Abstract
Objective:
The purpose of this study was to explore the accuracy of elevated liver function values, age, gender, pancreatitis and cholecystitis as predictors of common bile duct stones (CBDS).
Methods:
All patients operated on for gallstone disease over a period of 3 years in a Swedish county of 302 564 citizens were registered prospectively. Intraoperative cholangiography (IOC) was used to detect CBDS.
Results:
A total of 1171 patients were registered; 95% of these patients underwent IOC. Common bile duct stones were found in 42% of patients with elevated liver function values, 20% of patients with a history of pancreatitis and 9% of patients with cholecystitis. The presence of CBDS was significantly predicted by elevated liver function values, but not by age, gender, history of acute pancreatitis or cholecystitis. A total of 93% of patients with normal liver function tests had a normal IOC. The best agreement between elevated liver function values and CBDS was seen in patients undergoing elective surgery without a history of acute pancreatitis or cholecystitis.
Conclusions:
Although alkaline phosphatase (ALP) and bilirubin levels represented the most reliable predictors of CBDS, false positive and false negative values were common, especially in patients with a history of cholecystitis or pancreatitis, which indicates that other mechanisms were responsible for elevated liver function values in these patients.
Keywords: prediction, common bile duct stones, alkaline phosphatase (ALP), bilirubin, gallstone disease, cholecystectomy, dynamic intraoperative cholangiography, prospective population-based study, pancreatitis, cholecystitis
Introduction
Laparoscopic cholecystectomy can usually be performed with limited resources. The presence of common bile duct stones (CBDS), however, makes the procedure much more complicated and time-consuming. Preoperative awareness of CBDS enables better planning and facilitates the allocation of the necessary resources. In areas where more complicated procedures are centralized to units at which the optimal competence and equipment are available, it may be possible to avoid the need to perform surgery for CBDS at units that are not prepared for this procedure if the presence or absence of CBDS is known in advance.
A number of imaging techniques have been suggested as methods to predict the presence of CBDS preoperatively, including magnetic resonance cholangiopancreatography (MRCP) and endoscopic retrograde cholangiopancreatography (ERCP), as well as nomograms based on various preoperative data.1–3 However, these methods are either invasive, implying a certain incidence of morbidity or even mortality, as in ERCP, expensive, as in MRCP, or too complicated to gain acceptance in clinical practice, as in the case of nomograms. By contrast, biochemical markers, such as serum alkaline phosphatase (ALP) and bilirubin levels, are cheaply and easily sampled and thus commonly used clinically as predictors of CBDS. Despite the widespread use of these markers, their predictive value has not been fully assessed. Previous studies have often been based on selected patient samples or data assembled retrospectively.2,4–6 No prospective, non-selected, population-based studies on the ability of ALP and bilirubin to predict CBDS have been performed. Most studies have focused on patient groups with a high risk for CBDS (i.e. suspected CBDS based on ultrasound [US] findings),5,7,8 a history of previous acute biliary pancreatitis,5,8,9 jaundice,5,8 cholangitis5,8 or elevated liver function tests.5,7,8 The characteristics of such patient groups may affect the prevalence of CBDS and thereby also the negative and positive predictive values (NPVs and PPVs) of the predictor. As CBDS are encountered in only approximately 10% of an unselected population undergoing cholecystectomy, a very large number of patients is required to achieve a power sufficient to assess the ability of different markers to predict CBDS. Very few previous studies have included more than 100 patients.1–4 This has resulted in a wide range (17–92%) of PPVs regarding the ability of ALP and bilirubin levels to predict CBDS.1,5,8
If ALP and bilirubin levels are used as predictors, test results must be obtained a few weeks before surgery so that the cholecystectomy patient can be referred to the optimal unit in order to ensure the presence of appropriate equipment and to plan the time required for the procedure. However, CBDS may be passed spontaneously before surgery. Furthermore, some patients without CBDS at the time of blood sampling may develop CBDS as a result of stone migration from the gallbladder. The timing of sampling thus involves balancing the time required for planning against the risk for the occurrence of such events in the interval between the decision to perform surgery and the time of surgery. Most previous studies have been based on bilirubin and ALP sampled 1 or a few days before surgery. The predictive ability of ALP and bilirubin sampled at a time-point weeks before surgery has not been studied.
Therefore, this study aimed to explore the ability of bilirubin and ALP sampled 1–4 weeks prior to surgery to predict CBDS in a prospective, population-based setting, using intraoperative cholangiography (IOC) findings as a reference, and to analyse possible sources of false positive and false negative values. It also aimed to explore the use of previous history of acute biliary pancreatitis or cholecystitis, age and gender as predictive factors for CBDS and to study the effectiveness of using the preoperative prediction of CBDS as a basis for referring patients with CBDS to the unit most appropriate for their management.
