Abstract
Objective
There is overlap between an autistic and hyperactive-inattentive symptomatology when studied cross-sectionally. This study is the first to examine the longitudinal pattern of association between social-communication deficits and hyperactive-inattentive symptoms in the general population, from childhood through adolescence. We explored the interrelationship between trajectories of co-occurring symptoms, and sought evidence for shared prenatal/perinatal risk factors.
Method
Study participants were 5,383 singletons of white ethnicity from the Avon Longitudinal Study of Parents and Children (ALSPAC). Multiple measurements of hyperactive-inattentive traits (Strengths and Difficulties Questionnaire) and autistic social-communication impairment (Social Communication Disorder Checklist) were obtained between 4 and 17 years. Both traits and their trajectories were modeled in parallel using latent class growth analysis (LCGA). Trajectory membership was subsequently investigated with respect to prenatal/perinatal risk factors.
Results
LCGA analysis revealed two distinct social-communication trajectories (persistently impaired versus low-risk) and four hyperactive-inattentive trait trajectories (persistently impaired, intermediate, childhood-limited and low-risk). Autistic symptoms were more stable than those of attention-deficit/hyperactivity disorder (ADHD) behaviors, which showed greater variability. Trajectories for both traits were strongly but not reciprocally interlinked, such that the majority of children with a persistent hyperactive-inattentive symptomatology also showed persistent social-communication deficits but not vice versa. Shared predictors, especially for trajectories of persistent impairment, were maternal smoking during the first trimester, which included familial effects, and a teenage pregnancy.
Conclusions
Our longitudinal study reveals that a complex relationship exists between social-communication and hyperactive-inattentive traits. Patterns of association change over time, with corresponding implications for removing exclusivity criteria for ASD and ADHD, as proposed for DSM-5.
Key Words: social-communication trait, hyperactive-inattentive trait, maternal smoking, teenage pregnancy, ALSPAC
The expression of autism spectrum disorder (ASD) traits and attention-deficit/hyperactivity disorder (ADHD) traits in children from the general population is nonindependent.1-3 This is consistent with the high levels of comorbidity observed between ASD and ADHD, each of which is presumed to lie at the upper extreme of an underlying behavioral continuum.4,5 Children with ASD or pervasive developmental disorder (PDD) often have ADHD symptoms;6-11 recent reports indicate that 31% of children and adolescents with autism,6 45% with PDD–Not Otherwise Specified (NOS),7 and 28% to 53% with ASD8,11 meet ADHD criteria as outlined in the Diagnostic and Statistical Manual of Mental Disorders—4th edition (DSM-IV).12 Conversely, autistic symptoms are often found in children with ADHD,9,13,14 especially social interaction and communication impairments,9,13,14 but also repetitive behaviors.9 This phenotypic overlap is supported by family and twin research, which produced evidence for shared genetic influences between autistic and ADHD related symptoms,15 both throughout normal population variation1-3 and at the extreme.16,17
Little is known however, as to how the relationship between autistic and ADHD symptoms changes over time. Autistic traits18,19 and ASD diagnoses20 are in general persistent during the course of development, with DSM-IV–based diagnostic stability estimates ranging from 69 to 95%.21,22 In contrast, ADHD diagnoses23 and the expression of ADHD symptoms24 are more variable. There is some evidence for stability of ADHD diagnoses across time23,24 (DSM-IV–based diagnostic stability estimates, 15%–65%), especially for the combined hyperactive-impulsive/inattentive ADHD subtype.25 However, ADHD symptoms may decline with age,23,25 but may also increase during adolescence,24,26 suggesting heterogeneity in the underlying ADHD trajectories. The relationship between autistic and ADHD related symptoms may therefore vary over time, and some ADHD related trajectories might be more strongly related to autistic symptoms than others. Moreover, it is possible that, depending on this interrelationship, risk factors for both symptomatologies may indeed be shared.
Although genetic effects are strongly implicated in the co-development of ASD and ADHD traits, they do not account for all of their phenotypic covariation,1-3 implying that environmental risk factors could be common to both conditions. Support for this latter hypothesis comes from several epidemiological studies, which suggested the existence of overlapping prenatal/perinatal influences. Maternal immune activation by infections and maternal substance use during pregnancy, in particular maternal smoking, have been suggested as risk factors for both ADHD27-29 and autism.30,31 In addition, perinatal complications (such as being born prematurely or having a lower birth weight) may play a role in the development of both ADHD-related27,32 and autistic symptoms.33,34 Some research has also linked maternal age at birth to both symptomatologies, although it may reveal trait-specific risk patterns, as an advanced maternal age has been associated with autism33 and a younger maternal age with ADHD.32 Examining the influence of these risk factors upon jointly modeled autistic and hyperactive symptom trajectories with a view to identifying the antecedents of co-occurring symptoms will therefore facilitate the identification of common etiologies.
Using the Avon Longitudinal Study of Parents and Children (ALSPAC), a longitudinal population-based birth cohort, this work explored the developmental trajectories of autistic and ADHD traits in a general population sample. The selection of autistic symptoms focussed exclusively on the social-communication spectrum of ASD, which is likely to be etiologically distinct from the repetitive behavioral spectrum.35 Investigated ADHD symptoms comprised the entire ADHD triad, including symptoms of inattention, hyperactivity, and impulsivity.36 In the presented work, we a) interrogated the interrelationship between co-occurring social-communication and hyperactive-inattentive trait trajectories to explore links between trait-specific trajectory types; and b) investigated the evidence for shared prenatal/perinatal risk factors, in particular those that have been previously related to both social-communication and hyperactive-inattentive symptoms on an individual trait basis.
Method
Study Samples
ALSPAC is a population-based, longitudinal, pregnancy-ascertained birth cohort in southwest England with an estimated date of birth between April 1, 1991, and December 31, 1992;37 the initial cohort included 14,541 pregnancies and 13,971 children were alive at 12 months of age (>95% of white European origin). A detailed description of the cohort has been published previously.37 Ethical approval was obtained from the ALSPAC Law and Ethics Committee and the Local Research Ethics Committees. Eligible children for this analysis were white European singletons (12,627 of 13,971 children) with a total intelligence quotient (IQ) of ≥70 at 8 years of age (6,536 of 12,627 children had available intelligence scores, of which 6,418 were eligible). Children excluded because of lower cognitive functioning showed increased rates of both social-communication deficits (22%–31% between 8 and 17 years) and hyperactive-inattentive symptoms (18%–42% between 4 and 17 years, as defined below), and may share a common etiology primarily because of deficits in cognitive resources. Overall, ineligible white singleton children were more likely to have been born to mothers who had the following characteristics, when compared with eligible children: adolescent (<20 years; odds ratio [OR] 4.65, 95% confidence interval [95% CI] = 3.76–5.81); average (OR = 2.31, 95% CI = 2.12–2.50) or less than average education (OR = 2.31, 95% CI = 2.02–2.64), or in manual occupations (OR = 2.26, 95% CI = 2.05–2.50). As such ALSPAC, like other cohort studies, is prone to selective dropout, in particular with respect to socio-economic position. This may lead to an underestimation of the prevalence of a developmental trajectory, and will have an impact on power.38 However, a recent empirical study and simulations on the ALSPAC sample showed that this selective dropout only marginally affects regression models with respect to behavioral outcomes.38
Attrition rates among the 6,418 eligible children varied between 14.0% and 35.9% for social communication scores at 8 to 17 years of age, and between 11.2% and 36.1% for hyperactive-inattentive scores at 4 to 17 years of age. To further facilitate the identification of growth trajectories and the convergence of the complex statistical models (discussed below), all eligible individuals with more than 50% missing data for either social-communication or hyperactive-inattentive symptom scores were excluded, resulting in a total sample of 5,383 individuals (2,669 male and 2,714 female participants).
