Abstract
Uptake and compartmentation of reduced glutathione (GSH), oxidized glutathione (GSSG), and glutathione conjugates are important for many functions including sulfur transport, resistance against biotic and abiotic stresses, and developmental processes. Complementation of a yeast (Saccharomyces cerevisiae) mutant (hgt1) deficient in glutathione transport was used to characterize a glutathione transporter cDNA (OsGT1) from rice (Oryza sativa). The 2.58-kb full-length cDNA (AF393848, gi 27497095), which was obtained by screening of a cDNA library and 5′-rapid amplification of cDNA ends-polymerase chain reaction, contains an open reading frame encoding a 766-amino acid protein. Complementation of the hgt1 yeast mutant strain with the OsGT1 cDNA restored growth on a medium containing GSH as the sole sulfur source. The strain expressing OsGT1 mediated [3H]GSH uptake, and this uptake was significantly competed not only by unlabeled GSSG and GS conjugates but also by some amino acids and peptides, suggesting a wide substrate specificity. OsGT1 may be involved in the retrieval of GSSG, GS conjugates, and nitrogen-containing peptides from the cell wall.
Plants play a key role in the sulfur cycle because they are primary producers of organic sulfur. Together with S-methyl-Met (Bourgis et al., 1999), glutathione is one of the major forms of reduced sulfur in plants and other organisms (Leustek et al., 2000; Noctor et al., 2002). Glutathione is a tripeptide (γ-glutamyl-cysteinyl Gly) synthesized both in the cytosol and in the chloroplasts of plant cells, through the sequential action of γ-glutamyl Cys synthetase and glutathione synthetase. It plays numerous roles including storage and transport of reduced sulfur, control of sulfur assimilation, control of redox status, protection against biotic and abiotic stresses, protein folding, and in the cell cycle (May et al., 1998; Foyer et al., 2001).
Glutathione is a major form of long-distance transport of reduced sulfur, both in the xylem and in the phloem (Rennenberg et al., 1979; Herschbach et al., 2000). Split root experiments have shown that glutathione functions as a transported signal of plant sulfur status in oilseed rape (Brassica napus; Lappartient and Touraine, 1996; Lappartient et al., 1999). Sulfur deficiency of part of the root system induced ATP sulfurylase activity not only in the roots exposed to sulfur-deficient medium but also in those parts of the root that were normally fed with sulfate. In contrast, when parts of the roots were fed with reduced glutathione (GSH) or Cys instead of sulfate, the increase in sulfate uptake and ATP sulfurylase activity was prevented. However, use of buthionine sulfoximine, an inhibitor of γ-glutamyl-Cys synthetase, showed that Cys rather than GSH is the repressor signal in maize (Zea mays; Bolchi et al., 1999).
The intracellular medium is buffered in the reduced state by GSH. Upon oxidation, one GSH can react with another to produce the disulfide form (GSSG). GSH may be restored by NADPH-glutathione reductase and normally accounts for more than 90% of the total glutathione content (Noctor and Foyer, 1998). Participation of glutathione in the Halliwell-Asada cycle allows the destruction of hydrogen peroxide produced by oxidative stress (Kunert and Foyer, 1993). Various abiotic stresses (cold treatment, Esterbauer and Grill, 1978; drought and excess light, Schupp and Rennenberg, 1988; and variations in soil or atmospheric sulfur content, De Kok and Kuiper, 1986) induce oxidative stress and alter glutathione concentrations. In oat (Avena sativa) leaves, 1% or 2% of total cell glutathione leaks into the apoplast (Vanacker et al., 1999). Inoculation of barley (Hordeum vulgare) leaves with powdery mildew (Erysiphe graminus f. sp. hordei) leads to an increase in apoplastic GSH and modifications of the GSH to GSSG ratio. Glutathione may be involved in the induction of cell death responses (Vanacker et al., 1999), and GSH was suggested to promote the transcription of various genes related to defense reactions against fungi (Wingate et al., 1988).
Another important function of glutathione is the detoxification of heavy metals (Rauser, 1990; Salt and Rauser, 1995) and organic xenobiotics and the compartmentation of secondary metabolites (Martinoia et al., 1993; Lu et al., 1997, 1998; Rea et al., 1998; Tommasini et al., 1998).
Glutathione also participates in the control of flowering (Ogawa et al., 2001) and hair tip growth (Sanchez-Fernandez et al., 1997). It is required for the G1 → S phase transition of the cell cycle in roots, and the phenotype of the rml1 (rootmeristemless) mutant, lacking the first enzyme of glutathione biosynthesis, is relieved by supply of GSH, but not ascorbate, another antioxidant (Vernoux et al., 2000). Finally, the oxidation status of glutathione also may be involved in the aggregation of storage proteins in the endoplasmic reticulum of cereal seeds (Jung et al., 1997).
Transport and compartmentation are important for the various biological functions of glutathione, especially for recycling of the oxidized or conjugated forms. Although the biochemical and molecular basis of GS conjugate compartmentation into the vacuole have been extensively described (Rea et al., 1998), much less is known about the transporters mediating the uptake or efflux of glutathione across the plasma membrane, the plastid envelope, the mitochondrial envelope, or the endoplasmic reticulum membrane.
Experiments with leaf discs and protoplasts have characterized the glutathione uptake system in broad bean (Vicia faba) leaf tissues (Jamai et al., 1996). GSSG was taken up at about twice the rate of GSH. GSH uptake was inhibited by GSSG and GS conjugates; conversely, GSSG uptake was inhibited by GSH and GS conjugates. Various amino acids and peptides affected the transport of neither GSH nor GSSG. Altogether, the data suggested that GSH, GSSG, and GS conjugates may be absorbed by a common uptake system that differed from transporters for amino acids and for di- and tripeptides. Electrophysiological data and pH measurements indicated that glutathione uptake in leaf tissues was mediated with proton cotransport (Jamai et al., 1996).
Despite biochemical evidence for specific transport systems in other organisms, including bacteria (Sherrill and Fahey, 1998), yeasts (Lubkowitz et al., 1998), and mammalian cells (Iantomasi et al., 1997), the first successful identification of a glutathione transporter was only achieved recently in yeasts (Bourbouloux et al., 2000). A gene disruption strategy associated with studies of yeast growth on glutathione media and uptake assays with labeled glutathione unequivocally showed that the YJL212c open reading frame (ORF) from yeast (Saccharomyces cerevisiae) encodes a glutathione transporter. This transporter, called HGT1, exhibits high affinity for GSH, GSSG, and the glutathione-N-ethylmaleimide conjugate (GS-NEM). Independently, the same gene was identified to mediate uptake of tetra- and pentapeptides; therefore, it is also named OPT1 (oligopeptide transporter 1; Miyake et al., 2002). A close homolog of HGT1 in yeast, Ypr194c (OPT2), did not show any glutathione transport activity but had a peptide transport phenotype (Lubkowitz et al., 1998). No genes homologous to HGT1 were identified in animals, including Caenorhabditis elegans; however, HGT1 homologs are present in the Arabidopsis, rice (Oryza sativa), and cotton (Gossypium hirsutum) genomes. A recent report described a preliminary characterization of a glutathione transporter in Brassica juncea (Bogs et al., 2003). In the present paper, the hgt1 yeast mutant was used to characterize a rice transporter mediating uptake of GSH, GSSG, and GS conjugates.
