Abstract
Objective
The purpose of this study was to assess the safety of S-1 in Japanese in inoperable or recurrent breast cancer patients.
Methods
A prospective post-marketing surveillance was performed at 313 sites in Japan in patients with inoperable or recurrent breast cancer treated with S-1. We examined 1361 patients between January 2006 and December 2007 with regard to the incidence of adverse drug reactions graded by the Common Terminology Criteria for Adverse Events (CTCAE), version 3.0.
Results
At least one adverse drug reaction was encountered by 858 patients, with an overall incidence of 63.0% (858/1361). The incidence of Grade 3 or higher adverse drug reactions in a descending order was 14.7% (200/1361). In this study, the most common combination drug was trastuzumab. The overall incidence of adverse drug reactions was 63.5% (431/679 patients) in patients treated with S-1 alone, and 55.9% (66/118 patients) in patients treated with S-1 + trastuzumab.
Conclusions
Monotherapy with S-1 or combination therapy with S-1 + trastuzumab was well tolerated for inoperable or recurrent breast cancer patients.
Keywords: S-1, breast cancer, post-marketing surveillance, trastuzumab
INTRODUCTION
Breast cancer is currently curable if detected and treated early, with a better prognosis than other cancers. However, recurrent breast cancer is hard to cure, but can be treated to improve symptoms and quality of life and prolong survival time.
S-1 is a formulation comprising tegafur (FT), a prodrug of 5-fluorouracil (5-FU), gimeracil (CDHP), which inhibits dihydropyrimidine dehydrogenase (a catabolic enzyme of 5-FU) and oteracil potassium (Oxo), which inhibits orotate phosphoribosyltransferase (a kinase for 5-FU) at a molar ratio of 1:0.4:1 (FT:CDHP:Oxo). It is currently used for the treatment of breast cancer, gastric cancer, colorectal cancer, head and neck cancer, non-small cell lung cancer, pancreatic cancer and biliary cancer. S-1 is expected to be a therapeutic option that reduces the burden on patients because it can be administered orally on an outpatient basis, thereby reducing the number of hospital visits (1,2).
S-1 was approved for the treatment of inoperable or recurrent breast cancer in 2005 and had a response rate of 41.7% in a Phase II study involving patients previously treated with a single regimen and 21.8% in another Phase II study involving patients who were unresponsive to taxanes (3,4).
Trastuzumab is a humanized monoclonal antibody (4D5) designed to bind to the extracellular domain of human epidermal growth factor receptor 2 (HER2). The NCCN guidelines recommend trastuzumab, either with or without chemotherapy, as the first-line treatment for patients with HER2-overexpressing breast cancer (5). Trastuzumab can be combined with fluoropyrimidines, and the efficacy in combination with capecitabine has been reported (6). However, the safety of trastuzumab when used in combination with S-1 has not been studied in detail.
Data from the current post-marketing surveillance of S-1 involving Japanese patients with inoperable or recurrent breast cancer are presented. The safety of combined treatment with S-1 and trastuzumab was also evaluated in this article.
PATIENTS AND METHODS
Patients
Patients with inoperable or recurrent breast cancer to be treated with S-1 for 2 years from January 2006 to December 2007 were included in the surveillance.
Drug Administration
S-1 was administered according to the ‘Dosage and Administration’ section of the package insert: ‘A cycle consisting of repeated oral administration at an initial dose calculated from the body surface area twice daily (after breakfast and dinner) for 28 consecutive days followed by a 14-day washout period should be repeated’. After three cycles, a survey form was collected and each item on the form was assessed.
