMed] [Google Scholar]
- 13.Simo KA, Sereika SE, Newton KN, Gerber DA. Laparoscopic-assisted microwave ablation for hepatocellular carcinoma: Safety and efficacy in comparison with radiofrequency ablation. J Surg Oncol. 2011;Epub ahead of print doi: 10.1002/jso.21933. [DOI] [PubMed] [Google Scholar]
- 14.Huppert P. Current concepts in transarterial chemoembolization of hepatocellular carcinoma. Abdom Imaging. 2011 doi: 10.1007/s00261-011-9755-4. [DOI] [PubMed] [Google Scholar]
- 15.Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology. 2010;52:762–773. doi: 10.1002/hep.23725. [DOI] [PubMed] [Google Scholar]
- 16.Caja L, Sancho P, Bertran E, Iglesias-Serret D, Gil J, Fabregat I. Overactivation of the MEK/ERK pathway in liver tumor cells confers resistance to TGF-{beta}-induced cell death through impairing up-regulation of the NADPH oxidase NOX4. Cancer Res. 2009;69:7595–7602. doi: 10.1158/0008-5472.CAN-09-1482. [DOI] [PubMed] [Google Scholar]
- 17.Calvisi DF, Wang C, Ho C, Ladu S, Lee SA, Mattu S, Destefanis G, Delogu S, Zimmermann A, Ericsson J, et al. Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling, promotes development of human hepatocellular carcinoma. Gastroenterology. 2011;140:1071–1083. doi: 10.1053/j.gastro.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang Q, Zhang Y, Beezhold KJ, Bhatia D, Zhao H, Chen J, Castranova V, Shi X, Chen F. Sustained JNK1 activation is associated with altered histone H3 methylations in human liver cancer. J Hepatol. 2009;50:323–333. doi: 10.1016/j.jhep.2008.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He Y, Zhang H, Yin J, Xie J, Tan X, Liu S, Zhang Q, Li C, Zhao J, Wang H, et al. IkappaBalpha gene promoter polymorphisms are associated with hepatocarcinogenesis in patients infected with hepatitis B virus genotype C. Carcinogenesis. 2009;30:1916–1922. doi: 10.1093/carcin/bgp226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whittaker S, Marais R, Zhu AX. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene. 2010;29:4989–5005. doi: 10.1038/onc.2010.236. [DOI] [PubMed] [Google Scholar]
- 21.Apte U, Thompson MD, Cui S, Liu B, Cieply B, Monga SP. Wnt/beta-catenin signaling mediates oval cell response in rodents. Hepatology. 2008;47:288–295. doi: 10.1002/hep.21973. [DOI] [PubMed] [Google Scholar]
- 22.Nejak-Bowen KN, Monga SP. Beta-catenin signaling, liver regeneration and hepatocellular cancer: sorting the good from the bad. Semin Cancer Biol. 2011;21:44–58. doi: 10.1016/j.semcancer.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez FJ. Role of beta-catenin in the adult liver. Hepatology. 2006;43:650–653. doi: 10.1002/hep.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsieh A, Kim HS, Lim SO, Yu DY, Jung G. Hepatitis B viral X protein interacts with tumor suppressor adenomatous polyposis coli to activate Wnt/β-catenin signaling. Cancer Lett. 2011;300:162–172. doi: 10.1016/j.canlet.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 25.Cui J, Zhou X, Liu Y, Tang Z, Romeih M. Wnt signaling in hepatocellular carcinoma: analysis of mutation and expression of beta-catenin, T-cell factor-4 and glycogen synthase kinase 3-beta genes. J Gastroenterol Hepatol. 2003;18:280–287. doi: 10.1046/j.1440-1746.2003.02973.x. [DOI] [PubMed] [Google Scholar]
- 26.Calvisi DF, Factor VM, Ladu S, Conner EA, Thorgeirsson SS. Disruption of beta-catenin pathway or genomic instability define two distinct categories of liver cancer in transgenic mice. Gastroenterology. 2004;126:1374–1386. doi: 10.1053/j.gastro.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 27.Kemler R. From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet. 1993;9:317–321. doi: 10.1016/0168-9525(93)90250-l. [DOI] [PubMed] [Google Scholar]
- 28.Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakanaka C. Phosphorylation and regulation of beta-catenin by casein kinase I epsilon. J Biochem. 2002;132:697–703. doi: 10.1093/oxfordjournals.jbchem.a003276. [DOI] [PubMed] [Google Scholar]
- 30.Orford K, Crockett C, Jensen JP, Weissman AM, Byers SW. Serine phosphorylation-regulated ubiquitination and degradation of beta-catenin. J Biol Chem. 1997;272:24735–24738. doi: 10.1074/jbc.272.40.24735. [DOI] [PubMed] [Google Scholar]
- 31.Maher MT, Mo R, Flozak AS, Peled ON, Gottardi CJ. Beta-catenin phosphorylated at serine 45 is spatially uncoupled from beta-catenin phosphorylated in the GSK3 domain: implications for signaling. PLoS One. 2010;5:e10184. doi: 10.1371/journal.pone.0010184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng X, Huang H, Tamai K, Zhang X, Harada Y, Yokota C, Almeida K, Wang J, Doble B, Woodgett J, et al. Initiation of Wnt signaling: control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development. 2008;135:367–375. doi: 10.1242/dev.013540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeng X, Tamai K, Doble B, Li S, Huang H, Habas R, Okamura H, Woodgett J, He X. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438:873–877. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brantjes H, Barker N, van Es J, Clevers H. TCF: Lady Justice casting the final verdict on the outcome of Wnt signalling. Biol Chem. 2002;383:255–261. doi: 10.1515/BC.2002.027. [DOI] [PubMed] [Google Scholar]
