Abstract
Inactivation of the tumor suppressor phosphatase and tensin homolog deleted on chromosome 10 (PTEN) is heavily implicated in the tumorigenesis of prostate cancer. Conversely, the upregulation of the chemokine (CXC) receptor 4 (CXCR4) is associated with prostate cancer progression and metastasis. Studies have shown that loss of PTEN permits CXCR4-mediated functions in prostate cancer cells. Loss of PTEN function is typically due to genetic and epigenetic modulations, as well as active site oxidation by reactive oxygen species (ROS); likewise ROS upregulates CXCR4 expression. Herein, we show that ROS accumulation permitted CXCR4-mediated functions through PTEN catalytic inactivation. ROS increased p-AKT and CXCR4 expression, which were abrogated by a ROS scavenger in prostate cancer cells. ROS mediated PTEN inactivation but did not affect expression, yet enhanced cell migration and invasion in a CXCR4-dependent manner. Collectively, our studies add to the body of knowledge on the regulatory role of PTEN in CXCR4-mediated cancer progression, and hopefully, will contribute to the development of therapies that target the tumor microenvironment, which have great potential for the better management of a metastatic disease.
Keywords: Prostate cancer, PTEN, CXCR4, ROS, H2O2, AKT
Introduction
Prostate cancer is the most commonly diagnosed and second leading cause of cancer-related deaths among men in the United States, where 90% of cancer-related mortalities are due to a metastatic disease [1]. Emerging evidence has implicated the tumor microenvironment in cancer progression and metastasis. Most of the influences from the tumor microenvironment are due to secretion of various molecules from immune cells, such as cytokines and chemokines, which results in dysregulation of tumor suppressor genes and oncogenes [2]. The tumor microenvironment also secretes reactive oxygen species (ROS), which are associated with tumorigenesis by modulating anti-cancer and pro-cancer pathways [3].
ROS are a group of highly reactive oxygen containing molecules, such as hydrogen peroxide (H2O2) and O2- radicals, that are produced by the tumor microenvironment and cellular metabolic processes [4,5]. The accumulation of ROS typically regulates cell survival, proliferation and migration in cancer cells by functioning as a second messenger in intracellular signal transduction; however, excessive amounts of ROS in cells result in deleterious effects, such as genotoxicity and cell death [6]. Under physiologic conditions, ROS homeostasis is maintained by various neutralizing enzymes, such as catalase and superoxide dismutase [4]. An imbalance in ROS homeostasis is associated with prostate tumorigenesis by activating pro-cancer signaling pathways and inactivating tumor suppressor genes [7]. Furthermore, ROS has been linked to cell survival through activation of PI3K/AKT and ERK1/2 pathways [8]. ROS is also associated with inactivating the tumor suppressor PTEN, by forming a disulfide bond within the active site, rendering it catalytically inactive. Consequently, PTEN inactivation permits downstream survival signaling, such as PI3K/AKT, to support cancer progression [9].
Li et al. and Steck et al. first identified a high frequency of PTEN mutations and deletions in cancers of the brain, bladder, breast and prostate, implicating PTEN as a novel tumor suppressor in tumorigenesis [10,11]. Loss of PTEN by genetic or epigenetic modifications is an early event in prostate carcinogenesis, and is correlated with progression to an aggressive castration-resistant disease [12]. PTEN is involved in regulating a variety of cellular functions, including cell cycle, apoptosis, DNA repair, signal transduction and cell adhesion. Catalytically, PTEN functions by dephosphorylating PIP3, a product of PI3K, to form PIP2; thus negatively regulating the activation of AKT [13]. Consequently, loss of PTEN expression results in the accumulation of PIP3, and subsequently, constitutive activation of the PI3K/AKT signaling pathway. We have shown that reconstitution of PTEN expression negatively regulated CXCR4-mediated migration and proliferation in advanced prostate cancer cells, indicating that an absence of PTEN functioned as a permissive switch for CXCR4-mediated signaling and functions [9]. Furthermore, knockdown of PTEN by siRNA in DU145 cells enhanced CXCR4-mediated migration; thus indicating that PTEN serves as a regulator of CXCR4 function. PTEN did not directly regulate CXCR4; rather, their pathways converged at the level of cellular signaling [9].
