SUMMARY
While activation of BAX/BAK by BH3-only molecules (BH3s) is essential for mitochondrial apoptosis, the underlying mechanisms remain unsettled. Here, we demonstrate that BAX undergoes stepwise structural reorganization leading to mitochondrial targeting and homo-oligomerization. The α1 helix of BAX keeps the α9 helix engaged in the dimerization pocket, rendering BAX as a monomer in cytosol. The activator BH3s, tBID/BIM/PUMA, attack and expose the α1 helix of BAX, resulting in secondary disengagement of the α9 helix and thereby mitochondrial insertion. Activator BH3s remain associated with the N-terminally exposed BAX through the BH1 domain to drive homo-oligomerization. BAK, an integral mitochondrial membrane protein, has bypassed the first activation step, explaining its faster killing kinetics than BAX. Furthermore, death signals initiated at ER induce BIM and PUMA to activate mitochondrial apoptosis. Accordingly, deficiency of Bim/Puma impedes ER stress-induced BAX/BAK activation and apoptosis. Our study provides mechanistic insights regarding the spatiotemporal execution of BAX/BAK-governed cell death.
INTRODUCTION
Mammalian cell death proceeds through a highly regulated genetic program termed apoptosis that is heavily dependent on the mitochondria (Danial and Korsmeyer, 2004). Multiple apoptotic signals culminate in permeabilizing the mitochondrial outer membrane (MOM), resulting in the release of apoptogenic factors including cytochrome c and SMAC (Wang, 2001). Once released, cytochrome c activates Apaf-1 to assist the activation of caspases that cleave cellular proteins and thereby contribute to the morphological and biochemical changes associated with apoptosis. The permeabilization of the MOM not only couples the mitochondria to the activation of caspases but also initiates caspase-independent mitochondrial dysfunction (Cheng et al., 2001). The BCL-2 family proteins control a crucial checkpoint of apoptosis at the mitochondria (Cory and Adams, 2002; Korsmeyer et al., 2000). Multidomain proapoptotic BAX and BAK are essential effectors responsible for the permeabilization of the MOM, whereas anti-apoptotic BCL-2, BCL-XL, and MCL-1 preserve mitochondrial integrity and prevent cytochrome c efflux triggered by apoptotic stimuli. The third BCL-2 subfamily of proteins, BH3-only molecules (BH3s), promotes apoptosis by either activating BAX/BAK or inactivating BCL-2/BCL-XL/MCL-1 (Certo et al., 2006; Cheng et al., 2001; Kim et al., 2006; Kuwana et al., 2005; Letai et al., 2002; Wei et al., 2000; Wei et al., 2001). Upon apoptosis, the “activator” BH3s, including truncated BID (tBID), BIM, and PUMA, activate BAX and BAK to mediate cytochrome c efflux, leading to caspase activation (Cheng et al., 2001; Desagher et al., 1999; Kim et al., 2006; Wei et al., 2000; Wei et al., 2001). Conversely, the anti-apoptotic BCL-2, BCL-XL, and MCL-1 sequester activator BH3s into inert complexes, thus preventing BAX/BAK activation (Cheng et al., 2001; Kim et al., 2006). The remaining BH3s including BAD, NOXA, BMF, HRK, and BIK/BLK do not activate BAX/BAK directly, but instead prevent the anti-apoptotic BCL-2 members from sequestering the activator BH3s (Certo et al., 2006; Kim et al., 2006; Kuwana et al., 2005; Letai et al., 2002). Although it was proposed that activation of BAX and BAK occurred by default as long as all the anti-apoptotic BCL-2 proteins were neutralized by BH3s (Willis et al., 2007), the liposome studies clearly recapitulate the direct activation model in which tBID protein or BH3 domain peptides derived from BID or BIM induced BAX oligomerization and membrane permeabilization (Gavathiotis et al., 2008; Kuwana et al., 2002; Walensky et al., 2006). In addition, the temporal sequence of BAX activation by tBID was nicely demonstrated recently by a FRET (fluorescence resonance energy transfer)-based liposomal study (Lovell et al., 2008).