Measurement of Prenatal Risk Factors
The search for shared prenatal risk factors focused on the first trimester, as especially during this time-window risk factors for both autistic symptoms,30,31 reported during the earlier stages of pregnancy, and ADHD symptoms,27-29 reported throughout pregnancy, may overlap. Information on maternal substance use with respect to alcohol, tobacco (cigarette smoking) and cannabis use, influenza-like illnesses, and any infections was ascertained with questionnaires at 18 weeks of gestation (Supplement 1, available online).
Given the possibility that the effect of maternal smoking may manifest through familial influences,39,40 we also investigated the association with paternal smoking41 as part of a sensitivity analysis. Information on paternal (cigarette) smoking during early pregnancy was obtained with questionnaires at 18 weeks of gestation (Supplement 2, available online).
Measurement of Perinatal Information
Data on low birth weight, preterm birth, and maternal age at birth were collected at birth, and information on parity was ascertained with questionnaires at 18 weeks of gestation (Supplement 3, available online).
Measurement of Socio-Economic Position
Information on occupational social class and maternal education was obtained using questionnaires at 32 weeks of gestation (Supplement 4, available online).
Measurement of Social-Communication Traits
Social-communication skills were captured with the 12-item Social Communication Disorder Checklist (SCDC42; score range 0–24; Supplement 5, available online). The SCDC is a brief screening instrument of social reciprocity and verbal/nonverbal communication42 (age range 3–18 years) that has high sensitivity and specificity for autism43 with higher scores reflecting more social-communication deficits. Mother-reported SCDC scores for children and adolescents were assessed at 8, 11, 14, and 17 years of age, and all scores showed a high temporal stability (0.38<rho<0.58; Table S1, available online). For the presented work, high-scoring individuals were identified based on a cut-off at ≥9 scores, which has been previously shown to provide maximum discrimination between all PDD diagnoses and non-PDD diagnoses/normal comparisons.42
Measurement of Hyperactive-Inattentive Traits
The Strengths and Difficulties Questionnaire (SDQ)36 is a behavioral screening questionnaire (age range 4–16 years) with high reliability and validity with respect to the identification of a psychiatric diagnosis.44 The questionnaire assesses hyperactivity-inattention with a five-item subscale (score range 0–10), with higher scores indicating more behavioral problems (three hyperactive-impulsive and two inattentive items; Supplement 6, available online). Mother reports on their children's hyperactivity-inattention were obtained at 4, 7, 8, 10, 12, 13, and 17 years of age, and there was a high temporal stability among all symptom scores (0.34<rho<0.71; Table S2, available online). Pertinent to this analysis, high-scorers were identified using a cut-off at ≥7 scores, which is indicative of abnormal behavior.36
Sample characteristics for all outcome measurements, potential confounders and risk factors are given in Table S3, available online.
Statistical Analysis
The statistical analysis was carried out within two parts. Within the first part, trajectories of social-communication deficits and hyperactive-inattentive symptoms were modeled in parallel using latent class growth analysis (LCGA) as implemented within MPlus, v.6.1 (Muthén and Muthén, Los Angeles, CA). Similar to a growth mixture model (GMM), LCGA aims to identify a multinomial latent class variable, which corresponds to different underlying growth curve shapes of child behavior measured across multiple time points.45 In contrast to GMMs, however, all elements of the within-class covariance matrix of the growth factors are constrained to zero,45 and, as such, LCGA does not rely on the within-class normality assumption of random effects. LCGA allows the trajectory modeling of parallel outcomes with multiple measurements, including up to 7 repeated measures per trait as proposed within this study, which is computationally not yet feasible with GMM using dual-core Windows processors.
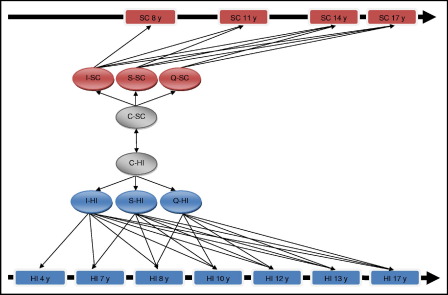
Using a parallel LCGA approach, trajectories for both social-communication and hyperactive-inattentive traits were each modeled using intercept, slope, and quadratic growth parameters. Specifically, a series of parallel LCGA models was fitted as a combination of one to three class models for social-communication traits and one to five class models (more classes did not provide stable model estimates) for hyperactive-inattentive traits. A graphical representation of the overall model structure is given in Figure 1. Missing data were accounted for through full information maximum likelihood. All models with two or more trajectories per trait were allowing for correlations between cross-trait trajectories through a log-linear link. As such social-communication trait trajectories and hyperactive-inattentive trait trajectories were modeled conditionally dependent on each other with the modeling of shared class membership being part of the same LCGA model. Posterior probabilities for combinations of social-communication and hyperactive-inattentive trait trajectories were based on joint posterior probabilities (i.e., the posterior probability of social-communication trait trajectories × the posterior probability of hyperactive-inattentive trait trajectories). The improvement in model fit was captured through the Bayesian information criterion (BIC), with lower BIC indicating a more parsimonious model fit. In addition, the model selection was guided through the evaluation of the model classification accuracy, with MPlus entropy values closer to 1 (range 0–1) indicating a higher precision. Sex-specific differences in developmental outcomes were captured as differences in trajectory proportions, i.e., including sex as a covariate in the regression equation. By contrast, models with sex-specific trajectories (allowing for sex-specific growth parameters) did not converge. Sex-specific analysis was eventually integrated within the regression models outlined below.
FIGURE 1.
Parallel latent class growth analysis model structure for social-communication and hyperactive-inattentive traits. Note: C = latent trajectory classes; HI = hyperactive-inattentive symptoms; I = intercept growth parameter; Q = quadratic growth parameter; S = slope growth parameter; SC = social-communication deficits; y = years (children's age at measurement).
Within the second part of the analysis, we used logistic and multinomial regression frameworks in STATA 11.1 (Stata Corporation, College Station, TX) to investigate the influence of potential risk factors on jointly modeled social-communication and hyperactive-inattentive trait trajectories respectively, using the best-fitting model from part 1. As MPlus-derived trajectory memberships are probabilistic, we generated 250 datasets in which assignments of trajectory classes for each individual were based on random draws from the distribution of joint posterior probabilities. The effect of each risk factor was studied using an individual regression model and adjusted for sex and potential confounders (i.e., social class and maternal education). Presented estimates were combined for each predictor across all datasets using the STATA mi command.
Results
Developmental Trajectories of Social-Communication and Hyperactive-Inattentive Traits
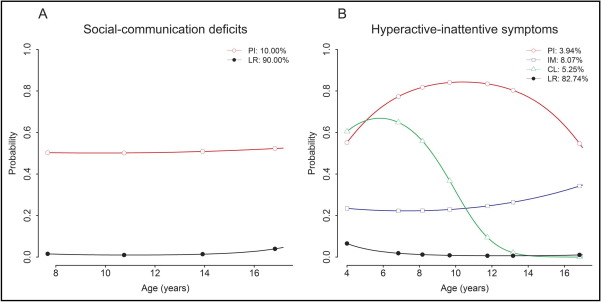
Parallel LCGA modeling showed that the most parsimonious model with the best BIC fit in combination with a high classification accuracy comprised two social-communication trait trajectories and four hyperactive-inattentive trait trajectories (Table S4, available online). Latent class trajectories for social-communication traits identified a persistently impaired group (10.00%) with a high probability of expressing deficits in social reciprocity and verbal/nonverbal communication throughout development, and a low-risk group (90.00%) (Figure 2A). ADHD-related developmental pathways during childhood and adolescence were described by four distinct trajectory classes (Figure 2B): 1) persistently impaired children with a high probability (probability >0.5) of expressing hyperactive-inattentive symptoms (3.94%); 2) children with an intermediate probability (0.2 < probability < 0.4) of expressing these symptoms (8.07%); 3) a group of children with a childhood-limited expression pattern of hyperactive-inattentive symptoms (5.25%); and 4) a low-risk group (82.75%). Joint probabilities for social-communication and hyperactive-inattentive trait trajectories revealed the tight interrelationship between both traits (Table 1). This link was strongest between the two persistently impaired groups, with respect to both magnitude and strength of the association (log-linear estimates and z-scores are detailed in Table 1). Most children of the persistently impaired social-communication group were either part of the persistently impaired hyperactive-inattentive group (32.29%) or the intermediate hyperactive-inattentive group (39.01%). Only a few of them fell into the childhood-limited hyperactive-inattentive (12.14%) or low-risk hyperactive-inattentive categories (16.56%). Children with a persistently high probability of hyperactive-inattentive symptoms by contrast were almost entirely included within the persistently impaired social communication group (82.04%). In addition, 48.30% children of the intermediate hyperactive-inattentive group, 23.09% children of the childhood-limited hyperactive-inattentive group, and 2.00% children of the low-risk hyperactive-inattentive group had an increased probability for persistent social-communication deficits (conditional probabilities are based on the log-linear estimates in Table 1). An adjustment for sex did not affect the observed trait interrelationships (Table S5, available online).