CXCR4 is a G-protein coupled receptor (GPCR) that exclusively binds to its ligand stromal cell-derived factor 1α (SDF1α or CXCL12). CXCR4 activity is involved in normal homeostasis, such as immune cell migration, embryonic development, growth, angiogenesis, and hematopoiesis [14]. Studies have also implicated CXCR4 in malignant cancer development by its involvement in cell motility, adhesion, secretion of matrix metalloproteinases (MMPs), angiogenesis and activation of survival signaling pathways (PI3K/AKT, ERK1/2, JAK/STAT, Src kinase and HER2) [15]. Thus, the CXCR4/SDF1α axis plays a crucial role in targeting solid tumor metastasis to sites outside of the primary tumor [16]. In cancer and stem cell models, CXCR4 was upregulated in high ROS environments, indicating that a ROS-enriched microenvironment may have a critical influence on CXCR4 expression and functions during cancer progression [17].
The tumor microenvironment influences the metastatic potential of various cancer models, including prostate cancer. These influences are in the form of various molecules, such as ROS, which can function as a secondary signaling molecule and negatively regulate tumor suppressors in favor of oncogenes for cancer survival. Studies have shown, independently, that ROS inactivates PTEN and up-regulates CXCR4; therefore, we investigated ROS-mediated PTEN inactivation, and subsequent upregulation of CXCR4, in prostate tumorigenesis and metastasis. We observed that ROS increased expression of phosphorylated AKT, while PTEN expression was stabilized. ROS inhibited PTEN catalytic function, while aberrantly regulating expression of phosphorylated ERK1/2. Upon treating prostate cancer cells with ROS, we observed an up- regulation of CXCR4 expression, and subsequent cell migration and invasion. Moreover, up-regulation of CXCR4 and subsequent functions were independent of its ligand, SDF1α. We have previously identified a functional relationship between PTEN and CXCR4 in prostate cancer [9]. Herein, we describe a putative mechanism by which PTEN function is lost in prostate cancer cells, resulting in enhanced CXCR4 expression and function, and overall tumorigenesis.
Materials and Methods
Cell culture, antibodies, and reagents
DU145 human prostate cancer cells were obtained from American Type Culture Collection (ATCC) and maintained in complete RPMI 1640 media (10% FBS, 1% nonessential amino acids and 1% antibiotic-antimycotic), or starvation media (RPMI only), at 37°C and 5% CO2. All cells were maintained at 60% to 80% confluency. Hydrogen peroxide (H2O2) was used as our model of ROS (Acros Organics). AMD3100 and N-acetyl-cysteine (NAC) were from Sigma Aldrich; N-ethylmaleimide was from EMD Chemicals. Cell culture supplies were from MediaTech and the following human antibodies were from Cell Signaling: anti-PTEN, anti-AKT, anti-phospho-AKT (p-AKT) and anti-phospho-ERK1/2 (p-ERK1/2). Anti-ERK1/2 was from Biosource; anti-Tubulin and anti-Fusin (CXCR4) were from Santa Cruz Biotech.
Western blot analysis
1×106 cells were harvested in lysis buffer (Cell Signaling), as previously described [9]. Equal concentrations of total cell lysate were resolved by 10% SDS-PAGE and transferred to a polyvinylidene fluoride (PVDF) transfer membrane. Nonspecific binding sites were blocked with 5% nonfat dry milk/0.1% Tween 20/1XTBS, followed by an incubation with primary antibodies for the proteins of interest in 3% bovine serum albumin–Tris buffered saline Tween 20 (BSA-TBST; p-ERK1/2, p-AKT, AKT, PTEN,) or 3% nonfat dry milk-TBST (ERK1/2). Protein complexes were detected with horseradish peroxidase-conjugated secondary antibodies (Jackson) and enhanced chemiluminescence reagents (Pierce). Exposed films were developed using an automated X-ray processor (Kodak X-OMAT M35A Processor).
Western blot analysis of alkylated PTEN
The oxidation state of PTEN was investigated using alkylating agents, as described by Lee et al. [18]. Briefly, 1×106 cells/well were treated with 0.25mM H2O2 and scraped into alkylating lysis buffer (20 mmol/L Tris-HCl (pH 7.5), 150 mmol/L NaCl, 1 mmol/L Na2EDTA, 1 mmol/L EGTA, 1% Triton, 2.5 mmol/L sodium pyrophosphate, 1 mmol/L b-glycerophosphate, 1 mmol/L Na3VO4, 1 mg/mL leupeptin, 1mmol/L PMSF, 2% SDS and 40 mM N-ethylmaleimide (NEM)). The cell lysate was sonicated, and equal amounts of protein (60 μg) were incubated at room temperature for 30 minutes. Total protein lysates were resolved by 10% SDS-PAGE under non-reducing conditions and transferred to a PVDF membrane. Reduced and non-reduced forms of PTEN were detected as described above.
