Abstract
Individuals living with HIV experience a much higher risk of progression from latent M. tuberculosis infection to active tuberculosis (TB) disease relative to individuals with intact immune systems. A several-month daily course of a single drug during latent infection (i.e. isoniazid preventive therapy (IPT)) has proved in clinical trials to substantially reduce an HIV-infected individual's risk of TB disease. As a result of these findings and ongoing studies, the World Health Organization has produced strong guidelines for implementing IPT on a community-wide scale for individuals with HIV at risk of TB disease. To date, there has been limited use of IPT at a community-wide level. In this paper, we present a new co-network model for HIV and TB co-epidemics to address questions about how the population-level impact of community-wide IPT may differ from the individual-level impact of IPT offered to selected individuals. In particular, we examine how the effect of clustering of contacts within high-TB incidence communities may affect the rates of re-infection with TB and how this clustering modifies the expected population-level effects of IPT. We find that populations with clustering of respiratory contacts experience aggregation of TB cases and high numbers of re-infection events. While, encouragingly, the overall population-level effects of community-wide IPT appear to be sustained regardless of network structure, we find that in populations where these contacts are highly clustered, there is dramatic heterogeneity in the impact of IPT: in some sub-regions of these populations, TB is nearly eliminated, while in others, repeated re-infection almost completely undermines the effect of IPT. Our findings imply that as IPT programmes are brought to scale, we should expect local heterogeneity of effectiveness as a result of the complex patterns of disease transmission within communities.
Keywords: mathematical model, co-infection, re-infection, clustering, epidemics, infectious disease
1. Introduction
The emergence of HIV over the past three decades has complicated the global control of tuberculosis (TB) [1]. In some sub-Saharan African countries with generalized (more than 1% of the population infected) HIV epidemics, TB incidence rates have increased over threefold in less than 20 years (for example, Lesotho and South Africa [2,3]).
Despite the widespread availability of effective anti-TB drug treatment, TB remains a leading cause of death for HIV-infected individuals. The latest estimates indicate that nearly a third of all HIV/AIDS-related deaths might be attributable to TB [4]. This grim statistic has motivated the adoption of new approaches for reducing the burden of HIV-associated TB through the use of intensive case identification, infection control to reduce nosocomial transmission and isoniazid preventive therapy (IPT) (the three I's [5]).
IPT reduces the risk of progression from latent infection with M. tuberculosis to active TB disease through the use of a single drug given for six to 12 months [6]; some recent trials include treatment arms of up to 36 months of therapy [7]. This beneficial effect has been demonstrated for both individuals with HIV [8] and without HIV/TB co-infection [9]. Since the primary mechanism by which HIV epidemics result in a rising incidence of TB is by increasing the rate at which co-infected individuals progress from latent M. tuberculosis infection to active TB disease, community-wide IPT (i.e. IPT delivered to all eligible HIV co-infected individuals in a community) promises to reduce the incidence of HIV-associated TB. Several mathematical models have identified IPT interventions as a important tool for long-term reductions in rates of TB incidence [10–12].
However, because of the complex dynamics underlying transmission of TB and HIV, it is not straightforward to extrapolate the observed effect of IPT delivered to individuals to the combined effect of IPT delivered to communities. Effects of interventions at the community level may not mirror those observed at the individual level. For example, the overall impact of IPT may be underestimated if IPT reduces onward TB transmission; in this case, even individuals not eligible for or not given IPT treatment may benefit. In contrast, the overall impact may be overestimated if individuals remain at high risk of re-infection after their IPT treatment is concluded.
The overall outcome after IPT is delivered to an individual will depend not only on the effectiveness of IPT in preventing disease in that individual, but also on the continuing risk of M. tuberculosis infection that persists beyond the time that IPT is administered. Assuming that prevalent cases of TB are treated, the continuing risk to an individual after IPT is a function of two factors: (i) the prevalence of latent infection among their respiratory contacts and (ii) the risk that these latent infections will progress to TB and result in re-infection.
The prevalence of latent infection among an individual's respiratory contacts in turn depends on the prevalence of latent M. tuberculosis infection in the population, and the extent to which these infections are aggregated or clustered in a community. The risk that such infections will progress to disease depends on the prevalence and aggregation of HIV infections (since HIV/TB co-infection is the largest known risk factor for progression) and on the probability that latent M. tuberculosis among these HIV co-infected contacts are also given IPT treatment. Thus, the overall effectiveness of IPT depends on the structure of the networks underlying disease transmission (which drives the clustering of HIV and TB on these networks), the prevalence of TB and HIV in the communities and the coverage levels and patterns of distribution of the IPT intervention.
Here we use a model to examine patterns of HIV and TB dynamics to relate the individual-level impact of IPT to its potential impact at the community level. The model uses two distinct contact networks representing respiratory and sexual transmission. Studies recording different types of contact over time, for example [13,14], can inform us about possible respiratory networks; however, not only are these studies in settings different from those we wish to represent (Europe instead of Africa), it is also difficult to extract from the results the contact patterns that would be sustained and close enough to allow TB transmission. In general, it is assumed that respiratory networks will have more local ‘clustering’ of contacts than sexual networks [15], since respiratory contacts will be shared within family, school and social groups to a greater extent than sexual contacts.
There is some evidence that TB cases are clustered, for example, a study documented TB disease patterns in an region of Cape Town, South Africa (an area of high TB incidence), and using spatial analysis techniques found there was clustering of active TB cases. They linked these ‘hot-spots’ of disease to other factors like overcrowding and Shebeen (informal bar) locations [38]. A more recent study of TB cases in The Gambia found that there was significant clustering of cases and suggested that using cluster detection techniques (such as contact tracing and case detection in the community) to identify clusters and their sources may have a positive impact on treatment programmes [16]. An analysis of the social network of the initial cases in a TB outbreak in British Columbia revealed that all the patients frequented common locations in the community and had shared contacts, even if they had no direct contact [17], again indicating a clustering of TB transmission. It is only recently that studies such as these have revealed the impact that network structures have on TB transmission; to investigate this further, we use varying types of respiratory networks to explore the effect on the epidemic.