Materials and methods
Study design and patients
The study was designed as a prospective, population-based study performed in the county of Uppsala in Sweden. In December 2004, the population of this county numbered 302 564.10 All patients who underwent cholecystectomy in Uppsala county from 1 January 2003 to 31 December 2005 were registered prospectively according to a standardized protocol. All variables related to indications for surgery, history of acute cholecystitis or gallstone pancreatitis, surgical intervention, IOC results, serum bilirubin and ALP levels, American Society of Anesthesiologists (ASA) physical status classification score and age were recorded by the surgeon undertaking the operation. Data related to the postoperative course, including additional investigations such as the results of possible postoperative cholangiography, were recorded by the surgeon responsible for discharging the patient.
Setting
All of the three hospitals in the county that used the same computer-based medical records participated in the study. These included one university hospital (Uppsala University Hospital), one short-stay clinic (Samariterhemmet) and one municipal hospital (Enköping Hospital). When a procedure was anticipated to be fairly complicated, as in cases of acute cholecystitis or suspected CBDS, the patient was generally referred to the university clinic, whereas procedures that were presumed to be uncomplicated were conducted at the short-stay clinic. Procedures of intermediate degrees of complicatedness were managed at the municipal hospital. Preoperative planning aimed to avoid the performance of surgery in patients with CBDS at the short-stay clinic because this clinic provided health care during weekdays only and lacked an intensive care unit.11 The short-stay clinic performed elective procedures only. Emergency procedures were usually performed only at the university hospital because this was the only hospital with a 24-h surgical emergency unit. Patients with acute ongoing cholecystitis of less than 3–5 days' duration were in general operated as soon as possible. However, if a patient showed symptoms of ongoing acute cholecystitis for more than 3–5 days, the surgery was generally postponed for 2–3 months until the inflammatory process had settled. The cholecystectomy was then performed at the municipal or the university hospital.
Common bile duct stones found during laparoscopic cholecystectomy were generally extracted during the primary operation. Randomized trials have shown this to be more cost-effective and to be associated with shorter hospital stays compared with extraction by postoperative endoscopic retrograde cholangiography.12–14
To ensure complete coverage, data collected were cross-checked against data in the electronic patient administrative system common to the three hospitals. This database contains data necessary for planning and financing health care. All operations are registered in this database. If any diagnosis or intervention code raising suspicion of gallstone-related events was registered in the electronic patient administrative system, that patient's computer-based medical records were reviewed. A second review of the records of all patients for whom a pathological finding at cholangiography was recorded in the register was also carried out. In the present study, patients who underwent cholecystectomy for suspected malignancy or as part of surgery carried out for other indications were excluded.
Intraoperative cholangiography
In accordance with local guidelines in Uppsala county, IOC is performed routinely in all patients undergoing cholecystectomy.11 The result of this IOC was used to test the predictive value of ALP and bilirubin levels. If IOC was not performed as a result of technical problems, the reasons for this were documented. An IOC technique similar to that described by MacFadyen15 was used. This method, which has been described in detail previously,16 resulted in a dynamic real-time intraoperative fluoroscopic cholangiogram obtained with a mobile C-arm X-ray apparatus (Ziehm Exposcop CB7-D; Ziehm Imaging GmbH, Nuremburg, Germany), using 10–40 ml Johexol (Omnipaque®; GE Healthcare, Little Chalfont, UK) (200 mg/ml) as contrast medium and 1 mg glucagon (Glucagon®; Novo Nordisk A/S, Bagsvaerd, Denmark) (1 mg/ml) i.v. to release any papillary spasm or, in patients with diabetes mellitus, 1–2 ml (20 mg/ml) i.v. butylscopolamine (Buscopan®; Boehringer Ingelheim, Ingelheim am Rhein, Germany).
The dynamic IOC was interpreted by the surgeon and a radiologist simultaneously using a two-way communication system.
The reliability of IOC as a method of diagnosing CBDS (i.e. the number of false positive and false negative findings on IOC) was assessed previously in the same cohort and presented in an earlier paper.16 The sensitivity and specificity of IOC were found to be 97% and 99%, respectively. The presence of CBDS was verified by common bile duct (CBD) exploration or ERCP in 95% of patients in whom IOC indicated CBDS. Only 0.4% of the patients with normal cholangiography were subsequently found to harbour CBDS over a 38-month follow-up.16
Statistical methods
Positive and negative odds ratios (ORs), sensitivity, specificity, PPVs, NPVs and accuracy were calculated for each predictor using IOC as the reference. Overall agreement (i.e. accuracy) was defined as the percentage of all patients in whom the predictor tested predicted correctly. Multivariate logistic regression analysis was performed in order to calculate the risk for CBDS associated with each respective predictive factor. The accuracy of risk factors that were found to be significantly associated with the presence of CBDS was analysed separately. All statistical analyses were performed using spss Version 16.0 for Windows (SSPS, Inc., Chicago, IL, USA). A P-value of <0.05 was considered to indicate statistical significance.
Values are given in numbers, percentages of all patients (n = 1171) or, in cases for which the predictive ability of a predictor was compared with findings on IOC, as the percentage of patients who underwent successful IOC (n = 1117). Continuous data are presented as medians and interquartile ranges.