Survey Method
A prospective post-marketing surveillance was performed at 313 sites in Japan from which approval of the director of the site was obtained and a contract was concluded. The prior registration form was faxed to the registration center through the central registration system no later than the first day of treatment. The survey items consisted of patient background, treatment state, concomitant medication/concurrent therapy, clinical laboratory test and adverse events. As the key survey items, the presence or absence of hand and foot syndrome at the start of TS-1 treatment and its onset in each course were surveyed. All adverse events that developed during three cycles of treatment with TS-1 were recorded on the case report form, and adverse events were recorded by the treating physician through interview and by laboratory tests. Adverse events were graded according to the Common Terminology Criteria for Adverse Events (CTCAE, version 3.0) and tabulated using MedDRA/J (ver.11.1) Preferred Term (PT). Red blood cell counts decreased were recorded as anemia for the assessment. Any medication or therapy used concomitantly was recorded on the survey form.
Statistics
Analyses were performed using the statistical package SAS 9.1 (SAS Institute, Cary, NC, USA). A logistic regression model was used to explore factors that may influence the development of adverse drug reactions. The significance level was set at 5%.
RESULTS
Patients
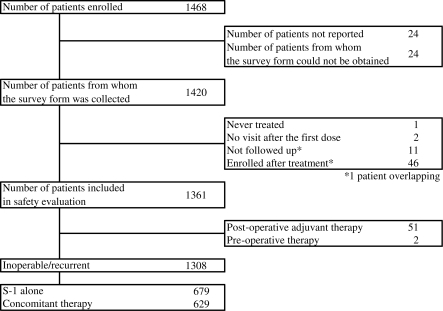
A total of 1468 patients from 313 institutions were enrolled between January 2006 and December 2007. Of these, 1444 patients were treated with S-1. A completed survey form was collected from 1420 patients. Patients who received their first dose before the contract of the surveillance study was concluded were excluded. Therefore, 1361 patients were included in the safety evaluation. The reasons for S-1 treatment were inoperable/recurrent disease in 1308 patients, adjuvant therapy in 51 patients and neoadjuvant therapy in 2 patients. Of the patients with inoperable/recurrent breast cancer, 679 patients were treated with S-1 alone and 629 patients were treated with S-1 in combination with other anticancer drug(s) (Fig. 1).
Figure 1.
Patients' background.
Adverse Drug Reactions
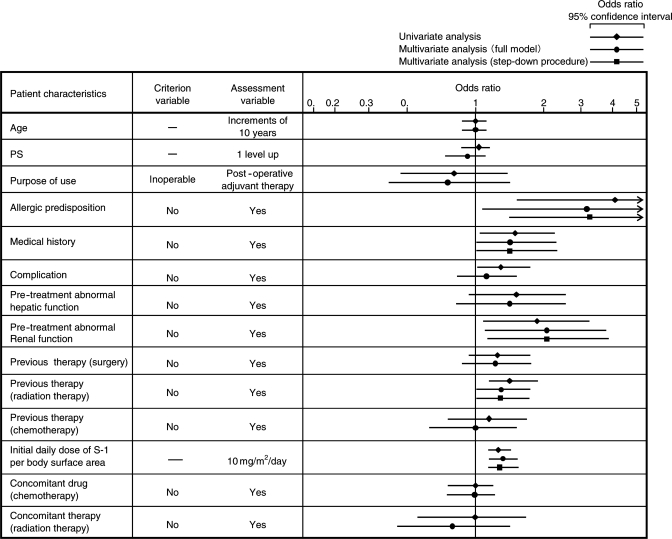
The number and incidence of common adverse drug reactions included in the safety evaluation are shown in Table 1. At least one adverse drug reaction was reported by 858 patients, with an overall incidence of 63.0% (858/1361). The incidence of Grade 3 or higher adverse drug reactions in a descending order was 14.7% (200/1361), including neutrophil count decreased (4.2%; 57/1361), white blood cell count decreased (3.4%; 46/1361), anemia (including red blood cell count decreased, 2.5%; 34/1361), diarrhea (1.5%; 21/1361) and platelet count decreased (1.4%; 19/1361). The incidences of other Grade 3 or higher adverse drug reactions were lower than 1%. The number and incidence of adverse drug reactions according to the patient characteristics are shown in Table 2. Multivariate analysis using a logistic regression model revealed that the factors such as allergic predisposition, medical history, pre-treatment abnormal renal function, previous therapy (radiation therapy) and initial daily dose of S-1 per body surface area were associated with the development of adverse drug reactions. The most influential factor was allergic predisposition, which was associated with ∼3.3-fold higher risk of adverse drug reactions (Fig. 2).