Migration assay
Assays were done using 8μm pores Transwell chambers (Costar). Briefly, cells were harvested in starvation media for 24 hours prior to detachment with 1× citric saline. 4×104 cells each were resuspended in RPMI media, and added to the upper Transwell chamber. RPMI containing various treatments were added to the lower chamber, and cells were allowed to migrate toward the lower chamber for 6 hours at 37°C. Cells that remained in the upper chamber were removed with a cotton swab, and the entire chamber was fixed and stained with Hemacolor Solution 3 Kit (EMD). Migrated cells were counted using a Zeiss Axiovert 200M light microscope. Results were quantified using GraphPad Prism 5 statistical program.
Invasion assay
Transwell chambers with 8μm pores (Costar) were coated with 40 μL of Matrigel (BD Scientific) gel mixture (1:4 in RPMI), and allowed to solidify for 1hour at 37°C. Briefly, cells were harvested in starvation media for 24 hours prior to detachment with 1× citric saline. 8×104 cells were resuspended in RPMI media, and added to the upper Transwell chamber. RPMI containing various treatments were added to the lower chamber, and cells were allowed to invade Matrigel towards the lower chamber for 24 hours at 37°C. Matrigel and cells that remained in the upper chamber were removed with a cotton swab, and the entire chamber was fixed and stained with Hemacolor Solution 3 Kit (EMD). Invaded cells were counted using a Zeiss Axiovert 200M light microscope. Results were quantified using GraphPad Prism 5 statistical program.
Statistics and quantifications
Data are presented as the mean ±SE of at least 3 independent experiments and were analyzed by 2-way ANOVA or Student's t test. All statistical analyses were done, and all graphs generated, using GraphPad Prism 5.0 software (GraphPad).
Results
H2O2 induced gradual expression of phosphorylated AKT
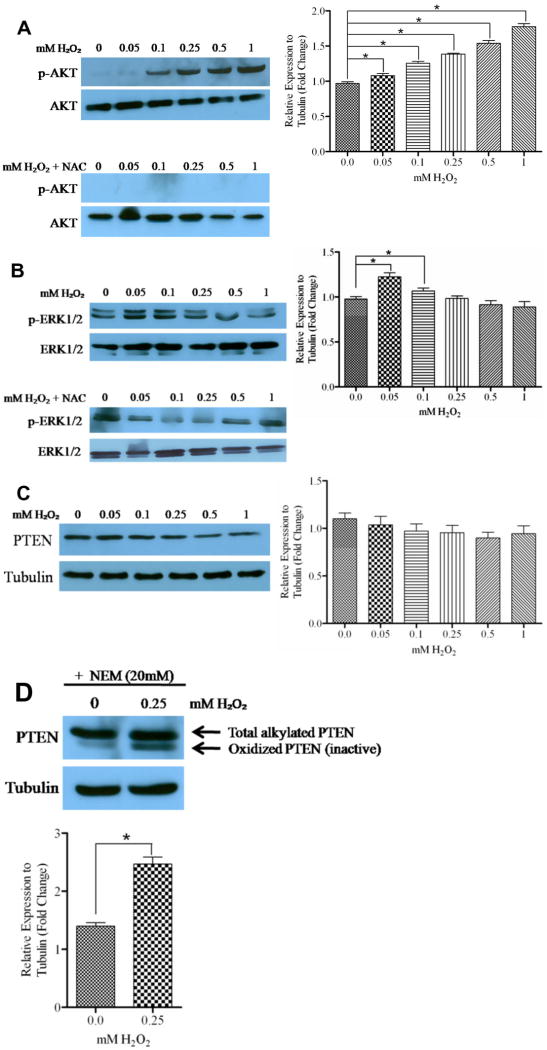
Generally, the presence of ROS increases expression of phosphorylated proteins, through activation of protein kinases and inhibition of protein phosphatases [7,19]. In prostate cancer, activation of survival proteins are the hallmark of the cancer progression [20]; therefore, we investigated whether H2O2, our model of ROS, increased expression of phosphorylated survival kinases. DU145 cells were treated with various concentrations of H2O2 for 1 hour and the expression of phosphorylated AKT (p-AKT) and phosphorylated ERK1/2 (p-ERK1/2) was determined by western blot analysis. We observed that H2O2 induced a gradual expression of p-AKT in a concentration dependent manner, which was abrogated by 10 mM NAC, a ROS scavenger (Fig. 1A). Furthermore, cells treated with H2O2 showed a maximum induction of p-ERK1/2 at 0.05mM H2O2, followed by a gradual decrease in expression. Likewise, 10mM NAC abrogated induction of p-ERK by H2O2 (Fig. 1B). These results show that the presence of ROS increases expression of survival kinases DU145 cells.
