Abstract
Endothelial cell (EC) barrier dysfunction results in increased vascular permeability, leading to increased mass transport across the vessel wall and leukocyte extravasation, the key mechanisms in pathogenesis of tissue inflammation and edema. We have previously demonstrated that OxPAPC (oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine) significantly enhances vascular endothelial barrier properties in vitro and in vivo and attenuates endothelial hyperpermeability induced by inflammatory and edemagenic agents via Rac and Cdc42 GTPase dependent mechanisms. These findings suggested potential important therapeutic value of barrier-protective oxidized phospholipids. In this study, we examined involvement of signaling complexes associated with caveolin-enriched microdomains (CEMs) in barrier-protective responses of human pulmonary ECs to OxPAPC. Immunoblotting from OxPAPC-treated ECs revealed OxPAPC-mediated rapid recruitment (5 minutes) to CEMs of the sphingosine 1-phosphate receptor (S1P1), the serine/threonine kinase Akt, and the Rac1 guanine nucleotide exchange factor Tiam1 and phosphorylation of caveolin-1, indicative of signaling activation in CEMs. Abolishing CEM formation (methyl-β-cyclodextrin) blocked OxPAPC-mediated Rac1 activation, cytoskeletal reorganization, and EC barrier enhancement. Silencing (small interfering RNA) Akt expression blocked OxPAPC-mediated S1P1 activation (threonine phosphorylation), whereas silencing S1P1 receptor expression blocked OxPAPC-mediated Tiam1 recruitment to CEMs, Rac1 activation, and EC barrier enhancement. To confirm our in vitro results in an in vivo murine model of acute lung injury with pulmonary vascular hyperpermeability, we observed that selective lung silencing of caveolin-1 or S1P1 receptor expression blocked OxPAPC-mediated protection from ventilator-induced lung injury. Taken together, these results suggest Akt-dependent transactivation of S1P1 within CEMs is important for OxPAPC-mediated cortical actin rearrangement and EC barrier protection.
Keywords: OxPAPC, Akt, S1P receptor, caveolin-enriched microdomain, endothelial barrier enhancement
Endothelial cells (ECs) provide a semiselective barrier between the blood and underlying tissue interstitium with barrier disruption resulting in increased vascular permeability and organ dysfunction.1,2 Therefore, agents that enhance EC barrier function are a desirable therapeutic strategy for a variety of inflammatory diseases, tumor angiogenesis and atherosclerosis.2,3 We have recently described that oxidized phospholipids (OxPLs) derived from OxPAPC (oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine) exhibited potent barrier-protective effects toward human pulmonary endothelial monolayers.4,5 However, the underlying signaling mechanisms by which OxPAPC increases vascular integrity remains poorly understood.
In endothelial cells, as in many other cell types, there exist specialized sterol- and sphingolipid-enriched domains, called lipid rafts, that have been implicated in OxPAPC signaling.6–9 In addition, there exists a subset of lipid rafts which are 50- to 100-nm plasma membrane microdomains containing a specific scaffolding protein called caveolin-1.10–12 We have previously shown that these caveolin-enriched microdomains (CEMs) regulate receptor interacts with the underlying actin cytoskeleton and EC barrier function.13–15 Furthermore, we have demonstrated that CEMs are critical for Rac1 activation of barrier enhancing stimuli including hyaluronan and hepatocyte growth factor.13,15 However, the role of CEMs in OxPAPC-mediated EC barrier regulation is incompletely defined.
The sphingosine-1-phosphate (S1P) receptor S1P1 resides in CEMs and is critically involved in EC barrier enhancement.13,14,16 Our published data indicate that certain EC barrier enhancing stimuli including hyaluronan13 induce S1P1 transactivation (threonine phosphorylation), which regulates Rac1 activation (Rac1-GTP formation) and rearrangement of the cortical actin cytoskeleton. We examined the role(s) of S1P1 receptor transactivation, CEMs, and Akt on OxPAPC-mediated cytoskeletal regulation and EC barrier enhancement in this study.
Materials and Methods
An expanded Materials and Methods section is available in the online data supplement at http://circres.ahajournals.org.
Cell Culture and Reagents
Human pulmonary artery ECs were obtained from Clonetics (Walkersville, Md) and cultured as previously described.16 Unless otherwise specified, reagents were obtained from Sigma (St Louis, Mo).
Lipid Oxidation and Analysis
PAPC (nonoxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine) was obtained from Avanti Polar Lipids (Alabaster, Ala) and oxidized and analyzed as previously described.17
CEM Isolation
CEMs were isolated from human lung ECs using Triton X-100 insolubility and centrifugation, as we have described previously.13–15
Measurement of EC Electric Resistance
ECs were grown to confluence in polycarbonate wells containing evaporated gold microelectrodes, and transendothelial cell electric resistance measurements performed using an electric cell-substrate impedance sensing system obtained from Applied Biophysics (Troy, NY), as described previously in detail.16
Delivery of Small Interfering RNA in Mice
Adult male C57BL/6J mice, 8 to 10 weeks old, with an average weight of 20 to 25 g (The Jackson Laboratory, Bar Harbor, Me) were bred at the University of Chicago animal care center. All experimental protocols involving the use of animals were approved by the University of Chicago Institutional Animal Care & Use Committee for the humane treatment of experimental animals. Small interfering (si)RNAs from Dharmacon (Lafayette, Colo) had the following sequences: siCaveolin1: 5′-ACGUAGACUCCGAGGGACA-3′; siS1P1 receptor: 5′-CUUGCUAACUAUUUGGAAA-3′; control siRNA (Luciferase): 5′-UAAGGCUAUGAAGAGAUA-3′. Polyethylenimine-22, which provides preferential RNA targeting to the lung,18 was used as a carrier in the in vivo experiments with siRNA-induced caveolin and S1P1 receptor knockdown in vivo. Obtained polyethylenimine-22–siRNA polyplexes (400 μL) were injected into the jugular vein of the 8- to 10-week-old C57BL/6 male mice under anesthesia. After 72 hours, the mice were subjected to mechanical ventilation or Evan’s blue dye and euthanized; lungs, livers, and hearts were collected and homogenized as previously described.19
Results
CEMs Regulate OxPAPC-Induced Human EC Barrier-Enhancing Events
OxPLs derived from OxPAPC promote a variety of important EC biological functions including vascular barrier enhancement.4,5 We explored the role of CEMs on OxPAPC-induced signaling and observed that OxPAPC induces tyrosine phosphorylation of the CEM scaffolding protein caveolin-1 (Figure 1A). Abolishing lipid raft formation with methyl-β-cyclodextrin (MβCD) (a cholesterol-depletion agent) inhibited several OxPAPC-mediated effects including EC barrier function (Figure 1B), Rac1 activation (Figure 1C), phosphorylation of caveolin-1 and the downstream Rac1 effector, PAK1 (Figure 1D), and actin cytoskeletal rearrangements (Figure 1E). These data demonstrate a crucial involvement of CEMs in OxPAPC-mediated signaling and EC barrier function.