While BAK and BAX are the essential effectors of mitochondrial apoptosis, their proapoptotic activity is tightly kept in check (Cheng et al., 2001; Lindsten et al., 2000; Wei et al., 2001). BAX exists in the cytosol as a monomer with its C-terminal α9 helix occupying the dimerization pocket formed by BH1-3 domains (Suzuki et al., 2000). This auto-inhibited BAX monomer may be further stabilized by associated proteins (Reed, 2006). By contrast, the C-terminal α9 helix of BAK is constitutively inserted in the MOM and its activity is inhibited by a mammalian restricted VDAC isoform, VDAC2, which occupies the dimerization pocket of BAK to restrict BAK in the monomeric inactive conformation (Cheng et al., 2003; Ren et al., 2009; Wei et al., 2000). Upon apoptosis, activator BH3s induce conformational changes of BAX to promote the targeting and homo-oligomerization of BAX at the MOM, and disrupt BAK-VDAC2 interaction to enable homo-oligomerization of BAK, leading to the efflux of apoptogenic factors (Cheng et al., 2003; Desagher et al., 1999; Kim et al., 2006; Ren et al., 2009; Wei et al., 2000). Therefore, the proapoptotic activity of BAX and BAK is triggered by BH3s, whose activity is in turn regulated either transcriptionally or posttranslationally by upstream death signaling cascades (Danial and Korsmeyer, 2004; Korsmeyer et al., 2000). By analogy, BH3s function as death ligands that allosterically regulate the mitochondrial death receptor BAX/BAK (Korsmeyer et al., 2000).
Conformational changes and homo-oligomerization are two critical events associated with the activation of BAX/BAK by BH3s (Korsmeyer et al., 2000; Reed, 2006). However, the underlying mechanisms remain unsettled. It is especially complex for BAX due to its change in the subcellular localization during apoptosis (Wolter et al., 1997). The N-terminal exposure of BAX is a commonly used marker for its activation of which the molecular basis is obscure (Desagher et al., 1999; Nechushtan et al., 1999). Although it was proposed that the intramolecular interaction between the N- and C-terminal regions of BAX might somehow regulate its activation (Goping et al., 1998; Nechushtan et al., 1999; Schinzel et al., 2004), direct experimental data supporting this model are missing. Interestingly, our recent study demonstrated that a novel interaction site involving the α1 and α6 helixes of BAX and the BH3 domain of BIM is required for BAX activation (Gavathiotis et al., 2008). The α1 helix of BAX was also implicated in its interaction with BID and PUMA (Cartron et al., 2004). However, it is unknown how this interaction contributes to BAX activation. BAK was reported to expose its BH3 domain to form dimers via BH3-groove interactions (Dewson et al., 2008). Unfortunately, this study did not examine higher-ordered BAK oligomers that are more functionally relevant since BAX tetramers but not dimers could form pores large enough for cytochrome c passage (Saito et al., 2000). To address these questions, we performed structural functional analyses to dissect the molecular mechanisms by which BH3s activate BAX/BAK.
We demonstrate that activation of BAX can be dissected into two sequential steps, mitochondrial targeting and homo-oligomerization, both of which require activator BH3s. The α1 helix of BAX stabilizes the binding of the α9 helix to the dimerization pocket, which in turn controls the mitochondrial targeting of BAX. Thus, tBID, BIM, and PUMA initiates the activation process of BAX by attacking the α1 helix, resulting in the N-terminal exposure of BAX and secondary disengagement of the α9 helix that becomes available for mitochondrial targeting. tBID, BIM, and PUMA remain associated with the N-terminally exposed BAX to drive the homo-oligomerization of mitochondrially localized BAX. Our data reveal dynamic interactions between activator BH3s and BAX, which helps explain the previous difficulty in detecting these interactions. BAK, an integral mitochondrial membrane protein, constitutively exposes its α1 helix and requires activator BH3s to trigger its homo-oligomerization. The BH1 and BH3 domains are required for the assembly of higher-ordered BAX or BAK oligomers, which is essential for the proapoptotic activity of BAX or BAK. Moreover, ER stress induces BIM and PUMA to activate BAX/BAK at the mitochondria, exemplifying how activator BH3s interconnect upstream death signaling cascades with downstream BAX/BAK-dependent mitochondrial death program. All together, our study supports the direct engagement of activator BH3s in activating BAX/BAK-dependent mitochondrial apoptosis.
