Abstract
Genetic diathesis to schizophrenia may involve alterations of adolescent neurodevelopment manifesting as cognitive deficits. Brain regions mediating executive function (fronto-striatal circuits) develop during adolescence while those supporting elementary aspects of attention (e.g. sustained focused attention) have a more protracted maturation beginning in childhood. We hence predicted that adolescents at risk for schizophrenia would show a failure of normal maturation of executive function. We prospectively assessed 18 offspring and 6 siblings of schizophrenia patients (HR) and 28 healthy controls at baseline, year-1 and year-2 follow-up using the Continuous Performance Test [visual-d′] and Wisconsin Card Sort Test (WCST). Perseverative errors on the WCST in HR remained stable but decreased in controls over the follow-up (study-group by assessment–time interaction, p = 0.01, controlling for IQ). No significant study-group by assessment-time interactions were seen for sustained attentional performance. HR may not improve while healthy subjects progressively improve on executive function during adolescence and early adulthood. Our results suggest an altered maturational trajectory of executive function during adolescence in individuals at familial risk for schizophrenia.
Keywords: Attention, Executive function, Relatives, Schizophrenia
1. Introduction
Schizophrenia may involve heritable alterations of neurodevelopment during adolescence and young adulthood (Keshavan et al., 2004). These alterations may manifest as cognitive deficits in areas including executive function and attention (Gur et al., 2007) and brain structural deficits (Keshavan et al., 2003, 1997) in schizophrenia patients, and in their genetically predisposed relatives. While maturation of the ability to sustain focused attention, as evaluated by continuous performance tests (Gur et al., 2007) begins in childhood (Levin et al., 1991; Lin et al., 1999), higher executive functions mature throughout adolescence and early adulthood (Levin et al., 1991). Executive function deficits are noted in schizophrenia patients, may precede the illness and have been shown to manifest in relatives of patients ‘at risk’ for the illness (Diwadkar et al., 2006; Shad et al., 2006). Executive function deficits in schizophrenia patients have been commonly indexed using perseverative error, non-perseverative error scores and lack of set-maintenance during performance of the Wisconsin Card Sorting test (WCST) (Bell et al., 1997). Of these measures, perseverative errors in relatives of patients, at familial risk for the illness, have been thought to represent vulnerability to the illness (Caldu et al., 2007; Egan et al., 2001; Franke et al., 1992; Gooding et al., 1999). As increased perseverative errors may be a relatively specific marker of schizophrenia, when compared to bipolar disorder (Wobrock et al., 2009) this deficit in relatives at risk may be an endophenotypic marker of schizophrenia (Prasad and Keshavan, 2008). Executive function, as assessed by perseverative errors on the WCST in normal individuals and schizophrenia patients may critically depend on prefrontal function and structure (Diwadkar et al., 2006; Kawada et al., 2009; Keshavan et al., 2002; Prasad and Keshavan, 2008; Shad et al., 2004, 2006). The number of perseverative errors has been linked to poor prefrontal regulation of striatal dopaminergic activity in patients (Meyer-Lindenberg et al., 2002) and may depend on prefrontal cortical dopaminergic and glutamatergic transmission (Caldu et al., 2007; Enomoto and Floresco, 2009; Krystal et al., 2000; Meyer-Lindenberg et al., 2002; Siegel et al., 1996). While executive function may depend on prefrontal and striatal function, sustained focused attention to visual information depends on a wider network including fronto-parietal regions (Posner and Dehaene, 1994). Brain structural studies show fronto-striatal regions to continue development during late adolescence and young adulthood (Sowell et al., 1999a), while parietal regions appear to mature earlier, during childhood (Gogtay et al., 2004; Sowell et al., 1999b). Functional imaging studies also suggest that while prefrontal cortical function begins in childhood and continues to mature through adolescence into young adulthood, maturation of parietal cortical function progresses more rapidly in childhood than in adolescence (Luna et al., 2001). The development of attention during childhood and of executive function throughout adolescence and young adulthood (Levin et al., 1991; Lin et al., 1999) may reflect maturation of parietal regions predominantly during childhood and the continuing maturation of frontal regions during adolescence. Executive function may however partly depend on extra-prefrontal regions (Abdullaev et al., 1998; Corbetta et al., 1995; Gur et al., 2007; Tomasi et al., 2008), hence undergoing some maturation during childhood.
Genetic diathesis for schizophrenia may involve neurodevelopmental alterations during adolescence (Eack et al., 2008; Keshavan et al., 2004). These may specifically compromise the maturation of prefrontal and fronto-striatal circuits during adolescence, sparing parietal cortical maturation which is completed by adolescence. Studies have shown altered prefrontal function and structure (Keshavan et al., 2003; Keshavan et al., 1997; Macdonald et al., 2008) in adolescent relatives of patients. Selective alterations of prefronto-striatal development in at-risk relatives may manifest as dysmaturation of executive function during adolescence and early adulthood in the context of a relatively less impaired development of sustained attention, the brain structures mediating which may mature before adolescence.
This prediction may be examined by prospective assessment of perseverative errors on the WCST, a putative marker of prefrontal cortical function (Caldu et al., 2007; Diwadkar et al., 2006; Egan et al., 2001; Enomoto and Floresco, 2009; Kawada et al., 2009; Keshavan et al., 2002; Krystal et al., 2000; Meyer-Lindenberg et al., 2002; Shad et al., 2004; Shad et al., 2006; Siegel et al., 1996) and of sustained attention, in first degree adolescent relatives of patients with familial liability to schizophrenia. Further, as perseverative errors on the WCST may not significantly correlate with attention (Chen et al., 1997), they may serve to assess executive function deficits per se, without the confound of attention deficits, common in relatives of patients (Keshavan et al., 2009). Cross-sectional deficits of executive function are noted in adult relatives of schizophrenia patients (Wolf et al., 2002). Longitudinal studies of attention and executive function have assessed adolescent relatives showing sub-threshold psychotic symptoms (Keefe et al., 2006) and post-adolescent relatives (Erlenmeyer-Kimling et al., 1995; Johnstone et al., 2005; Lencz et al., 2006; Niendam et al., 2007). Studies assessing relatives showing subthreshold psychotic symptoms may be confounded by interaction effects of symptoms with genetic risk on cognition and neurodevelopment (Eack et al., 2008; Keshavan et al., 2004). A study did not find a longitudinal change in attention or executive function but was limited by a low sample size and by assessment of second degree relatives who may have a reduced familial loading for schizophrenia compared to first-degree relatives (Whyte et al., 2006). Progressive decline of scholastic performance and IQ occurs in the premorbid phase, suggesting a declining course for executive function during this phase (Fuller et al., 2002; Kremen et al., 1998). Although cross sectional studies show poor attention and executive function in adolescent relatives of patients during asymptomatic phases of schizophrenia (Bove, 2008; Keefe et al., 1994a; Keshavan et al., 2004; Wood et al., 2003), the longitudinal course of these deficits has not been examined.
