Summary
Fascin is an actin bundling protein involved in filopodia assembly and cancer invasion and metastasis of multiple epithelial cancer types. Fascin forms stable actin bundles with slow dissociation kinetics in vitro [1] and is regulated by phosphorylation of serine 39 by protein kinase C (PKC) [2]. Cancer cells use invasive finger-like protrusions termed invadopodia to invade into and degrade extracellular matrix. Invadopodia have highly dynamic actin that is assembled by both Arp2/3 complex and formins [3, 4]; they also contain components of membrane trafficking machinery such as dynamin and cortactin [5] and have been compared with focal adhesions and podosomes [6, 7]. We show that fascin is an integral component of invadopodia and that it is important for the stability of actin in invadopodia. The phosphorylation state of fascin at S39, a PKC site, contributes to its regulation at invadopodia. We further implicate fascin in invasive migration into collagen I-Matrigel gels and particularly in cell types that use an elongated mesenchymal type of motility in 3D. We provide a potential molecular mechanism for how fascin increases the invasiveness of cancer cells and we compare invadopodia with invasive filopod-like structures in 3D.
Results and Discussion
Fascin is involved in microspike formation
Fascin is expressed in normal neonatal and adult human melanocytes and in several melanoma cell lines (Fig. S1A). Fascin co-localizes with filamentous actin at peripheral and dorsal spikes and filopodia in a highly metastatic cell line A375MM (Fig. S1B). Knockdown of fascin in A375MM cells (Fig. S1C) results in a >1.6-fold reduction in the formation of peripheral filopodia and dorsal microspikes without obviously affecting cell spreading or lamellipodia (Fig. S1D-E) consistent with [8]. Expression of GFP-tagged Xenopus tropicalis fascin (GFP-Xtfascin) [9], rescued filopodia formation in fascin siRNA cells (Fig. S1D-E). Thus we confirm a role for fascin in filopodia and we note its expression in normal melanocytes and melanoma cells.
Fascin is required for invadopodia formation and focal extracellular matrix degradation in human melanoma and breast cancer cells
Fascin is highly expressed at tumor invasive fronts [10, 11], suggesting a role in actively invading cancer cells in vitro. Endogenous and expressed GFP-fascin colocalized with actin and Arp2/3 complex, at invadopodia of CHL-1 melanoma cells (Figure 1A) and A375MM cells (Fig. S1F). A recent study of invadopodia dynamics revealed an actin comet-like organization, with cortactin, Arp2/3 complex and dynamin2 distributed throughout the comet structure and N-WASP at the comet head [3]. We observed numerous comet tails at degradation sites in A375MM cells, which appeared tethered near the head with a dynamic tail which spins somewhat reminiscent of a corkscrew (Fig. S2 and Movie S1). Only large invadopodia appeared as obvious comets. Arp2/3 complex, cortactin, and fascin localized throughout comet heads and tails (Fig. S2B-D, Movie S1) while N-WASP concentrated mostly in the head (Fig. S2A, Movie S1). N-WASP may be recruited to and activated at comet heads by phosphoinositides [12]. Similar comets appear on endocytic vesicles [13]. In addition to fascin, IRSP53, IRTKS and mDia2 [4], which are proteins that promote the formation of filopodia [14, 15], also localized at invadopodia (Fig. S2E-G and [4]). The provision of long parallel actin networks via DRFs and actin bundling proteins may provide both force production and structural stability at invadopodia. It isn’t clear why invadopodia use both Arp2/3 complex and DRFs to assemble actin, but the combined activity of Arp2/3 complex and fascin bundling can drive Listeria motility [16]. Invadopodia thus seem to be a hybrid structure with elements of comet tails and invasive filopodia.
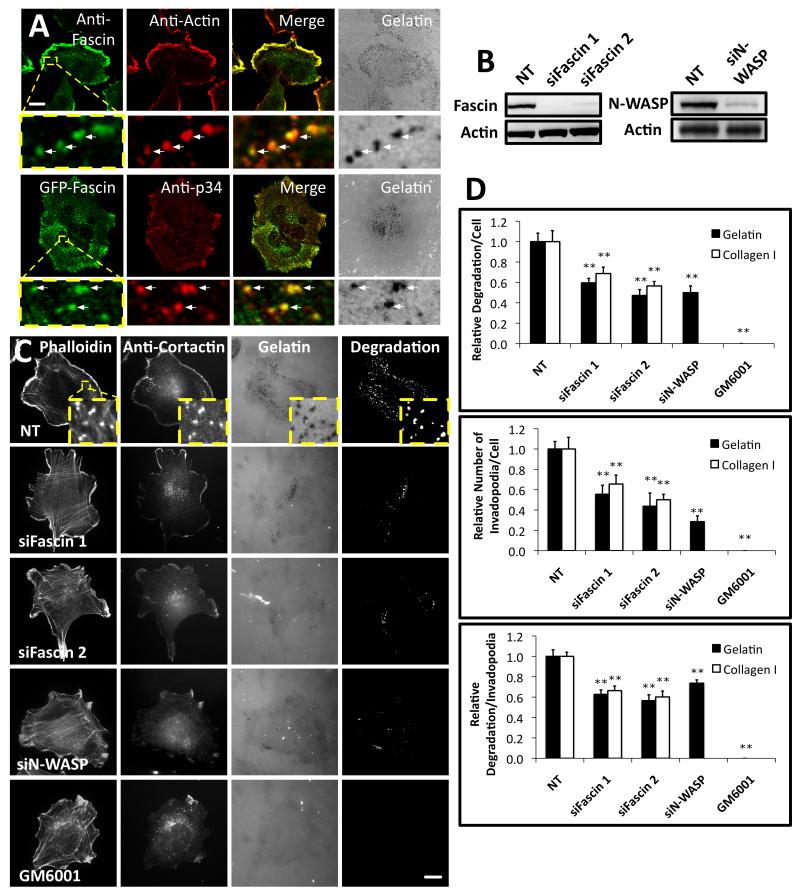
Figure 1. Fascin is important for invadopodia assembly and matrix degradation.