RESULTS
Isolation of a Glutathione Transporter cDNA from Rice
A 2.58-kb cDNA containing a 30-bp 5′-untranslated region and a 217-bp 3′-untranslated region was isolated by screening a cDNA library from rice seedlings and 5′-RACE-PCR. Complete sequencing of this clone (called OsGT1, for rice glutathione transporter, accession no. AF393848, gi 27497095) indicates that it contains an ORF that encodes a protein of 766-amino acid residue, with a predicted molecular mass of 86.1 kD and a pI of 6.5. The cDNA contains a stop codon (TGA) upstream and in frame with the start ATG. The nucleotide sequence at the start of translation, 5′-AACCATGAT-3′, is a consensus sequence for plant translation start site (5′-AACAATGGC-3′).
Hydropathy analysis using the method of Kyte and Doolittle (1982) suggests the presence of 13 predicted transmembrane helices and an external localization for the N terminus (TMPRED, http://www.ch.embnet.org/software/TMPRED_form.html). However, 12 transmembrane helices were predicted by TMHMM services (http://www.cbs.dtu.dk/services/TMHMM), with both the N and C termini being external (data not shown).
Two partially sequenced rice expressed sequence tags (ESTs; accession no. D25093, 330 pb; and accession no. AU082160, 265 bp) were found to be identical to a fragment of OsGT1. The corresponding complete EST cDNA (R3139) was obtained from the Rice Genomic Program and completely sequenced. However, the EST (R3139) contains only about 1.6 kb of the 3′ end of the OsGT1 cDNA.
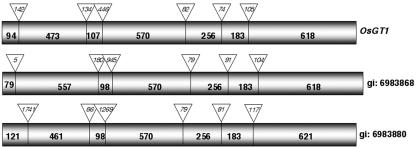
Alignment of the OsGT1 cDNA with sequences available from the rice genome, a yeast artificial chromosome (YAC)- and a phage artificial chromosome (PAC)-based rice transcript map (Wu et al., 2002) indicates that OsGT1 is located at 6 cM on the short arm of rice chromosome 6 and that there are three homologous copies on this PAC clone (accession no. AP001168). The ORF of the OsGT1 cDNA corresponds (100%) to the predicted ORF encoding protein BAA90804.1 (gi:6983869) on locus AP001168 (bacterial artificial chromosome clone P0425F02). The gene consists of seven exons and six introns (Fig. 1). The general organization of the other OsGT1 homologs is similar, with some differences concerning the lengths of intron 1 and exon 2 in the gene (gi: 6983868) encoding the protein BAA90803.1 and the lengths of introns 1 and 3 that were much longer in the gene (gi:6983880) encoding the protein BAA90815.1. Therefore, the latter gene (5.7 kb) is significantly longer than the two others (3.3 and 3.8 kb). At the amino acid level, similarities between OsGT1 and the two other cDNAs coding for the proteins BAA90803.1 and BAA90815.1 are 88.3% and 78.9%, respectively. Complex patterns obtained in Southern-blotting experiments confirm the existence of several homologs of OsGT1 in the rice genome (data not shown).
Figure 1.
Compared genomic structure of the OsGT1 homologs in rice. The relative positions and sizes of the introns and exons are indicated by triangles and boxes, respectively.
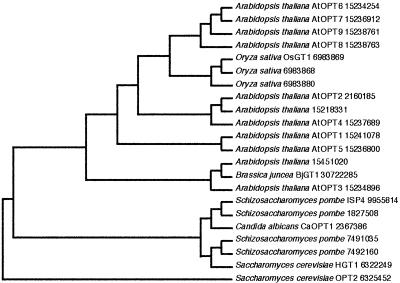
The amino acid sequences from OsGT1 and the two rice homologs were aligned with homologous sequences from different yeasts, Arabidopsis, and B. juncea, using the PAUP version 3.1 program (Sinauer Associates, Sunderland, MA; Fig. 2). In addition to HGT1 and its homologs in yeast and fission yeast (Schizosaccharomyces pombe), a BLAST search identified members of the AtOPT family and two proteins (accession nos. 15218331 and 15451020) from Arabidopsis as homologs of OsGT1. Although the AtOPT family from Arabidopsis was recently described as a tetra/pentapeptide transporter family (Koh et al., 2002), at least some members are able to mediate glutathione transport (O. Cagnac, A. Bourbouloux, M.Y. Zhang, V.C. Shrikanth, A.K. Bachhawat, and S. Delrot, unpublished data). Based on the deduced amino acid sequences, OsGT1 shows 27% to 88% similarity to other putative GSH transporters from rice, Arabidopsis, and yeasts. The plant sequences clearly cluster on a branch differing from the yeasts proteins. Among the plant sequences, the three rice genes are more closely related to AtOPT6-9 than to AtOPT1,4,5. Although BjGT1, a recently described glutathione transporter from B. juncea (Bogs et al., 2003), clusters with At 15451020 and AtOPT3, which is predicted to contain 10 transmembrane domains (http://aramemnon.botanik.uni-koeln.de), OsGT1 is closest to AtOPT7, which is predicted to contain 14 transmembrane domains. The predicted length of the OsGT1 protein is significantly longer than that of BjGT1 (766 versus 661 amino acids, respectively). Whether the pattern of clustering and the differences in molecular mass and in predicted topology reflects differences in transport properties will have to be investigated by functional assays.
Figure 2.
Phylogenetic analysis of glutathione and peptide transporter homologs from rice, Arabidopsis, yeasts, and Candida albicans. The acronyms indicate the function suggested by the experimental evidence published so far for glutathione (GT) or oligopeptide (OPT) transport. The most parsimonious tree was constructed by PAUP 3.1 using the protein sequences that are indicated (by accession nos.). Tree length, 3,856 steps; consistence, 0.75; retention index, 0.79. The YPR194 sequence was used as an outgroup.
