Abstract
A series of chalcone-based diarylpyrazoles containing a phenylsulphone or carbonitrile moiety was synthesized. Thus, 3-acetylpyrazoles 6a–c and 10a–c were used as useful substrates in facile synthesis of functional pyrazoles 7a–f and 11a–f, respectively. The anti-inflammatory activity and ulcerogenic effect were evaluated and some of the obtained products possessed a significant anti-inflammatory activity. 1-[1-(3-Methylphenyl)-5-phenyl-4-(phenylsulfonyl)-1H-pyrazol-3-yl]ethanone (6b) showed a high activity when compared with indomethacin as reference drug with lower gastrointestinal (GI) profile. Furthermore, molecular docking studies were performed in order to rationalize the obtained biological results.
Keywords: Diarylpyrazoles, Sulphones, Chalcones, Anti-inflammatory activity, Molecular docking
Introduction
Management of inflammatory disorders involves a stepwise approach to the use of therapeutic agents. Relieving of pain and reduction of inflammation are urgent goals to reduce the severity of symptoms. A generally accepted stepwise approach to treat the inflammation disorders includes physical therapy, non-steroidal anti-inflammatory drugs (NSAIDs), disease modifying anti-rheumatic drugs (DMARDs), corticosteroids and finally, immunosuppressive agents [1, 2]. However, NSAIDs remain among the most widely prescribed drugs worldwide; they have been generally considered as inhibitors of cyclooxygenases (COXs) [3, 4]. Most of NSAIDs act by reducing prostaglandin biosynthesis through the inhibition of the COX reaction [5]. Moreover, data have been accumulating through the years suggesting that NSAIDs also probably act on other targets to counteract pain. In spite of their beneficial action, their activity is associated with deleterious side effects, and continuous administration of these drugs leads to nephrotoxicity and gastric ulcerations. The therapeutic anti-inflammatory action of NSAIDs is produced by inhibition of COX-2, while the unwanted side effects arise from the inhibition of COX-1 activity. It is estimated that 25% of patients using NSAIDs experience some kind of side effect and about 5% develop serious health consequence (massive GI bleed, acute renal failure, etc.). Consequently, extensive research has been directed towards improving their pharmacological profile [6].
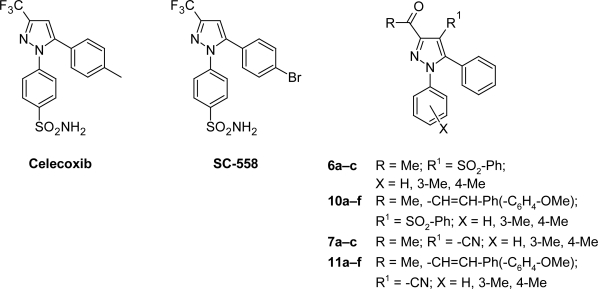
Recently, a novel class of selective COX-2 inhibitors has been discovered. Among this class, celecoxib (Figure 1), the potent and gastrointestinal (GI) safe anti-inflammatory agent. It is considered a typical model of sulphonamide-based diarylpyrazole template that is known to selectively inhibit COX-2 [7–15]. Furthermore, several arylpyrazoles are available as anti-inflammatory drugs in market such as valdecoxib [16], rofecoxib [17], etoricoxib [18, 19]. SC-558, diarylpyrazole derivative (Figure 1), was found to be a marvelous inhibitor with 1900-fold selectivity for COX-2 over COX-1. In addition, both SC-558 and celecoxib were published, co-crystallized with the active site of COX-2, with many investigations about their mechanism of action and selectivity to COX-2 [20–22].
Fig. 1.
Structure of Celecoxib, SC-558, 6a–c, 10a–f, 7a–c and 11a–f.
In view of the above facts and in continuation of our interest in the synthesis of bioactive heterocycles, especially pyrazole derivatives [23–32], we report here some di/triaryl-pyrazoles having phenylsulphone or carbonitrile moiety (Figure 1). The anti-inflammatory activity of the synthesized compounds was evaluated. Moreover, molecular docking studies of active compounds were carried out to rationalize their activity.
Results and Discussion
Chemistry
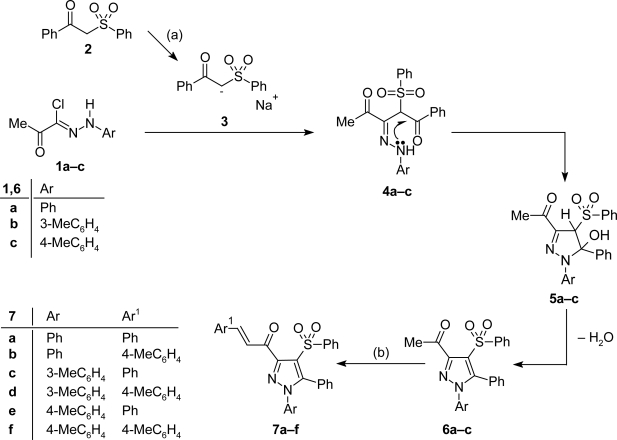
Cyclization reaction of hydrazonoyl chlorides 1a–c with 1-phenyl-2-(phenylsulfonyl)-ethanone (2) in ethanolic sodium ethoxide solution at room temperature furnished 1-(5-phenyl-4-(phenylsulfonyl)-1-aryl-1H-pyrazol-3-yl)ethanones (6a–c), respectively (Scheme 1). The latter pyrazoles were reacted with benzaldehyde or 4-anisaldehyde in ethanol containing 10% aqueous sodium hydroxide at room temperature to afford chalcones 7a–f. The IR spectra of the latter chalcones showed, in each case, the appearance of absorption band in the region 1640–1676 cm−1 corresponding to the carbonyl function. Their 1H NMR spectra showed the disappearance of COCH3 protons signal and displayed the signals of olefinic protons in the aromatic region.
Sch. 1.
Reagents and conditions: (a) EtONa/EtOH, stirring 12 h r.t.; (b) Ar1-CHO, EtOH, 10% NaOH, stirring 12 h, r.t.
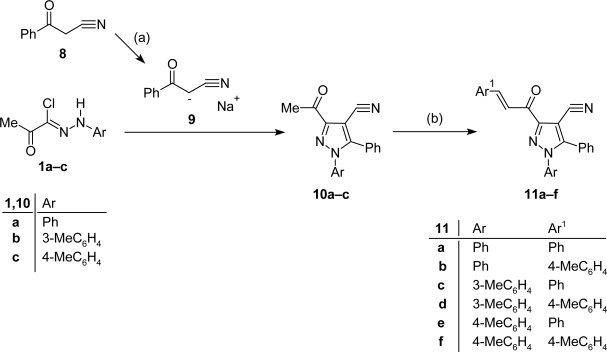
Similarly, the reaction of 3-acetyl-(1-aryl)-5-phenyl-1H-pyrazole-4-carbonitriles (10a–c) with benzaldehyde or 4-anisaldehyde, under the same reaction conditions for synthesis of 7a–f, afforded the corresponding chalcones 11a–f, respectively (Scheme 2). The structure of the latter chalcones was confirmed under the basis of their spectral data. For example, their IR spectra revealed the appearance of absorption band in the region 1662–1668 cm−1 due to the carbonyl group in addition to the appearance of sharp absorption peak of carbonitrile function around 2235 cm−1 and their mass spectra showed, in each case, a peak corresponding to their molecular ion.
