Abstract
Glucocorticoids, the end-products of the hypothalamic-pituitary-adrenal (HPA) axis, influence the functions of virtually all organs and tissues through the nuclear glucocorticoid receptor (GR). Circulating levels of glucocorticoids fluctuate naturally in a circadian fashion under the strong influence of the hypothalamic suprachiasmatic nucleus (SCN) circadian CLOCK system, and regulate the transcriptional activity of the GR in the brain and peripheral target tissues. We recently reported that the basic helix-loop-helix transcription factor Clock, which is a histone acetyltransferase and a central component of the self-oscillating transcription factor loop that generates circadian rhythms, represses GR transcriptional activity by acetylating lysine residues within the “lysine cluster” located in the hinge region of the receptor. This Clock-mediated repression of GR transcriptional activity oscillates in inverse phase to the HPA axis, acting as a target tissue counter regulatory mechanism to the diurnally fluctuating circulating glucocorticoids. Interestingly, mild evening elevations of cortisol, as occurs in chronic stress situations, and frequent uncoupling of the SCN CLOCK-directed HPA axis from the daily oscillation of target tissue sensitivity to glucocorticoids, as happens in trans-time zone travel and night shift work, produce functional hypercortisolism and, hence, multiple components of the metabolic syndrome with resultant cardiovascular complications.
Keywords: acetylation, CLOCK, circadian rhythm, hypothalamic-pituitary-adrenal (HPA) axis, glucocorticoid action
Introduction
Glucocorticoids, steroid hormones secreted by the adrenal gland cortices, have strong and diverse actions on every aspect of human physiology [1]. These hormones act as end-effectors of the hypothalamic-pituitary-adrenal (HPA) axis, which, together with the locus caeruleus/autonomic nervous system, plays a central role in both baseline homeostasis and the adaptive response to stress [2]. Glucocorticoids are necessary for proper functioning of virtually all organs and tissues, including the central nervous system (CNS), and the respiratory, cardiovascular, immune, and musculoskeletal systems [1]. Upon exposure to stress, glucocorticoids are secreted into the systemic circulation and alter target tissue activities, dramatically influencing the function of the CNS, shifting intermediary metabolism towards catabolism and modulating immune function [1-3].
The secretion of glucocorticoids is tightly regulated; indeed, the circadian, negative feedback and stress-related activities of the HPA axis are integrated by higher brain centers (Figure 1) [2, 4]. Circulating glucocorticoid levels, ie. cortisol in humans and corticosterone in rodents, are under the strong circadian influence of the suprachiasmatic nucleus (SCN) of the hypothalamus [4]. In humans, cortisol levels have a diurnal rhythm curve in which the zenith is reached in the early morning and the nadir at midnight, with the purpose of helping adjust the body’s activities to the regular periodicity of day/night changes. The overall sensitivity of tissues to glucocorticoids is also regulated by various physiologic and pathologic processes [5, 6]. For example, glucocorticoid action in cells is specifically adjusted during the different phases of the cell cycle [7, 8], while adrenomedullary cells exposed to very high concentrations of glucocorticoids directly diffusing from the adjacent adrenal cortex, are resistant to them [9, 10]. In addition, several autoimmune/allergic/inflammatory disorders, the metabolic syndrome, septic conditions and even infection with the human immunodeficiency virus type-1, have been associated with alterations in the responsiveness of specific organs and tissues to glucocorticoids [5, 6, 11]. Underlying mechanisms(s) for the alterations of local glucocorticoid actions, however, have not been well elucidated as yet. In this brief review we summarize recent findings demonstrating circadian regulation of the glucocorticoid signaling system by the biological CLOCK and discuss the potential clinical implications of this regulation.
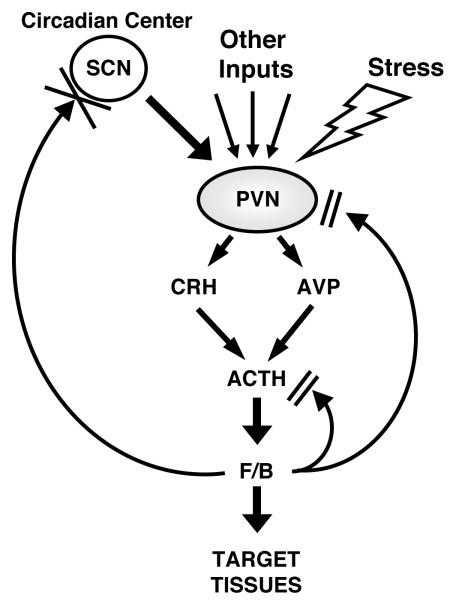
Figure 1.
