Abstract
Purpose: Most patients with chronic obstructive pulmonary disease (COPD) complain of dyspnea during and following exercise, and the development of intrinsic positive end-expiratory pressure (PEEP) is thought to contribute to lung hyperinflation and dyspnea. Many people with COPD use pursed lip breathing (PLB) in an attempt to produce extrinsic PEEP to reduce lung hyperinflation and dyspnea during and following exertion. We hypothesized that the use of a threshold, extrinsic PEEP device would reduce post-exercise dyspnea in people with COPD. Methods: A double blind, crossover study was conducted on post-exercise dyspnea in 8 patients with COPD whose exercise tolerance was limited by dyspnea. Subjects performed two identical 6-minute treadmill bouts that led to a Borg dyspnea rating of at least 5/10. Dyspnea, heart rate, and oxygen-hemoglobin saturation (SpO2) were recorded at rest, every 2 minutes during exercise and at 2, 5, and 10 minutes post-exercise. Immediately following the exercise bouts, the subjects used either a threshold PEEP device for 6 breaths at 10 cm H2O or a Sham device. Results: Heart rate and SpO2 were not different between treatments any time point before, during, or after exercise. Dyspnea ratings were not different between devices at rest or during exercise, but were lower in the post-exercise period following use of PEEP (p < 0.05). When asked which device, if any, the subjects would prefer to use to relieve post-exercise dyspnea, 7 of 8 chose the PEEP device and one had no preference. Conclusions: We found that the use of a PEEP device can help reduce postexercise dyspnea in patients with COPD.
Key Words: COPD, dyspnea, pursed lip breathing, PEEP, exertion
INTRODUCTION AND PURPOSE
Dyspnea during and following exertion is a common symptom reported by patients with chronic obstructive pulmonary disease (COPD), despite optimal medical management, the use of supplemental oxygen and pursed lip breathing (PLB). Many factors may contribute to the sense of dyspnea people with COPD experience during and following exercise including poor physical fitness,1 hypercapnia,2 hypoxemia,3 and dynamic hyperinflation (DH).4 Dynamic hyperinflation is the result of expiratory flow limitation. Most people with COPD are able to maintain a stable end expiratory lung volume (EELV) and inspiratory capacity (IC) at rest, but with the increased ventilatory demand imposed during exercise, expiratory flow limitations arise, leading to increased EELV and reduced IC. Increased EELV and the resultant reduced IC have been identified as major contributory factors to dyspnea in exercising people with COPD.5,6 Treatments to reduce airflow obstruction and/or DH include pursed lip breathing (PLB),7,8 anti-inflammatory drugs,9 breathing helium–oxygen mixtures,10,11 bronchodilators,9,12 placement of endobronchial valves,13,14 and lung volume reduction surgery.15,16
Pursed lip breathing has been recommended as a means to reduce dyspnea in people with COPD.7,17,18 One mechanism by which PLB could reduce dyspnea is reversing DH through an increased intraluminal pressure in the airways, resulting in a shift of the equal pressure point from distal to proximal airways, prolongation of exhalation, and preventing or attenuating dynamic airway collapse.19 While PLB increases mouth expiratory resistance and can increase intraluminal airway pressure, there are limitations on its performance. When a patient begins PLB, expired airflow begins as soon as there is a pressure gradient between the lungs and the atmosphere and the increase in intraluminal pressure is due to the increased mouth expiratory resistance, which varies as a function of the rate of airflow. The use of a threshold device to induce intraluminal airway pressure splinting has the potential advantage of generating an intraluminal pressure that is a function of the device, which means that intraluminal pressure is maintained at the set threshold pressure throughout the expiratory cycle, generating higher airway expiratory pressures, reducing airway collapse and DH, and thus possibly reducing dyspnea.