Table 1.
Number of patients with adverse drug reactions and incidence of adverse drug reactions (ADRs)
| ADRs | Number of patients with ADRs (%) | Number of patients with Grade 3 or more severe ADRs (%) |
|---|---|---|
| Overall | 858 (63.0) | 200 (14.7) |
| Hematologic toxicity | ||
| Anemia (including red blood cell count decreased) | 174 (12.8) | 34 (2.5) |
| Neutrophil count decreased | 159 (11.7) | 57 (4.2) |
| Platelet count decreased | 138 (10.1) | 19 (1.4) |
| White blood cell count decreased | 227 (16.7) | 46 (3.4) |
| Non-hematologic toxicity | ||
| Anorexia | 85 (6.2) | 10 (0.7) |
| Diarrhea | 139 (10.2) | 21 (1.5) |
| Nausea | 90 (6.6) | 7 (0.5) |
| Stomatitis | 64 (4.7) | 5 (0.4) |
| Vomiting | 48 (3.5) | 9 (0.7) |
| Hepatic function abnormal | 54 (4.0) | 7 (0.5) |
| Palmar–plantar erythrodysesthesia syndrome | 68 (5.0) | 2 (0.1) |
| Rash | 23 (1.7) | 1 (0.1) |
| Malaise | 31 (2.3) | 3 (0.2) |
| Alanine aminotransferase increased | 52 (3.8) | 4 (0.3) |
| Aspartate aminotransferase increased | 68 (5.0) | 6 (0.4) |
| Blood bilirubin increased | 123 (9.0) | 6 (0.4) |
| Blood alkaline phosphatase increased | 74 (5.4) | 8 (0.6) |
Table 2.
Number of patients with ADRs and incidence of ADRs in relation to patient characteristics
| Number of patients | Number of patients with ADRs (%) | |
|---|---|---|
| Age | ||
| ≥20 to <30 | 1 | 1 (100.0) |
| ≥30 to <40 | 44 | 23 (52.3) |
| ≥40 to <50 | 207 | 135 (65.2) |
| ≥50 to <60 | 459 | 285 (62.1) |
| ≥60 to <70 | 389 | 263 (67.6) |
| ≥70 to <80 | 216 | 126 (58.3) |
| ≥80 | 45 | 25(55.6) |
| PS | ||
| 0 | 835 | 517 (61.9) |
| 1 | 397 | 261 (65.7) |
| 2 | 100 | 61 (61.0) |
| 3 | 21 | 14 (66.7) |
| 4 | 8 | 5 (62.5) |
| Purpose of use | ||
| Inoperable | 137 | 81 (59.1) |
| Recurrent | 1171 | 746 (63.7) |
| Post-operative adjuvant therapy | 51 | 30 (58.8) |
| Pre-operative therapy | 2 | 1 (50.0) |
| Allergic predisposition | ||
| No | 1307 | 815 (62.4) |
| Yes | 39 | 34 (87.2) |
| Unknown | 15 | 9 (60.0) |
| Medical history | ||
| No | 1185 | 733 (61.9) |
| Yes | 149 | 107 (71.8) |
| Unknown | 27 | 18 (66.7) |
| Complications | ||
| No | 1052 | 646 (61.4) |
| Yes | 309 | 212 (68.6) |
| Pre-treatment abnormal hepatic function | ||
| No | 1145 | 723 (63.1) |
| Yes | 79 | 58 (73.4) |
| Unknown | 137 | 77 (56.2) |
| Pre-treatment abnormal renal function | ||
| No | 1189 | 754 (63.4) |
| Yes | 78 | 60 (76.9) |
| Unknown | 94 | 44 (46.8) |
| Previous therapy (surgery) | ||
| No | 167 | 96 (57.5) |