Biochemical parameters
Serum ALP and bilirubin were measured within 4 weeks preoperatively using standard analysis methods. Elevated ALP was defined as ALP > 5 µkat/l (normal range: 0.8–4.6 µkat/l). Elevated bilirubin was defined as total bilirubin > 50 mmol/l (normal range: 4–21 mmol/l). The cut-off levels for bilirubin were set relatively high in order to avoid too many false positive findings.7,8
The diameter of the CBD is not reported routinely on abdominal US in Sweden and thus was not documented in our protocol.
Definitions
Acute cholecystitis was defined by the demonstration of clinical symptoms typical of cholecystitis, such as right upper abdominal pain or tenderness, fever, elevated C-reactive protein (CRP) and stones present in a thickened, oedematous gallbladder on abdominal US or computed tomography (CT).
Acute pancreatitis was defined by the demonstration of elevated serum amylase of more than four times the normal value (normal range: 0.15–1.10 µkat/l), the presence of typical signs of pancreatitis on abdominal US or CT and typical clinical signs of pancreatitis, such as abdominal pain or tenderness, nausea or vomiting, in the absence of any history or radiological signs of chronic pancreatitis.
Biliary pancreatitis was represented by acute pancreatitis and the presence of gallstones on CT or abdominal US in the absence of any history, laboratory or radiological findings indicating another aetiology of pancreatitis.
Results
The study included 1171 patients. The process by which the cohort was assembled and the number of patients excluded are presented in Fig. 1. No protocols were discarded in the study.
Figure 1.
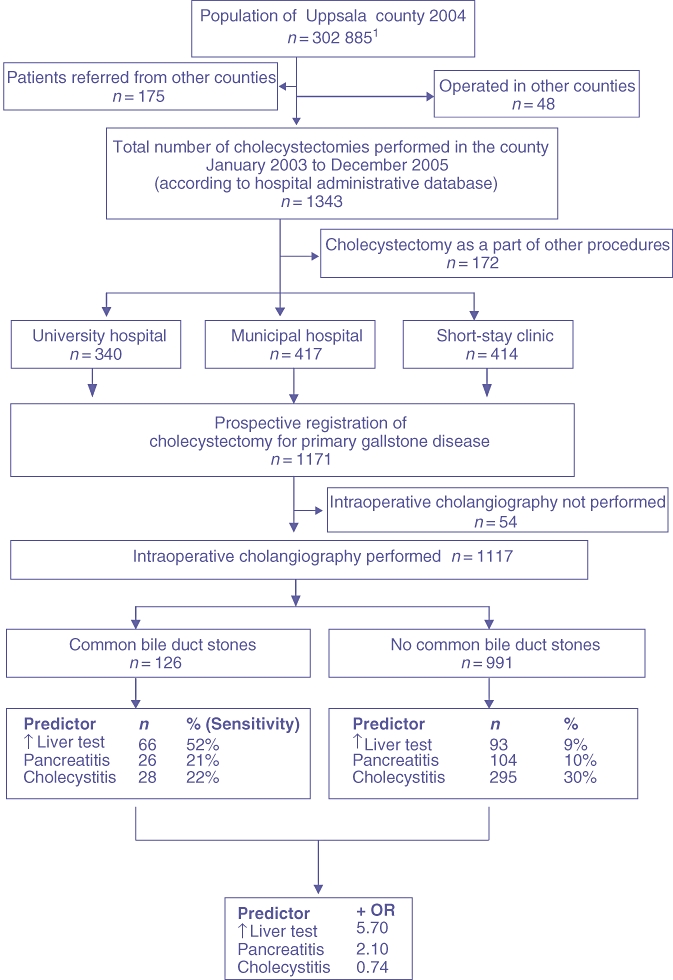
Flow chart describing the process of assembling the cohort under study
Baseline data for the population studied and for the different hospitals participating in the study, including indications for surgery, type of cholecystectomy, IOC findings and type of CBD exploration, are presented in Table 1.
Table 1.