Figure 1. ROS increased expression of p-AKT and p-ERK, but not PTEN in DU145 cells.
Forty μg of total protein were analyzed for (A) p-AKT, (B) p-ERK and (C) PTEN by western blot analysis using specific antibodies. Cells were treated at various time points with the indicated concentrations of H2O2 and 10mM NAC. Total protein levels of AKT, ERK1/2 and tubulin served as loading controls. (D) Sixty μg of total PTEN was analyzed for oxidized and reduced forms by western blot analysis using a PTEN specific antibody. Cells were exposed to H2O2 for 90 minutes, followed by alkylation as described. Tubulin served as a loading control. *, P < 0.05.
H2O2 inhibited PTEN catalytic domain in DU145 cells
Kumar et al. induced expression of endogenous ROS in prostate cancer cells, which resulted in increased expression of AKT [7]. It is well established that the tumor suppressor PTEN negatively regulates the PI3K/AKT pathway by dephosphorylating PIP3, a product of PI3K, to form PIP2; thus negatively regulating the activation of AKT [13]. Ha et al. found that exogenous treatment of hepatocellular carcinoma with H2O2 resulted in catalytic inactivation of PTEN [21]. Therefore, we determined whether the observed increased expression of p-AKT was due to H2O2-mediated inactivation of PTEN in DU145 cells, which have one functional allele of PTEN [10]. Cells were treated with various concentrations of H2O2 for 1 hour, and PTEN expression was determined by western blot analysis. We did not observe a significant change in PTEN expression upon treatment with H2O2 compared to control, except for a slight decrease at 0.5mM (Fig. 1C). Treatment with 10mM NAC showed similar results, with no change in PTEN expression (data not shown).
H2O2 oxidizes PTEN within its catalytic domain by forming a disulfide bond between Cys124 and Cys71 in the active site, thus inactivating its phosphatase function [18]. We investigated whether H2O2 inhibits the catalytic activity of PTEN, since there was no change observed in PTEN expression, but increased expression of p-AKT. Cells were treated with 0.25mM H2O2 for 90 minutes to induce oxidation within cysteine residues, which renders PTEN inactive. Cell extracts were then lysed in alkylating buffer (NEM) to block free sulfhydryls, and then subjected to western blot analysis with PTEN specific antibodies. Oxidized PTEN has two less cysteine residues available for alkylation, which results in a lower molecular weight form of the protein. We observed an increase in oxidized PTEN (inactive) in H2O2-treated cells compared to control (Fig. 1D). Collectively, these results suggest that H2O2 inactivates PTEN catalytically, which may allow the increased expression of p-AKT, in a concentration dependent manner.