Sch. 2.
Reagents and conditions: (a) EtONa/EtOH, stirring 12 h, r.t.; (b) Ar1-CHO, EtOH, 10% NaOH, stirring 12 h, r.t.
Pharmacology
Anti-inflammatory activity
The anti-inflammatory effect of tested compounds, meloxicam and indomethacin, on carrageenan-induced edema at 3 and 6h, is depicted in Table 1. Percent edema inhibition (Table 1) was calculated in regard to control group and the potency (%) was calculated respect to the indomethacin response. After 3h, the tested compounds 6a–c, 7b, 7d, 10a and 11b showed a reasonable decrease in the edema size ranging between 30.12% for compound 6c and 105.51% for the most active compound 6b. On the other hand, compounds 11c–f showed a weak activity in reduction of edema size after the same time (< 27.34 % for 11e) as shown in Table 1. After 6h, 6b was still the most potent anti-inflammatory compound in minimizing the inflammation size with 99.82% activity. However, compounds 7a, 7c, 7e, 7f, 10b, 10c and 11a showed no inhibitory effect (Table 1).
Tab. 1.
Inhibitory effect of indomethacin, meloxicam and the tested compounds on carrageenan-induced edema of the hind paw in rats
| Group | Before injection Edema vol. | 3 h | 6 h | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Edema vol. | % Edema inhibition | Pot. (%) | Edema vol. | % Edema inhibition | Pot. (%) | ||
| Control | 0.289 ± 0.018 | 0.475 ± 0.012 | – | 0 | 0.475 ± 0.012 | – | 0 |
| 6a | 0.243 ± 0.014 | 0.370 ± 0.017b | 31.39 | 68.53 | 0.378 ± 0.017 | 27.53 | 56.39 |
| 6b | 0.244 ± 0.017 | 0.340 ± 0.018c | 48.33 | 105.51 | 0.341 ± 0.021b | 48.74 | 99.82 |
| 6c | 0.256 ± 0.011 | 0.393 ± 0.018 | 13.80 | 30.12 | 0.356 ± 0.026a | 21.23 | 43.48 |
| 7a | 0.248 ± 0.010 | 0.437 ± 0.015 | 0.00 | 0.00 | 0.447 ± 0.014 | 0.00 | 0.00 |
| 7b | 0.249 ± 0.009 | 0.363 ± 0.020a | 38.38 | 83.77 | 0.366 ± 0.021a | 36.54 | 74.83 |
| 7c | 0.290 ± 0.015 | 0.481 ± 0.011 | 0.00 | 0.00 | 0.491 ± 0.008 | 0.00 | 0.00 |
| 7d | 0.257 ± 0.009 | 0.384 ± 0.011a | 32.05 | 69.96 | 0.368 ± 0.011a | 40.59 | 83.12 |
| 7e | 0.259 ± 0.012 | 0.448 ± 0.006 | 0.00 | 0.00 | 0.457 ± 0.021 | 0.00 | 0.00 |
| 7f | 0.260 ± 0.010 | 0.452 ± 0.007 | 0.00 | 0.00 | 0.458 ± 0.014 | 0.00 | 0.00 |
| 10a | 0.256 ± 0.013 | 0.379 ± 0.021a | 33.31 | 72.70 | 0.365 ± 0.017a | 41.00 | 83.97 |
| 10b | 0.241 ± 0.018 | 0.439 ± 0.005 | 0.00 | 0.00 | 0.443 ± 0.009 | 0.00 | 0.00 |
| 10c | 0.251 ± 0.010 | 0.447 ± 0.009 | 0.00 | 0.00 | 0.438 ± 0.004 | 0.00 | 0.00 |
| 11a | 0.248 ± 0.009 | 0.439 ± 0.011 | 0.00 | 0.00 | 0.446 ± 0.006 | 0.00 | 0.00 |
| 11b | 0.239 ± 0.009 | 0.391 ± 0.013 | 17.88 | 39.04 | 0.386 ± 0.045 | 20.50 | 41.99 |
| 11c | 0.252 ± 0.010 | 0.422 ± 0.019 | 8.08 | 17.64 | 0.414 ± 0.019 | 12.59 | 25.78 |
| 11d | 0.261 ± 0.009 | 0.451 ± 0.018 | 0.00 | 0.00 | 0.438 ± 0.020 | 4.94 | 10.11 |
| 11e | 0.253 ± 0.016 | 0.419 ± 0.016 | 10.85 | 23.69 | 0.410 ± 0.014 | 15.54 | 31.83 |
| 11f | 0.262 ± 0.011 | 0.424 ± 0.007 | 12.52 | 27.34 | 0.419 ± 0.011 | 15.36 | 31.46 |
| Meloxicam | 0.279 ± 0.009 | 0.385 ± 0.017a | 42.65 | 93.10 | 0.375 ± 0.018 | 48.25 | 98.82 |
| Indomethacin | 0.263 ± 0.016 | 0.364 ± 0.011b | 45.81 | 100 | 0.358 ± 0.017a | 48.83 | 100 |
Values represent means ± SEM of six animals for each group. The potency (pot.) was calculated comparedto the reference drug indomethacin. Statistical analysis using One-way ANOVA (Bonferroni’s multiple comparison test). Significance levels
P < 0.05;
P< 0.01and
P < 0.001as compared with control.
From the structure activity relationship (SAR) viewpoint, the anti-inflammatory activity of phenylsulfonylpyrazoles such as 6a, 6c, 7b and 7d were found to be higher than that of carbonitrile pyrazoles except 10a which achieved a greater activity. Additionally, the introducing of chalcone moiety to pyrazole ring was found to be variable in effectiveness of activity. However, these results imply that the phenyl sulphone moiety and carbonyl group attached to the pyrazole system is an essential pharmacophore responsible for activity. Furthermore, changing the position of 4-methyl substituent of N-phenyl ring in 6c to position 3 in 6b increased the activity. On the other hand, the phenylsulphonylpyrazoles that contains 4-methoxybenzylidine group, 7b and 7d, showed a higher activity while that with benzylidine group, 7a and 7c, have no activity. Taken together, the results of 6a–c and 7a–f provide evidence that the structural features of arylidine moieties and changes in the N-aryl group significantly affects their anti-inflammatory activity.
Ulcerogenic effects
The most active compounds 6b, 7b, 7d and 10a were selected for the ulcerogenic study as compared to the reference drugs meloxicam and indomethacin [33]. Compound 6b showed lower ulceration (1.5) respect to meloxicam (1.67) or to indomethacin (3.17) while compounds 7b, and 7d recorded ulcer index higher than meloxicam but still lower than indomethacin; compound 10a showed the highest ulcer index (2.83) (Table 2).
Tab. 2.