Positive end-expiratory pressure devices have been used to reduce ventilatory work, improve gas exchange and counteract intrinsic PEEP in patients receiving mechanical ventilation,20,21 but to our knowledge, this technique has not been used on an intermittent basis in nonventilated subjects. Petrof et al20 used continuous positive airway pressure (CPAP) to counteract intrinsic PEEP in people with COPD receiving mechanical ventilation. With 15 cm H2O of CPAP, the amount of inspiratory work performed per minute and per liter of ventilation decreased by 49.8% and 41.8%, respectively. van den Berg21 also found that CPAP reduced the work of breathing in mechanically ventilated patients. Henke22 used CPAP during exercise in cystic fibrosis patients and reported reduced dyspnea and noted that patients with the most severe obstruction received the greatest benefit.
These observations suggest that the use of an extrinsic threshold PEEP device could reduce post-exercise dyspnea in patients with COPD. Therefore, the purpose of this study was to determine if the short-term use of a PEEP device could reduce post-exercise dyspnea rating in people with COPD habitually accustomed to using PLB.
METHODS
Subjects
Subjects signed informed consent to participate in research and their rights were protected according to the procedures of the University of Florida Institutional Review Board (IRB-01). Entry criteria included a diagnosis of COPD with an FEV1.0 of < 50% of predicted, no orthopedic or neurological problems limiting exercise tolerance or affecting gait, familiarity with walking on a treadmill, exercise tolerance limited by dyspnea, habitual use of PLB during exercise to relieve dyspnea (by self report), and the ability to rate dyspnea levels on a Borg scale.23 Subjects were a sample of convenience and were recruited from local pulmonary rehabilitation programs and pulmonary medicine clinics. All subjects had a smoking history, but none had smoked in more than 3 months.
Subjects reported to the Physical Therapy exercise testing laboratory for 2 visits. The first visit included obtaining informed consent, pulmonary function testing, familiarization with the dyspnea scale, and familiarization with the Sham and PEEP devices. Based on the patients' self-reported exercise tolerance, an initial treadmill walking speed was determined and gradual increases were made in treadmill walking speed to determine a walking speed (at 0% grade) that would result in a Borg dyspnea rating of 5 at the end of a 6-minute walking bout. If subjects used supplemental oxygen during exercise, they continued to do so during observation.
Pulmonary function was measured with forced vital capacity spirometry (Future Med, Futuremed America, Inc., Granada Hills, CA) according to ATS/ERS standards.24 Subjects used their usual medications as directed prior to all testing and were administered a single breath of a fast acting bronchodilator (Albuterol120 mcg) 30 minutes prior to pulmonary function testing and exercise bouts.
The second visit started with the subjects using the bronchodilator inhaler followed by a 30 minute seated rest period. The subjects rated their dyspnea at rest and then performed a 6 minute walking bout on a treadmill at the previously determined speed. All subjects habitually used PLB during exercise, and were instructed to do so during the walking and post-exercise periods. Dyspnea ratings, heart rate, and SpO2 were obtained at rest, during exercise at minutes 2, 4, and 6 and at minutes 2, 5, and 10 during the post-exercise period.
After 6 minutes of exercise, the treadmill was stopped and the subjects immediately breathed through the Sham or PEEP device for 6 breaths. The investigator that oversaw the exercise bout and device used then left the room and an investigator blinded to the device used, obtained dyspnea ratings from the subjects at 2, 5, and 10 minutes post-exercise. The subjects then used the bronchodilator inhaler, rested for 30 minutes and the process was repeated with the alternate device. The order of presentation for Sham and PEEP devices was counterbalanced.
Dyspnea was rated by having the subjects point to a number on a large print Borg scale in the last 20 seconds of each time interval.23 The 0-10 Borg scale for rating dyspnea during exercise has been shown to be reproducible and responsive to change in people with COPD.4 The subjects were instructed to rate only their dyspnea and to not include any nonrespiratory sensations. Immediately following the 10 minute post-exercise dyspnea rating after the last walking bout, an investigator showed both devices to the subject and asked, “Which device, if any, did you prefer to use to reduce post-exercise shortness of breath?”