FIGURE 2.
Trajectories of social-communication deficits (A) and hyperactive-inattentive symptoms (B). Note: Each trait trajectory shows the probability of expressing social-communication deficits (A) or hyperactive-inattentive symptoms (B), with respect to the selected cut-off for high-scoring individuals. CL = childhood-limited; IM = intermediate; LR = low-risk; PI = persistently impaired.
TABLE 1.
Relationships Between Social-Communication and Hyperactive-Inattentive Trait Trajectories
| Log-linear Estimates β (SE) | ||||
|---|---|---|---|---|
| Hyperactive-Inattentive Trait Trajectories |
||||
| PIab | IMab | CLab | Interceptb | |
| Social-Communication Trait Trajectories | ||||
| PIab | 5.41 (0.36) (z = 14.87) |
3.82 (0.35) (z = 11.06) |
2.69 (0.42) (z = 6.38) |
−3.89 (0.28) |
| Intercepta | −4.74 (0.45) | −2.97 (0.25) | −3.00 (0.20) | |
| Joint Class Probabilities (%)c | |||||
|---|---|---|---|---|---|
| Hyperactive-Inattentive Trait Trajectories |
|||||
| PI | IM | CL | LR | Total | |
| Social-Communication Trait Trajectories | |||||
| PI | 3.23 | 3.90 | 1.21 | 1.66 | 10.00 |
| LR | 0.71 | 4.17 | 4.04 | 81.08 | 90.00 |
| Total | 3.94 | 8.07 | 5.25 | 82.74 | 100.00 |
Note: Log-linear estimates were derived from a parallel latent class growth analysis (LCGA) model linking hyperactive-inattentive trajectories with social-communication trajectories through jointly fitted multinomial and logit models. This provides identical estimates for β, but trait-specific intercepts. CL = childhood-limited; IM = intermediate; LR = low-risk; PI = persistently impaired; SE = standard error.
multinomial model (reference: hyperactive-inattentive low-risk group).
logit model (reference: social-communication low-risk group).
joint class probabilities were based on estimated posterior probabilities.
Predictors of Social-Communication and Hyperactive-Inattentive Trait Trajectories
The strongest predictor for both social-communication deficits and hyperactive-inattentive behavior was male sex (Table 2). This can be translated into sex-specific trajectory proportions: For hyperactive-inattentive trajectories, 5.92% of male participants were in the persistently impaired group, 9.64% in the intermediate group, 6.52% in the childhood-limited group, and 77.92% in the low-risk group. The proportions of these trajectories in females were 1.94%, 6.51%, 4.01%, and 87.54%, respectively. Furthermore, 12.74% of male participants versus 7.20% of female participants had persistently impaired social communication skills, whereas 87.26% of males versus 92.80% of females belonged to the social communication low-risk group. The strongest socio-economic predictor for both social-communication deficits and hyperactive-inattentive symptoms, especially for persistently impaired children, was a lower level of maternal education (Table 2).
TABLE 2.
Predictors of Social-Communication and Hyperactive-Inattentive Trait Trajectories
| Latent Trajectory Class | Social-Communicationg |
Hyperactive-Inattentiveg |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| PI |
PI |
IM |
CL |
Global |
|||||
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | p | |
| Sexa | |||||||||
| Male | 1.88 (1.53–2.32) | <.0001 | 3.43 (2.4–4.89) | <.0001 | 1.66 (1.3–2.12) | <.0001 | 1.82 (1.36–2.46) | <0.0001 | <0.0001 |
| Socio-economic positionb | |||||||||
| Nonmanual work | 1 (0.87–1.6) | .28 | 1.19 (0.73–1.93) | .48 | 1.33 (0.93–1.9) | .12 | 1.24 (0.79–1.95) | 0.34 | 0.32 |
| Materal education | |||||||||
| <O-levelc | 1.53 (1.08–2.17) | .017 | 1.93 (1.14–3.28) | .015 | 1.42 (0.92–2.2) | .12 | 1.44 (0.84–2.46) | 0.19 | 0.038 |
| O-levelc | 1.25 (1.01–1.55) | .038 | 1.47 (1.05–2.06) | .026 | 1.26 (0.97–1.63) | .084 | 1.34 (0.97–1.84) | 0.074 | 0.023 |
| Prenatal maternal risk factors (1st trimester)d | |||||||||
| Alcohol use | |||||||||
| ≥1 glass/wke | 1.28 (0.95–1.71) | .10 | 1.31 (0.84–2.05) | .23 | 1.02 (0.69–1.5) | .94 | 1.31 (0.84–2.05) | 0.24 | 0.48 |
| <1 glass/wke | 1.01 (0.81–1.28) | .91 | 1.04 (0.73–1.49) | .84 | 1.09 (0.83–1.42) | .54 | 1.34 (0.97–1.87) | 0.080 | 0.33 |
| Smoking | 1.45 (1.11–1.9) | .0063 | 1.95 (1.34–2.85) | .00052 | 1.31 (0.94–1.83) | .11 | 1.33 (0.91–1.96) | 0.15 | 0.0039 |
| Marijuana use | 1.25 (0.51–3.04) | .62 | NE | — | 1.72 (0.67–4.39) | .26 | 2.41 (0.97–5.98) | 0.057 | 0.20 |
| Influenza | 1.33 (0.94–1.88) | .11 | 1.30 (0.75–2.25) | .35 | 1.28 (0.83–1.96) | .26 | 1.20 (0.72–2.01) | 0.49 | 0.52 |
| Infections | 1.27 (0.99–1.61) | .058 | 1.27 (0.87–1.85) | .22 | 1.21 (0.9–1.62) | .21 | 1.09 (0.76–1.57) | 0.64 | 0.41 |
| Perinatal risk factorsd | |||||||||
| Low birth weight | 1.17 (0.68–2.01) | .57 | 1.53 (0.71–3.3) | 0.27 | 1.09 (0.53–2.24) | .81 | 1.69 (0.85–3.34) | 0.13 | 0.39 |
| Premature birth | 1.19 (0.76–1.88) | .45 | 1.44 (0.73–2.83) | 0.29 | 1.20 (0.68–2.11) | .54 | 1.69 (0.94–3.02) | 0.081 | 0.27 |
| Multiparous | 0.91 (0.74–1.12) | .38 | 0.85 (0.61–1.18) | 0.33 | 1.02 (0.79–1.32) | .89 | 0.99 (0.73–1.35) | 0.95 | 0.83 |
| Maternal age | |||||||||
| <20 yearsf | 2.16 (1.02–4.56) | 0.043 | 4.32 (1.77–10.54) | 0.0013 | 0.98 (0.24–3.93) | .97 | 1.54 (0.44–5.37) | 0.50 | 0.031 |
| >35 yearsf | 0.86 (0.62–1.20) | .37 | 0.94 (0.57–1.54) | 0.79 | 0.86 (0.58–1.28) | .44 | 0.76 (0.47–1.26) | 0.29 | 0.63 |
Note: Findings with p ≤ .05 are given in boldface. CL = childhood-limited; IM = intermediate; NE = not estimated; PI = persistently impaired.