However, previous studies using this strategy are limited by the cross-sectional nature of assessments (Keefe et al., 1994b) and inclusion of second degree relatives, which may dilute the genetic loading for schizophrenia (Whyte et al., 2006).
We prospectively assessed sustained attention and executive function using the Continuous Performance Test (CPT) (Gur et al., 2007; Lin et al., 1999) and WCST respectively in non-psychotic adolescent and young adult offspring and siblings of parents with schizophrenia (HR) at baseline, one-year and two-year follow-up. We predicted that executive function, but not attention in HR would fail to improve or decrease over time compared to controls suggesting, respectively, either a lag of the maturational improvement of executive function or an emerging decline.
2. Methods
2.1. Participants
The study was conducted at the Western Psychiatric Institute and Clinic, Pittsburgh. Participants were 18 young adult and adolescent offspring and 6 siblings [age (mean, S.D) = 14.94 years, 3.26 years, range = 6.4 to 24.7 years] of schizophrenia and schizoaffective probands (HR) and 28 healthy controls (HC) [age (mean, S.D) = 15.48 years, 3.04 years, range = 6.9 to 25.3 years]. The first-degree relatives (offspring and siblings) of patients with schizophrenia or schizoaffective disorder were recruited by approaching patients and through advertisements. Diagnoses of schizophrenia or schizoaffective disorder in the index relatives were confirmed using the Structured Clinical Interview for DSM Disorders (SCID) (First et al., 1995) and/or consensus meetings led by senior diagnosticians (M.K and D.M). Co-morbid with these psychotic disorders, 12 had major depressive disorder, one proband had depression not-otherwise-specified (NOS) and one proband was diagnosed with generalized anxiety, at baseline or within the past month. HR and HC were assessed at baseline, year 1 and year 2 using the SCID for the possible emergence of psychopathology. One offspring subject was diagnosed with psychotic illness at the year-1 follow-up and another converted to psychosis at the year-2 assessment. HC were included only if they did not have a first- or second-degree relative with psychotic illness. Those participants with DSM Mental Retardation, lifetime evidence of a psychotic disorder or lifetime exposure to antipsychotic medications at baseline, recent substance use disorder, neurological or medical condition were excluded. All participants signed informed consent after the study was fully explained to them. For participants <18 years of age, the consent was provided by the parent or guardian, and the subjects provided informed assent. The study was approved by the University of Pittsburgh Institutional Review Board.
2.2. Cognitive assessments
Percentage of perseverative errors committed on the WCST (Heaton et al., 1993)and visual d′ scores on the CPT-IP (Cornblatt et al., 1988)were used to assess executive function and sustained attention respectively. Assessments were conducted at baseline, and subjects were re-evaluated one and two years later. One of the subjects was briefly treated with an anti-psychotic (aripiprazole for 9 days) during the study.
2.3. Statistical analysis
Analysis of covariance (ANCOVA) and analysis of variance (ANOVA) were used to compare attention (visual d′ scores) and executive function (percentage of perseverative errors) between HR and controls at baseline. Repeated-measures tests with study-group (control/HR) as a between-subject factor and assessment-time (baseline/year 1/year 2) as a within-subject factor, were used to compare the longitudinal trajectories of attention and executive function between HR and controls. IQ deficits in schizophrenia may confound analyses of attention and executive function in schizophrenia (Dickinson et al., 2008). IQ decline is seen in schizophrenia, and controlling IQ when assessing cognitive deficits has been debated (Dickinson et al., 2008), as there are potential overcorrection and undercorrection problems with either strategy. Therefore, we conducted all comparisons using both ANCOVA and ANOVA, controlling and not controlling for IQ at baseline respectively. Significant interactions on the repeated-measures tests were interrogated by separate repeated-measures ANOVAs for controls and HR with assessment-time as the within-subject factor. Longitudinal analyses of executive function and sustained attention indices were repeated using repeated measures mixed-model designs with assessment-time as the random factor and group and gender as the fixed factors and age and IQ as covariates. This method might better account for correlations of the longitudinal, within-subject cognitive performance indices and has been used for longitudinal analyses of cognitive performance (Maurice-Stam et al., 2009).
3. Results
At baseline, HR had lower IQ than controls [Mean (S.D), controls: 113.6 (10.6), HR: 102.4 (11.2), F(1,50) = 12.09, p = .00] and did not differ from controls on education (in years educated) [Mean (S.D), controls: 9.82 (3.98), HR: 9.06 (3.55), F(1,50) = 0.58, p = 0.425], age (in years) [Mean (S.D), controls: 15.48 (3.04), HR: 14.94 (3.26), F(1,50) = 0.44, p = 0.43] or gender (45.2% males in HR, 43.6% males in control groups Chi-square = 0.04, p = 0.84). As the percentage of perseverative errors and visual-d' scores were not normally distributed (see Table 1), all raw scores were rank transformed before being analyzed by ANCOVA and ANOVA tests.
Table 1.