Ulcer index of indomethacin, meloxicam, 6b, 7b, 7d and 10a.
| Compound | Ulcer indexa |
|---|---|
| Control | 0.25 ± 0.274 |
| 6b | 1.5±1.000 |
| 7b | 2 ± 0.894b |
| 7d | 1.83 ± 0.983 |
| 10a | 2.83 ± 0.753c |
| Meloxicam | 1.67 ± 0.817 |
| Indomethacin | 3.17 ± 0.753c |
Values represent means ± SD (n = 6);
Significance level b, P < 0.05;
c, P < 0.01 as compared with the respective control.
Molecular docking
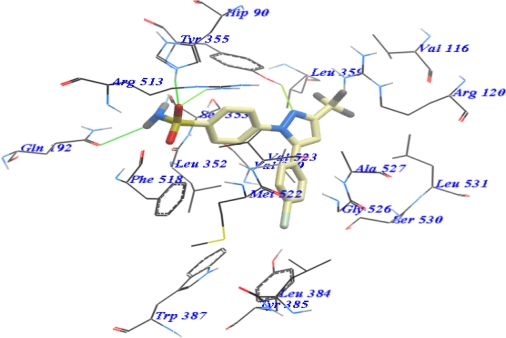
The docking runs were conducted using Molsoft ICM-pro software to rationalize the obtained biological results. Besides, the molecular docking studies helped in understanding the various interactions between the ligand and enzyme active sites. An automated docking study was carried out using the crystal structure of COX-2 (pdb ID: 1CX2) in which SC-558 co-crystallized as a ligand [20]. Both enzymes were submitted to regularization process to fit the protein model with the ideal covalent geometry of residues to the atom positions of a target PDB structure [34]. The regularized protein was used in determination of the important amino acids in ligand binding pocket (LBP) of COX-2 enzyme.
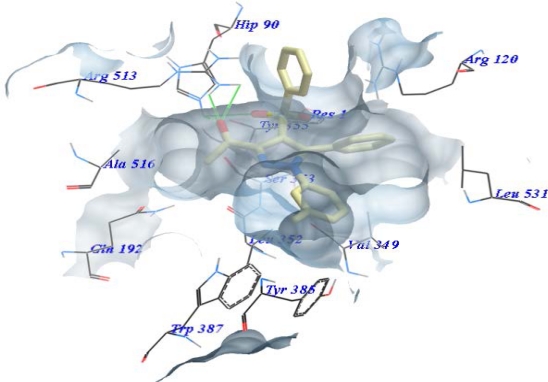
Re-docking of the SC-558 was done to investigate its interaction with active site of COX-2 enzyme. Binding mode of SC-558 showed the binding of bromophenyl ring in a hydrophobic cavity by Gly526, Leu384, Tyr385, Trp387, Met522, Phe518, Val523 and Ser530 interaction (Figure 2). The trifluoromethyl group was bounded in an adjacent pocket by Val116, Tyr355, Leu359 and Leu531. The sulphonamide group extends into a relatively polar region and interacts with His90, Arg513, Gln192, Leu352 and Ser353 [20, 35]. Interactive docking using Mol table ligand was carried out for all the conformers of all of the tested compounds with remarked biological activity to the selected active site of COX-2. Each docked compound was assigned by a score according to its fitting to LBP and its binding mode with different amino acid residues that have a role in its biological activity (Table 3).
Fig. 2.
Binding mode of SC-558 with COX-2 active site. The hydrogen bonds with amino acid residues His90, Tyr355, Arg513, Gln192 & Ser353 represented by green lines.
Tab. 3.
Docking results of SC-558 and the tested compounds with active site of COX-2.
| Cpd. | Conformational stack energy (kcal/mol) | H-bonds | Amino acid residue(s) of H-bond(s) (bond length Å) |
|---|---|---|---|
| SC-558 | –88.3709 | 5 | Arg513 (2.20), His90 (2.30), Gln192 (2.79), Ser353 (1.51) & Tyr355 (2.16) |
| 6a | −63.6787 | 5 | Tyr355 (1.03), His90 (2.51, 2.62) & Arg513 (1.76, 2.58) |
| 6b | −67.6756 | 5 | Tyr355 (1.09), His90 (2.39, 2.50) & Arg513 (1.82, 2.73) |
| 6c | −54.3336 | 5 | Tyr355 (1.07), His90 (2.45, 2.48) & Arg513 (1.62, 2.55) |
| 7b | −57.7361 | 3 | Tyr355 (0.95), His90 (2.66) & Arg513 (2.45) |
| 7d | −64.2746 | 3 | Tyr355 (1.01), His90 (2.59) & Arg513 (2.09) |
| 10a | −57.7051 | 2 | Arg120 (2.61) & Tyr355 (2.21) |
| 11b | −52.7570 | 2 | Arg120 (2.55) & Tyr355 (2.10) |
| 11c | −43.5693 | 3 | Arg513 (2.24) & Tyr355 (1.44) |
| 11e | −49.9558 | 2 | His90 (2.51) & Tyr355 (2.01) |
| 11f | −44.2973 | 3 | Arg513 (2.36) & Tyr355 (1.63) |
The interaction of 6b with COX-2 active site appeared that, sulphone function and carbonyl group were formed a hydrogen bonds with Tyr355, His90 and Arg513 (Figure 3). In addition, the acetyl group was imbedded in the hydrophobic pocket of Phe518, Ala516, Ser353 and Leu352. In addition, the m-methyl substituent in 6b was surrounded by a pocket of Gly526, Tyr385 and Trp387.
Fig. 3.
Docking of compound 6b inside the LBP of COX-2 showing the hydrogen bonds (green lines) and the stacks of aryl rings.
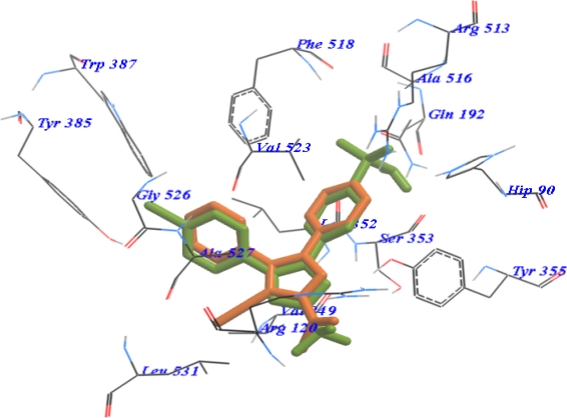
Next, the binding mode of interaction of compound 10a overlaid with SC-558 revealed the same alignment with SC-558. Nevertheless, compound 10a showed a deficient of sulphonamide binding mode (Figure 4). Compounds 7b and 7d gave good binding scores and binding modes with different amino acids in the receptor of COX-2 respect to their biological activity.
Fig. 4.
Overlay of SC-558 (green) and 10a (orange) inside the active site of COX-2.
Conclusion
In conclusion, we described a facile synthesis of poly-functionally pyrazoles. Some of synthesized pyrazoles showed a significant anti-inflammatory activity. 1-(5-Phenyl-4-(phenylsulfonyl)-1-(3-tolyl)-1H-pyrazol-3-yl)ethanone (6b) possessed a high activity, when compared with reference drugs, with lower gastrointestinal (GI) profiles while compounds 7b, 7d and 10a have a moderate anti-inflammatory activity. Molecular docking study gave us good information about the interaction mode of sulfone-based pyrazoles with COX-2 active site. They have the ability to make hydrogen bonds with amino acid residues Tyr355, His90 and Arg513 in COX-2 active site.