Figure 1.
CEMs regulate OxPAPC-mediated human EC barrier enhancement. A, ECs were grown to confluence, serum-starved for 1 hour, and either left untreated (control) or treated with 20 μg/mL OxPAPC for 5, 15, 30, 45, or 60 minutes. Cellular material was analyzed using immunoblotting with anti–phospho-tyrosine–caveolin-1 (a) or anti–caveolin-1 (b) antibody. B, ECs were plated on gold microelectrodes, serum-starved for 1 hour, and treated with either PBS (pH 7.4) (control) or 5 mmol/L MβCD (a cholesterol-depletion agent that abolishes CEM formation) 30 minutes before PBS (pH 7.4) or 20 μg/mL OxPAPC addition. The arrows indicate the times of MβCD and OxPAPC addition. C, ECs were grown to confluence, serum-starved for 1 hour, and either left untreated (control) or treated with 5 mmol/L MβCD 30 minutes before PBS (pH 7.4) or 20 μg/mL OxPAPC addition (5, 15, or 30 minutes). ECs were then solubilized and incubated with p21-binding domain (PBD)-conjugated beads to bind activated (GTP-bound form) Rac1. The PBD bead-associated material was analyzed using immunoblotting with anti-Rac1 antibody. D, ECs were grown to confluence, serum-starved for 1 hour, and either left untreated (control) or treated with 5 mmol/L MβCD 30 minutes before PBS (pH 7.4) or 20 μg/mL OxPAPC addition (5, 15, or 30 minutes). ECs were then solubilized and analyzed using immunoblotting with anti–phospho-tyrosine–caveolin-1 (a), anti–phospho-PAK1 (b), or anti–phospho-tubulin antibody (c). E, ECs were grown to confluence on glass coverslips, serum-starved for 1 hour, and either left untreated (control) or treated with 5 mmol/L MβCD 30 minutes before PBS (pH 7.4) or 20 μg/mL OxPAPC addition (15 minutes). Cells were then fixed and stained with TRITC-phalloidin (to visualize F-actin) and analyzed using fluorescent microscopy.
OxPAPC Induces S1P1 Receptor Transactivation
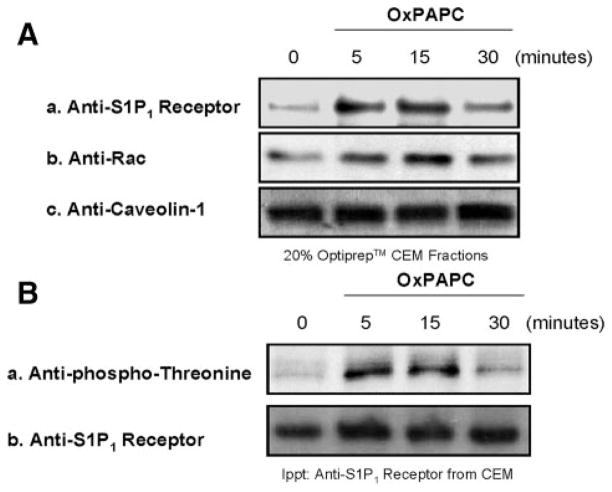
Activation of S1P1, which resides in CEMs, regulates EC barrier enhancement.13 Therefore, we examined whether OxPAPC is able to transactivate the S1P1 receptor within CEM structures. Isolation of CEMs from control and OxPAPC-treated human ECs followed by immunoblot analysis revealed that OxPAPC induces recruitment of S1P1 receptor and Rac1 to CEMs and OxPAPC-induced S1P1 receptor transactivation (threonine phosphorylation) within CEMs (Figure 2A and 2B).
Figure 2.
OxPAPC induces transactivation of the S1P1 receptor in human EC CEMs. A, ECs were grown to confluence, serum-starved for 1 hour, and either left untreated (control) or challenged with 20 μg/mL OxPAPC addition (5, 15, or 30 minutes). CEM fractions (20% Optiprep layer) were isolated, solubilized in immunoprecipitation buffer, and analyzed using immunoblotting with S1P1 receptor (a), anti-Rac1 (b), or anticaveolin-1 (c) anti-body. B, ECs were grown to confluence, serum-starved for 1 hour, and either left untreated (control) or challenged with 20 μg/mL OxPAPC addition (5, 15, or 30 minutes). CEM fractions were isolated, solubilized, and immunoprecipitated with anti-S1P1 receptor antibody. The immunoprecipitated material was analyzed using immunoblotting with anti–phospho-threonine (a) or anti-S1P1 receptor (b) antibody.
We and other have reported that the serine/threonine kinase Akt1 mediates S1P1 receptor transactivation in human ECs.13,20 OxPAPC challenge of human ECs induces recruitment of tyrosine phosphorylated Akt to CEMs, which is abolished with the Src family tyrosine kinase inhibitor protein phosphatase (PP)2 (Figure 3A). Silencing Src family members present in human pulmonary ECs21 revealed that Src and Fyn are responsible for OxPAPC-mediated Akt tyrosine phosphorylation (Figure 3B). In addition, Akt1 silencing (siRNA) in human ECs indicate that Akt1 expression is required for OxPAPC-mediated S1P1 receptor threonine phosphorylation (Figure 3C and 3D).