Shapiro–Wilk's statistics (W) and their p values are mentioned for raw scores of percentage of perseverative errors and visual-d' for HC and HR for each assessment time. p < 0.05 indicates a non-normal distribution of that dependent variable.
| HC (W,p) | HR (W,p) | |||||
|---|---|---|---|---|---|---|
| Baseline | Year 1 | Year 2 | Baseline | Year 1 | Year 2 | |
| Percentage of perseverative errors | 0.72, 0.00 | 0.87, 0.00 | 0.82, 0.00 | 0.86, 0.00 | 0.82, 0.00 | 0.77, 0.00 |
| Visual-d' scores | 0.97, 0.43 | 0.97, 0.85 | 0.98, 0.89 | 0.87, 0.00 | 0.94, 0.16 | 0.92, 0.04 |
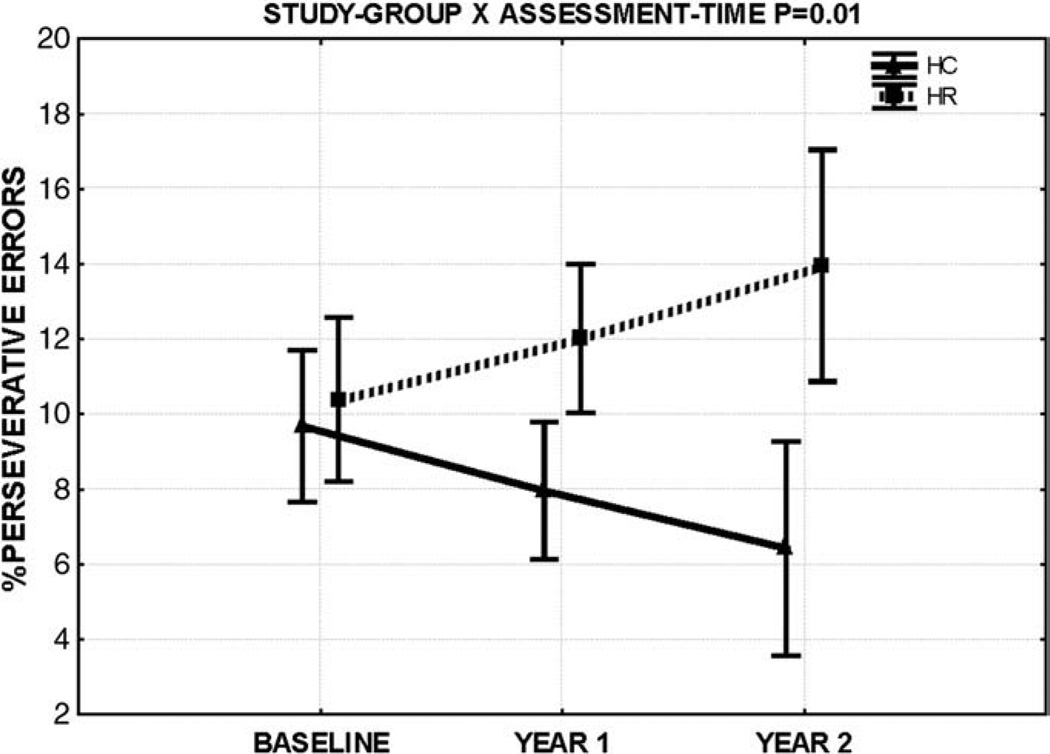
At baseline assessment, percentage of perseverative errors did not differ across groups. Repeated measures ANOVAs revealed a group by assessment-time interaction suggesting a differential longitudinal course for executive function in HR relative to controls. Main effects of assessment-time on the within-group repeated-measures ANOVAs revealed that controls progressively decreased [F(2,54) = 3.54, p = 0.035] while HR did not change [F(2,46) = 1.8, p = 0.17] on perseverative errors over follow-up (see Figs. 1 and 2). A main effect of study-group was also noted showing HR to commit more errors during the study. These findings for executive function deficits survived controlling for IQ (see Tables 2 and 3, Figs. 1 and 2). A repeated measures mixed-model ANCOVA with assessment-time as the random factor, group and gender as the fixed factors and age as the covariate also revealed a significant group × assessment-time interaction [F = 3.40, p = 0.035] but no main effect of group. The group × assessment-time interaction remained significant [F = 3.71, p = 0.026] after adding IQ as a covariate to this model. All above deficits, noted in HR using percentage of perseverative errors maintained statistical significance on reanalyses using the number of perseverative errors to index executive function. No deficits of attention were observed at baseline assessment or longitudinally. Without controlling for IQ, HR were seen to have poorer attention compared to controls although no longitudinal effects were noted (see Tables 2 and 3). Analyses using a mixed model design similar to that used for executive function did not reveal longitudinal sustained attention deficits [group × assessment-time, F = 1.12, p = 0.31 without controlling for IQ and F = 1.09, p = 0.31 after including IQ as a covariate] All above results maintained significance after controlling for age and gender.
Fig. 1.
Percentage of perseverative errors (raw scores) are plotted on the Y-axis against follow-up time. Bars denote 95% confidence intervals about the group mean. See Fig. 2 for subject-wise raw score distribution for the percentage of perseverative errors.
