Abstract
NK cells represent a large proportion of the lymphocyte population in the liver and are involved in early innate immunity to pathogen infection. As a result of liver endothelial cell fenestrations, parenchymal cells are not separated by a basal membrane, and thereby pathogen-infected hepatocytes are extensively capable of interacting with innate immune cells including NK cells. In addition, hepatic NK cells interact with surrounding DC and alter their differentiation and function. Recent studies reveal that NK cells exhibit a regulatory function that modulates T cell responses through their interaction with DC and/or direct effect on T cells. Thus, NK cells play a central role, not only in innate immunity, but also in shaping the adaptive immune response. During pathogen infection, there is a remarkable increase of hepatic NK cells, possibly due to the expansion of resident liver NK cells and/or recruitement of NK cells from the blood. The liver microenvironment is believed to modulate hepatic NK cell function through the induction of activating/inhibitory receptor expression and inflammatory cytokine secretion. Particularly, the liver maintains intrahepatic NK cells in a functionally hyporesponsive state compared to splenic NK cells: liver NK cells displayed a dampened IFN-γ response to IL-12/IL-18 stimulation. Notably, the liver contains a significant population of functionally hyporesponsive NK cells that express high levels of the inhibitory receptor NKG2A and lack expression of MHC class I-binding Ly49 receptors. Importantly, adoptively transferred splenic NK cells that migrate to the liver displayed phenotypic and functional changes, supporting a view that the liver environment modifies NK cell receptor expression and functional responsiveness. In this article, we will review studies on the regulation of NK cell repertoire and function in the hepatic environment and the impact of liver NK cell immunoregulatory function on influencing adaptive immunity.
Keywords: liver, NK cells, TLR activation, inflammatory cytokine stimulation, NK-DC/KC interaction
I. INTRODUCTION
The liver represents a unique immunological environment, as it is able to endure daily exposure to gut-derived foreign antigen without causing inflammation.1,2 The immune tolerance of the liver has been noted for having successful allograft transplants without the administration of immunosuppressive agents. Despite this tolerogenic environment, the liver rapidly and effectively switches from an environment where T cell engagement typically results in immune tolerance and/or cell death to a strong proinflammatory site upon infection, leading to pathogen clearance. However, several pathogens, including hepatitis B and hepatitis C viruses, and malaria exploit liver tolerance mechanisms in attempts to avoid immune clearance and establish persistent infection. An in-depth understanding of the regulatory mechanisms responsible for the initial state of immune tolerance may shed light on identifying potential factors targeted by persistent pathogens.
Natural killer (NK) cells constitute a large proportion of the lymphocyte population in the liver and are involved in regulating hepatic immunity to pathogen infection. Importantly, the phenotype and function of hepatic NK cells (both resident and recruited/blood-derived NK cells) are altered in the liver microenvironment: hepatic NK cells exhibit unique NK cell repertoire and cytokine profiles.3 Therefore, the alteration of hepatic NK cell subsets and their function may contribute to the establishment/maintenance of persistent infections. Furthemore, through cell-to-cell interaction and secretion of cytokines, NK cells can regulate the differentiation and function of dendritic cells (DCs), thereby affecting T cell responses. In this review, we will provide a brief discussion of liver NK cell subsets with distinct effector functions and describe the potential immunoregulatory role of NK cells in shaping the adaptive immunity.
II. NK CELL DEVELOPMENT AND DIFFERENTIATION
NK cells were initially defined as lymphocytes that could respond nonspecifically to transformed or virally infected target cells without prior sensitization. The majority of NK cells develop in the bone marrow from NK cell precursors (NKPs) while the development of NK cells also appears to occur at other lymphoid sites, such as the thymus and lymph nodes.4 NKPs are able to differentiate solely into NK cells, and both immature and mature NK cells express NK1.1. The earliest committed NKP population in the mouse bone marrow is defined as CD49b−CD161−CD122+.5 NKPs develop into immature NK cells, which express CD161 (NK1.1) and maintain CD122 expression.6,7 Immature NK cell development follows a stepwise acquisition of phenotypic markers, including the expression of CD94/NKG2 receptors, followed by Ly49 receptors and CD49b (Dx5). In addition, the expression of CD11b and CD43 increases as the maturation of NK cells progresses.8,9,10
Immature NK cells that develop into mature NK cells have recently been characterized as either educated (also termed “licensed”) or uneducated.11 An educated NK cell is defined as possessing full functional capacity; that is, NK cells respond to stimulation of activation receptor triggering. NK cell education appears to be regulated by signaling through MHC class I receptors, as NK cells lacking the expression of self-specific MHC class I receptors are functionally hyporesponsive.12,13 Indeed, NK cells isolated from MHC class I deficient mice display a lower functional capacity compared to NK cells from wild-type mice. Furthermore, the ITIM (located on the cytoplasmic tail of MHC class I inhibitory receptors) has been demonstrated to be critical for full functional capacity of NK cells.13 The engagement of self-specific MHC class I receptors is critical in maintaining NK cell tolerance in the face of activating receptor signaling. Taken together, host MHC class I molecules are crucial for regulating NK cell responsiveness and receptor repertoire formation.
At present, multiple models have been postulated for the functional maturation of NK cells (see Fig. 1).14,15 Two of these models, arming and disarming, are based on the idea that NK cell education, or licensing, is aimed at ensuring that a functional NK cell is equipped with an appropriate inhibitory receptor whose signaling acts as a balance to regulate signaling via activating receptors. The arming model suggests that direct signaling through a MHC class I receptor supplies a signal that conveys functional capacity on the NK cell. The disarming model, similar to the concept of T cell exhaustion, suggests that in the absence of inhibitory signaling via the MHC class I receptors, continual signaling through activating receptors renders the NK cell hyporesponsive. A more recently proposed third model, rheostat, satisfies both arming and disarming by suggesting that NK cells can be educated through the strength of inhibitory receptor signaling (Fig. 1). Tuning the NK cell with a higher inhibitory input can lead to better IFN-γ production, whereas potential granzyme release can be triggered at lower input.16 To date, the data is insufficient to determine the precise role of each model in acquring NK cell effector function.
