Abstract
Blood vessels are under constant mechanical loading from blood pressure and flow which cause internal stresses (endothelial shear stress and circumferential wall stress, respectively). The mechanical forces not only cause morphological changes of endothelium and blood vessel wall, but also trigger biochemical and biological events. There is considerable evidence that physiologic stresses and strains (stretch) exert vasoprotective roles via nitric oxide and provide a homeostatic oxidative balance. A perturbation of tissue stresses and strains can disturb biochemical homeostasis and lead to vascular remodelling and possible dysfunction (e.g. altered vasorelaxation, tone, stiffness, etc.). These distinct biological endpoints are caused by some common biochemical pathways. The focus of this brief review is to point out some possible commonalities in the molecular pathways in response to endothelial shear stress and circumferential wall stretch.
Keywords: mechanical transduction, laminar shear stress, circumferential stretch, endothelial cells, smooth muscle cells, oxidative stress
1. Introduction
It is well known that an artery consists of several layers of tissue: intima, media and adventitia. The abluminal side of endothelial cells layered on the intima makes contact with the blood flow whereas the media and adventitia provide mechanical support to oppose the blood pressure [1]. The pulsatile blood pressure originating from the heart drives blood circulation throughout the vasculature. The frictional force of the blood (wall shear stress) on the endothelial layer is opposed by tension and deformation in the endothelium while the circumferential distension of blood pressure is opposed by circumferential stress and strain (stretch) in the vessel wall (intima, media and adventitia). These applied loads (flow and pressure) and resulting internal stresses and deformation of the various vessel wall structure (fibres and cells) trigger and release biochemical reactants that maintain physiological function of blood vessels. The stresses and strains are intimately related through the material properties of the cells and fibres which can remodel in such conditions as hypertension [2] and diabetes [3,4].
Under physiologic conditions, a dynamic balance between mechanical or chemical stimulus and biological response is preserved to maintain homeostatic conditions [5]. A perturbation of this balance with mechanical stimuli that is too high or too low can either lead to physiological adaptation or possibly disease of the vessel wall. The most prominent perturbations involve changes in blood pressure and flow under physiologic (exercise, pregnancy, growth, development, etc.) or pathologic (hypertension, flow-reduction, flow-overload, etc.) conditions [6]. These physical as well as chemical perturbations such as in diabetes, hypercholesteremia, and cigarette smoking can change the stiffness of the vessel properties and hence alter the strain or stretch in response to the same loading (normotensive) or in hypertension (i.e. potential co-morbidity). A common mechanical feature inherent in both increase in pressure and flow is circumferential stretch. The former is induced by pressure distension and the latter by acute vasodilatation or chronic remodelling of vessel lumen.
How the vessel wall senses and transduces the mechanical stretch common to various haemodynamic perturbations remains a central topic of mechanobiology. A variety of receptors, integrins and extracellular matrix (ECM) components have been studied [6,7]. Various models of surface-sensor and nuclear-sensor have been proposed. In this brief review, we intend to identify the biochemical pathways that are common to both wall shear stress and pressure distension (i.e. circumferential stretch) as shown in figure 1, which summarizes the biological responses for endothelial cells and smooth muscle cells (SMC).
Figure 1.
A schematic diagram of cellular responses from the flow and pressure overload. The next level biochemical pathways induced by the wall shear stress and circumferential stretch are depicted in the graph. Two cell types, endothelial and SMC are included in the pathway. In the graph, solid arrows indicate activation while dash blunts indicate negative regulations.
2. Flow and pressure-mediated stretch
Both blood flow and pressure can induce vessels to stretch under different biological or physical mechanisms. Flow induces vasodilation whereas pressure causes mechanical distension (stretch). There is considerable literature on the role of wall shear stress in flow-mediated vasodilation. Nitric oxide (NO) generated from endothelial NO synthase (eNOS) is the critical biochemical that mediates the response to shear stress in cell culture and animal studies [8,9]. This is clinically supported by the association of hypertension and eNOS haplotypes in human studies [10]. In line with human data, chronic NOS inhibition in rat or eNOS gene deletion in mice results in significant blood pressure elevation [11,12]. Data from eNOS gene deficient mice, however, suggest that additional vasodilators are present because vessel reactivity from eNOS−/− mice remains fully functional as compared to those from wild-type mice [13]. In this regard, prostaglandin was demonstrated to be another important vasodilator [14].
NO bioavailability is also fundamental for regulation of the oxidative balance possibly by scavenging superoxide and inducing glutathion synthesis in the endothelium [15,16]. Another fundamental component of oxidative balance is nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase), a major vascular source of superoxide in addition to several other enzymes; e.g. xanthine oxidase, uncoupled eNOS, myeloperoxidase (MPO) and cyclooxygenase (COX). Chemically, superoxide generated from oxidase forms peroxynitrite, an oxidant and nitrating agent. Peroxynitrite can reduce vasodilation by directly reducing NO bioavailability and by reducing prostaglandin I2 (PGI2) content via nitration of PGI2 synthase [17]. In addition, peroxynitrite also oxidizes tetrahydrobiopterin, a cofactor of eNOS, which causes eNOS uncoupling [18]. Consequently, peroxynitrite can cause endothelial dysfunction through several mechanisms (figure 1). Regulation of NADPH oxidase activity can be achieved by modulation of angiotesin II (Ang II) receptor 1 (AT1R). Several AT1R antagonists have been shown to reduce reactive oxidative stress in human and animals [19,20]. Collectively, the oxidative balance regulates the degree of tone or vasodilatation and hence the stretch in the vessel wall.