| Yes | 1187 | 758 (63.9) |
| Unknown | 7 | 4 (57.1) |
| Previous therapy (radiation therapy) | ||
| No | 919 | 551 (60.0) |
| Yes | 426 | 296 (69.5) |
| Unknown | 16 | 11 (68.8) |
| Previous therapy (chemotherapy or hormone therapy) | ||
| No | 118 | 70 (59.3) |
| Yes | 1235 | 784 (63.5) |
| Unknown | 8 | 4 (50.0) |
| Initial daily dose of S-1 per body surface area (mg/m2) | ||
| ≤60 | 208 | 115 (55.3) |
| >60 to ≤70 | 516 | 311 (60.3) |
| >70 to ≤80 | 599 | 404 (67.4) |
| >80 | 38 | 28 (73.7) |
| Concomitant drug (chemotherapy or hormone therapy) | ||
| No | 707 | 445 (62.9) |
| Yes | 654 | 413 (63.1) |
| Concomitant therapy (radiation therapy) | ||
| No | 1304 | 822 (63.0) |
| Yes | 57 | 36 (63.2) |
PS, performance status.
Figure 2.
Logistic regression analysis of all adverse drug reactions. PS, performance status.
Hand and Foot Syndrome
A clinical trial suggested that the incidence of hand and foot syndrome was 21.8% (12/55 patients) in previously treated breast cancer patients (2); higher than that seen in other cancers. In addition to adverse drug reactions as study items, the presence or absence of hand and foot syndrome prior to S-1 treatment, as well as the occurrence of hand and foot syndrome during each treatment cycle, was investigated to determine whether a similar trend was observed in this survey. Hand and foot syndrome was reported by 113 patients during any one of the three cycles, with an incidence of 8.3% (113/1361 patients). Eighty patients with no pre-treatment findings experienced hand and foot syndrome after the initiation of S-1 treatment; an incidence of 6.2% (80/1300 patients).
SAFETY EVALUATION OF PATIENTS TREATED WITH S-1 + TRASTUZUMAB
Patient Characteristics
S-1 alone was used to treat 679 patients with inoperable/recurrent breast cancer and in combination with other anticancer drug(s) in 629 patients. The most common combination was S-1 + trastuzumab (118 patients), followed by anastrozole (84 patients), letrozole (80 patients) and exemestane (71 patients). The characteristics of the patients treated with S-1 alone and of those treated with S-1 + trastuzumab are shown in Table 3. There were significant differences in expression of hormone receptor and/or HER2, complications and previous therapy (chemotherapy or hormone therapy). The median duration of treatment with S-1 was not significantly different between patients treated with S-1 alone (59 days) and those treated with S-1 + trastuzumab (66 days).
Table 3.