(A) Top, CHL-1 human melanoma cells on Oregon Green® 488 gelatin matrix (grey) with anti-fascin (primary 55K2) and anti-actin. Bottom, cells expressing GFP-fascin on Alexa594 gelatin matrix (grey) with anti-p34-Arc (ARPC2). Arrows- invadopodia. Bar, 10μm. (B) Western blots of lysates from CHL-1 cells with anti-fascin (Left), anti-N-WASP (Right) or anti-actin (Loading control, left and right) (C) CHL-1 cells expressing siRNAs as indicated or treated with 5 μM GM6001 on Oregon Green® 488 gelatin matrix with rhodamine phalloidin (F-actin) and anti-cortactin. Bars, 10μm. (D) Top: Relative area of degradation per cell on gelatin (Black) and Collagen I (White). Middle: number of invadopodia per cell on gelatin (Black) and Collagen I (White). Bottom: area of degradation per invadopodium of the ten biggest degradations per cell on gelatin (Black) and Collagen I (White). All error bars indicate (Mean ± SEM). **, P< 0.01 by t-test. Invadopodia were defined as puncta enriched for actin, cortactin and gelatin degradation. Similar experiments done with A375MM or MDA-MB231 cells are shown in Figure S1. Detailed analysis of fascin localization at invadopodia is shown in Figure S2. Movie 1 shows dynamics of actin comets at invadopodia and 3D reconstruction of fascin localization in comets.
Fascin depletion in CHL-1 cells (Fig. 1B-D), A375MM cells (Fig. S1G-H) or MDA-MB-231 breast adenocarcinoma cells (Fig. S1J-M) by siRNA significantly reduced the number of invadopodia and extent of matrix degradation both on gelatin and collagen matrix. Thus, a requirement for fascin in invadopodia was neither cell-type specific nor matrix specific. In addition, siRNA against p34-Arc (ARPC2) also reduced invadopodia (Fig S1K-M). Together, our results demonstrate a general importance of fascin for invadopodia formation on various matrices. We suggest that fascin is involved in invasive protrusion and that it may provide a scaffold for force production in invadopodia.
Invadopodia formation depends on regulation of fascin at serine 39
Previous studies showed that the phosphorylation of fascin at serine 39 regulates its F-actin bundling activity [8, 17, 18]. To determine whether fascin phosphorylation is likely to regulate invadopodia formation, we made fascin knockdown cells with GFP, GFP-Xtfascin, or two different phosphorylation state mutants of fascin. We used GFP-Xtfascin S33A, an active dephospho-mimic which retains its actin bundling activity, or GFP-Xtfascin S33D, an inactive phospho-mimic which has lost its actin bundling activity but which retains PKC binding activity [9]. Knockdown of fascin in CHL-1 or A375MM cells did not alter the expression of Xtfascin (Fig. 2B and S3B). Invadopodia formation of fascin knockdown cells was fully restored by wild-type GFP-Xtfascin or GFP-Xtfascin S33A in both CHL-1 and A375MM cells (Fig. 2C, Fig. S3C). Both of these proteins localized to invadopodia together with cortactin (Fig 2A, Fig. S3A). GFP-Xtfascin S33D failed to rescue invadopodia formation and appeared diffuse with weak localization to residual invadopodia (Fig. 2 A and C and Fig. S3 A and C). In addition, expression of human GFP-fascin S39E in A375MM cells significantly decreased matrix degradation (Fig. S3D). However, expression of human GFP-fascin or GFP-fascin S39A did not significantly affect extracellular matrix (ECM) degradation (Fig. S3D). Together, these data suggest that the actin bundling activity of fascin may be required for invadopodia formation and that the inactive phospho-mimic mutant of fascin has a dominant negative effect. The weak localization of fascin S33D at residual invadopodia suggests that PKC could be involved with targeting fascin to invadopodia. A complex of PKCμ-cortactin-paxillin forms at invadopodia of MDA-MB231 cells [19], but no role for PKC-α or -γ has yet been described. Fascin phosphorylation may be important for the cycling of fascin on and off dynamic actin structures or for the localization of PKC complexes. Integrin engagement regulates fascin phosphorylation status via PKC [20], so different ECM substrates and rigidity of matrix may also affect fascin dynamics in vivo.