An elevated blood pressure also creates increased circumferential stretch on the vessel wall perpendicular to the direction of blood flow [21,22] (figure 1). Chronic hypertension imposes higher pressure on the vessel wall and induces compensatory vascular remodelling. The adaptive remodelling results in increased wall thickness, which can increase small vessel resistance and can increase large vessel compliance [23]. An increase in circumferential stretch due to pressure-overload can enhance endothelial permeability and vascular tone [24]. It is known that an increase in circumferential stretch can also enhance superoxide production in both endothelium and SMC [25,26]. Several sources may be responsible for the superoxide over-production, including upregulated NADPH oxidase, mitochondrial oxidant and xanthine oxidase. Although NADPH oxidase is implicated as the major source of superoxide, xanthine oxidase clearly plays a role in pressure-mediated stretch of endothelium [27].
3. Endothelium signalling pathway under shear stress
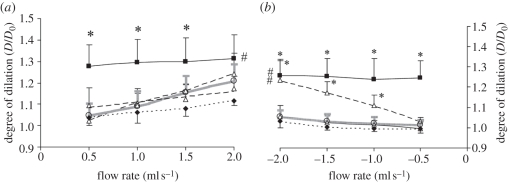
It has been well established that wall shear stress causes vessel dilation in an acute manner. The flow-mediated dilation has been shown to depend on the magnitude and the direction of blood flow and the activity of NADPH oxidase. In the presence of NADPH oxidase inhibitor, dilation induced by flow reversal was quantitatively comparable to that by the forward flow. In contrast, flow reversal induces negligible dilation in the absence of NADPH oxidase inhibitor [28,29] (figure 2).
Figure 2.
Vasodilations induced by forward or reverse flow conditions. This graph is revised from our previous work [28] and summarizes the degree of dilation as the function of flow rate. (a) Vessel dilation in forward flow condition (from proximal to distal) and (b) that in the reversal flow condition (from distal to proximal). Several superoxide pathway inhibitors were included in the experiment: solid line with filled black square, apocynin, a superoxide scavenger and NADPH oxidase inhibitor; dashed line with open triangle, gp91ds-tat, a NADPH oxidase inhibitor; small dashed line with filled black diamond, oxypurinol, a xanthine oxidase inhibitor; dashed-dotted line with open diamond, rotenone, a mitochondrial oxidase inhibitor; grey solid line with open circle, control. This graph highlights that reverse flow induces rate-dependent dilation in the similar degree as compared with forward flow in the presence of superoxide scavenger (apocynin) or a NADPH oxidase inhibitor (gp91ds-tat). The data suggest that flow direction and NADPH oxidase are essential to mediate the degree of dilation.
Multiple surface molecules are involved in mechanical transduction. Shear stress activates several pathways through endothelial surface molecules; e.g. platelet endothelial cell adhesion molecule (PECAM)-1, integrins, ion channels and tyrosine kinase receptor [30,31] (figure 1). Under shear stress, vascular NADPH oxidase is rapidly inactivated and superoxide production is reduced. Integrins can be activated by shear stress directly through tension or deformation on the endothelial surface (figure 1). The major pathways to activate integrins are the surface complex of PECAM-1, vascular endothelial cell cadherin (VE-cadherin) and vascular endothelial growth factor receptor 2 (VEGFR2) through the phosphoinositide 3-kinase (PI 3-kinase) [32,33].
Integrin pathway activation is important in shear-induced vasodilatation as evidenced by inhibition with Arg–Gly–Asp peptide which results in reduced flow-mediated dilation [34]. Shear stress-induced endothelium integrin activation can further phosphorylate eNOS at the site of serine-1179 which directly results in eNOS activation and enhanced NO production. The phosphorylation of serine-1179 can be mediated by AKT [35,36] or by protein kinase A [37]. The AKT activation is downstream from PI3 kinase [36] or from integrin-mediated phosphorylation [38]. eNOS generated NO can also modulate AT1R levels in addition to relaxation of SMC. Ramkhelawon et al. showed that eNOS knock-out mice express AT1R in both inner and outer curvatures of an aortic arch. eNOS transgenic mice, however, showed absence of AT1R expression in both inner and outer walls of the aortic arch as compared to the wild-type mice which expressed AT1R only at the inner curvature. They also demonstrated that shear stress reduces AT1R levels in an NO-dependent manner and the mechanism of action involves protein kinase A and protein kinase G. More specifically, protein kinase A plays an important role in eNOS activation and phosphorylation, followed by protein kinase G activation/downregulation of AT1R [39] (figure 1, dashed line on the left panel). Although more detailed molecular mechanisms are needed, this study sheds light on the additional role of NO since AT1R is a major regulator of endothelial NADPH oxidase.
As mentioned earlier, an additional interesting pathway involves COX-2 which results in the release of prostaglandin. One prostaglandin, PGI2, functions as an additional vasodilator in response to shear stress [40,41]. In eNOS-deficient mice, flow-mediated dilation is compensated by elevated prostaglandins relative to wild-type mice [40]. In Sun et al. [40] shear-mediated dilation was completely abolished in the presence of pathway inhibitors for NO and prostaglandin [42]. It was demonstrated that laminar shear stress-induced PGI2 can suppress tumour necrosis factor-α in endothelial cells. The mechanism in tumour necrosis factor-α reduction was mediated by downregulation of haem oxygenase-1 [43]. Although both COX-1 and COX-2 generate prostaglandins, additional studies demonstrate that COX-2 is essential for both shear stress response and to maintain flow-mediated dilation in the absence of eNOS [42,43]. The cross-talk between NO and prostaglandins is essential to maintain the normal tone of the vessel under physiologic conditions.