Figure 3.
Akt is required for OxPAPC-mediated S1P1 receptor transactivation. A, ECs were grown to confluence, serum-starved for 1 hour, and either left untreated (control) or treated with PP2 (250 nmol/L) 1 hour before addition of 20 μg/mL OxPAPC for 5 minutes. CEM fractions (20% Optiprep layer) were then isolated and analyzed using immunoblotting with anti–phospho-tyrosine (Y326) Akt (a) or anti-Akt (b) antibody. B, Graphic representation of the ratio of tyrosine (Y326) phosphorylated Akt to total Akt in EC lysates treated with 20 μg/mL OxPAPC (5 minutes) with or without scrambled siRNA, PP2, Src siRNA, Fyn siRNA, Yes siRNA, Lyn siRNA, Blk siRNA, or Src+Fyn siRNA. C, Cellular lysates from nontransfected (control, no siRNA), scrambled siRNA, or Akt1 siRNA13 transfection were analyzed using immunoblotting with anti-Akt1 (a) or anti-actin (b) antibody as described in Materials and Methods. D, ECs were either treated with scrambled siRNA or Akt1 siRNA, grown to confluence, serum-starved for 1 hour, and either left untreated (control) or treated with 20 μg/mL OxPAPC for 5 minutes. CEM fractions were then isolated, solubilized, and immunoprecipitated with anti-S1P1 receptor antibody. The immunoprecipitated material was analyzed using immunoblotting with anti–phospho-threonine (a) or anti-S1P1 receptor (b) antibody.
Akt1 and S1P1 Receptor Regulate OxPAPC-Mediated Rac1 Activation
As stated previously, OxPAPC-mediated EC barrier function is dependent on activation of the small G protein Rac1.4,5 Therefore, we examined whether Akt1 and/or the S1P1 receptor are upstream regulators of OxPAPC-mediated Rac1 activation. Silencing of Akt1 (Figure 3A) or the S1P1 receptor (Figure 4A) dramatically decreased OxPAPC-mediated activation of Rac1 (Rac1-GTP formation) in human ECs (Figure 4B).
Figure 4.
S1P1 receptor and Akt regulate OxPAPC-mediated Rac1 activation. A, Cellular lysates from nontransfected (control, no siRNA), scrambled siRNA, or S1P1 receptor siRNA13 transfection were analyzed using immunoblotting with anti-S1P1 receptor (a) or anti-actin (b) antibody. B, ECs were either treated with scrambled siRNA, S1P1 receptor siRNA, or Akt1 siRNA,13 grown to confluence, serum-starved for 1 hour, and either left untreated (control) or treated with 5 mmol/L MβCD before the addition of 20 μg/mL OxPAPC for 5 minutes. ECs were then solubilized and incubated with p21-binding domain (PBD)-conjugated beads to bind activated (GTP-bound form) Rac1. The PBD bead-associated material was analyzed using immunoblotting with anti-Rac1 antibody.
OxPAPC and S1P1 Receptor Regulate Akt Phosphorylation and EC Barrier Enhancement
Our results in Figures 3 and 4 indicate that Akt1 is tyrosine phosphorylated in CEMs and is required for OxPAPC-mediated S1P1 receptor transactivation and Rac1 activation. Akt is a serine/threonine kinase that is fully activated by serine, threonine, and tyrosine phosphorylation.22–24 Therefore, we examined the contributions of OxPAPC and the S1P1 receptor to the phosphorylation state of Akt. Interestingly, our results in Figure 5A indicate that OxPAPC-mediated tyrosine phosphorylation of Akt (by Src and Fyn) (Figure 3B) is S1P1 receptor-independent. However, serine and threonine phosphorylation of Akt is mediated by mTOR and the phosphatidylinositol (PI3)-kinase pathway and is S1P1 receptor–dependent (Figure 5B). Therefore, our results suggest that partial activation (tyrosine phosphorylation) of Akt appears to be sufficient for S1P1 receptor transactivation. However, full activation (serine, threonine, and tyrosine phosphorylation) of Akt appears to be required for Rac1 activation and EC barrier function (Figures 4 and 5). Measurements of EC barrier function in vitro (transendothelial cell electric resistance) revealed that silencing (siRNA) the expression of Akt1 (Figure 5C) or the S1P1 receptor (Figure 5D) reduced OxPAPC-induced EC barrier enhancement. In addition, silencing Src, Fyn, or mTOR or treatment with S1P1 receptor or PI3-kinase inhibitors attenuated OxPAPC-mediated EC barrier enhancement (Figure 5E).
Figure 5.

OxPAPC and S1P1 receptor regulate Akt phosphorylation and human EC barrier enhancement. A, ECs were either treated with scrambled siRNA or S1P1 receptor siRNA,7 grown to confluence, serum-starved for 1 hour, and either left untreated (control) or treated with 20 μg/mL OxPAPC for 5 minutes. ECs were then solubilized and analyzed using immunoblotting with anti–phospho-tyrosine (Y326) Akt (a), anti–phospho-serine (S473) Akt (b), anti–phospho-threonine (T308) Akt (c), or anti-Akt (d) antibody. B, Graphic representation of the ratio of tyrosine (Y326) phosphorylated Akt, serine phosphorylated (S473), or threonine phosphorylated (T308) to total Akt in EC lysates treated with 20 μg/mL OxPAPC (5 minutes) with or without scrambled siRNA, S1P1 receptor siRNA 1,13 S1P1 receptor siRNA 2 (Santa Cruz Biotechnology), S1P1 receptor antagonist W146 (250 nmol/L, 1 hour), mTOR siRNA, or PI3-kinase inhibitor LY294002 (10 μmol/L, 1 hour). C, ECs were plated on gold microelectrodes, treated with scrambled siRNA, or Akt1 siRNA,13 grown to confluence, serum-starved for 1 hour, and either left untreated (control) or treated with 20 μg/mL OxPAPC. The arrow indicates the time of OxPAPC addition. D, ECs were plated on gold microelectrodes, treated with scrambled siRNA, or S1P1 receptor siRNA,13 grown to confluence, serum-starved for 1 hour, and either left untreated (control) or treated with 20 μg/mL OxPAPC. The arrow indicates the time of OxPAPC addition. E, Graphic representation of the percentage maximal OxPAPC-induced transendothelial cell electric resistance (y axis) using siRNA and inhibitors (x axis) as we have described in Figure 3B and Figure 5B through 5D.