Functional Characterization of OsGT1 in Yeast
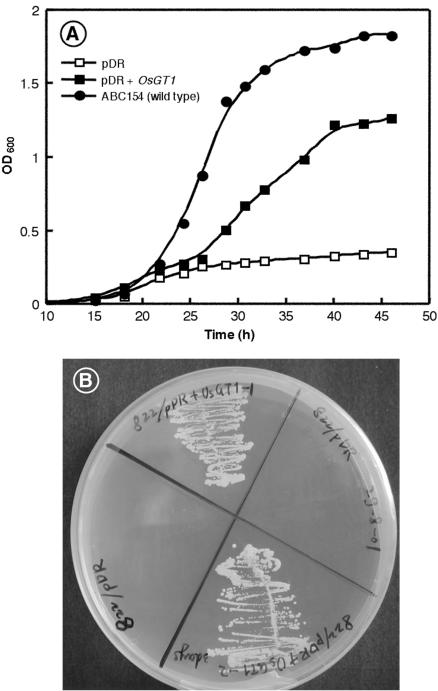
To investigate the function of the OsGT1 protein, a cDNA fragment containing the ORF (starting 10 nucleotides before the start codon) was amplified by PCR and inserted in the SmaI site of the vector pDR196, allowing expression under the control of the PMA1 promoter. This construct and the empty pDR196 vector were used to transform the yeast mutant ABC822, in which the HGT1 gene encoding the glutathione transporter is disrupted. This mutant has a low uptake capacity for glutathione and grows very poorly on a synthetic medium containing GSH as the sole sulfur source. Expression of OsGT1 restored growth of the hgt1 strain in liquid medium (Fig. 3A), and on a solid medium containing each 50 μm GSH as the sole sulfur source (Fig. 3B). The wild-type strain grew faster than the complemented strain under the same conditions, suggesting that the plant transporter may not be expressed at a high level and/or fully functional in yeast.
Figure 3.
Complementation of the ABC822 (hgt1Δ) strain by OsGT1. A, ABC822 strain transformed with either the empty vector pDR196 or the pDR+OsGT1 constructs was grown in synthetic dextrose (SD) medium to OD600 = 0.6, washed three times in cold sterile water, and diluted to OD600 = 0.001 in synthetic dextrose minus sulfur (SD-S) medium containing 50 μm GSH. Their growth was compared with that of the wild-type strain ABC154 under the same conditions. B, Compared growth of ABC822 strain after complementation by the empty vector pDR196 (left and right quarters) or pDR+OsGT1 (top and bottom quarters) on a medium containing 50 μm GSH as the sole sulfur source.
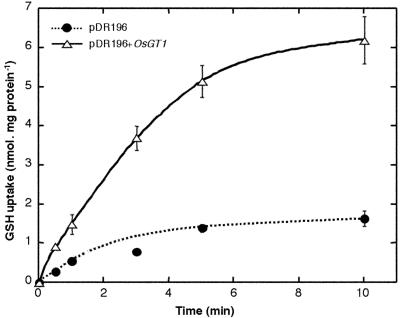
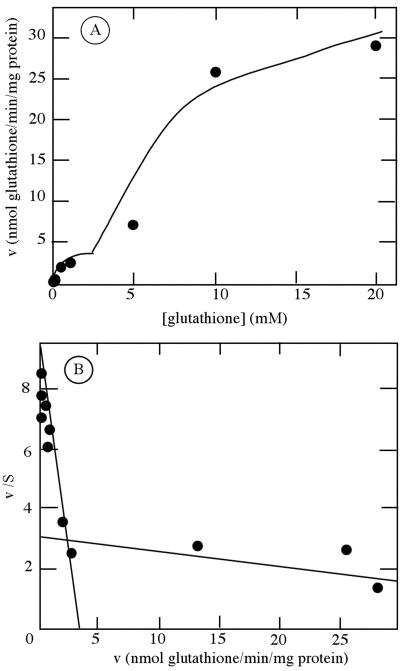
The ability of OsGT1 to mediate glutathione uptake was further checked by uptake measurements with [3H]glutathione. Under the conditions and during the incubation times used, no significant conversion of GSH to GSSG in the medium could be detected (data not shown). Expression of OsGT1 in the hgt1 mutant resulted in a strong increase in uptake of [3H]GSH compared with the strain transformed with the empty vector pDR196 (Fig. 4). Uptake activity was linear in the first 2 to 3 h and then slowly decreased. Therefore, in the following experiments, uptake activity was determined in the linear range of uptake. pH dependence studies showed that GSH uptake by the ABC822/pDR196+OsGT1 strain was maximal at pH 5.0 (data not shown). Uptake kinetics were studied at this pH by measuring the initial rates of uptake for external [3H]GSH concentration, ranging between 1 μm and 20 mm. Uptake kinetics mediated by OsGT1 did not obey simple Michaelis Menten kinetics even after subtraction of background uptake measured with the ABC822/pDR196 strain (Fig. 5A) Two saturable phases are apparent, and Eadie Hofstee plots (Fig. 5B) yield two straight lines corresponding to Km values of about 400 μm and 23 mm.
Figure 4.
Time course of [3H]GSH uptake by the ABC822 strain transformed with either the empty pDR196 vector or the pDR+OsGT1 construct in a medium containing 20 mm MES/KOH, 0.5 mm CaCl2, 0.25 mm MgCl2 (pH 5.0), and 100 μm [3H]GSH.
Figure 5.
Kinetic analysis of [3H]GSH uptake by yeast strain ABC822(hgt1) expressing OsGT1. Initial rates of uptake of GSH were measured with yeasts carrying either the pDR + OsGT1 construct or pDR alone. Background uptake measured with the strain carrying the empty plasmid was subtracted from uptake measured with the pDR + OsGT1 strain, and the data were plotted according to Michaelis Menten (A) and Eadie Hofstee (B). The latter representation yields straight lines of slope -1/Km and ordinal intercept v/Km. v is given is in nanomoles glutathione absorbed per minute and per milligram of protein.
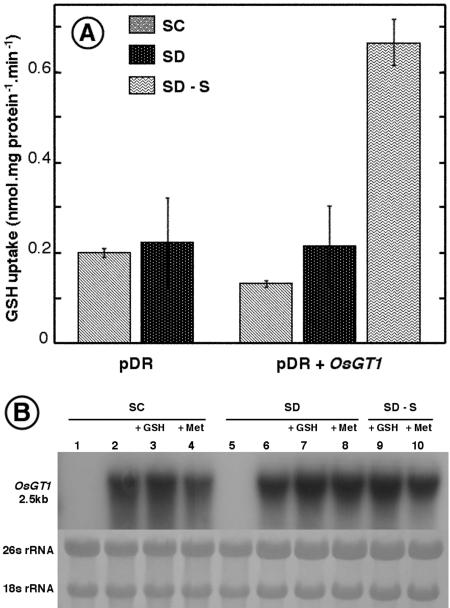
Interestingly, the glutathione uptake capacity seems to be strongly regulated by the sulfur content of the medium. Glutathione uptake capacity is very small in cells grown in synthetic complete (SC) medium containing ammonium sulfate and sulfur amino acids, whereas it is markedly enhanced when the only source of sulfur is glutathione (SD-S + GSH medium; Fig. 6A). Under the same conditions, the amounts of OsGT1 transcripts were not affected by the sulfur content of the medium (Fig. 6B). Altogether, these data suggest that glutathione transport activity in the yeast may be controlled by posttranscriptional processes.