The signalling pathway for PGI2 is much less understood than the laminar shear stress-induced NO release. Under shear stress, 6-keto-prostaglandin 1α (a stable metabolite of PGI2) increased 15-fold after 12 h in a system of human umbilical vein endothelial cells [44]. In this study, mRNA levels of COX-2 and PGI synthase only increased onefold, which suggests that other mechanisms may be responsible for the 15-fold PGI2 release. In addition, PGI2 release can occur in minutes upon shear stress stimulation [44,45]. This suggests that PGI2 also responds to shear stress in an acute manner. Since arachidonic acid is the precursor of prostaglandins, it is reasonable to assume that arachidonic acid shedding plays an important role in shear stress-induced PGI2 release. One study indicated that shear stress increases cytosolic phospholipase A2 activity and arachidonic acid release in human endothelial cells [45]. It is unclear how cytosolic phospholipase A2 senses the mechanical stimuli and whether there is an additional form of phospholipase A2 involved in this process. These open questions warrant additional future studies.
As discussed above, steady laminar shear stress is a determinant of normal vascular function through orchestration of activities of eNOS/COX-2 and consequently NO/PGI2 (two critical molecules for vasodilatations and anti-platelet aggregation). Steady wall shear stress is also important to downregulate pro-thrombotic molecules, such as tissue factor, an initiator of thrombus formation [46]. The major fibrinolytic molecule, tissue plasminogen activator (tPA) is also regulated by shear stress. Under steady wall shear stress, the endothelial secretion of tPA increases [47] with upregulated mRNA level [48]. In addition to tissue factor and tPA, the major anti-coagulatant cofactor (thrombomodulin) was also shown to be upregulated by high shear stress in human endothelium cell lines [49,50].
The low or reversing shear stress in disturbed flows contribute to atherosclerotic initiation and progression in conjunction with multiple risk factors involved in this pathological process. Low or reversing shear stress regions predispose the vessel wall atherosclerosis [51]. One detrimental effect of low or reversing shear stress is the elevation of oxidative stress due to increased oxidase activities and decreased superoxide scavenger [28,29]. In addition, reduced production of PGI2 due to low shear stress also contributes to higher susceptibility of atherosclerosis [52,53]. The global effect of low shear stress contributes to a high inflammatory and high prothrombotic state. The superposition of a detailed flow field (wall shear stress, wall shear stress gradient, oscillatory shear index, etc.) through computational modelling [54] and biological expression of various biochemicals should allow for better understanding of relation between mechanical stimulation and disease initiation and progression.
4. Signalling pathway under circumferential stretch in endothelium and vascular smooth muscle cells
Although blood flow imposes shear stress on the endothelial cells, cardiac pulsation generates circumferential stretch and imposes mechanical stimulation on both endothelial cells and SMC. One of the most significant biological consequences of circumferential stretch is Ang II release from the endothelium that is accompanied by elevated superoxide levels [55]. It appears that these events occur on the synthesis level because angiotensin converting enzyme inhibitor quinprilat abolishes Ang II release, but AT1R antagonist losartan does not. Stretch-induced superoxide production can be inhibited by quinprilat or losartan, which underscores the role of Ang II and AT1R in this process. In addition to Ang II release, studies also suggest that circumferential stretch directly activates AT1R in a ligand-independent manner [56] through a conformational switch on transmembrane seven which undergoes anticlockwise rotation and a shift. As an inverse agonist, candesartan inhibits stretch-induced AT1R activation by binding and stabilizing the receptor in the inactive conformation [57]. Hence, circumferential stretch appears to activate AT1R through ligand-dependent or -independent pathway. It remains to be determined whether both ligand-dependent and ligand-independent pathways coexist.
In addition to induction of vascular constriction, it is well established that AT1R activation upregulates NADPH oxidase activity and enhances superoxide production [58]. Assembled vascular NADPH oxidase complex consists of two membrane-bound subunits (Nox1 or Nox4, and p22phox) and three cytosolic subunits (p47phox, p67phox and Rac) [59]. Rac is a small G protein that plays a critical role in AT1R-mediated NADPH oxidase activation [60–62]. AT1R is the high affinity receptor for Ang II with Kd of 1 nM [63]. AT1R activation triggers multiple assembly and activation of G-proteins (including Gαq, Gβγ). The further downstream signals from AT1R include Rac activation via PI 3-kinase activation and PKC activation which causes p47phox phosphorylation [62]. Rac activation and p47phox phosphorylation enable the multimer recruitment and assembly of the active form of NADPH oxidase. As illustrated in figure 1, Ang II-mediated oxidative stress induced by increased circumferential stretch can be exerted by enhancement of NADPH oxidase activity which leads to higher superoxide production and lower NO bioavailability [64]. These two pathways synergistically increase oxidative stress and may lead to endothelial dysfunction. The functional complexity of Ang II was shown to activate Ang II receptor 2 (AT2R) [65] in an opposite manner to regulate superoxide generation which was shown by AT2R antagonist treatment to enhance endothelial superoxide generation in contrast to the AT1R antagonist which has the converse effect [66]. These data suggest that AT2R functionally antagonizes AT1R to regulate NADPH oxidase through tyrosine phosphatases [66]. An additional study indicates that AT2R activation enhances eNOS phosphorylation via bradykinin receptors and elevates NO generation [67]. In this regard, AT2R is a check point to balance the prooxidative/proinflammatory function of AT1R (figure 1).
In SMC, integrin signalling is an integral element of the response to the mechanical stress [68]. Circumferential stretch induces integrin activation through interactions with the ECM [69]. Integrin signals are trickled down to Rho kinase and focal adhesion kinase (FAK) phosphorylation pathways [70].