- 35.Daniels DL, Weis WI. Beta-catenin directly displaces Groucho/TLE repressors from Tcf/Lef in Wnt-mediated transcription activation. Nat Struct Mol Biol. 2005;12:364–371. doi: 10.1038/nsmb912. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto H, Komekado H, Kikuchi A. Caveolin is necessary for Wnt-3a-dependent internalization of LRP6 and accumulation of beta-catenin. Dev Cell. 2006;11:213–223. doi: 10.1016/j.devcel.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto H, Sakane H, Yamamoto H, Michiue T, Kikuchi A. Wnt3a and Dkk1 regulate distinct internalization pathways of LRP6 to tune the activation of beta-catenin signaling. Dev Cell. 2008;15:37–48. doi: 10.1016/j.devcel.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 38.Chairoungdua A, Smith DL, Pochard P, Hull M, Caplan MJ. Exosome release of β-catenin: a novel mechanism that antagonizes Wnt signaling. J Cell Biol. 2010;190:1079–1091. doi: 10.1083/jcb.201002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lotvall J, Valadi H. Cell to cell signalling via exosomes through esRNA. Cell Adh Migr. 2007;1:156–158. doi: 10.4161/cam.1.3.5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suzuki T, Yano H, Nakashima Y, Nakashima O, Kojiro M. Beta-catenin expression in hepatocellular carcinoma: a possible participation of beta-catenin in the dedifferentiation process. J Gastroenterol Hepatol. 2002;17:994–1000. doi: 10.1046/j.1440-1746.2002.02774.x. [DOI] [PubMed] [Google Scholar]
- 41.Wong CM, Fan ST, Ng IO. beta-Catenin mutation and overexpression in hepatocellular carcinoma: clinicopathologic and prognostic significance. Cancer. 2001;92:136–145. doi: 10.1002/1097-0142(20010701)92:1<136::aid-cncr1301>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 42.Huang H, Fujii H, Sankila A, Mahler-Araujo BM, Matsuda M, Cathomas G, Ohgaki H. Beta-catenin mutations are frequent in human hepatocellular carcinomas associated with hepatitis C virus infection. Am J Pathol. 1999;155:1795–1801. doi: 10.1016/s0002-9440(10)65496-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nhieu JT, Renard CA, Wei Y, Cherqui D, Zafrani ES, Buendia MA. Nuclear accumulation of mutated beta-catenin in hepatocellular carcinoma is associated with increased cell proliferation. Am J Pathol. 1999;155:703–710. doi: 10.1016/s0002-9440(10)65168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mao TL, Chu JS, Jeng YM, Lai PL, Hsu HC. Expression of mutant nuclear beta-catenin correlates with non-invasive hepatocellular carcinoma, absence of portal vein spread, and good prognosis. J Pathol. 2001;193:95–101. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH720>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 45.Inagawa S, Itabashi M, Adachi S, Kawamoto T, Hori M, Shimazaki J, Yoshimi F, Fukao K. Expression and prognostic roles of beta-catenin in hepatocellular carcinoma: correlation with tumor progression and postoperative survival. Clin Cancer Res. 2002;8:450–456. [PubMed] [Google Scholar]
- 46.Kondo Y, Kanai Y, Sakamoto M, Genda T, Mizokami M, Ueda R, Hirohashi S. Beta-catenin accumulation and mutation of exon 3 of the beta-catenin gene in hepatocellular carcinoma. Jpn J Cancer Res. 1999;90:1301–1309. doi: 10.1111/j.1349-7006.1999.tb00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cieply B, Zeng G, Proverbs-Singh T, Geller DA, Monga SP. Unique phenotype of hepatocellular cancers with exon-3 mutations in beta-catenin gene. Hepatology. 2009;49:821–831. doi: 10.1002/hep.22695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laurent-Puig P, Legoix P, Bluteau O, Belghiti J, Franco D, Binot F, Monges G, Thomas G, Bioulac-Sage P, Zucman-Rossi J. Genetic alterations associated with hepatocellular carcinomas define distinct pathways of hepatocarcinogenesis. Gastroenterology. 2001;120:1763–1773. doi: 10.1053/gast.2001.24798. [DOI] [PubMed] [Google Scholar]
- 49.Cadoret A, Ovejero C, Saadi-Kheddouci S, Souil E, Fabre M, Romagnolo B, Kahn A, Perret C. Hepatomegaly in transgenic mice expressing an oncogenic form of beta-catenin. Cancer Res. 2001;61:3245–3249. [PubMed] [Google Scholar]
- 50.Harada N, Miyoshi H, Murai N, Oshima H, Tamai Y, Oshima M, Taketo MM. Lack of tumorigenesis in the mouse liver after adenovirus-mediated expression of a dominant stable mutant of beta-catenin. Cancer Res. 2002;62:1971–1977. [PubMed] [Google Scholar]
- 51.Tan X, Apte U, Micsenyi A, Kotsagrelos E, Luo JH, Ranganathan S, Monga DK, Bell A, Michalopoulos GK, Monga SP. Epidermal growth factor receptor: a novel target of the Wnt/beta-catenin pathway in liver. Gastroenterology. 2005;129:285–302. doi: 10.1053/j.gastro.2005.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Colnot S, Decaens T, Niwa-Kawakita M, Godard C, Hamard G, Kahn A, Giovannini M, Perret C. Liver-targeted disruption of Apc in mice activates beta-catenin signaling and leads to hepatocellular carcinomas. Proc Natl Acad Sci U S A. 2004;101:17216–17221. doi: 10.1073/pnas.0404761101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prosperi JR, Becher KR, Willson TA, Collins MH, Witte DP, Goss KH. The APC tumor suppressor is required for epithelial integrity in the mouse mammary gland. J Cell Physiol. 2009;220:319–331. doi: 10.1002/jcp.21766. [DOI] [PubMed] [Google Scholar]
