Abstract
Averaged across many previous investigations, doubling the CO2 concentration ([CO2]) has frequently been reported to cause an instantaneous reduction of leaf dark respiration measured as CO2 efflux. No known mechanism accounts for this effect, and four recent studies have shown that the measurement of respiratory CO2 efflux is prone to experimental artifacts that could account for the reported response. Here, these artifacts are avoided by use of a high-resolution dual channel oxygen analyzer within an open gas exchange system to measure respiratory O2 uptake in normal air. Leaf O2 uptake was determined in response to instantaneous elevation of [CO2] in nine contrasting species and to long-term elevation in seven species from four field experiments. Over six hundred separate measurements of respiration failed to reveal any decrease in respiratory O2 uptake with an instantaneous increase in [CO2]. Respiration was found insensitive not only to doubling [CO2], but also to a 5-fold increase and to decrease to zero. Using a wide range of species and conditions, we confirm earlier reports that inhibition of respiration by instantaneous elevation of [CO2] is likely an experimental artifact. Instead of the expected decrease in respiration per unit leaf area in response to long-term growth in the field at elevated [CO2], there was a significant increase of 11% and 7% on an area and mass basis, respectively, averaged across all experiments. The findings suggest that leaf dark respiration will increase not decrease as atmospheric [CO2] rises.
A quantitative analysis of prior studies weighted for replication and experimental variation concluded that a doubling of atmospheric CO2 concentration would decrease respiratory CO2 efflux by 18% in woody plants (Curtis and Wang, 1998). This decrease is considered the result of a direct instantaneous effect of increased CO2 concentration and a longer term indirect effect due to changes in leaf composition with long-term growth at elevated CO2 (for review, see Drake et al., 1999). In an analysis of 45 species, 36 showed an average 15% instantaneous reduction in net respiratory CO2 efflux per unit leaf area (Rd,CO2) on transfer to elevated [CO2] (for review, see Amthor, 1997). Terrestrial plant respiration releases 10 times more carbon per annum than fossil fuel combustion (Amthor, 1997). Therefore, a 15% to 20% decrease in foliar respiration might increase the potential of terrestrial vegetation to sequester carbon into biomass, providing a partial amelioration to the rate of increase of atmospheric [CO2] (for review, see Gonzalez-Meler and Siedow, 1999).
However, there is considerable variation in the reported instantaneous effects of elevated [CO2] on Rd,CO2 with some studies reporting a large decrease and others reporting no change (Bunce, 2002; Bruhn et al., 2002). Furthermore, whereas some biochemical reactions within plant respiratory metabolic pathways are sensitive to [CO2], none of those identified exert sufficient metabolic control to account for the reported decreases in respiration (Gonzalez-Meler and Siedow, 1999). Four developments now suggest that the decreases observed when measuring Rd,CO2 from leaves in gas exchange systems incorporating infrared CO2 analyzers may be the result of artifacts in the measuring system. Amthor (2000) showed that very thorough sealing and use of an enlarged cuvette enclosing the leaf greatly reduced the apparent instantaneous effect of doubling [CO2] on Rd,CO2, although a small significant effect still remained. Jahnke (2001) systematically analyzed and eliminated potential artifacts within a gas exchange system and subsequently failed to detect any response in bean (Phaesoleus vulgaris) and poplar (Populus tremula) leaves when corrections were applied. Bouma et al. (1997) and Burton and Pregitzer (2002) similarly demonstrated that respiratory efflux of CO2 from roots was independent of measurement CO2 concentration. Assuming common dark respiratory metabolism between roots and leaves, this casts further doubt on the possibility of a direct response in the leaf. Avoiding the potential artifacts of CO2 exchange measurement by determining dark respiratory O2 uptake (Rd,O2), Amthor et al. (2001) showed no significant change in respiration of five Rumex crispus leaves in sharp contrast to earlier measurements of Rd,CO2 in the same species (Amthor et al., 1992).
Measurement of O2 uptake has three important advantages over measurement of CO2 efflux for an instantaneous effect of change in [CO2] on respiration. First, the gas being measured is not the gas being altered in concentration, avoiding any need for instrument recalibration. Second, the concentration gradient of [O2] between the cuvette enclosing the leaf and the surrounding air is unaltered when [CO2] is changed. Finally, O2, unlike CO2, is not easily absorbed and adsorbed by surfaces in the gas exchange system. Yet, even when the effect of elevated [CO2] on O2 uptake has been examined, findings have been variable. In contrast to Amthor et al. (2001), Reuveni et al. (1993) measured a decrease in respiratory O2 uptake under elevated [CO2]. Controversy over the presence or absence of a direct effect of [CO2] on Rd,CO2 continues. Some recent studies of Rd,CO2, despite controlling for errors likely to arise from leaks, continue to report a significant instantaneous decrease in Rd,CO2 on elevation of [CO2]. For example, decreases have been reported of 19% for rice (Oryza sativa) canopies on elevation of [CO2] from 350 to 700 μmol mol-1 (Baker et al., 2000); 31% to 79% for Scots pine (Pinus sylvestris) needles also on elevation from 370 to 700 μmol mol-1 (Jach and Ceulemans, 2000); 16% to 40% for eight species of crop and crop weeds on increase from 60 to 1,000 μmol mol-1 (Bunce, 2001); an 11% average for leaves of 12 grass species on elevation from 360 to 1,300 μmol mol-1 (Tjoelker et al., 2001); 18% in the second leaf of soybean (Glycine max) on elevation from 350 to 1,400 μmol mol-1 (Bunce, 2002); and 4.5% decrease for seedling shoots of the tropical rainforest tree Tectona grandis on elevation from 370 to 600 μmol mol-1 (Holtum and Winter, 2003). Precautions taken in measurement were sufficient for Bunce (2002) to rebut the idea that the direct effect of elevated [CO2] on respiration was an artifact: “These precautions coupled with observed effects of dark [CO2] treatments on mass accumulation and translocation, suggest that the observed changes in respiration were real.” Although these decreases are smaller than those suggested by earlier studies, and some use larger increases in [CO2] to elicit an effect, they continue to suggest direct instantaneous inhibition of leaf dark respiration by increase in [CO2]. An added uncertainty is whether an instantaneous suppression of respiration by elevated [CO2] is limited to certain species or conditions (Drake et al., 1999). Although maintaining the same [CO2] outside the cuvette to that inside will minimize diffusive leaks, it will not eliminate the substantial problem of CO2 absorption/desorption from surfaces in the gas exchange system (Bloom et al., 1980; Long et al., 1996; Jahnke, 2001) or the mass flow of CO2 between the cuvette and the outside through the intercellular air space when only part of the leaf is enclosed (Jahnke and Krewitt, 2002). Thus, some of these further artifacts may still affect even these very careful studies. Clearly, we are some way from any consensus over the existence, or otherwise, of a direct effect of increase in [CO2] on leaf respiration in the dark.