FIGURE 1. NK cell education models.
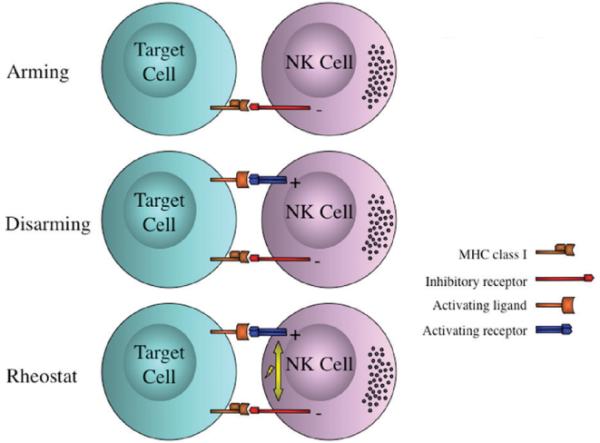
Models for NK cell education include the arming, the disarming, and the rheostat paradigms. Arming model indicates that a direct signaling through a MHC-I binding inhibitory receptor conveys functional capacity to the NK cell. Disarming model suggests that in the absence of inhibitory signaling while receiving an activating stimulus, NK cells will progress to hyporesponsivity. Rheostat model suggests that the strength of one or many inhibitory signals leads to NK cell functionality, where stronger inhibition correlates to higher INF-g production. (Modified from Hoglund and Brodin, Nature Reviews Immunology 2010).
III. UNIQUE FEATURE OF HEPATIC NK CELL SUBSETS
NK cells are relatively enriched in the liver and are embedded in the endothelial lining of the liver sinusoids. Early reports described a liver-specific NK cell population from rat as “pit cells,” due to the presence of a large number of intracellular granules. In addition, rat liver NK cells have been subclassified based on the density and size of their granules as 1) high density-large granules, and 2) low density-small granules. It appears that NK cells with high density-large granules derived from the blood migrate into the liver and further differentiate into low density-small granular hepatic NK cells.17 However, the mechanism(s) for NK cell migration to the liver and factor(s) involved in altering NK cell function are not well understood. Further studies are needed to define these questions.
Importantly, differential functional capacity has been attributed to distinct human NK cell subsets (CD16−CD56Bright or CD16+CD56Dim NK cells) based on the expression of CD16 and CD56. CD16-CD56Bright NK cells may give rise to CD16+CD56Dim NK cells, as suggested by shorter telomere length in CD16+CD56Dim NK cells.18 CD16+CD56Dim NK cells are highly cytotoxic, and make up the bulk of the NK cell population in human peripheral blood (>90%). In contrast, CD16−CD56Bright NK cells are poorly cytotoxic but produce a broad range of cytokines (IFN-γ, TNF-α, GM-CSF, IL-10).19,20 Recently, the surface expression of CD11b and CD27 have been used to define the maturation status of mouse NK cells.21 Adoptive transfer experiments indicated a linear progression of maturation from CD11b−CD27+, to CD11b+CD27+, to CD11b+CD27−. With regard to function, CD11b−CD27+ NK cells produce higher amounts of cytokines and show decreased cytotoxicity compared to CD11b+CD27+ NK cells. In contrast, CD11b+CD27− NK cells express higher levels of killer-cell lectin-like receptor subfamily G, member 1 (KLRG1), which is induced following NK cell activation and proliferation.22 In addition, CD11b+CD27− NK cells are predominant in the blood, while CD11b−CD27+ NK cells are found at a greater proportion in the liver.3
However, it is not known how unique NK cell subsets exist at different tissue sites, particularly in the liver compartment. Thus, future studies on determining factor(s) involved in NK cell differentiation and maturation at various tissue sites would be important for advancing our understanding of NK cell biology and their impact on mounting the immune response. In addition, it will provide useful insight into designing immunotherapeutics for treatment of infectious disease and cancer patients.
IV. NK CELL RECEPTORS AND FUNCTIONS
Besides the critical role for NK cell education through MHC class I receptors, NK cell activation is regulated by the balance of anti- and pro-activation signals mediated through the expression of inhibitory and activating receptors (Ly49 and NKG2) (see Fig. 2). In mice, several NK cell receptors have been identified in the Ly49 receptor family.23 Ly49 receptors are type II transmembrane glycoproteins that are expressed on NK cells and form disulfide linked homodimers, with the majority of NK cells expressing one to four individual Ly49 receptors.24 Most of the 19 Ly49 receptors identified are inhibitory receptors (incorporating one ITIM) and recognize MHC class I and MHC class I-like molecules.25 In contrast, many of the ligands for the activating Ly49 receptors remain unknown. While structurally different from Ly49 receptors, a family of functional homologues of the Ly49 receptor molecules is expressed on human NK cells, referred to as the killer cell immunoglobulin-like receptor (KIR) family, and includes both activating and inhibitory receptors. Importantly, there is a high genetic diversity in the KIR and Ly49 receptor families, where the genes vary in number, genomic organization, and allelic polymorphism among individual haplotypes.26
FIGURE 2. NK cell activating/inhibitory receptors and other surface molecules.
