Abstract
Objectives:
The reference standard technique for the reconstruction of the extrahepatic biliary tree is Roux-en-Y hepaticojejunostomy. This procedure is not without complications and may not be feasible in some patients. This project sought to evaluate a novel approach for repairing common bile duct injuries with a biosynthetic graft. This allows for the reconstruction of the anatomy without necessitating an intestinal bypass.
Methods:
Study subjects were 11 mongrel hounds. Utilizing an open approach, the common bile duct was transected in each animal. A 1-cm graft of a synthetic bioabsorbable prosthesis was interposed over a 5-Fr pancreatic stent and sewn in place as an interposition tube graft with absorbable sutures. Intraoperative cholangiograms and monthly liver function tests were completed. Animals were killed at 6, 7, 8, 10 and 12 months.
Results:
The first five animals were killed early in the process of protocol development. One animal developed obstructive symptoms and was killed on postoperative day 14. The next five animals were longterm survivors without evidence of clinically significant graft stenosis. Mean alkaline phosphatase and total bilirubin were normal, at 140 U/l and 0.2 mg/dl, respectively. Histology showed the complete replacement of the graft with native tissue at 6 months.
Conclusions:
Biliary reconstruction using a synthetic bioabsorbable prosthetic as an interposition tube graft is feasible based on initial results.
Keywords: basic science < liver, basic science < biliary
Introduction
The introduction of laparoscopic techniques to address biliary pathology has been associated with a marked increase in the number of iatrogenic common bile duct (CBD) injuries.1,2 Notwithstanding the technological advances in endoscopic and minimally invasive surgical techniques, these complications necessitate an open Roux-en-Y hepaticojejunostomy reconstruction. This surgery is not without morbidities, which can be both acute and longterm, and may not be feasible in patients with surgically altered anatomy. The ability to re-establish bilioenteric continuity in the absence of an enteric bypass offers potential therapeutic advantages to both patient and surgeon. Select institutions have attempted to identify alternative therapeutic approaches to reconstructing the extrahepatic biliary tree with variable degrees of success.3–8 In this study, a biosynthetic tube graft was interposed between the transected ends of the CBD, reconstituting the biliary anatomy without an intestinal bypass.
Materials and methods
Eleven canines, each weighing 20–25 kg, were anaesthetized and subjected to midline laparotomies. Prophylactic doses of i.v. cephazolin were administered preoperatively and for 5 days postoperatively. The gallbladder was removed in a standard dome-down fashion. In each animal, a transcystic cholangiogram was performed to ensure normal anatomy. The CBD was sharply transected 1 cm distal to the origin of the cystic duct. End-to-end anastomoses were created proximally and distally to the interposition graft with interrupted 6–0 absorbable monofilament sutures over a 5-Fr plastic stent (Fig. 1). Each graft was individually fashioned with an intraluminal diameter of 3 mm. The material used to construct the graft was a proprietary bioabsorbable mesh composed of a polymer of polyglycolic acid and trimethylene carbonate (PGA : TMC) and commercially available as Bio-a® (WL Gore & Associates, Inc., Newark, DE, USA). Following this, omentum was brought up to the right upper quadrant and placed over the repair. In animals 2–5, Tisseel® (Baxter International, Inc., Deerfield, IL, USA) was introduced around the anastomosis. This step was omitted in subjects 6–11. A layered closure of the abdomen was performed. No drains were placed.
Figure 1.