If the artifacts suggested by Amthor (2000), Jahnke (2001), and Jahnke and Krewitt (2002) apply to the effect of an instantaneous increase in [CO2] on Rd,CO2, then the same errors would at least in part apply when these systems are used to measure the effect of long-term acclimation to elevated [CO2] on Rd,CO2. Plants grown in elevated [CO2] in the long-term (1-10 years) typically show decreased nitrogen content and increased photosynthesis, growth, leaf mass per unit area, nonstructural carbohydrate concentrations (Drake et al., 1997), and large increases in leaf mitochondrial numbers (Robertson et al., 1995; Griffin et al., 2001; Tissue et al., 2002). With the exception of nitrogen content, these are all factors that might be expected to lead to increased leaf respiration. However, a quantitative survey using meta-analytical analysis concluded that long-term growth at elevated [CO2] decreased Rd,CO2 significantly and by 18% (Wang and Curtis, 2002).
Recently, high-sensitivity dual lead-gold (fuel cell) detectors have been developed that allow measurement of small differences in [O2] (approximately 1-2 μmol mol-1) between the inlet and outlet air streams of a leaf cuvette in normal air (Willms et al., 1997, 1999; Hunt, 2003). The objectives of this study were to use such a measurement system to answer two questions: Can the findings of Amthor et al. (2001) of no instantaneous effect of doubling of [CO2] on leaf Rd,O2 be extended to a wide range of species, growth conditions, and measurement [CO2]? Using a range of long-term experiments in which a wide range of plants have been grown under elevated [CO2] in the field (Table I) is Rd,O2 decreased or unaffected as a result of acclimation to elevated [CO2]?
Table I.
Species, [CO2] treatments, plant and leaf developmental stage at the time of measurement
Locations for material growing in the field and microclimate for controlled environment grown plants are given under “Materials and Methods”. References providing a description of each of the long-term field elevated [CO2] experiments used are included.
| Species | Growth [CO2] (μmol mol1) | Developmental Stage | Leaf Developmental Stage | Growth Conditions | Date of Measurement |
|---|---|---|---|---|---|
| Acer saccharum Marsh., Betula papyrifera Marsh. & Populus tremuloides Michx. | 368 and 560, respectively | 4 years old | Most recent fully expanded | FACE; At Rhinelander, Wisconsin in open soil conditions (Dickson et al. 2001) | July/August 2000a |
| Glycine max cv Williams | 368 | Vegetative, 36 d after emergence | Most recent fully expanded | Controlled environment; 14-h photoperiod, day/night temperature of 25°/20°C, 1000 μmol m−2 s−1Q, in 1.4 dm3 containers | May 2000b |
| Glycine max cv Pana | 369 | Podfill, 100 d after emergence | Most recent fully expanded | Controlled environment; 14-h photoperiod, day/night temperature of 25°/20°C, 1000 μmol m−2 s−1Q, in 1 m3 containers. | March 2001b |
| Glycine max cv Pana | 368 and 550 | Podfill, 90 d after emergence | Most recent fully expanded | FACE; Experiment Station (University of Illinois, Urbana-Champaign) in open soil conditions (Rogers et al., 2004) | August 2001a |
| Pinus taeda Seedlings | 368 | 3 years old | Current years needles | Experiment Station (University of Illinois, Urbana-Champaign) in 20 dm3 containers | June 2000b |
| P. taeda Mature | 368 and 560 | 18 years old | Previous years needles | FACE; at Duke Forest, North Carolina in open soil conditions (DeLucia et al., 1999) | April 2001a |
| Quercus rubra | 368 | 80 years old | Most recent fully expanded, 14 weeks after first leaf flush | Experiment Station (University of Illinois, Urbana-Champaign) in open soil conditions | July 2000b |
| Quercus geminata Willd. & Q. myrtifolia Small | 369 and 560 | 5-year-old re-growth trees after fire event | Previous years leaves just before new leaf flush | Open-top chambers; Merritt Island, Florida in open soil conditions. (Hymus et al., 2002; Ainsworth et al., 2003) | February/March 2001a |
| Zea mays cv IL 731 | 369 | 12 weeks after emergence | Most recent fully expanded | Experiment Station (University of Illinois, Urbana-Champaign) in open soil conditions | July 2000b |
a Measurements made in the first 2.5 h of the night. b Measurements made in the first and last 2.5 h of the night.