Devices
The PEEP device was a Respironics Threshold PEP® valve (model #HS735, Respironics, Inc, Cedar Grove, NJ) set at 10 cm H2O of pressure. Subjects were instructed to inhale through the nose and exhale slowly and as deeply as possible through the PEEP device 6 times. Subjects who were unable to exhale only through the devices were instructed to pinch their nose closed during exhalation. The number of breaths and pressure setting were set based on our prior clinical experience with the PEEP device and were found to provide the greatest effect on dyspnea in patients with COPD. The relationship between exhaled tidal volume, exhaled peak air flow and pressure in the PEEP device was measured previously on a custom manufactured lung simulator for 50 breaths. At an average exhaled tidal volume of 643 ± 17 ml with a peak expiratory flow of 61.6 ± 12.1 l/min, the pressure inside the PEEP device was 13.8 ± .6 cm H2O when the device was set at 10 cm H2O.
The Sham device was a Respironics Pflex® (model # HS553) inspiratory muscle trainer set at largest opening, position 1. A 3 mm hole was drilled into the device body to further reduce the resistance to airflow. Subjects were instructed to inhale through the nose and exhale slowly and as deeply as possible through the Sham device 6 times. Subjects who were unable to exhale only through the devices were instructed to pinch their nose closed during exhalation. As with the PEEP device, the relationship between exhaled tidal volume, exhaled peak airflow, and pressure in the Shamdevice was measured with a lung simulator for 50 breaths. At an average tidal volume of 650 ± 10 ml, with a peak expiratory flow of 59.5 ± 12.9 l/min, the pressure developed inside the modified Pflex® device was 2.2 ± 1.7cm H2O.
Analysis
Data were analyzed with ANOVA for repeated measures with two within subject factors, time and device using JMP statistical software (Version 7, SAS Institute Inc, Cary, NC). When significant interactions were found, simple effects were analyzed with cell means contrasts.25 Pre-planned contrasts were performed to compare variables at rest and during exercise between treatments. Significance was set at p < 0.05.
RESULTS
Eight subjects (4M/4F) completed all aspects of the study. Three additional subjects were recruited but were not studied because they were unable to tolerate repeated 6 minute walking bouts, were unable to reliably achieve a dyspnea rating of 5 during the trial visit or developed ventricular premature beats during the initial treadmill walking bout. Demographic data are given in Table 1. Five of 8 subjects used supplemental oxygen with a nasal canula during the rest, exercise, and post-exercise periods. All 8 subjects habitually used PLB and continued to do so during the exercise and post-exercise periods.
Table 1.
Subject Characteristics
| Gender | 4M/4F |
|---|---|
| Age (yrs) | 61.6 ± 11.0 |
| Height (cm) | 171.5 ± 12.7 |
| Weight (kg) | 79.0 ± 25.9 |
| FVC (L) | 2.07 ± 0.92 |
| FVC (% predicted) | 47.0 ± 9.1 |
| FEV1.0 (L) | 1.08 ± 0.56 |
| FEV1.0 (%predicted) | 34.6 ± 9.7 |
| FEV1.0/FVC (%) | 53.9 ± 15.1 |
| TM walking speed (m/min) | 60.6 ± 23.4 |
| Supplemental O2 use (L/min) during exercise, N = 5 | 2.20 ± 0.45 |
Data shown as mean ± SD.