Unadjusted.
Adjusted for sex.
Reference: >O-level.
Adjusted for sex, social class and maternal education.
Reference: never.
Reference: 20 to 35 years.
Reference: low-risk.
Shared predictors for both traits after accounting for the influences of sex and socio-economic position were maternal cigarette smoking during the first trimester and a teenage pregnancy (Table 2). For each predictor, the strongest relationships were observed with persistent social-communication deficits and persistent hyperactive-inattentive symptoms, respectively. Both risk factors are likely to act independently as the association was only marginally attenuated when their effects were controlled for each other (data not shown). Sensitivity analysis showed, however, that an adjustment for early paternal smoking during pregnancy41 weakened the association for maternal smoking with respect to both traits (Table 3), although a maternal risk effect for persistently impaired hyperactive-inattentive behavior was still present. On the other hand, there was no evidence for an association between paternal smoking and persistent behavioral problems independently of maternal smoking effects. To further characterize the influence of a teenage pregnancy with respect to social-communication deficits, children of the persistently impaired group were divided into individuals with and without co-occurring persistent hyperactive-inattentive behavior. This sensitivity analysis revealed that an adolescent pregnancy was a risk factor only for children with persistent social-communication deficits and persistent hyperactive-inattentive symptoms (OR = 4.57, 95% CI = 1.68–12.39; p = .003), but not for children with persistently impaired social-communication skills without a persistent hyperactive-inattentive symptomatology, i.e., in combination with an intermediate, childhood-limited, or low-risk hyperactive-inattentive profile (OR = 1.17, 95% CI = 0.29–4.68; p = .82; reference group: social-communication low-risk; data not shown).
TABLE 3.
Sensitivity Analysis for Exposure to Smoking During Early Pregnancy
| Latent Trajectory Class | Social-Communicationc |
Hyperactive-Inattentivec |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| PI |
PI |
IM |
CL |
Global | |||||
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | p | |
| Additional smoking predictor during early pregnancya | |||||||||
| Paternal smoking (18 wk) | 1.35 (1.08–1.70) | .0094 | 1.53 (1.08–2.15) | .016 | 1.22 (0.92–1.61) | .16 | 1.12 (0.81–1.55) | .51 | .076 |
| Adjusted analysisb | |||||||||
| Maternal smoking (first trimester) | 1.30 (0.98–1.74) | .072 | 1.67 (1.10–2.54) | .017 | 1.23 (0.86–1.78) | .26 | 1.33 (0.87–2.03) | .19 | .071 |
| Paternal smoking (18 wk) | 1.27 (0.99–1.61) | .057 | 1.32 (0.91–1.91) | .14 | 1.15 (0.86–1.55) | .35 | 1.04 (0.73–1.48) | .84 | .45 |
Note: Data with p ≤ .05 are in boldface. CL = childhood-limited; IM = intermediate; PI = persistently impaired; wk = weeks of gestation.
Adjusted for sex, social class, and maternal education.
In addition to a, paternal smoking (18 wk) and maternal smoking (first trimester) are adjusted for each other.
Reference: low-risk.
In addition, we observed a trend for an association between infections during the first trimester and persistent social communication deficits only (Table 2).
Discussion
Adopting a longitudinal perspective, this study observed strong links between co-occurring social-communication and hyperactive-inattentive traits in a general population sample. This developmental finding corroborates previous cross-sectional research.1-3,6-11,13-17 More importantly however, our findings reveal a novel temporal insight into the complexity of this trait interrelationship through the identification and association analysis of trait-specific developmental pathways. In particular, our longitudinal approach identified two social-communication domain related autistic trait trajectories (persistently impaired versus low-risk) and four hyperactive-inattentive trait trajectories (persistently impaired, intermediate, childhood-limited, and low-risk). This is consistent with the reported higher stability of autistic symptoms18-22 and the greater variability of ADHD-like behavior23,24,26 during the course of child development. Among the hyperactive-inattentive trajectories, the persistently impaired and childhood-limited groups each matched previous reports on stable ADHD symptoms23-25 and the decline of ADHD symptoms with progressing age,23,25 respectively. The hyperactive-inattentive intermediate trajectory with a trend for an increased probability of expressing symptoms during adolescence may also correspond to existing observations.24,26 Above all however, our study reported for the first time that the observed trait interrelationship between the most persistently impaired individuals is not reciprocal. Although the majority of children with persistently impaired social-communication skills were either part of the persistently impaired hyperactive-inattentive or the intermediate hyperactive-inattentive group, children with persistent hyperactive-inattentive symptoms were almost entirely subsumed within the persistently impaired social-communication group. In other words, almost all children who exhibited a high probability for persistent hyperactive-inattentive symptoms had also a high probability for persistent social communication deficits, but not vice versa. Interestingly, the ADHD combined subtype, based on both a DSM-IV diagnosis and population-derived latent classes, also had the highest stability across time25 and the highest autistic symptom scores46 in previous research.
The current DSM-IV-TR12 prohibits a diagnosis of ADHD when the symptoms occur during the course of a PDD, based on the rationale that these ADHD-related symptoms are primarily attributable to the autistic disorder. However, the strong trajectory links observed in our study, especially between the most persistently affected individuals, directly support the proposed revisions for the new DSM-5. These include changes with respect to the diagnostic ADHD criteria with ASD comorbidity, which will allow ASD and ADHD to be diagnosed in the same individual (http://www.dsm5.org/ProposedRevisions/). Our results may therefore be of clinical significance with respect to the refinement of the phenotypic ASD/ADHD overlap from a longitudinal perspective, and may indeed reflect the existence of a novel autistic/hyperactive-inattentive syndrome. This hypothesis finds general support through genetic analyses,15 including both general population traits1-3 and genetic studies of individuals with severe symptoms.16,17 Furthermore, several common genetic variants, such as recently identified ADHD and ASD genome-wide analysis signals,15 may have relevance for both conditions. Our findings are also consistent with the identification of clinical47 and distinct genetic17 subtypes of ADHD, with and without autistic symptoms. Latter may correspond to the observed weaker overlap between hyperactive-inattentive childhood-limited trajectories and persistently impaired social-communication skills. It is furthermore possible that children with a combined persistently impaired social-communication and hyperactive-inattentive phenotype may become more prominent clinically during the course of development, as other developmental pathways diverge. Thus, the hypothesis of a novel persistent autistic/hyperactive-inattentive phenotype could explain the previously reported increase in genetic correlation between autistic and ADHD traits with progressing age,3 rising from 0.23 to 0.26 in 2-year old children,3 to 0.54 to 0.57 during middle childhood2 to 0.72 in early adulthood.1
Finally, we also considered the hypothesis that the correlation between social-communication and hyperactive symptom trajectories could imply, beside genetic-factors, a nongenetic etiology. This is supported by our findings of shared prenatal/perinatal predictors for social-communication and hyperactive-inattentive trait trajectories, which included maternal tobacco smoking during the first trimester and a teenage pregnancy. These effects were most closely linked to the persistently impaired social-communication and persistently impaired hyperactive-inattentive trajectories.
Maternal smoking during pregnancy is a commonly reported predictor for both autistic and ADHD-related symptoms.27,29,30 In line with previous studies however, our results implied that the observed effect could to a considerable extent reflect familial influences,39,40 as we observed similar links between maternal and paternal smoking with respect to persistent behavioral problems (although we cannot exclude the possibility of passive smoking effects). Familial smoking effects may manifest as shared unaccounted social environmental but also as genetic factors,39-41 especially as paternal smoking has been suggested as a proxy for ADHD and/or smoking risk genes.48 On the other hand, maternal smoking was associated with persistent hyperactive-inattentive behavior independent of paternal smoking, and it is thus possible that some aspects of maternal smoking also influence as exposure in utero mental health across the life course.