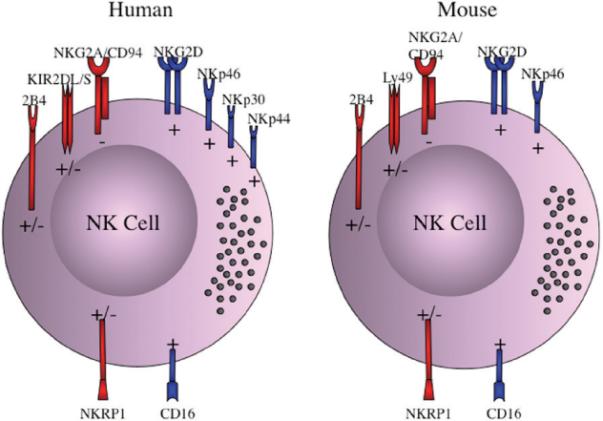
Numerous molecules including activating/inhibitory receptors and cytotoxicity receptors are expressed on the cell surface of NK cells and participate in acquiring NK cell effector function. The inhibitory receptors (−) are depicted as red with long intracellular protein domains; the activating receptors (+) are presented as blue with short intracellular protein domains. Both human and mouse NK cells express the inhibitory receptor NKG2A, and the activating receptors NKG2D, NKp46 and CD16. Moreover, 2B4 and NKRP1 are involved in the signaling to trigger NK cell development, and can induce either inhibitory or activating signals. While human NK cells have 15 inhibitory and activating KIR receptors, mouse NK cells contain at least 19 inhibitory and activating Ly49 receptors. The human natural cytotoxicity receptors (NCR) NKp30, NKp44, and NKp46 are all activating receptors that induce NK cell cytolytic function upon cell-cell contact.
The NKG2 group of receptors are C-type lectin-like receptors that are present in both humans and mice.27 The NKG2 receptors include NKG2A, C, and E, (with NKG2F also expressed in humans) and are expressed at the surface as heterodimers with CD94. NKG2A is an inhibitory receptor, with two ITIMs present in the cytoplasmic tail, while NKG2C and E receptor engagement results in an activating signal via the adaptor protein DAP12.28 In mice, NKG2A is expressed on most NK cells, while NKG2C and E are expressed at significantly lower frequencies. The ligand for NKG2A is the nonclassical MHC class I molecule Qa-1b (human HLA-E), whose expression is dependent on peptides derived from classical MHC class I molecules and thus acts as an overall measurement of MHC class I expression.29,30
NKG2 receptor expression is regulated by the inflammatory stimuli. In particular, IL-10 and TGF-β are able to induce the expression of NKG2A.31 Thereby, the local microenvironment may influence NK cells by modulating their response to infection. Within the liver, such NK cell modulation may contribute to liver tolerance, and may facilitate persistent infection by certain liver-tropic pathogens. Indeed, studies on the functional competence of liver NK cells by the expression of various NK inhibitory and activating receptors demonstrate that the liver contains a prominent subset of NKG2A+ NK cells that lack Ly49 receptor expression. Importantly, this NKG2A+Ly49− NK cell subset is hyporesponsive to IL-12/IL-18 stimulation in the liver compartment. Additionally, when adoptively transferred splenic NK cells migrate to the liver, they more closely resemble the phenotype and function of liver resident NK cells.3 These results support a view that the liver environment can modify NK cell receptor expression and responsiveness to cytokine stimulation, and/or may preferentially retain immature NK cells within the liver.
Although initially described as being part of the NKG2 group of receptors, NKG2D has very little homology to the other NKG2 receptors. NKG2D does not bind CD94, but is expressed as a homodimer on both mouse and human NK cells.32 NKG2D is an activating receptor, and recognizes ligands induced by cellular stress on virally infected or transformed cells.33 In mice, two isoforms of NKG2D are present, characterized by either a long or short cytoplasmic tail.34 The long-tailed isoform associates with the adaptor protein DAP10, while the short-tailed isoform can associate with DAP10 or DAP12. However, the expression of only the long-tailed isoform of NKG2D has been reported in humans. Notably, NKG2D is expressed by most NK cells and the cytokine environment can modulate the level of expression: IL-15 and TNF-α increase the expression of NKG2D, whereas TGF-β downregulates NKG2D expression. Importantly, the KIR, Ly49, and NKG2 receptors on NK cells are stochastically expressed, resulting in heterogenous expression of NK cell receptors on NK cells.35,36,37
The natural cytotoxicity receptors (NCR) are activating Ig-like transmembrane glycoproteins. While the NCRs consist of NKp30, NKp44, and NKp46 in humans, mice express only NKp46 and have a NKp30/Ncr3 pseudogene. NKp46 has two extracellular C2-type Ig-like domains, and a positively charged transmembrane region that associates with the ITAM containing CD3ζ and FcR′γ.38,39,40 It appears that the majority of normal resting target cells either express low-level or no NCR-ligands.39 Although no cellular NCR ligands have been formally identified, the extracellular form of the intermediate filament protein vimentin may be associated with NKp46 cross-linking.41,42,43 NKp46 may also bind membrane-associated heparan sulfate proteoglycans and has been shown to bind hemagglutinin-neuraminidase from influenza and sendai viruses.39,44
V. FACTOR(S) CRUCIAL FOR THE REGULATION OF HEPATIC NK CELL FUNCTION
The main NK cell effector functions include direct cell killing of infected or transformed cells by perforin, granzymes, and TRAIL as well as the secretion of a variety of pro- and anti-inflammatory cytokines.45,46 Notably, cytokine production by NK cells can be regulated through both activating and inhibitory receptors: activating receptor engagement leads to production of IFN-γ, which plays a critical role in shaping the subsequent adaptive immune response. In addition to the cross-linking of activating receptor, NK cell activation can also be induced by various cytokines, including type I IFNs, IL-2, IL-12, IL-15, and IL-18. Distinct NK cell functions have been attributed to stimulation with different cytokines having some synergistic effects. Type I IFNs lead to NK cytotoxic activity, while IL-12 and IL-18 are potent inducers of IFN-γ production.34,47 In addition, IL-15 is involved in NK cell proliferation and appears to be important in NK cell “priming” through trans-presentation of membrane-bound IL-15 on the surface of DCs.