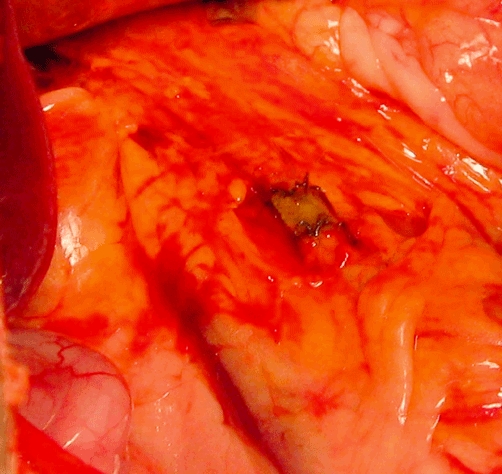
Post-implantation photograph of graft interposed between the two cut ends of the native common bile duct
Animals showing evidence of unrelieved pain or a suspected compromised repair were evaluated by the attending veterinarian whose recommendations in relation to the animal's suitability for continuation in the study were followed. Monthly liver function tests (alkaline phosphatase, total bilirubin, gamma-glutamyl transpeptidase [GGT], aspartate aminotransferase [AST] and alanine aminotransferase [ALT]) were drawn to assess the level of insult to the liver. At 6–8 weeks postoperatively, the animals were placed under general anaesthesia and endoscopic stent removal attempted.
One animal was killed at each of 6, 7, 8, 10 and 12 months, respectively. Following re-exploration of the abdomen, a cholangiogram was performed. The entirety of the extrahepatic biliary tree and a liver biopsy were then harvested from the animal, fixed in 10% neutral buffered formalin and sent to the histopathology laboratory for sectioning. Macroscopic assessment of the interposition graft was performed. Microscopic analysis included haematoxylin and eosin stains. The bile ducts were evaluated for evidence of inflammation, epithelialization of the neo-bile duct and incorporation of the graft into this structure.
Permission from the Ohio State University Medical Center Institutional Animal Care and Use Committee (IACUC) was obtained prior to beginning the study.
Results
Eleven animals were enrolled in the study. The first animal developed an anastomotic leak and was killed on postoperative day (PoD) 6. The next four dogs developed signs and symptoms consistent with cholangitis. They were killed at 3–6 days after the initial operation. In all four dogs, Tisseel® had been applied to the reconstruction. Necropsy showed the anastomoses to be intact, the stents in place and no evidence of bile extrusion. The sixth animal developed obstructive symptoms and was killed on PoD 14. At necropsy the stent was found to have migrated proximally and was no longer across the anastomosis, resulting in an acute anastomotic stenosis.
The final five animals survived to the predetermined dates of 6, 7, 8, 10 and 12 months post-surgery without complications or weight loss. In two animals the stent was verified to have passed spontaneously under fluoroscopy. In two animals the stent was successfully removed endoscopically. In a single animal the stent had migrated proximal to the anastomosis and could not be removed without disrupting the repair. In this instance, the stent was left in place. Necropsy revealed the abdominal cavities to be without bilious staining and showed that the graft had been grossly incorporated into the biliary tree (Fig. 2). Necropsy cholangiography showed some evidence of a dilated CBD, but no sign of a clinically significant anastomotic stricture as defined by laboratory abnormalities consistent with biliary obstruction (Fig. 3).
Figure 2.
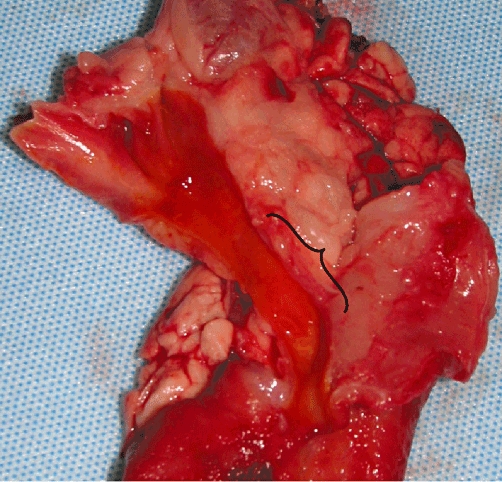
Explanted bile duct, pancreas and duodenum of a 6-month survivor without gross evidence of the interposed graft. The bracket denotes the area of graft implantation
Figure 3.