Organization of the HPA axis.
The PVN center of the HPA axis receives circadian inputs from the SCN and stress-related and other regulatory inputs from multiple brain and peripheral sites. CRH and AVP are secreted into the hypophyseal portal system and transported to the anterior pituitary gland where they synergistically stimulate ACTH secretion. ACTH released into the systemic circulation stimulates the zona fasciculata of the adrenal cortices to secrete cortisol or corticosterone in humans and rodents, respectively. The “master” circadian pacemaker of the SCN, thus, produces a circadian rhythm of glucocorticoid concentrations in the blood. Importantly, this site of the brain does not have GR and, hence, is not influenced by circulating glucocorticoids. (Modified from [16]) ACTH: adrenocorticotropic hormone, AVP, arginine-vasopressin, CRH: corticotropin-releasing hormone, F/B: cortisol/corticosterone, PVN: paraventricular nucleus, SCN: suprachiasmatic nucleus
Glucocorticoid signaling system
The diverse actions of glucocorticoids at their target tissues are mediated by the glucocorticoid receptor (GR) [2, 12]. This receptor, also called “nuclear receptor superfamily 3, group C, member 1 (NR3C1)”, expressed virtually in all the organs and tissues of the human body, and belongs to isthe steroid/sterol/thyroid/retinoid/orphan nuclear receptor superfamily, which consists of over 130 members expressed from the early metazoans to humans [5, 6]. The human GR gene, located in the short arm of chromosome 5 (5q31.3), is composed of 10 exons, and encodes two protein molecules GRα and GRβ through alternative use of specific exon 9 α and β [13].
GRα is the ubiquitously expressed classic receptor that binds to and mediates most of the known actions of glucocorticoids, while GRβ, although also expressed widely, cannot bind glucocorticoids and its physiologic actions have not been fully elucidated as yet [13]. It recently became evident that the GRα variant mRNA is translated from at least 8 initiation sites into multiple amino terminal GRα isoforms, termed A through D (A, B, C1-C3 and D1-D3), with distinct specific transcriptional activities on glucocorticoid-responsive genes [5, 14]. These GR molecules are also differentially expressed in several different cell lines and tissues [14]. Given that GRα and GRβ share a common mRNA domain that contains the same translation initiation sites [13], the GRβ variant mRNA is also most likely translated from the same initiation sites to a similar host of 8 β isoforms [5].
The human GRα consists of 777 amino acids and has 3 major distinct functional domains, the N-terminal or immunogenic (NTD), the DNA-binding (DBD) and the ligand-binding (LBD) domains (Figure 2A) [15]. GRα has also a hinge region (HD), located between the DBD and LBD and spanning amino acids 481 to 520, which plays a role in the affinity of the DBD to DNA recognition sequences and in the determination of the numbers of nucleotides spacing tandem response elements [16]. The LBD of GRα consists of 12 α-helices and 4 β-sheets, among which helices 3, 4, 11 and 12 form the glucocorticoid-binding pocket [12]. GRα is located primarily in the cytoplasm in the absence of glucocorticoid ligand, as part of hetero-oligomeric complexes containing heat shock proteins (HSPs) 90, 70, 50, 20 and, possibly, other proteins as well [5, 15]. After binding to its agonist ligand, GRα undergoes conformational changes, dissociates from the heat shock proteins, homo- or hetero-dimerizes, and translocates as a dimer and/or monomer into the nucleus through the nuclear pore, via an active ATP-dependent process mediated by its nuclear localization signals (NL)-1 and -2 [6, 17]. NL-1 is located in the junction of DBD and the hinge region, while NL-2 spans the entire LBD [17].
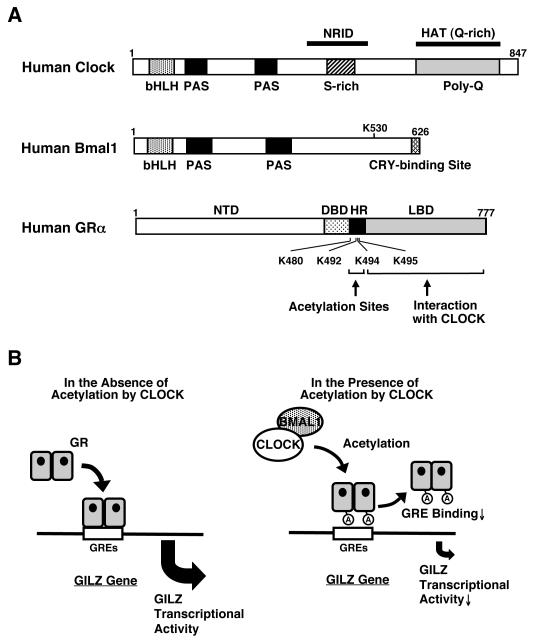
Figure 2.
