Abstract
Background:
Microwave ablation (MWA) is increasingly utilized in the treatment of hepatic tumours. Promising single-centre reports have demonstrated its safety and efficacy, but this modality has not been studied in a prospective, multicentre study.
Methods:
Eighteen international centres recorded operative and perioperative data for patients undergoing MWA for tumours of any origin in a voluntary Internet-based database. All patients underwent operative MWA using a 2.45-GHz generator with a 5-mm antenna.
Results:
Of the 140 patients, 114 (81.4%) were treated with MWA alone and 26 (18.6%) were treated with MWA combined with resection. Multiple tumours were treated with MWA in 40.0% of patients. A total of 299 tumours were treated in these 140 patients. The median size of ablated lesions was 2.5 cm (range: 0.5–9.5 cm). Tumours were treated with a median of one application (range: 1–6 applications) for a median of 4 min (range: 0.5–30.0 min). A power setting of 100 W was used in 78.9% of cases. Major morbidity was 8.3% and in-hospital mortality was 1.9%.
Conclusions:
These multi-institution data demonstrate rapid ablation time and low morbidity and mortality rates in patients undergoing operative MWA with a high rate of multiple ablations and concomitant hepatic resection. Longterm follow-up will be required to determine the efficacy of MWA relative to other forms of ablative therapy.
Keywords: microwave ablation, liver, operative
Introduction
Microwave ablation (MWA) represents the newest generation of thermal ablation technologies; it is increasingly utilized in the treatment of primary and metastatic hepatic malignancies and a variety of MWA systems are now clinically available. Microwave technology utilizes energy at frequencies ranging from 915 MHz to 9.2 GHz and differs from other thermal ablation modalities, most notably radiofrequency ablation (RFA), in many key aspects.1
Radiofrequency ablation requires a closed circuit between the ablation probe and the grounding pad for the flow of electrical current; therefore, RFA is subject to the distortion of the ablation zone when this current follows the path of least resistance, as well as increased tissue impedance if the tissue is heated too rapidly or to > 100 °C because the tissue chars and/or the tissue water boils.1 Radiofrequency ablation also appears susceptible to ‘heat sink’ whereby thermal energy is diverted from the target tissue by the flow of blood through adjacent vessels.2–5 Microwave ablation does not rely on the flow of current; rather, it causes coagulative necrosis by generating an electromagnetic field inside which a rapid and homogeneous heating of tissue occurs.6 The ablation of tissue within this field is not subject to the same limitations as RFA; however, outside the microwave near field, heat from the ablation zone is conducted through the surrounding tissue in a fashion similar to that seen in RFA, and the heat sink effect is apparent. In combination, these different properties of MWA allow a larger, more homogeneous and more predictable ablation zone to be accomplished in a shorter period of time. Microwave technology also allows for the simultaneous treatment of multiple tumours, in which RFA is technically limited.
Despite its apparent advantages, MWA is still a relatively new ablative modality and current clinical trials consist of single-institution case series, albeit with promising results.7–11 Moreover, new MWA technology is rapidly becoming available for clinical use, and each new combination of generator and ablation antenna cannot be presumed to provide equivalent results. Therefore, it is increasingly important that clinical validation models are in place as technological innovation in this field progresses. One method of resolving this challenge is to design a multicentre prospective database that facilitates patient enrolment and provides a platform from which to critically evaluate a new surgical modality based on its complication profile and efficacy.12
Current MWA technology uses frequencies between 915 MHz and 9.2 GHz. The first reported open surgical MWA was performed in Japan over two decades ago using a 2.45-GHz system (Microtaze; Heiwa Electronic Industrial Co. Ltd, Osaka, Japan).13 This frequency has been in use in Europe and Asia since the 1990s. By contrast, microwave technology was first used in the USA in 2003 and involved a 915-MHz system (Vivant Medical, Inc., Mountain View, CA, USA).14 Both 915-MHz and 2.45-GHz systems are now employed in the USA.
The focus of this international database was the evaluation of the safety and efficacy of MWA performed using the commercially available 2.45-GHz Acculis V generator with a 5-mm antenna (Acculis Ltd/Microsulis Medical Ltd, Denmead, UK) during open or laparoscopic operations.