RESULTS
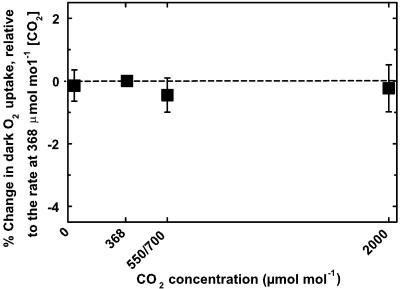
There was no significant instantaneous effect of [CO2] on leaf Rd,O2 in any of the nine species studied (Fig. 1; Tables II) and III). The results for the ANOVA were, for CO2, F3,332 = 0.032, P = 0.99; and for species x CO2, F24,332 = 0.528, P = 0.97. A complete lack of any effect of [CO2] was evident in all species not only on doubling [CO2], but when [CO2] was increased more than five times above the current ambient concentration or decreased to zero (Fig. 1; Tables II and III). The extremely high probabilities for accepting the null hypothesis (P > 0.97) eliminate any possibility of a Type II error, i.e. a difference undetected because of variability. This is also reflected in the combined regression of response relative to ambient [CO2] (Fig. 1), where the probability that the response is independent of [CO2] was P = 0.99 (F3,360 < 0.001). This lack of response of respiration to elevated [CO2] was independent of treatment method (F3,360 = 0.222, P = 0.88), developmental stage (F3,360 = 0.174, P = 0.92), beginning or end of night (F3,360 = 0.638, P = 0.59), and the [CO2] at which the plants had been grown (F3,100 = 0.080, P = 0.97). Although there was no interaction with [CO2], absolute rates of respiration generally decreased with time in the dark.
Figure 1.
The instantaneous effect of change in [CO2], from the current ambient concentration, on leaf respiratory O2 uptake in the dark. Respiration is plotted as the percentage of change (±1 SE) relative to the rate of O2 consumption at current ambient [CO2]. Each mean illustrated is based on measurements of 274 plants and nine species (Table I). Leaves were in darkness and their temperature was maintained at 25°C. Results for an analysis of variance (ANOVA) are given in the results text, and respiration rates for individual species contributing to the illustrated means are shown in Tables II and III.
Table II.
The response of leaf respiration to changes in [CO2] for plants grown at current [CO2] only.
The mean dark leaf O2 uptake on an area basis (micromoles per minute per second), and its SE (n = 6), are given for each of the four species at the beginning and end of the 10-h night.
| Species
|
Stage of Night
|
Measurement CO2 Concentration
|
|||
|---|---|---|---|---|---|
| 0 | 368 | 700 | 2000 | ||
| G. max cv Williams | 0−2.5 h | 1.02 ± 0.05 | 1.05 ± 0.04 | 1.02 ± 0.03 | 1.04 ± 0.03 |
| Vegetative | 7.5−10 h | 0.78 ± 0.04 | 0.79 ± 0.05 | 0.76 ± 0.05 | 0.77 ± 0.07 |
| G. max cv Pana | 0−2.5 h | 0.83 ± 0.13 | 0.81 ± 0.15 | 0.85 ± 0.17 | 0.85 ± 0.17 |
| Podfill | 7.5−10 h | 0.75 ± 0.08 | 0.73 ± 0.06 | 0.73 ± 0.05 | 0.77 ± 0.06 |
| P. taeda | 0−2.5 h | 0.89 ± 0.12 | 0.89 ± 0.11 | 0.91 ± 010 | 0.90 ± 0.11 |
| Seedlings | 7.5−10 h | 0.54 ± 0.10 | 0.55 ± 0.10 | 0.53 ± 0.10 | 0.55 ± 0.10 |
| Q. rubra | 0−2.5 h | 0.48 ± 0.04 | 0.47 ± 0.04 | 0.48 ± 0.04 | 0.49 ± 0.04 |
| 7.5−10 h | 0.37 ± 0.01 | 0.36 ± 0.01 | 0.36 ± 0.02 | 0.36 ± 0.02 | |
| Z. mays | 0−2.5 h | 1.58 ± 0.17 | 1.61 ± 0.16 | 1.58 ± 0.13 | 1.55 ± 0.12 |
| 7.5−10 h | 0.97 ± 0.09 | 0.96 ± 0.08 | 0.94 ± 0.09 | 0.94 ± 0.09 | |
Table III.
The response of leaf respiration to changes in (CO2) for species grown at long-term elevated (CO2) experiments
The mean respiration rate and its sem are given on an area basis and a dry weight basis. n indicates the number of plots or open-top chambers from which measurements of each treatment were made. Bold values show the critical long-term comparison of leaves grown and measured at their growth concentrations. An asterisk indicates that leaves grown and measured at elevated (CO2) have significantly different rates (P < 0.05) to those grown and measured at current ambient (CO2).
| a) Respiration on Leaf Area Basis (μmol O2 m−2 s−1)
| ||||||
|---|---|---|---|---|---|---|
|
|
n
|
Growth CO2 concentration (μmol mol−1)
|
Measurement CO2 concentration (μmol mol−1)
|
2000
|
||
| 0 | 368 | 550/700 | ||||
| Sugar maple | 3 | 368 | 0.54 ± 0.06 | 0.54 ± 0.06 | 0.54 ± 0.05 | 0.55 ± 0.06 |
| 560 | 0.55 ± 0.02 | 0.55 ± 0.03 | 0.54 ± 0.03 | 0.55 ± 0.04 | ||
| B. papyrifera | 3 | 368 | 0.86 ± 0.12 | 0.89 ± 0.11 | 0.82 ± 0.12 | 0.83 ± 0.10 |
| 560 | 0.85 ± 0.03 | 0.86 ± 0.04 | 0.82 ± 0.02 | 0.82 ± 0.01 | ||
| G. max cv pana | 4 | 368 | 1.27 ± 0.10 | 1.28 ± 0.10 | 1.29 ± 0.10 | 1.28 ± 0.09 |
| 550 | 1.54 ± 0.05 | 1.55 ± 0.06 | 1.56 ± 0.06* | 1.57 ± 0.05 | ||
| P. taeda | 3 | 368 | 0.76 ± 0.10 | 0.76 ± 0.10 | 0.76 ± 0.10 | 0.76 ± 0.09 |
| 550 | 0.78 ± 0.03 | 0.78 ± 0.03 | 0.78 ± 0.02 | 0.78 ± 0.02 | ||
| P. tremuloides | 3 | 368 | 1.37 ± 0.09 | 1.39 ± 0.08 | 1.39 ± 0.07 | 1.39 ± 0.07 |