Importantly, NK cells express TLRs crucial for the recognition of pathogen-associated molecular patterns (PAMPs).48 TLR stimulation on NK cells can lead to IFN-γ production, as well as the up-regulation of chemokines CCL3, CCL4, and CCL5.49 Particularly, TLR-3 activates NK cells and increases NK cytotoxicity along with induction of cytokine production.40 In addition, despite the upregulation of NKG2D by TLR stimulation, the induction of NKG2D ligands on macrophages is specific to TLR-4, but not TLR-3, stimulation.50 Because of the high concentration of incoming LPS from the gut, this could be potentially harmful to the liver and thereby it is likely that the counteractive mechanism would be of importance to keep the low level of NKG2D expression by NK cells in the liver. Recently, the crosstalk between complement and the TLRs has been reported to inhibit IL-12 production and this could play a role in dampening excessive NK cell activation. Given that the liver is a major site for the production of complement protein, the combinatorial effect of both TLR and complement could be of significant importance for influencing NK cell function in the liver.51
As hepatic tissue macrophages (ie, Kupffer cells) are critical in endotoxin removal from the blood, it is likely that Kupffer cells may play an important role in maintaining liver tolerance. Although they can be found throughout the liver, Kupffer cells are mainly localized around the periportal regions. A recent study demonstrated that LPS treatment of Kupffer cells resulted in the higher level of anti-inflammatory cytokine IL-10 production compared to that in polyI:C treatment, while IL-12 was produced at similar levels in response to both TLR ligands.52 This study also indicated an important role for IL-10 in maintaining liver tolerance, and further demonstrated that IL-10 production by Kupffer cells led to decreased NK cell activation. Based on the finding that NK cell killing of microglia in the CNS is regulated via NKG2A/Qa-1b interactions in the EAE mouse model,53 it is tempting to speculate that the high expression of NKG2A could play an equally important role in the regulation of liver NK cell function. Taken together, NK cells are regulated via a balance of potentially continuous sensing of their environment through various activating and inhibitory receptors, with activation occurring when activating signals overwhelm the inhibitory signals, either through decreased inhibitory signaling or increased activating signaling.
VI. REGULATION OF HEPATIC FIBROSIS BY NK CELLS
Hepatic fibrosis is a would-healing response to injury, commonly seen in chronic liver disease, and most often attributed to cell death within the liver. Fibrosis can be characterized by the formation of new blood vessels (angiogenesis), sinusoidal remodeling, and pericyte (stellate cell) expansion.54 Hepatic stellate cells (HSCs) are activated through the phagocytosis of apoptotic bodies and cell debris, leading to their differentiation, proliferation and pro-fibrotic progression. Therefore, HSC death is believed to deliver anti-inflammatory and anti-fibrotic responses. Primarily, NK cells appear to be involved in HSC death and thereby depletion of NK cells leads to greater observed fibrosis in the murine fibrosis model.
Quiescent HSCs are relatively resistant to apoptotic signals, whereas activated HSCs are more prone to cell death. Of the many pro-apoptotic molecules investigated in activated HSCs, the upregulation of TRAIL receptor may be an essential NK cell-mediated cytolytic ligand.55 Interestingly, activated HSCs also upregulate a variety of intermediate filaments, including desmin and vimentin, whose levels increase strongly between day 2 and 6 in primary cultures, but only vimentin levels remain consistently high.56 If extracellular vimentin is indeed an activating ligand for the NCR NKp46, it would be an intriguing mechanism behind NK cell-mediated killing of HSCs.
VII. IMMUNOREGULATORY FUNCTION OF NK CELLS
Recently, our understanding of the role of NK cells in regulating the overall host immunity has increased dramatically. Through the production of a wide range of cytokines, NK cells contribute to enhance the immune response by triggering the activation of other innate immune cells and inducing the proinflammatory responses. NK cells also play a critical role in shaping the adaptive immune response through optimal T cell activation via direct cell-to-cell interaction with DC. As a result of NK-DC crosstalk, DC mature and secrete cytokines/chemokines, resulting in productive T cell responses.47,57 In contrast, NK cells have been shown to dampen the adaptive immune responses by selective killing of macrophages, DC, and/or T cells.58,59 Thus, NK cells exhibit numerous functions to regulate host immunity. However, it is yet to be determined whether these multiple immunoregulatory functions of NK cells are exerted by specific NK cell subsets or functional changes of overall NK cells. Nevertheless, the understanding of the mechanism(s) involved in the regulation of host immunity by NK cells is crucial for designing immunotherapeutic agents to boost host immunity to viral infection and tumor.
Given a large proportion of NK cells in the liver lymphocyte populations, NK cells may help maintain liver tolerance through their interactions with various cell types. Interestingly, NK cells cocultured with hepatocytes appear to alter the ability of DC to prime CD4+ T cells, resulting in a regulatory T cell phenotype and function.31 In addition, DC induction of a T cell regulatory phenotype is dependent on NKG2A engagement on NK cells during coculture with hepatocytes.60 NKG2A expression is reportedly increased on NK cells from chronic HCV patients, suggesting a role for NKG2A in persistent viral infection.61,62 These results indicate that liver NK cells might lead to inhibition of antiviral T cell responses and facilitate pathogen persistence.
Although there are conflicting reports, several studies have demonstrated the alteration in NK cell population from chronic HCV patients, including frequency, phenotype and function. It has also been reported that freshly isolated NK cells from HCV patients show significant production of IL-10.63 In addition, NK cells from chronic HCV patients are less capable in activating DCs, compared to control NK cells.64 Recently, it was suggested that NK cells may play a central role in the ability of HCV to establish persistent liver infection, and that the NK-DC interaction may be at the core of the immunomodulatory effects targeted by HCV.64 Furthermore, there has been recent evidence suggesting that NKp30 expressing NK cells may contribute to innate resistance and help reduce HCV infection.65 Given the central role that DC play in driving a strong and competent antiviral immune response, and the ability of NK cells to influence DC maturation, dysregulation of the NK-DC interaction in favor of a weakened T cell response would be an effective means of establishing a persistent infection. It is plausible that HCV and other persistent liver pathogens may take advantage of tolerance mechanisms already in place within the naïve liver, thus emphasizing the importance of investigating such mechanisms that contribute to a tolerogenic liver environment. Therefore, it is possible that NK cells are capable of influencing adaptive immunity via two potential mechanisms: 1) affecting APC (eg, DC) function, 2) direct effect on T cell function (see Fig. 3).