Materials and methods
A secure Internet-based database was created in which de-identified data on MWAs could be voluntarily and independently recorded. All ablations were performed using the commercially available Microsulis 2.45-GHz Acculis V generator with a 5-mm antenna (Fig. 1). Thirty-six centres originally enrolled for participation in this study; however, data were recorded at the 18 international centres listed in Table 1. Patients with hepatic tumours (primary or metastatic) treated with this 2.45-GHz MWA system were enrolled between January 2008 and March 2010. Each centre adhered to the specific policies mandated by the institutional review board of its respective institution. The database website consisted of four separate data entry sections for the following categories of information: indications for ablation; operative data; morbidity and mortality, and recurrence and follow-up. Data input included indications for ablation (tumour aetiology) and operative variables (type of treatment, ablation power setting, duration of application, number of applications, lesion diameter). Outcome variables included major and minor complications, as well as in-hospital, 30-day and 90-day mortality rates. Follow-up data included length of follow-up and recurrence as identified by post-ablation radiological imaging consisting of either magnetic resonance imaging (MRI) or computed tomography (CT) according to the standard of the particular institution. Recurrence was categorized according to the site at which it occurred: Local recurrence was defined as viable tumour at the previous ablation site noted on follow-up and incomplete ablation was represented by residual tumour at the ablation zone discovered on the first post-treatment radiological imaging. Regional recurrence was defined as the appearance of a new lesion in the liver located at a site distinct from the ablation site.
Figure 1.
(A) The 2.45-GHz generator and (B) 5-mm antenna
Table 1.
Institutions contributing patient data to the study database (n = 18)
| Leicester Royal Infirmary, Leicester, UK | Jersey Shore Hospital, Jersey Shore, NJ, USA |
| North Manchester General Hospital, Manchester, UK | Hospital Beaujon, Paris, France |
| North Hampshire Hospital, Basingstoke, UK | Bern University Hospital, Bern, Switzerland |
| Mater Hospital, Belfast, UK | Agia Olga Hospital, Athens, Greece |
| Johns Hopkins University Hospital, Baltimore, MD, USA | Haut Pierre Hospital, Strasbourg, France |
| Renown Regional Medical Center, Reno, NV, USA | University Hospital Groningen, Groningen, the Netherlands |
| Hospital of Saint Raphael, New Haven, CT, USA | McCallum Cancer Centre, Melbourne, Vic, Australia |
| Roger Williams Cancer Center, Providence, RI, USA | Prince of Wales Hospital, Hong Kong SAR, China |
| Carolinas Medical Center, Charlotte, NC, USA | Canberra Hospital, Canberra, ACT, Australia |
Descriptive statistics (tumour size, number of applications/lesions, duration of application, follow-up, mortality and recurrence) were compared. sas Version 9.2 (SAS Institute, Inc., Cary, NC, USA) was used for analysis. A P-value of <0.05 was considered to indicate statistical significance.
Results
A total of 162 patients were enrolled in the database. Data on indications for ablation were recorded for 126 patients, complete operative details were recorded for 140 patients, morbidity and mortality were recorded for 117 patients, and follow-up imaging data were recorded for 83 patients. In order to appropriately assess this 2.45-GHz generator with 5-mm antenna MWA system, only the 140 patients for whom complete operative details were available were included in this analysis.
Tumour aetiology was recorded for 111 of these 140 patients (Table 2). Colorectal hepatic metastases (CRHM) represented the most prevalent aetiology (50.5%). The CRHM group demonstrated a median tumour size of 2.0 cm (mean: 2.27 cm; range: 0.3–7.0 cm) and a median of two tumours per patient (mean: 2.82; range: 1–8 tumours). The second most prevalent aetiology (34.2%) was hepatocellular carcinoma (HCC). In the HCC group, median tumour size was 2.8 cm (mean: 3.16 cm; range: 0.5–7.0 cm) and patients demonstrated a median of one tumour each (mean: 1.45; range: 1–6 tumours).
Table 2.