We use a dynamic model of HIV and TB transmission over these coupled networks and investigate the effects of local network structure on the effectiveness of IPT interventions at the population and the individual level. At the population level, we examine the impact of IPT on TB incidence and prevalence over time, while at the individual level, we compare cohorts of individuals receiving no IPT with those receiving varying-length IPT courses. To further elucidate the impact of network structure, we examine patterns of spatial clustering in TB infection events and explore whether spatial heterogeneity can explain variability in the impact of IPT.
2. Methods
2.1. Network generation
The respiratory contact networks were generated using the method described by Read & Keeling [18], with an average degree of 12 and a low variance in the degree distribution. In this algorithm, every individual is assigned a location and connected to other nodes with a probability proportional to their distance apart. On more local networks, nodes form connections preferentially with those closer to them, thereby forming a more clustered structure, while on global graphs, connections are, on average, longer and there are fewer cliques. The ‘location’ represents both geographical and social location: we wish to represent that some people are at higher risk than others for exposure to TB, both because of their shared environment and because of whom they are in contact with. This network generation method allows us to investigate how changing these risks affects the epidemic: we investigated dynamics on local, intermediate and more global networks.
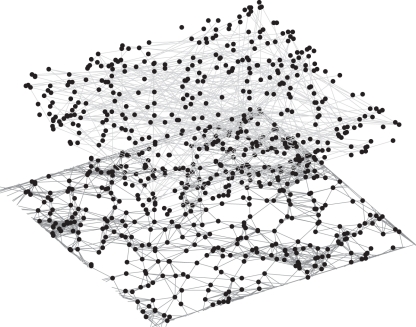
In contrast, in the sexual contact network, most individuals have a small degree and there are a few highly connected individuals. The network was generated following the configuration model method [19] using a power law degree distribution [20]. The network is static, so the degree represents the total number of sexual contacts over the duration of the simulation. The mean degree of an individual is around 1.8, with 5 per cent of individuals having no sexual contacts. Examples of the networks are shown in figure 1.
Figure 1.
Examples of idealized contact networks of 400 individuals generated using the methods outlined in §2.1. The lower network (dark grey) represents a respiratory contact network, generated with a local structure and average degree set to 12; the top network (light grey) is a sexual contact network with 5% of individuals unconnected. The respiratory network clearly shows clustering with some connecting edges between the clusters, whereas the sexual network is the same set of individuals connected randomly.
2.2. Disease transmission
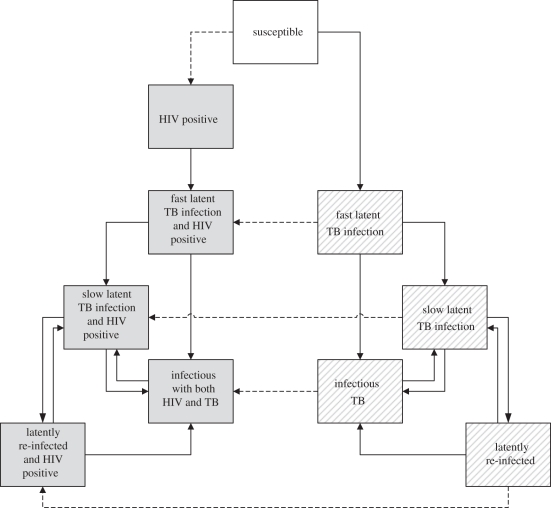
From the respiratory and sexual contact networks, we create the co-network by combining them in a multi-graph, so every individual may have contacts in both networks. Each individual is in one of seven states: susceptible to both diseases, infected with latent TB (exposed), infected with active TB, HIV-positive, infected with latent TB and HIV-positive, infected with active TB and HIV-positive (co-infected), dead. Each state (except if dead) has corresponding parameters reflecting mortality, TB progression and recovery from active TB (table 1). At each (monthly) time step, the status change for each individual is determined stochastically, based on the probability of infection with HIV and/or TB by infectious contacts on the networks and the relevant mortality, progression and recovery rates. The possible transitions between states in the network model are illustrated in figure 2 and described in detail in appendix A.
Table 1.
Parameters for the network model. Note that probabilities are per year.
| probability-based parameters | probability per year | source |
|---|---|---|
| natural death | 0.020 | consistent with a 50 year lifespan |
| death owing to active TB | 0.20 | from Cohen et al. [21] |
| death owing to HIV | 0.070 | average from World Health Organization [22] |
| death owing to HIV/TB co-infection | 0.78 | six to seven times more than without HIV, double HIV alone |
| HIV transmission | 0.13–0.22 | fit to appropriate epidemic |
| TB transmission | 0.30–0.50 | fit to appropriate epidemic |
| fast progression to infectious TB | 0.020 | risk is 10% in first 5 years [23] |
| slow progression to infectious TB | 0.004 | risk is 10% over rest of lifetime (30 years) [23] |
| fast progression to infectious TB with HIV | 0.19 | risk is approximately 45% in first 5 years |
| slow progression to infectious TB with HIV | 0.030 | risk is approximately 45% over next 30 years |
| recovery from infectious to latent TB | 0.18 | [24,25] |
| recovery from infectious to latent TB, with HIV | 0.05 | assumption and Cohen et al. [10] |
| other parameters | value | source |
| partial immunity, no HIV | 0.65 | from Dye et al. [26] |
| partial immunity, with HIV | 0.25 | from Dye et al. [26] |
| reduction in transmission of TB over the HIV network | 1/9 | much less than 1, reflecting less sustained contact |
| months in fast progression | 60 | as in Vynnycky & Fine [23] |
Figure 2.