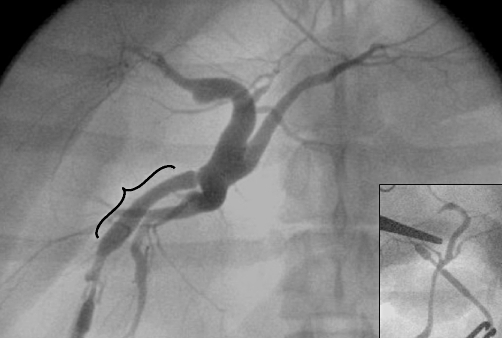
Necropsy cholangiogram with evidence of dilation secondary to biliary stasis and injection artefact, but without a focal graft-related stenosis. The bracket denotes the area of graft implantation. Inset: pre-implantation cholangiogram
In the animals that completed the study, median total bilirubin and alkaline phosphatase, AST and GGT remained within normal limits throughout the study. Median ALT was within normal ranges except for a minor increase during the third postoperative month (Table 1).
Table 1.
Median values in liver function tests in the final five animals
| Months post-surgery | Total bilirubin (0.3–0.5 mg/dla) | Alkaline phosphatase (5–131 IU/la) | Alanine aminotransferase (12–118 IU/la) | Aspartate aminotransferase (15–66 IU/la) | γ-glutamyl transpeptidase (1–12 IU/la) |
|---|---|---|---|---|---|
| 1 | 0.2 | 191 | 45 | 25 | 7 |
| 3 | 0.2 | 75 | 124 | 51 | 5 |
| 6 | 0.2 | 92 | 73 | 31 | 7 |
| 7 | 0.1 | 104 | 88 | 31 | 12 |
| 8 | 0.1 | 90 | 78 | 29 | 7 |
| 10 | 0.2 | 45 | 53 | 26 | 4 |
| 12 | 0.1 | 69 | 45 | 26 | 6 |
Normal range
Histological analysis of the graft at 2 weeks showed increased thickness of the wall with marked transmural neutrophile and lymphoplasma cell infiltrate and granulation tissue (Fig. 4A, B). In the grafted area, ulceration with remaining undigested synthetic polymers graft admixed with foreign body-type multinucleated giant cell formation was present. No re-epithelialization was identified at this time-point. The grafted bile duct at 6 months showed quite different morphological findings (Fig. 4C). Although focal erosion with some chronic lymphoplasma cell infiltrate was present, the majority of the area was lined with a single layer of re-epithelialized tall columnar cells overlying collagenous connective tissue. At 8, 10 and 12 months, the bile duct appeared to be no different from a normal benign bile duct (Fig. 4D, E). Biopsies of the liver showed no significant pathological changes.
Figure 4.
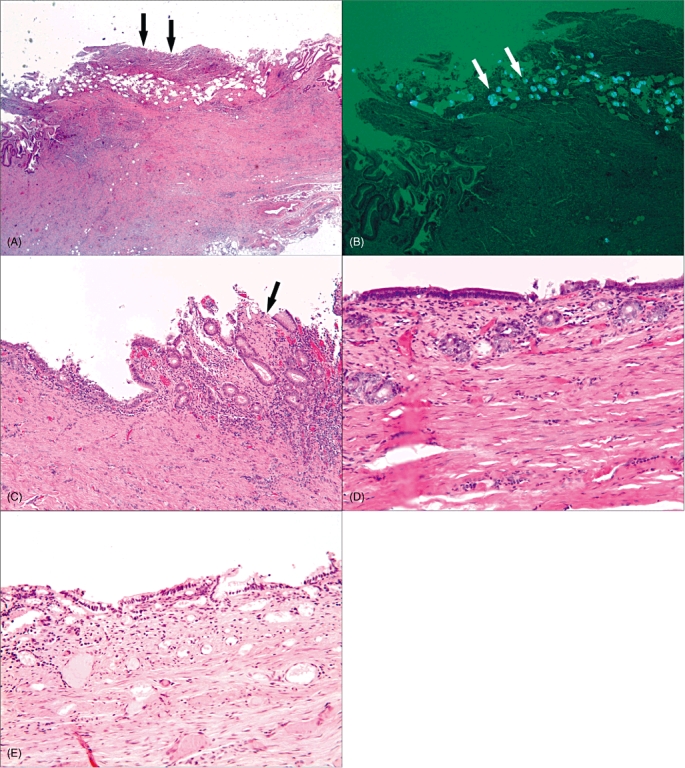
(A, B) Two-week specimen showing a thickened wall with transmural acute and chronic inflammation and granulation tissue. An ulcer with partial synthetic graft polymers surrounded by foreign body-type multinucleated giant cells is indicated by arrowheads (haematoxylin and eosin stain; original magnification ×4). (B) Polarized light microscopy shows synthetic graft polymers and interconnected pores. (C) At 6 months, the graft is replaced by collagenous connective tissue and lined with a single layer of tall columnar epithelium. Focal erosion with chronic inflammation of lymphoplasma cell infiltrate remains in the right upper corner (arrow) (H&E stain; ×10). (D, E) At 8 months, the graft area is completely replaced by collagenous connective tissue lined with a single layer of tall columnar epithelium. There is little inflammation (H&E stain; [D]×20, [E]×20)
Discussion