Caveolin-1 and S1P1 Receptor Regulate OxPAPC-Mediated Protection From Ventilator-Induced Lung Injury
We used a ventilator-induced lung injury (VILI) model to test the role of CEMs and the S1P1 receptor on OxPAPC-mediated protection from vascular hyperpermeability associated with acute lung injury (ALI) in vivo. Our results in Figure 6 indicate that selective lung silencing of caveolin-1 expression (intravenous administration of caveolin-1 siRNA/polyethylenimine-22 polyplexes18; Figure 6A) blocked OxPAPC-mediated protection from VILI as measured by Evans blue dye leakage (Figure 6B) and bronchoalveolar lavage (BAL) total cell count and protein content (Figure 6C). In addition, using the same procedures as above with S1P1 receptor siRNA indicate that selective pulmonary silencing of the S1P1 receptor blocked OxPAPC-mediated protection from VILI, as measured by BAL total cell count (Figure 7A) and BAL protein content (Figure 7B). These findings indicate the importance of the S1P1 receptor and CEMs in OxPAPC-mediated protection of pulmonary vascular integrity.
Figure 6.
Depletion of pulmonary caveolin-1 expression impairs the protective effects of OxPAPC on VILI. A, Polyethylenimine-mediated lung specific transfection18 and depletion of caveolin-1 in dose-dependent manner. Mice were transfected with nonspecific RNA (nsRNA) or caveolin-1 siRNA at dose of 4, 6, or 10 mg/kg. Depletion of caveolin in different organs (lung, liver, and heart) was verified by Western blot 72 hours after transfection. B, Effects of caveolin-1 depletion on the attenuation of lung vascular leak by OxPAPC in response to HTV. After 4 hours of ventilation, Evans blue dye (30 mL/kg) was injected into the external jugular vein 2 hours before termination of ventilation to assess vascular leak. Lungs were harvested and imaged against white background. Spectrophotometry measurement of extravagated Evans blue was performed as described in Materials and Methods. Insets depict the quantitative measurement of Evans blue–labeled albumin extravasation in the shown lung preparation. Evans blue accumulation in the lungs from small nuclear RNA VILI animals was taken as 100% (n=4 per condition). C, Depletion of caveolin-1 impairs protective effects of OxPAPC on VILI. HTV (30 mL/kg, 4 hours) induced a dramatic increase in BAL total cell count and protein content, which was significantly attenuated by intravenous injection of 1.5 mg/kg OxPAPC in control mice transfected with nonspecific RNA knockdown of caveolin abolished the protective effects of OxPAPC. *P<0.05 ND-no difference, n=4 per group.
Figure 7.
Inhibiting pulmonary S1P1 receptor expression attenuates the protective effects of OxPAPC on VILI. A, Polyethylenimine-mediated lung specific transfection18 and depletion of S1P1 receptor impairs the protective effects of OxPAPC on VILI. HTV (30 mL/kg, 4 hours) induced a dramatic increase in BAL total cell count and protein content, which was significantly attenuated by intravenous injection of 1.5 mg/kg OxPAPC in control mice transfected with nonspecific RNA. Knockdown of caveolin abolished the protective effects of OxPAPC. NS indicates no significant difference (n=5 per group). B, Depletion of S1P1 receptor impairs protective effects of OxPAPC on VILI. HTV (30 mL/kg, 4 hours) induced a dramatic increase in BAL total cell count and protein content, which was significantly attenuated by intravenous injection of 1.5 mg/kg OxPAPC in control mice transfected with nonspecific RNA. Knockdown of caveolin abolished the protective effects of OxPAPC. NS indicates no significant difference (n=6 per group). C, Mice were transfected with nonspecific RNA (nsRNA) or S1P1 receptor siRNA at a dose of 10 mg/kg. Depletion of S1P1 receptor expression in lung was verified by immunoblot 72 hours after transfection.
Discussion
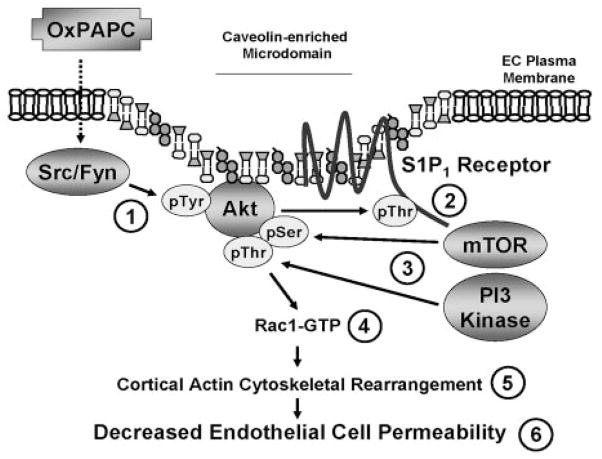
Agents that exhibit the capacity to reverse increases in vascular permeability, a prominent feature in diverse inflammatory syndromes, tumor angiogenesis, and atherosclerosis, have obvious therapeutic applications.25,26 OxPAPC decreases EC permeability both in vitro and in vivo.5,27 As the upstream mechanisms of OxPAPC-mediated GTPase regulation and endothelial barrier protection remain poorly understood, we examined the role of CEMs and S1P1 receptor transactivation in OxPAPC-mediated signaling and human EC barrier regulation. Our novel results indicate that Ox-PAPC induces partial activation (Src- and Fyn-dependent tyrosine phosphorylation) of Akt, resulting in Akt-mediated S1P1 receptor transactivation (threonine phosphorylation) in CEMs. Activated S1P1 receptor induces full activation (mTOR and PI3-kinase--dependent serine and threonine phosphorylation) of Akt required for Rac1 activation, cortical actin cytoskeletal rearrangement, and consequent OxPAPC-mediated EC barrier enhancement (Figure 8).