Clinical data for the populations studied by whole group and by different hospital populations
| Clinical data for the population studied | ||||
|---|---|---|---|---|
| All | University hospital | Municipal hospital | Short-stay clinic | |
| Patient data | ||||
| Patients, n (%) | 1171 | 340 (29) | 417 (36) | 414 (35) |
| Female, n (%) | 776 (66) | 190 (60) | 296 (71) | 290 (70) |
| Male, n (%) | 395 (34) | 150 (44) | 121 (29) | 124 (30) |
| Age, years, median (IQR) | 48 (36–59) | 51 (36–63) | 50 (36–61) | 44 (35–56) |
| BMI, kg/m2, median (IQR) | 26 (24–30) | 27 (24–31) | 26 (24–30) | 26 (23–29) |
| Elevated ALP or bilirubin, n (%) | 164 (14) | 92 (27) | 64 (15) | 8 (2) |
| Pathological IOC, n (%) | 152 (13) | 94 (29) | 72 (18) | 49 (13) |
| CBDS, n (%) | 134 (11) | 70 (21) | 44 (11) | 20 (5) |
| Operation time, min, median (IQR) | 105 (80–135) | 120 (90–150) | 110 (85–140) | 85 (70–110) |
| Successful IOC, n (%) | 1117 (95) | 325 (96) | 400 (96) | 392 (95) |
| Mortality, n (%) | 4 (0.3) | 4 (1) | 0 | 0 |
| Type of surgery, n (%) | ||||
| Elective procedure | 986 (84) | 210 (62) | 363 (87) | 412 (99) |
| Acute procedure | 186 (16) | 130 (38) | 54 (13) | 2 (0.5) |
| Indications for surgery, n (%) | ||||
| Gallstone-related prandial pain | 695 (60) | 121 (36) | 277 (66) | 297 (72) |
| Pancreatitis | 125 (11) | 76 (22) | 37 (9) | 12 (3) |
| Ongoing cholecystitis | 99 (8) | 73 (22) | 23 (6) | 3 (0.7) |
| Previous cholecystitis | 219 (19) | 46 (14) | 71 (17) | 102 (25) |
| Ongoing and previous pancreatitis | 8 (0.7) | 5 (2) | 3 (0.7) | 0 |
| Cholecystitis with imminent threat of perforation | 25 (2) | 19 (6) | 6 (1) | 0 |
| Type of cholecystectomy, n (%) | ||||
| Laparoscopic | 922 (79) | 169 (50) | 361 (87) | 392 (95) |
| Conventional | 138 (12) | 110 (32) | 24 (6) | 4 (1) |
| Conversion from laparoscopic to conventional | 111 (10) | 61 (18) | 32 (8) | 18 (4) |
| CBD exploration, n (%)a | ||||
| Open bile duct exploration | 77 (59) | 56 (86) | 16 | 5 |
| Laparoscopic transcystic exploration | 24 (18) | 9 (14) | 0 | 15 |
| Laparoscopic bile duct exploration | 28 (21) | 0 | 28 | 0 |
| Open transcystic exploration | 1 (0.8) | 0 | 0 | 1 |
| All, n | 130 | 65 | 44 | 21 |
Figures in brackets represent the percentage of the total number of CBD explorations performed at the actual hospital
IQR, interquartile range; BMI, body mass index; ALP, alkaline phosphatase; IOC, intraoperative cholangiography; CBD, common bile duct
Prediction of common bile duct stones
In univariate and multivariate logistic analyses of factors predicting the presence of CBDS, elevated liver function values were found to be significantly and independently associated with the presence of CBDS, whereas age, gender and history of acute biliary pancreatitis or acute cholecystitis were not (Table 2). Agreements between elevated liver function values, history of acute biliary pancreatitis, cholecystitis and the presence of CBDS are presented in Table 3.
Table 2.
Multivariate logistic analysis of risk factors predicting pathologic cholangiography
| Factor | Univariate model | Final multivariate model | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Age | ||||
| <median (48 years) | 1 | Reference | 1 | Reference |
| ≥median (48 years) | 1.48 | 1.03–2.13 | 1.49 | 1.002–2.22 |
| Gender | ||||
| Female | 1 | Reference | ||
| Male | 1.29 | 0.88–1.87 | ||
| Biochemical markers | ||||
| Bilirubin < 50 mmol/l and ALP < 5 µkat/l | 1 | Reference | 1 | Reference |
| Bilirubin > 50 mmol/l and ALP > 5 µkat/l | 11.27 | 7.54–16.86 | 11.29 | 7.54–16.92 |
| History of cholecystitis | ||||
| No cholecystitis | 1 | Reference | ||
| Previous or ongoing cholecystitis | 0.70 | 0.46–1.08 | ||
| History of pancreatitis | ||||
| No pancreatitis | 1 | Reference | ||
| Pancreatitis | 2.13 | 1.34–3.41 | ||
Stepwise selection method with entry testing based on the significance of the score statistic, and removal testing based on the probability of a likelihood ratio statistic based on maximum partial likelihood estimates
OR, odds ratio; 95% CI, 95% confidence interval; ALP, alkaline phosphatase
Table 3.
Agreement between elevated ALP or bilirubin levels, pancreatitis, cholecystitis and findings of common bile duct stones on intraoperative cholangiography
| Predictor | Total | No CBDS | CBDS | PPV/NPV |
|---|---|---|---|---|
| ALP and bilirubin | ||||
| Normal ALP and bilirubin | 958 | 898 | 60 | 94% (NPV) |
| Elevated ALP and bilirubin | 159 | 93 | 66 | 42% (PPV) |
| Specificity | 91% | |||
| Sensitivity | 52% | |||
| Total agreement | 86% | |||
| Pancreatitis | ||||
| No pancreatitis | 987 | 887 | 100 | 90% (NPV) |
| Pancreatitis | 130 | 104 | 26 | 20% (PPV) |
| Specificity | 90% | |||
| Sensitivity | 21% | |||
| Total agreement | 82% | |||
| Cholecystitis | ||||
| No cholecystitis | 794 | 696 | 98 | 88% (NPV) |
| Cholecystitis | 323 | 295 | 28 | 9% (PPV) |
| Specificity | 70% | |||
| Sensitivity | 22% | |||
| Total agreement | 65% | |||
| Total, n | 1117 | 991 | 126 | |
PPV, positive predictive value; NPV, negative predictive value; ALP, alkaline phosphatase; CBDS, common bile duct stones
Subgroup analysis
Levels of ALP and bilirubin may be elevated by mechanisms that are not related to CBDS. We therefore calculated the ORs and agreements between elevated bilirubin and/or ALP and the presence of CBDS separately for the different hospitals, for different ASA classes and according to whether the patient was operated electively or for acute disease. These data are presented in Table 4.