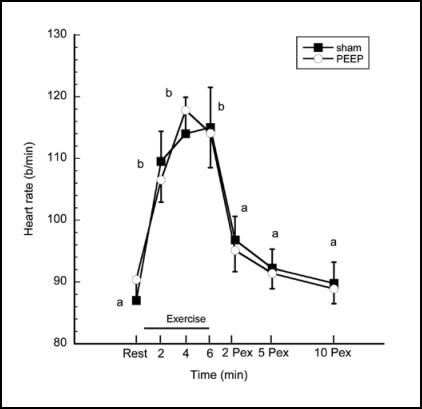
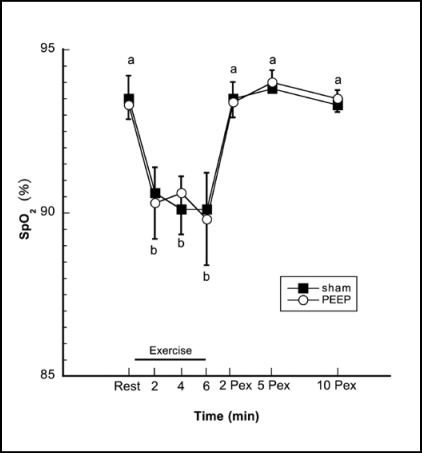
Heart rates and SpO2 at rest, during exercise and postexercise are shown in Figures 1 and 2. ANOVA revealed no statistically significant treatment or interaction effects in either variable. Planned contrasts revealed that, as expected, heart rates were higher during exercise minutes 2, 4, and 6 compared to resting or post-exercise periods, p < 0.0001, but were not different between treatments. SpO2 was lower during exercise minutes 2, 4, and 6 compared to rest or post-exercise, p < 0.0001, but again, did not differ between treatment conditions.
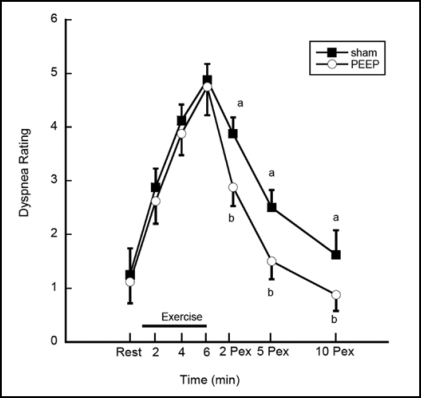
Dyspnea ratings are shown in Figure 3. There was a significant device and time interaction for dyspnea (p = 0.003), thus simple effects were performed. A contrast comparing pooled dyspnea ratings between devices at rest and during exercise was not significant (p = 0.37). A contrast comparing the pooled dyspnea ratings between devices during the post-exercise period (pooled average of post exercise minutes 2, 5 and 10) revealed lower dyspnea ratings after the PEEP treatment compared to Sham. The average pooled post-exercise dyspnea ratings for the Sham versus PEEP treatments respectively were 2.6 ± 1.4 vs. 1.8 ± 1.2 (p < 0.0001). The mean post-exercise Sham minus PEEP dyspnea rating difference was 0.83 ± 0.87 (95% CI 0.58 − 1.09).
Figure 3.
Dyspnea ratings. Dyspnea ratings were lower following the use of PEEP (b) compared to the Sham condition (a) at 2, 5 and 10 minutes post-exercise, p < 0.001. Data shown as mean ± SE.
DISCUSSION
The novel finding of this study is that 6 breaths through an extrinsic threshold PEEP device set at 10 cm H2O immediately after exercise reduced dyspnea in the post-exercise period in patients with moderate to severe COPD that habitually used PLB. The mean difference (0.83 ± 0.87) in dyspnea between treatments was statistically significant and approached the minimally clinical significant difference of 1 unit.
In the normal healthy lung, the filled alveoli exert outward radial traction on collapsible airways and help hold the airways open during exhalation. In patients with significant COPD, alveolar destruction reduces outward radial traction on the airways, allowing airway collapse, trapping air in alveoli, and resulting in increased EELV or DH and reduced IC.26 During exercise, when ventilation and respiratory rate are increased and expiratory time is deceased, the air trapping worsens, EELV increases, IC decreases, and patients report dyspnea that they characterized as an inability to breathe deeply.27 Despite optimal pharmacological management for bronchodilation and inflammation control, supplemental oxygen and PLB, most patients with moderate to severe COPD continue to report significant dyspnea during and following exercise. While pharmacological treatment can reduce or prevent bronchoconstriction and inflammation, bronchodilator and anti-inflammatory drugs are unlikely to prevent dynamic airway collapse due to a loss of radial traction on the collapsible airways and the resulting DH. Endobronchial valves are a promising new treatment modality for patients with COPD that are thought to mechanically reduce or prevent DH in specific regions of the lung and thus reduce dyspnea and increase exercise tolerance.13 However, placement of endobronchial valves requires a minimally invasive procedure, bronchoscopy. Optimal benefit from endobronchial valves requires careful selection of airways and skillful insertion of numerous valves in the airways. Intermittent use of extrinsic PEEP may offer a temporary, noninvasive treatment to reduce post-exercise dyspnea in patients with COPD.