Figure 2. Fascin regulation at serine 39 is important for invaopodia formation.
(A) CHL-1 cells stably expressing GFP, GFP-X.tropicalis Fascin, GFP-X. tropicalis Fascin S33A and GFP-X. tropicalis Fascin S33D were transfected with NT control siRNA or Fascin siRNA (siFascin 1), cultured on Alexa594 gelatin matrix and labeled with anti-cortactin. Arrows- invadopodia. Bars, 10μm (B) Western blot showing stable expression of GFP-X.tropicalis Fascin or mutants following fascin knockdown in CHL-1 cells (C) Top: Relative area of degradation on gelatin per cell (as in Figure 1). Middle: area of degradation per invadopod (as in Figure 1). Bottom: number of invadopodia per cell (as in Figure 1). Invadopodia were defined as cortactin puncta colocalizing with area of matrix degradation and error bars show (Mean ± SEM). **, P< 0.01 by t-test. See Figure S3 for related data with A375MM cells.
Fascin regulates actin stability at invadopodia
To understand how fascin controls actin dynamics at invadopodia, we examined the turnover rates of GFP-tagged actin, cortactin, neuronal Wiskott-Aldrich Syndrome Protein (N-WASP), p21-Arc (ARPC3) and fascin at invadopodia (Fig. S4A and Movie S2) using FRAP (fluorescence recovery after photobleaching). Although invadopodia persist for hours, cortactin, N-WASP and p21-Arc all rapidly recover to more than 80% of pre-bleach values, indicating a dynamic association with invadopodia. Actin, however, only recovered to about 70% pre-bleach level, suggesting 30% of actin is trapped (Fig. 3A). Although, fascin at invadopodia recovered to nearly pre-bleach level, the recovery rate of fascin was >10-fold slower than that of actin, cortactin, N-WASP and p21-Arc. Fascin turnover in filopodia was more rapid, with a half time of recovery of about 9s (Fig. 3A) and 96% recovery (Fig. 3B and S4A and in agreement with [8]). Our kinetics agree fairly closely with previously published values for turnover of actin, cortactin and Arp2/3 complex in lamellipodia [21] and suggest that fascin is particularly stable at invadopodia, perhaps to provide a platform for force production.
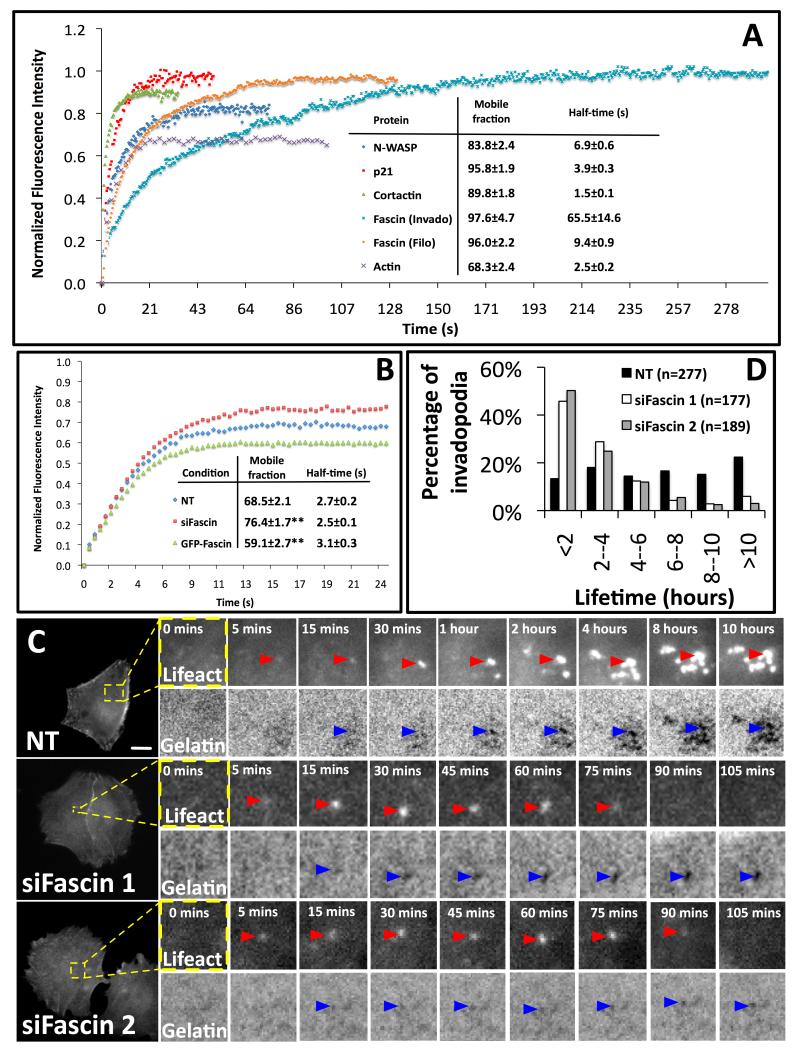
Figure 3. Fascin is stably associated with invadopodia and promotes long lifetime.