Experimental
Chemistry
Melting points (°C, uncorrected) were determined using a Gallenkamp melting point apparatus. Elemental analytical data were obtained from the microanalytical unit, Cairo University, Giza, Egypt. The IR spectra (KBr) were recorded on a PerkinElmer FT/IR spectrometer. At 400 MHz, the NMR spectra were recorded on a Jeol spectrometer using tetramethylsilane as an internal standard. 1H and 13C spectra were run at 400 and 100 MHz, respectively. Splitting patterns are designated as follows: s, singlet; d, doublet; t, triplet; m, multiplet. Chemical shift (δ) values are given in parts per million and coupling constants (J) in Hertz. The mass spectra were performed using a Varian MAT CH-5 spectrometer (70 eV).
1-(5-Phenyl-4-(phenylsulfonyl)-1-aryl-1H-pyrazol-3-yl)ethanone (6a–c)
1-Phenyl-2-(phenylsulfonyl)ethanone (2) (2.6 g, 10 mmol) was added to a stirred ethanolic sodium ethoxide solution [prepared from sodium metal (0.23 g, 10 mmol) and 50 mL of absolute ethanol]. After stirring for 20 min, the appropriate 2-oxo-N'-arylpropane-hydrazonoyl chloride (1a–c) (10 mmol) was added and the reaction mixture was left to stir at room temperature for 12 h. Then added to cold water, the solid product was collected by filtration, washed with water and dried. Recrystallization from ethanol afforded pyrazoles 6a–c. Synthesis of pyrazoles 6a and 6c, using ultrasonic, was reported earlier [36].
1-[1-(3-Methylphenyl)-5-phenyl-4-(phenylsulfonyl)-1H-pyrazol-3-yl]ethanone (6b)
Pale yellow crystals, 74% yield; mp 166–168 °C; IR (KBr) νmax/cm−1 1708 (C=O), 1610 (C=N); 1H NMR (DMSO-d6) δ 2.24 (s, 3H, m-CH3), 2.56 (s, 3H, -COCH3), 7.09–7.42 (m, 9H, ArH), 7.57–7.69 (m, 3H, ArH), 7.89 (d, 2H, J = 7.92 Hz, ArH); 13C NMR δ 21.21 (m-CH3), 28.73 (-COCH3), 127.44, 127.92, 128.36, 129.18, 129.43, 130.25, 130.46, 131.19, 133.83, 138.43, 139.25, 142.49, 148.37, 192.70 (-COCH3); MS m/z (%) 416 (M+, 1.93), 207 (100.0). Anal. Calcd for C24H20N2O3S (416.49): C, 69.21; H, 4.84; N, 6.73; S, 7.70. Found: C, 69.42; H, 4.93; N, 6.59; S, 7.53.
Synthesis of chalcones 7a–f
To a stirred solution of pyrazole 6a–c (10 mmol) and the appropriate aldehyde (10 mmol) in ethanol (30 mL), 10% aqueous sodium hydroxide (5 mL) was added portion-wise at room temperature for 10 min, the reaction mixture was further stirred for 12 h. The resulting solid was filtered off, washed with water, dried and crystallized from EtOH/DMF to afford chalcones 7a–f.
(2E)-1-(1,5-Diphenyl-4-(phenylsulfonyl)-1H-pyrazol-3-yl)-3-phenylprop-2-en-1-one (7a)
White powder, 79% yield; mp 186–188 °C; IR (KBr) νmax/cm−1 1646 (C=O), 1607 (C=N); 1H NMR (DMSO-d6) δ 7.38–7.90 (m, 22H, ArH); 13C NMR δ 121.89, 124.82, 127.00, 127.70, 127.91, 128.47, 129.53, 129.65, 129.79, 130.37, 131.22, 131,74, 133.92, 134.92, 138.59, 142.52, 146.80, 148.95, 185.21 (-C=O); MS m/z (%) 490 (M+, 0.95), 207 (100). Anal. Calcd for C30H22N2O3S (490.57): C, 73.45; H, 4.52; N, 5.71; S, 6.54. Found: C, 73.31; H, 4.57; N, 5.84; S, 6.42.
(2E)-1-(1,5-Diphenyl-4-(phenylsulfonyl)-1H-pyrazol-3-yl)-3-(4-methoxyphenyl)prop-2-en-1-one (7b)
White powder, 62% yield; mp 176–178 °C; IR (KBr) νmax/cm−1 1676 (C=O), 1589 (C=N); 1H NMR (DMSO-d6) δ 3.82 (s, 3H, -OCH3), 7.01–7.04 (d, 2H, J = 8.8 Hz, ArH), 7.36–7.44 (m, 11H, ArH), 7.57–7.89 (m, 8H, ArH); 13C NMR δ 56.00 (-OCH3), 115.15, 121.78, 122.61, 126.96, 127.30, 127.73, 127.91, 128.47, 129.51, 129.74, 130.35, 131.22, 131.54, 133.88, 138.60, 142.58, 146.93, 147.58, 149.28, 185.31 (-C=O); MS m/z (%) 521 (M++1, 0.70), 281 (36.82), 207 (100), 73 (41.93). Anal. Calcd for C31H24N2O4S (520.60): C, 71.52; H, 4.65; N, 5.38; S, 6.16. Found: C, 71.35; H, 4.48; N, 5.31; S, 6.30.
(2E)-1-[1-(3-Methylphenyl)-5-phenyl-4-(phenylsulfonyl)-1H-pyrazol-3-yl]-3-phenylprop-2-en-1-one (7c)
White powder, 65% yield; mp 110–112 °C; IR (KBr) νmax/cm−1 1676 (C=O), 1610 (C=N); 1H NMR (DMSO-d6) δ 2.24 (s, 3H, m-CH3), 7.14–7.88 (m, 21H, ArH); 13C NMR δ 21.23 (m-CH3), 123.97, 124.81, 127.42, 127.72, 127.86, 128.44, 129.19, 129.51, 129.63, 130.38, 131.21, 131.72, 133.89, 134.67, 138.50, 139.24, 146.67, 147.69, 185.40 (-C=O); MS m/z (%) 504 (M+, 6.87), 207 (100). Anal. Calcd for C31H24N2O3S (504.60): C, 73.79; H, 4.79; N, 5.55; S, 6.35. Found: C, 73.85; H, 4.75; N, 5.47; S, 6.48.