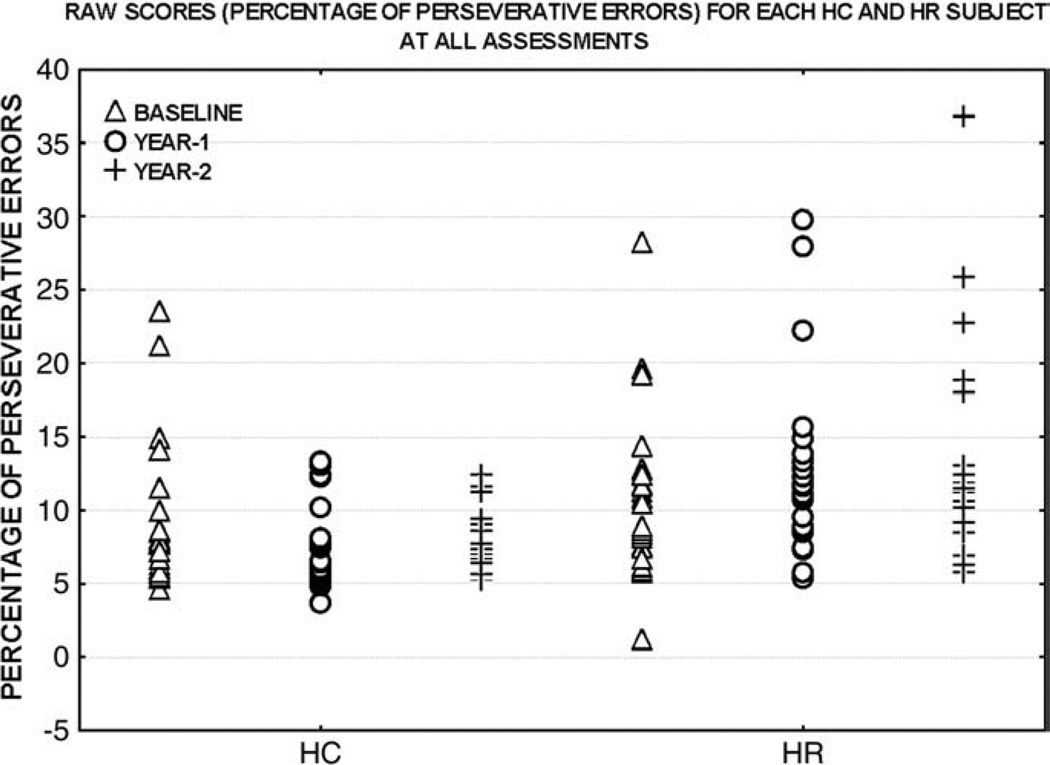
Fig. 2.
Raw scores (percentage of perseverative errors) for each HC and HR subject are plotted. Baseline, year-1 and year-2 raw scores for each subject are plotted on the Y-axis and are presented from left to right for each group.
Table 2.
Baseline assessments used ANOVA (left columns) and ANCOVA (right columns) with I.Q as covariate. Repeated measures ANOVA (left columns) and ANCOVA (right columns), with I.Q as covariate were used to examine main effect of study-group and study-group × time.
| Baseline | Main effect: study-group | Study-group × time | ||||
|---|---|---|---|---|---|---|
| ANOVA/ANCOVA | F(1,50),p | F(1,49),p | F(1,50),p | F(1,49),p | F(1,100),p | F(1,98),p |
| WCST | 0.98, 0.440 | 0.26, 0.612 | 12.81, 0.000 | 5.42, 0.026 | 4.9, 0.009 | 4.5, 0.011 |
| CPT | 3.38, 0.071 | 1.79, 0.250 | 5.24, 0.026 | 1.21, 0.426 | 0.20, 0.681 | 0.16, 0.832 |
Table 3.
Mean and standard deviations (S.D) for raw scores of percentage of perseverative errors and visual-d' scores for HC and HR at baseline, year 1 and year 2 assessments are tabulated.
| HC | HR | |||||
|---|---|---|---|---|---|---|
| Baseline Mean, S.D | Year 1 Mean, S.D | Year 2 Mean, S.D | Baseline Mean, S.D | Year 1 Mean, S.D | Year 2 Mean, S.D | |
| Percentage of perseverative errors | 9.51, 5.21 | 7.84, 2.77 | 7.14, 1.96 | 10.57, 5.33 | 12.10, 6.41 | 13.25, 7.35 |
| Visual-d' scores | 1.61, 0.87 | 1.94, 1.01 | 2.42, 0.93 | 1.12, 0.97 | 1.48, 1.04 | 1.78, 1.18 |
4. Discussion
We show an altered maturational trajectory of executive performance in adolescent and young adult non-psychotic offspring and siblings of schizophrenia patients compared to controls. Executive function in controls progressively improved and that in HR remained stable over the follow-up. This finding survived controlling for IQ suggesting that the arrested development of executive function in HR is independent of generalized intellectual deficit (Byrne et al., 1999; Dickinson et al., 2008). Although we note a failure of executive function maturation, inspection of means (Fig. 1 and Table 3) suggested a non-significant decline of executive function in HR. A subtle decline in executive function in HR could have missed statistical significance due to the low sample size. Reduced ability to sustain focused attention in HR did not survive controlling for IQ (Dickinson et al., 2008) and changed similarly for HR and controls over time.
Neurodevelopmental alterations in schizophrenia emerge during adolescence and may have genetic bases (Harrison, 2007). Maturation of fronto-striatal dopaminergic circuits, including the progressively increasing prefrontal cortical regulation of striatal dopaminergic transmission may occur during adolescence and young adulthood (Sowell et al., 1999a). The maturation of these circuits involves a progressive increase of top–down prefrontal influences on striatal dopaminergic transmission (Meyer-Lindenberg et al., 2002; Sowell et al., 1999a).The fronto-striatal circuits subserve executive function and the progressive maturation of prefrontal regulation of striatal processing (Sowell et al., 1999a) may contribute to the normative development and emergence of executive function during adolescence. We have found structural, functional and metabolic abnormalities (Diwadkar et al., 2001; Keshavan et al., 2003, 2002, 1997) of prefrontal regions in adolescent relatives of schizophrenia patients suggesting that enduring prefrontal neuropathology may play a critical role in the familial diathesis to schizophrenia. Alterations of adolescent neurodevelopment are implicated in genetic diathesis to schizophrenia and may interfere with fronto-striatal and consequently executive function maturation. Adolescent carriers of genetic polymorphisms thought to interfere with fronto-striatal dopaminergic activity show compromised executive performance (Harrison, 2007; Weinberger et al., 2001). Altered maturation of prefrontal and striatal regions may explain the altered developmental trajectory of executive function we observed in HR.
Maturation of parietal and thalamic circuits mediating sustained attention may be completed by adolescence (Gogtay et al., 2004; Sowell et al., 1999b), hence pre-dating the neurodevelopmental alterations in adolescent HR subjects. This may explain the absence of longitudinal alterations in attention in adolescent HR subjects as suggested by a non-significant study-group × time effect for CPT scores. Further, while attention deficits in schizophrenia are moderately heritable (heritability = 0.48–0.62 (Gur et al., 2007)), executive function may correlate with both genetic and environmental influences (Gur et al., 2007) and may be vulnerable to environmental stressors and gene–environment interaction effects during adolescence (Harrison, 2007).