- 54.Qian J, Sarnaik AA, Bonney TM, Keirsey J, Combs KA, Steigerwald K, Acharya S, Behbehani GK, Barton MC, Lowy AM, et al. The APC tumor suppressor inhibits DNA replication by directly binding to DNA via its carboxyl terminus. Gastroenterology. 2008;135:152–162. doi: 10.1053/j.gastro.2008.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kawate S, Fukusato T, Ohwada S, Watanuki A, Morishita Y. Amplification of c-myc in hepatocellular carcinoma: correlation with clinicopathologic features, proliferative activity and p53 overexpression. Oncology. 1999;57:157–163. doi: 10.1159/000012024. [DOI] [PubMed] [Google Scholar]
- 56.Gotoh J, Obata M, Yoshie M, Kasai S, Ogawa K. Cyclin D1 over-expression correlates with beta-catenin activation, but not with H-ras mutations, and phosphorylation of Akt, GSK3 beta and ERK1/2 in mouse hepatic carcinogenesis. Carcinogenesis. 2003;24:435–442. doi: 10.1093/carcin/24.3.435. [DOI] [PubMed] [Google Scholar]
- 57.Chan KL, Guan XY, Ng IO. High-throughput tissue microarray analysis of c-myc activation in chronic liver diseases and hepatocellular carcinoma. Hum Pathol. 2004;35:1324–1331. doi: 10.1016/j.humpath.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 58.Sato Y, Itoh F, Hareyama M, Satoh M, Hinoda Y, Seto M, Ueda R, Imai K. Association of cyclin D1 expression with factors correlated with tumor progression in human hepatocellular carcinoma. J Gastroenterol. 1999;34:486–493. doi: 10.1007/s005350050301. [DOI] [PubMed] [Google Scholar]
- 59.Nishida N, Fukuda Y, Komeda T, Kita R, Sando T, Furukawa M, Amenomori M, Shibagaki I, Nakao K, Ikenaga M. Amplification and overexpression of the cyclin D1 gene in aggressive human hepatocellular carcinoma. Cancer Res. 1994;54:3107–3110. [PubMed] [Google Scholar]
- 60.Joo M, Kang YK, Kim MR, Lee HK, Jang JJ. Cyclin D1 overexpression in hepatocellular carcinoma. Liver. 2001;21:89–95. doi: 10.1034/j.1600-0676.2001.021002089.x. [DOI] [PubMed] [Google Scholar]
- 61.Ueta T, Ikeguchi M, Hirooka Y, Kaibara N, Terada T. Beta-catenin and cyclin D1 expression in human hepatocellular carcinoma. Oncol Rep. 2002;9:1197–1203. [PubMed] [Google Scholar]
- 62.de La Coste A, Romagnolo B, Billuart P, Renard CA, Buendia MA, Soubrane O, Fabre M, Chelly J, Beldjord C, Kahn A, et al. Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Natl Acad Sci U S A. 1998;95:8847–8851. doi: 10.1073/pnas.95.15.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cadoret A, Ovejero C, Terris B, Souil E, Lévy L, Lamers WH, Kitajewski J, Kahn A, Perret C. New targets of beta-catenin signaling in the liver are involved in the glutamine metabolism. Oncogene. 2002;21:8293–8301. doi: 10.1038/sj.onc.1206118. [DOI] [PubMed] [Google Scholar]
- 64.Yamamoto Y, Sakamoto M, Fujii G, Tsuiji H, Kenetaka K, Asaka M, Hirohashi S. Overexpression of orphan G-protein-coupled receptor, Gpr49, in human hepatocellular carcinomas with beta-catenin mutations. Hepatology. 2003;37:528–533. doi: 10.1053/jhep.2003.50029. [DOI] [PubMed] [Google Scholar]
- 65.Pang R, Yuen J, Yuen MF, Lai CL, Lee TK, Man K, Poon RT, Fan ST, Wong CM, Ng IO, et al. PIN1 overexpression and beta-catenin gene mutations are distinct oncogenic events in human hepatocellular carcinoma. Oncogene. 2004;23:4182–4186. doi: 10.1038/sj.onc.1207493. [DOI] [PubMed] [Google Scholar]
- 66.Nusse R, Varmus HE. Wnt genes. Cell. 1992;69:1073–1087. doi: 10.1016/0092-8674(92)90630-u. [DOI] [PubMed] [Google Scholar]
- 67.Moon RT, Brown JD, Torres M. WNTs modulate cell fate and behavior during vertebrate development. Trends Genet. 1997;13:157–162. doi: 10.1016/s0168-9525(97)01093-7. [DOI] [PubMed] [Google Scholar]
- 68.Kolligs FT, Bommer G, Göke B. Wnt/beta-catenin/tcf signaling: a critical pathway in gastrointestinal tumorigenesis. Digestion. 2002;66:131–144. doi: 10.1159/000066755. [DOI] [PubMed] [Google Scholar]
- 69.Lee HH, Uen YH, Tian YF, Sun CS, Sheu MJ, Kuo HT, Koay LB, Lin CY, Tzeng CC, Cheng CJ, et al. Wnt-1 protein as a prognostic biomarker for hepatitis B-related and hepatitis C-related hepatocellular carcinoma after surgery. Cancer Epidemiol Biomarkers Prev. 2009;18:1562–1569. doi: 10.1158/1055-9965.EPI-09-0039. [DOI] [PubMed] [Google Scholar]