Figure 6.
Effects of sulfur content on glutathione uptake and expression of OsGT1 in yeasts. A, GSH uptake by ABC822/pDR and ABC822/pDR+OsGT1 strains. The strains were grown in SC, SD, or SD-S media, all containing 100 μm GSH, and then transferred to the uptake medium described in Figure 6. B, Northern-blot analysis of OsGT1 expressed in the ABC822 strain grown in media differing by their sulfur content. Met and GSH were provided at 100 μm. Each lane was loaded with 10 μg of total RNA. The methylene blue-stained ribosomal bands are shown as a control for equal loading of the different lanes. All lanes were loaded with RNA from ABC822/pDR+OsGT1, except lanes 1 and 5 (RNA from ABC822/pDR).
Energy Requirement and Substrate Specificity of OsGT1 Expressed in Yeast
GSH uptake mediated by OsGT1 expression in yeast was strongly sensitive to low temperature (4°C) and to the protonophore carbonyl cyanide m-chlorophenylhydrazone (CCCP), indicating that the transport was an active process that may depend on the transmembrane pH gradient (Table I).
Table I.
Effect of low temperature and various compounds on GSH uptake in ABC822/pDR + OsGT1
The initial rates of uptake from a 50 μm [3H]-GSH solution were measured in the presence of various amino acids or peptides (500 μm). Results are the mean (±se) of 12 or 16 samples from three or four independent experiments.
| Treatment | GSH Uptake | % of Control |
|---|---|---|
| pmol mg protein−1min−1 | ||
| Control | 253 ± 12 | 100 |
| CCCP (10 μm) | 127 ± 40 | 50 |
| 4°C | 13 ± 10 | 5 |
| GSSG | 67 ± 11 | 27 |
| GS-NEM | 138 ± 14 | 55 |
| l-Glu | 140 ± 9 | 55 |
| Gly | 215 ± 10 | 85 |
| l-Gln | 83 ± 12 | 33 |
| l-Met | 102 ± 7 | 40 |
| l-Pro | 195 ± 11 | 77 |
| γ-Glu-Cys | 176 ± 13 | 69 |
| Gly-Gly | 192 ± 20 | 76 |
| Gly-Glu | 131 ± 15 | 52 |
| Leu-Leu | 219 ± 10 | 86 |
| Gly-Gly-Gly | 141 ± 14 | 55 |
| KLLLG | 144 ± 10 | 57 |
| AALLG | 208 ± 26 | 83 |
| YGGFL | 235 ± 28 | 93 |
To further analyze the transport characteristics of OsGT1, GSH uptake by the ABC822/pDR+OsGT1 strain was measured in the presence of a 10-fold excess of amino acids, peptides, or GSH derivatives. Uptake of GSH was significantly reduced by several compounds, i.e. by decreasing order of efficiency, GSSG, Gln, Met, Gly-Glu, and GS-NEM/l-Glu. The ability of OSGT1 to mediate Met uptake was directly tested by complementation of the yeast strains CD 150 (Matα, ade2, his3, leu2, trp1, ura3, and mup1) and CD152 (MATa, his3, leu2, ura3, ade2, trp1, mup1::HIS3, and mup2::LEU2), which are deficient for Met uptake (Isnard et al., 1996). No significant increase of Met uptake could be detected after complementation by the pDR196+OsGT1 construct (data not shown). Because OsGT1 exhibits significant sequence homology with the AtOPT transporter family, among which some members may transport Leuenkephalin (YGGFL; Koh et al., 2002), this compound and other pentapeptides were also tested in competition experiments. Various dipeptides or pentapeptides (Leu-Leu, Gly-Gly, YGGFL, and AALLG) did not compete significantly with GSH for uptake, whereas other peptides (Gly-Glu, Gly-Gly-Gly, and KLLLG) reduced GSH transport to some extent (Table I). Excess unlabeled Pro or adenine, taken as negative controls, did not affect GSH transport (data not shown).
Uptake of GS-NEM
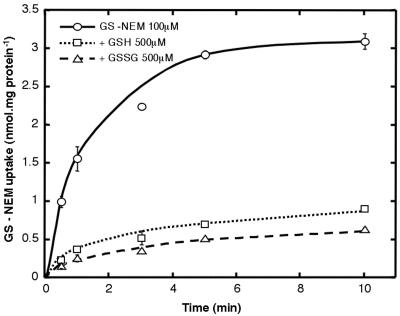
Competition by unlabeled compounds only gives indirect evidence that the substrate is actually taken up by a given transporter. More direct evidence that OsGT1 is able to mediate transport of glutathione conjugates was sought, therefore, by studying uptake of labeled GS-NEM. The ABC822/pDR+OsGT1 strain mediates transport of [3H] GS-NEM, and this transport is strongly inhibited by an excess of either GSH or GSSG (Fig. 7).
Figure 7.
Uptake of [3H]GS-NEM (100 μm) by the ABC822/pDR+OsGT1 strain. Unlabeled GSH or GSSG were added at 500 μm (final concentration).
DISCUSSION
Transport and compartmentation of glutathione and its derivatives are important for the numerous functions played by this compound. Control of the redox potential depends on a constant recycling and reduction of GSSG. In the case of fungal attack, for example, excess GSSG produced in the apoplast (Vanacker et al., 1999) cannot be reduced in this compartment because of the lack of necessary enzymes (Foyer et al., 2001) and, therefore, must be retrieved by the cell before being reduced. GSH may also be conjugated to various electrophilic organic xenobiotics and secondary metabolites. This conjugation may be either spontaneous or mediated by GSTs (Kreuz et al., 1996), some of which are expressed in the cell wall (Flury et al., 1996). The GS conjugates synthesized in the cell wall should also be retrieved by the plasma membrane, as shown by the ability of plant tissues to take up GS conjugates (Jamai et al., 1996), but the molecular identity of the transporter involved in this process was still unknown. A recent report (Bogs et al., 2003) showed that BjGT1, an OPT member cloned from B. juncea, was able to mediate uptake of labeled glutathione in yeast, but the specificity of this transporter was not studied.