Patient characteristics (S-1 alone vs. S-1 in combination with trastuzumab)
| Patient characteristics | Number of patients (%) |
Fisher's exact test | |
|---|---|---|---|
| S-1 alone (n= 679) | S-1 + trastuzumab (n= 118) | ||
| Age | |||
| <65 | 459 (67.6) | 89 (75.4) | P= 0.106 |
| ≥65 | 220 (32.4) | 29 (24.6) | |
| Median | 60 | 56 | — |
| Minimum–maximum | 29–92 | 34–80 | |
| PS | |||
| 0 | 416 (61.3) | 64 (54.2) | P= 0.089 |
| 1 | 200 (29.5) | 44 (37.3) | |
| 2 | 52 (7.7) | 5 (4.2) | |
| 3 | 8 (1.2) | 4 (3.4) | |
| 4 | 3 (0.4) | 1 (0.8) | |
| Histology | |||
| Papillotubular carcinoma | 187 (27.5) | 29 (24.6) | P= 0.731 |
| Solid-tubular carcinoma | 149 (21.9) | 29 (24.6) | |
| Scirrhous carcinoma | 236 (34.8) | 40 (33.9) | |
| Other | 72 (10.6) | 11 (9.3) | |
| Unknown | 35 (5.2) | 9 (7.6) | |
| Menopause | |||
| Before | 84 (12.4) | 14 (11.9) | P= 0.554 |
| After | 578 (85.1) | 99 (83.9) | |
| Unknown | 17 (2.5) | 5 (4.2) | |
| Hormone receptor | |||
| ER(−) and PgR(−) | 303 (44.6) | 75 (63.6) | P< 0.001 |
| Other | 376 (55.4) | 43 (36.4) | |
| HER2 | |||
| 0–2 | 506 (74.5) | 22 (18.6) | P< 0.001 |
| 3 | 73 (10.8) | 95 (80.5) | |
| Unknown | 100 (14.7) | 1 (0.8) | |
| Complication | |||
| No | 543 (80.0) | 84 (71.2) | P= 0.038 |
| Yes | 136 (20.0) | 34 (28.8) | |
| Previous therapy (chemotherapy or hormone therapy) | |||
| No | 69 (10.2) | 2 (1.7) | P= 0.003 |
| Yes | 603 (88.8) | 115 (97.5) | |
| Unknown | 7 (1.0) | 1 (0.8) | |
| Initial daily dose of S-1 per body surface area (mg/m2) | |||
| ≤60 | 87 (12.8) | 14 (11.9) | P= 0.745 |
| >60 to ≤70 | 264 (38.9) | 52 (44.1) | |
| >70 to ≤80 | 305 (44.9) | 49 (41.5) | |
| >80 | 23 (3.4) | 3 (2.5) | |
| Number of days of actual treatment with S-1 (days) | |||
| Median | 59 | 66 | P= 0.084 |
| Mean | 58 | 62 | |
| 25th–75th percentile | 29–84 | 42–84 | |
| Minimum–maximum | 1–132 | 5–142 | |
| Number of days of actual treatment with trastuzumab (days) | |||
| Median | — | 8 | — |
| Mean | 8 | ||
| 25th–75th percentile | 3–11 | ||
| Minimum–maximum | 1–23 | ||
The P-value from Wilcoxon's rank-sum test is shown for PS, initial daily dose of S-1 per body surface area and duration of treatment with S-1. ER, estrogen; PgR, progesterone; HER2, human epidermal growth factor receptor 2.
Comparison of Adverse Drug Reactions Between S-1 Alone and S-1 + Trastuzumab
The most common adverse drug reactions recorded in patients treated with S-1 alone or with S-1 + trastuzumab are shown in Table 4. The overall incidence of adverse drug reactions was 63.5% (431/679 patients) in patients treated with S-1 alone and 55.9% (66/118 patients) in patients treated with S-1 + trastuzumab. There were no marked differences in the incidence of other adverse drug reactions between the two groups.
Table 4.