- 70.Kim M, Lee HC, Tsedensodnom O, Hartley R, Lim YS, Yu E, Merle P, Wands JR. Functional interaction between Wnt3 and Frizzled-7 leads to activation of the Wnt/beta-catenin signaling pathway in hepatocellular carcinoma cells. J Hepatol. 2008;48:780–791. doi: 10.1016/j.jhep.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bengochea A, de Souza MM, Lefrançois L, Le Roux E, Galy O, Chemin I, Kim M, Wands JR, Trepo C, Hainaut P, et al. Common dysregulation of Wnt/Frizzled receptor elements in human hepatocellular carcinoma. Br J Cancer. 2008;99:143–150. doi: 10.1038/sj.bjc.6604422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Merle P, de la Monte S, Kim M, Herrmann M, Tanaka S, Von Dem Bussche A, Kew MC, Trepo C, Wands JR. Functional consequences of frizzled-7 receptor overexpression in human hepatocellular carcinoma. Gastroenterology. 2004;127:1110–1122. doi: 10.1053/j.gastro.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 73.Ban KC, Singh H, Krishnan R, Seow HF. GSK-3beta phosphorylation and alteration of beta-catenin in hepatocellular carcinoma. Cancer Lett. 2003;199:201–208. doi: 10.1016/s0304-3835(03)00421-x. [DOI] [PubMed] [Google Scholar]
- 74.Desbois-Mouthon C, Blivet-Van Eggelpoël MJ, Beurel E, Boissan M, Delélo R, Cadoret A, Capeau J. Dysregulation of glycogen synthase kinase-3beta signaling in hepatocellular carcinoma cells. Hepatology. 2002;36:1528–1536. doi: 10.1053/jhep.2002.37192. [DOI] [PubMed] [Google Scholar]
- 75.Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim YD, Park CH, Kim HS, Choi SK, Rew JS, Kim DY, Koh YS, Jeung KW, Lee KH, Lee JS, et al. Genetic alterations of Wnt signaling pathway-associated genes in hepatocellular carcinoma. J Gastroenterol Hepatol. 2008;23:110–118. doi: 10.1111/j.1440-1746.2007.05250.x. [DOI] [PubMed] [Google Scholar]
- 77.Taniguchi K, Roberts LR, Aderca IN, Dong X, Qian C, Murphy LM, Nagorney DM, Burgart LJ, Roche PC, Smith DI, et al. Mutational spectrum of beta-catenin, AXIN1, and AXIN2 in hepatocellular carcinomas and hepatoblastomas. Oncogene. 2002;21:4863–4871. doi: 10.1038/sj.onc.1205591. [DOI] [PubMed] [Google Scholar]
- 78.Satoh S, Daigo Y, Furukawa Y, Kato T, Miwa N, Nishiwaki T, Kawasoe T, Ishiguro H, Fujita M, Tokino T, et al. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat Genet. 2000;24:245–250. doi: 10.1038/73448. [DOI] [PubMed] [Google Scholar]
- 79.Zucman-Rossi J, Benhamouche S, Godard C, Boyault S, Grimber G, Balabaud C, Cunha AS, Bioulac-Sage P, Perret C. Differential effects of inactivated Axin1 and activated beta-catenin mutations in human hepatocellular carcinomas. Oncogene. 2007;26:774–780. doi: 10.1038/sj.onc.1209824. [DOI] [PubMed] [Google Scholar]
- 80.Um TH, Kim H, Oh BK, Kim MS, Kim KS, Jung G, Park YN. Aberrant CpG island hypermethylation in dysplastic nodules and early HCC of hepatitis B virus-related human multistep hepatocarcinogenesis. J Hepatol. 2011;54:939–947. doi: 10.1016/j.jhep.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 81.Kishida S, Yamamoto H, Hino S, Ikeda S, Kishida M, Kikuchi A. DIX domains of Dvl and axin are necessary for protein interactions and their ability to regulate beta-catenin stability. Mol Cell Biol. 1999;19:4414–4422. doi: 10.1128/mcb.19.6.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chan DW, Chan CY, Yam JW, Ching YP, Ng IO. Prickle-1 negatively regulates Wnt/beta-catenin pathway by promoting Dishevelled ubiquitination/degradation in liver cancer. Gastroenterology. 2006;131:1218–1227. doi: 10.1053/j.gastro.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 83.Yau TO, Chan CY, Chan KL, Lee MF, Wong CM, Fan ST, Ng IO. HDPR1, a novel inhibitor of the WNT/beta-catenin signaling, is frequently downregulated in hepatocellular carcinoma: involvement of methylation-mediated gene silencing. Oncogene. 2005;24:1607–1614. doi: 10.1038/sj.onc.1208340. [DOI] [PubMed] [Google Scholar]
- 84.Ryo A, Nakamura M, Wulf G, Liou YC, Lu KP. Pin1 regulates turnover and subcellular localization of beta-catenin by inhibiting its interaction with APC. Nat Cell Biol. 2001;3:793–801. doi: 10.1038/ncb0901-793. [DOI] [PubMed] [Google Scholar]
- 85.Waterman ML. Lymphoid enhancer factor/T cell factor expression in colorectal cancer. Cancer Metastasis Rev. 2004;23:41–52. doi: 10.1023/a:1025858928620. [DOI] [PubMed] [Google Scholar]
- 86.Van de Wetering M, Castrop J, Korinek V, Clevers H. Extensive alternative splicing and dual promoter usage generate Tcf-1 protein isoforms with differential transcription control properties. Mol Cell Biol. 1996;16:745–752. doi: 10.1128/mcb.16.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jiang Y, Zhou XD, Liu YK, Wu X, Huang XW. Association of hTcf-4 gene expression and mutation with clinicopathological characteristics of hepatocellular carcinoma. World J Gastroenterol. 2002;8:804–807. doi: 10.3748/wjg.v8.i5.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schmitt-Graeff A, Ertelt-Heitzmann V, Allgaier HP, Olschewski M, Nitschke R, Haxelmans S, Koelble K, Behrens J, Blum HE. Coordinated expression of cyclin D1 and LEF-1/TCF transcription factor is restricted to a subset of hepatocellular carcinoma. Liver Int. 2005;25:839–847. doi: 10.1111/j.1478-3231.2005.01069.x. [DOI] [PubMed] [Google Scholar]
- 89.Lee NP, Poon RT, Shek FH, Ng IO, Luk JM. Role of cadherin-17 in oncogenesis and potential therapeutic implications in hepatocellular carcinoma. Biochim Biophys Acta. 2010;1806:138–145. doi: 10.1016/j.bbcan.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 90.Wong BW, Luk JM, Ng IO, Hu MY, Liu KD, Fan ST. Identification of liver-intestine cadherin in hepatocellular carcinoma--a potential disease marker. Biochem Biophys Res Commun. 2003;311:618–624. doi: 10.1016/j.bbrc.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 91.Wang XQ, Luk JM, Leung PP, Wong BW, Stanbridge EJ, Fan ST. Alternative mRNA splicing of liver intestine-cadherin in hepatocellular carcinoma. Clin Cancer Res. 2005;11:483–489. [PubMed] [Google Scholar]