| 560 | 1.36 ± 0.06 | 1.37 ± 0.06 | 1.37 ± 0.06 | 1.38 ± 0.06 | ||
| Q. geminata | 8 | 368 | 0.59 ± 0.04 | 0.60 ± 0.04 | 0.59 ± 0.04 | 0.60 ± 0.04 |
| 700 | 0.73 ± 0.03 | 0.75 ± 0.04 | 0.74 ± 0.05* | 0.75 ± 0.05 | ||
| Q. myrtifolia | 8 | 368 | 0.66 ± 0.06 | 0.65 ± 0.06 | 0.62 ± 0.05 | 0.63 ± 0.05 |
| 700 | 0.72 ± 0.08 | 0.72 ± 0.07 | 0.73 ± 0.08 | 0.72 ± 0.08 | ||
| b) Respiration on leaf dry mass basis (μmol O2 kg−1 s−1)
| ||||||
|---|---|---|---|---|---|---|
| Species
|
n
|
Growth CO2 concentration (μmol mol−1)
|
Measurement CO2 concentration (μmol mol−1)
|
|||
| 0 | 368 | 550/700 | 2000 | |||
| Sugar maple | 3 | 368 | 9.01 ± 1.12 | 8.92 ± 1.1 | 8.87 ± 1.05 | 9.14 ± 1.1 |
| 560 | 8.27 ± 1.3 | 8.31 ± 0.21 | 8.30 ± 0.22 | 8.29 ± 0.34 | ||
| B. papyrifera | 3 | 368 | 11.5 ± 1.4 | 12.0 ± 1.2 | 11.1 ± 1.5 | 11.2 ± 1.3 |
| 560 | 11.5 ± 0.1 | 11.6 ± 0.2 | 11.2 ± 0.1 | 11.2 ± 0.3 | ||
| G. max cv pana | 4 | 368 | 20.5 ± 1.7 | 20.6 ± 1.7 | 20.7 ± 1.7 | 20.7 ± 1.6 |
| 560 | 25.0 ± 0.9 | 25.1 ± 0.9 | 25.3 ± 0.9* | 25.5 ± 0.8 | ||
| P. taeda | 3 | 368 | 5.07 ± 0.24 | 5.08 ± 0.19 | 5.08 ± 0.15 | 5.09 ± 0.11 |
| 550 | 5.56 ± 0.05 | 5.56 ± 0.06 | 5.59 ± 0.08* | 5.55 ± 0.10 | ||
| P. tremuloides | 3 | 368 | 15.1 ± 2.0 | 15.1 ± 2.1 | 15.2 ± 2.1 | 15.2 ± 2.2 |
| 560 | 15.4 ± 0.7 | 15.6 ± 0.7 | 15.7 ± 0.6 | 15.7 ± 0.6 | ||
| Q. geminata | 8 | 368 | 3.07 ± 0.23 | 3.14 ± 0.22 | 3.12 ± 0.22 | 3.13 ± 0.24 |
| 700 | 3.28 ± 0.21 | 3.34 ± 0.21 | 3.28 ± 0.20 | 3.34 ± 0.24 | ||
| Q. myrtifolia | 8 | 368 | 4.57 ± 0.47 | 4.54 ± 0.43 | 4.31 ± 0.38 | 4.41 ± 0.40 |
| 700 | 4.75 ± 0.63 | 4.72 ± 0.60 | 4.79 ± 0.64 | 4.76 ± 0.68 | ||
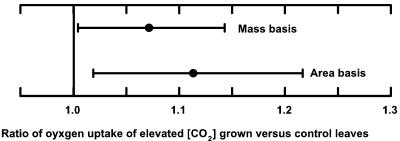
Despite the lack of an instantaneous effect, long-term growth at elevated [CO2] did affect respiration. Leaves grown and measured at elevated [CO2] had the same or a significantly higher Rd,O2 than those grown and measured at current ambient [CO2] (Table III). The largest increases in respiration were for Quercus geminata (23%) and soybean (22%) on a leaf area basis. By contrast, there was no increase in Acer saccharum, Betula papyrifera, and Populus tremuloides (Table IIIa). When all values from the long-term experiments were combined, there was a significant increase in Rd,O2 of 11% and 7% on a leaf area and mass basis, respectively, and relative to controls (Fig. 2).
Figure 2.
The effect of long-term (lifetime or >3 years) growth at elevated [CO2] in the field on leaf respiration. The data points show mean leaf respiratory O2 uptake (±90% confidence intervals) on an area and mass basis for leaves grown at elevated [CO2] relative to controls grown at current ambient [CO2]. The points are means for seven species (Table I) weighted for replication and variance using meta-analytical statistical methods.
DISCUSSION
Six hundred separate measurements of different leaves, encompassing four long-term field CO2 enrichment experiments revealed no evidence of any decrease in Rd,O2 in response to instantaneous elevation of [CO2]. This was consistent for seven tree and two crop species, the majority sampled from long-term elevated [CO2] experiments where plants have developed throughout their life or for several seasons under elevated [CO2]. This lack of response was irrespective of developmental stage, growth conditions, and growth [CO2]. Bunce (2001) reported that the sensitivity of Rd,CO2 to an instantaneous elevation of [CO2] increased with duration of the dark period. However, no effect on Rd,O2 was evident here (Tables II and III), regardless of whether measurements were made at the start or end of the natural dark period. Jahnke (2001) similarly found no effect of [CO2] on Rd,CO2 even after 72 h of darkness. Tjoelker et al. (2001) noted that the instantaneous decrease in Rd,CO2 with elevated [CO2], although minor (1.8%) in response to increase from 360 to 700 μmol mol-1, was far more substantial (11%) on increase from 360 to 1,300 μmol mol-1. However, a 5-fold increase above current ambient [CO2] or removal of CO2 from the atmosphere around the leaf did not alter Rd,O2 (Fig. 1). These findings support the conclusions of Amthor et al. (2001) and of Jahnke (2001) and extend them to more species and to major long-term [CO2] field manipulation studies. Although the results show no effect of instantaneous elevation of [CO2] on Rd,O2, this cannot rule out some effect on apparent Rd,CO2 due to non-respiratory processes, for example, CO2 uptake into carboxylic acids. However, in one of the very few studies to measure CO2 and O2 exchange at different [CO2], Amthor et al. (2001) found no effect of elevated [CO2] on respiratory quotient.