Diagnoses of patients treated with microwave ablation
| Aetiology | Patients, n (%) |
|---|---|
| Colorectal hepatic metastases | 56 (50.5%) |
| Hepatocellular carcinoma | 38 (34.2%) |
| Neuroendocrine tumour | 6 (5.4%) |
| Other | 11 (9.9%) |
| Total | 111 |
Microwave ablation alone was performed in 114 patients (81.4%) who exhibited a median of one tumour per patient (mean: 2.14; range: 1–9 tumours). Of these, 56 patients underwent multiple ablations performed for separate lesions; these constituted 40.0% of all patients and 49.1% of patients undergoing ablation alone. Twenty-one patients (15.0%) were treated with MWA and concomitant resection of separate lesions. Three patients (2.1%) were treated with a combination of MWA and resection of the same lesion and two patients (1.4%) were treated with a combination of ablation, resection and combined ablation/resection of multiple lesions.
A total of 299 individual tumours were treated in these 140 patients, of which 247 (82.6%) were treated with ablation alone, 47 (15.7%) were treated with resection alone and five (1.7%) were treated with combined ablation and resection. The median diameter of those tumours treated with ablation alone was 2.5 cm (mean: 2.7 cm; range: 0.5–9.5 cm), whereas the median diameter of resected tumours was 1.2 cm (mean: 2.1 cm; range: 0.3–9.0 cm). The median number of applications per tumour was 1.0 (mean: 1.59; range: 1–6 applications); 125 tumours (50.6%) were treated with only one application and another 63 tumours (25.5%) were treated with two applications. Of the 247 ablations, 195 (78.9%) were performed using the standard 100-W energy setting, and the median total ablation time per tumour was 4 min (mean: 5.25 min; range: 0.5–30 min). A comparison of energy deposition per tumour ablation (power × duration) with the diameter of the lesions demonstrated a significant increase in energy deposition with increasing tumour diameter (P < 0.0001) (Fig. 2).
Figure 2.
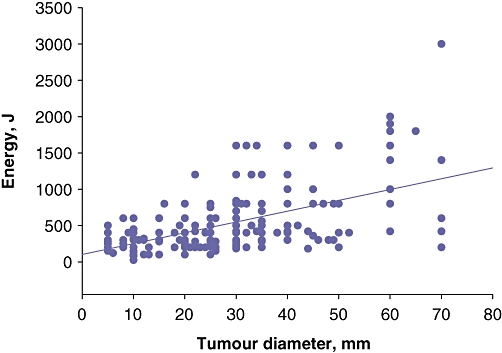
Correlation between energy deposition and tumour diameter
Morbidity data were available for 108 (77.1%) of the 140 patients, of whom 15 underwent concomitant liver resection and 93 underwent ablation alone (Table 3). Median length of stay was 4 days (range: 0–100 days). Twenty-four individual complications were noted, of which 11 were considered major and 13 were minor. Nineteen patients (17.6%) experienced at least one complication, and nine of these patients (8.3%) experienced at least one major complication. Five of the 15 patients (33.3%) who underwent concomitant liver resection and 14 of the 93 patients (15.1%) who underwent MWA alone experienced at least one complication.
Table 3.
Morbidity data for the 19 patients who experienced at least one complication following hepatic microwave ablation administered alone or in combination with hepatic resection
| Patient | Diagnosis | Tumour treatment | Complication | Mortality | ||
|---|---|---|---|---|---|---|
| Resection, n | Ablation, n | Combined, n | ||||
| 1 | NR | 1 | Liver failure chest infection | |||
| 2 | NR | 1 | Liver failure ischaemic bowel | In-hospital | ||
| 3 | CRHM | 1 | 2 | Bacteraemia | ||
| 4 | CRHM | 2 | 4 | Chest infection | ||
| 5 | HCC | 1 | Percutaneous drainage of liver and pelvic abscesses | |||
| 6 | CRHM | 2 | Chest infection | |||
| 7 | CRHM | 4 | 4 | Chest infection urinary tract infection | ||
| 8 | CRHM | 9 | Chest infection | |||
| 9 | IHC | 1 | Percutaneous drainage of fluid collection | 90-day | ||
| 10 | CRHM | 4 | Seroma aspirated | |||
| 11 | HCC | 2 | Postoperative haemorrhage | |||
| 12 | CRHM | 6 | Percutaneous drainage of fluid collection | |||
| 13 | HCC | 3 | 3 | Wound infection | 90-day | |
| 14 | HCC | 3 | Ascites controlled with diuretics | |||
| 15 | CRHM | 3 | Cardiac arrhythmia | |||
| 16 | NR | 1 | Percutaneous drainage of fluid collection | |||
| 17 | HCC | 1 | ARDS | |||
| 18 | Other | 1 | Chest infection, wound infection | |||
| 19 | HCC | 1 | NR | In-hospital | ||
NR, data not recorded; CRHM, colorectal hepatic metastases; HCC, hepatocellular carcinoma; IHC, intrahepatic cholangiocarcinoma; ARDS, adult respiratory distress syndrome.