Table 4.
Correlation between elevated liver function tests and common bile duct stones in different subgroups of patients
| IOC | CBDS | Elevated liver test | PPV, % | NPV, % | Sens., % | Spec., % | Agr., % | +OR | −OR | |
|---|---|---|---|---|---|---|---|---|---|---|
| Hospital | ||||||||||
| University clinic | 325 | 62 | 90 | 44 | 91 | 64 | 81 | 78 | 3.39 | 0.44 |
| Municipal clinic | 400 | 44 | 61 | 38 | 94 | 52 | 89 | 85 | 4.89 | 0.53 |
| Short-stay clinic | 392 | 20 | 8 | 38 | 96 | 15 | 99 | 94 | 11.53 | 0.86 |
| Operation | ||||||||||
| Emergency | 172 | 44 | 72 | 49 | 91 | 80 | 71 | 73 | 2.75 | 0.29 |
| Elective | 945 | 82 | 87 | 36 | 94 | 38 | 94 | 89 | 5.81 | 0.66 |
| ASA score | ||||||||||
| 1–2 | 1032 | 109 | 144 | 40 | 94 | 52 | 91 | 87 | 5.56 | 0.53 |
| 3–5 | 82 | 15 | 14 | 57 | 90 | 53 | 91 | 84 | 5.92 | 0.51 |
| Pancreatitis | ||||||||||
| Pancreatitis | 130 | 26 | 42 | 31 | 85 | 50 | 72 | 68 | 1.79 | 0.69 |
| No pancreatitis | 987 | 100 | 117 | 45 | 95 | 53 | 93 | 89 | 7.36 | 0.51 |
| Cholecystitis | ||||||||||
| Acute cholecystitis | 130 | 19 | 35 | 37 | 94 | 68 | 80 | 78 | 3.45 | 0.39 |
| No cholecystitis | 794 | 98 | 116 | 44 | 93 | 52 | 91 | 86 | 5.59 | 0.53 |
| All | 1117 | 126 | 159 | 42 | 94 | 52 | 91 | 86 | 5.57 | 0.52 |
The table displays how well elevated bilirubin or ALP predicted CBDS in different subgroups of patients. Values are calculated from the number of patients with successful intraoperative cholangiograms in each group. A high positive odds ratio (+OR) and agreement (Agr.) signify the high predictive ability of a test. Thus, elevated liver function values predicted CBDS best among electively operated patients without a history of biliary pancreatitis or cholecystitis operated at the short-stay clinic, indicating that other mechanisms may have been responsible for elevating liver function values in acutely operated patients with a history of biliary pancreatitis or acute cholecystitis
IOC, intraoperative cholangiography; CBDS, common bile duct stones; PPV, positive predictive value; NPV, negative predictive value; Sens., sensitivity; Spec., specificity; Agr., agreement; +OR, positive odds ratio; −OR, negative odds ratio; ASA, American Society of Anesthesiologists; ALP, alkaline phosphatase
The best agreement between elevated liver function values and presence of CBDS was seen in patients without acute pancreatitis or cholecystitis and operated electively at the municipal hospital or short-stay clinic. These groups also presented the highest ORs (i.e. the relationship between patients with CBDS and elevated liver function values compared with patients without CBDS and with elevated liver function values was most distinct in these groups).
Discussion
The prevalence of CBDS was found to be only 42% in patients with elevated bilirubin or ALP levels. Although the risk for CBDS in patients with normal ALP and bilirubin levels was only 6%, the group was very large and thus almost half (48%) of the patients with CBDS had normal ALP and bilirubin values. Thus, using findings of normal ALP and bilirubin as indicators of the absence of CBDS would imply that half of CBDS patients would remain undetected.
There are several mechanisms that may explain the limited value of ALP and bilirubin levels as predictors of CBDS. Figure 2 summarizes some of them. Common bile duct stones that are only partially obstructive may not cause elevated bilirubin levels and may generate false negative values. Stones may enter or pass from the CBD spontaneously in the interval between blood sampling and surgery, thereby generating both false positive and false negative values. Microlithiasis and sludge in the CBD may increase the viscosity of the bile, causing levels of both ALP and bilirubin to rise, but may pass undetected on IOC because they are flushed out to the duodenum by the contrast medium, thus allowing a normal IOC.17,18 Sphincter of Oddi dysfunction caused by stenosis or spasticity of the sphincter of Oddi may result in elevated liver function values and biliary pain in the absence of CBDS,19–22 and conjugation defects, such as Gilbert's syndrome, a common cause of unconjugated hyperbilirubinaemia found in 5% of a healthy population, may cause false positive values.23,24
Figure 2.