Our study adds to recent evidence implicating a familial basis for deficits in WCST performance in schizophrenia. A study showed relatives with a family history of schizophrenia patients to commit more errors on the WCST compared to those without familial predisposition, implicating genetic risk in executive function deficits (Birkett et al., 2008). Executive function as assessed by the trail-making test may also be a heritable marker of schizophrenia (Quinones et al., 2009). Recent studies have suggested that genes, including those on chromosome 22 and genetic variants of the catechol-O-methyl-transferase (COMT) gene may be involved in WCST performance abnormalities (Liao et al., 2009; Liu et al., 2008) in schizophrenia. Dopamine transporter genetic polymorphisms in schizophrenia may also be related to WCST performance deficits (Rybakowski et al., 2005), as would be expected from the central role for dopaminergic transmission in the fronto-striatal circuits which may subserve executive performance (Meyer-Lindenberg et al., 2002). WCST performance deficits, in light of no CPT performance abnormalities in HR subjects may support previous evidence of differential deficits of specific neurotransmitter systems in schizophrenia (Bosia et al., 2009). Our study is limited by lack of data regarding sub-threshold (prodromal) psychotic symptoms, which may emerge in young subjects at familial risk for schizophrenia and which may be related to executive function deficits (Berman et al., 1997). Our results of executive function deficits may be confounded by working memory and general intelligence impairments, common in HR (Gur et al., 2007). As working memory performance was not assessed, it remains unclear whether our findings of increased perseverative errors in HR represent working memory alterations rather than executive function deficits per se (Hartman et al., 2003). Although our findings survived controlling for IQ, the role of general intelligence deficits in executive function deficits cannot be ruled out, as statistically controlling for IQ may undercorrect the findings for those executive function deficits correlated with general intelligence (IQ) deficits (Dickinson et al., 2008). Practice effects may be seen for the WCST and may occur due to processes like development of memory for the testing procedures and materials, procedural learning and increasing familiarity with the testing settings due to repeated exposure to the test (Goldberg et al., 2007). These effects lead to a progressive improvement in performance on repeatedly (e.g. longitudinally) administered cognitive tests. Differential practice effects in control compared to HR subjects may have confounded our findings. Schizophrenia patients may show smaller practice effects compared to healthy individuals possibly due poor initial encoding (Gold et al., 2000; Goldberg et al., 2007) of information and reduced non-declarative (procedural) learning capacities (Weickert et al., 2002). The evidence for reduced practice effects in schizophrenia is however conflicting, as a study showed procedural (non-declarative) memory capacities to be relatively spared in patients (Goldberg et al., 2007; Heaton et al., 1994). Further, although the WCST performance decline we show in HR subjects may reflect reduced efficacy of practice effects rather than a deterioration of executive function per se, reduced practice effects have not been as yet documented in relatives of schizophrenia patients to our knowledge.
Our findings suggest an impaired development of executive function in adolescent offspring and siblings at genetic risk for schizophrenia. This may reflect dysmaturation of prefrontal cortices and fronto-striatal circuits in the genetic diathesis to schizophrenia (Harrison, 2007; Krystal et al., 2000; Weinberger et al., 2001). However, a progressive, albeit subtle, decline in executive functioning during the premorbid phase of schizophrenia consistent with a neurodegenerative model cannot be ruled out (DeLisi, 2008).
Acknowledgements
We thank Diana Mermon for her help with the recruitment and Shreedhar Kulkarni and Jean Miewald for their help with data management.
Role of funding source
National Institute of Mental Health (MH 64023 and 01180 to MK); National Alliance for Research on Schizophrenia and Depression (Independent Investigator award to MK).
Abbreviations
- WCST
Wisconsin Card Sorting test
- CPT-IP
Continuous Performance Test-Identical Pairs version
- IQ
Intelligence Quotient
- HC
Healthy control subjects
- HR
Subjects at high risk for schizophrenia
- SD
Standard Deviation
- ANCOVA
Analysis of covariance
- ANOVA
Analysis of variance
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- SCID
Structured Clinical Interview for DSM Disorders
Footnotes
Conflict of interest statement
This research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Abdullaev Y, Bechtereva N, Melnichuk K. Neuronal activity of human caudate nucleus and prefrontal cortex in cognitive tasks. Behav Brain Res. 1998;97:159–177. doi: 10.1016/s0166-4328(98)00037-0. [DOI] [PubMed] [Google Scholar]
- Bell MD, Greig TC, Kaplan E, Bryson G. Wisconsin Card Sorting Test dimensions in schizophrenia: factorial, predictive, and divergent validity. J Clin Exp Neuropsychol. 1997;19:933–941. doi: 10.1080/01688639708403774. [DOI] [PubMed] [Google Scholar]
- Berman I, Viegner B, Merson A, Allan E, Pappas D, Green AI. Differential relationships between positive and negative symptoms and neuropsychological deficits in schizophrenia. Schizophr Res. 1997;25:1–10. doi: 10.1016/S0920-9964(96)00098-9. DOI: S0920-9964(96)00098-9 [pii] [DOI] [PubMed] [Google Scholar]