Cholelithiasis is a disease process that affects more than 30 million Americans, resulting in approximately 750 000 surgical interventions for symptomatic relief each year.9 In 1990, Dubois et al. published a seminal paper describing laparoscopic cholecystectomy as a new and less invasive method to treat symptomatic gallstone disease.10 This adoption of the laparoscopic approach to biliary disease was associated with a concurrent increase in the number of CBD injuries.1,2,11 Furthermore, as the indications and techniques for liver transplantation have evolved, the number of biliary tract reconstructions performed annually has continued to increase.12 It is clear that the reconstruction of the biliary tree in transplant surgery and extrahepatic biliary pathology resulting from iatrogenic injury remains a therapeutic challenge for the hepatobiliary surgeon.
Notwithstanding this, surgical innovation has only nominally improved the repair of this pathology. A primary end-to-end anastomosis is commonly eschewed as a result of the stricture rate associated with a compromised blood supply and potential tension on the repair. The Roux-en-Y hepaticojejunostomy remains the reference standard repair and, although this does effectively reconstitute the bilioenteric anatomy, it requires a mobile, healthy small bowel and is not without its own complications, including acute and chronic cholangitis, recurrent strictures, anastomotic leaks and an increased risk for cholangiocarcinoma.3,6,9
A review of the literature shows that a small number of institutions are conducting novel research in an effort to redefine the manner in which this complication is addressed. Several groups have utilized their experience with vascular repair and transitioned that knowledge to the reconstruction of CBD injuries.4,5,13–16 Many of these studies were complicated by low patency rates and short lengths of follow-up.4,7,13 Others have employed different biosynthetic materials as patches or interposition grafts to bridge the defect in the biliary tree.6–8,17 Similarly to the aforementioned studies, most were of short duration, which makes extrapolation to a longterm model difficult.
This study evaluated the use of a new biosynthetic absorbable graft to bridge a defect in the CBD, reconstituting the extrahepatic biliary anatomy without an enteric bypass. The initial five animals were killed early in the process of protocol development. The first animal experienced an early anastomotic dehiscence, representing an error in surgical technique. In animals 2–5, an intact and patent anastomosis was noted on necropsy. In these animals, Tisseel® was used to help prevent early leaks through the porous graft material until neoepithelialization could begin. Given the appearance of the intact anastomoses and the clinical presentation of the animals, it is hypothesized that the fibrin sealant acted as an infectious nidus for enteric flora, resulting in the development of acute cholangitis. For this reason, the fibrin sealant was omitted from the final six animals. The sixth animal experienced an acute anastomotic obstruction secondary to stent migration. In this animal, the stenosis is presumed to have occurred secondary to normal postoperative oedema following biliary manipulation and suture placement. In this case, the oedema is hypothesized to have been significant enough to occlude drainage through the neo-bile duct. This animal may have convalesced without complication if not for the stent movement.