(2E)-3-(4-Methoxyphenyl)-1-[1-(3-methylphenyl)-5-phenyl-4-(phenylsulfonyl)-1H-pyrazol-3-yl]prop-2-en-1-one (7d)
White powder, 63% yield; mp 169–171 °C; IR (KBr) νmax/cm−1 1640 (C=O), 1597 (C=N); 1H NMR (DMSO-d6) δ 2.24 (s, 3H, m-CH3), 3.83 (s, 3H, -OCH3), 7.02–7.86 (m, 20H, ArH); 13C NMR δ 21.23 (m-CH3), 56.01 (-OCH3), 115.15, 121.70, 122.58, 123.94, 127.30, 127.39, 127.75, 127.85, 128.44, 129.18, 129.49, 130.33, 131.21, 131.55, 133.85, 138.51, 139.23, 142.59, 146.81, 147.50, 149.23, 185.30 (-C=O); MS m/z (%) 534 (M+, 2.56), 207 (100). Anal. Calcd for C32H26N2O4S (534.62): C, 71.89; H, 4.90; N, 5.24; S, 6.00. Found: C, 71.77; H, 4.91; N, 5.38; S, 5.93.
(2E)-1-[1-(4-Methylphenyl)-5-phenyl-4-(phenylsulfonyl)-1H-pyrazol-3-yl]-3-phenylprop-2-en-1-one (7e)
White fibers, 60% yield; mp 173–174 °C; IR (KBr) νmax/cm−1 1642 (C=O), 1602 (C=N); 1H NMR (DMSO-d6) δ 2.26 (s, 3H, p-CH3), 7.17–7.89 (m, 21H, ArH); 13C NMR δ 21.15 (p-CH3), 121.80, 124.86, 126.74, 127.78, 127.89, 128.47, 129.51, 129.64, 129.93, 130.34, 131.20, 131.71, 133.88, 134.69, 136.18, 139.50, 142.56, 146.70, 147.69, 148.87, 185.22 (-C=O); MS m/z (%) 504 (M+, 0.57), 281 (39.65), 207 (100), 96 (24.96), 73 (45.89). Anal. Calcd for C31H24N2O3S (504.60): C, 73.79; H, 4.79; N, 5.55; S, 6.35. Found: C, 73.66; H, 4.91; N, 5.42; S, 6.38.
(2E)-3-(4-Methoxyphenyl)-1-[1-(4-methylphenyl)-5-phenyl-4-(phenylsulfonyl)-1H-pyrazol-3-yl]prop-2-en-1-one (7f)
White fibers, 61% yield; mp 178–180 °C; IR (KBr) νmax/cm−1 1676 (C=O), 1593 (C=N); 1H NMR (DMSO-d6) δ (s, 3H, p-CH3), 3.82 (s, 3H, -OCH3), 7.01–7.88 (m, 20H, ArH); 13C NMR δ 21.23 (p-CH3), 56.00 (-OCH3), 115.15, 121.68, 122.66, 126.70, 127.32, 127.81, 127.88, 128.47, 129.50, 129.92, 130.31, 131.20131.51, 133.85, 136.20, 139.44, 142.62, 146.82, 147,49, 149.20, 185.32 (-C=O); MS m/z (%) 533 (M+, 0.50), 281 (17.83), 207 (100), 73 (29.26). Anal. Calcd for C32H26N2O4S (534.62): C, 71.89; H, 4.90; N, 5.24; S, 6.00. Found: C, 72.04; H, 5.06; N, 5.37; S, 5.88.
Synthesis of pyrazoles 10a–c
Those compounds were synthesized using the method that described for synthesis of pyrazoles 6a–c using benzoylacetonitrile 8 instead of sulphone 3. pyrazoles 10a and 10c were described in literature [37].
3-Acetyl-1-(3-methylphenyl)-5-phenyl-1H-pyrazole-4-carbonitrile (10b)
Pale yellow crystals, 70% yield; mp 160–162 °C; IR (KBr) νmax/cm−1 2233 (C≡N); 1693 (C=O), 1610 (C=N); 1H NMR (DMSO-d6) δ 2.30 (s, 3H, m-CH3), 2.61 (s, 3H, -COCH3), 7.09–7.52 (m, 9H, ArH); 13C NMR δ 21.29 (m-CH3), 26.99 (-COCH3), 92.64, 123.58, 126.37, 126.83, 129.52, 129.62, 129.90, 130.81, 131.12, 138.39, 139.80, 151.01, 151.10, 192.30 (-COCH3); MS m/z (%) 301 (M+, 3.79), 207 (100). Anal. Calcd for C19H15N3O (301.34): C, 75.73; H, 5.02; N, 13.94. Found: C, 75.85; H, 4.89; N, 14.03.
Synthesis of chalcones 11a–f
This reaction was carried out by the same procedure described in the synthesis of compounds 7a–f using pyrazoles 10a–c instead of 6a–c.
1,5-Diphenyl-3-[(2E)-3-phenylprop-2-enoyl]-1H-pyrazole-4-carbonitrile (11a)
White powder, 66% yield; mp 220–222 °C; IR (KBr) νmax/cm−1 2237 (C≡N); 1664 (C=O), 1597 (C=N); 1H NMR (DMSO-d6) δ 7.42–7.98 (m, 17H, ArH); 13C NMR δ 92.60, 113.73, 121.65, 126.39, 126.64, 129.53, 129.56 129.69, 129.94, 130.00, 131.16, 131.80, 182.39 (-C=O); MS m/z (%) 375 (M+, 0.37), 281 (39.68), 207 (100), 91 (37.51), 73 (79.69). Anal. Calcd for C25H17N3O (375.42): C, 79.98; H, 4.56; N, 11.19. Found: C, 80.07; H, 4.58; N, 11.08.
3-[(2E)-3-(4-Methoxyphenyl)prop-2-enoyl]-1,5-diphenyl-1H-pyrazole-4-carbonitrile (11b)
Pale yellow powder, 60% yield; mp 209–211 °C; IR (KBr) νmax/cm−1 2235 (C≡N); 1667 (C=O), 1595 (C=N); 1H NMR (DMSO-d6) δ 3.82 (s, 3H, -OCH3), 7.02 (d, 2H, J = 8.8 Hz, ArH), 7.41–7.52 (m, 11H, ArH), 7.6–7.95 (m, 3H, ArH); 13C NMR δ 56.01 (-OCH3), 93.62, 113.83, 115.18, 119.04, 126.65, 129.52, 129.93, 130.00, 130.18, 131.62, 138.54, 145.52, 151.17, 151.65, 162.42, 182.19 (-C=O); MS m/z (%) 406 (M++1, 0.22), 272 (74.44), 207 (100), 73 (36.91). Anal. Calcd for C26H19N3O2 (405.45): C, 77.02; H, 4.72; N, 10.36. Found: C, 77.17; H, 4.67; N, 10.28.
1-(3-Methylphenyl)-5-phenyl-3-[(2E)-3-phenylprop-2-enoyl]-1H-pyrazole-4-carbonitrile (11c)
Pale yellow powder, 69% yield; mp 181–183 °C; IR (KBr) νmax/cm−1 2234 (C≡N); 1663 (C=O), 1602 (C=N); 1H NMR (DMSO-d6) δ 2.32 (s, 3H, m-CH3), 7.16–7.53 (m, 13H, ArH), 7.80–7.98 (m, 3H, ArH); 13C NMR δ 21.32 (m-CH3), 93.62, 113.75, 121.63, 123.71, 126.41, 127.00, 129.11, 129.50, 129.58, 129.69, 129.97, 130.84, 131.80, 134.66, 138.47, 139.78, 145.49, 151.21, 151.36, 182.37 (-C=O); MS m/z (%) 389 (M+, 5.28), 207 (100). Anal. Calcd for C26H19N3O (389.45): C, 80.18; H, 4.92; N, 10.79. Found: C, 80.31; H, 5.08; N, 10.74.