It has been suggested that the variability of response to instantaneous increase in [CO2] apparent in the literature may be the result of species differences (Drake et al., 1999; Baker et al., 2000; Hamilton et al., 2001). Without definitive testing, it is impossible to be certain on this point. However, this study contains a diverse range of species and functional types (including C3 and C4 photosynthetic types, herbaceous and woody forms, two major crops, and seven forest dominant gymnosperm and angiosperm species) that individual exceptions exist now seem somewhat improbable. Moreover, we deliberately selected species previously reported to show some of the largest inhibitions of respiration by elevated CO2 with previous methods and technology: soybean 40% (Bunce, 1995), Pinus taeda 14% (Teskey, 1995), Quercus rubra 5.6% (Amthor, 2000), and maize (Zea mays) 46% (Cornic and Jarvis, 1972).
Any errors associated with measuring the instantaneous response of respiratory CO2 efflux to elevated [CO2] may be equally applicable to measurement of the long-term effects of growth at elevated [CO2] on Rd,CO2. The significant 7% increase in Rd,O2 on a mass basis, averaged across seven species from four long-term experiments (Fig. 2), contrasts sharply with the average 18% decrease reported by Wang and Curtis (2002) in their meta-analysis of Rd,CO2 on a mass basis measured in long-term experiments. The significant increase on a mass, as well as an area basis, suggests that increased respiration per unit leaf area is not just the result of the increased mass per unit area that is commonly observed in leaves grown at elevated [CO2] (Drake et al., 1999). However, as noted above, this mean increase masks marked differences between species in this long-term response (Table III).
To our knowledge, this is the first study to use this alternative method to investigate the effects of long-term growth at elevated [CO2] on respiration. The increase in respiration shown here is consistent with the widely reported increase in leaf-soluble carbohydrates with long-term growth at elevated [CO2] (Moore et al., 1999). Azcon-Bieto and Osmond (1983) showed that increase in leaf nonstructural carbohydrate content produced by manipulation of photosynthesis during the photoperiod increased subsequent dark respiration rates. Therefore, a similar response might be expected when elevated [CO2] increases photosynthesis and leaf nonstructural carbohydrate content during the photoperiod.
In conclusion, instantaneous elevation of [CO2] had no effect on leaf O2 uptake in these nine species. This further confirms the suggestion that reports of respiratory inhibition, based on measurements of CO2 uptake, are the result of experimental artifacts and not the result of any sensitivity of plant respiration to the [CO2] at the time of measurement. A 15% to 20% reduction of terrestrial plant respiration with a doubling of atmospheric [CO2] concentration would represent some amelioration, at least temporarily, in the rate of rise in the global atmospheric [CO2] (for review, see Gonzalez-Meler and Siedow, 1999). It can no longer be assumed that the direct effect of elevated [CO2] on plant respiration will reduce future ecosystem CO2 efflux. On the basis of the long-term field manipulations of [CO2] studied here, a small increase in respiration per unit leaf area should be expected—this will amplify the effect of any increase in leaf area due to growth at elevated [CO2].
MATERIALS AND METHODS
Growth Conditions
The species, developmental stage, growth conditions, and experimental site are listed in Table I. Seven out of nine species were grown at current ambient (368 μmol mol-1) and at an elevated [CO2] (550 or 700 μmol mol-1). Two of the species were measured at more than one developmental stage; soybean (Glycine max) at vegetative and podfill stages, and Pinus taeda at juvenile and mature stages. Soybean was grown in controlled environment- and open-field conditions (FACE).
Oxygen Analysis System
Leaf O2 uptake rate was determined in an open gas exchange system incorporating a dual-cell oxygen analyzer (S-3A/DOX; AEI Technologies, Pittsburgh) described by Willms et al. (1997), and incorporating a stainless steel and glass leaf gas exchange cuvette (MPH-1000; Campbell Scientific, Logan, UT). The analyzer incorporates a novel differential oxygen sensor obtained by modifying two electrochemical fuel cells (model KE-25; Figaro USA, Wilmette, IL), placing one in a reference gas stream and the other in an analytical gas stream, and then ensuring that the two gas sensors were exposed to an identical temperature and pressure (Willms et al., 1997). The O2 fuel cells contain a Teflon membrane through which O2 can diffuse, and a weak acid electrolyte bathes a lead anode and a gold cathode electrode. In the environment of the sensor, the O2 oxidizes the lead, forming PbO, which dissolves into the solution and generates a small current. The difference in output of the two sensors is directly proportional to the δ[O2] between the air streams. The selective diffusive properties of Teflon and the specific electrochemistry ensure that the differential sensor responds directly only to O2 in air. However, changes in partial pressures of CO2 and water vapor due to respiration and transpiration will affect that of O2 causing an indirect effect (Willms et al., 1999). For this reason, these gases are removed from the air stream before the O2 sensors (Hunt, 2003). The analyzer was secured to a vibrationless table top and additional layers of insulation were wound around the O2 measurement cells and instrument casing to further improve resolution. The differential resolution of the modified dual-cell O2 analyzer was 1 to 2 μmol mol-1 against a background [O2] of 210 mmol mol-1. The cuvette was large enough to enclose the entire leaf, leaflet, or cohort of needles; typically, 50 to 120 cm2 of leaf were enclosed. This avoided errors associated with gas flow between the cuvette and surrounding air via the intercellular air space that Jahnke and Krewitt (2002) have shown to result from the use of cuvettes that only enclose part of the leaf. The cuvette (MPH-1000; Campbell Scientific) has been described in detail previously (Bingham et al., 1980), adapted from the earlier design of Bingham and Coyne (1977). Typical boundary layer conductances to water vapor are 2.5 to 4 mol m-2 s-1 (calculated from Bingham and Coyne, 1977; Bingham et al., 1980). A gas exchange system (LI-6400; LI-COR, Lincoln, NE) was used to provide a controlled [CO2] to the cuvette, and air flow rate was measured by a pair of flow meters (F350; AEI Technologies), calibrated as described previously (Long et al., 1996). The gas flow across the leaf was maintained at 2.5 cm3 s-1. This flow rate and the enclosed leaf area resulted in a δ[O2] of 40 to 80 μmol mol-1. The entry air streams to the oxygen analyzer were scrubbed of water and CO2 by magnesium perchlorate and soda lime. Column sizes were calculated to remove all CO2 and to be sufficient to dry the air stream to the equilibrium vapor pressure of magnesium perchlorate (approximately 0.1 Pa). The efficacy of these columns was tested by switching dry air (<0.01 kPa water vapor) with a humidified air stream (2.0 kPa water vapor) at the cuvette inlet; no change in δ[O2] from 0 μmol mol-1 was recorded. Similar tests showed that the soda lime column was effective in preventing interference from differences in [CO2] between the cuvette and reference air streams.