Integrin activation is important for regulation of Rho family GTPase. Importance of Rho protein in actin polymerization was highlighted by RhoA RNA interference. In RhoA knock-down arteries, actin polymerization was significantly reduced [71]. Rho-GTP activates Rho kinase which in turn blocks myosin light chain phosphatase activity. The net activity from Rho/Rho kinase activation is the elevation of myosin light chain phosphorylation that further induces actin polymerization [72].
Integrin activation is also involved in Src-family tyrosine kinase activation by the direct protein–protein interactions [73]. Activated Src kinase can further activate FAK by phosphorylation. Paxillin is the target of activated FAK for phosphorylation in addition to multiple other kinases. The phosphorylated paxillin interacts with multiple proteins, including vinculin- and actin-related proteins (Arp2/3) to form the complex paxillin ‘interactome’ [74]. In swine carotid arteries, the regulatory role in sustained phase of contraction was demonstrated between tyrosine-118 phosphorylation on paxillin and actin polymerization [75].
5. Overlap of biochemical pathways in response to shear and stretch
Common molecules may be activated by laminar shear stress or circumferential stretch as shown in figure 1. Shear stress activates integrin on the endothelium surface to exert upregulation of eNOS activity through the molecular sensor PECAM-1 complex. In contrast, stretch-mediated integrin activation on SMC is modulated by ECM interactions and results in actin polymerization. Shear stress and stretch differentially regulate AT1R and induce opposite effects on superoxide generation. Shear stress may inactivate AT1R via the NO-dependent pathway. Circumferential stretch-induced Ang II release, on the other hand, can activate AT1R in a ligand-independent manner. Activation of AT1R leads to the NADPH oxidase-dependent superoxide production, and endothelial and SMC dysfunction. The basis for the seemingly opposing responses is unclear and requires further study. A full understanding of the commonality and differences in these biochemical pathways in response to perturbed physical environment may lead to additional pharmacological interventions and therapies for treatment of vascular diseases. Since the majority of current cardiovascular drugs are directed at lowering the risk factors (e.g. hypertension, diabetes, cigarette smoking, etc.), direct vascular pharmacological therapy may provide an add-on benefit. For example, as diabetes becomes a world pandemic healthcare challenge, treatment of diabetic vascular complications is essentially lacking. The dissection of vascular response in diabetes and related co-morbidities (e.g. altered haemodynamic conditions) may provide great opportunities to identify novel pharmacological therapies.
Acknowledgements
This research was supported in part by National Heart, Lung, and Blood Institute Grant HL-084 529 and HL-055 554-12. This work was supported in part by National Institute of Health Grants HL084 529 and HL055 554-11.
References
- 1.Califano J. P., Reinhart-King C. A. 2010. Exogenous and endogenous force regulation of endothelial cell behavior. J. Biomech. 43, 79–86 10.1016/j.jbiomech.2009.09.012 (doi:10.1016/j.jbiomech.2009.09.012) [DOI] [PubMed] [Google Scholar]
- 2.Agabiti-Rosei E., Rizzoni D. 2010. Regression of small resistance artery structural alterations in hypertension by appropriate antihypertensive treatment. Curr. Hypertens. Rep. 12, 80–85 10.1007/s11906-010-0093-7 (doi:10.1007/s11906-010-0093-7) [DOI] [PubMed] [Google Scholar]
- 3.Sachidanandam K., Hutchinson J. R., Elgebaly M. M., Mezzetti E. M., Dorrance A. M., Motamed K., Ergul A. 2009. Glycemic control prevents microvascular remodeling and increased tone in type 2 diabetes: link to endothelin-1. Am. J. Physiol. Regulatory, Integr. Comp. Physiol. 296, R952–R959 10.1152/ajpregu.90537.2008 (doi:10.1152/ajpregu.90537.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Creager M. A., Luscher T. F., Cosentino F., Beckman J. A. 2003. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy—part I. Circulation 108, 1527–1532 10.1161/01.CIR.0000091257.27563.32 (doi:10.1161/01.CIR.0000091257.27563.32) [DOI] [PubMed] [Google Scholar]
- 5.Chien S. 2007. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am. J. Physiol. Heart Circ. Physiol. 292, H1209–H1224 10.1152/ajpheart.01047.2006 (doi:10.1152/ajpheart.01047.2006) [DOI] [PubMed] [Google Scholar]