Linearized structures of the human Clock, Bmal1 and GR molecules and the regulation GR transcriptional activity by Clock .
(A) Human Clock and Bmal1 are 847 and 626 amino acid proteins, respectively, that contain one basic helix-loop-helix (bHLH) and two Per-Amt-Sim (PAS) domains in their N-terminal portion. Clock has also one nuclear receptor interacting domain (NRID) in its middle portion and a histone acetyltransferase domain (HAT) in its C-terminal area, while Bmal1 has a CRY-binding site in its C-terminal end. A serine-rich (S-rich) and a glutamine-rich (Q-rich) region, the latter containing a poly-glutamine (polyQ) stretch, are also shown. The GR has 3 main functional domains, the amino terminal, DNA-binding and ligand-binding domains and a lysine cluster in its hinge region.
(B) Clock physically interacts with the ligand-binding domain of the GR through the domain enclosed in its C-terminal part and suppresses GR-induced transcriptional activity by acetylating via its intrinsic HAT activity a lysine cluster located in the GR hinge region. Acetylation by Clock reduces the affinity of the GR to its cognate DNA sequences, the GREs.
A: acetylation, Bmal1: brain-muscle-arnt-like protein 1, DBD: DNA-binding domain, GR: glucocorticoid receptor, GRE: glucocorticoid response element, HR: hinge region, K: lysine residue, LBD: ligand-binding domain, NTD: N-terminal domain C: A heuristic model of the physiologic implications of this study.
Inside the nucleus, the ligand-activated GRα directly interacts as a homo- or hetero-dimer with specific DNA sequences, the glucocorticoid response elements (GREs), in the promoter regions of target genes, or as a monomer or dimer with other transcription factors via protein-protein interactions, indirectly influencing the activity of the latter on their own target genes [5, 6]. GR contains two transactivation domains, activation function (AF)-1 and -2, located at its NTD and LBD, respectively, through which it interacts with many proteins and protein complexes, such as the nuclear receptor coactivator [p160, p300/CREB-binding protein (CBP) and p300/CBP-associated factor (p/CAF)] complexes and the SWI/SNF and vitamin D receptor-interacting protein/thyroid hormone receptor-associated protein (DRIP/TRAP) chromatin-remodeling complexes, eventually influencing the activity of RNA polymerase II and its ancillary factors, ultimately altering the transcription rates of glucocorticoid-responsive genes [5, 15, 18].
GRα also interacts with the nuclear receptor corepressor (NCoR) and its homolog silencing mediator of retinoic acid and thyroid hormone receptor (SMRT), which are macromolecular docking platforms for nuclear receptors and many transcription factors, repressing the transcriptional activity of the GR by attracting histone deacetylase/Sin3 complexes and other repressing agents [18]. The p160 type coactivators and the NCoR/SMRT type corepressors establish equilibrium in their interaction with the GR to respectively facilitate or hinder its transcriptional activity [19].
In addition to transactivation or transrepression of the glucocorticoid-responsive genes explained above, GRα modulates other signal transduction cascades through mutual protein-protein interactions with specific transcription factors, by influencing the ability of these factors to stimulate or inhibit the transcription rates of their respective target genes [1, 5]. This activity may be more important than the GRE-mediated one, granted that mice harboring a mutant GRα, which is active in terms of protein-protein interactions but inactive in terms of dimerization and transactivation via DNA GREs, survive and procreate, in contrast to mice with a deletion of the entire GR gene that die immediately after birth from severe respiratory distress syndrome [20, 21]. The former mouse model and additional in vitro results indicate that GR interacts with and influences other transcription factors primarily as a monomer [20, 22].
The protein-protein interactions of GRα with other transcription factors may take place on promoters that do not contain GREs, as well as on promoters that have both GRE(s) and responsive element(s) of transcription factors that interact with GRα [23]. Suppression of transactivation of other transcription factors through protein-protein interactions may be particularly important in the regulation of immune function and suppression of inflammation by glucocorticoids [20, 22]. A substantial part of the effects of glucocorticoids on the immune system may be explained by the interaction between GRα with nuclear factor-κB (NF-κB), activator protein-1 (AP-1), the signal transducers and activators of transcription (STATs) and, probably, interferon regulatory factors [5, 6, 24].