Two of these 108 patients (1.9%) died while in hospital. In the total sample of 140 patients, 30- and 90-day mortality data were recorded for only 86 (61.4%) and 79 (56.4%) patients, respectively. Based on this limited data accrual, 30- and 90-day mortality rates were found to be 2.3% (n = 2) and 5.1% (n = 4), respectively.
Follow-up imaging data were recorded for 68 (48.6%) of the 140 patients for whom operative data were available. The median follow-up was 4 months (mean: 5.9 months; range: 0.5–21 months). Two patients (2.9%) were found to have incomplete ablations on the first follow-up radiological imaging. Hepatic recurrence was noted in 24 of these patients over this limited follow-up period; seven of these patients were noted to have local recurrence alone, eight were noted to have regional recurrence alone and two were found to have both local and regional recurrence. In seven patients, hepatic recurrence was noted; however, its relationship with the original ablation site was not detailed adequately, which makes the true incidence of local recurrence indeterminate.
Discussion
Hepatic resection and, in select patients, liver transplantation represent the standard treatments for primary liver tumours and hepatic resection is considered the standard treatment modality for patients with an increasing range of hepatic metastases. Multiple advances over the previous 30 years have expanded the indications for hepatic resection; however, despite these advances, many patients still do not meet criteria for resection or transplantation.15,16 Tumour-related issues such as tumour location and size, and extrahepatic disease, as well as patient-related concerns such as advanced underlying liver dysfunction and comorbid conditions, often preclude resection. For these patients, ablation provides a less morbid yet still potentially curative treatment option.17
In select patients with early HCC and cirrhosis, multiple large case series have demonstrated low perioperative morbidity and exceptional longterm outcomes with thermal ablation, of which RFA is the most utilized and studied ablative modality.18–21 With improving ablative options, some authors have even advocated the application of thermal ablation rather than hepatic resection in select patients. Two randomized controlled trials in patients with resectable early HCC with preserved hepatic function showed no survival difference between hepatic resection and RFA;22,23 however, study design issues in these trials have left the issue of equivalence in this highly selected patient population open to debate.24
Similarly, resection is the standard treatment for resectable CRHM; however, ablation is now routinely employed as one aspect of a multimodal, often staged, treatment algorithm aimed at achieving complete hepatic tumour clearance.25 For patients with advanced CRHM who do not meet resection criteria, thermal ablation also provides a low-morbidity treatment option that facilitates better survival than chemotherapy alone.26
Although ablative modalities have become an essential component of the liver-directed therapy arsenal, the inability to consistently predict the exact size and shape of the ablation zone limits their efficacy. Radiofrequency ablation, the most commonly used ablation modality, is particularly susceptible to this inconsistency as it requires the flow of an electrical current, which can be diverted away from the target tissue if a path of lower impedance is present; this is termed ‘electrical sink’. Similarly, when RFA is employed near large blood vessels, blood flow can divert thermal energy away from the targeted tissue in a process termed ‘heat sink’.2–5 Complete tumour eradication with RFA often requires multiple treatment sessions, probably because of the technical limitations described above.18,27 Furthermore, a recent meta-analysis of RFA for hepatic lesions has shown that local recurrence rates of HCC and CRHM are as high as 14.9%, again indicating that these technical limitations may lead to the under-treatment of tumours.27
Microwave technology differs fundamentally from radiofrequency technology and may address the shortcomings of RFA.1 Microwave ablation does not rely upon a flow of electrical current as in RFA; rather, microwave energy is broadcast from the ablation antenna to create an electromagnetic field within the surrounding tissue. Water molecules within this field oscillate rapidly in accordance with the microwave frequency, causing molecular friction and a rapid rise in thermal energy throughout the field.6 The result is a homogeneous, larger and more predictable ablation zone in which distortion by the heat sink effect is limited.5,28,29 Direct comparisons of percutaneous MWA and RFA in the treatment of HCC have demonstrated lower local recurrence rates for MWA.30–32