The observed risk effects associated with a teenage pregnancy are compatible with previous reports linking younger maternal age and an ADHD symptomatology,32 but contrast with autism-associated risk and advanced maternal age at birth.33 Closer examination showed, however, that a teenage pregnancy was predominantly associated with persistent social-communication deficits in combination with persistent hyperactive-inattentive symptoms, but not without a persistent hyperactive-inattentive symptomatology.
In line with the observed trajectory interrelationships, the identification of shared predictors highlights therefore the possibility that a common comorbidity between social-communication and hyperactive-inattentive traits might only be inherent to specific trajectory combinations. On the other hand, children may vary in combinations of continuously distributed phenomena without the presence of specific endophenotypes. Testing the hypothesis of an underlying autistic/hyperactive-inattentive syndrome will require the adoption of a new research perspective, which understands social-communication deficits and hyperactive-inattentive symptoms not only as correlated single traits but as part of a joint phenotype. Modeling the developmental pathways of such a presumed combined syndrome by identifying classes of conditionally independent joint social-communication and hyperactive-inattentive trajectories will then facilitate the identification of endophenotypic characteristics.
These findings must be interpreted within the context of potential limitations. First, measures of social-communication traits, hyperactive-inattentive traits, and prenatal risk factors were predominantly based on mother-report and may have contributed to a greater variance sharing. Second, autistic trajectories were explored only with respect to the social-communication domain, and it is possible that the repetitive behavioral spectrum of ASD has different developmental outcomes and relationships with co-occurring hyperactive-inattentive symptom trajectories. Likewise, the pattern of association might differ for symptoms of the ADHD combined type, compared with the predominantly inattentive or predominantly hyperactive-impulsive type,10 thus setting new targets for future research into linked trajectories of ASD and ADHD domains. Third, some of the assessed social-communication deficits may reflect social impairments that are the consequence of hyperactive-inattentive behavior itself. Concurrent validity analysis of the SCDC, however, convincingly demonstrated strong differences in SCDC scores between ASD groups and clinical control groups, including children with ADHD.42 Fourth, although we focused on the analysis of social-communication deficit and hyperactive-inattentive symptom trajectories using validated and standardized psychological instruments, and although the observed trait links were supported by independent genetic and observational research, we cannot exclude the possibility that other trait combinations may show similar trajectory associations in relation to the ASD domain. Finally, social-communication traits have been assessed only from age 8 years onward, and it is possible that earlier measures would have contributed to greater trajectory variability.
In summary, our study provided evidence for a strong interrelationship between social-communication and hyperactive-inattentive traits and their developmental pathways in childhood and adolescence, especially for persistently impaired children.
Acknowledgments
The authors are grateful to all of the families who took part in ALSPAC, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses. The authors also thank L. Muthén of Muthén and Muthén, Los Angeles, CA, for statistical advice on MPlus-related modeling. This publication is the work of the authors, who will serve as guarantors for the contents of the paper.
Footnotes
This work was supported by UK Medical Research Council grant 74882, Wellcome Trust grant 076467/Z05/z, and the University of Bristol, which provided core support for the Avon Longitudinal Study of Parents and Children (ALSPAC) and this work.
Disclosure: Drs. St. Pourcain, Mandy, Heron, Golding, Davey Smith, and Skuse report no biomedical financial interests or potential conflicts of interest.
This article is discussed in an editorial by Dr. Angela Reiersen on page 857.
Supplemental material cited in this article is available online.
Supplement 1: Assessment of Maternal Risk Factors During the First Trimester
Information on maternal tobacco use (cigarette smoking) was obtained with the question, “Did you smoke regularly at any of the following times?” and answered by mothers with respect to the first 3 months of the pregnancy. Maternal marijuana use during pregnancy was measured with the question, “How often did you smoke marijuana/grass/cannabis/ganja in the first 3 months of pregnancy?” (Response options: Every day, 2–4 times a week, Once a week, Less than once a week, Not at all). These response options were combined into No (Not at all) and Yes (Remaining options). The frequency of the mother's alcohol consumption was assessed with the question, “How often have you drunk alcoholic drinks?” with respect to the first 3 months of this pregnancy (Response options: Never, <1 glass per week, 1+ glasses per week, 1–2 glasses per day, 3–9 glasses per day, 10+ glasses per day). Because of their lower frequency, response options of 1–2 glasses per day, 3–9 glasses per day, and 10+ glasses per day were combined into 1+ glasses per week.
Information on influenza-like illnesses was measured with the question: “During this pregnancy so far have you had any of the following?” which was answered by mothers with respect to “Influenza in the first 3 months” (Response options: Yes or No). The presence of any infection was indicated if the mothers answered the above question with respect to any urinary infection, influenza, rubella, candida, genital herpes, or other infections during the first 3 months of pregnancy with Yes.
Supplement 2: Assessment of Additional Smoking Predictors
Mothers' partners were asked, “How many times per day did you smoke at the start of your partner's pregnancy?” (Response options: None, 1–4, 5–9, 10–14, 15–19, 20–24, 25–29, 30+). In addition, mothers were asked about their partner's smoking behaviour at 18 weeks of gestation (“Does your partner smoke?”). Information on paternal smoking from both questions was collapsed into presence or absence of smoking (Response options: Yes and No). Partners who were not the father of the child (or unsure) were excluded from the analysis (0.55%).
Supplement 3: Assessment of Perinatal Information
Information on parity (number of previous pregnancies resulting in a live birth or still-birth) was collected at 18 weeks of gestation and recoded into “Primiparous” and “Multiparous” mothers. Children's birth weight and gestational age was assessed at birth, and recoded into low birth weight (Yes, <2,500 g; No, ≥2,500 g) and preterm birth (Yes, <37 weeks of gestation; No, ≥37 weeks of gestation). Maternal age at birth was categorized with respect to a teenage pregnancy (<20 years), a normal age range (20–35 years), and an advanced maternal age (>35 years).
Supplement 4: Assessment of Socio-Economic Position
Data were collected from mothers and their partners at 32 weeks of gestation using questionnaires. Occupational social class was derived as the lower of either maternal or paternal social class, and dichotomized into “Nonmanual” (I, II, III nonmanual) and “Manual” (III manual, IV, V) work.1 Information on maternal education was available with respect to O-levels, which are school tests taken approximately at age 16 years in England, and categorized into “Below O-level” (basic school unfinished or some vocational training), “O-level” (minimum school qualifications), and “Above O-level” (higher school-based qualifications).
Supplement 5: Item Description of the Social Communication Disorder Checklist (SCDC)2
The SCDC contains both items that address specific autism spectrum disorder (ASD) symptoms of social impairment and that relate to difficulties with empathy and social insight, as well as other items that are related to the consequences of a lack of social-communicative skill. The former include questions about whether the child is “Aware of other people's feelings”; “Does not realize when others are upset or angry”; “Does not notice the effect of his/her behavior on other members of the family”; “Does not seem to understand social skills”; and “Does not pick up on body language.” The latter items comprise questions such as, “Behaviour often disrupts family life”, “Does not understand how to behave when out”, and “Does not realize if behavior is offensive to other people.”
Supplement 6: Description of Hyperactive-Inattentive Items on the Strengths and Difficulties Questionnaire (SDQ)3
The SDQ assesses hyperactivity-inattention with a five-item subscale (score range 0–10) comprising the following items: “Restless, overactive. Cannot stay still for long” (hyperactive-impulsive), “Constantly fidgeting or squirming” (hyperactive-impulsive), “Easily distracted, concentration wanders” (inattentive), “Thinks things out before acting” (hyperactive-impulsive, reverse coded), and “Sees tasks through to the end. Good attention span” (inattentive, reverse coded). These items are rated as “Not true” (0), “Somewhat true” (1), and “Certainly true” (2) and are added to a summary score. All five items have primary loadings on their intended factor, i.e. hyperactivity-inattention, with minimal cross-loadings on other factors measured by the SDQ.3,4
TABLE S1.