RESULTS
Previous mutagenesis studies of BAX and BAK often generate conflicting results due to overexpression of BAX/BAK mutants in cells containing wild-type (wt) BAX/BAK, which likely assess their function as BH3-like death ligands that could activate endogenous BAX/BAK (Chittenden et al., 1995; Simonen et al., 1997; Wang et al., 1998). To fully address the role of BAX and BAK as mitochondrial death receptors, we reconstituted mutants of BAX or BAK into Bax/Bak double knockout (DKO) mouse embryonic fibroblasts (MEFs) to a physiological level similar to wt cells and examined the ability of each mutant to restore the apoptotic sensitivity of death-resistant DKO cells (Fig. S1).
Mitochondrial targeting and homo-oligomerization are two separable, essential steps of BAX activation
Although BAX contains a conserved, putative transmembrane domain at the C-terminal α9 helix (Wolter et al., 1997), it was reported that its N-terminus also bears a mitochondrial targeting signal (Cartron et al., 2003). To determine whether the C-terminal transmembrane domain is required for the mitochondrial targeting and proapoptotic activity of BAX, GFP-BAX or GFP-BAXΔC (BAX mutant with deletion of the C-terminal transmembrane domain) was reconstituted into DKO cells. In accordance with BAX, GFP-BAX restored the apoptotic sensitivity of DKO cells and translocated from cytosol to mitochondria in response to various intrinsic death signals (Fig. 1A, 1C and data not shown). On the contrary, GFP-BAXΔC remained in the cytosol upon death stimuli and BAXΔC failed to induce apoptosis, supporting the essential role of α9 helix for both mitochondrial targeting and death induction (Fig. 1A and 1C).
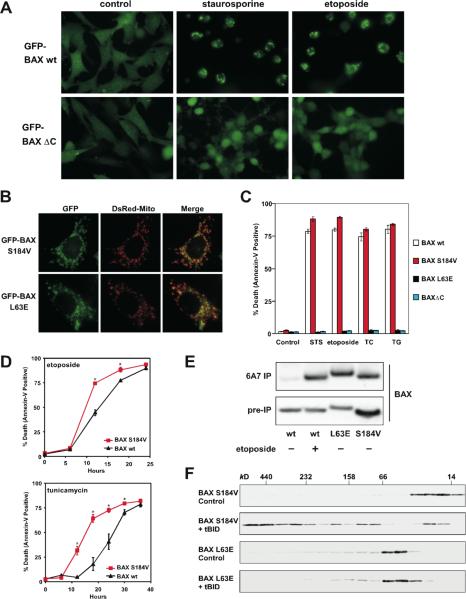
Figure 1. Mitochondrial targeting and homo-oligomerization are two separable, essential steps of BAX activation.
(A) C-terminal α9 helix targets BAX to the mitochondria. Fluorescence microscopy of Bax/Bak DKO MEFs reconstituted with GFP-BAX or GFP-BAXΔC before or after treatment with staurosporine (12 hr) or etoposide (15 hr).
(B) A single amino acid substitution at the BH3 domain or α9 helix of BAX constitutively targets BAX to the mitochondria. Fluorescence microscopy of DKO MEFs stably reconstituted with GFP-BAX S184V or L63E followed by retroviral transduction of DsRed-Mito.
(C) Mitochondrially localized BAX is not constitutively active. DKO MEFs reconstituted with wt or mutant BAX were treated with staurosporine (18 hr), etoposide (18 hr), tunicamycin (TC, 30 hr), or thapsigargin (TG, 30 hr) to induce apoptosis.