Another major limitation of RFA refers to the time required for individual applications, as well as the length of overall ablation time. Because RFA relies on current flow, rapid heating will cause charring of tissue and heating to temperatures > 100 °C will cause tissue water immediately adjacent to the RFA probe to boil, both of which events result in increased tissue impedance and either the overheating of the probe or the diminishing of the ablation zone.1 Hence, RFA requires a controlled increase in temperature and prolonged ablation times (routinely > 10 min per application), despite multiple innovations intended to control probe and tissue temperature.33,34 By contrast, the properties of MWA allow for a rapid increase in temperature throughout the microwave field without the concern of increased impedance, which, in turn, results in a much shorter application time.6 In addition, the current requirement of RFA does not allow for the simultaneous treatment of multiple lesions. Conversely, MWA allows for the use of multiple antennae, which enables lesions to be treated in parallel rather than in series, thus greatly reducing operative time in some cases.
This multi-institution, international, web-based database of uses of MWA administered with the Microsulis 2.45-GHz Acculis V generator with a 5-mm antenna provides important information regarding intraoperative variables and perioperative morbidity and mortality, although conclusions on the completeness of ablation and recurrence are limited.
Most previously reported data regarding MWA outcomes derive from single-institution reviews, in which patient- and surgeon-related variability may be limited.7–11 This is the first international collaborative effort to evaluate outcomes of MWA, incorporating multiple institutions and surgeons, as well as widely variable tumour aetiologies.
The operative details provided in this study demonstrate that this MWA system is routinely employed for multiple ablations as well as in concert with resection to treat a wide range of tumour types. The advantages provided by this system are evident in the short total ablation time per tumour (median: 4 min/tumour) and the low number of applications required to treat each tumour (over 50% can be treated with one application and over 75% can be treated with two applications). Clearly, its shorter ablation time gives MWA an advantage over other thermal ablation modalities by allowing for the rapid simultaneous ablation of multiple tumours, as well as for combined resection and ablation, which is often precluded by prolonged ablation times.
The manner in which this system was employed varied considerably in this study, probably as a result of the respective surgeons' assessments of energy requirements for complete ablation and their judgements of the potential for damage to adjacent structures. Over 20% of ablations were performed at a power setting below the standard 100 W and the range of total ablation time per tumour varied from 30 s to 30 min. The extent of tissue coagulation obtained with any thermal ablation system varies significantly depending upon the type of technology, as well as the generator and antenna design; therefore, in order to provide an estimation of ablation volume at any given power and time setting, each system requires thorough preclinical evaluation.35,36 This MWA system has been validated in a variety of tissue types and at a variety of power and time settings in animal studies.37,38 However, the full range of acceptable variations in power and time in a clinical setting remains to be defined. Extreme variations from the tested power and time settings may result in under-treatment and should be carefully considered. Unfortunately, this dataset does not allow for a more in-depth analysis of the relationship between power and time settings and local recurrence at each individual ablation site. It was observed, however, that both incomplete ablations occurred at tumour sites that were treated with the recommended 100-W power setting for 8 min.
This MWA system utilizing a 5-mm diameter antenna is designed for operative, rather than percutaneous, ablation. All MWAs included in this dataset were operative: nearly 20% of patients underwent concomitant liver resection and 40% of patients underwent MWA of multiple lesions; thus this case mix differs significantly from those of many of the larger series employing percutaneous MWA.10,31,32,39 The operative mortality rate of 1.9% and major morbidity rate of 8.3% in this setting appear to be comparable with those of single-institution series with comparable case mixes.9,11 One noteworthy major complication that has not been reported in prior series was the development of adult respiratory distress syndrome in one patient with HCC treated with MWA, which may have resulted from a concomitant fresh frozen plasma transfusion. Also important is the lack of certain complications thought to be specific to MWA, such as adjacent organ injury, skin burn at the antenna track and hepatic vein thrombosis.