The response time of the gas exchange system was assessed using two cylinders of compressed air found to differ in [O2] by 80 μmol mol-1. Air from one of these cylinders was fed to the empty cuvette inlet and also the reference air stream until a constant δ[O2] of 0 μmol mol-1 was obtained at the analyzer. Air from the second cylinder was then switched in at the cuvette inlet and the time taken to obtain a constant δ[O2] of 80 μmol mol-1 at the analyzer was recorded. The change was 98% complete within 10 min and complete within the resolution of the analyzer at 15 min. Therefore, it was assumed that 20 min was adequate to record any response of respiratory O2 efflux to an instantaneous change in [CO2].
Respiration Measurements
Respiration was measured in the last 2.5 h of the night in all species and additional measurements were made in the first 2.5 h of the night for three species (Table I). Measurements were made at each location listed in Table I. However, because of its size and stability, the gas exchange system was housed in a field laboratory at each site and leaves were detached shortly before measurement. Petioles were cut under water and their cut ends were kept immersed in water until measurements were complete. The leaves remained turgid throughout. For pot-grown soybean (Table I), parallel measurements on attached and detached leaves were made; no significant differences in respiration or its response to [CO2] were found. Leaves were equilibrated in the dark to 25°C before measurement. For a single replicate measurement, respiration was measured at a [CO2] of 2,000 μmol mol-1, and then again at 700, 550, 360, and 0 μmol mol-1 CO2. To avoid systematic error, each alternate replicate measurement was started at 0 μmol mol-1 CO2 and was then switched to 368, 700, 550, and 2,000 μmol mol-1 CO2. Species from the FACE experiments at Duke Forest, Rhinelander, and the University of Illinois, and the open-top chamber experiment at Merritt Island were measured at 550 μmol mol-1 [CO2] rather than at 700 μmol mol-1 [CO2] because this was the growth concentration (Table I). After each step-wise change in [CO2], a 20-min waiting period ensured adequate time for any instantaneous response in Rd,O2. Each set of measurements took 80 min to complete, and leaves were maintained in the dark at a leaf temperature of 25°C for the entire measurement period. After each set of measurements, leaf area was measured by digital imaging and the leaves were then dried to a constant weight at 80°C. Leaf O2 evolution rates were calculated on a leaf area and a dry mass basis by adapting the CO2 uptake equations of von Caemmerer and Farquhar (1981) for open system gas exchange measurements.
Statistical Analysis
In the four long-term field experiments, replicate number was determined by the number of treatment plots (Table III). This avoided pseudoreplication. A minimum of three repeat measurements were taken in each treatment plot of each experiment, and their pooled values provided the individual sample measure. Only these data were used to assess the effect of long-term growth at elevated [CO2] on respiratory O2 uptake. Outside of these long-term elevated [CO2] studies, measurements were made from six randomly selected plants each of P. taeda seedlings, Q. rubra mature trees, and maize flowering plants growing under current ambient [CO2] in the open. Soybean was grown in two separate controlled environment studies, and here, replicates represent the number of separate chambers used (Table I).
ANOVA was used to test the effect of immediate changes of [CO2] on leaf respiration rate for all nine species combined (SYSTAT, Evanston, IL). This tested the effect of species, growth conditions, developmental stage, time of measurement, and growth [CO2] on the instantaneous response to elevated [CO2]. In addition, a regression analysis of response relative to ambient [CO2] was tested (SYSTAT).
Because of the very different experimental designs, durations, and treatment procedures of the experiments in which plants were grown at elevated [CO2], and the possibility that species may respond differently to long-term growth in elevated [CO2], the pooled effect of all field studies was assessed by meta-analytical statistical techniques (Hedges et al., 1999; Ainsworth et al., 2002; Morgan et al., 2003) using a software package (MetaWin, Rosenberg et al., 2000). This allows determination of the consensus of studies differing in design. The natural log of the response ratio (respiratory O2 uptake for leaves grown at elevated [CO2]/respiratory O2 uptake for leaves grown at the current ambient [CO2]) was used as the metric for analysis (Hedges et al., 1999). A mixed-model analysis was used based on the assumption of random variation in effect sizes between individual measurements. A weighted parametric analysis was used (Gurevitch and Hedges, 1999), and each individual observation was weighted by the reciprocal of the mixed-model variance (Hedges et al., 1999). If the response ratio was not significantly different from unity, growth at elevated [CO2] failed to change leaf O2 uptake from that at current ambient [CO2]. However, if significantly different from 1, growth at elevated [CO2] on average altered O2 uptake across the studies.