(D) BAX S184V displays faster proapoptotic kinetics than wt BAX. DKO MEFs reconstituted with wt BAX or BAX S184V were treated with etoposide or tunicamycin for the indicated time. Asterisk, P<0.05.
(E) Mitochondrially localized BAX mutants or activated BAX expose the N-terminal α1 helix. DKO MEFs reconstituted with wt BAX before or after treatment with etoposide for 15 hr, or reconstituted with indicated BAX mutants were lysed in 1% CHAPS and then immunoprecipitated with the 6A7 antibody. Immunoprecipitates were analyzed by anti-BAX (N20) immunoblots.
(F) BH3 domain is required for the homo-oligomerization of BAX. Mitochondria isolated from DKO MEFs reconstituted with BAX S184V or BAX L63E were incubated with recombinant tBID (1 ng/μl) and solubilized in 2 % CHAPS buffer. Protein lysates (200 μg) were subjected to Superdex 200 (HR 10/30) gel filtration chromatography and fractions were analyzed by anti-BAX immunoblots.
Data shown in (C) and (D) are mean ± s.d. from three independent experiments. Cell death was quantified by Annexin-V.
The S184V mutation at the α9 helix of BAX constitutively targeted BAX to mitochondria, which is consistent with a previous report (Nechushtan et al., 1999)(Fig. 1B). Remarkably, BAX S184V did not spontaneously induce apoptosis in the absence of death signals (Fig. 1C). DKO cells reconstituted with BAX S184V did die faster than those expressing wt BAX (Fig. 1D), consistent with the notion that BAX S184V has bypassed the activation process required for mitochondrial targeting. This might help explain why Bax-deficient cells (BAK-dependent apoptosis) died faster than Bak-deficient cells (BAX-dependent apoptosis) (Fig. S2). Interestingly, L63E mutation at the BH3 domain also rendered BAX localized at the mitochondria, but it totally abolished the proapoptotic activity (Fig. 1B and 1C). To determine the molecular basis underlying the functional differences between these two mitochondria-targeted BAX mutants, we examined whether they differ in the ability to undergo conformational changes. The N-terminal BAX epitope recognized by the 6A7 antibody (aa residues 12–24) becomes exposed after an apoptotic stimulus (Hsu and Youle, 1997; Nechushtan et al., 1999). Thus, anti-6A7 antibody could only immunoprecipitate BAX from cells following DNA damage but not from viable cells using CHAPS buffer (Fig. 1E). It was reported that NP-40 and Triton-X 100 but not CHAPS could induce the conformational changes of BAX (Hsu and Youle, 1997). Surprisingly, both L63E and S184V mutants of BAX have already exposed their N-termini even in the absence of apoptotic stimuli (Fig. 1E). These data suggest that the N-terminal exposure indicates mitochondrial targeting but not full activation of BAX. We next performed gel filtration assays to assess the formation of BAX homo-oligomers triggered by tBID. Like wt BAX, BAX S184V was eluted as a monomer (molecular weight ~20 kD) before activation and formed higher-ordered oligomers in response to tBID (Fig. 1F). By contrast, BAX L63E failed to undergo homo-oligomerization and was eluted around 50–60 kD range irrespective of tBID treatment (Fig. 1F). It is possible that BAX L63E formed dimer, or associated with other proteins or detergent micelles, which accounts for its elution at almost twice of its molecular weight. Nevertheless, these findings clearly demonstrate the importance of BH3 domain for the homo-oligomerization and thereby the proapoptotic activity of BAX.