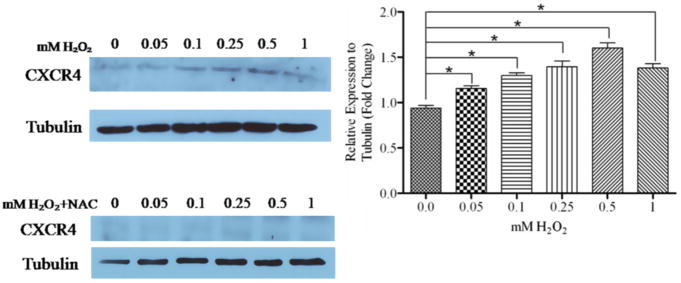
H2O2 induced expression of CXCR4 in DU145 cells
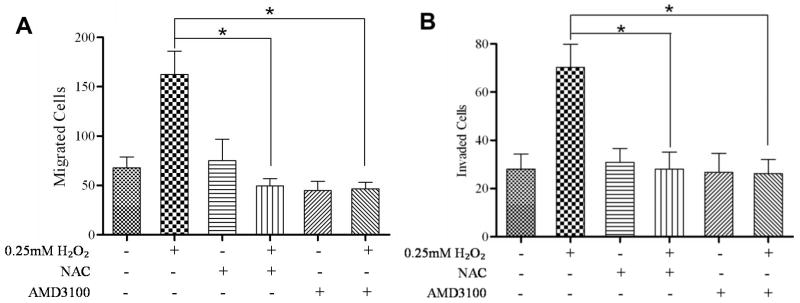
In mesenchymal stem cells, H2O2 induced CXCR4 expression [17]. We observed that various concentrations of H2O2 gradually increased CXCR4 expression, up to 0.5 mM H2O2 (Fig. 2A). 10mM NAC abrogated H2O2-induced expression of CXCR4 (Fig. 2B). Previously, we have shown that PTEN regulates CXCR4 signaling, and an absence of PTEN permits CXCR4-mediated functions [9]. We wanted to evaluate the importance of H2O2-mediated inactivation of PTEN, and up-regulation of CXCR4. We analyzed whether CXCR4-mediated functions were enhanced in the presence of H2O2. We evaluated DU145 cells for migration towards a combination of ligands and inhibitors (0.25mM H2O2, 10mM NAC and 10mM AMD3100) in the lower chamber. By transwell assay, we observed a significant increase in cells migrating towards H2O2 compared to control (Fig. 3 A), which was not observed with NAC or AMD3100 (CXCR4 antagonist) in the lower chamber. Moreover, H2O2 failed to induce migration in the simultaneous presence of NAC (Fig. 3 A). Finally, using a CXCR4 antagonist, we confirmed that ROS-mediated migration was through CXCR4. Likewise, ROS induced invasion through matrigel, which was inhibited by NAC and AMD3100 (Fig. 3 B).
Figure 2. ROS induced expression of CXCR4.
Fourty μg of total protein were analyzed for CXCR4 expression by western blot analysis using a specific antibody. Cells were treated with various concentrations of H2O2 and 10 mM NAC. Tubulin served as a loading control. *, P < 0.05.
Figure 3. ROS enhanced migration and invasion in a CXCR4-dependent manner.
Migration assays: 4×104 cells each were seeded into the upper transwell chamber and allowed to migrate towards various treatments in the lower chanber for 6 hours at 37°C, 5% CO2. Five fields of each transwell insert were randomly selected and counted for migrated cells at 10× magnification using a Zeiss Axiovert 200M light microscope and graphed. (A) A graphical representation of total migrated cells. Experiments were repeated thrice, and data represents the averages of 3 independent experiments. Invasion assays: 8×104 cells each were seeded into the upper transwell chamber layered with matrigel and allowed to invade towards the indicated treatments in the lower chamber for 24 hours at 37°C, 5% CO2. Five fields of each transwell insert were randomly selected and counted for invaded cells at 10× magnification using a Zeiss Axiovert 200M light microscope. (B) A graphical representation of total invaded cells. Experiments were repeated thrice, and data represents the averages of 3 independent experiments. *, P < 0.05.
Discussion
A large body of evidence suggests that the tumor microenvironment influences tumor development and progression. The gradual accumulation of reactive oxygen species (ROS) is commonly involved in promoting tumorigenesis through dysregulation of various anti-cancer and pro-cancer pathways [7]. Leslie et al. observed that ROS inactivated PTEN catalytic function, which failed to inhibit cell survival signaling pathways and correlated with cancer progression toward a metastatic phenotype [22]. In a non-cancer model, ROS induced expression of CXCR4, which has been shown to influence carcinogenesis and play a critical role in directing cancer metastasis, including prostate [16]. Previously, we observed that reconstitution of PTEN in PTEN-null prostate cancer cells inhibited CXCR4-mediated functions, suggesting that PTEN can serve as a regulatory switch in CXCR4-mediated events [9]. Loss of PTEN is commonly due to point mutations, genetic deletions and epigenetic silencing; however, the effects of the tumor microenvironment on PTEN expression and subsequent CXCR4 activation have yet to be explored [23]. Herein, we describe that ROS may negatively affect PTEN activity, and conversely, induce the expression of CXCR4 and survival signaling pathways.