(A) The recovery kinetics of GFP-cortactin (green triangles) (n=28), GFP-actin (purple crosses) (n=31), GFP-p21-Arc (red squares) (n=30), GFP-N-WASP (blue diamond) (n=30) at invadopodia and GFP-fascin at invadopodia (light blue x) (n=22) and filopodia (orange dots) (n=25) after photobleaching. Mobile fraction and half time of recovery are indicated. (Mean ± SEM). (B) Recovery kinetics of mRFP-actin at invadopodia in cells expressing NT control siRNA (blue diamond) (n=50), GFP-fascin and control siRNA (EGFP-fascin) (green triangles) (n=47) or fascin siRNA (siFascin) (red squares) (n=45). Percentage mobile fraction and half time of recovery for each condition are indicated. (Mean ± SEM) **, P< 0.01 by t-test. (C) Timelapse image sequence of A375MM cells stably expressing GFP-Lifeact expressing control or fascin siRNA on gelatin matrix. Bars, 10μm. Red arrowheads - invadopodia and blue arrowheads - gelatin degradation. D, Time-lapse lifetime of invadopodia was calculated with data from 30 cells from at least three independent experiments. (n=the total number of invadopodia analyzed). See Figure S4 and Movies 2 and 3 for photobleaching and timelapse of invadopodia lifetime.
We also investigated whether the level of fascin in cells could change the actin dynamics at invadopodia. The actin dynamics were compared in fascin depleted and overexpressing cells using FRAP (Fig. S4B). The mobile fraction of actin at invadopodia was significantly increased in fascin knockdown cells and decreased in GFP-fascin overexpressing cells (Fig. 3B). However, the bulk turnover rates of actin at invadopodia were almost unaffected (Fig. 3B recovery half-time). Thus the amount the actin trapped at invadopodia is at least partially dependent on fascin, but rapidly cycling actin may not be bundled by fascin. This is consistent with the presence of unbundled highly dynamic actin, which is likely being rapidly turned over to produce force for motility and more stable actin in association with fascin to provide longevity of protrusions.
Since fascin stabilizes the filamentous actin at invadopodia and itself exchanges slowly at invadopodia, we postulated that fascin might function at invadopodia to provide a more stable platform for degradation of matrix. Most (>50%) invadopodia in A375MM cells persisted for up to and beyond 6 to 8 hours (Figure 3C-D and Movie S3). Fascin knockdown cells formed less invadopodia and invadopodia were generally smaller and more short-lived (approx. 50% invadopodia persist less than 2 h) (Figure 3C-D and Movie S3). Together, this data indicates that fascin is important for invadopodia stability and function by affecting the number and size of invadopodia, as well as their lifetime.
Mesenchymal type cancer cells selectively require fascin during cell invasion in a 3D environment
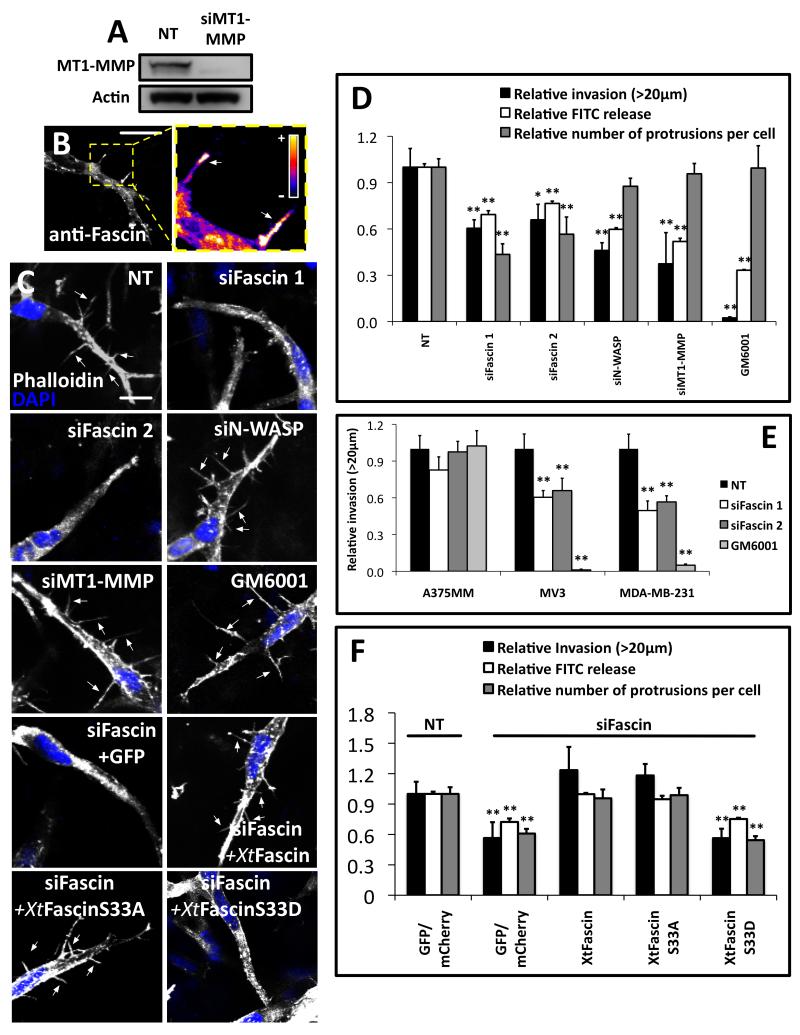
Since fascin stabilizes actin at invadopodia, we investigated a role for fascin in invasion into 3D matrix. Consistent with previous reports, migrating A375MM cells in collagen I-Matrigel appeared rounded, characteristic of the so-called amoeboid type of migration (Figure S1I) [22, 23], whereas CHL-1 cells appeared elongated and very spiky, with many long fine actin-rich protrusions, many of which showed abundant fascin (Figure 4B, Movie S4). A375MM cell invasion into 3D matrix was not significantly inhibited by fascin depletion (Fig. 4E), while CHL-1 cells depended on fascin for efficient matrix invasion and 3D collagen degradation (Fig. 4D). MV3 human melanoma