- 6.Chiu J.-J., Chien S. 2011. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol. Rev. 91, 327–387 10.1152/physrev.00047.2009 (doi:10.1152/physrev.00047.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies P. F. 2009. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat. Clin. Pract. Cardiovasc. Med. 6, 16–26 10.1038/ncpcardio1397 (doi:10.1038/ncpcardio1397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. 1987. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl Acad. Sci. USA 84, 9265–9269 10.1073/pnas.84.24.9265 (doi:10.1073/pnas.84.24.9265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang P. L., Huang Z., Mashimo H., Bloch K. D., Moskowitz M. A., Bevan J. A., Fishman M. C. 1995. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature 377, 239–242 10.1038/377239a0 (doi:10.1038/377239a0) [DOI] [PubMed] [Google Scholar]
- 10.Vasconcellos V., Lacchini R., Jacob-Ferreira A. L. B., Sales M. L., Ferreira-Sae M. C., Schreiber R., Nadruz W., Tanus-Santos J. E. 2010. Endothelial nitric oxide synthase haplotypes associated with hypertension do not predispose to cardiac hypertrophy. DNA Cell Biol. 29, 171–176 10.1089/dna.2009.0955 (doi:10.1089/dna.2009.0955) [DOI] [PubMed] [Google Scholar]
- 11.Ribeiro M., Antunes E., de Nucci G., Lovisolo S., Zatz R. 1992. Chronic inhibition of nitric oxide synthesis. A new model of arterial hypertension. Hypertension 20, 298–303 [DOI] [PubMed] [Google Scholar]
- 12.Shesely E. G., Maeda N., Kim H.-S., Desai K. M., Krege J. H., Laubach V. E., Sherman P. A., Sessa W. C., Smithies O. 1996. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc. Natl Acad. Sci. USA 93, 13 176–13 181 10.1073/pnas.93.23.13176 (doi:10.1073/pnas.93.23.13176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamping K. G., Nuno D. W., Shesely E. G., Maeda N., Faraci F. M. 2000. Vasodilator mechanisms in the coronary circulation of endothelial nitric oxide synthase-deficient mice. Am. J. Physiol. Heart Circ. Physiol. 279, H1906–H1912 [DOI] [PubMed] [Google Scholar]
- 14.Kadowitz P. J., Chapnick B. M., Feigen L. P., Hyman A. L., Nelson P. K., Spannhake E. W. 1978. Pulmonary and systemic vasodilator effects of the newly discovered prostaglandin, PGI2. J. Appl. Physiol. 45, 408–413 [DOI] [PubMed] [Google Scholar]
- 15.Moellering D., Mc Andrew J., Patel R. P., Forman H. J., Mulcahy R. T., Jo H., Darley-Usmar V. M. 1999. The induction of GSH synthesis by nanomolar concentrations of NO in endothelial cells: a role for γ-glutamylcysteine synthetase and γ-glutamyl transpeptidase. FEBS Lett. 448, 292–296 10.1016/S0014-5793(99)00371-3 (doi:10.1016/S0014-5793(99)00371-3) [DOI] [PubMed] [Google Scholar]
- 16.Moellering D., et al. 1998. Nitric oxide-dependent induction of glutathione synthesis through increased expression of γ-glutaphymylcysteine synthetase. Arch. Biochem. Biophys. 358, 74–82 10.1006/abbi.1998.0854 (doi:10.1006/abbi.1998.0854) [DOI] [PubMed] [Google Scholar]
- 17.Ullrich V., Daiber A., Bachschmid M., Zou M.-H. 2002. Nitration of prostacyclin synthase: mechanism and physiological implications. Int. Congr. Ser. 1233, 405–414 10.1016/S0531-5131(02)00375-8 (doi:10.1016/S0531-5131(02)00375-8) [DOI] [Google Scholar]
- 18.Kohnen S. L., Mouithys-Mickalad A. A., Deby-Dupont G. P., Deby C. M. T., Lamy M. L., Noels A. F. 2001. Oxidation of tetrahydrobiopterin by peroxynitrite or oxoferryl species occurs by a radical pathway. Free Rad. Res. 35, 709–721 10.1080/10715760100301221 (doi:10.1080/10715760100301221) [DOI] [PubMed] [Google Scholar]
- 19.Landmesser U., Spiekermann S., Preuss C., Sorrentino S., Fischer D., Manes C., Mueller M., Drexler H. 2007. Angiotensin II induces endothelial xanthine oxidase activation: role for endothelial dysfunction in patients with coronary disease. Arterioscler. Thromb. Vasc. Biol. 27, 943–948 10.1161/01.ATV.0000258415.32883.bf (doi:10.1161/01.ATV.0000258415.32883.bf) [DOI] [PubMed] [Google Scholar]
- 20.Welch W. J. 2008. Angiotensin II-dependent superoxide: effects on hypertension and vascular dysfunction. Hypertension 52, 51–56 10.1161/HYPERTENSIONAHA.107.090472 (doi:10.1161/HYPERTENSIONAHA.107.090472) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang W., Liu Y., Kassab G. S. 2007. Flow-induced shear strain in intima of porcine coronary arteries. J. Appl. Physiol. 103, 587–593 10.1152/japplphysiol.00199.2007 (doi:10.1152/japplphysiol.00199.2007) [DOI] [PubMed] [Google Scholar]
- 22.Kim J., Baek S. 2011. Circumferential variations of mechanical behavior of the porcine thoracic aorta during the inflation test. J. Biomech. 44, 1941–1947 10.1016/j.jbiomech.2011.04.022 (doi:10.1016/j.jbiomech.2011.04.022) [DOI] [PubMed] [Google Scholar]
- 23.Mulvany M. J. 1999. Vascular remodelling of resistance vessels: can we define this? Cardiovasc. Res. 41, 9–13 10.1016/S0008-6363(98)00289-2 (doi:10.1016/S0008-6363(98)00289-2) [DOI] [PubMed] [Google Scholar]