Functionally, PTEN is involved in regulating a variety of cellular processes, including cell cycle, apoptosis, DNA repair, signal transduction and cell adhesion. These functions are mediated by the N-terminus phosphatase domain, which has dual-specificity activity that dephosphorylates protein and phosphoinositide substrates [24]. The lipid phosphatase activity of PTEN dephosphorylates PIP3, a product of PI3K, to form PIP2, thus negatively regulating the activation of AKT [13]. Consequently, loss of PTEN results in the accumulation of PIP3, and subsequently, constitutive activation of the PI3K/AKT signaling pathway [25]. Nearly, 60% of advanced prostate cancers exhibit a loss of PTEN, suggesting that the decreased expression of PTEN plays a crucial role in prostate cancer development and progression [10]. Previously, we have shown that PTEN expression negatively regulated CXCR4-mediated migration and proliferation in advance prostate cancer cells, indicating that loss of PTEN functioned as a permissive switch to promote CXCR4-mediated functions (Fig. 2) [9]. Furthermore, knock-down of PTEN in Du145 cells enhanced CXCR4-mediated migration, compared to control, thus indicated that PTEN served as a control for CXCR4 functions (Fig. 3). The regulation of CXCR4 by PTEN is indirect, as PTEN and CXCR4 converge at the signaling pathways that they both regulate, PI3K/AKT and ERK1/2 [9].
We were interested in investigating mechanisms by which PTEN is loss in advanced prostate cancer. ROS has been linked to cell survival and proliferation through the activation of ERK1/2 and PI3K/AKT pathways [26]. Therefore, we investigated the effects of ROS (H2O2) on the phosphorylation of AKT. We observed a gradual increase in phospho-AKT expression, but aberrant expression of phospho-ERK1/2, in a concentration dependent manner. Furthermore, ROS is associated with PTEN inactivation; consequently, PTEN inactivation permits downstream survival pathways, such as PI3K/AKT to support cancer progression [27]. DU145 cells express a functional PTEN; therefore, we investigated whether up-regulation of phospho-AKT was due to PTEN catalytic inactivation, mediated by H2O2. There was no significant change observed in the expression levels of PTEN upon treatment with H2O2, with a slight decrease at 0.5mM. The catalytic domain of PTEN can form a disulfide bond between Cys124 and Cys71 in the active site [18,28]. Lee et al. described that that H2O2 induced disulfide bonding within the active site of PTEN, rendering it inactive [18]. We, too, observed an increase in this inactive form of PTEN, suggesting that ROS-mediated upregulation in signaling was not due to diminished levels of PTEN. Instead, ROS abrogated PTEN regulation through catalytic inactivation, thus allowing increased expression of the activated survival kinase, phospho-AKT.
Prostate cancer mortality is often a result of metastasis to secondary organs. Taichman et al. initially observed that CXCR4 facilitated prostate cancer metastasis to the bone, the primary site of distal prostate cancer colonization [29]. SDF1α was constitutively expressed in the bone marrow by osetoblasts, fibroblasts, and endothelial cells, which directed cell migration, by attracting prostate cancer cells that expressed CXCR4 on the surface. CXCR4 is involved in cell survival through activation of requisite pathways, which signals to downstream targets for metastasis and anti-apoptosis [15]. In cancer and stem cells models, CXCR4 and SDF1α are up-regulated in hypoxic and high ROS environments [17]. We observed that H2O2 increased expression of CXCR4, which was inhibited by a ROS scavenger. Likewise, DU145 cell migration and invasion were enhanced in the presence H2O2, in a CXCR4-dependent manner.
Oxidative stress is higher in the epithelium of prostate cancer patients compared to normal men, suggesting that ROS plays a critical role in prostate cancer development and progression, which has not been fully elucidated [4]. ROS is classically known to induce genomic alterations (point mutations or deletions), inhibit tumor suppressor genes and/or activate oncogenes [30]. As we observed with PTEN, ROS inhibited the tumor suppressor, protein tyrosine phosphatase (PTP) 1B, by inducing disulphide bonding in the active site [19]. Likewise, Cao et al. suggested that ROS directly regulated AKT activation in human leukemia cells [31]. We also observed that ROS induced expression of CXCR4 and enhanced subsequent functions, independent of SDF1α. We have not explored, however, whether increased expression of phospho-AKT was a direct result of ROS, or a result of increased CXCR4, as AKT is a downstream target [15]. Thus, upregulation of phospho-AKT and CXCR4 by ROS may coordinate ensuing metastatic events for cancer survival.