Increasing DH results in greater threshold and elastic loads to breathing and flattening of the diaphragm,28 leading to diaphragm weakness.29 As DH progresses, IC is reduced, patients experience increased drive to breathe and since they are unable to generate increased tidal volumes, they increase their breathing frequency. Increased breathing frequency exacerbates DH as it limits the time for exhalation and a positive feedback cycle ensues in which DH leads to decreased IC, leading to increased breathing frequency leading to intrinsic PEEP, leading to further DH and dyspnea.
We believe that the application of PEEP to these patients ameliorates the DH by moving the equal pressure point from the more collapsible distal airways into the less collapsible proximal airways. This occurs as the result of an increased backpressure of at least 10 cm H2O generated during expiration through the PEEP device. When breathing through the PEEP device set at 10 cm H2O, airflow can only result if the lung-to-atmosphere expiratory pressure gradient is greater than 10 cm H2O. The PEEP generates a net increase in intraluminal pressure thereby increasing the transpulmonary pressure. The intraluminal pressure exceeds the airway collapsing pressure further along the airway length with PEEP present. This moves the equal pressure point proximally to less collapsible airways, preventing dynamic airway collapse and gas trapping during exhalation. The 10 cm H2O of PEEP will allow deflation of the lung even with the shorter post-exercise related expiratory durations. The prevention of DH reduces lung inflation related dyspnea commonly reported in patients with COPD.5,6 In addition, the effect of the PEEP device lasts at least 10 minutes during post-exercise rest.
Many patients with COPD use an interval training approach to rehabilitation in which they exercise for a few minutes, become short of breath, rest until their dyspnea level returns to near baseline, and then resume exercising. The use of extrinsic PEEP may reduce the time that patients require to “catch their breath” between exercise bouts, and thus possibly reduce the recovery time between exercise bouts. Future work should evaluate the use of extrinsic PEEP during exercise.
There are some limitations to our work. We elected to not make serial measures of IC during the post-exercise period, because we feared that repeated IC measures might lead to DH and obscure any potential therapeutic effect of PEEP on post-exercise dyspnea. Future studies examining the impact of PEEP on IC and lung mechanics during and following exercise are warranted. Our sample size was small, but we were able to detect a statistically significant effect on post-exercise dyspnea, indicating a robust treatment effect. The use of the PEEP device resulted in a mean Borg scale change in dyspnea at 2 and 5 minutes post-exercise of 1.00 ± 0.01 units, which meets the criteria for a minimally clinically important difference.30 As a safety factor, we propose that recent thoracic surgery and/or a history of spontaneous pneumothorax are relative contraindications to the use of extrinsic PEEP.
CONCLUSIONS
In summary, we found that 6 breaths through a threshold PEEP device set at 10 cm H2O reduced post-exercise dyspnea in patients with moderate to severe COPD compared to the Sham device plus PLB condition. This treatment will enable patients to recover from dyspnea more rapidly than when using PLB alone. Further studies examining the effects of intermittent extrinsic PEEP on dyspnea, lung mechanics, and gas exchange during and following exercise in patients with COPD are warranted.
Figure 1.
Heart rate response. HR was higher (p< 0.05) at minutes 2, 4 and 6 of exercise compared to rest and 2, 5 and 10 minutes post-exercise (Pex). Time intervals with the same letter designation are not different (p > 0.05). There were no significant differences between the PEEP and Sham treatments.
Figure 2.
SpO2(%) response. SpO2(%) was lower (p< 0.05) at minutes 2,4 and 6 of exercise compared to rest and 2, 5 and 10 minutes post-exercise. Time intervals with the same letter designation are not different (p > 0.05). There were no significant differences between the PEEP and Sham treatments, p > 0.05. Data shown as mean ± SE.