The N-terminal exposure of BAX correlates with mitochondrial targeting rather than homo-oligomerization
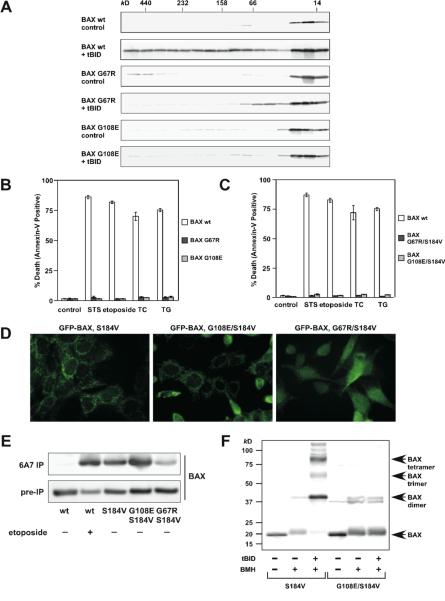
To further investigate the importance of BAX homo-oligomerization, we performed extensive mutagenesis and identified two additional BAX mutants that failed to undergo homo-oligomerization. BH1 (G108E) and BH3 (G67R) domain mutants failed to form higher-ordered oligomers detected by gel filtration in response to tBID (Fig. 2A). In addition, HA-tagged BAX G67R or G108E was unable to co-precipitate non-tagged corresponding mutant by anti-HA immunoprecipitation (Fig. S3). Accordingly, these two mutants were unable to rescue the apoptotic defect of DKO cells (Fig. 2B). The S184V substitution was introduced into these BAX mutants to enforce mitochondrial localization. These mutants remained inactive in triggering apoptosis upon death signals (Fig. 2C). GFP-BAX G108E/S184V targeted at the mitochondria constitutively, whereas GFP-BAX G67R/S184V only exhibited partial mitochondrial localization (Fig. 2D). Since our initial study suggests that the N-terminal exposure of BAX likely reflects mitochondrial targeting, we next tested whether these two double mutants could be pulled down by the 6A7 antibody. Indeed, the N-terminus of BAX G108E/S184V was readily exposed in the absence of apoptotic stimuli whereas that of BAX G67R/S184V was partially exposed (Fig. 2E). By analogy to BAX L63E, the mitochondria-targeted BAX G108E/S184V could not be activated by tBID to undergo homo-oligomerization detected by protein cross-linking, and thereby failed to induce apoptosis (Fig. 2C and 2F). We also tested a previously reported BAX P168A mutant that was defective in mitochondrial targeting (Schinzel et al., 2004). This mutant when stably reconstituted in DKO cells could not be immunoprecipitated by the 6A7 antibody (Fig. S4). Of note, it was previously reported that the N-terminus of this mutant was exposed when it was over-expressed in HeLa cells that contain wt BAX/BAK (Schinzel et al., 2004). Collectively, the N-terminal exposure of BAX is the conformational change associated with mitochondrial targeting rather than homo-oligomerization. More importantly, our data indicate that mitochondrial targeting and homo-oligomerization are two separable, distinct steps of BAX activation. Homo-oligomerization of BAX is apparently not required for BAX to translocate to mitochondria.
Figure 2. Characterization of homo-oligomerization and mitochondrial targeting of BAX.
(A) BH1 and BH3 domain mutants of BAX fail to undergo homo-oligomerization in response to tBID. Mitochondria isolated from DKO MEFs reconstituted with wt or mutant BAX were treated with recombinant tBID (1 ng/μl) and solubilized in 2 % CHAPS buffer. Protein lysates (200 μg) were subjected to Superdex 200 (HR 10/30) gel filtration chromatography and fractions were analyzed by anti-BAX immunoblots.
(B and C) BH1 and BH3 domain mutants of BAX fail to trigger apoptosis. DKO MEFs reconstituted with wt or mutant BAX were treated with staurosporine (18 hr), etoposide (18 hr), tunicamycin (30 hr) or thapsigargin (30 hr) to induce apoptosis. Cell death was quantified by Annexin-V. Data shown are mean ± s.d. from three independent experiments.
(D) S184V mutation fully targets BAX G108E but only partially targets BAX G67R to the mitochondria. Fluorescence microscopy of DKO MEFs reconstituted with the indicated GFP-tagged BAX mutants.