In addition to co-regulators and other transcription factors that modulate GR-induced transcriptional activity, several distinct signaling pathways influence the transcriptional activity of the GR via post-translational modifications of the receptor protein [5]. These include methylation, acetylation, nitrosylation, sumoylation and ubiquitination, as well as phosphorylation, which has been studied best. Indeed, several kinases, such as the cell-cycle-related kinases, mitogen-activated kinases (MAPKs) and the glycogen synthase kinases, phosphorylate specific serine or threonine residues of the GR, while energy sensing AMP-activated protein kinase indirectly phosphorylates the GR through p38 MAPK [13, 25, 26]. Interestingly, the majority of these residues are located in the AF-1 domain of the human GR NTD, thus, phosphorylation of some or all of these amino acids modulates GR-induced transcriptional activity through alteration of co-regulator attraction to the promoter region of glucocorticoid-responsive genes, possibly by changing their affinity and overall interaction with the AF-1 domain of the GR [25, 27].
The Circadian CLOCK and its interactions with the glucocorticoid signaling system
We recently found that the circadian rhythm transcription factor Clock acetylates the GR at a multiple lysine cluster located in its hinge region, and represses GR-induced transcriptional activity of several glucocorticoid-responsive genes (Figure 2) [28]. Clock, the “circadian locomotor output cycle kaput”, and its heterodimer partner “brain-muscle-arnt-like protein 1” (Bmal1) belong to the basic helix-loop-helix (bHLH)-PER-ARNT-SIM (PAS) superfamily of transcription factors [29, 30], and play an essential role in the formation of the circadian oscillation rhythm of both hypothalamic SCN and peripheral CLOCK systems that function as internal circadian time keepers [31]. The CLOCK system located in the SCN of the hypothalamus, acts as, the “master” oscillator and generator of the body’s circadian rhythm [32, 33] while the peripheral CLOCK system virtually distributed in all organs and tissues, including the CNS outside the SCN, acts generally as a “slave” CLOCK under the influence of the SCN . Human Clock and Bmal1 are 847 and 626 amino acid proteins, respectively, and contain one bHLH and two PAS domains in their N-terminal portion (Figure 2A). In addition, Clock has one nuclear receptor interacting domain (NRID) in its middle portion and the histone acetyltransferase domain (HAT) in the C-terminal area, while Bmal1 has a CRY-binding site in its C-terminal end [34, 35].
The Clock/Bmal1 heterodimer binds to the E-box response elements located in the promoter region of other essential CLOCK genes, such as Periods (Per1, Per2 and Per3) and Cryptochromes (Cry1 and Cry2) and stimulates their transcription. Accumulated proteins Pers and Crys then form a complex with caseine kinase 1ε and δ, are phosphorylated, translocate into the cell nucleus and repress the transcriptional activity of the Clock/Bmal1 heterodimer by inhibiting its binding to the E-box response elements located in their own promoters, thus ultimately forming a negative feedback transcriptional loop that maintains an oscillation of their gene expression with a period of approximately every 24 hours [31, 36, 37]. The HAT activity of Clock is essential for creating diurnal oscillation of this transcription factor loop, possibly by acetylating histones associated with its responsive genes, as well as by acetylating its partner Bmal1, a change that increases the attraction of Cry, facilitating thus the repressive effect of this molecule [35, 38].
In addition to the regulation of this principal transcriptional loop, Clock/Bmal1 stimulates expression of other clock-related proteins, such as Rev-erbα, retinoic acid receptor-related orphan receptor α (RORα), Dec1, Dec2 and albumin gene D site-binding protein (Dbp), which form an auxiliary loop that stabilizes the main regulatory loop composed of Clock/Bmal1, Pers and Crys. Importantly, the transcription factors of the main and auxiliary loops control numerous “downstream” clock-responsive genes to influence a variety of biological activities [30, 31, 39], so that the circadian CLOCK system can directly adjust body’s fundamental activities, such as feeding, thermoregulation, energy expenditure and intermediary metabolism, synchronizing the organism with the day/night changes of the environment linked to the rotation of the planet.