- 24.Birukova A. A., Chatchavalvanich S., Rios A., Kawkitinarong K., Garcia J. G. N., Birukov K. G. 2006. Differential regulation of pulmonary endothelial monolayer integrity by varying degrees of cyclic stretch. Am. J. Pathol. 168, 1749–1761 10.2353/ajpath.2006.050431 (doi:10.2353/ajpath.2006.050431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wung B. S., Cheng J. J., Hsieh H. J., Shyy Y. J., Wang D. L. 1997. Cyclic strain-induced monocyte chemotactic protein-1 gene expression in endothelial cells involves reactive oxygen species activation of activator protein 1. Circ. Res. 81, 1–7 [DOI] [PubMed] [Google Scholar]
- 26.Inoue N., Kawashima S., Hirata K.-I., Rikitake Y., Takeshita S., Yamochi W., Akita H., Yokoyama M. 1998. Stretch force on vascular smooth muscle cells enhances oxidation of LDL via superoxide production. Am. J. Physiol. Heart Circ. Physiol. 274, H1928–H1932 [DOI] [PubMed] [Google Scholar]
- 27.Abdulnour R.-E. E., et al. 2006. Mechanical stress activates xanthine oxidoreductase through MAP kinase-dependent pathways. Am. J. Physiol. Lung Cell. Mol. Physiol. 291, L345–L353 10.1152/ajplung.00453.2005 (doi:10.1152/ajplung.00453.2005) [DOI] [PubMed] [Google Scholar]
- 28.Godbole A. S., Lu X., Guo X., Kassab G. S. 2009. NADPH oxidase has a directional response to shear stress. Am. J. Physiol. Heart Circ. Physiol. 296, H152–H158 10.1152/ajpheart.01251.2007 (doi:10.1152/ajpheart.01251.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu X., Kassab G. S. 2004. Nitric oxide is significantly reduced in ex vivo porcine arteries during reverse flow because of increased superoxide production. J. Physiol. 561, 575–582 10.1113/jphysiol.2004.075218 (doi:10.1113/jphysiol.2004.075218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujiwara K. 2006. Platelet endothelial cell adhesion molecule-1 and mechanotransduction in vascular endothelial cells. J. Intern. Med. 259, 373–380 10.1111/j.1365-2796.2006.01623.x (doi:10.1111/j.1365-2796.2006.01623.x) [DOI] [PubMed] [Google Scholar]
- 31.Shyy J. Y.-J., Chien S. 2002. Role of integrins in endothelial mechanosensing of shear stress. Circ Res. 91, 769–775 10.1161/01.RES.0000038487.19924.18 (doi:10.1161/01.RES.0000038487.19924.18) [DOI] [PubMed] [Google Scholar]
- 32.Miao H., Yuan S., Wang Y., Tsygankov A., Chien S. 2002. Role of Cbl in shear-activation of PI 3-kinase and JNK in endothelial cells. Biochem. Biophys. Res. Commun. 292, 892–899 10.1006/bbrc.2002.6750 (doi:10.1006/bbrc.2002.6750) [DOI] [PubMed] [Google Scholar]
- 33.Tzima E., Irani-Tehrani M., Kiosses W. B., Dejana E., Schultz D. A., Engelhardt B., Cao G., DeLisser H., Schwartz M. A. 2005. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437, 426–431 10.1038/nature03952 (doi:10.1038/nature03952) [DOI] [PubMed] [Google Scholar]
- 34.Muller J. M., Chilian W. M., Davis M. J. 1997. Integrin signaling transduces shear stress–dependent vasodilation of coronary arterioles. Circ. Res. 80, 320–326 [DOI] [PubMed] [Google Scholar]
- 35.Dimmeler S., Fleming I., Fisslthaler B., Hermann C., Busse R., Zeiher A. M. 1999. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399, 601–605 10.1038/21224 (doi:10.1038/21224) [DOI] [PubMed] [Google Scholar]
- 36.Fisslthaler B., Dimmeler S., Hermann C., Busse R., Fleming I. 2000. Phosphorylation and activation of the endothelial nitric oxide synthase by fluid shear stress. Acta Physiol. Scand. 168, 81–88 10.1046/j.1365-201x.2000.00627.x (doi:10.1046/j.1365-201x.2000.00627.x) [DOI] [PubMed] [Google Scholar]
- 37.Boo Y. C., Sorescu G., Boyd N., Shiojima I., Walsh K., Du J., Jo H. 2002. Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Ser1179 by Akt-independent mechanisms. J. Biol. Chem. 277, 3388–3396 10.1074/jbc.M108789200 (doi:10.1074/jbc.M108789200) [DOI] [PubMed] [Google Scholar]
- 38.Koshida R., Rocic P., Saito S., Kiyooka T., Zhang C., Chilian W. M. 2005. Role of focal adhesion kinase in flow-induced dilation of coronary arterioles. Arterioscler. Thromb. Vasc. Biol. 25, 2548–2553 10.1161/01.ATV.0000188511.84138.9b (doi:10.1161/01.ATV.0000188511.84138.9b) [DOI] [PubMed] [Google Scholar]
- 39.Ramkhelawon B., Vilar J., Rivas D., Mees B., de Crom R., Tedgui A., Lehoux S. 2009. Shear stress regulates angiotensin type 1 receptor expression in endothelial cells. Circ. Res. 105, 869–875 10.1161/CIRCRESAHA.109.204040 (doi:10.1161/CIRCRESAHA.109.204040) [DOI] [PubMed] [Google Scholar]
- 40.Sun D., Huang A., Smith C. J., Stackpole C. J., Connetta J. A., Shesely E. G., Koller A., Kaley G. 1999. Enhanced release of prostaglandins contributes to flow-induced arteriolar dilation in eNOS knockout mice. Circ. Res. 85, 288–293 [DOI] [PubMed] [Google Scholar]
- 41.Koller A., Kaley G. 1990. Prostaglandins mediate arteriolar dilation to increased blood flow velocity in skeletal muscle microcirculation. Circ. Res. 67, 529–534 [DOI] [PubMed] [Google Scholar]