Figure 8.
Proposed model of OxPAPC-induced human EC barrier enhancement. OxPAPC induces partial activation (Src- and Fyn-dependent tyrosine phosphorylation) of Akt,1 resulting in Akt-mediated S1P1 receptor transactivation (threonine phosphorylation) in CEMs.2 Activated S1P1 receptor can induce full activation (mTOR- and PI3-kinase–dependent serine and threonine phosphorylation) of Akt3 required for Rac1 activation,4 cortical actin cytoskeletal rearrangement,5 and consequent OxPAPC-mediated EC barrier enhancement.6
CEMs, a subset of lipid rafts containing caveolin-1, have been implicated in EC migration, proliferation, adhesion, endocytosis, cholesterol, and calcium regulation and signal transduction.11,12,28 Deletion of caveolin-1 expression in mice inhibits CEM (caveolae) formation in ECs and promotes lung fibrosis and microvascular hyperpermeability.29 We observed that OxPAPC requires the existence of CEM fractions for Akt-mediated S1P1 receptor transactivation, Rac1 signaling, and EC barrier enhancement. Targeted use of siRNA to differentially reduce expression of either S1P1 or Akt1 revealed that OxPAPC transactivation of S1P1 is responsible for subsequent signaling to the EC cytoskeleton and barrier enhancement.
Our data indicate that Akt activation is required for OxPAPC-mediated EC barrier function. Activation of Akt1 can occur through threonine phosphorylation (T308) in the catalytic domain by PI3-kinase–dependent PDK-1 and by serine phosphorylation (S473) in the hydrophobic motif by various kinases including mTOR.30–33 In addition, Akt can be activated by tyrosine phosphorylation by Src family kinases,34 an event we observe with OxPAPC treatment of human ECs. Activated Akt can directly phosphorylate threonine residues within the S1P1 receptor (T236),20 which promotes S1P-mediated EC Tiam1/Rac1 activation and cortical actin reorganization and migration. Our data indicate that activated S1P1 receptor is required for OxPAPC-mediated serine and threonine phosphorylation of Akt1. Because full activation of Akt occurs with serine, threonine, and tyrosine phosphorylation,30–34 our data indicate that Src and Fyn are responsible for OxPAPC-mediated Akt tyrosine phosphorylation, whereas S1P1 receptor– dependent activation of mTOR and PI3-kinase pathways promote Akt serine/threonine phosphorylation.
Using pulmonary targeting of caveolin-1 and S1P1 receptor siRNA, our data indicate that OxPAPC protection from VILI is dependent on caveolin-1 and S1P1 expression. Furthermore, our intravenous administration of OxPAPC and intravenous pulmonary targeting of caveolin-1 siRNA suggest OxPAPC-induced protection from VILI act via a preferential endothelial, rather than epithelial, mechanism.18,35,36
OxPLs play a dual role in vascular inflammation.4 In hyperlipidemic states, OxPLs contained in minimally modified LDL activate monocyte adhesion and transmigration through EC monolayer, stimulate production of chemotactic and inflammatory mediators, promote foam cell formation, and lead to progression of atherosclerotic vascular inflammation.37,38 In turn, various models of acute sepsis or inflammation (lipopolysaccharide [LPS]-induced lung injury, CpG, VILI, necrotizing pancreatitis) show potent antiinflammatory effects of transient OxPAPC elevation via blocking of signaling by nuclear factor κB and stress kinases, and direct barrier-protective effects on vascular endothelium.27,39–41 These reports show that antiinflammatory effects of OxPLs (OxPAPC or OxPAPS) administered at specific doses intravenously, subcutaneously, or intratracheally far exceed potential adverse proinflammatory effects.
Because OxPLs may possess beneficial or detrimental effects under different circumstances, these conditions need to be clearly defined. Protective effects of exogenously oxidized synthetic PAPC include: (1) inhibition of “sterile” ALI and sepsis induced by viral and bacterial derived Toll-like receptor (TLR)4/TLR9 ligands27,40; (2) inhibition of “aseptic” ALI induced by injurious mechanical ventilation27; (3) inhibition of stress signaling, inflammation, and tissue injury in the model of chemically induced acute necrotizing pancreatitis41; and (4) inhibition of dendritic cell maturation,42 which may prevent excessive immune reactions.
One protective mechanism of OxPAPC is via antagonistic interaction with the LPS coreceptors LPS-binding protein and CD14, leading to competitive blockage of the ability of LPS to bind its receptor, TLR4.43 Such inhibition blunts the nuclear factor κB–mediated inflammatory cascade. OxPAPC administration decreased inflammatory cell recruitment and even protected against LPS-mediated lethal shock.39
In agreement with our previous report using aseptic rat and murine models of VILI,27 the protective effects reported in this study do not appear to be mediated by an inhibition of LPS action. What are the mechanisms of OxPAPC protective effects in VILI model? One such mechanism involves direct protective effects of OxPAPC on vascular endothelial monolayers and Rac/Cdc42-dependent attenuation of barrier-disruptive Rho signaling.4,5 Indeed, OxPAPC attenuated Rho pathway of barrier disruption in pulmonary ECs subjected to thrombin and high-magnitude cyclic stretch in vitro, promoted Rac-dependent barrier recovery, and markedly reduced lung barrier dysfunction in mice exposed to high tidal volume mechanical ventilation and Rho activator TRAP (thrombin receptor activating peptide) in vivo.27 Similar protective effects were achieved by intravenous injection of Rho kinase inhibitor Y-27632.27 The results of the present study further support this model and strongly suggest a CEM/S1P1-mediated pathway of Rac activation underlying OxPAPC barrier protective effects in vitro and in vivo.