The glucocorticoid signaling system is also a target of the circadian CLOCK system [4]. In addition to the diurnal secretion of circulating glucocorticoids controlled by the master CLOCK, peripheral CLOCK negatively regulates GR-induced transcriptional activity through acetylation of the GR [16]. Indeed, Clock physically interacts with the LBD of the GR through its NRID and acetylates lysine residues located at amino acid positions 480, 492, 494 and 495 of the human GR in its hinge region. The acetylation of GR further suppresses binding of this receptor to GREs, explaining the mechanism of its repressive effect [16] (Figure 2 B). It is likely that acetylation-mediated repression of GR transcriptional activity by Clock functions as a local counter regulatory mechanism to diurnally oscillating circulating glucocorticoids [4]. Since phosphorylation-mediated transcriptional regulation of GR, the most well-examined epigenetic regulation of GR activity, influences GR transcriptional activity in a gene- and tissue-specific fashion [25], it is quite probable that acetylation of the GR also acts gene-, and possibly, tissue-specifically.
Pathophysiologic implications of the circadian CLOCK-mediated GR signaling system
This presumed gene- and tissue-specific negative regulation of GR transcriptional activity by Clock indicates that diurnal oscillating of gene expression may be necessary for some glucocorticoid-responsive genes to maintain their proper biologic actions. It is known that subjects under chronic stress, who have a flattened cortisol circadian rhythm because of increased hormone levels at night, frequently develop symptoms and signs of chronic hypercortisolism, i.e. components of the metabolic syndrome, such as central obesity, dyslipidemia, insulin resistance and hypertension, and, hence, are at an increased risk of developing cardiovascular complications [40, 41] (Figure 3). This evidence indicates that proper gene- and tissue-specific diurnal regulation of glucocorticoid hormone action is essential for a healthy life. Interestingly, a fraction (~10%) of the energy-controlling genes are also under circadian regulation in a tissue-specific fashion [42-45].
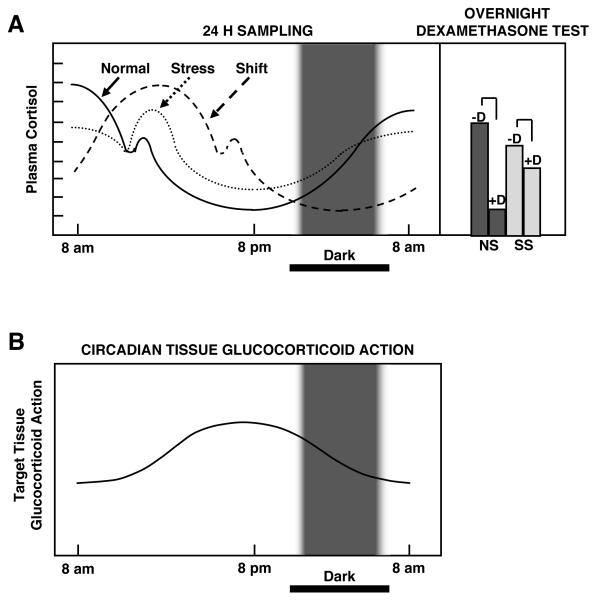
Figure 3.
A heuristic scheme of the circadian secretion of cortisol in normal, chronically stressed and phase-shifted humans (top left panel) and the responses of chronically stressed individuals to midnight dexamethasone administration (top right panel). The corresponding circadian change of target tissue sensitivity to glucocorticoids (bottom panel).
It is obvious that even mild evening cortisol elevations, as those seen in chronically stressed individuals, will exert disproportionately increased glucocorticoid effects because of the natural circadian target tissue sensitivity increase at this time of the day. Frequent abrupt phase-shifts in circulating cortisol levels due to night-shift work or trans-time zone travel causes uncoupling of circulating cortisol levels and tissue glucocorticoid sensitivity of target tissues, influenced by the peripheral CLOCK, increase the overall glucocorticoid effect and, hence, the risk for metabolic syndrome and associated cardiovascular complications.
CS: Chronically stressed individuals, D: midnight dexamethasone administration, NS: non-stressed individuals.
Modified from [49].
Uncoupling of the cortisol circadian rhythm, under the influence of the SCN CLOCK system, and the circadian peripheral sensitivity to glucocorticoids, under the influence of the peripheral CLOCK, also leads to development of components of the metabolic syndrome and its cardiovascular complications, as it occurs with chronic hypercortisolism (Figure 3) [4]. For example, rotating shift workers, whose circadian system is repetitively reset by night-time activity/day-time sleep, and persons exposed to frequent jet lag, because of traveling across time zones, are at an increased risk for cardiometabolic disease [46, 47]. Thus, the HPA axis and the SCN and peripheral circadian CLOCK systems are in close cooperation in the regulation of mental and physical activities of human beings, possibly by regulating a substantial number of shared genes.