3-[(2E)-3-(4-Methoxyphenyl)prop-2-enoyl]-1-(3-methylphenyl)-5-phenyl-1H-pyrazole-4-carbonitrile (11d)
Pale yellow powder, 58% yield; mp 170–172 °C; IR (KBr) νmax/cm−1 2234 (C≡N); 1667 (C=O), 1598 (C=N); 1H NMR (DMSO-d6) δ 2.32 (s, 3H, m-CH3), 3.83 (s, 3H, -OCH3), 7.01–7.94 (m, 15H, ArH); 13C NMR δ 21.32 (m-CH3), 56.01 (-OCH3), 93.55, 113.84, 115.18, 123.71, 126.47, 127.01, 127.32, 129.50, 129.59, 129.96, 130.80, 131.10, 131.62, 138.49, 139.76, 145.48, 151.11, 151.60, 162.43, 182.18 (-C=O); MS m/z (%) 419 (M+, 6.37), 207 (100). Anal. Calcd for C27H21N3O2 (419.47): C, 77.31; H, 5.05; N, 10.02. Found: C, 77.22; H, 4.93; N, 9.95.
1-(4-Methylphenyl)-5-phenyl-3-[(2E)-3-phenylprop-2-enoyl]-1H-pyrazole-4-carbonitrile (11e)
Pale yellow powder, 64% yield; mp 262–264 °C; IR (KBr) νmax/cm−1 2236 (C≡N); 1668 (C=O), 1597 (C=N); 1H NMR (DMSO-d6) δ 2.35 (s, 3H, p-CH3), 7.28–7.51 (m, 12H, ArH), 7.79–7.98 (m, 4H, ArH); 13C NMR δ 21.25 (p-CH3), 93.60, 113.83, 115.46, 121.58 124.65, 126.41, 127.00, 129.53, 129.69, 129.93, 129.99, 130.33, 131.90, 131.80, 134.12, 139.84, 146.50, 147.69, 151.20, 182.20 (-C=O); MS m/z (%) 389 (M+, 9.65), 207 (200). Anal. Calcd for C26H19N3O (389.45): C, 80.18; H, 4.92; N, 10.79. Found: C, 80.34; H, 4.98; N, 10.85.
3-[(2E)-3-(4-Methoxyphenyl)prop-2-enoyl]-1-(4-methylphenyl)-5-phenyl-1H-pyrazole-4-carbonitrile (11f) [38, 39]
Pale yellow crystals, 66% yield; mp 215–217 °C; IR (KBr) νmax/cm−1 2237 (C≡N); 1662 (C=O), 1589 (C=N); 1H NMR (DMSO-d6) δ (s, 3H, p-CH3), 3.83 (s, 3H, -OCH3), 7,03 (d, 2H, J = 9.16 Hz, ArH), 7.28–7.50 (m, 9H, ArH), 7.65–7.95 (m, 4H, ArH); 13C NMR δ 21.25 (p-CH3), 56.02 (-OCH3), 93.60, 113.84, 115.18, 119.13, 126.41, 126.52, 127.33, 129.51, 129.98, 130.31, 131.60, 136.16, 139.93, 145.48, 151.09, 162.42, 182.21 (-C=O); MS m/z (%) 419 (M+, 4.24), 207 (100). Anal. Calcd for C27H21N3O2 (419.47): C, 77.31; H, 5.05; N, 10.02. Found: C, 77.40; H, 4.97; N, 10.16.
Pharmacology
Anti-inflammatory Activity
Adult albino rats of both sexes weighing 120–150 g were obtained from animal house laboratory of Nile Company, Cairo, Egypt and acclimatized for 1 week in the animal facility that has 12 h light/dark cycles with the temperature controlled at 21–23 °C. Normal rat chow and water were made available. The tested compounds and the reference standards were completely dissolved in DMSO. The administered oral dose of the tested compounds was 10 mg/kg body weight with analogy of a reported procedure. Ninety rats were divided into 15 groups each of six animals. All rats were deprived from food and water for 18 h before the experiment and were injected orally by 5 mL water to avoid fluid variation during the process of edema. Two groups received the reference standards; 12 groups received the tested compounds dissolved in DMSO and one group left as negative control group which given 0.2 mL DMSO by oral tube. Indomethacin was obtained from Nile Company for Pharmaceuticals and Chemical Industries, Cairo, Egypt and meloxicam was obtained from Memphis Company for Pharmaceuticals and Chemical Industries, Cairo, Egypt. The tested compounds and meloxicam were given by oral route at doses of 10 mg/kg body weight while indomethacin was given by oral rout at 5 mg/kg body weight. This dose of indomethacin was considered as a positive control for experiments with any new chemical entity [40]. Then, sublunary of 0.1 mL of 2% carrageenan sodium (Sigma, USA) was injected in the right hind paw. The volume of the paw was measured immediately after injection and after administration of the compounds at time intervals 3 and 6 h by using Dial micrometer model (120–1206) Baty, Sussex, England). The results were expressed as volume of edema at each time interval, percentage inhibition of edema volume at each time with respect to control and potency which was calculated compared to indomethacin.
Ulcerogenic effects
Ulcerogenic activity of meloxicam, indomethacin and tested compounds were studied in Albino rats of wistar strain weighing 150–200 g of either sex and divided into seven groups each of six animals. The first group served as control group treated with 0.2 mL DMSO. Indomethacin was given by a dose of 5 mg/kg/day while the dose of meloxicam and other tested compounds was 10 mg/kg/day. All groups were treated for three consecutive days by oral tube. During three days of dosage treatment, the animals were starved for 18 h but water was provided ad libitum. Food was allowed 2 hours post administration of the drugs. Two hours following the last doses, rats were sacrificed. The stomach of each rat were removed, opened along the greater curvature, rinsed with 0.9% sodium chloride (isotonic solution) and stretched by pins on a cork board. The lesions in gastric mucosa were determined by using stereoscopic microscope. Hemorrhagic lesions were evaluated by scores: 0.0, Normal (no injury, bleeding and latent injury); 0.5, Latent injury or widespread bleeding; 1.0, Slight injury (2 to 3 dotted lines); 2.0, Severe injury (5–6 dotted injuries); 3.0, Very severe injury (several continuous lined injuries) and 4.0, Widespread lined injury or widened injury [33].
Statistics
In anti-inflammatory study, data are expressed as value ± SEM. Results of carrageenan-induced paw edema experiments are also expressed as percentage of change from control (pre-drug) values. Differences between vehicle control and treatment groups were tested using one-way ANOVA followed by multiple comparisons by the Bonferroni’s test. In ulcerogenic study, Data are presented as mean ± SD and were subjected to one way ANOVA, followed by multiple comparisons by the Bonferroni’s test.
Authors’ statements
Competing interests
The authors declare no conflict of interest.