Outline of the possible transitions in the network model, fully described in appendix A. Grey boxes indicate HIV-seropositive states and lined boxes indicate HIV-seronegative states. The susceptible state is white. The dashed arrows indicate becoming infected with HIV.
While the natural history of TB is modelled in some detail, we include only a single state of HIV infection for simplicity, recognizing that in reality, the state of HIV infection is heterogeneous [27].
2.3. IPT cohort trials
We generated populations of 10 000 individuals with either more or less clustered respiratory networks. We scaled the TB and HIV transmission parameters to allow for stable and comparable epidemics on each network structure. We then simulated IPT trials by running three simulations, ‘enrolling’ a cohort of 200 HIV-infected, IPT-eligible individuals in each: the control cohort received no IPT (and served as our reference group), the first intervention cohort received six months of IPT and the second intervention cohort received 36 months of IPT. We then followed these cohorts for 10 years to examine the simulated effects of these interventions. Each simulation was repeated 10 times on each network structure. Because we knew precise timing of infection and disease events in these cohorts, we could construct Kaplan–Meier curves for displaying event-free survival. To examine whether there was spatial variability in the effects of IPT, for each simulation, we split the population of individuals into four regions and plotted separate Kaplan–Meier curves for each sub-area.
2.4. Spatial analysis
Heat maps and spatial statistical analysis were used to find significant spatial clusters in the number of TB re-infection events per individual. We used SatScan1 to identify statistically significant circular clusters of individuals re-infected three or more times. Finally, we calculated Ripley's K-statistic and its derivative, the L-statistic. The K-statistic identifies clustering in point patterns [28] and the L-statistic is used to identify deviations from complete spatial randomness; these were examined only for individuals re-infected three or more times. We then used a Monte Carlo approach to determine the significance of the results.
3. Results
The model simulations recreate realistic HIV and TB trends from typical settings in sub-Saharan Africa. The initial conditions were chosen to approximately match those of high-TB settings such as Botswana in 1990 [2,3]. With no intervention, the prevalence of HIV and latent TB in the population grows steadily, slowing after 20 years. As is seen in regions of the world with high TB and HIV incidence, for all networks in our model, the co-infection rate is very high; up to 75 per cent of those with active TB are co-infected with HIV.
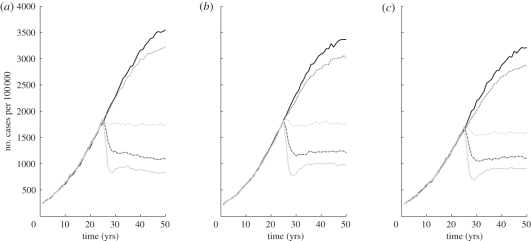
IPT has a dramatic impact on TB in the simulations, an effect that increases with IPT coverage (figure 3). These effects at the population level do not exhibit a strong dependence on the respiratory contact structure of the population. The effect of increased coverage is not linear; while there is a substantial difference in TB incidence between 10 and 25 per cent coverage with IPT, there is little difference between 25 and 50 per cent coverage. Each line in the figure represents an average over 50 simulations, and the percentage by which the individual runs varied was on average 6 per cent for locally and globally connected networks (results not shown).
Figure 3.
The effect on TB incidence by changing the coverage level for IPT and ART in the population. Treatment programmes begin 25 years into the simulation; the coverage level was altered by changing the percentage of eligible individuals who receive treatment each year. The IPT course length was fixed at nine months and ART is given continuously. More local networks on the left, and most global on the right. Each graph is averaged over 50 simulations. Solid black line, 0%; dark grey dotted line, 1%; light grey dotted line, 10%; dark grey dashed line, 25%; solid light grey line, 50%.
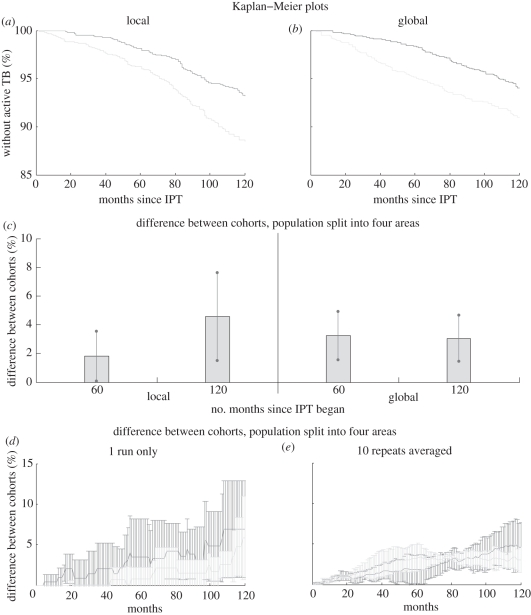
Figure 4 illustrates the result of our individual-level (cohort-based) comparisons of the performance of IPT in the different networks. In figure 4a,b, the Kaplan–Meier survival plots show the per cent of the cohort that has not developed active TB since IPT began. While the endpoints at 120 months are similar on both network topographies, the difference between the IPT and non-IPT cohorts emerges more rapidly in the more global network. Indeed, in the more local networks, the overall disease dynamics are slower [29], but after 10 years, the performance of IPT is better than in the more global networks. Unsurprisingly, we find that a strategy that uses a 36 month course of IPT has a more pronounced effect than a six month course of IPT.
Figure 4.