REFERENCES
- 1.Rochester CL. Exercise training in chronic obstructive pulmonary disease. J Rehabil Res Dev. 2003;40:59–80. doi: 10.1682/jrrd.2003.10.0059. [DOI] [PubMed] [Google Scholar]
- 2.Grazzini M, Stendardi L, Gigliotti F, et al. Pathophysiology of exercise dyspnea in healthy subjects and in patients with chronic obstructive pulmonary disease (COPD) Respir Med. 2005;99:1403–1412. doi: 10.1016/j.rmed.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Hoo GW. Nonpharmacologic adjuncts to training during pulmonary rehabilitation: the role of supplemental oxygen and noninvasive ventilation. J Rehabil Res Dev. 2003;40:81–97. doi: 10.1682/jrrd.2003.10.0081. [DOI] [PubMed] [Google Scholar]
- 4.O'Donnell DE, Lam M, Webb KA. Measurement of symptoms, lung hyperinflation, and endurance during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158:1557–1565. doi: 10.1164/ajrccm.158.5.9804004. [DOI] [PubMed] [Google Scholar]
- 5.O'Donnell DE, Lam M, Webb KA. Spirometric correlates of improvement in exercise performance after anticholinergic therapy in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:542–549. doi: 10.1164/ajrccm.160.2.9901038. [DOI] [PubMed] [Google Scholar]
- 6.Marin JM, Carrizo SJ, Gascon M, et al. Inspiratory capacity, dynamic hyperinflation, breathlessness, and exercise performance during the 6-minute-walk test in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:1395–1399. doi: 10.1164/ajrccm.163.6.2003172. [DOI] [PubMed] [Google Scholar]
- 7.Gosselink R. Breathing techniques in patients with chronic obstructive pulmonary disease (COPD) Chron Respir Dis. 2004;1:163–172. doi: 10.1191/1479972304cd020rs. [DOI] [PubMed] [Google Scholar]
- 8.Spahija J, de Marchie M, Grassino A. Effects of imposed pursed-lips breathing on respiratory mechanics and dyspnea at rest and during exercise in COPD. Chest. 2005;128:640–650. doi: 10.1378/chest.128.2.640. [DOI] [PubMed] [Google Scholar]
- 9.Grimes GC, Manning JL, Patel P, et al. Medications for COPD: a review of effectiveness. Am Fam Physician. 2007;76:1141–1148. [PubMed] [Google Scholar]
- 10.Eves ND, Petersen SR, Haykowsky MJ, et al. Helium-hyperoxia, exercise, and respiratory mechanics in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;174:763–771. doi: 10.1164/rccm.200509-1533OC. [DOI] [PubMed] [Google Scholar]
- 11.Laude EA, Duffy NC, Baveystock C, et al. The effect of helium and oxygen on exercise performance in chronic obstructive pulmonary disease: a randomized crossover trial. Am J Respir Crit Care Med. 2006;173:865–870. doi: 10.1164/rccm.200506-925OC. [DOI] [PubMed] [Google Scholar]
- 12.Rice KL, Kunisaki KM, Niewoehner DE. Role of tiotropium in the treatment of COPD. Int J Chron Obstruct Pulmon Dis. 2007;2:95–105. [PMC free article] [PubMed] [Google Scholar]
- 13.Hopkinson NS, Toma TP, Hansell DM, et al. Effect of bronchoscopic lung volume reduction on dynamic hyperinflation and exercise in emphysema. Am J Respir Crit Care Med. 2005;171:453–460. doi: 10.1164/rccm.200407-961OC. [DOI] [PubMed] [Google Scholar]
- 14.Wan IY, Toma TP, Geddes DM, et al. Bronchoscopic lung volume reduction for end-stage emphysema: report on the first 98 patients. Chest. 2006;129:518–526. doi: 10.1378/chest.129.3.518. [DOI] [PubMed] [Google Scholar]