FIGURE 3. Immunoregulatory role of NK cells.
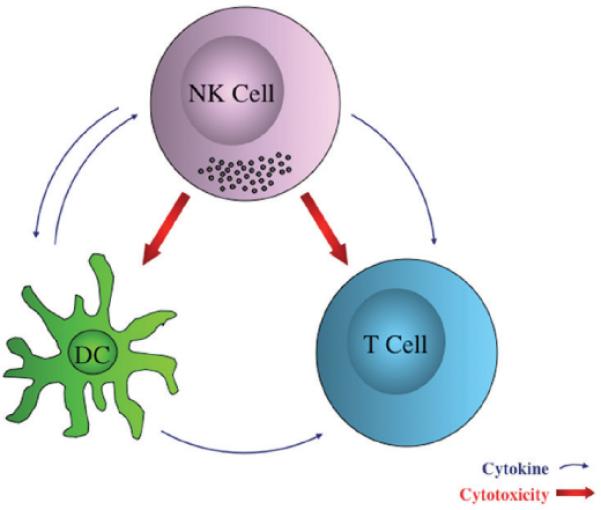
NK cells play a role in shaping adaptive immune responses by either NK-DC interaction or direct effect on T cells. NK-DC crosstalk is implicated in the potential for DC's ability to successfully prime T cells. Cytokines from both NK cells and DC counter-regulate each other, resulting in affecting T cell function. Furthermore, NK cells exhibit direct cytolytic function toward both DC and T cells ex vivo.
As a first possibility for the T cell modulation by NK cells, several studies have indicated that the liver DC population display an immature phenotype and are poor T cell activators. Human liver DC have been shown to secrete more IL-10, as compared to skin DC, and were found to be less capable in stimulating T cell proliferation, leading to IL-10-producing T cells.66 In contrast, skin DC activation of T cells resulted in greater proliferation and more IFN-γ production. Interestingly, DC isolated from human hepatic lymph nodes have also been characterized by high IL-10 production and low allogenic stimulatory capacity, compared to human inguinal lymph node DC.67 Murine liver DC have also been described as less capable of stimulating T cells, compared to spleen DC.68 This may be due in part to differences in subset composition. Indeed, the liver reportedly contains a large proportion of plasmacytoid DC, as well as a B220-CD11chigh and B220-CD-11clow DC subsets, expressing high and low levels of costimulatory molecules, respectively.69 Secondly, recent evidence has given a direct role of NK cells to the cytolysis of activated T cells. Interestingly, apoptotic cells can upregulate NKp46 expression by NK cells, leading to increased natural cytotoxicity toward vementin+ CD4 T cells, which could be interesting due to the high numbers of apoptotic T cells found in the liver.43 On the other hand, IL-10 is able to block vementin secretion by macrophages.41 However, a direct effect of liver resident NK cells on killing T cells in vivo has yet to be determined.
VIII. CONCLUSIONS
The tolerogenic nature of the liver allows daily exposure to gut-derived foreign antigen without causing inflammation, but may facilitate persistent infection in the liver. Natural killer (NK) cells play a central role, not only in innate immunity, but also in shaping the adaptive immune response. The liver maintains intrahepatic NK cells in a functionally hyporesponsive state. Compared to splenic NK cells, liver NK cells display a dampened IFN-γ response to IL-12/IL-18 stimulation. In addition, the liver contains a significant population of functionally hyporesponsive NK cells that express high levels of the inhibitory receptor NKG2A and lack expression of MHC class I-binding Ly49 receptors. Based on the finding that adoptively transferred splenic NK cells that migrate to the liver displayed phenotypic and functional changes, the liver environment modifies NK cell receptor expression and functional responsiveness. Future studies need to be done to define key factor(s) contributing to the regulation of liver NK cells in part by maintaining a greater percentage of the hyporesponsive NKG2A+Ly49− NK cells.
ACKNOWLEDGEMENTS
We thank the members of Hahn laboratory for helpful discussions. This work was supported by the grants from NIH (R01DK063222, U19AI083024) to YSH.