Our results contribute to the decipherment of molecular mechanisms that exist between the tumor microenvironment, and the imbalance of tumor suppressor and oncogenic functions. Our studies add to the body of knowledge on the role of PTEN in CXCR4-mediated cancer progression, and hopefully, will contribute to the development of therapies that target the tumor microenvironment, which have great potential for the better management of metastatic prostate cancer.
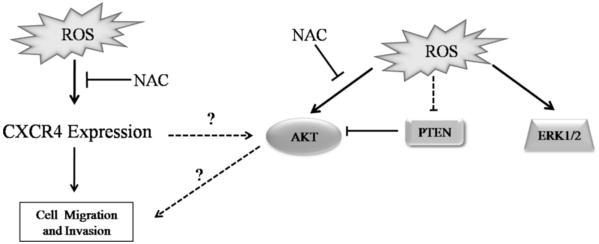
Figure 4. Proposed mechanism for ROS-mediated regulation of PTEN and CXCR4.
ROS mediated disulfide bonding within the active site of PTEN, rendering it inactive, and increased expression of CXCR4 and phosphorylated AKT.
Acknowledgments
Research in this laboratory is supported, in part, by National Institutes of Health grants RCMI22622A (MAC), 2G12RR003062-22(CVH) and 1 P20 MD002285 (CVH).
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Liu J, Zhang Y, Zhao J, Yang Z, Li D, Katirai F, Huang B. Mast cell: insight into remodeling a tumor microenvironment. Cancer Metastasis Rev. 2011 doi: 10.1007/s10555-011-9276-1. [DOI] [PubMed] [Google Scholar]
- 3.Cook JA, Gius D, Wink DA, Krishna MC, Russo A, Mitchell JB. Oxidative stress, redox, and the tumor microenvironment. Semin Radiat Oncol. 2004;14:259–266. doi: 10.1016/j.semradonc.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Khandrika L, Kumar B, Koul S, Maroni P, Koul HK. Oxidative stress in prostate cancer. Cancer Lett. 2009;282:125–136. doi: 10.1016/j.canlet.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finger EC, Giaccia AJ. Hypoxia, inflammation, and the tumor microenvironment in metastatic disease. Cancer Metastasis Rev. 2010;29:285–293. doi: 10.1007/s10555-010-9224-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Autréaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 7.Kumar B, Koul S, Khandrika L, Meacham R, Koul H. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 2008;68:1777–1785. doi: 10.1158/0008-5472.CAN-07-5259. [DOI] [PubMed] [Google Scholar]
- 8.Gao N, Shen L, Zhang Z, Leonard SS, He H, Zhang XG, Shi X, Jiang BH. Arsenite induces HIF-1alpha and VEGF through PI3K, Akt and reactive oxygen species in DU145 human prostate carcinoma cells. Mol Cell Biochem. 2004;255:33–45. doi: 10.1023/b:mcbi.0000007259.65742.16. [DOI] [PubMed] [Google Scholar]
- 9.Chetram MA, Odero-Marah V, Hinton CV. Loss of PTEN Permits CXCR4-Mediated Tumorigenesis through ERK1/2 in Prostate Cancer Cells. Mol Cancer Res. 2011;9:90–102. doi: 10.1158/1541-7786.MCR-10-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, Ittmann M, Tycko B, Hibshoosh H, Wigler MH, Parsons R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 11.Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T, Frye C, Hu R, Swedlund B, Teng DH, Tavtigian SV. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- 12.Rasheed BK, Stenzel TT, McLendon RE, Parsons R, Friedman AH, Friedman HS, Bigner DD, Bigner SH. PTEN gene mutations are seen in high-grade but not in low-grade gliomas. Cancer Res. 1997;57:4187–4190. [PubMed] [Google Scholar]
- 13.Wang X, Jiang X. PTEN: a default gate-keeping tumor suppressor with a versatile tail. Cell Res. 2008;18:807–816. doi: 10.1038/cr.2008.83. [DOI] [PubMed] [Google Scholar]
- 14.Kucia M, Jankowski K, Reca R, Wysoczynski M, Bandura L, Allendorf D, Zhang J, Ratajczak J, Ratajczak M. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol Histol. 2004;35:233–245. doi: 10.1023/b:hijo.0000032355.66152.b8. [DOI] [PubMed] [Google Scholar]