In the present work, we used the sequence information and the yeast mutant available from our previous work on the cloning of the yeast glutathione transporter HGT1/OPT1 to characterize a rice glutathione transporter, able to transport GSH and GS conjugates, and whose structural features significantly differ from BjGT1 and HGT1/OPT1. The molecular mass, protein length, and pI of OsGT1 are 86 kD, 766 amino acids, and 6.5 versus 74 kD, 661 amino acids, and 9.29 for BjGT1 (Bogs et al., 2003) and 91 kD, 799 amino acids, and 9.00 for HGT1 (Bourbouloux et al., 2000). Phylogenetic analysis indicates that OsGT1 and its rice homologs cluster on a different branch, whereas BjGT1 is close to AtOPT3 (Fig. 2). OsGT1 and HGT1 are expected to contain 12 to 14 transmembrane domains versus 10 for BjGT1.
That OsGT1 is able to mediate GSH uptake is clearly shown by its ability to mediate growth of the hgt1 yeast mutant on medium with GSH as the sole source of sulfur in solid and liquid medium (Fig. 3) and by direct measurements of [3H-GSH uptake (Fig. 4). In addition to GSH, OsGT1 also mediates transport of GSSG and GS conjugates. In this respect, the transport properties of OsGT1 differ from that of the AtMRP1 and AtMRP2 transporters that mediate transfer of GS conjugates into the vacuoles but have very low affinity for GSH (Tommasini et al., 1993; Rea et al., 1998). Furthermore, analysis of OsGT1 sequence failed to detect any of the signature sequences (Walker motifs) characteristic for ABC transporters.
The substrate specificity of OsGT1 was studied indirectly by measuring the uptake of [3H]GSH in the presence of an excess of various compounds. The best competitors were l-Met, GS-NEM, l-Gln, and GSSG. That GS-NEM was transported by OsGT1 and was checked by uptake experiments with labeled GS-NEM. The ability of OsGT1 to transport the GS conjugate GS-NEM (Table I; Fig. 7) is in good agreement with previous physiological characterization of glutathione transport in plants (Jamai et al., 1996) and also with the properties of the yeast glutathione transporter (Bourbouloux et al., 2000). In contrast, although Met appeared as a strong competitor of GSH uptake (Table I), we were unable to find any evidence that labeled Met was transported by OsGT1. This, and the fact that Met does not affect the amount of OsGT1 transcripts (Fig. 6B), suggest that this amino acid may act as an allosteric repressor of OsGT1. Competition by l-Gln and l-Glu may be because of structural similarity with the γ-Glu part of the GSH molecule. However, γ-Glu-Cys only moderately decreased GSH uptake. Although Gly-Gly and Gly-Gly-Gly to some extent affected GSH uptake, poor inhibition by Leu-Leu, which is recognized with high affinity by peptide transporters (Rentsch et al., 1995; Song et al., 1996) and the strong inhibition by GS-NEM and GSSG suggest that the primary function of OsGT1 is the transport of the various forms of glutathione (conjugated, oxidized, and reduced). However, the substrate specificity of OsGT1 is broader than that of HGT1 because the only good competitors for GSH transport mediated by HGT1 were GSSG and GS-NEM, the other compounds having weaker effects than that described here for OsGT1. Although HGT1 (OPT1) expressed in a Met- Leu- yeast mutant enabled growth on Leuenkephalin (YGGFL; Hauser et al., 2000), Leuenkephalin does not compete for GSH uptake mediated by OsGT1 (Table I) and is not able to support growth in yeasts expressing any of the various AtOPTs (Koh et al., 2002). Strong inhibition of GSH uptake by KLLLG (Table I) agrees well with the fact that various AtOPTs may support yeast growth on this compound (Koh et al., 2002).
Several possible explanations may be relevant for the fact that GSH uptake mediated by OsGT1 in yeast does not obey simple saturation kinetics (Fig. 5), like BjGT1 (Bogs et al., 2003) but unlike the yeast HGT1 (Bourbouloux et al., 2000). Eadie-Hofstee plots yielded two phases with Km values that markedly differ (about 410 μm and 23 mm). Although the Km value found for the high-affinity phase is in good agreement with that measured for GSH uptake in broad bean protoplasts (0.4 mm), no low-affinity phase was apparent in the latter material (Jamai et al., 1996). In contrast, GSSG uptake in broad bean protoplasts was mediated by two apparent saturable phases with apparent Km of 0.7 and 4 mm. Complex kinetics have been described for GSH and GSSG uptake in plant cells (Schneider et al., 1992; Jamai et al., 1996) and chloroplasts (Noctor et al., 2002). These kinetics may be because of the presence of several transporters with different affinities, but this explanation cannot hold for the kinetics observed by expressing a single transporter in yeast. One possible explanation is that this pattern actually reflects a true intrinsic property of the glutathione transporter. Biphasic kinetics with a high- and a low-affinity component were described for the potassium AtKUP transporter, for example (Fu and Luan, 1998), and the low-affinity nitrate transporter CHL1 (Liu et al., 1999). An alternative explanation would be the presence in the protein of two recognition sites with different affinities, for example in the case of GSH/GSSG or GSH/GS conjugate exchange, because the internal glutathione (mainly GSH) concentration is in the millimolar range, whereas the external glutathione (mainly oxidized and conjugated) concentration is in the micromolar range (Vanacker et al., 1999). In human (Homo sapiens) intestinal epithelial cells, pre-loading of the cells with GSH increases subsequent GSH uptake (Iantomasi et al., 1997). This trans-stimulation effect, also found in rat hepatocytes and rabbit brush border membrane vesicles, would fit with an exchange mechanism. Finally, because OsGT1 also seems able to transport peptides to some extent, the protein could have a site for peptide transport and a site for glutathione transport, thus giving a bifunctional transporter, such as that described for BnNRT1,2, which may transport nitrate or His (Zhou et al., 1998). In both cases (exchanger or bifunctional enzyme), because only one radioactive substrate is tested in transport assays, interaction of this substrate with the “good” site would be with high affinity, and interaction with the second, “wrong” site would be with low affinity. A final explanation may be because of the use of yeasts as a heterologous expression system. Codon bias and mistargeting of plant membrane proteins are often cited as potential pitfalls. Both OsGT1 (this report) and BjGT1 (Bogs et al., 2003) have a transport activity for labeled glutathione that seems significantly lower than HGT1 when they are expressed in yeast (about 2-fold lower). That OsGT1 is not completely efficient in yeast is also suggested by the growth curves in liquid medium (Fig. 3A). This low activity may be because of poor targeting to the plasma membrane.