- 42.Sun D., Liu H., Yan C., Jacobson A., Ojaimi C., Huang A., Kaley G. 2006. COX-2 contributes to the maintenance of flow-induced dilation in arterioles of eNOS-knockout mice. Am. J. Physiol. Heart Circ. Physiol. 291, H1429–H1435 10.1152/ajpheart.01130.2005 (doi:10.1152/ajpheart.01130.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Francesco L., et al. 2009. Induction of prostacyclin by steady laminar shear stress suppresses tumor necrosis factor-alpha biosynthesis via heme oxygenase-1 in human endothelial cells. Circ. Res. 104, 506–513 10.1161/CIRCRESAHA.108.191114 (doi:10.1161/CIRCRESAHA.108.191114) [DOI] [PubMed] [Google Scholar]
- 44.Okahara K., Sun B., Kambayashi J.-i. 1998. Upregulation of prostacyclin synthesis-related gene expression by shear stress in vascular endothelial cells. Arterioscler. Thromb. Vasc. Biol. 18, 1922–1926 10.1161/01.ATV.18.12.1922 (doi:10.1161/01.ATV.18.12.1922) [DOI] [PubMed] [Google Scholar]
- 45.Pearce M. J., McIntyre T. M., Prescott S. M., Zimmerman G. A., Whatley R. E. 1996. Shear stress activates cytosolic phospholipase A2(cPLA2) and MAP kinase in human endothelial cells. Biochem. Biophys. Res. Commun. 218, 500–504 10.1006/bbrc.1996.0089 (doi:10.1006/bbrc.1996.0089) [DOI] [PubMed] [Google Scholar]
- 46.Rochier A., Nixon A., Yamashita N., Abe R., Madri J. A., Sumpio B. E. In press Laminar shear, but not orbital shear, has a synergistic effect with thrombin stimulation on tissue factor expression in human umbilical vein endothelial cells. J. Vasc. Surg. [DOI] [PubMed] [Google Scholar]
- 47.Kawai Y., Matsumoto Y., Watanabe K., Yamamoto H., Satoh K., Murata M., Handa M., Ikeda Y. 1996. Hemodynamic forces modulate the effects of cytokines on fibrinolytic activity of endothelial cells. Blood 87, 2314–2321 [PubMed] [Google Scholar]
- 48.Malek A., Jackman R., Rosenberg R., Izumo S. 1994. Endothelial expression of thrombomodulin is reversibly regulated by fluid shear stress. Circ. Res. 74, 852–860 [DOI] [PubMed] [Google Scholar]
- 49.Takada Y., Shinkai F., Kondo S., Yamamoto S., Tsuboi H., Korenaga R., Ando J. 1994. Fluid shear stress increases the expression of thrombomodulin by cultured human endothelial cells. Biochem. Biophys. Res. Commun. 205, 1345–1352 10.1006/bbrc.1994.2813 (doi:10.1006/bbrc.1994.2813) [DOI] [PubMed] [Google Scholar]
- 50.Bergh N., Ulfhammer E., Glise K., Jern S., Karlsson L. 2009. Influence of TNF-α and biomechanical stress on endothelial anti- and prothrombotic genes. Biochem. Biophys. Res. Commun. 385, 314–318 10.1016/j.bbrc.2009.05.046 (doi:10.1016/j.bbrc.2009.05.046) [DOI] [PubMed] [Google Scholar]
- 51.Caro C. G., Fitz-Gerald J. M., Schroter R. C. 1969. Arterial wall shear and distribution of early atheroma in man. Nature 223, 1159–1161 10.1038/2231159a0 (doi:10.1038/2231159a0) [DOI] [PubMed] [Google Scholar]
- 52.Sinzinger H., Clopath P., Silberbauer K., Winter M. 1980. Is the variation in the susceptibility of various species to atherosclerosis due to inborn differences in prostacyclin (PGI2) formation? Cell. Mol. Life Sci. 36, 321–323 10.1007/BF01952302 (doi:10.1007/BF01952302) [DOI] [PubMed] [Google Scholar]
- 53.Smith D. D., Tan X., Tawfik O., Milne G., Stechschulte D. J., Dileepan K. N. 2010. Increased aortic atherosclerotic plaque development in female apolipoprotein E-null mice is associated with elevated thromboxane A2 and decreased prostacyclin production. J. Physiol. Pharmacol. 61, 309–316 [PMC free article] [PubMed] [Google Scholar]
- 54.Huo Y., Guo X., Kassab G. 2008. The flow field along the entire length of mouse aorta and primary branches. Ann. Biomed. Eng. 36, 685–699 10.1007/s10439-008-9473-4 (doi:10.1007/s10439-008-9473-4) [DOI] [PubMed] [Google Scholar]
- 55.Gatti C. D., Osto E., Kouroedov A., Eto M., Shaw S., Volpe M., Lüscher T. F., Cosentino F. 2008. Pulsatile stretch induces release of angiotensin II and oxidative stress in human endothelial cells: effects of ACE inhibition and AT1 receptor antagonism. Clin. Exp. Hypertens. 30, 616–627 10.1080/10641960802443183 (doi:10.1080/10641960802443183) [DOI] [PubMed] [Google Scholar]
- 56.Zou Y., et al. 2004. Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat. Cell Biol. 6, 499–506 10.1038/ncb1137 (doi:10.1038/ncb1137) [DOI] [PubMed] [Google Scholar]
- 57.Yasuda N., et al. 2008. Conformational switch of angiotensin II type 1 receptor underlying mechanical stress-induced activation. EMBO Rep. 9, 179–186 10.1038/sj.embor.7401157 (doi:10.1038/sj.embor.7401157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Warnholtz A., et al. 1999. Increased NADH-oxidase-mediated superoxide production in the early stages of atherosclerosis: evidence for involvement of the renin-angiotensin system. Circulation 99, 2027–2033 10.1161/01.CIR.99.15.2027 (doi:10.1161/01.CIR.99.15.2027) [DOI] [PubMed] [Google Scholar]