- 15.Benditt JO, Wood DE, McCool FD, et al. Changes in breathing and ventilatory muscle recruitment patterns induced by lung volume reduction surgery. Am J Respir Crit Care Med. 1997;155:279–284. doi: 10.1164/ajrccm.155.1.9001325. [DOI] [PubMed] [Google Scholar]
- 16.Lederer DJ, Thomashow BM, Ginsburg ME, et al. Lungvolume reduction surgery for pulmonary emphysema: Improvement in body mass index, airflow obstruction, dyspnea, and exercise capacity index after 1 year. J Thorac Cardiovasc Surg. 2007;133:1434–1438. doi: 10.1016/j.jtcvs.2006.12.062. [DOI] [PubMed] [Google Scholar]
- 17.Nield MA, Soo Hoo GW, Roper JM, et al. Efficacy of pursed-lips breathing: a breathing pattern retraining strategy for dyspnea reduction. J Cardiopulm Rehabil Prev. 2007;27:237–244. doi: 10.1097/01.HCR.0000281770.82652.cb. [DOI] [PubMed] [Google Scholar]
- 18.Dechman G, Wilson CR. Evidence underlying breathing retraining in people with stable chronic obstructive pulmonary disease. Phys Ther. 2004;84:1189–1197. [PubMed] [Google Scholar]
- 19.Zach MS. The physiology of forced expiration. Paediatr Respir Rev. 2000;1:36–39. doi: 10.1053/prrv.2000.0010. [DOI] [PubMed] [Google Scholar]
- 20.Petrof BJ, Legare M, Goldberg P, et al. Continuous positive airway pressure reduces work of breathing and dyspnea during weaning from mechanical ventilation in severe chronic obstructive pulmonary disease. Am Rev Respir Dis. 1990;141:281–289. doi: 10.1164/ajrccm/141.2.281. [DOI] [PubMed] [Google Scholar]
- 21.van den Berg B, Aerts JG, Bogaard JM. Effect of continuous positive airway pressure (CPAP) in patients with chronic obstructive pulmonary disease (COPD) depending on intrinsic PEEP levels. Acta Anaesthesiol Scand. 1995;39:1097–1102. doi: 10.1111/j.1399-6576.1995.tb04237.x. [DOI] [PubMed] [Google Scholar]
- 22.Henke KG, Regnis JA, Bye PT. Benefits of continuous positive airway pressure during exercise in cystic fibrosis and relationship to disease severity. Am Rev Respir Dis. 1993;148:1272–1276. doi: 10.1164/ajrccm/148.5.1272. [DOI] [PubMed] [Google Scholar]
- 23.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 24.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 25.Freund RJ. SAS for linear models: A guide to the ANOVA and GLM procedures. Cary, NC: SAS Institute, Inc; 1981. pp. 171–176. [Google Scholar]
- 26.Puente-Maestu L, Stringer WW. Hyperinflation and its management in COPD. Int J Chron Obstruct Pulmon Dis. 2006;1:381–400. doi: 10.2147/copd.2006.1.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Donnell DE, Bertley JC, Chau LK, et al. Qualitative aspects of exertional breathlessness in chronic airflow limitation: pathophysiologic mechanisms. Am J Respir Crit Care Med. 1997;155:109–115. doi: 10.1164/ajrccm.155.1.9001298. [DOI] [PubMed] [Google Scholar]
- 28.O'Donnell DE. Hyperinflation, dyspnea, and exercise intolerance in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:180–184. doi: 10.1513/pats.200508-093DO. [DOI] [PubMed] [Google Scholar]
- 29.O'Donnell DE. Dynamic lung hyperinflation and its clinical implication in COPD. Rev Mal Respir. 2008;25:1305–1318. doi: 10.1016/s0761-8425(08)75094-0. [DOI] [PubMed] [Google Scholar]
- 30.Ries AL. Minimally clinically important difference for the UCSD Shortness of Breath Questionnaire, Borg Scale, and Visual Analog Scale. COPD. 2005;2:105–110. doi: 10.1081/copd-200050655. [DOI] [PubMed] [Google Scholar]