The glutathione transport activity of OsGT1 in yeasts appears only if the growth medium is depleted of any other sulfur source than glutathione (Fig. 6), confirming recent data with BjGT1 (Bogs et al., 2003). This indicates that this transport activity in yeast is repressed (or not induced) when other sulfur sources are available. A cis-regulator involved in the repression of HGT1 (OPT1) by Cys in yeast was described recently (Miyake et al., 2002). In the construct used here, OsGT1 is under the control of the PMA1 promoter and is not expected to be controlled at the transcriptional level by the sulfur content of the medium, which is demonstrated by Figure 6B. Likewise, the activity of the yeast HGT1 expressed in a plasmid containing a TEF promoter (and not the HGT1 promoter) also depended on the sulfur composition of the medium (Bourbouloux et al., 2000). These data suggests that in addition to the transcriptional control of glutathione transport activity (Miyake et al., 2002), posttranscriptional controls may also occur, at least in yeasts. In rice plants grown under normal conditions, OsGT1 transcripts cannot be detected by northern blot with total RNA, suggesting that it is normally poorly expressed. However, reverse transcriptase-PCR data indicate that it is expressed to some extent in various organs of vegetative or heading rice plants (data not shown).
In conclusion, OsGT1 is able to transport GSH, GS conjugates, and most likely also GSSG and some nitrogen-containing peptides. It corresponds to a new family of proteins able to mediate the transport of GS conjugates in plants, in addition to the tonoplast ABC transporters of the MRP subclass studied earlier in detail (Martinoia et al., 1993; Lu et al., 1997, 1998; Rea et al., 1998; Tommasini et al., 1998). At least three OsGT1 homologs with similar genomic structure are present in the rice genome. Whether they all encode glutathione transporters remains to be tested. No signal sequence for organelles could be found by computer analysis. The fact that expression of OsGT1 in yeast confers the ability to take up exogenous glutathione and the lack of targeting sequence for organelles suggests that the protein is targeted to the plasma membrane. OsGT1 is only weakly expressed in various parts or rice plants, which suggests that it does not play a key role in sulfur transport under normal growth conditions. Its function may be to retrieve GSSG, GS conjugates, and peptides under biotic or abiotic stress.
MATERIALS AND METHODS
Isolation of OsGT1
Sequence alignments between the yeast (Saccharomyces cerevisiae) glutathione transporter HGT1 and its homologs in Arabidopsis allowed the design of the following degenerated primers: forward KLGD, 5′-AAGCTWGGYCAYTACATGAARATT-3′; and reverse DASV, 5′-ARYCCCCAWATMACYGAWGCRTC-3′. These primers were used to amplify a 210-bp fragment from the rice (Oryza sativa) seedling cDNA library. This library, prepared from whole plants grown under normal conditions, was a kind gift from Dr. Minyong Zhang (South China Institute of Botany, Chinese Academy of Sciences, Guranzhou, China). Sequencing of the DNA fragment showed high similarity to Hgt1p and other putative GSH transporters from Arabidopsis. A 2.1-kb cDNA fragment was isolated by screening of a rice seedling cDNA library with this 210-bp PCR fragment as a probe. Subsequently, a full-length cDNA of 2.58 kb (accession no. AF 393848) was obtained by 5′-RACE-PCR using a cDNA-specific primer (NM5, 5′-CCCACCACCTGAGCCATAAACATTG-3′) with the Marathon cDNA Amplification Kit (CLONTECH Laboratories, Palo Alto, CA) according to the manufacturer's instructions.
RNA Isolation and Northern Blot
Total RNA was isolated from yeasts as described by Logemann et al. (1987). Northern blots were performed following standard protocols (Sambrook et al., 1989), with 10 μg of total RNA in each lane. The full-length OsGT1 cDNA was labeled by random primer labeling with 32P-dCTP and used as a probe.
Yeast Strains and Growth
The yeast strain ABC822 bearing a deletion in HGT1 (Matα ura3-52 leu2-Δ1 lys2-801 his3-Δ200 trp1-Δ63 ade2-101 hgt1 Δ::LEU2; Bourbouloux et al., 2000) is deficient in glutathione uptake and was used for complementation studies.
SD minus sulfur medium (SD-S) was prepared according to the YNB recipe (Bacto Yeast Nitrogen Base without amino acids and ammonium sulfate, DIFCO Laboratories, Detroit), with the modification that all sulfur-containing reagents in macroelements, microelements, and vitamins were substituted with equal amounts of the corresponding chloride salt (i.e. CuCl2, MgCl2, ZnCl2, MnCl2, and NH4Cl) and free adenine base. The yeast strain ABC822 and strain ABC822 transformed with empty vector pDR196 (ABC822/pDR) grew poorly in SD-S medium supplemented with the required amino acids (His, Trp, and Lys), adenine, and GSH; therefore, these strains were grown in synthetic minimal medium (SD; DIFCO Laboratories) with ammonium sulfate or in SC medium (Sherman, 1991), which contains ammonium sulfate and sulfur amino acids.
Heterologous Expression of OsGT1 in a Yeast hgt1Δ Mutant
The OsGT1 cDNA was amplified by PCR (forward primer, 5′-ACACACAACCATGATGCTCC-3′; and reverse primer, SP6 primer in pGEM-T easy vector) and cloned into the SmaI site of the yeast-Escherichia coli shuttle vector pDR196 (Rentsch et al., 1995). The nucleotide sequence of the PCR fragment was verified by sequencing, and the yeast strain ABC822 was transformed with either pDR+OsGT1 or the empty pDR196 vector by the LiOAc/polyethylene glycol method (Gietz and Schiestl, 1991). The transformants growing on a selection medium (SC) lacking uracile were further selected for growth on an SD-S medium containing 50 μm GSH.
Transport Experiments
For growth assays in liquid and on solid medium, cells of ABC822/pDR and ABC822/pDR+OsGT1 were grown overnight to an OD600 = 0.6 in minimal liquid medium SD containing ammonium sulfate and 2% (w/v) Glc and the necessary amino acids. For most of the uptake experiments, the yeast strain ABC822 transformed with pDR+OsGT1 (ABC822/pDR+OsGT1) was grown in liquid SD-S medium containing 100 μm GSH. Cells were incubated at 28°C for 12 h and rotary shaken at 200 rpm. Cells were harvested at OD600 = 0.6, washed with the same volume of sterile water (4°C), and then with the washing buffer containing 20 mm MES/KOH, 0.5 mm CaCl2, and 0.25 mm MgCl2 (pH 5.0), unless otherwise stated. They were finally resuspended in the transport medium (the washing buffer plus 2% [w/v] Glc), and 100-μL samples were kept on ice until the uptake experiment.
After a 5-min incubation of the cells at 28°C, [3H] GSH (1.9 TBq mmol-1, Amersham France, Les Ulis) was added to the transport-buffered medium to a final concentration of 0.1 mm GSH (final specific activity 38 MBq mmol-1). At selected times, uptake was stopped by diluting the medium with a 20-fold volume of water (4°C), and cells were separated from the medium by filtering through a glass fiber filter (Sartorius AG, Goettingen, Germany). The cells trapped on the filter were washed twice with the same volume of cold water. The filter was dried and placed in a scintillation vial containing 4 mL of Ecolite (ICN, Orsay, France). The radioactivity was counted after correction for background and quenching (Packard Instruments, Les Ulis, France).