The data presented in this study represent an important start in documenting the patterns of use, safety and efficacy of this MWA system as used in liver-directed therapy; however, several limitations to the study must be considered. Firstly, this database includes only patients treated in an operative setting with one type of MWA generator and one ablation antenna design; therefore, these results should not be generalized to all MWA procedures. Secondly, this multi-institution database consists of self-reported data, and the degree of access afforded to patient charts and imaging was institution-specific. Moreover, the voluntary nature of this study resulted in incomplete data collection, rendering some comparisons difficult, particularly when assessing follow-up imaging. This lack of information regarding disease status within a month of ablation for all patients made the confirmation of ablation success difficult and detracted from the potential benefit of this data collection system.
A solution to this problem would be to establish a single comprehensive data model in which incomplete data entry is limited by designating personnel at each centre to ensure thorough data capture. Alternatively, a prospective multi-institution trial with centralized imaging analysis and data collection would be advantageous.
Despite the limitations of this data collection model, the analysis of these multi-institution data does provide important information regarding the patterns of use, safety and efficacy of this MWA system as it is currently used clinically around the world. In summary, these data demonstrate that operative MWA utilizing this Microsulis 2.45-GHz Acculis V generator with a 5-mm antenna system is being performed, often for multiple lesions or in conjunction with hepatic resection, with a rapid ablation time, low morbidity and mortality profile, and high complete ablation rate for a variety of tumours across multiple institutions. Further collaborative efforts to combine outcomes-based data should focus on defining the ideal energy settings for different patient and lesion characteristics, improving follow-up imaging analysis to define recurrence rates, and investigating longterm outcomes. A randomized controlled trial is warranted to compare microwave technology with other ablation modalities in order to further delineate its utility in treating liver tumours.
Acknowledgments
Data collection for this study was supported by an education grant from Microsulis Medical Ltd to the Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland (AUGIS).
The authors submit this report on behalf of all members of the International Microwave Tumour Ablation Group (IMTAG). Data included in this analysis were provided by the authors and by IMTAG members Jacques Belghiti, Koert P. de Jong, Christos Dervenis, Safi Dokmak, Omar Farges, Christos Galanopoulos, Ellen Hagopian, Scott Helton, Derek A. O'Reilly and Ponnandai S. Somasundar.
Conflicts of interest
DML holds stock certificates in Microsulis Medical Ltd.
References
- 1.Brace CL. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: what are the differences? Curr Probl Diagn Radiol. 2009;38:135–143. doi: 10.1067/j.cpradiol.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim SK, Rhim H, Kim YS, Koh BH, Cho OK, Seo HS, et al. Radiofrequency thermal ablation of hepatic tumours: pitfalls and challenges. Abdom Imaging. 2005;30:727–733. doi: 10.1007/s00261-005-0304-x. [DOI] [PubMed] [Google Scholar]
- 3.Patterson EJ, Scudamore CH, Owen DA, Nagy AG, Buczkowski AK. Radiofrequency ablation of porcine liver in vivo: effects of blood flow and treatment time on lesion size. Ann Surg. 1998;227:559–565. doi: 10.1097/00000658-199804000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu DS, Raman SS, Limanond P, Aziz D, Economou J, Busuttil R, et al. Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumours. J Vasc Interv Radiol. 2003;14:1267–1274. doi: 10.1097/01.rvi.0000092666.72261.6b. [DOI] [PubMed] [Google Scholar]