Results for simulations of a six month trial of IPT in a cohort of 200 HIV-infected individuals, compared to a control cohort for the most local and most global respiratory networks. The cohorts are followed for 10 years from the start of IPT. (a,b) Kaplan–Meier plots for the cohorts, for a more local network (a) and a more global network (b). (c) The population is split into four areas and the differences between the KM estimates for the cohorts in each area found for 60 and 120 months after the start of IPT. The average difference (bars) and the standard deviation (error lines) between areas are plotted. (d,e) The population is again split into four areas and the average difference and variability (standard deviation) between cohorts are calculated for one run (d) and 10 runs (e). (a,b) Light grey lines, no IPT; dark grey lines, with IPT. (d,e) Dark grey lines, local; light grey lines, global.
When we now examine the more local effects of IPT by splitting the population into spatial regions (figure 4c), we see considerably more variation in the effect of IPT between areas on the more local networks. On the local networks, a 36 month course of IPT had a more consistent effect in the four regions than the six month course. However, on more global networks, there was little variability across the regions for both the six and 36 month courses.
The spatial analysis reveals the origin of the high heterogeneity between regions in local networks. We find that the total number of re-infection events throughout the simulation is far lower for more global networks, consistent with the idea that TB infection is contained within tightly linked clusters of contacts and within these more infection events are likely to be re-infections. However, similar numbers of individuals are re-infected in each network. This means that there are more individuals suffering multiple re-infections on local networks; these individuals were re-infected more often (3.3 times on average) than those re-infected on global networks (2.1 times on average) (table 2).
Table 2.
Re-infection statistics for example simulation runs for varying network structures. The numbers are totalled over the entire simulation run. The average is over those who have been re-infected at least once during the simulation.
| clustering | local | intermediate | global |
|---|---|---|---|
| number of re-infection events | 41 249 | 24 055 | 19 674 |
| number of individuals re-infected at least once | 12 562 | 10 595 | 9355 |
| average number of re-infection events | 3.3 | 2.3 | 2.1 |
| number of individuals with two or more re-infection events | 8382 | 5751 | 4771 |
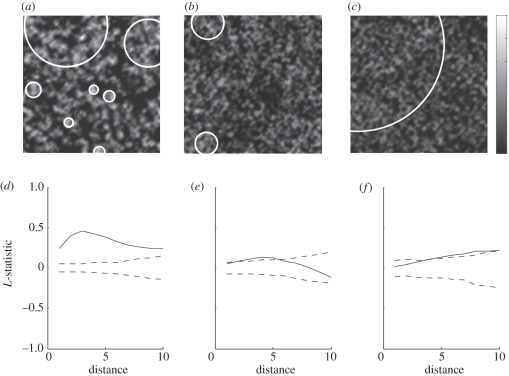
On the local network (figure 5a), there are obvious clusters of high numbers of re-infection events (lightly shaded areas), and there is a low level of re-infection events elsewhere (dark shaded areas) (online version in colour). The circular clusters found by SatScan correlate with the areas highlighted in the heat map, implying that in local networks, individuals with multiple re-infection events are grouped with other re-infected individuals. In the more global networks (figure 5b,c), there are fewer clusters, with a lower number of re-infection events per individual in these clusters, and the distribution of re-infection events is more uniform. Results from the L-statistic confirm this interpretation (figure 5d–f).
Figure 5.
Most local network on the left, more global in the middle and most global on the right. (a–c) Heat maps illustrating the distribution of multiply re-infected individuals (the locations are those assigned during the network generation). Lighter shading indicates over 20 re-infection events per individual, dark grey shading indicates no re-infection events. The white circles are circular clusters of individuals that have been re-infected at least three times identified using SatScan. (d–f) Plots of the L-statistics for these simulations (solid line) together with the upper and lower bounds calculated from Monte Carlo simulations (dashed line). A deviation of the data outside of the Monte Carlo bounds indicates an attraction (or repulsion) of cases (re-infection events) in the population [30]. The distance on the x-axis is in the same ‘units’ as the distance between nodes (see method). There is a peak at distance 3 for the local network (d), indicating a strong clustering of re-infection cases at this distance, and the decline following this implies decreasing clustering at higher distances. For the intermediate network (e), there is some clustering at distances less than 6, and for the most global network in (f), there is only weak clustering at large distances.
4. Discussion
Despite the anticipated population benefits of IPT, this intervention has only been implemented in a limited number of settings and in 2007, a mere 0.1 per cent of eligible individuals actually began a course of IPT [31]. Possible reasons for the slow pace of adoption of IPT interventions include the challenges associated with administering daily single drug preventive therapy for a very large number of individuals without symptomatic TB disease [32] and the difficulty in ruling out the presence of active TB disease in HIV co-infected individuals [33]. Increasing the uptake of IPT in communities with high HIV prevalence may require projections of the cost-effectiveness of such interventions and further demonstration that available diagnostic tools can effectively rule out subclinical TB disease before monotherapy is used. To encourage access and usage of IPT, the World Health Organization (WHO) recently revised IPT guidelines to suggest that the absence of symptoms (current cough, fever, weight loss or night sweats) is sufficient for ruling out active disease and initiating preventive therapy [34]. They strongly recommend that eligible individuals complete a six month course of IPT, and in high-transmission areas, a 36 month course is advised.
However, recent studies aiming to assess the effectiveness and optimal duration of IPT have come to varying conclusions. A study in Botswana of HIV-positive individuals compared six and 36 months of IPT. Among those who tested positive for latent TB, in the six month cohort, the TB rate was 2.22 per 100 person years compared with 0.57 per 100 person years in the 36 month cohort. In individuals who tested negative for latent TB, there was little difference between the six and 36 month courses [7,35]. By contrast, a trial in India of HIV-positive patients without active TB concluded that six and 36 month courses of IPT were equally effective [35,36].
