Several patterns of cutaneous hyperpigmentation in patients receiving the multikinase inhibitor vandetanib (ZD6474) were recently described.1 We describe a patient with perifollicular dark blue-gray macular hyperpigmentation secondary to vandetanib who was successfully treated with 755 nm Q-switched alexandrite laser.
Report of a Case. A 38 year old patient with Fitzpatrick Type II skin and Von Hippel-Lindau syndrome with associated clear cell renal cell carcinoma enrolled onto a phase 2 study and received once-daily oral vandetanib for treatment of her renal tumors. Within two weeks of initiating treatment, the patient developed an acneiform eruption of scattered erythematous papules and pustules on the face and trunk. After 7 months, the patient also developed multiple perifollicular 1-2 mm slate blue macules on the forehead, cheeks, neck and conchal bowls at sites of existing scars and vandetanib-induced acneiform lesions (Figure 1a). She was treated with doxycycline (100 mg twice daily) and strict photoprotection. Her acneiform lesions improved significantly on doxycycline but recurred when she discontinued therapy.
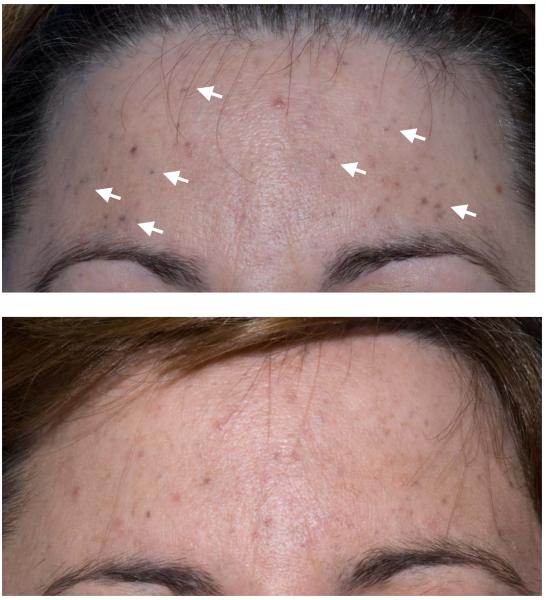
FIGURE 1.
Vandetanib-associated perifollicular blue-gray macules. A, Scattered on forehead prior to laser treatment. B, Decreased macules on the forehead after four laser treatments.
The patient was offered treatment with monthly Q-switched alexandrite laser therapy to address the blue facial pigmentation. A test region on the patient’s forehead was treated with the 755 nm Q-switched alexandrite laser using the following settings: 7.5 J/cm2, 3 mm collimated spot size and 50-ns pulse duration with demonstrable lightening of the blue macules. The entire face was treated on subsequent visits with marked improvement (Figure 1b). After four monthly sessions, the treatments were temporarily discontinued, and the blue hyperpigmentation returned. The patient remains on therapy with vandetanib approximately 22 months following enrollment on protocol.
Comment
The tyrosine kinase inhibitor vandetanib targets vascular endothelial growth factor receptor, epidermal growth factor receptor and the RET protooncogene. Vandetanib is currently being investigated as a treatment in patients with several tumor types. This agent has been associated with acneiform lesions, eczematous skin changes, photosensitivity and pigmentation.1 Hyperpigmentation can present as blue pigmentation at sites of prior scars, diffuse blue-gray pigmentation, diffuse brown pigmentation, and perifollicular dark blue-gray macules. Biopsy of the perifollicular blue hyperpigmentation shows Perls negative, Fontana-Mason positive dermal melanophages.1
Laser therapy has been used with variable success in the treatment of drug-induced hyperpigmentation. The Q-switched ruby, alexandrite, and Nd-Yag lasers have been used successfully for minocycline-induced and amiodarone-induced hyperpigmentation.2,3,4 We hypothesized that given the clinical similarity to Type I minocycline hyperpigmentation and Fontana-Masson positivity, vandetanib-induced hyperpigmentation might respond similarly. The Q-Switched 755 nm alexandrite laser was selected in this case because of its longer wavelength and greater dermal penetration as compared with ruby or frequency-doubled Q-switched Nd-Yag lasers. Previously described laser energy, spot size and pulse duration settings were used. 2
The etiology of the pigmentary changes induced by vandetanib has yet to be elucidated. Similarly, the mechanism by which laser reduces hyperpigmentation is unclear. Laser treatment of vandetanib-associated blue macular pigmentation appears to be an effective, yet temporizing measure. The pigmentation can recur while patients remain on the drug. For patients who receive long-term vandetanib treatment to control their neoplasms and experience associated pigmentation, laser therapy can be a therapeutic option.
Acknowledgments
Funding/Support: This work was supported by the Intramural Research Program of the NIH, National Cancer Institute.
Footnotes
Author Contributions: Drs Brooks, Linehan, Srinivasan and Kong had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Brooks and Kong. Acquisition of data: Brooks and Kong. Analysis and interpretation of data: Brooks, Linehan, Srinivasan, and Kong. Drafting of the manuscript: Brooks and Kong. Critical revision of the manuscript for important intellectual content: Brooks, Linehan, Srinivasan, and Kong. Administrative, technical, or material support: Brooks, Linehan, Srinivasan, and Kong. Study supervision: Srinivasan and Kong.
Financial Disclosure: None reported
References
- 1.Kong HH, Fine HA, Stern JB, Turner ML Chanco. Cutaneous pigmentation after photosensitivity induced by Vandetanib therapy. Arch Dermatolol. 2009;145(8):923–926. doi: 10.1001/archdermatol.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green D, Friedman KJ. Treatment of minocycline-induced pigmentation with the Q-switched Alexandrite laser and a review of the literature. J Am Acad Dermatol. 2001;44(2):342–347. doi: 10.1067/mjd.2001.103036. [DOI] [PubMed] [Google Scholar]
- 3.Alster TS, Gupta SN. Minocycline-induced hyperpigmentation treated with a 755-nm Q-switched alexandrite laser. Dermatol Surg. 2004;30(9):1201–1204. doi: 10.1111/j.1524-4725.2004.30372.x. [DOI] [PubMed] [Google Scholar]
- 4.Tsao H, Busam K, Barnhill RL, Dover JS. Treatment of minocycline-induced hyperpigmentation with the Q-switched ruby laser. Arch Dermatol. 1996;132:1250–1251. [PubMed] [Google Scholar]