Concluding remarks
During the last decade, significant progress has been made in the field of chronobiology and the molecular functions of CLOCK System, which helps adjust the body’s daily activities to the predictable regular day/night changes of the environment. The adaptive response to unpredicted, acute changes in the environment, on the other hand, is serviced on demand by the Stress System including the HPA axis. Interestingly, the SCN master CLOCK, which creates this diurnal oscillation of circulating glucocorticoids, at the same time moderates their peripheral actions via the peripheral ubiquitous CLOCK, which suppresses GR action by acetylation.
ACKNOWLEDGEMENTS
Literary work of this article was funded partly by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD and the University of Athens, Athens, Greece.
REFERENCES
- [1].Kino T, Chrousos GP. Glucocorticoid effects on gene expression. In: Steckler T, Kalin NH, Reul JMHM, editors. Handbook on Stress and the Brain. Elsevier BV; Amsterdam: 2005. pp. 295–312. [Google Scholar]
- [2].Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332(20):1351–1362. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- [3].Chrousos GP. Glucocorticoid therapy. In: Felig P, Frohman LA, editors. Endocrinology & Metabolism. McGraw-Hill; New York: 2001. pp. 609–632. [Google Scholar]
- [4].Nader N, Chrousos GP, Kino T. Interactions of the circadian CLOCK system and the HPA axis. Trends in Endocrinology & Metabolism. 2010 doi: 10.1016/j.tem.2009.12.011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chrousos GP, Kino T. Intracellular glucocorticoid signaling: a formerly simple system turns stochastic. Sci STKE. 2005;2005(304):e48. doi: 10.1126/stke.3042005pe48. [DOI] [PubMed] [Google Scholar]
- [6].Kino T, De Martino MU, Charmandari E, Mirani M, Chrousos GP. Tissue glucocorticoid resistance/hypersensitivity syndromes. J Steroid Biochem Mol Biol. 2003;85(2-5):457–467. doi: 10.1016/s0960-0760(03)00218-8. [DOI] [PubMed] [Google Scholar]
- [7].Abel GA, Wochnik GM, Ruegg J, Rouyer A, Holsboer F, Rein T. Activity of the GR in G2 and mitosis. Mol Endocrinol. 2002;16(6):1352–1366. doi: 10.1210/mend.16.6.0842. [DOI] [PubMed] [Google Scholar]
- [8].Cidlowski JA, Michaels GA. Alteration in glucocorticoid binding site number during the cell cycle in HeLa cells. Nature. 1977;266(5603):643–645. doi: 10.1038/266643a0. [DOI] [PubMed] [Google Scholar]
- [9].Wurtman RJ. Stress and the adrenocortical control of epinephrine synthesis. Metabolism. 2002;51(6 Suppl 1):11–14. doi: 10.1053/meta.2002.33185. [DOI] [PubMed] [Google Scholar]
- [10].Ehrhart-Bornstein M, Bornstein SR. Cross-talk between adrenal medulla and adrenal cortex in stress. Ann N Y Acad Sci. 2008;1148:112–117. doi: 10.1196/annals.1410.053. [DOI] [PubMed] [Google Scholar]
- [11].Chrousos GP, Kino T. Glucocorticoid action networks and complex psychiatric and/or somatic disorders. Stress. 2007;10(2):213–219. doi: 10.1080/10253890701292119. [DOI] [PubMed] [Google Scholar]
- [12].Nicolaides NC, Galata Z, Kino T, Chrousos GP, Charmandari E. The human glucocorticoid receptor: molecular basis of biologic function. Steroids. 75(1):1–12. doi: 10.1016/j.steroids.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kino T, Su YA, Chrousos GP. Human glucocorticoid receptor isoform β: recent understanding of its potential implications in physiology and pathophysiology. Cell Mol Life Sci. 2009;66(21):3435–3448. doi: 10.1007/s00018-009-0098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lu NZ, Cidlowski JA. Translational regulatory mechanisms generate N-terminal glucocorticoid receptor isoforms with unique transcriptional target genes. Mol Cell. 2005;18(3):331–342. doi: 10.1016/j.molcel.2005.03.025. [DOI] [PubMed] [Google Scholar]
- [15].Kino T, Chrousos GP. Glucocorticoid and mineralocorticoid receptors and associated diseases. Essays Biochem. 2004;40:137–155. doi: 10.1042/bse0400137. [DOI] [PubMed] [Google Scholar]