The final five animals all survived for the predetermined periods of 6, 7, 8, 10 and 12 months, respectively, without complications. In four subjects the stents were removed or verified as having passed. In the last animal the stent had migrated to an intrahepatic position and was no longer across the anastomosis. As this did not influence the maturation of the repair, this stent positioning was not addressed further. This is a critical issue as a retained trans-anastomotic stent would mask a clinically significant stricture, potentially invalidating conclusions drawn from the study.
Once the technique was refined, there was no evidence of graft rejection or clinically significant anastomotic stenosis in the final five animals. Results of initial liver function tests (LFTs) were elevated in each of these animals. This can be attributed to normal postoperative surgical oedema at both the anastomosis and dissection bed. In the preponderance of cases, LFTs indicated normal function within 3 weeks (Table 1). This finding is consistent with the premise that dissection-related oedema was the initiating factor for the LFT abnormalities. Liver function tests in two animals showed more persistent minor elevations. However, these values trended towards normalization to the predetermined time of death. Perhaps most importantly, no animals showed clinical evidence of hyperbilirubinaemia or lack of incorporation of the interposition graft.
In each of the final six animals, the graft successfully functioned as a tissue scaffold, allowing for ingrowth of native tissues to reconstitute the bilioenteric anatomy. As illustrated in animal 6, within 2 weeks of implantation the graft was almost completely incorporated into a neo-bile duct with evidence of acute inflammation and granulation tissue. At 6 months, only minimal chronic inflammation was present. Perhaps most importantly, the majority of the bile duct was lined with a single layer of re-epithelialized tall columnar cells overlying collagenous connective tissue. Histological assessments of the final three specimens resembled that of a normal bile duct. In the final five animals, liver biopsies failed to demonstrate any intrahepatic biliary pathology whatsoever. This would suggest that the graft was successfully incorporated without negative sequelae around the anastomosis and within the liver parenchyma distant to the implantation site.
Conclusions
Biliary reconstruction using a synthetic bioabsorbable prosthetic as an interposition tube graft is feasible. Anastomotic stenosis does not appear to be clinically significant, but remains a concern. Additional study is necessary to ascertain the significance of this complication and the durability of these preliminary results.
Acknowledgments
This study was supported in part by WL Gore & Associates, Inc. (Newark, DE, USA) and the American Hepato-Pancreato-Biliary Association Surgical Technology Assessment and Outcomes Research Grant for 2009.
Conflicts of interest
None declared.
References
- 1.Connor S, Garden OJ. Bile duct injury in the era of laparoscopic cholecystectomy. Br J Surg. 2006;93:158–168. doi: 10.1002/bjs.5266. [DOI] [PubMed] [Google Scholar]
- 2.Gigot J, Etienne J, Aerts R, Wibin E, Dallemagne B, Deweer F, et al. The dramatic reality of biliary tract injury during laparoscopic cholecystectomy. An anonymous multicentre Belgian survey of 65 patients. Surg Endosc. 1997;11:1171–1178. doi: 10.1007/s004649900563. [DOI] [PubMed] [Google Scholar]
- 3.Seeliger H, Furst A, Zulke C, Jauch KW. Surgical management of bile duct injuries following laparoscopic cholecystectomy: analysis and follow-up of 28 cases. Langenbecks Arch Surg. 2002;389:286–293. doi: 10.1007/s00423-002-0330-x. [DOI] [PubMed] [Google Scholar]