Acknowledgments
We thank Henry Ginsberg and Joseph Veltre of AEI Technologies, and Nicholas Dowling of Qubit Systems for technical help and modifications to the O2 analysis system; Jeff Amthor, Miquel Gonzalez-Meler, Andrew Leakey, and Shawna Naidu for comments on draft versions of the manuscript; Carlos Pimentel and Kate George for the supply of plant material; and Lisa Ainsworth for advice on the meta-analysis.
This work was supported by the U.S. Department of Energy's Office of Science (to B.E.R.), as were the long-term elevated [CO2] experiments in Florida, North Carolina, and Wisconsin.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.030569.
References
- Ainsworth EA, Davey PA, Bernacchi CJ, Dermody OC, Heaton EA, Moore DJ, Morgan PB, Naidu SL, Yoo Ra HS, Zhu XG et al. (2002) A meta-analysis of elevated [CO2] effects on soybean (Glycine max) physiology, growth and yield. Glob Change Biol 8: 695-709 [Google Scholar]
- Ainsworth EA, Tranel PJ, Drake BG, Long SP (2003) The clonal structure of Quercus geminata revealed by conserved microsatellite loci. Mol Ecol 12: 527-532 [DOI] [PubMed] [Google Scholar]
- Amthor JS (1997) Plant respiratory responses to elevated CO2 partial pressure. In LH Allen, MB Kirkham, DM Olszyk, CE Whitman, eds, Advances in Carbon Dioxide Effects Research. American Society of Agronomy Special Publication, Madison, WI, pp 35-77
- Amthor JS (2000) Direct effect of elevated CO2 on nocturnal in situ leaf respiration in nine temperate deciduous tree species is small. Tree Physiol 20: 139-144 [DOI] [PubMed] [Google Scholar]
- Amthor JS, Koch GW, Bloom AJ (1992) CO2 inhibits respiration in leaves of Rumex crispus L. Plant Physiol 98: 757-760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amthor JS, Koch GW, Willms JR, Layzell DB (2001) Leaf O2 uptake in the dark is independent of coincident CO2 partial pressure. J Exp Bot 52: 2235-2238 [DOI] [PubMed] [Google Scholar]
- Azcon-Bieto L, Osmond CB (1983) Relationship between photosynthesis and respiration: the effect of carbohydrate status on the rate of CO2 production by respiration in darkened and illuminated wheat leaves. Plant Physiol 71: 574-581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JT, Allen LH, Boote KJ, Pickering NB (2000) Direct effects of atmospheric carbon dioxide concentration on whole canopy dark respiration of rice. Glob Change Biol 6: 275-286 [Google Scholar]
- Bingham GE, Coyne PI (1977) A portable, temperature-controlled, steady-state porometer for field measurements of transpiration and photosynthesis. Photosynthetica 11: 148-160 [Google Scholar]
- Bingham GE, Coyne PI, Kennedy RB, Jackson WL (1980) Design and fabrication of a portable minicuvette system for measuring leaf photosynthesis and stomatal conductance under controlled conditions. UCRL-52895. Lawrence Livermore National Laboratory, Livermore, CA
- Bloom AJ, Mooney HA, Bjorkman O, Berry JA (1980) Materials and methods for carbon dioxide and water exchange analysis. Plant Cell Environ 3: 371-376 [Google Scholar]
- Bouma TJ, Nielsen KL, Eissenstat DM, Lynch JP (1997) Soil CO2 concentration does not affect growth or root respiration in bean or citrus. Plant Cell Environ 20: 1495-1505 [Google Scholar]
- Bruhn D, Mikkelsen TN, Atkin OK (2002) Does the direct effect of atmospheric CO2 concentration on leaf respiration vary with temperature? Responses in two species of Plantago that differ in relative growth rate. Physiol Plant 114: 57-64 [PubMed] [Google Scholar]
- Bunce JA (1995) The effect of carbon dioxide concentration on respiration of growing and mature soybean leaves. Plant Cell Environ 18: 575-581 [Google Scholar]
- Bunce JA (2001) Effects of prolonged darkness on the sensitivity of leaf respiration to carbon dioxide concentration in C3 and C4 species. Ann Bot 87: 463-468 [Google Scholar]
- Bunce JA (2002) Carbon dioxide concentration at night affects translocation from soybean leaves. Ann Bot 90: 399-403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton AJ, Pregitzer KS (2002) Measurement carbon dioxide concentration does not affect root respiration of nine tree species in the field. Tree Physiol 22: 67-72 [DOI] [PubMed] [Google Scholar]
- Cornic G, Jarvis PG (1972) Effects of oxygen on CO2 exchange and stomatal resistance in Sitka spruce and maize at low irradiances. Photosynthetica 6: 225-239 [Google Scholar]
- Curtis PS, Wang X (1998) A meta-analysis of elevated CO2 effects on woody plant mass, form and physiology. Oecologia 113: 299-313 [DOI] [PubMed] [Google Scholar]
- DeLucia EH, Hamilton JG, Naidu SL, Thomas RB, Andrews JA, Finzi A, Lavine M, Matamala R, Mohan JE, Hendrey GR et al. (1999) Net primary production of a forest ecosystem with experimental CO2 enrichment. Science 284: 1177-1179 [DOI] [PubMed] [Google Scholar]
- Dickson RE, Lewin KF, Isebrands JG, Coleman MD, Heilman WE, Riemenschneider DE, Sober J, Host GE, Hendrey GR, Pregitzer KS et al. (2001) Forest atmosphere carbon transfer storage-II (FACTS II): the aspen free-air CO2 and O3 enrichment (FACE) project in an overview. General Tech. Rep. NC-214. U.S. Department of Agriculture Forest Service North Central Experiment Station, Rhinelander, WI.