Our work suggests that differences between respiratory contact patterns and TB incidence between these settings may help explain why IPT strategies may have variable impact in different settings. While IPT treatment given to individuals prevents progression to disease, the impact of IPT at the community level depends on (i) the prevalence of TB infection among an individual's respiratory contacts, and (ii) the incidence of disease among those contacts, resulting in re-infection occurring after the IPT course is complete. The first of these is related to the extent of clustering of TB among shared groups of respiratory contacts, together with the overall prevalence of TB infection in the population. The second is mediated by the level of HIV/TB co-infection and the level of IPT coverage.
We have shown that the effect of IPT is highly variable in locally clustered networks compared with more globally connected ones. This is because in locally clustered networks, latent and incident TB infections occur in clusters, so that some areas suffer very high rates of incidence and re-infection, while other clusters are effectively protected from disease. In these protected clusters, treatment of a sufficient number of TB cases results in a reduction or elimination of onward TB transmission in the area. This occurs as a result of IPT when (i) a sufficient portion of the local TB burden is among HIV-infected individuals, and (ii) IPT coverage of eligible individuals is sufficiently high. We would therefore expect IPT to be most undermined by network and dynamical effects when TB infections are clustered among local groups of shared respiratory contacts, when HIV/TB co-infection is low among prevalent TB cases and/or when IPT coverage levels are too low to prevent onward transmission. This effect is exacerbated by local clustering of respiratory contacts, because it is in these circumstances that individuals who have been infected with TB once (whether or not they received IPT) are likely to be re-infected rapidly after their IPT treatment ends.
In terms of policy guidelines, our results imply that IPT will perform best if entire clusters of infected individuals at high risk of progression can be treated simultaneously. The benefits of IPT may be eroded if infection is not cleared from these clusters. This provides support to the conclusions from studies on clustering of TB cases in Africa [16], advising that cluster detection techniques (of both high and low case numbers) may help TB control programmes use their resources efficiently and effectively. Contact patterns and clustering are difficult to measure, particularly because relationships are dynamic and contacts may occur in casual settings where individuals are not known. However, techniques such as contact tracing from known TB cases have proved useful for identifying possible clusters (covering both past and current contacts) [37]; these efforts to identify clustered risk may be supported by social network analysis [17] and targeted investigation within settings of possible transmission such as bars [38].
It has previously been observed that clustering of contacts reduces disease spread over a network [39,40]; we also saw this effect in our simulations. Indeed, a high degree of clustering reduces disease spread compared with what would be expected from the initial growth rate of an epidemic or outbreak [41]. Because epidemics spread first within a community and then (typically later) between them, networks with community structure can maintain epidemics at quite low incidence levels overall, but with a large number of generations of infectives over a relatively long period of time. By contrast, globally connected networks tend to have relatively fast, high-incidence, but short epidemics [42]. This can make estimating the efficiency of control measures very difficult.
Our original hypothesis was that high levels of re-infection in locally clustered networks would undermine IPT programmes by allowing those individuals who had recently received IPT to be at risk of TB disease despite their treatment. However, instead we saw that the same mechanism that exposed some individuals to high levels of re-infection (clustering) also worked to protect other individuals from re-infection, via some clusters enjoying the near-elimination of TB after the introduction of IPT. As the protective effect of clustering is much weaker on globally connected networks, IPT performance on local networks can actually be better than on global ones at the population level, despite substantial regional variability.
The model is limited by the network structures we assumed. It is difficult to obtain comprehensive network data for populations, so our networks are caricatures of the structures of contact networks. In addition, for simplicity, we assumed that the networks were static; this assumption probably alters the transmission dynamics across the population. However, these limitations do not alter the overall qualitative insights from the model.
In this model, we have investigated single, fixed-length courses of IPT, which is consistent with current policy guidelines. We showed that the network structure affects both the frequency and distribution of TB re-infection, and thereby may modify the expected effectiveness of the impact of these types of IPT interventions. To mitigate the effect of re-infection, it may be useful to consider continuous or multiple courses of IPT, as suggested by some studies [7]. These policies could potentially improve the impact of IPT by providing a longer period of protection from re-infection and progression, but there are concerns that longer or multiple courses of IPT may provide increased selective pressure and may facilitate the emergence of drug-resistant strains of TB (despite there being no evidence that IPT causes acquired resistance in individual patients [10]). In the recent publication of TB guidelines, the WHO concluded that IPT does not increase the risk of developing drug-resistant TB, so this should not be a barrier to its provision. Drug-resistant strains have been identified in most high-TB incidence areas [43–45], and IPT would be expected to exert selective pressure at the population level if given in the context of co-circulating resistance and sensitive strains [46,47]. Our model assumes only one, drug-sensitive strain of TB and does not consider the evolution of new drug-resistant strains or the effect on existing levels of resistance.
In conclusion, we have developed a new multi-network model for HIV and TB and find that local network structure can induce high levels of repeated re-infection that may undermine the projected effectiveness of IPT. At the same time, strong local clustering may provide a protective effect wherein TB is essentially eliminated from some areas and re-introduction of TB is limited by a scarcity of long-range connections. The combination of these dynamics means that IPT's effectiveness in clustered networks may be variable; this insight will be useful as we attempt to interpret and reconcile results from ongoing IPT trials.
Acknowledgements
T.C. was funded by NIH grants U54 GM088558-01 (MIDAS), R01 AI083036 and DP2OD006663 from the Office of the Director, US National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Office of the Director of the US NIH, or the NIH. H.L.M. was supported by the Bristol Centre for Complexity Sciences and EPSRC grant EP/5011214.