ABBREVIATIONS
- NK
natural killer cells
- DC
dendritic cells
- KC
Kupffer cells
- HSC
hepatic stellate cells
REFERENCES
- 1.Adams DH, Eksteen B, Curbishley SM. Immunology of the gut and liver: a love/hate relationship. Gut. 2008;57:838–48. doi: 10.1136/gut.2007.122168. [DOI] [PubMed] [Google Scholar]
- 2.Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol. 2003;3:51–62. doi: 10.1038/nri981. [DOI] [PubMed] [Google Scholar]
- 3.Lassen MG, Lukens JR, Dolina JS, Brown MG, Hahn YS. Intrahepatic IL-10 maintains NKG2A+Ly49− liver NK cells in a functionally hyporesponsive state. J Immunol. 2010;184:2693–701. doi: 10.4049/jimmunol.0901362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Santo JP. Natural killer cell developmental pathways: a question of balance. Annu Rev Immunol. 2006;24:257–86. doi: 10.1146/annurev.immunol.24.021605.090700. [DOI] [PubMed] [Google Scholar]
- 5.Rosmaraki EE, Douagi I, Roth C, Colucci F, Cumano A, Di Santo JP. Identification of committed NK cell progenitors in adult murine bone marrow. Eur J Immunol. 2001;31:1900–9. doi: 10.1002/1521-4141(200106)31:6<1900::aid-immu1900>3.0.co;2-m. 2001. [DOI] [PubMed] [Google Scholar]
- 6.Vosshenrich CA, Samson-Villeger SI, Di Santo JP. Distinguishing features of developing natural killer cells. Curr Opin Immunol. 2005;17:151–8. doi: 10.1016/j.coi.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Kim S, Iizuka K, Kang HS, Dokun A, French AR, Greco S, Yokoyama WM. In vivo developmental stages in murine natural killer cell maturation. Nat Immunol. 2002;3:523–8. doi: 10.1038/ni796. [DOI] [PubMed] [Google Scholar]
- 8.Dorfman JR, Raulet DH. Acquisition of Ly49 receptor expression by developing natural killer cells. J Exp Med. 1998;187:609–18. doi: 10.1084/jem.187.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sivakumar PV, Gunturi A, Salcedo M, Schatzle JD, Lai WC, Kurepa Z, Pitcher L, Seaman MS, Lemonnier FA, Bennett M, Forman J, Kumar V. Cutting edge: expression of functional CD94/NKG2A inhibitory receptors on fetal NK1.1+Ly-49− cells: a possible mechanism of tolerance during NK cell development. J Immunol. 1999;162:6976–80. [PubMed] [Google Scholar]
- 10.Williams NS, Kubota A, Bennett M, Kumar V, Takei F. Clonal analysis of NK cell development from bone marrow progenitors in vitro: orderly acquisition of receptor gene expression. Eur J Immunol. 2000;30:2074–82. doi: 10.1002/1521-4141(200007)30:7<2074::AID-IMMU2074>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 11.Huntington ND, Vosshenrich CA, Di Santo JP. Developmental pathways that generate natural-killer-cell diversity in mice and humans. Nat Rev Immunol. 2007;7:703–14. doi: 10.1038/nri2154. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez NC, Treiner E, Vance RE, Jamieson AM, Lemieux S, Raulet DH. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 2005;105:4416–23. doi: 10.1182/blood-2004-08-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, French AR, Sunwoo JB, Lemieux S, Hansen TH, Yokoyama WM. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–13. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 14.Raulet DH, Vance RE. Self-tolerance of natural killer cells. Nat Rev Immunol. 2006;6:520–31. doi: 10.1038/nri1863. [DOI] [PubMed] [Google Scholar]
- 15.Yokoyama WM, Kim S. Licensing of natural killer cells by self-major histocompatibility complex class I. Immunol Rev. 2006;214:143–54. doi: 10.1111/j.1600-065X.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- 16.Brodin P, Karre K, Hoglund P. NK cell education: not an on-off switch but a tunable rheostat. Trends Immunol. 2009;30:143–9. doi: 10.1016/j.it.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Nakatani K, Kaneda K, Seki S, Nakajima Y. Pit cells as liver-associated natural killer cells: morphology and function. Med Electron Microsc. 2004;37:29–36. doi: 10.1007/s00795-003-0229-9. [DOI] [PubMed] [Google Scholar]
- 18.Ouyang Q, Baerlocher G, Vulto I, Lansdorp PM. Telomere length in human natural killer cell subsets. Ann N Y Acad Sci. 2007;1106:240–52. doi: 10.1196/annals.1392.001. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs R, Hintzen G, Kemper A, Beul K, Kempf S, Behrens G, Sykora KW, Schmidt RE. CD56bright cells differ in their KIR repertoire and cytotoxic features from CD-56dim NK cells. Eur J Immunol. 2001;31:3121–7. doi: 10.1002/1521-4141(2001010)31:10<3121::aid-immu3121>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 20.Lanier LL, Le AM, Civin CI, Loken MR, Phillips JH. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol. 1986;136:4480–6. [PubMed] [Google Scholar]
- 21.Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol. 2006;176:1517–24. doi: 10.4049/jimmunol.176.3.1517. [DOI] [PubMed] [Google Scholar]
- 22.Robbins SH, Nguyen KB, Takahashi N, Mikayama T, Biron CA, Brossay L. Cutting edge: inhibitory functions of the killer cell lectin-like receptor G1 molecule during the activation of mouse NK cells. J Immunol. 2002;168:2585–9. doi: 10.4049/jimmunol.168.6.2585. [DOI] [PubMed] [Google Scholar]
- 23.Brown MG, Scalzo AA. NK gene complex dynamics and selection for NK cell receptors. Semin Immunol. 2008;20:361–8. doi: 10.1016/j.smim.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanke T, Takizawa H, Raulet DH. MHC-dependent shaping of the inhibitory Ly49 receptor repertoire on NK cells: evidence for a regulated sequential model. Eur J Immunol. 2001;31:3370–9. doi: 10.1002/1521-4141(200111)31:11<3370::aid-immu3370>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 25.Biassoni R. Human natural killer receptors, co-receptors, and their ligands. Curr Protoc Immunol. 2009;Chapter 14(Unit 14):10. doi: 10.1002/0471142735.im1410s84. [DOI] [PubMed] [Google Scholar]