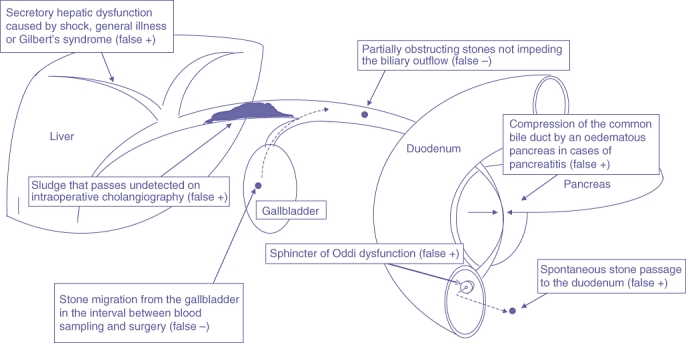
Causes of false positive or false negative findings of elevated alkaline phosphatase (ALP) or bilirubin
The association between elevated liver function values and findings of CBDS on IOC was weaker in patients with a history of acute pancreatitis compared with patients without a history of pancreatitis (Table 4). A suggested explanation for this difference may be that the CBD is obstructed by the inflammatory process of the pancreatitis itself, which generates oedema that compresses it, rather than by the CBDS.25,26 Furthermore, the secretory process of expelling conjugated bilirubin into the bile is highly energy-dependent and sensitive to hepatic insult.27 Acute pancreatitis and cholangitis may thus cause dysfunction of the hepatic secretory process as a result of inflammatory reactions and diminished blood flow secondary to shock.
Thus, when dealing patients with acute pancreatitis and elevated liver function tests, it is worth noting that although CBDS may be a cause of pancreatitis, the predictive values of ALP and bilirubin levels are rather poor in this group of patients.
A history of acute biliary pancreatitis correlated poorly with the presence of CBDS. The risk for CBDS was only 20% among patients with a history of acute biliary pancreatitis.
This low correlation between CBDS and a recent history of acute biliary pancreatitis was also observed in a previous study4 and is most probably explained by the likelihood that the stones that induce acute pancreatitis are often too small to remain trapped in the CBD. When they are expelled into the distal CBD from the gallbladder, these stones may only temporarily obstruct the flow of pancreatic juice into the duodenum, thereby giving rise to acute pancreatitis, and then continue their journey to the duodenum. Larger stones, however, seem to become trapped more proximally in the CBD where they do not interfere with the exocrine function of the pancreas and thus never cause pancreatitis. This theory is supported by findings of gallstones in the stools of 97% of patients with biliary pancreatitis.28
A history of acute cholecystitis was not significantly associated with an increased risk for CBDS. In fact, only 8.7% of patients with a history of ongoing or previous acute cholecystitis were diagnosed with CBDS, compared with 12% of patients without a history of cholecystitis. Common bile duct stones were thus more common in patients without a history of acute cholecystitis. This may be because the impacted stone that provokes the cholecystitis may also have a protective effect on the CBD by preventing additional gallstones from entering the bile ducts from the gallbladder, such as may occur during manipulation in surgery.
Common bile duct stones were found in 22% of patients at the university clinic, 11% of patients at the municipal clinic and 5% of patients at the short-stay clinic. Operating time, conversion rate and the frequency of primary open operations were all considerably higher at the university clinic than at the short-stay clinic, which indicates that the preoperative planning succeeded in directing the more complicated procedures to the university clinic (Table 1).
A common problem in many previous studies refers to the incomplete description of the patient sample. This may be a cause of selection bias. Furthermore, a reference standard for diagnosing CBDS, such as IOC, is not always described. A large meta-analysis performed in 1996 and based on 2221 articles dealing with predictors of CBDS was able to include only 22 studies.29
This is the first prospective, population-based study dedicated to this subject, and the only study to assess the predictive ability of bilirubin and ALP levels sampled weeks before surgery, as is necessary in order to facilitate the planning of the procedure. The time from sampling of liver function tests to surgery varied from a few days to 4 weeks. Thus, it is possible that patients with CBDS passed their stones into the duodenum before surgery, or that stones may have migrated into the CBD after the samples were obtained. The migration of CBDS is probably a more dynamic process than previously thought. In a study by Collins et al., in which all patients with asymptomatic CBDS diagnosed by IOC during cholecystectomy were managed conservatively and followed with repeated postoperative cholangiography through a transcystic catheter, up to 30% of the CBDS were observed to pass spontaneously within 6 weeks of cholecystectomy.30 However, this study protocol was not designed to analyse the predictive values of ALP and bilirubin levels sampled at different time intervals before surgery.
A weakness of the present study refers to the fixed cut-off levels for ALP and bilirubin. Our original reason for using fixed cut-off levels referred to the fact that obstructing processes in the CBD should be suspected if only one of the predictors was elevated. We also used total bilirubin and not conjugated bilirubin as a predictor, principally because, by tradition, conjugated bilirubin is not a standard analysis in many Swedish hospitals and has to be ordered separately. By differentiating between conjugated and unconjugated bilirubin, it is possible to distinguish between elevated levels caused by a mechanical CBD obstruction, as in the case of CBDS, or by increased production of haem, as seen in haemolysis, or defective conjugation capacity, as in Gilbert's syndrome. Both mechanisms, however, cause total bilirubin to be elevated.