- 92.Wang XQ, Luk JM, Garcia-Barcelo M, Miao X, Leung PP, Ho DW, Cheung ST, Lam BY, Cheung CK, Wong AS, et al. Liver intestine-cadherin (CDH17) haplotype is associated with increased risk of hepatocellular carcinoma. Clin Cancer Res. 2006;12:5248–5252. doi: 10.1158/1078-0432.CCR-06-0558. [DOI] [PubMed] [Google Scholar]
- 93.Liu LX, Lee NP, Chan VW, Xue W, Zender L, Zhang C, Mao M, Dai H, Wang XL, Xu MZ, et al. Targeting cadherin-17 inactivates Wnt signaling and inhibits tumor growth in liver carcinoma. Hepatology. 2009;50:1453–1463. doi: 10.1002/hep.23143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zöller M. Tetraspanins: push and pull in suppressing and promoting metastasis. Nat Rev Cancer. 2009;9:40–55. doi: 10.1038/nrc2543. [DOI] [PubMed] [Google Scholar]
- 95.Kanetaka K, Sakamoto M, Yamamoto Y, Yamasaki S, Lanza F, Kanematsu T, Hirohashi S. Overexpression of tetraspanin CO-029 in hepatocellular carcinoma. J Hepatol. 2001;35:637–642. doi: 10.1016/s0168-8278(01)00183-0. [DOI] [PubMed] [Google Scholar]
- 96.Ji J, Wang XW. New kids on the block: diagnostic and prognostic microRNAs in hepatocellular carcinoma. Cancer Biol Ther. 2009;8:1686–1693. doi: 10.4161/cbt.8.18.8898. [DOI] [PubMed] [Google Scholar]
- 97.Boutz DR, Collins PJ, Suresh U, Lu M, Ramírez CM, Fernández-Hernando C, Huang Y, Abreu Rde S, Le SY, Shapiro BA, et al. Two-tiered approach identifies a network of cancer and liver disease-related genes regulated by miR-122. J Biol Chem. 2011;286:18066–18078. doi: 10.1074/jbc.M110.196451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Burchard J, Zhang C, Liu AM, Poon RT, Lee NP, Wong KF, Sham PC, Lam BY, Ferguson MD, Tokiwa G, et al. microRNA-122 as a regulator of mitochondrial metabolic gene network in hepatocellular carcinoma. Mol Syst Biol. 2010;6:402. doi: 10.1038/msb.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu AM, Zhang C, Burchard J, Fan ST, Wong KF, Dai H, Poon RT, Luk JM. Global regulation on microRNA in hepatitis B virus-associated hepatocellular carcinoma. OMICS. 2011;15:187–191. doi: 10.1089/omi.2010.0098. [DOI] [PubMed] [Google Scholar]
- 100.Pineau P, Volinia S, McJunkin K, Marchio A, Battiston C, Terris B, Mazzaferro V, Lowe SW, Croce CM, Dejean A. miR-221 overexpression contributes to liver tumorigenesis. Proc Natl Acad Sci U S A. 2010;107:264–269. doi: 10.1073/pnas.0907904107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wong CC, Wong CM, Tung EK, Au SL, Lee JM, Poon RT, Man K, Ng IO. The microRNA miR-139 suppresses metastasis and progression of hepatocellular carcinoma by down-regulating Rho-kinase 2. Gastroenterology. 2011;140:322–331. doi: 10.1053/j.gastro.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 102.Huang K, Zhang JX, Han L, You YP, Jiang T, Pu PY, Kang CS. MicroRNA roles in beta-catenin pathway. Mol Cancer. 2010;9:252. doi: 10.1186/1476-4598-9-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ji J, Yamashita T, Budhu A, Forgues M, Jia HL, Li C, Deng C, Wauthier E, Reid LM, Ye QH, et al. Identification of microRNA-181 by genome-wide screening as a critical player in EpCAM-positive hepatic cancer stem cells. Hepatology. 2009;50:472–480. doi: 10.1002/hep.22989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ladeiro Y, Couchy G, Balabaud C, Bioulac-Sage P, Pelletier L, Rebouissou S, Zucman-Rossi J. MicroRNA profiling in hepatocellular tumors is associated with clinical features and oncogene/tumor suppressor gene mutations. Hepatology. 2008;47:1955–1963. doi: 10.1002/hep.22256. [DOI] [PubMed] [Google Scholar]
- 105.Liu AM, Xu MZ, Chen J, Poon RT, Luk JM. Targeting YAP and Hippo signaling pathway in liver cancer. Expert Opin Ther Targets. 2010;14:855–868. doi: 10.1517/14728222.2010.499361. [DOI] [PubMed] [Google Scholar]
- 106.Varelas X, Miller BW, Sopko R, Song S, Gregorieff A, Fellouse FA, Sakuma R, Pawson T, Hunziker W, McNeill H, et al. The Hippo pathway regulates Wnt/beta-catenin signaling. Dev Cell. 2010;18:579–591. doi: 10.1016/j.devcel.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 107.Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, Martin JF. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–461. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xu MZ, Yao TJ, Lee NP, Ng IO, Chan YT, Zender L, Lowe SW, Poon RT, Luk JM. Yes-associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer. 2009;115:4576–4585. doi: 10.1002/cncr.24495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 110.Krupnik VE, Sharp JD, Jiang C, Robison K, Chickering TW, Amaravadi L, Brown DE, Guyot D, Mays G, Leiby K, et al. Functional and structural diversity of the human Dickkopf gene family. Gene. 1999;238:301–313. doi: 10.1016/s0378-1119(99)00365-0. [DOI] [PubMed] [Google Scholar]