List of ADRs
| ADRs | S-1 alone (n= 679) |
S-1 + trastuzumab (n= 118) |
Fisher's exact test |
|||
|---|---|---|---|---|---|---|
| Number of patients with ADRs (%) | Number of patients with Grade 3 or more severe ADRs (%) | Number of patients with ADRs (%) | Number of patients with Grade 3 or more severe ADRs (%) | Incidence of ADRs | Incidence of Grade 3 or more severe ADRs | |
| Overall | 431 (63.5) | 99 (14.6) | 66 (55.9) | 19 (16.1) | P= 0.124 | P= 0.674 |
| Hematologic toxicity | ||||||
| Anemia (including red blood cell count decreased) | 77 (11.3) | 19 (2.8) | 17 (14.4) | 3 (2.5) | P= 0.354 | P= 1.000 |
| Neutrophil count decreased | 74 (10.9) | 28 (4.1) | 17 (14.4) | 6 (5.1) | P= 0.273 | P= 0.622 |
| Platelet count decreased | 67 (9.9) | 11 (1.6) | 11 (9.3) | 0 (0.0) | P= 1.000 | P= 0.384 |
| White blood cell count decreased | 105 (15.5) | 22 (3.2) | 23 (19.5) | 6 (5.1) | P= 0.278 | P= 0.286 |
| Non-hematologic toxicity | ||||||
| Anorexia | 44 (6.5) | 4 (0.6) | 9 (7.6) | 1 (0.8) | P= 0.688 | P= 0.552 |
| Diarrhea | 66 (9.7) | 11 (1.6) | 18 (15.3) | 3 (2.5) | P= 0.075 | P= 0.448 |
| Nausea | 47 (6.9) | 3 (0.4) | 5 (4.2) | 0 (0.0) | P= 0.417 | P= 1.000 |
| Stomatitis | 36 (5.3) | 3 (0.4) | 5 (4.2) | 1 (0.8) | P= 0.822 | P= 0.474 |
| Vomiting | 25 (3.7) | 6 (0.9) | 6 (5.1) | 2 (1.7) | P= 0.441 | P= 0.337 |
| Hepatic function abnormal | 28 (4.1) | 5 (0.7) | 3 (2.5) | 0 (0.0) | P= 0.606 | P= 1.000 |
| Palmar–plantar erythrodysesthesia syndrome | 42 (6.2) | 1 (0.1) | 3 (2.5) | 1 (0.8) | P= 0.133 | P= 0.274 |
| Rash | 10 (1.5) | 0 (0.0) | 3 (2.5) | 0 (0.0) | P= 0.423 | — |
| Malaise | 13 (1.9) | 1 (0.1) | 2 (1.7) | 0 (0.0) | P= 1.000 | P= 1.000 |
| Alanine aminotransferase increased | 31 (4.6) | 2 (0.3) | 7 (5.9) | 1 (0.8) | P= 0.486 | P= 0.382 |
| Aspartate aminotransferase increased | 43 (6.3) | 5 (0.7) | 3 (2.5) | 0 (0.0) | P= 0.133 | P= 1.000 |
| Blood bilirubin increased | 56 (8.2) | 4 (0.6) | 13 (11.0) | 1 (0.8) | P= 0.374 | P= 0.552 |
| Blood alkaline phosphatase increased | 31 (4.6) | 3 (0.4) | 7 (5.9) | 0 (0.0) | P= 0.486 | P= 1.000 |
DISCUSSION
We set out to determine whether monotherapy with S-1 or combination therapy of S-1 and trastuzumab would be effective in the treatment of breast cancer. With the remarkable progress in cancer therapy over recent years, a variety of therapeutic options are now available. For example, molecular-targeted therapy is increasingly used, emphasizing tailored medicine, and various combination therapies are being administered to further enhance the antitumor effects. However, it is impossible to evaluate the efficacy and safety of all possible drug combinations before marketing. Post-marketing surveillance can be used to collect data from patients who have received a variety of combination therapies because there are not as many restrictions on the administration methods used as there are in clinical studies. Therefore, stratified analysis of patients given combined drug treatments using post-marketing surveillance may provide significant information that can complement the pre-marketing data. In addition, post-marketing surveillance may help to obtain information from patient subgroups that have been excluded from clinical studies and highlight previously unknown adverse drug reactions.
Therefore, we reported the results of the current post-marketing surveillance of S-1 for the treatment of inoperable or recurrent breast cancer (covering 2 years from 2006), and also explored the safety of S-1 when used in combination with trastuzumab (the most commonly used combination drug).