Another possible protective mechanism of OxPAPC in aseptic ALI models including VILI may be inhibition of TLR-mediated inflammatory signaling triggered by endogenous TLR ligands generated in the course of ALI.44 For example, murine model of bleomycin-induced ALI showed increased generation of low-molecular-mass hyaluronan, which engaged MyD88 and both TLR4 and TLR2 and initiated inflammatory responses in the lung.43
The following negative OxPAPC effects in acute sepsis and lung injury models were reported: (1) high doses of exogenous OxPAPC caused pulmonary endothelial barrier disruption4 and increased lung elastance as a parameter of rapid impairment of lung function44; (2) products of advanced endogenous phospholipid oxidation such as 4-hydroxy-2-nonenal, ozone-oxidized surfactant phospholipids stimulate inflammatory reactions, cause dysfunction of lung mechanical properties and lung endothelial permeability,45,46 and fragmented PAPC oxidation products induce endothelial barrier disruption in vitro.4,5; (3) OxPAPC suppressed bacterial phagocytosis and pinocytosis by peritoneal macrophages and neutrophils in the model of bacterial peritonitis, leading to propagation of bacteriemia.47
A study by Imai et al44 showed accumulation of oxidized phosphatidylcholine products in human and animal lungs infected with SARS (severe acute respiratory syndrome), anthrax, and H5N1 avian influenza virus and in a mouse model of acid-induced lung injury judged by increased immunoreactivity with monoclonal EO6 antibody. In vitro, BSA-conjugated PAPC oxidation products stimulated interleukin-6 production by alveolar macrophages in a TLR4/TRIF-dependent fashion.44
Apparently conflicting reports regarding a role of OxPL in acute inflammation and lung barrier function may be explained by differences in composition and concentrations of OxPLs used in different studies. Immunologic OxPL detection using EO6 antibody has certain limitations. EO6 specifically binds to OxPL containing the PC head group, such as POVPC, but not to native nonoxidized PLs. EO6 recognizes products of aldol condensation of PAPC oxidation products, such as P(POVPC)VPC, diLysoPC-C9, and di-OVPC.48 This antibody also reacts with Schiff bases forming covalent bonds between protein lysine residues and aldehyde groups of fragmented OxPLs such as POVPC-BSA.48 Therefore, EO6 does not discriminate between fragmented and oxygenated products of PAPC oxidation, and specific profiles of OxPLs generated in various ALI models remain to be determined.
OxPL exhibits dose-dependent, biphasic effects on the endothelial permeability. Low OxPL concentrations protect endothelial barrier, whereas high concentrations of the same OxPLs induced barrier-disruptive effects.4 Previous in vitro studies have described caveolar disruption with Ox-PAPC at 50 μg/mL.8 Because the present studies demonstrate that the S1P1 transactivation leading to EC barrier enhancement depends on intact caveolae, such caveolae disruption at high OxPAPC concentrations may help explain barrier-disruptive effects of OxPAPC at higher concentrations.
A similar explanation can be applied to the animal models. The OxPAPC doses used for intratracheal instillation by Imai et al44 (20 μg/g body weight) were 5 to 10 times higher compared to the protective doses of intratracheal OxPAPC against of LPS- and CpG DNA–induced ALI.40 Whether these OxPL treatments44 quantitatively and qualitatively represent endogenous OxPL generation during acid-, SARS-, anthrax-, or H5N1 influenza–induced lung injury in vivo is a subject of further studies. It also appears that, if administered intravenously, even higher OxPAPC doses (up to 40 mg/kg) may be well tolerated and exhibit protective effects in animal models of LPS-induced lung injury, VILI, and acute necrotizing pancreatitis.27,40,41
Thus, careful analysis of tissue OxPL levels using more elaborate techniques such as mass spectrometry is essential for precise characterization of the composition and amounts of endogenous OxPL generated in different pathological conditions. These studies will allow better understanding of the OxPL role in the pathogenesis of ALI.
In summary, although putative receptor(s) mediating Ox-PAPC barrier protection remain elusive, we now show that OxPAPC-mediated Rac1 activation, cortical actin rearrangement, and barrier regulation are critically dependent on S1P1 transactivation within CEMs. The recruitment of S1P1, Akt1, and Rac GTPases within CEM fractions may be a common feature of barrier enhancing stimuli. These results further indicate that OxPAPC may serve as a potentially useful therapeutic treatment for diseases characterized by high permeability states.
Supplementary Material
Acknowledgments
Sources of Funding
This research was supported by the American Heart Association National Scientist Development Grant 0730277N; American Lung Association National Biomedical Research grant RG-75229-N; National Heart, Lung, and Blood Institute awards HL58094 and HL76259; and NIH grant 2-R37-EB000244-29.
Footnotes
Disclosures
None.