- 111.Aravind L, Koonin EV. A colipase fold in the carboxy-terminal domain of the Wnt antagonists--the Dickkopfs. Curr Biol. 1998;8:R477–R478. doi: 10.1016/s0960-9822(98)70309-4. [DOI] [PubMed] [Google Scholar]
- 112.van Tilbeurgh H, Bezzine S, Cambillau C, Verger R, Carrière F. Colipase: structure and interaction with pancreatic lipase. Biochim Biophys Acta. 1999;1441:173–184. doi: 10.1016/s1388-1981(99)00149-3. [DOI] [PubMed] [Google Scholar]
- 113.Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25:7469–7481. doi: 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- 114.Nakayama K. Furin: a mammalian subtilisin/Kex2p-like endoprotease involved in processing of a wide variety of precursor proteins. Biochem J. 1997;327(Pt 3):625–635. doi: 10.1042/bj3270625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- 116.Mukhopadhyay M, Shtrom S, Rodriguez-Esteban C, Chen L, Tsukui T, Gomer L, Dorward DW, Glinka A, Grinberg A, Huang SP, et al. Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell. 2001;1:423–434. doi: 10.1016/s1534-5807(01)00041-7. [DOI] [PubMed] [Google Scholar]
- 117.Semënov MV, Tamai K, Brott BK, Kühl M, Sokol S, He X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol. 2001;11:951–961. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- 118.Blitzer JT, Nusse R. A critical role for endocytosis in Wnt signaling. BMC Cell Biol. 2006;7:28. doi: 10.1186/1471-2121-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nakamura T, Nakamura T, Matsumoto K. The functions and possible significance of Kremen as the gatekeeper of Wnt signalling in development and pathology. J Cell Mol Med. 2008;12:391–408. doi: 10.1111/j.1582-4934.2007.00201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM, Delius H, Hoppe D, Stannek P, Walter C, et al. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature. 2002;417:664–667. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- 121.Davidson G, Mao B, del Barco Barrantes I, Niehrs C. Kremen proteins interact with Dickkopf1 to regulate anteroposterior CNS patterning. Development. 2002;129:5587–5596. doi: 10.1242/dev.00154. [DOI] [PubMed] [Google Scholar]
- 122.Ellwanger K, Saito H, Clément-Lacroix P, Maltry N, Niedermeyer J, Lee WK, Baron R, Rawadi G, Westphal H, Niehrs C. Targeted disruption of the Wnt regulator Kremen induces limb defects and high bone density. Mol Cell Biol. 2008;28:4875–4882. doi: 10.1128/MCB.00222-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang K, Zhang Y, Li X, Chen L, Wang H, Wu J, Zheng J, Wu D. Characterization of the Kremen-binding site on Dkk1 and elucidation of the role of Kremen in Dkk-mediated Wnt antagonism. J Biol Chem. 2008;283:23371–23375. doi: 10.1074/jbc.M802376200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.González-Sancho JM, Aguilera O, García JM, Pendás-Franco N, Peña C, Cal S, García de Herreros A, Bonilla F, Muñoz A. The Wnt antagonist DICKKOPF-1 gene is a downstream target of beta-catenin/TCF and is downregulated in human colon cancer. Oncogene. 2005;24:1098–1103. doi: 10.1038/sj.onc.1208303. [DOI] [PubMed] [Google Scholar]
- 125.Pendás-Franco N, García JM, Peña C, Valle N, Pálmer HG, Heinäniemi M, Carlberg C, Jiménez B, Bonilla F, Muñoz A, et al. DICKKOPF-4 is induced by TCF/beta-catenin and upregulated in human colon cancer, promotes tumour cell invasion and angiogenesis and is repressed by 1alpha,25-dihydroxyvitamin D3. Oncogene. 2008;27:4467–4477. doi: 10.1038/onc.2008.88. [DOI] [PubMed] [Google Scholar]
- 126.Yu B, Yang X, Xu Y, Yao G, Shu H, Lin B, Hood L, Wang H, Yang S, Gu J, et al. Elevated expression of DKK1 is associated with cytoplasmic/nuclear beta-catenin accumulation and poor prognosis in hepatocellular carcinomas. J Hepatol. 2009;50:948–957. doi: 10.1016/j.jhep.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 127.Mao B, Niehrs C. Kremen2 modulates Dickkopf2 activity during Wnt/LRP6 signaling. Gene. 2003;302:179–183. doi: 10.1016/s0378-1119(02)01106-x. [DOI] [PubMed] [Google Scholar]
- 128.Chen L, Wang K, Shao Y, Huang J, Li X, Shan J, Wu D, Zheng JJ. Structural insight into the mechanisms of Wnt signaling antagonism by Dkk. J Biol Chem. 2008;283:23364–23370. doi: 10.1074/jbc.M802375200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hoang BH, Kubo T, Healey JH, Yang R, Nathan SS, Kolb EA, Mazza B, Meyers PA, Gorlick R. Dickkopf 3 inhibits invasion and motility of Saos-2 osteosarcoma cells by modulating the Wnt-beta-catenin pathway. Cancer Res. 2004;64:2734–2739. doi: 10.1158/0008-5472.can-03-1952. [DOI] [PubMed] [Google Scholar]
- 130.Lee EJ, Jo M, Rho SB, Park K, Yoo YN, Park J, Chae M, Zhang W, Lee JH. Dkk3, downregulated in cervical cancer, functions as a negative regulator of beta-catenin. Int J Cancer. 2009;124:287–297. doi: 10.1002/ijc.23913. [DOI] [PubMed] [Google Scholar]
- 131.Yue W, Sun Q, Dacic S, Landreneau RJ, Siegfried JM, Yu J, Zhang L. Downregulation of Dkk3 activates beta-catenin/TCF-4 signaling in lung cancer. Carcinogenesis. 2008;29:84–92. doi: 10.1093/carcin/bgm267. [DOI] [PubMed] [Google Scholar]