The most common Grade 3, or higher, severe adverse drug reactions were: neutrophil count decreased (4.2%), white blood cell count decreased (3.4%), anemia (including red blood cell count decreased; 2.5%), diarrhea (1.5%) and platelet count decreased (1.4%). In a Phase II clinical study of S-1 in patients with taxane-resistant inoperable or recurrent breast cancer, the incidence of common Grade 3, or higher, adverse drug reactions were: neutrophil count decreased (10.9%), white blood cell count decreased (9.1%), anorexia (5.5%) and diarrhea (5.5%) (4), illustrating that the incidence of adverse reactions was lower in the current study.
The presence or absence of allergic disposition was shown in Fig. 2 as the factor having the strongest impact on the onset of adverse drug reactions. This was possibly because many of the subjects with allergic disposition had a history of hypersensitivity to drugs. The factor with the second highest odds ratio was baseline renal dysfunction. Correlationship between lowered renal function and onset of adverse events was also shown in the risk analysis of adverse events in the drug use investigation (3758 subjects with gastric cancer) performed in 1999 (7). It is presumed that gimeracil is not excreted in patients with renal dysfunction, because the route of excretion of gimeracil, the active ingredient of this drug, was via the kidney, and thereby the blood concentration of 5-FU is maintained at a higher level compared with patients with normal renal function.
The package insert states that a Phase II clinical study of S-1 showed the incidence of hand and foot syndrome to be 21.8% (12/55 patients); higher than that for other cancers (4). Therefore, we examined whether a similar trend was observed in the current surveillance. The incidence of palmar–plantar erythrodysesthesia syndrome was 5.0% higher than that in previous post-marketing surveillances for other cancers [0.2% (8/3808 patients) for gastric cancer, 0% (0/375 patients) for head and neck cancer and 0.06% (1/1669 patients) for non-small cell lung cancer]. In agreement with the clinical study, the current surveillance study suggests that the incidence of hand and foot syndrome may be higher in inoperable or recurrent breast cancer than in other cancers. In addition, hand and foot syndrome occurred in 37, 30 and 13 out of 80 patients after the start Cycles 1, 2 and 3, respectively. The incidence was the highest after Cycle 1, but almost as high after Cycle 2, indicating that patients should be carefully monitored up to after Cycle 2.
For inoperable or recurrent breast cancer, anthracyclines and taxanes are the standard chemotherapeutic agents for HER2-negative breast cancer (5). Chemotherapy combined with trastuzumab improves survival times in HER2-positive breast cancer. Response rate with trastuzumab was 55.9% when used in combination with AC therapy and 41.3% when used in combination with paclitaxel, in an overseas Phase III study (8). However, due to the high incidence of peripheral nerve disorders associated with taxanes (9,10), and bone marrow depression and phlebitis associated with vinorelbine (11), there is a need for new regimens that cause fewer adverse drug reactions.
In the current surveillance study, the most common combination drug was trastuzumab. The overall incidence of adverse drug reactions was 63.5% (431/679 patients) in patients treated with S-1 alone and 55.9% (66/118 patients) in patients treated with S-1 + trastuzumab. This suggested that combination therapy did not increase the incidence of adverse drug reactions. In addition, the incidence of adverse drug reactions analyzed according to the patient characteristics, revealing no particular characteristic resulting in a significant difference. In our hospital, a combination of S-1 and trastuzumab was administered to patients with HER2-positive metastatic breast cancer, and trastuzumab was found to have little effect on the pharmacokinetics of S-1 and caused no serious adverse drug reactions (12).
In conclusion, the current surveillance showed that monotherapy with S-1 or combination therapy with S-1 + trastuzumab was well tolerated for inoperable or recurrent breast cancer patients. However, since this was a post-marketing surveillance study, efficacy could not be evaluated according to the RECIST criteria as is common for clinical studies. Further studies are needed to evaluate the safety and efficacy of S-1 with or without trastuzumab.
Funding
This surveillance study was sponsored by Taiho Pharmaceutical Co., Ltd.