- 4.Ren W. Experimental study on repair of bile duct defects with expanded polytetrafluoroethylene. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2001;15:305–307. [PubMed] [Google Scholar]
- 5.Heistermann H, Palmes D, Stratmann U, Hohlbach G, Hierlemann H, Langer M, et al. A new technique for reconstruction of the common bile duct by an autologous vein graft and a biodegradable endoluminal stent. J Invest Surg. 2006;19:57–60. doi: 10.1080/08941930500444560. [DOI] [PubMed] [Google Scholar]
- 6.Rosen M, Ponsky J, Petras R, Fanning A, Brody F, Duperier F. Small intestinal submucosa as a bioscaffold for biliary tract regeneration. Surgery. 2002;132:480–486. doi: 10.1067/msy.2002.126505. [DOI] [PubMed] [Google Scholar]
- 7.Aikawa M, Miyazawa M, Okamoto K, Toshimitsu Y, Torii T, Okada K, et al. A novel treatment for bile duct injury with a tissue-engineered bioabsorbable polymer patch. Surgery. 2010;147:575–580. doi: 10.1016/j.surg.2009.10.049. [DOI] [PubMed] [Google Scholar]
- 8.Miyazawa M, Torii T, Toshimitsu Y, Okada K, Koyama I, Ikada Y. A tissue-engineered artificial bile duct grown to resemble the native bile duct. Am J Transplant. 2005;5:1541–1547. doi: 10.1111/j.1600-6143.2005.00845.x. [DOI] [PubMed] [Google Scholar]
- 9.Sicklick JK, Camp MS, Lillemoe KD, Melton GB, Yeo CJ, Campbell KA, et al. Surgical management of bile duct injuries sustained during laparoscopic cholecystectomy: perioperative results in 200 patients. Ann Surg. 2005;241:786–792. doi: 10.1097/01.sla.0000161029.27410.71. discussion 793–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubois F, Icard P, Berthelot G, Levard H. Coeliscopic cholecystectomy. Preliminary report of 36 cases. Ann Surg. 1990;211:60–62. doi: 10.1097/00000658-199001000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melton GB, Lillemoe KD, Cameron JL, Sauter PA, Coleman J, Yeo CJ. Major bile duct injuries associated with laparoscopic cholecystectomy: effect of surgical repair on quality of life. Ann Surg. 2002;235:888–895. doi: 10.1097/00000658-200206000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merion RM. Current status and future of liver transplantation. Semin Liver Dis. 2010;30:411–421. doi: 10.1055/s-0030-1267541. [DOI] [PubMed] [Google Scholar]
- 13.Heistermann HP, Palmes D, Hierlemann H. Reconstruction of bile duct lesions by an autologous vein graft and a biodegradable endoluminal stent in an animal model: technique and clinical impact. Zentralbl Chir. 2003;128:952–957. doi: 10.1055/s-2003-44803. [DOI] [PubMed] [Google Scholar]
- 14.Palmes D, Wolters H, Spiegel HU, M Ller E, Minin E, Heistermann HP. Morphological changes during creation of a neo-bile duct using a vein and a biodegradable endoluminal stent. J Invest Surg. 2009;22:435–444. doi: 10.3109/08941930903410791. [DOI] [PubMed] [Google Scholar]
- 15.Gomez NA, Alvarez LR, Mite A, Andrade JP, Alvarez JR, Vargas PE, et al. Repair of bile duct injuries with Gore-Tex vascular grafts: experimental study in dogs. J Gastrointest Surg. 2002;6:116–120. doi: 10.1016/s1091-255x(01)00038-5. [DOI] [PubMed] [Google Scholar]
- 16.Christensen M, Laursen HB, Rokkjaer M, Jensen PF, Yasuda Y, Mortensen FV. Reconstruction of the common bile duct by a vascular prosthetic graft: an experimental study in pigs. J Hepatobiliary Pancreat Surg. 2005;12:231–234. doi: 10.1007/s00534-005-0976-1. [DOI] [PubMed] [Google Scholar]
- 17.Nakashima S, Nakamura T, Miyagawa K, Yoshikawa T, Kin S, Kuriu Y, et al. In situ tissue engineering of the bile duct using polypropylene mesh-collagen tubes. Int J Artif Organs. 2007;30:75–85. doi: 10.1177/039139880703000110. [DOI] [PubMed] [Google Scholar]