- Birkett P, Sigmundsson T, Sharma T, Toulopoulou T, Griffiths TD, Reveley A, et al. Executive function and genetic predisposition to schizophrenia—the Maudsley family study. Am J Med Genet B Neuropsychiatr Genet. 2008;147:285–293. doi: 10.1002/ajmg.b.30594. [DOI] [PubMed] [Google Scholar]
- Bosia M, Anselmetti S, Pirovano A, Ermoli E, Marino E, Bramanti P, et al. HTTLPR functional polymorphismin schizophrenia: executive functions vs. sustained attention dissociation. Prog Neuropsychopharmacol Biol Psychiatry. 2009 doi: 10.1016/j.pnpbp.2009.10.001. DOI: S0278-5846(09)00346-7 [pii] [DOI] [PubMed] [Google Scholar]
- Bove E. Cognitive performance and basic symptoms in first-degree relatives of schizophrenic patients. Compr Psychiatry. 2008;49:321–329. doi: 10.1016/j.comppsych.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Byrne M, Hodges A, Grant E, Owens DC, Johnstone EC. Neuropsychological assessment of young people at high genetic risk for developing schizophrenia compared with controls: preliminary findings of the Edinburgh High Risk Study (EHRS) Psychol Med. 1999;29:1161–1173. doi: 10.1017/s0033291799001002. [DOI] [PubMed] [Google Scholar]
- Caldu X, Vendrell P, Bartres-Faz D, Clemente I, Bargallo N, Jurado MA, et al. Impact of the COMT Val108/158 Met and DAT genotypes on prefrontal function in healthy subjects. Neuroimage. 2007;37:1437–1444. doi: 10.1016/j.neuroimage.2007.06.021. DOI: S1053-8119(07)00565-4 [pii] [DOI] [PubMed] [Google Scholar]
- Chen EY, Lam LC, Chen RY, Nguyen DG, Chan CK, Wilkins AJ. Neuropsychological correlates of sustained attention in schizophrenia. Schizophr Res. 1997;24:299–310. doi: 10.1016/s0920-9964(96)00120-x. DOI: S092099649600120X [pii] [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman G, Miezin F, Petersen S. Superior parietal cortex activation during spatial attention shifts and visual feature conjunction. Science. 1995;270:802. doi: 10.1126/science.270.5237.802. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Risch NJ, Faris G, Friedman D, Erlenmeyer-Kimling L. The Continuous Performance Test, identical pairs version (CPT-IP): I: new findings about sustained attention in normal families. Psychiatry Res. 1988;26:223–238. doi: 10.1016/0165-1781(88)90076-5. DOI: 0165-1781(88)90076-5 [pii] [DOI] [PubMed] [Google Scholar]
- DeLisi LE. The concept of progressive brain change in schizophrenia: implications for understanding schizophrenia. Schizophr Bull. 2008;34:312–321. doi: 10.1093/schbul/sbm164. DOI: sbm164 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D, Ragland JD, Gold JM, Gur RC. General and specific cognitive deficits in schizophrenia: Goliath defeats David? Biol Psychiatry. 2008;64:823–827. doi: 10.1016/j.biopsych.2008.04.005. DOI: S0006-3223(08)00401-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar VA, Sweeney JA, Boarts D, Montrose DM, Keshavan MS. Oculomotor delayed response abnormalities in young offspring and siblings at risk for schizophrenia. CNS Spectr. 2001;6:899–903. doi: 10.1017/s109285290000095x. [DOI] [PubMed] [Google Scholar]
- Diwadkar VA, Montrose DM, Dworakowski D, Sweeney JA, Keshavan MS. Genetically predisposed offspring with schizotypal features: an ultra high-risk group for schizophrenia? Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:230–238. doi: 10.1016/j.pnpbp.2005.10.019. DOI: S0278-5846(05)00318-0 [pii] [DOI] [PubMed] [Google Scholar]
- Eack SM, Prasad KM, Montrose DM, Goradia DD, Dworakowski D, Miewald J, et al. An integrated psychobiological predictive model of emergent psychopathology among young relatives at risk for schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1873–1878. doi: 10.1016/j.pnpbp.2008.08.024. DOI: S0278-5846(08)00257-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto T, Floresco SB. Disruptions in spatial working memory, but not short-term memory, induced by repeated ketamine exposure. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:668–675. doi: 10.1016/j.pnpbp.2009.03.013. DOI: S0278-5846(09)00084-0 [pii] [DOI] [PubMed] [Google Scholar]
- Erlenmeyer-Kimling L, Squires-Wheeler E, Adamo U, Bassett A, Cornblatt B, Kestenbaum C, et al. The New York High-Risk Project. Psychoses and cluster A personality disorders in offspring of schizophrenic parents at 23 years of follow-up. Arch Gen Psychiatry. 1995;52:857. doi: 10.1001/archpsyc.1995.03950220067013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Williams J, Gibbon M. Structured clinical interview for DSM-IV (SCID) Washington, DC: American Psychiatric Association; 1995. [Google Scholar]
- Franke P, Maier W, Hain C, Klingler T. Wisconsin Card Sorting Test: an indicator of vulnerability to schizophrenia? Schizophr Res. 1992;6:243–249. doi: 10.1016/0920-9964(92)90007-r. [DOI] [PubMed] [Google Scholar]
- Fuller R, Nopoulos P, Arndt S, O'Leary D, Ho B, Andreasen N. Longitudinal assessment of premorbid cognitive functioning in patients with schizophrenia through examination of standardized scholastic test performance. Am J Psychiatry. 2002;159:1183–1189. doi: 10.1176/appi.ajp.159.7.1183. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamicmapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Rehkemper G, Binks SW, III, Carpenter CJ, Fleming K, Goldberg TE, et al. Learning and forgetting in schizophrenia. J Abnorm Psychology. 2000;109:534–538. [PubMed] [Google Scholar]
- Goldberg TE, Goldman RS, Burdick KE, Malhotra AK, Lencz T, Patel RC, et al. Cognitive improvement after treatment with second-generation antipsychotic medications in first-episode schizophrenia: is it a practice effect? Arch Gen Psychiatry. 2007;64:1115–1122. doi: 10.1001/archpsyc.64.10.1115. DOI: 64/10/1115 [pii] [DOI] [PubMed] [Google Scholar]
- Gooding DC, Kwapil TR, Tallent KA. Wisconsin Card Sorting Test deficits in schizotypic individuals. Schizophr Res. 1999;40:201–209. doi: 10.1016/s0920-9964(99)00124-3. DOI: S0920996499001243 [pii] [DOI] [PubMed] [Google Scholar]