References
- 1.Pearson JD. Endothelial cell biology. Radiology. 1991;179:9–14. doi: 10.1148/radiology.179.1.2006310. [DOI] [PubMed] [Google Scholar]
- 2.Dudek SM, Garcia JGN. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol. 2001;91:1487–1500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- 3.Felmeden DC, Lip GY. Endothelial function and its assessment. Expert Opin Investig Drugs. 2005;14:1319–1336. doi: 10.1517/13543784.14.11.1319. [DOI] [PubMed] [Google Scholar]
- 4.Birukov KG. Oxidized lipids: the two faces of vascular inflammation. Curr Atheroscler Rep. 2006;8:223–231. doi: 10.1007/s11883-006-0077-x. [DOI] [PubMed] [Google Scholar]
- 5.Birukova AA, Chatchavalvanich S, Oskolkova O, Bochkov VN, Birukov KG. Signaling pathways involved in OxPAPC-induced pulmonary endothelial barrier protection. Microvasc Res. 2007;73:173–181. doi: 10.1016/j.mvr.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singleton PA, Bourguignon LY. CD44 interaction with ankyrin and IP3 receptor in lipid rafts promotes hyaluronan-mediated Ca2+ signaling leading to nitric oxide production and endothelial cell adhesion and proliferation. Exp Cell Res. 2004;295:102–118. doi: 10.1016/j.yexcr.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 7.Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol. 2007;8:185–194. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- 8.Yeh M, Cole AL, Choi J, Liu Y, Tulchinsky D, Qiao JH, Fishbein MC, Dooley AN, Hovnanian T, Mouilleseaux K, Vora DK, Yang WP, Gargalovic P, Kirchgessner T, Shyy JY, Berliner JA. Role for sterol regulatory element-binding protein in activation of endothelial cells by phospholipid oxidation products. Circ Res. 2004;95:780–788. doi: 10.1161/01.RES.0000146030.53089.18. [DOI] [PubMed] [Google Scholar]
- 9.Walton KA, Gugiu BG, Thomas M, Basseri RJ, Eliav DR, Salomon RG, Berliner JA. A role for neutral sphingomyelinase activation in the inhibition of LPS action by phospholipid oxidation products. J Lipid Res. 2006;47:1967–1974. doi: 10.1194/jlr.M600060-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Harder T, Simons K. Caveolae, DIGs, and the dynamics of sphingolipid-cholesterol microdomains. Curr Opin Cell Biol. 1997;9:534–542. doi: 10.1016/s0955-0674(97)80030-0. [DOI] [PubMed] [Google Scholar]
- 11.Schwencke C, Braun-Dullaeus RC, Wunderlich C, Strasser RH. Caveolae and caveolin in transmembrane signaling: implications for human disease. Cardiovasc Res. 2006;70:42–49. doi: 10.1016/j.cardiores.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 12.Li XA, Everson WV, Smart EJ. Caveolae, lipid rafts, and vascular disease. Trends Cardiovasc Med. 2005;15:92–96. doi: 10.1016/j.tcm.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Singleton PA, Dudek SM, Ma SF, Garcia JGN. Transactivation of sphingosine 1-phosphate receptors is essential for vascular barrier regulation. Novel role for hyaluronan and CD44 receptor family. J Biol Chem. 2006;281:34381–34393. doi: 10.1074/jbc.M603680200. [DOI] [PubMed] [Google Scholar]
- 14.Singleton PA, Dudek SM, Chiang ET, Garcia JGN. Regulation of sphingosine 1-phosphate-induced endothelial cytoskeletal rearrangement and barrier enhancement by S1P1 receptor, PI3 kinase, Tiam1/Rac1, and alpha-actinin. FASEB J. 2005;19:1646–1656. doi: 10.1096/fj.05-3928com. [DOI] [PubMed] [Google Scholar]
- 15.Singleton PA, Salgia R, Moreno-Vinasco L, Moitra J, Sammani S, Mirzapoiazova T, Garcia JGN. CD44 regulates hepatocyte growth factor-mediated vascular integrity: role of c-Met, Tiam1/Rac1, dynamin 2 and cortactin. J Biol Chem. 2007;282:30643–30657. doi: 10.1074/jbc.M702573200. [DOI] [PubMed] [Google Scholar]
- 16.Garcia JGN, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamburg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest. 2001;108:689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watson AD, Leitinger N, Navab M, Faull KF, Horkko S, Witztum JL, Palinski W, Schwenke D, Salomon RG, Sha W, Subbanagounder G, Fogelman AM, Berliner JA. Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J Biol Chem. 1997;272:13597–13607. doi: 10.1074/jbc.272.21.13597. [DOI] [PubMed] [Google Scholar]
- 18.Thomas M, Lu JJ, Ge Q, Zhang C, Chen J, Klibanov AM. Full deacylation of polyethylenimine dramatically boosts its gene delivery efficiency and specificity to mouse lung. Proc Natl Acad Sci U S A. 2005;102:5679–5684. doi: 10.1073/pnas.0502067102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nonas S, Birukova AA, Fu P, Xing J, Chatchavalvanich S, Bochkov VN, Leitinger N, Garcia JG, Birukov KG. Oxidized phospholipids reduce ventilator-induced vascular leak and inflammation in vivo. Crit Care. 2008;12:R27. doi: 10.1186/cc6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee MJ, Thangada S, Paik JH, Sapkota GP, Ancellin N, Chae SS, Wu M, Morales-Ruiz M, Sessa WC, Alessi DR, Hla T. Akt-mediated phosphorylation of the G protein-coupled receptor EDG-1 is required for endothelial cell chemotaxis. Mol Cell. 2001;8:693–604. doi: 10.1016/s1097-2765(01)00324-0. [DOI] [PubMed] [Google Scholar]
- 21.Chang R, Chicoine LG, Cui H, Kanagy NL, Walker BR, Liu Y, English BK, Nelin LD. Cytokine-induced arginase activity in pulmonary endothelial cells is dependent on Src family tyrosine kinase activity. Am J Physiol Lung Cell Mol Physiol. 2008;295:L688–L697. doi: 10.1152/ajplung.00504.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang BH, Liu LZ. PI3K/PTEN signaling in tumorigenesis and angiogenesis. Biochim Biophys Acta. 2008;1784:150–158. doi: 10.1016/j.bbapap.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frost RA, Lang CH. Protein kinase B/Akt: a nexus of growth factor and cytokine signaling in determining muscle mass. J Appl Physiol. 2007;103:378–387. doi: 10.1152/japplphysiol.00089.2007. [DOI] [PubMed] [Google Scholar]