Synthesis of [3H] GS-NEM was as described by Bourbouloux et al. (2000). Protein content was measured by the method of Bradford (1976) using bovine serum albumin as a standard. Amino acids and peptides were obtained from Sigma France (Saint-Quentin Fallavier, France) at the highest purity available, except KLLLG and AALLA, which were custom synthesized on a 333A synthesizer (PE-Applied Biosystems, Foster City, CA) and were used at 90% (w/v) purity.
Acknowledgments
We thank Dr. Takuji Sasaki (Rice Genome Research Program, STAFF Institute, Tsukuba, Japan) for providing the EST clone R3139, Dr. Michael Jackson (International Rice Research Institute, Manila, Philippines) for the gift of rice seeds, Dr. Ming Yong Zhang (South China Institute of Botany, Chinese Academy of Sciences, Guanzhou, China) for the gift of the rice library, Daniel Guyonnet (University of Poitiers, France) for synthesis of oligopeptides, and Dr. W. Frommer (University of Tübingen, Tübingen, Germany) for critical reading of the manuscript.
This work was supported by grants from the Indo-French Centre for the Promotion of Advanced Research and the Association Franco-Chinoise pour la Recherche Scientifique et Technique.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.030940.
References
- Bogs J, Bourbouloux A, Cagnac O, Wachter A, Rausch T, Delrot S (2003) Functional characterization and expression analysis of a glutathione transporter, BjGT1, from Brassica juncea: evidence for regulation by heavy metal exposure. Plant Cell Environ 26: 1703-1711 [Google Scholar]
- Bolchi A, Petrucco S, Tenca PL, Foroni C, Ottonello S (1999) Coordinate modulation of maize sulfate permease and ATP sulfurylase mRNAs in response to variations in sulfur nutritional status: stereospecific down-regulation by l-cysteine. Plant Mol Biol 39: 527-537 [DOI] [PubMed] [Google Scholar]
- Bourbouloux A, Shahi P, Chakladar A, Delrot S, Bachhawat AK (2000) Hgt1p, a high affinity glutathione transporter from the yeast Saccharomyces cerevisiae. J Biol Chem 275: 13259-13265 [DOI] [PubMed] [Google Scholar]
- Bourgis F, Roje S, Nuccio ML, Fisher DB, Tarczynski MC, Li C, Herschbach C, Rennenberg H, Pimenta MJ, Shen TL et al. (1999) S-methylmethionine plays a major role in phloem sulfur transport and is synthesized by a novel type of methyltransferase. Plant Cell 11: 1485-1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254 [DOI] [PubMed] [Google Scholar]
- De Kok LJ, Kuiper PJC (1986) Effect of short term dark incubation with sulfate, chloride and selenate on the glutathione content of spinach leaf discs. Physiol Plant 68: 477-482 [Google Scholar]
- Esterbauer H, Grill D (1978) Seasonal variation in glutathione and glutathione reductase in needles of Picea abies. Plant Physiol 61: 119-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flury T, Wagner E, Kreuz K (1996) An inducible glutathione-S-transferase in soybean hypocotyl is localized in the apoplast. Plant Physiol 112: 1185-1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Theodoulou F, Delrot S (2001) The functions of inter- and intracellular glutathione transport systems in plants. Trends Plant Sci 6: 486-492 [DOI] [PubMed] [Google Scholar]
- Fu HH, Luan S (1998) AtKUP1: a dual affinity K+ transporter from Arabidopsis. Plant Cell 10: 63-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz RD, Schiestl RH (1991) Applications of high efficiency transformation of intact yeast cells using single stranded nucleic acids as carrier. Yeast 7: 253-263 [DOI] [PubMed] [Google Scholar]
- Hauser M, Donhardt AM, Barnes D, Naider F, Becker JM (2000) Enkephalins are transported by a novel eukaryotic peptide uptake system. J Biol Chem 275: 3037-3041 [DOI] [PubMed] [Google Scholar]
- Herschbach C, van Der Zalm E, Schneider A, Jouanin L, De Kok LJ, Rennenberg H (2000) Regulation of sulfur nutrition in wild-type and transgenic poplar by over-expressing γ-glutamylcysteine synthetase in the cytosol as affected by atmospheric H2S. Plant Physiol 124: 461-473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iantomasi T, Favilli F, Marraccini P, Magaldi T, Bruni P, Vincenzini MT (1997) Glutathione transport system in human small intestine epithelial cells. Biochim Biophys Acta 1330: 274-283 [DOI] [PubMed] [Google Scholar]
- Isnard AD, Thomas D, Surdin-Kerjan Y (1996) The study of methionine uptake in Saccharomyces cerevisiae reveals a new family of amino acid permeases. J Mol Biol 262: 473-484 [DOI] [PubMed] [Google Scholar]
- Jamai A, Tommasini R, Martinoia E, Delrot S (1996) Characterization of glutathione uptake in broad bean leaf protoplasts. Plant Physiol 111: 1145-1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung R, Nam YW, Saalbach I, Muntz K, Nielsen NC (1997) Role of the sulfhydryl redox state and disulfide bonds in processing and assembly of 11S seed globulins. Plant Cell 9: 2037-2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh S, Wiles AM, Sharp JS, Naider FR, Becker JM, Stacey G (2002) An oligopeptide transporter gene family in Arabidopsis. Plant Physiol 128: 21-29 [PMC free article] [PubMed] [Google Scholar]
- Kreuz K, Tommasini R, Martinoia E (1996) Old enzymes for a new job: herbicide detoxication in plants. Plant Physiol 111: 339-353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunert KJ, Foyer CH (1993) Thiol/disulfide exchange in plants. In LJ De Kok, I Stulen, H Rennenberg, C Brunold, WE Rauser, eds, Sulfur Nutrition and Assimilation in Higher Plants: Regulatory, Agricultural and Environmental Aspects. SBP Academic Publishing, The Hague, The Netherlands, pp 139-151
- Kyte J, Doolittle RF (1982) A simple method for displaying a hydropathic character of a protein. J Mol Biol 157: 105-132 [DOI] [PubMed] [Google Scholar]
- Lappartient A, Touraine B (1996) Demand-driven control of root ATP sulfurylase activity and SO42- uptake in intact canola. Plant Physiol 111: 147-157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappartient A, Vidmar JJ, Leustek T, Glass AD, Touraine B (1999) Interorgan signaling in plants: regulation of ATP sulfurylase and sulphate transporter genes expression in roots mediated by phloem-translocated compound. Plant J 18: 89-95 [DOI] [PubMed] [Google Scholar]