- 59.Dusting G. J., Selemidis S., Jiang F. 2005. Mechanisms for suppressing NADPH oxidase in the vascular wall. Memórias do Instituto Oswaldo Cruz. 100, 97–103 10.1590/S0074-02762005000900016 (doi:10.1590/S0074-02762005000900016) [DOI] [PubMed] [Google Scholar]
- 60.Seshiah P. N., Weber D. S., Rocic P., Valppu L., Taniyama Y., Griendling K. K. 2002. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ. Res. 91, 406–413 10.1161/01.RES.0000033523.08033.16 (doi:10.1161/01.RES.0000033523.08033.16) [DOI] [PubMed] [Google Scholar]
- 61.Wassmann S., Laufs U., Bäumer A. T., Konkol C., Sauer H., Bäohm M., Nickenig G. 2001. Inhibition of geranylgeranylation reduces angiotensin II-mediated free radical production in vascular smooth muscle cells: involvement of angiotensin AT1 receptor expression and Rac1 GTPase. Mol. Pharmacol. 59, 646–654 [DOI] [PubMed] [Google Scholar]
- 62.Choi H., Leto T. L., Hunyady L. s, Catt K. J., Bae Y. S., Rhee S. G. 2008. Mechanism of angiotensin II-induced superoxide production in cells reconstituted with angiotensin type 1 receptor and the components of NADPH oxidase. J. Biol. Chem. 283, 255–267 10.1074/jbc.M708000200 (doi:10.1074/jbc.M708000200) [DOI] [PubMed] [Google Scholar]
- 63.Shukla A. K., Reinhart C., Michel H. 2006. Comparative analysis of the human angiotensin II type 1a receptor heterologously produced in insect cells and mammalian cells. Biochem. Biophys. Res. Commun. 349, 6–14 10.1016/j.bbrc.2006.07.210 (doi:10.1016/j.bbrc.2006.07.210) [DOI] [PubMed] [Google Scholar]
- 64.Zhang Q., et al. 2008. Paradoxical activation of endothelial nitric oxide synthase by NADPH oxidase. Arterioscler. Thromb. Vasc. Biol. 28, 1627–1633 10.1161/ATVBAHA.108.168278 (doi:10.1161/ATVBAHA.108.168278) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schulman I., Raij L. 2008. The angiotensin II type 2 receptor: what is its clinical significance? Curr. Hypertens. Rep. 10, 188–193 10.1007/s11906-008-0036-8 (doi:10.1007/s11906-008-0036-8) [DOI] [PubMed] [Google Scholar]
- 66.Sohn H. Y., Raff U., Hoffmann A., Gloe T., Heermeier K., Galle J., Pohl U. 2000. Differential role of angiotensin II receptor subtypes on endothelial superoxide formation. Br. J. Pharmacol. 131, 667–672 10.1038/sj.bjp.0703566 (doi:10.1038/sj.bjp.0703566) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yayama K., Hiyoshi H., Imazu D., Okamoto H. 2006. Angiotensin II stimulates endothelial NO synthase phosphorylation in thoracic aorta of mice with abdominal aortic banding via type 2 receptor. Hypertension 48, 958–964 10.1161/01.HYP.0000244108.30909.27 (doi:10.1161/01.HYP.0000244108.30909.27) [DOI] [PubMed] [Google Scholar]
- 68.Harburger D. S., Calderwood D. A. 2009. Integrin signalling at a glance. J. Cell Sci. 122, 159–163 10.1242/jcs.018093 (doi:10.1242/jcs.018093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schwartz M. A. 2010. Integrins and extracellular matrix in mechanotransduction. Cold Spring Harbor Perspectives in Biology. 2(12):ARTN a005066. (doi:10.1101/cshperspect.a005066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tang D. D., Anfinogenova Y. 2008. Physiologic properties and regulation of the actin cytoskeleton in vascular smooth muscle. J. Cardiovasc. Pharmacol. Therapeut. 13, 130–140 10.1177/1074248407313737 (doi:10.1177/1074248407313737) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Corteling R. L., Brett S. E., Yin H., Zheng X.-L., Walsh M. P., Welsh D. G. 2007. The functional consequence of RhoA knockdown by RNA interference in rat cerebral arteries. Am. J. Physiol. Heart Circ. Physiol. 293, H440–H447 10.1152/ajpheart.01374.2006 (doi:10.1152/ajpheart.01374.2006) [DOI] [PubMed] [Google Scholar]
- 72.Chen X., Pavlish K., Benoit J. N. 2008. Myosin phosphorylation triggers actin polymerization in vascular smooth muscle. Am. J. Physiol. Heart Circ. Physiol. 295, H2172–H2177 10.1152/ajpheart.91437.2007 (doi:10.1152/ajpheart.91437.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arias-Salgado E. G., Lizano S., Sarkar S., Brugge J. S., Ginsberg M. H., Shattil S. J. 2003. Src kinase activation by direct interaction with the integrin beta cytoplasmic domain. Proc. Natl Acad. Sci. USA 100, 13 298–13 302 10.1073/pnas.2336149100 (doi:10.1073/pnas.2336149100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Deakin N. O., Turner C. E. 2008. Paxillin comes of age. J. Cell Sci. 121, 2435–2444 10.1242/jcs.018044 (doi:10.1242/jcs.018044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rembold C. M., Tejani A. D., Ripley M. L., Han S. 2007. Paxillin phosphorylation, actin polymerization, noise temperature, and the sustained phase of swine carotid artery contraction. Am. J. Physiol. Cell Physiol. 293, C993–C1002 10.1152/ajpcell.00090.2007 (doi:10.1152/ajpcell.00090.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]