- Gur R, Calkins M, Gur R, Horan W, Nuechterlein K, Seidman L, et al. The consortium on the genetics of schizophrenia: neurocognitive endophenotypes. Schizophr Bull. 2007;33:49. doi: 10.1093/schbul/sbl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ. Schizophrenia susceptibility genes and neurodevelopment. Biol Psychiatry. 2007;61:1119–1120. doi: 10.1016/j.biopsych.2007.02.021. DOI: S0006-3223(07)00194-1 [pii] [DOI] [PubMed] [Google Scholar]
- Hartman M, Steketee MC, Silva S, Lanning K, Andersson C. Wisconsin Card Sorting Test performance in schizophrenia: the role of working memory. Schizophr Res. 2003;63:201–217. doi: 10.1016/s0920-9964(02)00353-5. DOI: S0920996402003535 [pii] [DOI] [PubMed] [Google Scholar]
- Heaton R, Chelune G, Talley J, Kay G, Curtiss G. Wisconsin Card Sorting Test manual: revised and expanded. Odessa, FL: Psychological Assessment Resources; 1993. [Google Scholar]
- Heaton R, Paulsen JS, McAdams LA, Kuck J, Zisook S, Braff D, et al. Neuropsychological deficits in schizophrenics. Relationship to age, chronicity, and dementia. Arch Gen Psychiatry. 1994;51:469–476. doi: 10.1001/archpsyc.1994.03950060033003. [DOI] [PubMed] [Google Scholar]
- Johnstone E, Ebmeier K, Miller P, Owens D, Lawrie S. Predicting schizophrenia: findings from the Edinburgh high-risk study. Br J Psychiatry. 2005;186:18–25. doi: 10.1192/bjp.186.1.18. [DOI] [PubMed] [Google Scholar]
- Kawada R, Yoshizumi M, Hirao K, Fujiwara H, Miyata J, Shimizu M, et al. Brain volume and dysexecutive behavior in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1255–1260. doi: 10.1016/j.pnpbp.2009.07.014. DOI: S0278-5846(09)00242-5 [pii] [DOI] [PubMed] [Google Scholar]
- Keefe R, Silverman J, Roitman S, Harvey P, Duncan M, Alroy D, et al. Performance of nonpsychotic relatives of schizophrenic patients on cognitive tests. Psychiatry Res. 1994a;53:1–12. doi: 10.1016/0165-1781(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Silverman JM, Roitman SE, Harvey PD, Duncan MA, Alroy D, et al. Performance of nonpsychotic relatives of schizophrenic patients on cognitive tests. Psychiatry Res. 1994b;53:1–12. doi: 10.1016/0165-1781(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Perkins DO, Gu H, Zipursky RB, Christensen BK, Lieberman JA. A longitudinal study of neurocognitive function in individuals at-risk for psychosis. Schizophr Res. 2006;88:26–35. doi: 10.1016/j.schres.2006.06.041. DOI: S0920-9964(06)00309-4 [pii] [DOI] [PubMed] [Google Scholar]
- Keshavan M, Diwadkar V, Montrose D, Stanley J, Pettegrew J. Premorbid characterization in schizophrenia: the Pittsburgh High Risk Study. World Psychiatry. 2004;3:163–168. [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Montrose DM, Pierri JN, Dick EL, Rosenberg D, Talagala L, et al. Magnetic resonance imaging and spectroscopy in offspring at risk for schizophrenia: preliminary studies. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:1285–1295. doi: 10.1016/s0278-5846(97)00164-4. DOI: S0278584697001644 [pii] [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Diwadkar VA, Spencer SM, Harenski KA, Luna B, Sweeney JA. A preliminary functional magnetic resonance imaging study in offspring of schizophrenic parents. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:1143–1149. doi: 10.1016/s0278-5846(02)00249-x. DOI: S0278-5846(02)00249-X [pii] [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Stanley JA, Montrose DM, Minshew NJ, Pettegrew JW. Prefrontal membrane phospholipid metabolism of child and adolescent offspring at risk for schizophrenia or schizoaffective disorder: an in vivo 31P MRS study. Mol Psychiatry. 2003;8:316–323. doi: 10.1038/sj.mp.4001325. 251. [pii] [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Diwadkar VA, Montrose DM, Stanley JA, Pettegrew JW. Premorbid characterization in schizophrenia: the Pittsburgh High Risk Study. World Psychol. 2004;3:163–168. [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Kulkarni S, Bhojraj T, Francis A, Diwadkar V, Montrose DM, et al. Premorbid cognitive deficits in young relatives of schizophrenia patients. Front Hum Nutr. 2009;3 doi: 10.3389/neuro.09.062.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen W, Buka S, Seidman L, Goldstein J, Koren D, Tsuang M. IQ Decline during Childhood and Adult Psychotic Symptoms in a Community Sample: A 19-year Longitudinal Study. Am Psychiatric Assoc. 1998:672–677. doi: 10.1176/ajp.155.5.672. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Bennett A, Abi-Saab D, Belger A, Karper LP, D'Souza DC, et al. Dissociation of ketamine effects on rule acquisition and rule implementation: possible relevance to NMDA receptor contributions to executive cognitive functions. Biol Psychiatry. 2000;47:137–143. doi: 10.1016/s0006-3223(99)00097-9. DOI: S0006-3223(99)00097-9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencz T, Smith C, McLaughlin D, Auther A, Nakayama E, Hovey L, et al. Generalized and specific neurocognitive deficits in prodromal schizophrenia. Biol Psychiatry. 2006;59:863–871. doi: 10.1016/j.biopsych.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Levin H, Culhane K, Hartmann J, Evankovich K, Mattson A, Harward H, et al. Developmental changes in performance on tests of purported frontal lobe functioning. Dev Neuropsychol. 1991;7:377–395. [Google Scholar]
- Liao SY, Lin SH, Liu CM, Hsieh MH, Hwang TJ, Liu SK, et al. Genetic variants in COMT and neurocognitive impairment in families of patients with schizophrenia. Genes Brain Behav. 2009;8:228–237. doi: 10.1111/j.1601-183X.2008.00467.x. DOI: GBB467 [pii] [DOI] [PubMed] [Google Scholar]
- Lin C, Hsiao C, Chen W. Development of sustained attention assessed using the continuous performance test among children 6–15 years of age. J Abnorm Child Psychol. 1999;27:403–412. doi: 10.1023/a:1021932119311. [DOI] [PubMed] [Google Scholar]