Temporal Stability of Social-Communication Deficits
| Age (y) | 8 | 11 | 14 | 17 |
|---|---|---|---|---|
| 8 | 1.00 | — | — | — |
| 11 | 0.58 | 1.00 | — | — |
| 14 | 0.49 | 0.58 | 1.00 | — |
| 17 | 0.38 | 0.45 | 0.55 | 1.00 |
Note: Temporal stability of social-communication deficits was assessed using Spearman's rank correlation coefficient. The analysis was based on a sample of eligible individuals with complete information on social-communication deficits across all time points (N = 3,511).
TABLE S2.
Temporal Stability of Hyperactive-Inattentive Symptoms
| Age (y) | 4 | 7 | 8 | 10 | 12 | 13 | 17 |
|---|---|---|---|---|---|---|---|
| 4 | 1.00 | — | — | — | — | — | — |
| 7 | 0.54 | 1.00 | — | — | — | — | — |
| 8 | 0.52 | 0.70 | 1.00 | — | — | — | — |
| 10 | 0.48 | 0.67 | 0.70 | 1.00 | — | — | — |
| 12 | 0.43 | 0.61 | 0.66 | 0.69 | 1.00 | — | — |
| 13 | 0.40 | 0.57 | 0.60 | 0.64 | 0.71 | 1.00 | — |
| 17 | 0.34 | 0.45 | 0.49 | 0.54 | 0.58 | 0.60 | 1.00 |
Note: Temporal stability of hyperactive-inattentive symptoms was assessed using Spearman's rank correlation coefficient. The analysis was based on a sample of eligible individuals with complete information on hyperactive-inattentive symptoms across all time points (N = 3,182).
TABLE S3.
Sample Characteristics (max N = 5,383)
| Trait | Males (N = 2,669) |
Females (N = 2,714) |
||||
|---|---|---|---|---|---|---|
| n (%) | Age, y | Total | n (%) | Age, y | Total | |
| Mean (SD) | Mean (SD) | |||||
| Social-communication deficits (High-scorer) | 220 (8.7) | 7.6 (0.1) | 2,535 | 110 (4.3) | 7.6 (0.1) | 2,567 |
| 188 (7.5) | 10.7 (0.1) | 2,511 | 97 (3.8) | 10.7 (0.1) | 2,545 | |
| 177 (7.6) | 13.9 (0.1) | 2,341 | 122 (5.1) | 13.9 (0.2) | 2,394 | |
| 161 (8.2) | 16.8 (0.4) | 1,953 | 179 (8.5) | 16.8 (0.4) | 2,105 | |
| 388 (15.3) | 4.0 (0.3) | 2,532 | 261 (10.2) | 4.0 (0.4) | 2,554 | |
| Hyperactive-inattentive symptoms (High-scorer) | 303 (12.1) | 6.8 (0.1) | 2,502 | 151 (6.0) | 6.8 (0.1) | 2,535 |
| 345 (14.1) | 8.2 (0.2) | 2,454 | 164 (6.6) | 8.2 (0.2) | 2,492 | |
| 222 (8.8) | 9.6 (0.1) | 2,518 | 109 (4.2) | 9.6 (0.1) | 2,566 | |
| 213 (8.9) | 11.7 (0.1) | 2,391 | 85 (3.5) | 11.7 (0.1) | 2,453 | |
| 203 (8.8) | 13.2 (0.2) | 2,319 | 89 (3.8) | 13.1 (0.2) | 2,365 | |
| 141 (7.2) | 16.8 (0.4) | 1,946 | 73 (3.5) | 16.8 (0.4) | 2,103 | |
| Socio-Economic Position | n (%) | Total | n (%) | Total | ||
|---|---|---|---|---|---|---|
| Nonmanual work | 2,244 (88.9) | 2,525 | 2,264 (87.4) | 2,591 | ||
| Maternal education | 2,627 | 2,669 | ||||
| < O-level | 220 (8.4) | 202 (7.5) | ||||
| O-level | 1,199 (45.6) | 1,211 (45.4) | ||||
| > O-level (R) | 1,208 (46.0) | 1,256 (47.1) |
| Prenatal Maternal Risk Factors (1st Trimester) | n (%) | Total | n (%) | Total | ||
|---|---|---|---|---|---|---|
| Alcohol use | 2635 | 2677 | ||||
| ≥1 glass/wk | >1128 (42.8) | >1168 (43.6) | ||||
| <1 glass/wk | 1089 (41.3) | 1116 (41.7) | ||||
| Never (R) | 418 (15.9) | 393 (14.7) | ||||
| Smoking | 432 (16.3) | 2645 | 409 (15.2) | 2686 | ||
| Marijuana use | 42 (1.6) | 2553 | 38 (1.5) | 2582 | ||
| Influenza | 208 (8.3) | 2507 | 238 (9.4) | 2529 | ||
| Infections | 577 (23) | 2507 | 595 (23.5) | 2529 |
| Perinatal Risk Factors | n (%) | Total | n (%) | Total | ||
|---|---|---|---|---|---|---|
| Low birth weight | >101 (3.8) | 2639 | >76 (2.8) | 2677 | ||
| Premature birth | 140 (5.2) | 2669 | 101 (3.7) | 2714 | ||
| Multiparous | 1372 (52.4) | 2619 | 1390 (52.5) | 2646 | ||
| Maternal age | 2669 | 2714 | ||||
| <20 years | 31 (1.2) | 35 (1.3) | ||||
| 20–35 years (R) | 2253 (84.4) | 2363 (87.1) | ||||
| >35 years | 385 (14.4) | 316 (11.6) |
Note: R = reference.
TABLE S4.
Parallel Latent Class Growth Analysis Model Fit Indices
| No. of Fitted Latent Class Trajectories(Social-Communication Traits, Hyperactive-Inattentive Traits) | LL | BIC | Entropy | No. of Parameters |
|---|---|---|---|---|
| 1,1a | −1,3999 | 28,049.52 | — | 6 |
| 1,2 | −12,236.5 | 24,576.07 | 0.904 | 12 |
| 1,3 | −12,140 | 24,417.53 | 0.859 | 16 |
| 1,4 | −12,094.8 | 24,361.43 | 0.866 | 20 |
| 1,5 | −12075.9 | 24,358.07 | 0.657 | 24 |
| 2,1 | −13,348.4 | 26,799.94 | 0.883 | 12 |
| 2,2 | −11,250.6 | 22,647.22 | 0.902 | 17 |
| 2,3 | −11,128.5 | 22,445.99 | 0.869 | 22 |
| 2,4 | −11,067 | 22,365.92 | 0.860 | 27 |
| 2,5 | −11,046.3 | 22,367.47 | 0.869 | 32 |
| 3,1 | −13,325.1 | 26,787.73 | 0.904 | 16 |
| 3,2 | −11,215.6 | 22,620.17 | 0.884 | 22 |
| 3,3 | −11,080.6 | 22,401.7 | 0.809 | 28 |
| 3,4b | −11,003.8 | 22,299.73 | 0.847 | 34 |
| 3,5 | NE | NE | NE | NE |
Note: Some data not estimated (NE) because of unstable model estimates (nonpositive definite first-order derivative product matrix when using 250 random starts). The selected latent class growth analysis (LCGA) model is indicated in boldface. BIC = Bayesian information criterion; LL = log-likelihood.
The 1,1 model is identical to a LCGA model with one class only.
Note that the 3,4 model had a lower BIC but also less classification accuracy compared with the more parsimonious 2,4 model.
TABLE S5.