Appendix A. Supplementary methods
The probability per time step of an individual with KHIV HIV-positive contacts becoming infected with HIV is equal to pHIV = 1 − (1 − αHIV)KHIV, where αHIV is the probability of transmission of HIV per infected contact, per month. The probability per time step of an individual becoming latently infected with TB is equal to pTB = 1 − (1−αTB)KTB(1 − εαTB)KTB,HIV, where αTB is the probability of transmission of TB and where the individual has KTB respiratory contacts with active TB and KTB,HIV sexual contacts with active TB. Sexual contacts are also respiratory contacts, so TB may be transmitted over the sexual contact network. The probability of transmission is reduced by ε over the sexual network to reflect the fact that these contacts are generally likely to be seen less often than those in the respiratory network.
An individual can progress from latent to active TB infection with a higher probability of progressing in the first 5 years after the initial infection [23]. Individuals may be re-infected by infectious contacts, which is modelled as follows: if the re-infected individual is in the fast progression stage (within 5 years of initial infection), there is no change to its progression probability; however, if they are in the slow progression stage, they will have an elevated progression probability. Owing to some partial immunity acquired from the initial infection, this probability is lower than the fast progression probability. HIV-positive individuals have 25 per cent partial immunity and individuals not infected with HIV have 65 per cent partial immunity [26]. The elevated progression probability caused by re-infection lasts for 5 years.
In the network model, transmission is more efficient on global networks, causing more active TB and more re-infection. To allow comparison of IPT on different networks in the same epidemiological setting, the transmission of TB was scaled between the more local and global networks so that the incidence of active TB was the same in each network.
Finally, to allow for the possibility of relapse and represent the immunity acquired after recovering from an active infection, recovery from active TB is modelled as moving to the slow progressing latent class.
If an individual dies, it is replaced in the following time step by a susceptible individual. This individual has the same respiratory contacts as the dead individual, but forms new contacts at random in the sexual network while retaining the sexual degree of the dead individual. The rearrangement of sexual contacts means old contacts do not immediately form a new sexual contact and the new individual is not automatically placed in an area of high HIV prevalence.
A.1. IPT and antiretroviral therapy
An IPT programme can be introduced in the simulation at any time step. The probability of an individual receiving IPT at any time step is such that over a year of the programme, a set percentage of those eligible will receive IPT (an eligible individual is HIV positive without active TB). The IPT course lasts for a set time period (here, nine months) during which the individual loses any latent TB infection and will not become infected again, although after the course of IPT is complete and they are susceptible to TB, they may become latently infected. An individual only receives IPT once in their lifetime, reflecting the current policy guidelines.
In the model, every individual who receives IPT also receives antiretroviral therapy (ART). It is assumed that ART reduces the infectivity of the individual by 80 per cent per contact (trial results vary between 67 and 92% [48–51]), reduces their mortality rate by 50 per cent and continues for the rest of their life.
Footnotes
SatScan is freely available software that was developed under the joint auspices of (i) Martin Kulldorff, (ii) the National Cancer Institute, and (iii) Farzad Mostashari of the New York City Department of Health and Mental Hygiene.
References
- 1.Corbett E. L., Watt C. J., Walker N., Maher D., Williams B. G., Raviglione M. C., Dye C. 2003. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch. Int. Med. 163, 1009–1021 10.1001/archinte.163.9.1009 (doi:10.1001/archinte.163.9.1009) [DOI] [PubMed] [Google Scholar]
- 2.UNAIDS 2008. Report on the global AIDS epidemic, August 2008. See http://data.unaids.org/pub/GlobalReport/2008/jc1510_2008globalreport_en.pdf. [Google Scholar]
- 3.World Health Organization 2009. Global tuberculosis control: a short update to the 2009 report, March. See http://whqlibdoc.who.int/publications/2009/9789241598866_eng.pdf. [Google Scholar]
- 4.Lawn S. D., Wood R., Wilkinson R. J. 2011. Changing concepts of ‘latent tuberculosis infection’ in patients living with HIV infection. Clin. Dev. Immunol. 2011, 980594 (doi:10.1155/2011/980594) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization 2008. WHO Three I's Meeting, April 2008. Report of a joint WHO HIV/AIDS and TB Department meeting, Geneva, Switzerland, 2–4 April 2008. See http://www.who.int/hiv/pub/meetingreports/WHO_3Is_meeting_report.pdf. [Google Scholar]
- 6.World Health Organization 2009. Isoniazid preventive therapy (IPT) for people living with HIV, Consensus statement of the Core Group of the TB/HIV Working Group of the Stop TB Partnership. See http://www.who.int/tb/features_archive/ipt_statement_tbhiv_coregroup.pdf [Google Scholar]