- 26.Long EO. Negative signaling by inhibitory receptors: the NK cell paradigm. Immunol Rev. 2008;224:70–84. doi: 10.1111/j.1600-065X.2008.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gunturi A, Berg RE, Forman J. The role of CD94/NKG2 in innate and adaptive immunity. Immunol Res. 2004;30:29–34. doi: 10.1385/IR:30:1:029. [DOI] [PubMed] [Google Scholar]
- 28.Lanier LL, Corliss B, Wu J, Phillips JH. Association of DAP12 with activating CD94/NKG2C NK cell receptors. Immunity. 1998;8:693–701. doi: 10.1016/s1074-7613(00)80574-9. [DOI] [PubMed] [Google Scholar]
- 29.Le Drean E, Vely F, Olcese L, Cambiaggi A, Guia S, Krystal G, Gervois N, Moretta A, Jotereau F, Vivier E. Inhibition of antigen-induced T cell response and antibody-induced NK cell cytotoxicity by NKG2A: association of NKG2A with SHP-1 and SHP-2 protein-tyrosine phosphatases. Eur J Immunol. 1998;28:264–76. doi: 10.1002/(SICI)1521-4141(199801)28:01<264::AID-IMMU264>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 30.Vance RE, Jamieson AM, Raulet DH. Recognition of the class Ib molecule Qa-1(b) by putative activating receptors CD94/NKG2C and CD94/NKG2E on mouse natural killer cells. J Exp Med. 1999;190:1801–12. doi: 10.1084/jem.190.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jinushi M, Takehara T, Tatsumi T, Yamaguchi S, Sakamori R, Hiramatsu N, Kanto T, Ohkawa K, Hayashi N. Natural killer cell and hepatic cell interaction via NKG2A leads to dendritic cell-mediated induction of CD4 CD25 T cells with PD-1-dependent regulatory activities. Immunology. 2007;120:73–82. doi: 10.1111/j.1365-2567.2006.02479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu J, Song Y, Bakker AB, Bauer S, Spies T, Lanier LL, Phillips JH. An activating immunoreceptor complex formed by NKG2D and DAP10. Science. 1999;285:730–2. doi: 10.1126/science.285.5428.730. [DOI] [PubMed] [Google Scholar]
- 33.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–9. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 34.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–74. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 35.Castriconi R, Cantoni C, Della Chiesa M, Vitale M, Marcenaro E, Conte R, Biassoni R, Bottino C, Moretta L, Moretta A. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: consequences for the NK-mediated killing of dendritic cells. Proc Natl Acad Sci U S A. 2003;100:4120–5. doi: 10.1073/pnas.0730640100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roberts AI, Lee L, Schwarz E, Groh V, Spies T, Ebert EC, Jabri B. NKG2D receptors induced by IL-15 costimulate CD28-negative effector CTL in the tissue microenvironment. J Immunol. 2001;167:5527–30. doi: 10.4049/jimmunol.167.10.5527. [DOI] [PubMed] [Google Scholar]
- 37.Sutherland CL, Chalupny NJ, Schooley K, VandenBos T, Kubin M, Cosman D. UL16-binding proteins, novel MHC class I-related proteins, bind to NKG2D and activate multiple signaling pathways in primary NK cells. J Immunol. 2002;168:671–9. doi: 10.4049/jimmunol.168.2.671. [DOI] [PubMed] [Google Scholar]
- 38.Mandelboim O, Porgador A. NKp46. Int J Biochem Cell Biol. 2001;33:1147–50. doi: 10.1016/s1357-2725(01)00078-4. [DOI] [PubMed] [Google Scholar]
- 39.Walzer T, Blery M, Chaix J, Fuseri N, Chasson L, Robbins SH, Jaeger S, Andre P, Gauthier L, Daniel L, Chemin K, Morel Y, Dalod M, Imbert J, Pierres M, Moretta A, Romagne F, Vivier E. Identification, activation, and selective in vivo ablation of mouse NK cells via NKp46. Proc Natl Acad Sci U S A. 2007;104:3384–9. doi: 10.1073/pnas.0609692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moretta L, Bottino C, Pende D, Castriconi R, Mingari MC, Moretta A. Surface NK receptors and their ligands on tumor cells. Semin Immunol. 2006;18:151–8. doi: 10.1016/j.smim.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Mor-Vaknin N, Punturieri A, Sitwala K, Markovitz DM. Vimentin is secreted by activated macrophages. Nat Cell Biol. 2003;5:59–63. doi: 10.1038/ncb898. [DOI] [PubMed] [Google Scholar]
- 42.Garg A, Barnes PF, Porgador A, Roy S, Wu S, Nanda JS, Griffith DE, Girard WM, Rawal N, Shetty S, Vankayalapati R. Vimentin expressed on Mycobacterium tuberculosis-infected human monocytes is involved in binding to the NKp46 receptor. J Immunol. 2006;177:6192–8. doi: 10.4049/jimmunol.177.9.6192. [DOI] [PubMed] [Google Scholar]
- 43.Chong WP, Zhou J, Law HK, Tu W, Lau YL. Natural killer cells become tolerogenic after interaction with apoptotic cells. Eur J Immunol. 2010;40:1718–27. doi: 10.1002/eji.200939768. [DOI] [PubMed] [Google Scholar]
- 44.Bloushtain N, Qimron U, Bar-Ilan A, Hershkovitz O, Gazit R, Fima E, Korc M, Vlodavsky I, Bovin NV, Porgador A. Membrane-associated heparan sulfate proteoglycans are involved in the recognition of cellular targets by NKp30 and NKp46. J Immunol. 2004;173:2392–401. doi: 10.4049/jimmunol.173.4.2392. [DOI] [PubMed] [Google Scholar]
- 45.Hamerman JA, Ogasawara K, Lanier LL. NK cells in innate immunity. Curr Opin Immunol. 2005;17:29–35. doi: 10.1016/j.coi.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 46.Takeda K, Hayakawa Y, Smyth MJ, Kayagaki N, Yamaguchi N, Kakuta S, Iwakura Y, Yagita H, Okumura K. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med. 2001;7:94–100. doi: 10.1038/83416. [DOI] [PubMed] [Google Scholar]