- [16].Nader N, Chrousos GP, Kino T. Circadian rhythm transcription factor CLOCK regulates the transcriptional activity of the glucocorticoid receptor by acetylating its hinge region lysine cluster: potential physiological implications. FASEB J. 2009;23(5):1572–1583. doi: 10.1096/fj.08-117697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Savory JG, Hsu B, Laquian IR, Giffin W, Reich T, Hache RJ, Lefebvre YA. Discrimination between NL1- and NL2-mediated nuclear localization of the glucocorticoid receptor. Mol Cell Biol. 1999;19(2):1025–1037. doi: 10.1128/mcb.19.2.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rosenfeld MG, Lunyak VV, Glass CK. Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev. 2006;20(11):1405–1428. doi: 10.1101/gad.1424806. [DOI] [PubMed] [Google Scholar]
- [19].Wang Q, Blackford JA, Jr., Song LN, Huang Y, Cho S, Simons SS., Jr. Equilibrium interactions of corepressors and coactivators with agonist and antagonist complexes of glucocorticoid receptors. Mol Endocrinol. 2004;18(6):1376–1395. doi: 10.1210/me.2003-0421. [DOI] [PubMed] [Google Scholar]
- [20].Reichardt HM, Kaestner KH, Tuckermann J, Kretz O, Wessely O, Bock R, Gass P, Schmid W, Herrlich P, Angel P, Schutz G. DNA binding of the glucocorticoid receptor is not essential for survival. Cell. 1998;93(4):531–541. doi: 10.1016/s0092-8674(00)81183-6. [DOI] [PubMed] [Google Scholar]
- [21].Cole TJ, Blendy JA, Monaghan AP, Krieglstein K, Schmid W, Aguzzi A, Fantuzzi G, Hummler E, Unsicker K, Schutz G. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev. 1995;9(13):1608–1621. doi: 10.1101/gad.9.13.1608. [DOI] [PubMed] [Google Scholar]
- [22].Reichardt HM, Tuckermann JP, Gottlicher M, Vujic M, Weih F, Angel P, Herrlich P, Schutz G. Repression of inflammatory responses in the absence of DNA binding by the glucocorticoid receptor. EMBO J. 2001;20(24):7168–7173. doi: 10.1093/emboj/20.24.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Miner JN, Yamamoto KR. Regulatory crosstalk at composite response elements. Trends Biochem Sci. 1991;16(11):423–426. doi: 10.1016/0968-0004(91)90168-u. [DOI] [PubMed] [Google Scholar]
- [24].Ogawa S, Lozach J, Benner C, Pascual G, Tangirala RK, Westin S, Hoffmann A, Subramaniam S, David M, Rosenfeld MG, Glass CK. Molecular determinants of crosstalk between nuclear receptors and toll-like receptors. Cell. 2005;122(5):707–721. doi: 10.1016/j.cell.2005.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kino T, Ichijo T, Amin ND, Kesavapany S, Wang Y, Kim N, Rao S, Player A, Zheng YL, Garabedian MJ, Kawasaki E, Pant HC, Chrousos GP. Cyclin-dependent kinase 5 differentially regulates the transcriptional activity of the glucocorticoid receptor through phosphorylation: clinical implications for the nervous system response to glucocorticoids and stress. Mol Endocrinol. 2007;21(7):1552–1568. doi: 10.1210/me.2006-0345. [DOI] [PubMed] [Google Scholar]
- [26].Nader N, Ng SM, Lambrou GI, Pervanidou P, Wang YH, Chrousos GP, Kino T. AMPK regulates metabolic actions of glucocorticoids by phosphorylating the glucocorticoid receptor through p38 MAPK. Mol Endocrinol. 2010 doi: 10.1210/me.2010-0192. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kino T. Tissue glucocorticoid sensitivity: beyond stochastic regulation on the diverse actions of glucocorticoids. Horm Metab Res. 2007;39(6):420–424. doi: 10.1055/s-2007-980193. [DOI] [PubMed] [Google Scholar]
- [28].Nader N, Chrousos GP, Kino T. Circadian rhythm transcription factor CLOCK regulates the transcriptional activity of the glucocorticoid receptor by acetylating its hinge region lysine cluster: potential physiological implications. FASEB J. 2009;23(5):1572–1583. doi: 10.1096/fj.08-117697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cermakian N, Sassone-Corsi P. Multilevel regulation of the circadian clock. Nat Rev Mol Cell Biol. 2000;1(1):59–67. doi: 10.1038/35036078. [DOI] [PubMed] [Google Scholar]