- 7.Samandari T., et al. 2010. Randomized, placebo-controlled trial of 6 vs 36 months isoniazid TB preventive therapy for HIV-infected adults in Botswana. In 17th Conf. on Retroviruses and Opportunistic Infections; San Francisco, CA, USA, 16–19 February 2010, Abstract 104LB. [Google Scholar]
- 8.Akolo C., Adetifa I., Shepperd S., Volmink J. 2010. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst. Rev. 1, CD000171. 10.1002/14651858.CD000171.pub3 (doi:10.1002/14651858.CD000171.pub3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smieja M., Marchetti C., Cook D., Smaill F. M. 1999. Isoniazid for preventing tuberculosis in non-HIV infected persons. Cochrane Database Syst. Rev. 1, CD001363. 10.1002/14651858.cd001363 (doi:10.1002/14651858.cd001363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen T., Lipsitch M., Walensky R. P., Murray M. 2006. Beneficial and perverse effects of isoniazid preventive therapy for latent tuberculosis infection in HIV–tuberculosis coinfected populations. Proc. Natl Acad. Sci. USA 103, 7042–7047 10.1073/pnas.0600349103 (doi:10.1073/pnas.0600349103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Long E. F., Vaidya N. K., Brandeau M. L. 2008. Controlling co-epidemics: analysis of HIV and tuberculosis infection dynamics. Oper. Res. 56, 1366–1381 10.1287/opre.1080.0571 (doi:10.1287/opre.1080.0571) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhunu C. P., Garira W., Mukandavire Z. 2009. Modeling HIV/AIDS and tuberculosis coinfection. Bull. Math. Biol. 71, 1745–1780 10.1007/s11538-009-9423-9 (doi:10.1007/s11538-009-9423-9) [DOI] [PubMed] [Google Scholar]
- 13.Mossong J., et al. 2008. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 5, e74. 10.1371/journal.pmed.0050074 (doi:10.1371/journal.pmed.0050074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hens N., Goeyvaerts N., Aerts M., Shkedy Z., Van Damme P., Beutels P. 2009. Mining social mixing patterns for infectious disease models based on a two-day population survey in Belgium. BMC Infect. Dis. 9, 5 10.1186/1471-2334-9-5 (doi:10.1186/1471-2334-9-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keeling M. J., Eames K. T. D. 2005. Networks and epidemic models. J. R. Soc. Interface 2, 295–307 10.1098/rsif.2005.0051 (doi:10.1098/rsif.2005.0051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Touray K., Adetifa I. M., Jallow A., Rigby J., Jeffries D., Cheung Y. B., Donkor S., Adegbola R. A., Hill P. C. 2010. Spatial analysis of tuberculosis in an Urban West African setting: is there evidence of clustering? Trop. Med. Int. Health 15, 664–672 10.1111/j.1365-3156.2010.02533.x (doi:10.1111/j.1365-3156.2010.02533.x) [DOI] [PubMed] [Google Scholar]
- 17.Gardy J. L., et al. 2011. Whole-genome sequencing and social-network analysis of a tuberculosis outbreak. N. Engl. J. Med. 364, 730–739 [DOI] [PubMed] [Google Scholar]
- 18.Read J. M., Keeling M. J. 2003. Disease evolution on networks: the role of contact structure. Proc. R. Soc. Lond. B 270, 699–708 10.1098/rspb.2002.2305 (doi:10.1098/rspb.2002.2305) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newman M. E. J. 2002. Spread of epidemic disease on networks. Phys. Rev. E 66, 016128. 10.1103/PhysRevE.66.016128 (doi:10.1103/PhysRevE.66.016128) [DOI] [PubMed] [Google Scholar]
- 20.Liljeros F., Edling C. R., Amaral L. A. N., Stanley H. E., Åberg Y. 2001. The web of human sexual contacts. Nature 411, 907–908 10.1038/35082140 (doi:10.1038/35082140) [DOI] [PubMed] [Google Scholar]
- 21.Cohen T., Colijn C., Finklea B., Murray M. 2007. Exogenous re-infection and the dynamics of tuberculosis epidemics: local effects in a network model of transmission. J. R. Soc. Interface 4, 523–531 10.1098/rsif.2006.0193 (doi:10.1098/rsif.2006.0193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization 2009. AIDS epidemic update. See http://data.unaids.org/pub/Report/2009/JC1700_Epi_Update_2009_en.pdf. [Google Scholar]
- 23.Vynnycky E., Fine P. E. M. 1997. The natural history of tuberculosis: the implications of age-dependent risks of disease and the role of reinfection. Epidemiol. Infect. 119, 183–201 10.1017/S0950268897007917 (doi:10.1017/S0950268897007917) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Springett V. H. 1971. Ten-year results during the introduction of chemotherapy for tuberculosis. Tubercle 52, 73–87 [DOI] [PubMed] [Google Scholar]
- 25.Enarson D. A., Rouillon A. 1998. The epidemiological basis of tuberculosis control. In Clinical tuberculosis, 2nd edn (ed. Davies P. D. O.), pp. 35–52 London, UK: Chapman and Hall Medical [Google Scholar]
- 26.Dye C., Garnett G. P., Sleeman K., Williams B. G. 1998. Prospects for worldwide tuberculosis control under the WHO DOTS strategy. Lancet Infect. Dis. 352, 1886–1891 [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization 2006. WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children, August 2006. See http://www.who.int/hiv/pub/guidelines/HIVstaging150307.pdf. [Google Scholar]
- 28.Ripley B. D. 1976. The second-order analysis of stationary point processes. J. Appl. Probab. 13, 255–266 10.2307/3212829 (doi:10.2307/3212829) [DOI] [Google Scholar]
- 29.Newman M. E. J. 2003. Properties of highly clustered networks. Phys. Rev. E 68, 026121. 10.1103/PhysRevE.68.026121 (doi:10.1103/PhysRevE.68.026121) [DOI] [PubMed] [Google Scholar]
- 30.Dixon P. M. 2002. Ripley's K function. Encycl. Environmetr. 3, 1796–1803 [Google Scholar]