- 132.Patil MA, Chua MS, Pan KH, Lin R, Lih CJ, Cheung ST, Ho C, Li R, Fan ST, Cohen SN, et al. An integrated data analysis approach to characterize genes highly expressed in hepatocellular carcinoma. Oncogene. 2005;24:3737–3747. doi: 10.1038/sj.onc.1208479. [DOI] [PubMed] [Google Scholar]
- 133.Kwack MH, Hwang SY, Jang IS, Im SU, Kim JO, Kim MK, Kim JC, Sung YK. Analysis of cellular changes resulting from forced expression of Dickkopf-1 in hepatocellular carcinoma cells. Cancer Res Treat. 2007;39:30–36. doi: 10.4143/crt.2007.39.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nouso K, Kobayashi Y, Nakamura S, Kobayashi S, Takayama H, Toshimori J, Kuwaki K, Hagihara H, Onishi H, Miyake Y, et al. Prognostic importance of fucosylated alpha-fetoprotein in hepatocellular carcinoma patients with low alpha-fetoprotein. J Gastroenterol Hepatol. 2011;26:1195–1200. doi: 10.1111/j.1440-1746.2011.06720.x. [DOI] [PubMed] [Google Scholar]
- 135.Koike Y, Shiratori Y, Sato S, Obi S, Teratani T, Imamura M, Yoshida H, Shiina S, Omata M. Des-gamma-carboxy prothrombin as a useful predisposing factor for the development of portal venous invasion in patients with hepatocellular carcinoma: a prospective analysis of 227 patients. Cancer. 2001;91:561–569. doi: 10.1002/1097-0142(20010201)91:3<561::aid-cncr1035>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 136.Makino T, Yamasaki M, Takemasa I, Takeno A, Nakamura Y, Miyata H, Takiguchi S, Fujiwara Y, Matsuura N, Mori M, et al. Dickkopf-1 expression as a marker for predicting clinical outcome in esophageal squamous cell carcinoma. Ann Surg Oncol. 2009;16:2058–2064. doi: 10.1245/s10434-009-0476-7. [DOI] [PubMed] [Google Scholar]
- 137.Yamabuki T, Takano A, Hayama S, Ishikawa N, Kato T, Miyamoto M, Ito T, Ito H, Miyagi Y, Nakayama H, et al. Dikkopf-1 as a novel serologic and prognostic biomarker for lung and esophageal carcinomas. Cancer Res. 2007;67:2517–2525. doi: 10.1158/0008-5472.CAN-06-3369. [DOI] [PubMed] [Google Scholar]
- 138.Sato N, Yamabuki T, Takano A, Koinuma J, Aragaki M, Masuda K, Ishikawa N, Kohno N, Ito H, Miyamoto M, et al. Wnt inhibitor Dickkopf-1 as a target for passive cancer immunotherapy. Cancer Res. 2010;70:5326–5336. doi: 10.1158/0008-5472.CAN-09-3879. [DOI] [PubMed] [Google Scholar]
- 139.Kaiser M, Mieth M, Liebisch P, Oberländer R, Rademacher J, Jakob C, Kleeberg L, Fleissner C, Braendle E, Peters M, et al. Serum concentrations of DKK-1 correlate with the extent of bone disease in patients with multiple myeloma. Eur J Haematol. 2008;80:490–494. doi: 10.1111/j.1600-0609.2008.01065.x. [DOI] [PubMed] [Google Scholar]
- 140.Heider U, Kaiser M, Mieth M, Lamottke B, Rademacher J, Jakob C, Braendle E, Stover D, Sezer O. Serum concentrations of DKK-1 decrease in patients with multiple myeloma responding to anti-myeloma treatment. Eur J Haematol. 2009;82:31–38. doi: 10.1111/j.1600-0609.2008.01164.x. [DOI] [PubMed] [Google Scholar]
- 141.Fulciniti M, Tassone P, Hideshima T, Vallet S, Nanjappa P, Ettenberg SA, Shen Z, Patel N, Tai YT, Chauhan D, et al. Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood. 2009;114:371–379. doi: 10.1182/blood-2008-11-191577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Katyal S, Oliver JH, Peterson MS, Ferris JV, Carr BS, Baron RL. Extrahepatic metastases of hepatocellular carcinoma. Radiology. 2000;216:698–703. doi: 10.1148/radiology.216.3.r00se24698. [DOI] [PubMed] [Google Scholar]
- 143.Yang B, Du Z, Gao YT, Lou C, Zhang SG, Bai T, Wang YJ, Song WQ. Methylation of Dickkopf-3 as a prognostic factor in cirrhosis-related hepatocellular carcinoma. World J Gastroenterol. 2010;16:755–763. doi: 10.3748/wjg.v16.i6.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sato H, Suzuki H, Toyota M, Nojima M, Maruyama R, Sasaki S, Takagi H, Sogabe Y, Sasaki Y, Idogawa M, et al. Frequent epigenetic inactivation of DICKKOPF family genes in human gastrointestinal tumors. Carcinogenesis. 2007;28:2459–2466. doi: 10.1093/carcin/bgm178. [DOI] [PubMed] [Google Scholar]
- 145.Maehata T, Taniguchi H, Yamamoto H, Nosho K, Adachi Y, Miyamoto N, Miyamoto C, Akutsu N, Yamaoka S, Itoh F. Transcriptional silencing of Dickkopf gene family by CpG island hypermethylation in human gastrointestinal cancer. World J Gastroenterol. 2008;14:2702–2714. doi: 10.3748/wjg.14.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hirata H, Hinoda Y, Nakajima K, Kawamoto K, Kikuno N, Kawakami K, Yamamura S, Ueno K, Majid S, Saini S, et al. Wnt antagonist gene DKK2 is epigenetically silenced and inhibits renal cancer progression through apoptotic and cell cycle pathways. Clin Cancer Res. 2009;15:5678–5687. doi: 10.1158/1078-0432.CCR-09-0558. [DOI] [PubMed] [Google Scholar]
- 147.Ding Z, Qian YB, Zhu LX, Xiong QR. Promoter methylation and mRNA expression of DKK-3 and WIF-1 in hepatocellular carcinoma. World J Gastroenterol. 2009;15:2595–2601. doi: 10.3748/wjg.15.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jiang T, Huang L, Wang S, Zhang S. Clinical significance of serum Dkk-3 in patients with gynecological cancer. J Obstet Gynaecol Res. 2010;36:769–773. doi: 10.1111/j.1447-0756.2010.01234.x. [DOI] [PubMed] [Google Scholar]