cells and MDA-MB-231 cells which both display mesenchymal migration [24, 25] also depended on fascin for efficient invasion (Fig. 4E). Fascin knockdown changed the shape of CHL-1 cells migrating in 3D gels, with a decrease in filopod-like protrusions but some remaining small puncta and stubby protrusions (Figure 4C and Movie S4) but fascin knockdown had no apparent effect on the rounded shape of A375MM cells (Figure S1I). We reasoned that at least some of the finger-like protrusions in CHL-1 cells could be invasive filopodia and could aid the movement of mesenchymal type cells in 3D matrix. We therefore probed the requirements for other components of invadopodia, including N-WASP, which organizes Arp2/3 complex mediated actin assembly and the transmembrane matrix metalloprotease MT1-MMP, which controls matrix degradation at invadopodia. Knockdown of N-WASP, which causes a reduction of invadopodia (Figure 1 and [26, 27]), but not a change in the filopod-like protrusions in 3D, both reduced invasion into collagen I-Matrigel matrix and and 3D collagen degradation (Figure 4C and D). Likewise, the MMP inhibitor GM6001 or siRNA reduction of MT1-MMP (Figure 4A, C, D and E) did not affect filopod-like protrusions in 3D matrix, but significantly reduced invasion of mesenchymal-type cells and 3D collagen degradation. Treatments to inhibit protease activity did not affect invasion of A375MM cells (Figure 4D). Our results show that filopod-like protrusions alone are not sufficient for 3D invasion and suggest that invadopodia or their equivalents in 3D are required for mesenchymal type cells to degrade the matrix during 3D invasion in our conditions. We next examined the ability of Xtfascin S33A and Xtfascin S33D to rescue fascin knockdown cells. Xtfascin S33A restored the normal number of protrusions and relative invasion and also 3D collagen degradation while S33D did not (Figure 4C and F). Taken together, our data suggest that fascin is important for the formation of stable actin-based degradative structures (invadopodia on 2D matrix and their equivalent in 3D) and for filopod-like protrusions that contribute to invasion in 2D and in 3D. Formation of filopodia alone is not sufficient for optimal migration in 3D (in our experimental conditions) as knockdown of N-WASP and MT1-MMP reduced invasion, but didn’t reduce the formation of filopod-like protrusions. Previous studies suggest that cell leading edges are not necessarily major places of matrix degradation, but rather degradation happens at lateral points where the matrix restricts the cell movement and at structures which have been called “lytic protrusions” [28]. Some of the filopod-like protrusions in CHL-1 cells may be involved in matrix remodeling as they all contained GFP-MT1-MMP (Figure S4C, >100 filopod-like protrusions in > 10 cells). However, our data strongly suggest that matrix remodeling promoted by “invadopodia equivalents” is more important for efficient cell invasion than filopod-like protrusions. “Invadopodia equivalents” may be smaller and more transient in 3D than in 2D on very stiff matrix, analogous to focal adhesions [29]. Historically, invadopodia were first described as invasive finger-like protrusions into gelatin beads [30, 31], but invadopodia with obvious actin comet tails have not yet been visualized in 3D gels. We find that fascin promotes long protrusions in 3D and stabilizes actin in invadopodia and that this enhanced stability allows for more efficient invasion by mesenchymal cells both in 2D and in 3D.