- 5.Wright AS, Sampson LA, Warner TF, Mahvi DM, Lee FT., Jr Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology. 2005;236:132–139. doi: 10.1148/radiol.2361031249. [DOI] [PubMed] [Google Scholar]
- 6.Brace CL. Microwave ablation technology: what every user should know. Curr Probl Diagn Radiol. 2009;38:61–67. doi: 10.1067/j.cpradiol.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawamoto C, Ido K, Isoda N, Hozumi M, Nagamine N, Ono K, et al. Longterm outcomes for patients with solitary hepatocellular carcinoma treated by laparoscopic microwave coagulation. Cancer. 2005;103:985–993. doi: 10.1002/cncr.20880. [DOI] [PubMed] [Google Scholar]
- 8.Jagad RB, Koshariya M, Kawamoto J, Papastratis P, Kefalourous H, Patris V, et al. Laparoscopic microwave ablation of liver tumours: our experience. Hepatogastroenterology. 2008;55:27–32. [PubMed] [Google Scholar]
- 9.Martin RC, Scoggins CR, McMasters KM. Safety and efficacy of microwave ablation of hepatic tumours: a prospective review of a 5-year experience. Ann Surg Oncol. 2010;17:171–178. doi: 10.1245/s10434-009-0686-z. [DOI] [PubMed] [Google Scholar]
- 10.Liang P, Wang Y, Yu X, Dong B. Malignant liver tumours: treatment with percutaneous microwave ablation – complications among cohort of 1136 patients. Radiology. 2009;251:933–940. doi: 10.1148/radiol.2513081740. [DOI] [PubMed] [Google Scholar]
- 11.Iannitti DA, Martin RC, Simon CJ, Hope WW, Newcomb WL, McMasters KM, et al. Hepatic tumour ablation with clustered microwave antennae: the US Phase II Trial. HPB (Oxford) 2007;9:120–124. doi: 10.1080/13651820701222677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barkun JS, Aronson JK, Feldman LS, Maddern GJ, Strasberg SM, Balliol Collaboration et al. Evaluation and stages of surgical innovations. Lancet. 2009;374:1089–1096. doi: 10.1016/S0140-6736(09)61083-7. [DOI] [PubMed] [Google Scholar]
- 13.Seki T, Wakabayashi M, Nakagawa T, Itho T, Shiro T, Kunieda K, et al. Ultrasonically guided percutaneous microwave coagulation therapy for small hepatocellular carcinoma. Cancer. 1994;74:817–825. doi: 10.1002/1097-0142(19940801)74:3<817::aid-cncr2820740306>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 14.Simon CJ, Dupuy DE, Iannitti DA, Lu DS, Yu NC, Aswad BI, et al. Intraoperative triple antenna hepatic microwave ablation. AJR Am J Roentgenol. 2006;187:333–340. doi: 10.2214/AJR.05.0804. [DOI] [PubMed] [Google Scholar]
- 15.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 16.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 17.Sindram D, Lau KN, Martinie JB, Iannitti DA. Hepatic tumour ablation. Surg Clin North Am. 2010;90:863–876. doi: 10.1016/j.suc.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Lencioni R, Cioni D, Crocetti L, Franchini C, Pina CD, Lera J, et al. Early-stage hepatocellular carcinoma in patients with cirrhosis: longterm results of percutaneous image-guided radiofrequency ablation. Radiology. 2005;234:961–967. doi: 10.1148/radiol.2343040350. [DOI] [PubMed] [Google Scholar]
- 19.Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology. 2008;47:82–89. doi: 10.1002/hep.21933. [DOI] [PubMed] [Google Scholar]
- 20.Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Lerace T, Solbiati L, et al. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000;214:761–768. doi: 10.1148/radiology.214.3.r00mr02761. [DOI] [PubMed] [Google Scholar]
- 21.Tateishi R, Shiina S, Teratani T, Obi S, Sato S, Koike Y, et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer. 2005;103:1201–1209. doi: 10.1002/cncr.20892. [DOI] [PubMed] [Google Scholar]
- 22.Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu MD, Kuang M, Liang LJ, Xie XY, Peng BG, Lui GJ, et al. [Surgical resection versus percutaneous thermal ablation for early-stage hepatocellular carcinoma: a randomized clinical trial.] Zhonghua Yi Xue Za Zhi. 2006;86:801–805. [PubMed] [Google Scholar]