- [30].Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15(2):R271–277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- [31].Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9(10):764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247(4945):975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- [33].Rusak B, Zucker I. Neural regulation of circadian rhythms. Physiol Rev. 1979;59(3):449–526. doi: 10.1152/physrev.1979.59.3.449. [DOI] [PubMed] [Google Scholar]
- [34].Grimaldi B, Nakahata Y, Sahar S, Kaluzova M, Gauthier D, Pham K, Patel N, Hirayama J, Sassone-Corsi P. Chromatin remodeling and circadian control: master regulator CLOCK is an enzyme. Cold Spring Harb Symp Quant Biol. 2007;72:105–112. doi: 10.1101/sqb.2007.72.049. [DOI] [PubMed] [Google Scholar]
- [35].Hirayama J, Sahar S, Grimaldi B, Tamaru T, Takamatsu K, Nakahata Y, Sassone-Corsi P. CLOCK-mediated acetylation of BMAL1 controls circadian function. Nature. 2007;450(7172):1086–1090. doi: 10.1038/nature06394. [DOI] [PubMed] [Google Scholar]
- [36].Kondratov RV, Kondratova AA, Lee C, Gorbacheva VY, Chernov MV, Antoch MP. Post-translational regulation of circadian transcriptional CLOCK(NPAS2)/BMAL1 complex by CRYPTOCHROMES. Cell Cycle. 2006;5(8):890–895. doi: 10.4161/cc.5.8.2684. [DOI] [PubMed] [Google Scholar]
- [37].Kiyohara YB, Tagao S, Tamanini F, Morita A, Sugisawa Y, Yasuda M, Yamanaka I, Ueda HR, van der Horst GT, Kondo T, Yagita K. The BMAL1 C terminus regulates the circadian transcription feedback loop. Proc Natl Acad Sci U S A. 2006;103(26):10074–10079. doi: 10.1073/pnas.0601416103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Doi M, Hirayama J, Sassone-Corsi P. Circadian regulator CLOCK is a histone acetyltransferase. Cell. 2006;125(3):497–508. doi: 10.1016/j.cell.2006.03.033. [DOI] [PubMed] [Google Scholar]
- [39].Ripperger JA, Schibler U. Rhythmic CLOCK-BMAL1 binding to multiple E-box motifs drives circadian Dbp transcription and chromatin transitions. Nat Genet. 2006;38(3):369–374. doi: 10.1038/ng1738. [DOI] [PubMed] [Google Scholar]
- [40].Chrousos GP, Kino T. Glucocorticoid action networks and complex psychiatric and/or somatic disorders. Stress. 2007;10(2):213–219. doi: 10.1080/10253890701292119. [DOI] [PubMed] [Google Scholar]
- [41].Chrousos GP, Kino T. Glucocorticoid signaling in the cell. Expanding clinical implications to complex human behavioral and somatic disorders. Ann N Y Acad Sci. 2009;1179:153–166. doi: 10.1111/j.1749-6632.2009.04988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Yang X, Downes M, Yu RT, Bookout AL, He W, Straume M, Mangelsdorf DJ, Evans RM. Nuclear receptor expression links the circadian clock to metabolism. Cell. 2006;126(4):801–810. doi: 10.1016/j.cell.2006.06.050. [DOI] [PubMed] [Google Scholar]
- [43].Staels B. When the Clock stops ticking, metabolic syndrome explodes. Nat Med. 2006;12(1):54–55. doi: 10.1038/nm0106-54. [DOI] [PubMed] [Google Scholar]
- [44].Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109(3):307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- [45].Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417(6884):78–83. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- [46].Ekstrand K, Bostrom PA, Arborelius M, Nilsson JA, Lindell SE. Cardiovascular risk factors in commercial flight aircrew officers compared with those in the general population. Angiology. 1996;47(11):1089–1094. doi: 10.1177/000331979604701109. [DOI] [PubMed] [Google Scholar]
- [47].Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106(11):4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hirayama J, Sahar S, Grimaldi B, Tamaru T, Takamatsu K, Nakahata Y, Sassone-Corsi P. CLOCK-mediated acetylation of BMAL1 controls circadian function. Nature. 2007;450(7172):1086–1090. doi: 10.1038/nature06394. [DOI] [PubMed] [Google Scholar]
- [49].Chrousos GP. The role of stress and the hypothalamic-pituitary-adrenal axis in the pathogenesis of the metabolic syndrome: neuro-endocrine and target tissue-related causes. Int J Obes Relat Metab Disord. 2000;24(Suppl 2):S50–55. doi: 10.1038/sj.ijo.0801278. [DOI] [PubMed] [Google Scholar]