- Liu YL, Fann CS, Liu CM, Chen WJ, Wu JY, Hung SI, et al. RASD2, MYH9, and CACNG2 genes at chromosome 22q12 associated with the subgroup of schizophrenia with non-deficit in sustained attention and executive function. Biol Psychiatry. 2008;64:789–796. doi: 10.1016/j.biopsych.2008.04.035. DOI: S0006-3223(08)00567-2 [pii] [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, et al. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [pii] S1053-8119(00)90743-2. [DOI] [PubMed] [Google Scholar]
- Macdonald AW, III, Thermenos HW, Barch DM, Seidman LJ. Imaging genetic liability to schizophrenia: systematic review of fMRI studies of patients' nonpsychotic relatives. Schizophr Bull. 2008 doi: 10.1093/schbul/sbn053. DOI: sbn053 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice-Stam H, Oort FJ, Last BF, Brons PP, Caron HN, Grootenhuis MA. School-aged children after the end of successful treatment of non-central nervous system cancer: longitudinal assessment of health-related quality of life, anxiety and coping. Eur J Cancer Care (Engl) 2009;18:401–410. doi: 10.1111/j.1365-2354.2008.01041.x. DOI: ECC1041 [pii] [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Miletich RS, Kohn PD, Esposito G, Carson RE, Quarantelli M, et al. Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat Neurosci. 2002;5:267–271. doi: 10.1038/nn804. [pii] [DOI] [PubMed] [Google Scholar]
- Niendam T, Bearden C, Zinberg J, Johnson J, O'Brien M, Cannon T. The course of neurocognition and social functioning in individuals at ultra high risk for psychosis. Schizophr Bull. 2007;33:772. doi: 10.1093/schbul/sbm020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Dehaene S. Attentional networks. Trends Neurosci. 1994;17:75–79. doi: 10.1016/0166-2236(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Prasad KM, Keshavan MS. Structural cerebral variations as useful endophenotypes in schizophrenia: do they help construct “extended endophenotypes”? Schizophr Bull. 2008;34:774–790. doi: 10.1093/schbul/sbn017. DOI: sbn017 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinones RM, Calderin YC, Dominguez M, Bravo TM, Berazain AR, Garcia A, et al. Heritability of Trail Making Test performance in multiplex schizophrenia families: implications for the search for an endophenotype. Eur Arch Psychiatry Clin Neurosci. 2009;259:475–481. doi: 10.1007/s00406-009-0012-6. [DOI] [PubMed] [Google Scholar]
- Rybakowski JK, Borkowska A, Czerski PM, Kapelski P, Dmitrzak-Weglarz M, Hauser J. An association study of dopamine receptors polymorphisms and the Wisconsin Card Sorting Test in schizophrenia. J Neural Transm. 2005;112:1575–1582. doi: 10.1007/s00702-005-0292-6. [DOI] [PubMed] [Google Scholar]
- Shad MU, Muddasani S, Prasad K, Sweeney JA, Keshavan MS. Insight and prefrontal cortex in first-episode schizophrenia. Neuroimage. 2004;22:1315–1320. doi: 10.1016/j.neuroimage.2004.03.016. [pii]S1053811904001715. [DOI] [PubMed] [Google Scholar]
- Shad MU, Tamminga CA, Cullum M, Haas GL, Keshavan MS. Insight and frontal cortical function in schizophrenia: a review. Schizophr Res. 2006;86:54–70. doi: 10.1016/j.schres.2006.06.006. DOI: S0920-9964(06)00274-X [pii] [DOI] [PubMed] [Google Scholar]
- Siegel BV, Jr, Trestman RL, O'Flaithbheartaigh S, Mitropoulou V, Amin F, Kirrane R, et al. D-amphetamine challenge effects on Wisconsin Card Sort Test. Performance in schizotypal personality disorder. Schizophr Res. 1996;20:29–32. doi: 10.1016/0920-9964(95)00002-x. DOI: 0920-9964(95)00002-X [pii] [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci. 1999a;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Batth R, Jernigan TL, Toga AW. Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. Neuroimage. 1999b;9:587–597. doi: 10.1006/nimg.1999.0436. DOI: S1053-8119(99)90436-6 [pii] [DOI] [PubMed] [Google Scholar]
- Tomasi D, Wang R, Telang F, Boronikolas V, Jayne M, Wang G, et al. Impairment of attentional networks after 1 night of sleep deprivation. Cereb Cortex. 2008 doi: 10.1093/cercor/bhn073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weickert TW, Terrazas A, Bigelow LB, Malley JD, Hyde T, Egan MF, et al. Habit and skill learning in schizophrenia: evidence of normal striatal processing with abnormal cortical input. Learn Mem. 2002;9:430–442. doi: 10.1101/lm.49102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR, Egan MF, Bertolino A, Callicott JH, Mattay VS, Lipska BK, et al. Prefrontal neurons and the genetics of schizophrenia. Biol Psychiatry. 2001;50:825–844. doi: 10.1016/s0006-3223(01)01252-5. S0006322301012525 [pii] [DOI] [PubMed] [Google Scholar]
- Whyte M, Brett C, Harrison L, Byrne M, Miller P, Lawrie S, et al. Neuropsychological performance over time in people at high risk of developing schizophrenia and controls. Biol Psychiatry. 2006;59:730–739. doi: 10.1016/j.biopsych.2005.08.028. [DOI] [PubMed] [Google Scholar]
- Wobrock T, Ecker UK, Scherk H, Schneider-Axmann T, Falkai P, Gruber O. Cognitive impairment of executive function as a core symptom of schizophrenia. World J Biol Psychiatry. 2009;10:442–451. doi: 10.1080/15622970701849986. 790041372 [pii] [DOI] [PubMed] [Google Scholar]
- Wolf LE, Cornblatt BA, Roberts SA, Shapiro BM, Erlenmeyer-Kimling L. Wisconsin Card Sorting deficits in the offspring of schizophrenics in the New York High-Risk Project. Schizophr Res. 2002;57:173. doi: 10.1016/s0920-9964(01)00301-2. S0920996401003012 [pii] [DOI] [PubMed] [Google Scholar]
- Wood S, Pantelis C, Proffitt T, Phillips L, Stuart G, Buchanan J, et al. Spatial working memory ability is a marker of risk-for-psychosis. Psychol Med. 2003;33:1239–1247. doi: 10.1017/s0033291703008067. [DOI] [PubMed] [Google Scholar]