Relationships Between Social-Communication and Hyperactive-Inattentive Trait Trajectories Adjusted for Sex
| Log-linear Estimates β (SE) | |||
|---|---|---|---|
| Hyperactive-Inattentive Trait Trajectories |
|||
| PIa,b | IMa,b | CLa,b | |
| Social-Communication Trait Trajectories | |||
| PIa,b | 5.56 (0.40) (z = 13.98) |
3.81 (0.0.35) (z = 11.05) |
2.81 (0.45) (z = 6.18) |
| Joint Class Probabilities (%)c | |||||
|---|---|---|---|---|---|
| Hyperactive-Inattentive Trait Trajectories |
|||||
| PI | IM | CL | LR | Total | |
| Social-Communication Trait Trajectories | |||||
| PI | 3.26 | 3.92 | 1.27 | 1.55 | 10.00 |
| LR | 0.70 | 4.66 | 4.13 | 80.51 | 90.00 |
| Total | 3.96 | 8.58 | 5.40 | 82.06 | 100.00 |
Note: Log-linear estimates were derived from a parallel latent class growth analysis (LCGA) model linking hyperactive-inattentive trajectories with social-communication trajectories through jointly fitted multinomial and logit models. This provides identical estimates for β, but trait-specific intercepts. CL = childhood-limited; IM = intermediate; LR = low-risk; PI = persistently impaired; SE = standard error.
multinomial model (reference: hyperactive-inattentive low-risk group).
logit model (reference: social-communication low-risk group).
joint class probabilities were based on estimated posterior probabilities.
References
- 1.Reiersen A.M., Constantino J.N., Grimmer M., Martin N.G., Todd R.D. Evidence for shared genetic influences on self-reported ADHD and autistic symptoms in young adult Australian twins. Twin Res Hum Genet. 2008;11:579–585. doi: 10.1375/twin.11.6.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ronald A., Simonoff E., Kuntsi J., Asherson P., Plomin R. Evidence for overlapping genetic influences on autistic and ADHD behaviors in a community twin sample. J Child Psychol Psychiatry. 2008;49:535–542. doi: 10.1111/j.1469-7610.2007.01857.x. [DOI] [PubMed] [Google Scholar]
- 3.Ronald A., Edelson L.R., Asherson P., Saudino K.J. Exploring the relationship between autistic-like traits and ADHD behaviors in early childhood: findings from a community twin study of 2-year-olds. J Abnorm Child Psychol. 2010;38:185–196. doi: 10.1007/s10802-009-9366-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Constantino J.N., Todd R.D. Intergenerational transmission of subthreshold autistic traits in the general population. Biol Psychiatry. 2005;57:655–660. doi: 10.1016/j.biopsych.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 5.Levy F., Hay D.A., McStephen M., Wood C., Waldman I. Attention-deficit hyperactivity disorder: a category or a continuum?: Genetic analysis of a large-scale twin study. J Am Acad Child Adolesc Psychiatry. 1997;36:737–744. doi: 10.1097/00004583-199706000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Leyfer O.T., Folstein S.E., Bacalman S. Comorbid psychiatric disorders in children with autism: interview development and rates of disorders. J Autism Dev Disord. 2006;36:849–861. doi: 10.1007/s10803-006-0123-0. [DOI] [PubMed] [Google Scholar]
- 7.de Bruin E.I., Ferdinand R.F., Meester S., de Nijs P.F.A., Verheij F. High rates of psychiatric co-morbidity in PDD-NOS. J Autism Dev Disord. 2007;37:877–886. doi: 10.1007/s10803-006-0215-x. [DOI] [PubMed] [Google Scholar]
- 8.Simonoff E., Pickles A., Charman T., Chandler S., Loucas T., Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry. 2008;47:921–929. doi: 10.1097/CHI.0b013e318179964f. [DOI] [PubMed] [Google Scholar]
- 9.Hattori J., Ogino T., Abiru K., Nakano K., Oka M., Ohtsuka Y. Are pervasive developmental disorders and attention-deficit/hyperactivity disorder distinct disorders? Brain Dev. 2006;28:371–374. doi: 10.1016/j.braindev.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Gadow K.D., DeVincent C.J., Pomeroy J. ADHD symptom subtypes in children with pervasive developmental disorder. J Autism Dev Disord. 2006;36:271–283. doi: 10.1007/s10803-005-0060-3. [DOI] [PubMed] [Google Scholar]
- 11.Sinzig J., Walter D., Doepfner M. Attention deficit/hyperactivity disorder in children and adolescents with autism spectrum disorder: symptom or syndrome? J Attention Disord. 2009;13:117–126. doi: 10.1177/1087054708326261. [DOI] [PubMed] [Google Scholar]
- 12.American Psychiatric Association . Text Revision, 4th Edition. American Psychiatric Association; Washington, DC: 2000. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 13.Santosh P., Mijovic A. Social impairment in hyperkinetic disorder. Eur Child Adolesc Psychiatry. 2004;13:141–150. doi: 10.1007/s00787-004-0372-4. [DOI] [PubMed] [Google Scholar]
- 14.Geurts H.M., Verté S., Oosterlaan J. Can the Children's Communication Checklist differentiate between children with autism, children with ADHD, and normal controls? J Child Psychol Psychiatry. 2004;45:1437–1453. doi: 10.1111/j.1469-7610.2004.00850.x. [DOI] [PubMed] [Google Scholar]
- 15.Rommelse N., Franke B., Geurts H., Hartman C., Buitelaar J. Shared heritability of attention-deficit/hyperactivity disorder and autism spectrum disorder. Eur Child Adolesc Psychiatry. 2010;19:281–295. doi: 10.1007/s00787-010-0092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lichtenstein P., Carlstrom E., Rastam M., Gillberg C., Anckarsater H. The genetics of autism spectrum disorders and related neuropsychiatric disorders in childhood. Am J Psychiatry. 2010;167:1357–1363. doi: 10.1176/appi.ajp.2010.10020223. [DOI] [PubMed] [Google Scholar]
- 17.Mulligan A., Anney R.J.L., O'Regan M. Autism symptoms in attention-deficit/hyperactivity disorder: a familial trait which correlates with conduct, oppositional defiant, language and motor disorders. J Autism Dev Disord. 2009;39:197–209. doi: 10.1007/s10803-008-0621-3. [DOI] [PubMed] [Google Scholar]
- 18.Constantino J.N., Abbacchi A.M., Lavesser P.D. Developmental course of autistic social impairment in males. Dev Psychopathol. 2009;21:127–138. doi: 10.1017/S095457940900008X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson E.B., Munir K., Munafò M.R., Hughes M., McCormick M.C., Koenen K.C. Stability of autistic traits in the general population: further evidence for a continuum of impairment. J Am Acad Child Adolesc Psychiatry. 2011;50:376–384. doi: 10.1016/j.jaac.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daniels A.M., Rosenberg R.E., Law J.K., Lord C., Kaufmann W.E., Law P.A. Stability of initial autism spectrum disorder diagnoses in community settings. J Autism Dev Disord. 2010;41:110–121. doi: 10.1007/s10803-010-1031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charman T., Taylor E., Drew A., Cockerill H., Brown J., Baird G. Outcome at 7 years of children diagnosed with autism at age 2: predictive validity of assessments conducted at 2 and 3 years of age and pattern of symptom change over time. J Child Psychol Psychiatry. 2005;46:500–513. doi: 10.1111/j.1469-7610.2004.00377.x. [DOI] [PubMed] [Google Scholar]
- 22.Lord C., Risi S., DiLavore P.S., Shulman C., Thurm A., Pickles A. Autism from 2 to 9 years of age. Arch Gen Psychiatry. 2006;63:694–701. doi: 10.1001/archpsyc.63.6.694. [DOI] [PubMed] [Google Scholar]