- 24.Livraghi T. Single HCC smaller than 2 cm: surgery or ablation: interventional oncologist's perspective. J Hepatobiliary Pancreat Sci. 2010;17:425–429. doi: 10.1007/s00534-009-0244-x. [DOI] [PubMed] [Google Scholar]
- 25.Abdalla EK, Adam R, Bilchik AJ, Jaeck D, Vauthey JN, Mahvi D. Improving resectability of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol. 2006;13:1271–1280. doi: 10.1245/s10434-006-9045-5. [DOI] [PubMed] [Google Scholar]
- 26.Siperstein AE, Berber E, Ballem N, Parikh RT. Survival after radiofrequency ablation of colorectal liver metastases: 10-year experience. Ann Surg. 2007;246:559–565. doi: 10.1097/SLA.0b013e318155a7b6. discussion 565–567. [DOI] [PubMed] [Google Scholar]
- 27.Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg. 2005;242:158–171. doi: 10.1097/01.sla.0000171032.99149.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: principles and applications. Radiographics. 2005;25(Suppl 1):69–83. doi: 10.1148/rg.25si055501. [DOI] [PubMed] [Google Scholar]
- 29.Yu NC, Raman SS, Kim YJ, Lassman C, Chang X, Lu DS. Microwave liver ablation: influence of hepatic vein size on heat-sink effect in a porcine model. J Vasc Interv Radiol. 2008;19:1087–1092. doi: 10.1016/j.jvir.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 30.Bhardwaj N, Strickland AD, Ahmad F, Dennison AR, Lloyd DM. Liver ablation techniques: a review. Surg Endosc. 2010;24:254–265. doi: 10.1007/s00464-009-0590-4. [DOI] [PubMed] [Google Scholar]
- 31.Lu MD, Xu HX, Xie XY, Yin XY, Chen JW, Kuang M, et al. Percutaneous microwave and radiofrequency ablation for hepatocellular carcinoma: a retrospective comparative study. J Gastroenterol. 2005;40:1054–1060. doi: 10.1007/s00535-005-1671-3. [DOI] [PubMed] [Google Scholar]
- 32.Xu HX, Xie XY, Lu MD, Chen JW, Yin XY, Xu ZF, et al. Ultrasound-guided percutaneous thermal ablation of hepatocellular carcinoma using microwave and radiofrequency ablation. Clin Radiol. 2004;59:53–61. doi: 10.1016/j.crad.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Hope WW, Arru JM, McKee JQ, Vrochides D, Aswad B, Simon CJ, et al. Evaluation of multiprobe radiofrequency technology in a porcine model. HPB (Oxford) 2007;9:363–367. doi: 10.1080/13651820701611218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solazzo SA, Ahmed M, Liu Z, Hines-Peralta AU, Goldberg SN. High-power generator for radiofrequency ablation: larger electrodes and pulsing algorithms in bovine ex vivo and porcine in vivo settings. Radiology. 2007;242:743–750. doi: 10.1148/radiol.2423052039. [DOI] [PubMed] [Google Scholar]
- 35.Hope WW, Schmelzer TM, Newcomb WL, Heath JJ, Lincourt AE, Norton HJ, et al. Guidelines for power and time variables for microwave ablation in a porcine liver. J Gastrointest Surg. 2008;12:463–467. doi: 10.1007/s11605-007-0248-2. [DOI] [PubMed] [Google Scholar]
- 36.Hope WW, Schmelzer TM, Newcomb WL, Heath JJ, Lincourt AE, Norton HJ, et al. Guidelines for power and time variables for microwave ablation in an in vivo porcine kidney. J Surg Res. 2009;153:263–267. doi: 10.1016/j.jss.2008.03.044. [DOI] [PubMed] [Google Scholar]
- 37.Hines-Peralta AU, Pirani N, Clegg P, Cronin N, Ryan TP, Liu Z, et al. Microwave ablation: results with a 2.45-GHz applicator in ex vivo bovine and in vivo porcine liver. Radiology. 2006;239:94–102. doi: 10.1148/radiol.2383050262. [DOI] [PubMed] [Google Scholar]
- 38.Awad MM, Devgan L, Kamel IR, Torbensen M, Choti MA. Microwave ablation in a hepatic porcine model: correlation of CT and histopathologic findings. HPB (Oxford) 2007;9:357–362. doi: 10.1080/13651820701646222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boutros C, Somasundar P, Garrean S, Saied A, Espat NJ. Microwave coagulation therapy for hepatic tumours: review of the literature and critical analysis. Surg Oncol. 2010;19:22–32. doi: 10.1016/j.suronc.2009.02.001. [DOI] [PubMed] [Google Scholar]