Conflict of interest statement
None declared.
Acknowledgements
We express our sincere gratitude to the physicians in each medical institution and to all those involved in this post-marketing surveillance. We also thank Professor J. Patrick Barron of the Department of International Medical Communications Centre of Tokyo Medical University (Tokyo, Japan), a remunerated consultant of Taiho Pharmaceutical for his review of this report.
References
- 1.Shirasaka T, Shimamoto Y, Fukushima M. Inhibition by oxonic acid of gastrointestinal toxicity of 5-fluorouracil without loss of its antitumor activity in rats. Cancer Res. 1993;53:4004–9. [PubMed] [Google Scholar]
- 2.Shirasaka T, Shimamato Y, Ohshimo H, Yamaguchi M, Kato T, Yonekura K, et al. Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drugs. 1996;7:548–57. doi: 10.1097/00001813-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Saek T, Takashima S, Sano M, Horikoshi N, Miura S, Shimizu S, et al. A phase II study of S-1 in patients with metastatic breast cancer, a Japanese trial by the S-1 Cooperative Study Group (Breast Cancer Working Group) Breast Cancer. 2004;11:194–202. doi: 10.1007/BF02968301. [DOI] [PubMed] [Google Scholar]
- 4.Hino M, Saeki T, Sato Y, Sano M S-1 Cooperative Study Group (Breast Cancer Working Group) Late phase II study of S-1 in patients with taxane resistant breast cancer. J Clin Oncol. 2004;22(14S):745. (ASCO Annual Meeting Proceedings) [Google Scholar]
- 5.NCCN Clinical Practice Guidelines in Oncology™. Breast Cancer. 2010. NCCN® Practice Guidelines in Oncology. Version 2.
- 6.Yamamoto D, Iwase S, Kitamura K, Odagiri H, Yamamoto C, Nagumo Y. A phase II study of trastuzumab and capecitabine for patients with HER2-overexpressing metastatic breast cancer Japan Breast Cancer Research Network (JBCRN) 00 Trial. Cancer Chemother Pharmacol. 2008;61:509–14. doi: 10.1007/s00280-007-0497-5. [DOI] [PubMed] [Google Scholar]
- 7.Yamanaka T, Matsumoto S, Teramukai S, Ishiwata R, Nagai Y, Fukushima M. Analysis of risk factors for severe adverse effects of oral 5-fluorouracil S-1 in patients with advanced gastric cancer. Gastric Cancer. 2007;10:129–34. doi: 10.1007/s10120-007-0422-y. [DOI] [PubMed] [Google Scholar]
- 8.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 9.Seidman AD, Fornier MN, Esteva FJ, Tan L, Kaptain S, Bach A, et al. Weekly trastuzumab and paclitaxel therapy for metastatic breast cancer with analysis of efficacy by HER2 immunophenotype and gene amplification. J Clin Oncol. 2001;19:2587–95. doi: 10.1200/JCO.2001.19.10.2587. [DOI] [PubMed] [Google Scholar]
- 10.Esteva FJ, Valero V, Booser D, Guerra LT, Murray JL, Pusztai L, et al. Phase II study of weekly docetaxel and trastuzumab for patients with HER-2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:1800–8. doi: 10.1200/JCO.2002.07.058. [DOI] [PubMed] [Google Scholar]
- 11.Burstein HJ, Kuter I, Campos SM, Gelman RS, Tribou L, Parker LM, et al. Clinical activity of trastuzumab and vinorelbine in women with HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2001;15:2722–30. doi: 10.1200/JCO.2001.19.10.2722. [DOI] [PubMed] [Google Scholar]
- 12.Yasuhiro S, Yuki S, Takuho O, Banri T, Mayako T, Mizuho T, et al. Study of S-1/trastuzumab combination therapy for HER2-positive metastatic breast cancer. Global Breast Cancer Conference; 2009. (PO1-033) [Google Scholar]