Figure 4. Fascin is required for efficient mesenchymal type invasion in 3D.
(A) Western blot of CHL-1 cells expressing NT control siRNA, MT1-MMP siRNA probed with anti-MT1-MMP and anti-actin loading control. (B) Left, CHL-1 in collagen I-Matrigel matrix fixed and stained with anti-fascin (55K2). Serial z-stack images (0.5 μm) were combined and shown here. Bars, 10μm. Right, densitometric analysis of fascin signal with arrows at peak fascin intensities (C) CHL-1 cells in 3D collagen I-Matrigel matrix treated with siRNA, inhibitor or rescued with Xtfascin constructs (as indicated) were fixed and stained with rhodamine phalloidin and DAPI. Serial z-stack images (0.5 μm interval) were combined. Bars, 10μm. Filopod-like protrusions were arrowed. (D) Relative invasion >20μM into collagen I-Matrigel (Black); relative release of soluble FITC from collagenolysis caused by CHL-1 cells within 3D FITC–collagen lattices (White) and relative number of filopod-like protrusions per cell (Grey). (E) Summaries of invasion assays of A375MM, MV3 and MDA-MB-231 cells treated with NT, Fascin siRNA or 5μM GM6001 using collagen I-Matrigel matrix. (F) Relative invasion into 3D collagen I-Matrigel, relative release of soluble FITC from collagenolysis caused by cells within 3D FITC–collagen lattices (White) and relative number of filopod-like protrusions per cell (Grey) in cells expressing GFP and NT siRNA, GFP and siFascin, or XtFascin and mutants as indicated on the figure. All error bars show (Mean ± SEM). **, P< 0.01; *, P<0.05 by t-test. Relative invasion and filopod-like protrusions were quantified as described in Supplementary Experimental Procedures. Figure S4C shows the localization of GFP-MT1-MMP to filopod-like protrusions in 3D collagen I-Matrigel matrix. Movie S4 shows the dynamics of filopod-like protrusions in 3D and a comparison of NT and fascin knockdown cells.
In summary, we implicate fascin in stabilizing the actin stuctures in invadopodia and also in aiding cell invasion into 3D matricies using a mesenchymal elongated protrusive mechanism. We suggest that invadopodia represent invasive filopodia, yet are distinct from filopodia in that they have actin comet dynamics as well. Our data build on previous literature establishing fascin as an important protein for cancer invasion and metastasis and elucidate a role for fascin and invadopodia in mesenchymal type invasion.
Methods
DNA and antibody reagents, immunoblotting, cell culture and transfections
Details of all commercially obtained antibody reagents and gift sources of plasmids are given in the supplementary information. Non-targeting (NT) control siRNA, ON-TARGETplus SMARTpool siRNA targeting N-WASP or MT1-MMP and siRNA targeting human fascin 1 (siFascin 1), target sequence: GAGCATGGCTTCATCGGCT) was from Dharmacon (Lafayette, USA). Another siRNA targeting human fascin 1 (siFascin 2, target sequence: CACGGGCACCCTGGACGCCAA) and siRNA targeting human p34-Arc was (sip43, target sequence: AAGGATTCCATTGTGCATCAA from Qiagen (Crawley, UK). Standard methods were used for immunoblotting cell culture and transfections. The details are given in Supplementary Information.
Fluorescent gelatin degradation assay
Gelatin degradation assay [32] and collagenolysis in 3D [28] were done as previously described, and modifications are described in Supplementary Information. Area of gelatin degradation in invadopodia assays was done using an Image J plug-in that was designed for this purpose and which will be described elsewhere. We will make this available to readers on request.
Confocal imaging and FRAP analysis
A375MM cells expressing green fluorescent protein (GFP) or red fluorescent protein (RFP) expression constructs were cultured on crosslinked gelatin for 16 hours. Culture medium was replaced with CO2 independent low fluorescence medium with 10% FCS before imaging. All FRAP experiments were performed using an Olympus FV1000 confocal microscope with a simultaneous illumination (SIM) scanner allowing simultaneous bleaching and image acquisition at 37 °C. Detailed explanations are given in Supplementary Information.
Inverted Invasion Assays and Live Cell Imaging
Inverted invasion assays were preformed essentially as previously described [33], except that we used a mixture of Matrigel and collagen I yielding a final collagen concentration of approximately 4.4 mg/ml−1 and a final Matrigel concentration of approximately 2.2 mg/ml−1 in transwell inserts containing 8μm pore-size micropore polycarbonate membrane filters. Details are given in the Supplementary Information.
Supplementary Material
Highlights.