Animal Rights
The conducted research followed the international ethical standards for the care and use of laboratory animals and was approved by the ethical committee for use of experimental animals.
References
- [1].Vane J, Bottling R. Inflammation and the mechanism of action of anti-inflammatory drugs. FASEB J. 1987;1:89–96. [PubMed] [Google Scholar]
- [2].Lemke TL, Williams DA, Roche VF, Zito SW. Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins; 2007. [Google Scholar]
- [3].Vane JR, Botting RM. Mechanism of action of nonsteroidal anti-inflammatory drugs. New York, NY: ETATS-UNIS: Elsevier; 1998. [DOI] [PubMed] [Google Scholar]
- [4].Walker JS. NSAID: An update on their analgesic effects. Clin Exp Pharm Physiol. 1995;22:855–860. doi: 10.1111/j.1440-1681.1995.tb01950.x. [DOI] [PubMed] [Google Scholar]
- [5].Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- [6].Munroe DG, Lau CY. Turning down the heat: new routes to inhibition of inflammatory signaling by prostaglandin H2 synthases. Chem Biol. 1995;2:343–350. doi: 10.1016/1074-5521(95)90212-0. [DOI] [PubMed] [Google Scholar]
- [7].Gökhan-Kelekçi N, Yabanoglu S, Küpeli E, SalgIn U, Özgen Ö, Uçar G, Yesilada E, Kendi E, Yesilada A, Bilgin AA. A new therapeutic approach in Alzheimer disease: Some novel pyrazole derivatives as dual MAO-B inhibitors and antiinflammatory analgesics. Bioorg Med Chem. 2007;15:5775–5786. doi: 10.1016/j.bmc.2007.06.004. [DOI] [PubMed] [Google Scholar]
- [8].Maggio B, Daidone G, Raffa D, Plescia S, Mantione L, Cutuli VMC, Mangano NG, Caruso A. Synthesis and pharmacological study of ethyl 1-methyl-5-(substituted 3,4-dihydro-4-oxoquinazolin-3-yl)-1H-pyrazole-4-acetates. Eur J Med Chem. 2001;36:737–742. doi: 10.1016/S0223-5234(01)01259-4. [DOI] [PubMed] [Google Scholar]
- [9].Rapposelli S, Lapucci A, Minutolo F, Orlandini E, Ortore G, Pinza M, Balsamo A. Synthesis and COX-2 inhibitory properties of N-phenyl- and N-benzyl-substituted amides of 2-(4-methylsulfonylphenyl)cyclopent-1-ene-1-carboxylic acid and of their pyrazole, thiophene and isoxazole analogs. Farmaco. 2004;59:25–31. doi: 10.1016/j.farmac.2003.09.003. [DOI] [PubMed] [Google Scholar]
- [10].Bekhit AA, Ashour HMA, Guemei AA. Novel pyrazole derivatives as potential promising anti-inflammatory antimicrobial agents. Arch Pharm. 2005;338:167–174. doi: 10.1002/ardp.200400940. [DOI] [PubMed] [Google Scholar]
- [11].Ochi T, Jobo-Magari K, Yonezawa A, Matsumori K, Fujii T. Anti-inflammatory and analgesic effects of a novel pyrazole derivative, FR140423. Eur J Pharmacol. 1999;365:259–266. doi: 10.1016/S0014-2999(98)00868-1. [DOI] [PubMed] [Google Scholar]
- [12].Gadad AK, Kittur BS, Kapsi SG, Mahajanshetti CS, Rajur SB. Synthesis, analgesic and anti-inflammatory activities of some 1-acyl/aracyl-5-aminopyrazole derivatives. Arzneimittelforschung. 1996;46:1082–1085. [PubMed] [Google Scholar]
- [13].Penning TD, Talley JJ, Bertenshaw SR, Carter JS, Collins PW, Docter S, Graneto MJ, Lee LF, Malecha JW, Miyashiro JM, Rogers RS, Rogier DJ, Yu SS, Anderson GD, Burton EG, Cogburn JN, Gregory SA, Koboldt CM, Perkins WE, Seibert K, Veenhuizen AW, Zhang YY, Isakson PC. Synthesis and biological evaluation of the 1,5-diarylpyrazole class of cyclooxygenase-2 inhibitors: Identification of 4-[5-(4-methylphenyl)-3- (trifluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide (SC-58635, Celecoxib) J Med Chem. 1997;40:1347–1365. doi: 10.1021/jm960803q. [DOI] [PubMed] [Google Scholar]
- [14].Tsuji K, Nakamura K, Ogino T, Konishi N, Tojo T, Ochi T, Seki N, Matsuo M. Studies on anti-inflammatory agents. VI. Synthesis and pharmacological properties of 2,3-diarylthiophenes. Chem Pharm Bull. 1998;46:279–286. doi: 10.1248/cpb.46.279. [DOI] [PubMed] [Google Scholar]
- [15].Bing RJ, Lomnicka M. Why do cyclo-oxygenase-2 inhibitors cause cardiovascular events. J Am Coll Cardiol. 2002;39:521–522. doi: 10.1016/S0735-1097(01)01749-1. [DOI] [PubMed] [Google Scholar]
- [16].Sikes DH, Agrawal NM, Zhao WW, Kent JD, Recker DP, Verburg KM. Incidence of gastroduodenal ulcers associated with valdecoxib compared with that of ibuprofen and diclofenac in patients with osteoarthritis. Eur J Gastroenterol Hepatol. 2002;14:1101–1111. doi: 10.1097/00042737-200210000-00011. [DOI] [PubMed] [Google Scholar]
- [17].Langman MJ, Jensen DM, Watson DJ, Harper SE, Zhao P-L, Quan H, Bolognese JA, Simon TJ. Adverse upper gastrointestinal effects of rofecoxib compared with NSAIDs. JAMA. 1999;282:1929–1933. doi: 10.1001/jama.282.20.1929. [DOI] [PubMed] [Google Scholar]
- [18].Cochrane DJ, Jarvis B, Keating GM. Etoricoxib. Drugs. 2002;62:2637–2651. doi: 10.2165/00003495-200262180-00006. [DOI] [PubMed] [Google Scholar]
- [19].Dallob A, Hawkey CJ, Greenberg H, Wight N, De Schepper P, Waldman S, Wong P, DeTora L, Gertz B, Agrawal N, Wagner J, Gottesdiener K. Characterization of etoricoxib, a novel, selective COX-2 inhibitor. J Clin Pharmacol. 2003;43:573–585. doi: 10.1177/0091270003253703. [DOI] [PubMed] [Google Scholar]
- [20].Kurumbail RG, Stevens AM, Gierse JK, McDonald JJ, Stegeman RA, Pak JY, Gildehaus D, Iyashiro JM, Penning TD, Seibert K, Isakson PC, Stallings WC. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature. 1996;384:644–648. doi: 10.1038/384644a0. [DOI] [PubMed] [Google Scholar]