- Drake BG, Azcon-Bieto J, Berry J, Bunce J, Dijkstra J, Farrar J, Gifford RM, Gonzàlez-Meler MA, Koch G, Lambers H et al. (1999) Does elevated atmospheric CO2 concentration inhibit mitochondrial respiration in green plants? Plant Cell Environ 22: 649-657 [Google Scholar]
- Drake BG, Gonzalez-Meler M, Long SP (1997) More efficient plants: a consequence of rising atmospheric CO2. Annu Rev Plant Physiol Plant Mol Biol 48: 607-637 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Meler MA, Siedow JN (1999) Direct inhibition of mitochondrial respiratory enzymes by elevated CO2: Does it matter at the tissue or whole-plant level? Tree Physiol 19: 253-259 [DOI] [PubMed] [Google Scholar]
- Griffin KL, Anderson OR, Gastrich MD, Lewis JD, Lin GH, Schuster W, Seemann JR, Tissue DT, Turnbull MH, Whitehead D (2001) Plant growth in elevated CO2 alters mitochondrial number and chloroplast fine structure. Proc Natl Acad Sci USA 98: 2473-2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevitch J, Hedges LV (1999) Statistical issues in ecological meta-analyses (meta-analysis in ecology). Ecology 80: 1142-1150 [Google Scholar]
- Hamilton JG, Thomas RB, Delucia EH (2001) Direct and indirect effects of elevated CO2 on leaf respiration in a forest ecosystem. Plant Cell Environ 24: 975-982 [Google Scholar]
- Hedges LV, Gurevitch J, Curtis PS (1999) The meta-analysis of response ratios in experimental ecology. Ecology 80: 1150-1156 [Google Scholar]
- Holtum JAM, Winter K (2003) Photosynthetic CO2 uptake in seedlings of two tropical tree species exposed to oscillating elevated concentrations of CO2. Planta 218: 152-158 [DOI] [PubMed] [Google Scholar]
- Hunt S (2003) Measurements of photosynthesis and respiration in plants. Physiol Plant 117: 314-325 [DOI] [PubMed] [Google Scholar]
- Hymus GJ, Snead TG, Johnson DP, Hungate BA, Drake BG (2002) Acclimation of photosynthesis and respiration to elevated atmospheric CO2 in two Scrub Oaks. Glob Change Biol 8: 317-328 [Google Scholar]
- Jach ME, Ceulemans R (2000) Short-versus long-term effects of elevated CO2 on night-time respiration of needles of Scots pine (Pinus sylvestris L.). Photosynthetica 38: 57-67 [Google Scholar]
- Jahnke S (2001) Atmospheric CO2 concentration does not directly affect leaf respiration in bean or poplar. Plant Cell Environ 24: 1139-1151 [Google Scholar]
- Jahnke S, Krewitt M (2002) Atmospheric CO2 concentration may directly affect leaf respiration measurement in tobacco, but not respiration itself. Plant Cell Environ 25: 641-651 [Google Scholar]
- Long SP, Farage PK, Garcia RL (1996) Measurement of leaf and canopy photosynthetic CO2 exchange in the field. J Exp Bot 47: 1629-1642 [Google Scholar]
- Moore BD, Cheng SH, Sims D, Seemann JR (1999) The biochemical and molecular basis for photosynthetic acclimation to elevated atmospheric CO2. Plant Cell Environ 22: 567-582 [Google Scholar]
- Morgan PB, Ainsworth EA, Long SP (2003) How does elevated ozone impact soybean? A meta-analysis of photosynthesis, growth and yield. Plant Cell Environ 26: 1317-1328 [Google Scholar]
- Reuveni J, Gale J, Mayer AM (1993) Reduction of respiration by high ambient CO2 and the resulting error in measurement of respiration made with O2 electrodes. Ann Bot 72: 129-131 [Google Scholar]
- Robertson EJ, Williams M, Harwood JL, Lindsay JG, Leaver CJ, Leech RM (1995) Mitochondria increase 3-fold and mitochondrial proteins and lipid change dramatically in postmeristematic cells in young wheat leaves grown in elevated CO2. Plant Physiol 108: 469-474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers A, Allen DJ, Davey PA, Morgan PB, Ainsworth EA, Bernacchi CJ, Cornic G, Dermody O, Heaton EA, Mahoney J et al. (2004) Leaf photosynthesis and carbohydrate dynamics of soybeans grown throughout their life-cycle under free-air carbon dioxide enrichment. Plant Cell Environ 27 (in press)
- Rosenburg MS, Adams DC, Gurevitch J (2000) MetaWin: Statistical Software for Meta-Analysis, Version 2.0. Sinauer Associates, Sunderland, MA
- Teskey RO (1995) A field study of the effects of elevated CO2 on carbon assimilation, stomatal conductance and leaf and branch growth of Pinus taeda trees. Plant Cell Environ 18: 565-573 [Google Scholar]
- Tissue DT, Lewis JD, Wullschleger SD, Amthor JS, Griffin KL, Anderson R (2002) Leaf respiration at different canopy positions in sweetgum (Liquidambar styraciflua) grown in ambient and elevated concentrations of carbon dioxide in the field. Tree Physiol 22: 1157-1166 [DOI] [PubMed] [Google Scholar]
- Tjoelker MG, Oleksyn J, Lee TD, Reich PB (2001) Direct inhibition of leaf dark respiration by elevated CO2 is minor in 12 grassland species. New Phytol 150: 419-424 [Google Scholar]
- von Caemmerer S, Farquhar GD (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153: 376-387 [DOI] [PubMed] [Google Scholar]
- Wang XZ, Curtis P (2002) A meta-analytical test of elevated CO2 effects on plant respiration. Plant Ecol 161: 251-261 [Google Scholar]
- Willms JR, Dowling AN, Dong ZM, Hunt S, Shelp BJ, Layzell DB (1997) The simultaneous measurement of low rates of CO2 and O2 exchange in biological systems. Anal Biochem 254: 272-282 [DOI] [PubMed] [Google Scholar]
- Willms JR, Salon C, Layzell DB (1999) Evidence for light-stimulated fatty acid synthesis in soybean fruit. Plant Physiol 120: 1117-1127 [DOI] [PMC free article] [PubMed] [Google Scholar]