Fascin is a component of invadopodia and is important for their assembly and stability
Matrix remodeling by cancer cells relies on fascin stabilizing actin structures
Fascin is important for invasion of mesenchymal type cancer cells
Acknowledgements
A.L., H.J.S., X.Y. and I.K. are supported by core Cancer Research UK grants to L.M.M. and K.A. and an Medical Research Council UK Senior research Fellowship to L.M.M. (G117/569) and J.D. is supported by an Association for international Cancer Research project grant (07-0438) to L.M.M. We thank Margaret O’Prey and Tom Gilbey for excellent microscope and FACS assistance. We thank Eric Sahai, Peter Friedl and Katarina Wolf for helpful discussions.
References
- 1.Tseng Y, Fedorov E, McCaffery JM, Almo SC, Wirtz D. Micromechanics and ultrastructure of actin filament networks crosslinked by human fascin: a comparison with alpha-actinin. J Mol Biol. 2001;310:351–366. doi: 10.1006/jmbi.2001.4716. [DOI] [PubMed] [Google Scholar]
- 2.Adams JC. Roles of fascin in cell adhesion and motility. Curr Opin Cell Biol. 2004;16:590–596. doi: 10.1016/j.ceb.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Baldassarre M, Ayala I, Beznoussenko G, Giacchetti G, Machesky LM, Luini A, Buccione R. Actin dynamics at sites of extracellular matrix degradation. Eur J Cell Biol. 2006;85:1217–1231. doi: 10.1016/j.ejcb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Lizarraga F, Poincloux R, Romao M, Montagnac G, Le Dez G, Bonne I, Rigaill G, Raposo G, Chavrier P. Diaphanous-related formins are required for invadopodia formation and invasion of breast tumor cells. Cancer Res. 2009;69:2792–2800. doi: 10.1158/0008-5472.CAN-08-3709. [DOI] [PubMed] [Google Scholar]
- 5.Buccione R, Caldieri G, Ayala I. Invadopodia: specialized tumor cell structures for the focal degradation of the extracellular matrix. Cancer Metastasis Rev. 2009;28:137–149. doi: 10.1007/s10555-008-9176-1. [DOI] [PubMed] [Google Scholar]
- 6.Buccione R, Orth JD, McNiven MA. Foot and mouth: podosomes, invadopodia and circular dorsal ruffles. Nat Rev Mol Cell Biol. 2004;5:647–657. doi: 10.1038/nrm1436. [DOI] [PubMed] [Google Scholar]
- 7.Gimona M, Buccione R, Courtneidge SA, Linder S. Assembly and biological role of podosomes and invadopodia. Curr Opin Cell Biol. 2008;20:235–241. doi: 10.1016/j.ceb.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Vignjevic D, Kojima S, Aratyn Y, Danciu O, Svitkina T, Borisy GG. Role of fascin in filopodial protrusion. J Cell Biol. 2006;174:863–875. doi: 10.1083/jcb.200603013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hashimoto Y, Parsons M, Adams JC. Dual actin-bundling and protein kinase C-binding activities of fascin regulate carcinoma cell migration downstream of Rac and contribute to metastasis. Mol Biol Cell. 2007;18:4591–4602. doi: 10.1091/mbc.E07-02-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hashimoto Y, Skacel M, Adams JC. Roles of fascin in human carcinoma motility and signaling: prospects for a novel biomarker? Int J Biochem Cell Biol. 2005;37:1787–1804. doi: 10.1016/j.biocel.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Vignjevic D, Schoumacher M, Gavert N, Janssen KP, Jih G, Lae M, Louvard D, Ben-Ze’ev A, Robine S. Fascin, a novel target of beta-catenin-TCF signaling, is expressed at the invasive front of human colon cancer. Cancer Res. 2007;67:6844–6853. doi: 10.1158/0008-5472.CAN-07-0929. [DOI] [PubMed] [Google Scholar]
- 12.Caldieri G, Giacchetti G, Beznoussenko G, Attanasio F, Ayala I, Buccione R. Invadopodia Biogenesis Is Regulated by Caveolin-Mediated Modulation of Membrane Cholesterol Levels. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaksonen M, Toret CP, Drubin DG. Harnessing actin dynamics for clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2006;7:404–414. doi: 10.1038/nrm1940. [DOI] [PubMed] [Google Scholar]
- 14.Chhabra ES, Higgs HN. The many faces of actin: matching assembly factors with cellular structures. Nat Cell Biol. 2007;9:1110–1121. doi: 10.1038/ncb1007-1110. [DOI] [PubMed] [Google Scholar]
- 15.Millard TH, Dawson J, Machesky LM. Characterisation of IRTKS, a novel IRSp53/MIM family actin regulator with distinct filament bundling properties. J Cell Sci. 2007;120:1663–1672. doi: 10.1242/jcs.001776. [DOI] [PubMed] [Google Scholar]