- [21].Sai Ram KVVM, Rambabu G, Sarma JARP, Desiraju GR. Ligand coordinate analysis of Sc-558 from the active site to the the surface of COX-2: A molecular dynamics study. J Chem Inf Mod. 2006;46:1784–1794. doi: 10.1021/ci050142i. [DOI] [PubMed] [Google Scholar]
- [22].Wang JL, Limburg D, Graneto MJ, Springer J, Hamper JRB, Liao S, Pawlitz JL, Kurumbail RG, Maziasz T, Talley JJ, Kiefer JR, Carter J. The novel benzopyran class of selective cyclooxygenase-2 inhibitors. Part 2: The second clinical candidate having a shorter and favorable human half-life. Bioorg Med Chem Lett. 2010;20:7159–7163. doi: 10.1016/j.bmcl.2010.07.054. [DOI] [PubMed] [Google Scholar]
- [23].Abdel-Aziz HA, El-Zahabi HSA, Dawood KM. Regioselective synthesis and in-vitro anti-tumor activity of 1,3,4-triaryl-5-N-arylpyrazole-carboxamides. Eur J Med Chem. 2010;45:2427–2432. doi: 10.1016/j.ejmech.2010.02.026. [DOI] [PubMed] [Google Scholar]
- [24].Abdel-Aziz HA, Abdel-Wahab BF, Badria FA. Stereoselective synthesis and antiviral activity of (1E,2Z,3E)-1-(piperidin-1-yl)-1-(arylhydrazono)-2-(benzoyl/benzothiazol-2-oylhydrazono)-4-aryl1but-3-ene. Arch Pharm. 2010;343:152–159. doi: 10.1002/ardp.200900195. [DOI] [PubMed] [Google Scholar]
- [25].Abdel-Aziz HA, Saleh TS, El-Zahabi HSA. Facile synthesis and in-vitro anti-tumor activity of some pyrazolo[3,4-b]pyridines and pyrazolo[1,5-a]pyrimidines linked to thiazolo[3,2-a]benzimidazole moiety. Arch Pharm. 2010;343:24–30. doi: 10.1002/ardp.200900082. [DOI] [PubMed] [Google Scholar]
- [26].Hamdy NA, Gamal-Eldeen AM, Abdel-Aziz HA, Fakhr IMI. Modulation of carcinogen metabolizing enzymes by new fused heterocycles pendant to 5,6,7,8-tetrahydronaphthalene derivatives. Eur J Med Chem. 2010;45:463–470. doi: 10.1016/j.ejmech.2009.10.027. [DOI] [PubMed] [Google Scholar]
- [27].Abdel-Aziz HA, Mekawey AAI. Stereoselective synthesis and antimicrobial activity of benzofuran-based (1E)-1-(piperidin-1-yl)-N2-arylamidrazones. Eur J Med Chem. 2009;44:3985–3997. doi: 10.1016/j.ejmech.2009.02.020. [DOI] [PubMed] [Google Scholar]
- [28].Abdel-Aziz HA, Mekawey AAI, Dawood KM. Convenient synthesis and antimicrobial evaluation of some novel 2-substituted-3-methylbenzofuran derivatives. Eur J Med Chem. 2009;44:3637–3644. doi: 10.1016/j.ejmech.2009.02.020. [DOI] [PubMed] [Google Scholar]
- [29].Abdel-Wahab BF, Abdel-Aziz HA, Ahmed EM. Synthesis and antimicrobial evaluation of 1-(benzofuran-2-yl)-4-nitro-3-arylbutan-1-ones and 3-(benzofuran-2-yl)-4,5-dihydro-5-aryl-1-[4-(aryl)-1,3-thiazol-2-yl]-1H-pyrazoles. Eur J Med Chem. 2009;44:2632–2635. doi: 10.1016/j.ejmech.2008.09.029. [DOI] [PubMed] [Google Scholar]
- [30].Abdel-Aziz HA, Gamal-Eldeen AM, Hamdy NA, Fakhr IMI. Immunomodulatory and anti-cancer activity of some novel 2-substituted-6-bromo-3-methyl-thiazolo[3,2-a]benzimidazole derivatives. Arch Pharm. 2009;342:230–237. doi: 10.1002/ardp.200800189. [DOI] [PubMed] [Google Scholar]
- [31].Abdel-Aziz HA, Hamdy NA, Farag AM, Fakhr IMI. Synthesis of some novel pyrazolo[1,5-a]pyrimidine, 1,2,4-triazolo[1,5-a]pyrimidine, pyrido[2,3-d]pyrimidine, pyrazolo[5,1-c]-1,2,4-triazine and 1,2,4-triazolo[5,1-c]-1,2,4-triazine derivatives incorporating a thiazolo[3,2-a]benzimidazole moiety. J Heterocycl Chem. 2008;45:1–5. doi: 10.1002/jhet.5570450413. [DOI] [Google Scholar]
- [32].Shaaban MR, Saleh TS, Mayhoub AS, Mansour A, Farag AM. Synthesis and analgesic/ anti-inflammatory evaluation of fused heterocyclic ring systems incorporating phenylsulfonyl moiety. Bioorg Med Chem. 2008;16:6344–6352. doi: 10.1016/j.bmc.2008.05.011. [DOI] [PubMed] [Google Scholar]
- [33].Kumar SGV, Mishra DN. Analgesic, antiinflammatory, and ulcerogenic studies of meloxicam solid dispersion prepared with polyethylene glycol 6000. Methods Find Exp Clin Pharmacol. 2006;28:419–422. doi: 10.1358/mf.2006.28.7.1003549. [DOI] [PubMed] [Google Scholar]
- [34].Maiorov V, Abagyan R. Energy strain in three-dimensional protein structures. Fold Des. 1998;3:259–269. doi: 10.1016/S1359-0278(98)00037-6. [DOI] [PubMed] [Google Scholar]
- [35].Llorens O, Perez JJ, Palomer A, Mauleon D. Differential binding mode of diversy cyclooxygenase inhibitors. J Mol Graph Model. 2002;20:359–371. doi: 10.1016/S1093-3263(01)00135-8. [DOI] [PubMed] [Google Scholar]
- [36].Saleh TS, Abd EL-Rahman NM. Ultrasound promoted synthesis of substituted pyrazoles and isoxazoles containing sulphone moiety. Ultrasonics Sonochem. 2009;16:237–242. doi: 10.1016/j.ultsonch.2008.07.012. [DOI] [PubMed] [Google Scholar]
- [37].Twari RS, Parihar P. Studies on nitrile imines: Synthesis of pyrazoles using active methylene compounds. Indian J Chem. 1980;19B:217–218. [Google Scholar]
- [38].Abdel-Aziz HA, Bari A, Weng Ng S. 3-[(E)-3-(4-Methoxyphenyl)prop-2-enoyl]-1-(4-methylphenyl)-5-phenyl-1H-pyrazole-4-carbonitrile. Acta Cryst. 2011;E67:o694. doi: 10.1107/S1600536811005770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39]. Supplementary data and X-ray figures for compound 11f are available from the IUCR electronic archives (XU5162).
- [40].Whiteley PE, Dalrymple SA. Models of Inflammation: Carrageenan-Induced Paw Edema in the Rat. John Wiley & Sons, Inc; 2001. [Google Scholar]