- 47.Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 2004;5:1260–5. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 48.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–84. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 49.Katsargyris A, Klonaris C, Alexandrou A, Giakoustidis AE, Vasileiou I, Theocharis S. Toll-like receptors in liver ischemia reperfusion injury: a novel target for therapeutic modulation? Expert Opin Ther Targets. 2009;13:427–42. doi: 10.1517/14728220902794939. [DOI] [PubMed] [Google Scholar]
- 50.Eissmann P, Evans JH, Mehrabi M, Rose EL, Nedvetzki S, Davis DM. Multiple mechanisms downstream of TLR-4 stimulation allow expression of NKG2D ligands to facilitate macrophage/NK cell crosstalk. J Immunol. 2010;184:6901–9. doi: 10.4049/jimmunol.0903985. [DOI] [PubMed] [Google Scholar]
- 51.Hajishengallis G, Lambris JD. Crosstalk pathways between Toll-like receptors and the complement system. Trends Immunol. 2010;31:154–63. doi: 10.1016/j.it.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tu Z, Bozorgzadeh A, Pierce RH, Kurtis J, Crispe IN, Orloff MS. TLR-dependent cross talk between human Kupffer cells and NK cells. J Exp Med. 2008;205:233–44. doi: 10.1084/jem.20072195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hao J, Liu R, Piao W, Zhou Q, Vollmer TL, Campagnolo DI, Xiang R, La Cava A, Van Kaer L, Shi FD. Central nervous system (CNS)-resident natural killer cells suppress Th17 responses and CNS autoimmune pathology. J Exp Med. 2010;207:1907–21. doi: 10.1084/jem.20092749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–69. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mehal W, Imaeda A. Cell death and fibrogenesis. Semin Liver Dis. 2010;30:226–31. doi: 10.1055/s-0030-1255352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Niki T, Pekny M, Hellemans K, Bleser PD, Berg KV, Vaeyens F, Quartier E, Schuit F, Geerts A. Class VI intermediate filament protein nestin is induced during activation of rat hepatic stellate cells. Hepatology. 1999;29:520–7. doi: 10.1002/hep.510290232. [DOI] [PubMed] [Google Scholar]
- 57.Andrews DM, Scalzo AA, Yokoyama WM, Smyth MJ, Degli-Esposti MA. Functional interactions between dendritic cells and NK cells during viral infection. Nat Immunol. 2003;4:175–81. doi: 10.1038/ni880. [DOI] [PubMed] [Google Scholar]
- 58.Lu L, Ikizawa K, Hu D, Werneck MB, Wucherpfennig KW, Cantor H. Regulation of activated CD4+ T cells by NK cells via the Qa-1-NKG2A inhibitory pathway. Immunity. 2007;26:593–604. doi: 10.1016/j.immuni.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moretta A. Natural killer cells and dendritic cells: rendezvous in abused tissues. Nat Rev Immunol. 2002;2:957–64. doi: 10.1038/nri956. [DOI] [PubMed] [Google Scholar]
- 60.Roy S, Barnes PF, Garg A, Wu S, Cosman D, Vankayalapati R. NK cells lyse T regulatory cells that expand in response to an intracellular pathogen. J Immunol. 2008;180:1729–36. doi: 10.4049/jimmunol.180.3.1729. [DOI] [PubMed] [Google Scholar]
- 61.Jinushi M, Takehara T, Tatsumi T, Kanto T, Miyagi T, Suzuki T, Kanazawa Y, Hiramatsu N, Hayashi N. Negative regulation of NK cell activities by inhibitory receptor CD94/NKG2A leads to altered NK cell-induced modulation of dendritic cell functions in chronic hepatitis C virus infection. J Immunol. 2004;173:6072–81. doi: 10.4049/jimmunol.173.10.6072. [DOI] [PubMed] [Google Scholar]
- 62.Nattermann J, Feldmann G, Ahlenstiel G, Langhans B, Sauerbruch T, Spengler U. Surface expression and cytolytic function of natural killer cell receptors is altered in chronic hepatitis C. Gut. 2006;55:869–77. doi: 10.1136/gut.2005.076463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De Maria A, Fogli M, Mazza S, Basso M, Picciotto A, Costa P, Congia S, Mingari MC, Moretta L. Increased natural cytotoxicity receptor expression and relevant IL-10 production in NK cells from chronically infected viremic HCV patients. Eur J Immunol. 2007;37:445–55. doi: 10.1002/eji.200635989. [DOI] [PubMed] [Google Scholar]
- 64.Golden-Mason L, Rosen HR. Natural killer cells: primary target for hepatitis C virus immune evasion strategies? Liver Transpl. 2006;12:363–72. doi: 10.1002/lt.20708. [DOI] [PubMed] [Google Scholar]
- 65.Golden-Mason L, Cox AL, Randall JA, Cheng L, Rosen HR. Increased natural killer cell cytotoxicity and NKp30 expression protects against hepatitis C virus infection in high-risk individuals and inhibits replication in vitro. Hepatology. 2010;52:1581–9. doi: 10.1002/hep.23896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goddard S, Youster J, Morgan E, Adams DH. Interleukin-10 secretion differentiates dendritic cells from human liver and skin. Am J Pathol. 2004;164:511–9. doi: 10.1016/S0002-9440(10)63141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kwekkeboom J, Boor PP, Sen E, Kusters JG, Drexhage HA, de Jong EC, Tilanus HW, Ijzermans JN, Metselaar HJ. Human liver myeloid dendritic cells maturate in vivo into effector DC with a poor allogeneic T-cell stimulatory capacity. Transplant Proc. 2005;37:15–6. doi: 10.1016/j.transproceed.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 68.Pillarisetty VG, Shah AB, Miller G, Bleier JI, DeMatteo RP. Liver dendritic cells are less immunogenic than spleen dendritic cells because of differences in subtype composition. J Immunol. 2004;172:1009–17. doi: 10.4049/jimmunol.172.2.1009. [DOI] [PubMed] [Google Scholar]
- 69.Jomantaite I, Dikopoulos N, Kroger A, Leithauser F, Hauser H, Schirmbeck R, Reimann J. Hepatic dendritic cell subsets in the mouse. Eur J Immunol. 2004;34:355–65. doi: 10.1002/eji.200324336. [DOI] [PubMed] [Google Scholar]