- 31.Date A. A., Vitoria M., Granich R., Banda M., Fox M. Y., Gilks C. 2010. Implementation of co-trimoxazole prophylaxis and isoniazid preventive therapy for people living with HIV. Bull. World Health Organ. 88, 253–259 10.2471/BLT.09.066522 (doi:10.2471/BLT.09.066522) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mosimaneotsile B., Mathoma A., Chengeta B., Nyirenda S., Agizew T. B., Tedla Z., Motsamai O. I., Kilmarx P. H., Wells C. D., Samandari T. 2010. Isoniazid tuberculosis preventive therapy in HIV-infected adults accessing antiretroviral therapy: a Botswana experience, 2004–2006. J. Acquir. Immune Defic. Syndr. 54, 71–77 [DOI] [PubMed] [Google Scholar]
- 33.Reid M. J. A., Shah N. S. 2009. Approaches to tuberculosis screening and diagnosis in people with HIV in resource-limited settings. Lancet Infect. Dis. 9, 173–184 10.1016/S1473-3099(09)70043-x (doi:10.1016/S1473-3099(09)70043-x) [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization 2010. Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings, December. See http://whqlibdoc.who.int/publications/2011/9789241500708_eng.pdf. [Google Scholar]
- 35.Boughton B. 2010. Two studies, 2 conclusions about duration of TB prevention therapy for HIV-infected patients. Medscape Medical News, February 2010 (online) [Google Scholar]
- 36.Swaminathan S., et al. 2010. Efficacy of a 6-month vs a 36-month regimen for prevention of tuberculosis in HIV-infected persons in India: a randomized clinical trial. In 17th Conf. on Retroviruses and Opportunistic Infections; San Francisco, CA, USA, 16–19 February 2010, Abstract 103 [Google Scholar]
- 37.Chin D. P., Crane C. M., Diul M. Y., Sun S. J., Agraz R., Taylor S., Desmond E., Wise F. 2000. Spread of Mycobacterium tuberculosis in a community implementing recommended elements of tuberculosis control. J. Am. Med. Assoc. 283, 2968–2974 10.1001/jama.283.22.2968 (doi:10.1001/jama.283.22.2968) [DOI] [PubMed] [Google Scholar]
- 38.Munch Z., Van Lill S. W. P., Booysen C. N., Zietsman H. L., Enarson D. A., Beyers N. 2003. Tuberculosis transmission patterns in a high-incidence area: a spatial analysis. Int. J. Tuberc. Lung Dis. 7, 271–277 [PubMed] [Google Scholar]
- 39.KTD Eames 2008. Modelling disease spread through random and regular contacts in clustered populations. Theor. Popul. Biol. 73, 104–111 10.1016/j.tpb.2007.09.007 (doi:10.1016/j.tpb.2007.09.007) [DOI] [PubMed] [Google Scholar]
- 40.Miller J. C. 2009. Spread of infectious disease through clustered populations. J. R. Soc. Interface 6, 1121–1134 10.1098/rsif.2008.0524 (doi:10.1098/rsif.2008.0524) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keeling M. 2005. The implications of network structure for epidemic dynamics. Theor. Popul. Biol. 67, 1–8 10.1016/j.tpb.2004.08.002 (doi:10.1016/j.tpb.2004.08.002) [DOI] [PubMed] [Google Scholar]
- 42.Salathé M., Jones J. H. 2010. Dynamics and control of diseases in networks with community structure. PLoS Comput. Biol. 6, e1000736. 10.1371/journal.pcbi.1000736 (doi:10.1371/journal.pcbi.1000736) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh J. A., Upshur R., Padayatchi N. 2007. XDR-TB in South Africa: no time for denial or complacency. PLoS Med. 4, e50. 10.1371/journal.pmed.0040050 (doi:10.1371/journal.pmed.0040050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dye C. 2009. Doomsday postponed? Preventing and reversing epidemics of drug-resistant tuberculosis. Nat. Rev. Microbiol. 7, 81–87 10.1038/nrmicro2048 (doi:10.1038/nrmicro2048) [DOI] [PubMed] [Google Scholar]
- 45.Jassal M., Bishai W. R. 2009. Extensively drug-resistant tuberculosis. Lancet Infect. Dis. 9, 19–30 10.1016/S1473-3099(08)70260-3 (doi:10.1016/S1473-3099(08)70260-3) [DOI] [PubMed] [Google Scholar]
- 46.Cohen T., Colijn C., Murray M. 2008. Modeling the effects of strain diversity and mechanisms of strain competition on the potential performance of new tuberculosis vaccines. Proc. Natl Acad. Sci. USA 105, 16 302–16 307 10.1073/pnas.0808746105 (doi:10.1073/pnas.0808746105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Colijn C., Cohen T., Murray M. 2009. Latent coinfection and the maintenance of strain diversity. Bull. Math. Biol. 71, 247–263 10.1007/s11538-008-9361-y (doi:10.1007/s11538-008-9361-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Porco T. C., Martin J. N., Page-Shafer K. A., Cheng A., Charlebois E., Grant R. M., Osmond D. H. 2004. Decline in HIV infectivity following the introduction of highly active antiretroviral therapy. AIDS 18, 81–88 10.1097/00002030-200401020-00010 (doi:10.1097/00002030-200401020-00010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Calmy A., Pinoges L., Szumilin E., Zachariah R., Ford N., Ferradini L. 2006. Generic fixed-dose combination antiretroviral treatment in resource-poor settings: multicentric observational cohort. AIDS 20, 1163–1169. 10.1097/01.aids.0000226957.79847.d6 (doi:10.1097/01.aids.0000226957.79847.d6) [DOI] [PubMed] [Google Scholar]
- 50.Salomon J. A., Hogan D. R. 2008. Evaluating the impact of antiretroviral therapy on HIV transmission. AIDS 22, S149–S159 10.1097/01.aids.0000327636.82542.87 (doi:10.1097/01.aids.0000327636.82542.87) [DOI] [PubMed] [Google Scholar]
- 51.Donnell D., et al. 2010. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet Infect. Dis. 375, 2092–2098 10.1016/S0140-6736(10)60705-2 (doi:10.1016/S0140-6736(10)60705-2) [DOI] [PMC free article] [PubMed] [Google Scholar]