- Leustek T, Martin MN, Bick JA, Davies JP (2000) Pathways and regulation of sulfur metabolism revealed through molecular and genetic studies. Annu Rev Plant Physiol Plant Mol Biol 51: 141-165 [DOI] [PubMed] [Google Scholar]
- Liu KH, Huang CY, Tasy YF (1999) CHL1 is a dual-affinity nitrate transporter of Arabidopsis involved in multiple phases of nitrate uptake. Plant Cell 11: 865-874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logemann J, Schell J, Willmitzer L (1987) Improved method for the isolation of RNA from plant tissues. Anal Biochem 163: 16-20 [DOI] [PubMed] [Google Scholar]
- Lu YP, Li ZS, Drozdowicz YM, Hörtensteiner S, Martinoia E, Rea PA (1998) AtMRP2, an Arabidopsis ATP binding cassette transporter able to transport glutathione S-conjugates and chlorophyll catabolites: functional comparisons with AtMRP1. Plant Cell 10: 267-282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YP, Li ZS, Rea PA (1997) AtMRP1 gene of Arabidopsis encodes a glutathione S-conjugate pump: isolation and functional definition of a plant ATP binding cassette transporter gene. Proc Natl Acad Sci USA 94: 8243-8248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubkowitz MA, Barnes D, Breslav M, Burchfield A, Naider F, Becker JM (1998) Schizosaccharomyces pombe isp4 encodes a transporter representing a novel family of oligopeptide transporters. Mol Microbiol 28: 729-741 [DOI] [PubMed] [Google Scholar]
- Martinoia E, Grill E, Tommasini R, Kreuz K, Amrhein N (1993) ATP-dependent glutathione S-conjugate “export” pump in the vacuolar membrane of plants. Nature 634: 247-249 [Google Scholar]
- May MJ, Vernoux T, Leaver C, Van Montagu M, Inzé D (1998) Glutathione homeostasis in plants: implications for environmental sensing and plant development. J Exp Bot 49: 649-667 [Google Scholar]
- Miyake T, Kanayama M, Sammoto H, Ono B (2002) A novel cis-acting cysteine-responsive regulatory element of the gene for the high-affinity glutathione transporter of Saccharomyces cerevisiae. Mol Genet Genomics 266: 1004-1011 [DOI] [PubMed] [Google Scholar]
- Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Biochem 49: 249-279 [DOI] [PubMed] [Google Scholar]
- Noctor G, Gomez L, Vanacker H, Foyer CH (2002) Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signalling. J Exp Bot 53: 1283-1304 [DOI] [PubMed] [Google Scholar]
- Ogawa KI, Tasaka Y, Mino M, Tanaka Y, Iwabuchi M (2001) Association of glutathione with flowering in Arabidopsis thaliana. Plant Cell Physiol 42: 524-530 [DOI] [PubMed] [Google Scholar]
- Rauser WE (1990) Phytochelatins. Annu Rev Plant Physiol Plant Mol Biol 59: 61-86 [DOI] [PubMed] [Google Scholar]
- Rea PA, Li ZS, Lu YP, Drozdowicz YM, Martinoia E (1998) From vacuolar GS-X pumps to multispecific ABC transporters. Annu Rev Plant Physiol Plant Mol Biol 49: 727-760 [DOI] [PubMed] [Google Scholar]
- Rennenberg H, Schmitz K, Bergmann L (1979) Long distance transport of sulfur in Nicotiana tabacum. Planta 147: 57-82 [DOI] [PubMed] [Google Scholar]
- Rentsch D, Laloi M, Rouhara I, Schmelzer E, Delrot S, Frommer WB (1995) NTR1 encodes a high affinity oligopeptide transporter in Arabidopsis. FEBS Lett 370: 264-268 [DOI] [PubMed] [Google Scholar]
- Salt DE, Rauser WE (1995) MgATP-dependent transport of phytochelatins across the tonoplast of oat roots. Plant Physiol 107: 1293-1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual, Ed 2. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Sanchez-Fernandez R, Fricker M, Corben LB, White NS, Sheard N, Leaver CJ, Van Montagu M, Inze D, May MJ (1997) Cell proliferation and hair tip growth in the Arabidopsis root are mechanistically different forms of redox control. Proc Natl Acad Sci USA 94: 2745-2750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Martini N, Rennenberg H (1992) Reduced glutathione (GSH) transport in cultured tobacco cells. Plant Physiol Biochem 30: 29-38 [Google Scholar]
- Schupp R, Rennenberg H (1988) Diurnal changes in the glutathione concentration of spruce needles (Picea abies L.). Plant Sci 57: 113-117 [Google Scholar]
- Sherman F (1991) Getting started with yeast. In C Guthrie, GR Fink, eds, Guide to Yeast Genetics and Molecular Biology. Academic Press, New York, pp 3-21
- Sherrill C, Fahey RC (1998) Import and metabolism of glutathione by Streptococcus mutans. J Bacteriol 180: 1454-1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Steiner HY, Zang L, Naider F, Becker JM, Stacey G (1996) Cloning of a second Arabidopsis peptide transport gene. Plant Physiol 110: 171-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommasini R, Martinoia E, Grill E, Dietz KJ, Amrhein N (1993) Transport of oxidized glutathione into barley vacuoles: evidence for the involvement of the glutathione-S-conjugate ATPase. Z Naturforsch 48: 867-871 [Google Scholar]
- Tommasini R, Vogt E, Fromenteau M, Hortensteiner S, Matile P, Amrhein N, Martinoia E (1998) An ABC-transporter of Arabidopsis thaliana has both glutathione-conjugate and chlorophyll catabolite transport activity. Plant J 13: 773-780 [DOI] [PubMed] [Google Scholar]
- Vanacker H, Foyer CH, Carver TLW (1999) Changes in apoplastic antioxidants induced by powdery mildew attack in oat genotypes with race non-specific resistance. Planta 208: 444-452 [Google Scholar]
- Vernoux T, Wilson RC, Seeley KA, Reichheld JP, Muroy S, Brown S, Maughan SC, Cobbett CS, Van Montagu M, Inze D et al. (2000) The root meristemless1/cadmium sensitive 2 gene defines a glutathione dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. Plant Cell 12: 97-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingate VPM, Lawton MA, Lamb CJ (1988) Glutathione causes a massive and selective induction of plant defense genes. Plant Physiol 87: 206-210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Maehara T, Shimokawa T, Yamamoto S, Harada C, Takazaki Y, Ono N, Mukai Y, Koike K, Yazaki J et al. (2002) A comprehensive rice transcript map containing 6591 expressed sequence tag sites. Plant Cell 14: 525-535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JJ, Theodoulou F, Muldin I, Ingemarsson B, Miller AJ (1998) Cloning and functional characterization of a Brassica napus transporter that is able to transport nitrate and histidine. J Biol Chem 273: 12017-12023 [DOI] [PubMed] [Google Scholar]