- 16.Brieher WM, Coughlin M, Mitchison TJ. Fascin-mediated propulsion of Listeria monocytogenes independent of frequent nucleation by the Arp2/3 complex. J Cell Biol. 2004;165:233–242. doi: 10.1083/jcb.200311040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ono S, Yamakita Y, Yamashiro S, Matsudaira PT, Gnarra JR, Obinata T, Matsumura F. Identification of an actin binding region and a protein kinase C phosphorylation site on human fascin. J Biol Chem. 1997;272:2527–2533. doi: 10.1074/jbc.272.4.2527. [DOI] [PubMed] [Google Scholar]
- 18.Yamakita Y, Ono S, Matsumura F, Yamashiro S. Phosphorylation of human fascin inhibits its actin binding and bundling activities. J Biol Chem. 1996;271:12632–12638. doi: 10.1074/jbc.271.21.12632. [DOI] [PubMed] [Google Scholar]
- 19.Bowden ET, Barth M, Thomas D, Glazer RI, Mueller SC. An invasion-related complex of cortactin, paxillin and PKCmu associates with invadopodia at sites of extracellular matrix degradation. Oncogene. 1999;18:4440–4449. doi: 10.1038/sj.onc.1202827. [DOI] [PubMed] [Google Scholar]
- 20.Parsons M, Adams JC. Rac regulates the interaction of fascin with protein kinase C in cell migration. J Cell Sci. 2008;121:2805–2813. doi: 10.1242/jcs.022509. [DOI] [PubMed] [Google Scholar]
- 21.Lai FP, Szczodrak M, Block J, Faix J, Breitsprecher D, Mannherz HG, Stradal TE, Dunn GA, Small JV, Rottner K. Arp2/3 complex interactions and actin network turnover in lamellipodia. EMBO J. 2008;27:982–992. doi: 10.1038/emboj.2008.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaggioli C, Sahai E. Melanoma invasion - current knowledge and future directions. Pigment Cell Res. 2007;20:161–172. doi: 10.1111/j.1600-0749.2007.00378.x. [DOI] [PubMed] [Google Scholar]
- 23.Sahai E, Marshall CJ. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol. 2003;5:711–719. doi: 10.1038/ncb1019. [DOI] [PubMed] [Google Scholar]
- 24.Friedl P, Maaser K, Klein CE, Niggemann B, Krohne G, Zanker KS. Migration of highly aggressive MV3 melanoma cells in 3-dimensional collagen lattices results in local matrix reorganization and shedding of alpha2 and beta1 integrins and CD44. Cancer Res. 1997;57:2061–2070. [PubMed] [Google Scholar]
- 25.Wolf K, Wu YI, Liu Y, Geiger J, Tam E, Overall C, Stack MS, Friedl P. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol. 2007;9:893–904. doi: 10.1038/ncb1616. [DOI] [PubMed] [Google Scholar]
- 26.Desmarais V, Yamaguchi H, Oser M, Soon L, Mouneimne G, Sarmiento C, Eddy R, Condeelis J. N-WASP and cortactin are involved in invadopodium-dependent chemotaxis to EGF in breast tumor cells. Cell Motil Cytoskeleton. 2009 doi: 10.1002/cm.20361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamaguchi H, Lorenz M, Kempiak S, Sarmiento C, Coniglio S, Symons M, Segall J, Eddy R, Miki H, Takenawa T, et al. Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin. J Cell Biol. 2005;168:441–452. doi: 10.1083/jcb.200407076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolf K, Friedl P. Mapping proteolytic cancer cell-extracellular matrix interfaces. Clin Exp Metastasis. 2009;26:289–298. doi: 10.1007/s10585-008-9190-2. [DOI] [PubMed] [Google Scholar]
- 29.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 30.Chen WT. Proteolytic activity of specialized surface protrusions formed at rosette contact sites of transformed cells. J Exp Zool. 1989;251:167–185. doi: 10.1002/jez.1402510206. [DOI] [PubMed] [Google Scholar]
- 31.Mueller SC, Chen WT. Cellular invasion into matrix beads: localization of beta 1 integrins and fibronectin to the invadopodia. J Cell Sci. 1991;99(Pt 2):213–225. doi: 10.1242/jcs.99.2.213. [DOI] [PubMed] [Google Scholar]
- 32.Artym VV, Zhang Y, Seillier-Moiseiwitsch F, Yamada KM, Mueller SC. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 2006;66:3034–3043. doi: 10.1158/0008-5472.CAN-05-2177. [DOI] [PubMed] [Google Scholar]
- 33.Caswell PT, Spence HJ, Parsons M, White DP, Clark K, Cheng KW, Mills GB, Humphries MJ, Messent AJ, Anderson KI, et al. Rab25 associates with alpha5beta1 integrin to promote invasive migration in 3D microenvironments. Dev Cell. 2007;13:496–510. doi: 10.1016/j.devcel.2007.08.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.