- 15.Busillo JM, Benovic JL. Regulation of CXCR4 signaling. Biochim Biophys Acta. 2007;1768:952–963. doi: 10.1016/j.bbamem.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akashi T, Koizumi K, Tsuneyama K, Saiki I, Takano Y, Fuse H. Chemokine receptor CXCR4 expression and prognosis in patients with metastatic prostate cancer. Cancer Sci. 2008;99:539–542. doi: 10.1111/j.1349-7006.2007.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li S, Deng Y, Feng J, Ye W. Oxidative preconditioning promotes bone marrow mesenchymal stem cells migration and prevents apoptosis. Cell Biol Int. 2009;33:411–418. doi: 10.1016/j.cellbi.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 18.Lee S, Yang K, Kwon J, Lee C, Jeong W, Rhee S. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 2002;277:20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- 19.Barrett WC, DeGnore JP, Keng YF, Zhang ZY, Yim MB, Chock PB. Roles of superoxide radical anion in signal transduction mediated by reversible regulation of protein-tyrosine phosphatase 1B. J Biol Chem. 1999;274:34543–34546. doi: 10.1074/jbc.274.49.34543. [DOI] [PubMed] [Google Scholar]
- 20.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 21.Ha HL, Yu DY. HBx-induced reactive oxygen species activates hepatocellular carcinogenesis via dysregulation of PTEN/Akt pathway. World J Gastroenterol. 2010;16:4932–4937. doi: 10.3748/wjg.v16.i39.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leslie N, Bennett D, Lindsay Y, Stewart H, Gray A, Downes C. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J. 2003;22:5501–5510. doi: 10.1093/emboj/cdg513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu J, Zhang SS, Saito K, Williams S, Arimura Y, Ma Y, Ke Y, Baron V, Mercola D, Feng GS, Adamson E, Mustelin T. PTEN regulation by Akt-EGR1-ARF-PTEN axis. EMBO J. 2009;28:21–33. doi: 10.1038/emboj.2008.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JO, Yang H, Georgescu MM, Di Cristofano A, Maehama T, Shi Y, Dixon JE, Pandolfi P, Pavletich NP. Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell. 1999;99:323–334. doi: 10.1016/s0092-8674(00)81663-3. [DOI] [PubMed] [Google Scholar]
- 25.Wu Z, Cho H, Hampton GM, Theodorescu D. Cdc6 and cyclin E2 are PTEN-regulated genes associated with human prostate cancer metastasis. Neoplasia. 2009;11:66–76. doi: 10.1593/neo.81048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fruehauf JP, Meyskens FL. Reactive oxygen species: a breath of life or death? Clin Cancer Res. 2007;13:789–794. doi: 10.1158/1078-0432.CCR-06-2082. [DOI] [PubMed] [Google Scholar]
- 27.Hernandez-Aya LF, Gonzalez-Angulo AM. Targeting the phosphatidylinositol 3-kinase signaling pathway in breast cancer. Oncologist. 2011;16:404–414. doi: 10.1634/theoncologist.2010-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maehama T, Taylor GS, Dixon JE. PTEN and myotubularin: novel phosphoinositide phosphatases. Annu Rev Biochem. 2001;70:247–279. doi: 10.1146/annurev.biochem.70.1.247. [DOI] [PubMed] [Google Scholar]
- 29.Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002;62:1832–1837. [PubMed] [Google Scholar]
- 30.Oliveira AM, Ross JS, Fletcher JA. Tumor suppressor genes in breast cancer: the gatekeepers and the caretakers. Am J Clin Pathol. 2005;124 Suppl:S16–28. doi: 10.1309/5XW3L8LU445QWGQR. [DOI] [PubMed] [Google Scholar]
- 31.Cao J, Xu D, Wang D, Wu R, Zhang L, Zhu H, He Q, Yang B. ROS-driven Akt dephosphorylation at Ser-473 is involved in 4-HPR-mediated apoptosis in NB4 cells. Free Radic Biol Med. 2009;47:536–547. doi: 10.1016/j.freeradbiomed.2009.05.024. [DOI] [PubMed] [Google Scholar]