Conclusions
Although elevated ALP and bilirubin levels were the best predictors of CBDS in the present study, the number of false positive and false negative findings was relatively high and less than half of the patients with elevated bilirubin or ALP were found to have CBDS on IOC. The best agreement was seen in patients who underwent elective surgery, which indicates that mechanisms other than CBDS were responsible for elevating ALP and/or bilirubin in patients with acute cholecystitis or a history of acute biliary pancreatitis. Normal bilirubin or ALP levels seen in patients with CBDS verified at surgery may reflect the presence of partially obstructive stones or the migration of stones from the gallbladder to the CBD in the interval between blood sampling and surgery, or even during surgery when the gallbladder is manipulated. Elevated liver function values in the absence of CBDS may reflect the migration of stones from the CBD to the duodenum, sludge, CBD compression caused by pancreatitis, or hepatic dysfunction caused by shock or Gilbert's syndrome. Pancreatitis was not found to be a significant predictor of CBDS. Common bile duct stones were less common in patients with cholecystitis, indicating that an impacted stone provoking cholecystitis may have a protective effect against CBDS by preventing additional stones from translocating from the gallbladder into the CBD.
Conflicts of interest
None declared.
References
- 1.Menezes N, Marson LP, Debeaux AC, Muir IM, Auld CD. Prospective analysis of a scoring system to predict choledocholithiasis. Br J Surg. 2000;87:1176–1181. doi: 10.1046/j.1365-2168.2000.01511.x. [DOI] [PubMed] [Google Scholar]
- 2.Trondsen E, Edwin B, Reiertsen O, Faerden AE, Fagertun H, Rosseland AR. Prediction of common bile duct stones prior to cholecystectomy: a prospective validation of a discriminant analysis function. Arch Surg. 1998;133:162–166. doi: 10.1001/archsurg.133.2.162. [DOI] [PubMed] [Google Scholar]
- 3.Trondsen E, Edwin B, Reiertsen O, Fagertun H, Rosseland AR. Selection criteria for endoscopic retrograde cholangiopancreaticography (ERCP) in patients with gallstone disease. World J Surg. 1995;19:852–856. doi: 10.1007/BF00299784. discussion 857. [DOI] [PubMed] [Google Scholar]
- 4.Cohen ME, Slezak L, Wells CK, Andersen DK, Topazian M. Prediction of bile duct stones and complications in gallstone pancreatitis using early laboratory trends. Am J Gastroenterol. 2001;96:3305–3311. doi: 10.1111/j.1572-0241.2001.05330.x. [DOI] [PubMed] [Google Scholar]
- 5.Yang MH, Chen TH, Wang SE, Tsai YF, Su CH, Wu CW, et al. Biochemical predictors for absence of common bile duct stones in patients undergoing laparoscopic cholecystectomy. Surg Endosc. 2008;22:1620–1624. doi: 10.1007/s00464-007-9665-2. [DOI] [PubMed] [Google Scholar]
- 6.Notash AY, Salimi J, Golfam F, Habibi G, Alizadeh K. Preoperative clinical and paraclinical predictors of choledocholithiasis. Hepatobiliary Pancreat Dis Int. 2008;7:304–307. [PubMed] [Google Scholar]
- 7.Sgourakis G, Dedemadi G, Stamatelopoulos A, Leandros E, Voros D, Karaliotas K. Predictors of common bile duct lithiasis in laparoscopic era. World J Gastroenterol. 2005;11:3267–3272. doi: 10.3748/wjg.v11.i21.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prat F, Meduri B, Ducot B, Chiche R, Salimbeni-Bartolini R, Pelletier G. Prediction of common bile duct stones by non-invasive tests. Ann Surg. 1999;229:362–368. doi: 10.1097/00000658-199903000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Santvoort HC, Bakker OJ, Besselink MG, Bollen TL, Fischer K, Nieuwenhuijs VB, et al. Dutch Pancreatitis Study Group. Prediction of common bile duct stones in the earliest stages of acute biliary pancreatitis. Endoscopy. 2011;43:8–13. doi: 10.1055/s-0030-1255866. [DOI] [PubMed] [Google Scholar]
- 10.Statistics Sweden. 2007. http://www.scb.se/[Accessed 26 April 2011]
- 11.Henricsson R, Karlsson B-M, Rasmussen C, Sandblom G, Darkahi B, Liljeholm H, et al. Management programme for gallstone disease in Uppsala County, Sweden. 2005. http://www.akademiska.se/upload/33480/Anvisningar%20f%C3%B6r%20behandling%20av%20patienter%20med%20gallstenssjukd%E2%80%A6.pdf[Accessed 19 September 2007]