- 23.Faraone S., Biederman J., Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med. 2006;36(2):159–165. doi: 10.1017/S003329170500471X. [DOI] [PubMed] [Google Scholar]
- 24.Larsson J., Larsson H., Lichtenstein P. Genetic and environmental contributions to stability and change of ADHD symptoms between 8 and 13 years of age: a longitudinal twin study. J Am Acad Child Adolesc Psychiatry. 2004;43:1267–1275. doi: 10.1097/01.chi.0000135622.05219.bf. [DOI] [PubMed] [Google Scholar]
- 25.Lahey B.B., Pelham W.E., Loney J., Lee S.S., Willcutt E. Instability of the DSM-IV subtypes of ADHD from preschool through elementary school. Arch Gen Psychiatry. 2005;62:896–902. doi: 10.1001/archpsyc.62.8.896. [DOI] [PubMed] [Google Scholar]
- 26.Willoughby M.T., Curran P.J., Costello E.J., Angold A. Implications of early versus late onset of attention-deficit/hyperactivity disorder symptoms. J Am Acad Child Adolesc Psychiatry. 2000;39:1512–1519. doi: 10.1097/00004583-200012000-00013. [DOI] [PubMed] [Google Scholar]
- 27.Banerjee T., Middleton F., Faraone S. Environmental risk factors for attention-deficit hyperactivity disorder. Acta Paediatr. 2007;96:1269–1274. doi: 10.1111/j.1651-2227.2007.00430.x. [DOI] [PubMed] [Google Scholar]
- 28.Mann JR, McDermott S. Are maternal genitourinary infection and pre-eclampsia associated with ADHD in school aged children? [published online ahead of print September 13, 2010]. J Atten Disord. doi: 10.1177/1087054710370566. [DOI] [PubMed]
- 29.Froehlich T.E., Lanphear B.P., Auinger P. Association of tobacco and lead exposures with attention-deficit/hyperactivity disorder. Pediatrics. 2009;124:e1054–e1063. doi: 10.1542/peds.2009-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hultman C.M., Sparén P., Cnattingius S. Perinatal risk factors for infantile autism. Epidemiology. 2002;13:417–423. doi: 10.1097/00001648-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Atladóttir H.Ó., Thorsen P., Østergaard L. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord. 2010;40:1423–1430. doi: 10.1007/s10803-010-1006-y. [DOI] [PubMed] [Google Scholar]
- 32.Gustafsson P., Källén K. Perinatal, maternal, and fetal characteristics of children diagnosed with attention-deficit-hyperactivity disorder: results from a population-based study utilizing the Swedish Medical Birth Register. Dev Med Child Neurol. 2010;53:263–268. doi: 10.1111/j.1469-8749.2010.03820.x. [DOI] [PubMed] [Google Scholar]
- 33.Kolevzon A., Gross R., Reichenberg A. Prenatal and Perinatal Risk Factors for Autism: A Review and Integration of Findings. Arch Pediatr Adolesc Med. 2007;161(4):326–333. doi: 10.1001/archpedi.161.4.326. [DOI] [PubMed] [Google Scholar]
- 34.Larsson H., Eaton W., Madsrn K. Risk factors for autism: perinatal factors, parental psychiatric history, and socioeconomic status. Am J Epidemiol. 2005;161:916–925. doi: 10.1093/aje/kwi123. [DOI] [PubMed] [Google Scholar]
- 35.Mandy W., Skuse D. Research review: what is the association between the social-communication element of autism and repetitive interests, behaviours and activities? J Child Psychol Psychiatry. 2008;49:795–808. doi: 10.1111/j.1469-7610.2008.01911.x. [DOI] [PubMed] [Google Scholar]
- 36.Goodman R. The Strengths and Difficulties Questionnaire: a research note. J Child Psychol Psychiatry. 1997;38:581–586. doi: 10.1111/j.1469-7610.1997.tb01545.x. [DOI] [PubMed] [Google Scholar]
- 37.Golding J., Pembrey M., Jones R. ALSPAC—the Avon Longitudinal Study of Parents and Children: I. Study methodology. Paediatr Perinat Epidemiol. 2001;15:74–87. doi: 10.1046/j.1365-3016.2001.00325.x. [DOI] [PubMed] [Google Scholar]
- 38.Wolke D., Waylen A., Samara M. Selective drop-out in longitudinal studies and non-biased prediction of behaviour disorders. Br J Psychiatry. 2009;195:249–256. doi: 10.1192/bjp.bp.108.053751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thapar A., Rice F., Hay D. Prenatal smoking might not cause attention-deficit/hyperactivity disorder: evidence from a novel design. Biol Psychiatry. 2009;66:722–727. doi: 10.1016/j.biopsych.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Obel C, Olsen J, Henriksen TB, et al. Is maternal smoking during pregnancy a risk factor for hyperkinetic disorder? Findings from a sibling design [published online ahead of print March 18, 2011]. Int J Epidemiol. doi: 10.1093/ije/dyq185. [DOI] [PubMed]
- 41.Davey Smith G. Assessing intrauterine influences on offspring health outcomes: can epidemiological studies yield robust findings? Basic Clin Pharmacol Toxicol. 2008;102:245–256. doi: 10.1111/j.1742-7843.2007.00191.x. [DOI] [PubMed] [Google Scholar]
- 42.Skuse D., Mandy W., Scourfield J. Measuring autistic traits: heritability, reliability and validity of the Social and Communication Disorders Checklist. Br J Psychiatry. 2005;187:568–572. doi: 10.1192/bjp.187.6.568. [DOI] [PubMed] [Google Scholar]
- 43.Bolte S., Westerwald E., Holtmann M., Freitag C., Poustka F. Autistic traits and autism spectrum disorders: the clinical validity of two measures presuming a continuum of social communication skills. J Autism Dev Disord. 2011;41:66–72. doi: 10.1007/s10803-010-1024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goodman R., Ford T., Simmons H., Gatward R., Meltzer H. Using the Strengths and Difficulties Questionnaire (SDQ) to screen for child psychiatric disorders in a community sample. Br J Psychiatry. 2000;177:534–539. doi: 10.1192/bjp.177.6.534. [DOI] [PubMed] [Google Scholar]
- 45.Muthén B., Muthén L.K. Integrating person-centered and variable-centered analyses: growth mixture modeling with latent trajectory classes. Alcohol Clin Exp Res. 2000;24:882–891. [PubMed] [Google Scholar]
- 46.Reiersen A.M., Constantino J.N., Volk H.E., Todd R.D. Autistic traits in a population-based ADHD twin sample. J Child Psychol Psychiatry. 2007;48:464–472. doi: 10.1111/j.1469-7610.2006.01720.x. [DOI] [PubMed] [Google Scholar]
- 47.Grzadzinski R, Di Martino A, Brady E, et al. Examining autistic traits in children with ADHD: does the autism spectrum extend to ADHD? [published online ahead of print November 25, 2010]. J Autism Dev Disord. doi: 10.1007/s10803-010-1135-3. [DOI] [PMC free article] [PubMed]
- 48.Altink M.E., Slaats-Willemse D.I.E., Rommelse N.N.J. Effects of maternal and paternal smoking on attentional control in children with and without ADHD. Eur Child Adolesc Psychiatry. 2009;18:465–475. doi: 10.1007/s00787-009-0001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- 1.Dale A., Marsh C. Office for National Statistics; 1993. The 1991 Census User's Guide. [Google Scholar]
- 2.Skuse D., Mandy W., Scourfield J. Measuring autistic traits: heritability, reliability and validity of the Social and Communication Disorders Checklist. Br J Psychiatry. 2005;187:568–572. doi: 10.1192/bjp.187.6.568. [DOI] [PubMed] [Google Scholar]
- 3.Goodman R. The Strengths and Difficulties Questionnaire: a research note. J Child Psychol Psychiatry. 1997;38:581–586. doi: 10.1111/j.1469-7610.1997.tb01545.x. [DOI] [PubMed] [Google Scholar]
- 4.Goodman R. Psychometric properties of the Strengths and Difficulties Questionnaire. J Am Acad Child Psychiatry. 2001;40:1337–1345. doi: 10.1097/00004583-200111000-00015. [DOI] [PubMed] [Google Scholar]