- 25.Tesfamariam B, DeFelice AF. Endothelial injury in the initiation and progression of vascular disorders. Vascul Pharmacol. 2007;46:229–237. doi: 10.1016/j.vph.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Serne EH, de Jongh RT, Eringa EC, Ijzerman RG, de Boer MP, Stehouwer CD. Microvascular dysfunction: causative role in the association between hypertension, insulin resistance and the metabolic syndrome? Essays Biochem. 2006;42:163–176. doi: 10.1042/bse0420163. [DOI] [PubMed] [Google Scholar]
- 27.Nonas S, Miller I, Kawkitinarong K, Chatchavalvanich S, Gorshkova I, Bochkov VN, Leitinger N, Natarajan V, Garcia JGN, Birukov KG. Oxidized phospholipids reduce vascular leak and inflammation in rat model of acute lung injury. Am J Respir Crit Care Med. 2006;173:1130–1138. doi: 10.1164/rccm.200511-1737OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomez-Mouton C, Lacalle RA, Mira E, Jimenez-Baranda S, Barber DF, Carrera AC, Martinez AC, Manes S. Dynamic redistribution of raft domains as an organizing platform for signaling during cell chemotaxis. J Cell Biol. 2004;164:759–768. doi: 10.1083/jcb.200309101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 30.Somanath PR, Razorenova OV, Chen J, Byzova TV. Akt1 in endothelial cell and angiogenesis. Cell Cycle. 2006;5:512–518. doi: 10.4161/cc.5.5.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris TK. PDK1 and PKB/Akt: ideal targets for development of new strategies to structure-based drug design. IUBMB Life. 2003;55:117–126. doi: 10.1080/1521654031000115951. [DOI] [PubMed] [Google Scholar]
- 32.Du K, Tsichlis PN. Regulation of the Akt kinase by interacting proteins. Oncogene. 2005;24:7401–7409. doi: 10.1038/sj.onc.1209099. [DOI] [PubMed] [Google Scholar]
- 33.Woodgett JR. Recent advances in the protein kinase B signaling pathway. Curr Opin Cell Biol. 2005;17:150–157. doi: 10.1016/j.ceb.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 34.Chen R, Kim O, Yang J, Sato K, Eisenmann KM, McCarthy J, Chen H, Qiu Y. Regulation of Akt/PKB activation by tyrosine phosphorylation. J Biol Chem. 2001;276:31858–31862. doi: 10.1074/jbc.C100271200. [DOI] [PubMed] [Google Scholar]
- 35.Murata T, Lin MI, Huang Y, Yu J, Bauer PM, Giordano FJ, Sessa WC. Reexpression of caveolin-1 in endothelium rescues the vascular, cardiac, and pulmonary defects in global caveolin-1 knockout mice. J Exp Med. 2007;204:2373–2382. doi: 10.1084/jem.20062340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wunderlich C, Schober K, Schmeisser A, Heerwagen C, Tausche AK, Steinbronn N, Brandt A, Kasper M, Schwencke C, Braun-Dullaeus RC, Strasser RH. The adverse cardiopulmonary phenotype of caveolin-1 deficient mice is mediated by a dysfunctional endothelium. J Mol Cell Cardiol. 2008;44:938–947. doi: 10.1016/j.yjmcc.2008.02.275. [DOI] [PubMed] [Google Scholar]
- 37.Ninio E. Phospholipid mediators in the vessel wall: involvement in atherosclerosis. Curr Opin Clin Nutr Metab Care. 2005;8:123–131. doi: 10.1097/00075197-200503000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Leitinger N. Oxidized phospholipids as modulators of inflammation in atherosclerosis. Curr Opin Lipidol. 2003;14:421–430. doi: 10.1097/00041433-200310000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Bochkov VN, Kadl A, Huber J, Gruber F, Binder BR, Leitinger N. Protective role of phospholipid oxidation products in endotoxin-induced tissue damage. Nature. 2002;419:77–81. doi: 10.1038/nature01023. [DOI] [PubMed] [Google Scholar]
- 40.Ma Z, Li J, Yang L, Mu Y, Xie W, Pitt B, Li S. Inhibition of LPS- and CpG DNA-induced TNF-alpha response by oxidized phospholipids. Am J Physiol Lung Cell Mol Physiol. 2004;286:L808–L816. doi: 10.1152/ajplung.00220.2003. [DOI] [PubMed] [Google Scholar]
- 41.Li L, Wang XP, Wu K. The therapeutic effect of oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine in rodents with acute necrotizing pancreatitis and its mechanism. Pancreas. 2007;35:e27–36. doi: 10.1097/mpa.0b013e3181525855. [DOI] [PubMed] [Google Scholar]
- 42.Blüml S, Kirchberger S, Bochkov VN, Krönke G, Stuhlmeier K, Majdic O, Zlabinger GJ, Knapp W, Binder BR, Stöckl J, Leitinger N. Oxidized phospholipids negatively regulate dendritic cell maturation induced by TLRs and CD40. J Immunol. 2005;175:501–508. doi: 10.4049/jimmunol.175.1.501. [DOI] [PubMed] [Google Scholar]
- 43.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 44.Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G, Ermolaeva M, Veldhuizen R, Leung YH, Wang H, Liu H, Sun Y, Pasparakis M, Kopf M, Mech C, Bavari S, Peiris JS, Slutsky AS, Akira S, Hultqvist M, Holmdahl R, Nicholls J, Jiang C, Binder CJ, Penninger JM. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Usatyuk PV, Natarajan V. Role of mitogen-activated protein kinases in 4-hydroxy-2-nonenal-induced actin remodeling and barrier function in endothelial cells. J Biol Chem. 2004;279:11789–11797. doi: 10.1074/jbc.M311184200. [DOI] [PubMed] [Google Scholar]
- 46.Janssen LJ. Isoprostanes and lung vascular pathology. Am J Respir Cell Mol Biol. 2008;39:383–389. doi: 10.1165/rcmb.2008-0109TR. [DOI] [PubMed] [Google Scholar]
- 47.Knapp S, Matt U, Leitinger N, van der Poll T. Oxidized phospholipids inhibit phagocytosis and impair outcome in gram-negative sepsis in vivo. J Immunol. 2007;178:993–1001. doi: 10.4049/jimmunol.178.2.993. [DOI] [PubMed] [Google Scholar]
- 48.Friedman P, Horkko S, Steinberg D, Witztum JL, Dennis EA. Correlation of antiphospholipid antibody recognition with the structure of synthetic oxidized phospholipids. Importance of Schiff base formation and aldol concentration. J Biol Chem. 2002;277:7010–7020. doi: 10.1074/jbc.M108860200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.