- 12.Cuschieri A, Lezoche E, Morino M, Croce E, Lacy A, Toouli J, et al. EAES multicentre prospective randomized trial comparing two-stage vs single-stage management of patients with gallstone disease and ductal calculi. Surg Endosc. 1999;13:952–957. doi: 10.1007/s004649901145. [DOI] [PubMed] [Google Scholar]
- 13.Suc B, Escat J, Cherqui D, Fourtanier G, Hay JM, Fingerhut A, et al. Surgery vs endoscopy as primary treatment in symptomatic patients with suspected common bile duct stones: a multicentre randomized trial. French Association for Surgical Research. Arch Surg. 1998;133:702–708. doi: 10.1001/archsurg.133.7.702. [DOI] [PubMed] [Google Scholar]
- 14.Urbach DR, Khajanchee YS, Jobe BA, Standage BA, Hansen PD, Swanstrom LL. Cost-effective management of common bile duct stones: a decision analysis of the use of endoscopic retrograde cholangiopancreatography (ERCP), intraoperative cholangiography, and laparoscopic bile duct exploration. Surg Endosc. 2001;15:4–13. doi: 10.1007/s004640000322. [DOI] [PubMed] [Google Scholar]
- 15.MacFadyen BV. Intraoperative cholangiography: past, present, and future. Surg Endosc. 2006;2:436–440. doi: 10.1007/s00464-006-0053-0. [DOI] [PubMed] [Google Scholar]
- 16.Videhult P, Sandblom G, Rasmussen IC. How reliable is intraoperative cholangiography as a method for detecting common bile duct stones? A prospective population-based study on 1171 patients. Surg Endosc. 2009;23:304–312. doi: 10.1007/s00464-008-9883-2. [DOI] [PubMed] [Google Scholar]
- 17.Kelly DA. Intestinal failure-associated liver disease: what do we know today? Gastroenterology. 2006;130(2) Suppl. 1:70–77. doi: 10.1053/j.gastro.2005.10.066. [DOI] [PubMed] [Google Scholar]
- 18.Thorbøll J, Vilmann P, Jacobsen B, Hassan H. Endoscopic ultrasonography in detection of cholelithiasis in patients with biliary pain and negative transabdominal ultrasonography. Scand J Gastroenterol. 2004;39:267–269. doi: 10.1080/00365520310008377. [DOI] [PubMed] [Google Scholar]
- 19.Elta GH. Sphincter of Oddi dysfunction and bile duct microlithiasis in acute idiopathic pancreatitis. World J Gastroenterol. 2008;14:1023–1026. doi: 10.3748/wjg.14.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen BT. Sphincter of Oddi dysfunction, part 2: evidence-based review of the presentations, with ‘objective’ pancreatic findings (types I and II) and of presumptive type III. Gastrointest Endosc. 2004;59:670–687. doi: 10.1016/s0016-5107(04)00297-4. [DOI] [PubMed] [Google Scholar]
- 21.Petersen BT. An evidence-based review of sphincter of Oddi dysfunction: part 1: presentations with ‘objective’ biliary findings (types I and II) Gastrointest Endosc. 2004;59:525–534. doi: 10.1016/s0016-5107(04)00012-4. [DOI] [PubMed] [Google Scholar]
- 22.McLoughlin MT, Mitchell RM. Sphincter of Oddi dysfunction and pancreatitis. World J Gastroenterol. 2007;13:6333–6343. doi: 10.3748/wjg.v13.i47.6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bosma PJ. Inherited disorders of bilirubin metabolism. J Hepatol. 2003;38:107–117. doi: 10.1016/s0168-8278(02)00359-8. [DOI] [PubMed] [Google Scholar]
- 24.Radu P, Atsmon J. Gilbert's syndrome – clinical and pharmacological implications. Isr Med Assoc J. 2001;3:593–598. [PubMed] [Google Scholar]
- 25.Tung BY, Carithers RL., Jr Cholestasis and alcoholic liver disease. Clin Liver Dis. 1999;3:585–601. doi: 10.1016/s1089-3261(05)70086-6. [DOI] [PubMed] [Google Scholar]
- 26.Roche SP, Kobos R. Jaundice in the adult patient. Am Fam Physician. 2004;69:299–304. [PubMed] [Google Scholar]
- 27.Weisiger RA. Hyperbilirubinemia, conjugated. 2007. http://www.emedicine.com/med/TOPIC1065.HTM[Accessed 26 September 2008]
- 28.Acosta MJ, Rossi R, Ledesma CL. The usefulness of stool screening for diagnosing cholelithiasis in acute pancreatitis. A description of the technique. Am J Dig Dis. 1977;22:168–172. doi: 10.1007/BF01072962. [DOI] [PubMed] [Google Scholar]
- 29.Abboud PA, Malet PF, Berlin JA, Staroscik R, Cabana MD, Clarke JR, et al. Predictors of common bile duct stones prior to cholecystectomy: a meta-analysis. Gastrointest Endosc. 1996;44:450–455. doi: 10.1016/s0016-5107(96)70098-6. [DOI] [PubMed] [Google Scholar]
- 30.Collins C, Maguire D, Ireland A, Fitzgerald E, O'Sullivan GC. A prospective study of common bile duct calculi in patients undergoing laparoscopic cholecystectomy: natural history of choledocholithiasis revisited. Ann Surg. 2004;239:28–33. doi: 10.1097/01.sla.0000103069.00170.9c. [DOI] [PMC free article] [PubMed] [Google Scholar]