- 149.Urakami S, Shiina H, Enokida H, Hirata H, Kawamoto K, Kawakami T, Kikuno N, Tanaka Y, Majid S, Nakagawa M, et al. Wnt antagonist family genes as biomarkers for diagnosis, staging, and prognosis of renal cell carcinoma using tumor and serum DNA. Clin Cancer Res. 2006;12:6989–6997. doi: 10.1158/1078-0432.CCR-06-1194. [DOI] [PubMed] [Google Scholar]
- 150.Pineau P, Nagai H, Prigent S, Wei Y, Gyapay G, Weissenbach J, Tiollais P, Buendia MA, Dejean A. Identification of three distinct regions of allelic deletions on the short arm of chromosome 8 in hepatocellular carcinoma. Oncogene. 1999;18:3127–3134. doi: 10.1038/sj.onc.1202648. [DOI] [PubMed] [Google Scholar]
- 151.Hirata H, Hinoda Y, Majid S, Chen Y, Zaman MS, Ueno K, Nakajima K, Tabatabai ZL, Ishii N, Dahiya R. DICKKOPF-4 activates the noncanonical c-Jun-NH2 kinase signaling pathway while inhibiting the Wnt-canonical pathway in human renal cell carcinoma. Cancer. 2011;117:1649–1660. doi: 10.1002/cncr.25666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Austinat M, Dunsch R, Wittekind C, Tannapfel A, Gebhardt R, Gaunitz F. Correlation between beta-catenin mutations and expression of Wnt-signaling target genes in hepatocellular carcinoma. Mol Cancer. 2008;7:21. doi: 10.1186/1476-4598-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lepourcelet M, Chen YN, France DS, Wang H, Crews P, Petersen F, Bruseo C, Wood AW, Shivdasani RA. Small-molecule antagonists of the oncogenic Tcf/beta-catenin protein complex. Cancer Cell. 2004;5:91–102. doi: 10.1016/s1535-6108(03)00334-9. [DOI] [PubMed] [Google Scholar]
- 154.Emami KH, Nguyen C, Ma H, Kim DH, Jeong KW, Eguchi M, Moon RT, Teo JL, Kim HY, Moon SH, et al. A small molecule inhibitor of beta-catenin/CREB-binding protein transcription [corrected] Proc Natl Acad Sci U S A. 2004;101:12682–12687. doi: 10.1073/pnas.0404875101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Thorne CA, Hanson AJ, Schneider J, Tahinci E, Orton D, Cselenyi CS, Jernigan KK, Meyers KC, Hang BI, Waterson AG, et al. Small-molecule inhibition of Wnt signaling through activation of casein kinase 1α. Nat Chem Biol. 2010;6:829–836. doi: 10.1038/nchembio.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.He B, You L, Uematsu K, Xu Z, Lee AY, Matsangou M, McCormick F, Jablons DM. A monoclonal antibody against Wnt-1 induces apoptosis in human cancer cells. Neoplasia. 2004;6:7–14. doi: 10.1016/s1476-5586(04)80048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.You L, He B, Xu Z, Uematsu K, Mazieres J, Fujii N, Mikami I, Reguart N, McIntosh JK, Kashani-Sabet M, et al. An anti-Wnt-2 monoclonal antibody induces apoptosis in malignant melanoma cells and inhibits tumor growth. Cancer Res. 2004;64:5385–5389. doi: 10.1158/0008-5472.CAN-04-1227. [DOI] [PubMed] [Google Scholar]
- 158.Behari J, Zeng G, Otruba W, Thompson MD, Muller P, Micsenyi A, Sekhon SS, Leoni L, Monga SP. R-Etodolac decreases beta-catenin levels along with survival and proliferation of hepatoma cells. J Hepatol. 2007;46:849–857. doi: 10.1016/j.jhep.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Prange W, Breuhahn K, Fischer F, Zilkens C, Pietsch T, Petmecky K, Eilers R, Dienes HP, Schirmacher P. Beta-catenin accumulation in the progression of human hepatocarcinogenesis correlates with loss of E-cadherin and accumulation of p53, but not with expression of conventional WNT-1 target genes. J Pathol. 2003;201:250–259. doi: 10.1002/path.1448. [DOI] [PubMed] [Google Scholar]
- 160.Ovejero C, Cavard C, Périanin A, Hakvoort T, Vermeulen J, Godard C, Fabre M, Chafey P, Suzuki K, Romagnolo B, et al. Identification of the leukocyte cell-derived chemotaxin 2 as a direct target gene of beta-catenin in the liver. Hepatology. 2004;40:167–176. doi: 10.1002/hep.20286. [DOI] [PubMed] [Google Scholar]
- 161.Cavard C, Terris B, Grimber G, Christa L, Audard V, Radenen-Bussiere B, Simon MT, Renard CA, Buendia MA, Perret C. Overexpression of regenerating islet-derived 1 alpha and 3 alpha genes in human primary liver tumors with beta-catenin mutations. Oncogene. 2006;25:599–608. doi: 10.1038/sj.onc.1208860. [DOI] [PubMed] [Google Scholar]
- 162.Mukhopadhyay M, Gorivodsky M, Shtrom S, Grinberg A, Niehrs C, Morasso MI, Westphal H. Dkk2 plays an essential role in the corneal fate of the ocular surface epithelium. Development. 2006;133:2149–2154. doi: 10.1242/dev.02381. [DOI] [PubMed] [Google Scholar]
- 163.Li X, Liu P, Liu W, Maye P, Zhang J, Zhang Y, Hurley M, Guo C, Boskey A, Sun L, et al. Dkk2 has a role in terminal osteoblast differentiation and mineralized matrix formation. Nat Genet. 2005;37:945–952. doi: 10.1038/ng1614. [DOI] [PubMed] [Google Scholar]
- 164.Barrantes Idel B, Montero-Pedrazuela A, Guadaño-Ferraz A, Obregon MJ, Martinez de Mena R, Gailus-Durner V, Fuchs H, Franz TJ, Kalaydjiev S, Klempt M, et al. Generation and characterization of dickkopf3 mutant mice. Mol Cell Biol. 2006;26:2317–2326. doi: 10.1128/MCB.26.6.2317-2326.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]