(E) The N-terminal exposure of BAX correlates with mitochondrial targeting. DKO MEFs reconstituted with wt BAX before or after treatment with etoposide (15 hr), or reconstituted with indicated BAX mutants were lysed in 1% CHAPS and then immunoprecipitated with the 6A7 antibody. Immunoprecipitates were analyzed by anti-BAX (N20) immunoblots.
(F) BH1 domain is required for the homo-oligomerization of BAX. Mitochondria isolated from DKO MEFs reconstituted with BAX S184V or BAX G108E/S184V were incubated with recombinant tBID for 30 min and then treated with BMH crosslinker. The BAX oligomers were detected by an anti-BAX immunoblot.
The BH1 and BH3 domains of BAX are required for its activation
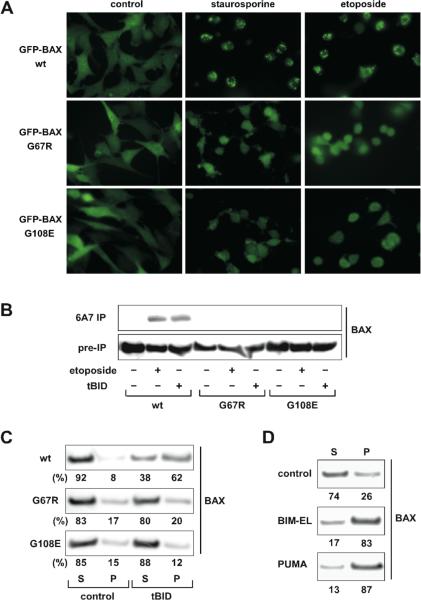
Although both BAX G67R and G108E mutants failed to undergo homo-oligomerization driven by tBID, it remains possible that they have defects even in the earlier step of conformational changes. Indeed, both mutants failed to translocate to mitochondria following intrinsic death signals and were unable to expose their N-terminal epitope in response to DNA damage or tBID (Fig. 3A and 3B), indicating that they have defects in the first step of BAX activation. In addition to visualizing mitochondrial targeting, we performed alkaline extraction to quantify the percentage of wt or mutant BAX integrating into the MOM (Goping et al., 1998). BAX was loosely associated with mitochondria and could be extracted by alkali (Fig. 3C). Once activated by tBID, BAX inserted into the MOM and became resistant to alkaline extraction (Fig. 3C). By contrast, both G67R and G108E mutants were still sensitive to alkaline extraction, indicating that they failed to insert into the MOM upon tBID treatment. Similar to tBID, BIM and PUMA also directly activated BAX to induce its mitochondrial insertion (Fig. 3D). Collectively, intact BH1 and BH3 domains are essential for the activation of BAX by activator BH3s.
Figure 3. BH1 and BH3 domains of BAX are required for its activation.
(A) BH1 and BH3 domain mutants of BAX fail to translocate to mitochondria upon apoptotic signals. Fluorescence microscopy of DKO MEFs reconstituted with GFP-tagged wt or mutant BAX before or after treatment with staurosporine (12 hr) or etoposide (15 hr).
(B) BH1 and BH3 domain mutants of BAX fail to expose the α1 helix in response to DNA damage or tBID. DKO MEFs reconstituted with wt or mutant BAX were untreated, treated with etoposide, or transduced with tBID by retrovirus. Cells lysed in 1 % CHAPS were subject to the 6A7 immunoprecipitation, followed by an anti-BAX (N20) immunoblot.
(C) BH1 and BH3 domain mutants of BAX fail to insert into the MOM in response to tBID. Mitochondria isolated from DKO MEFs reconstituted wt or mutant BAX were mock treated or treated with IVTT tBID, followed by alkaline extraction. The alkali-sensitive supernatant (S) and alkali-resistant pellet (P) fractions were analyzed by anti-BAX immunoblots. The numbers shown denote the percent of BAX quantified by densitometry.
(D) BIM and PUMA induce the mitochondrial insertion of BAX. Mitochondria isolated from DKO MEFs reconstituted with wt BAX were mock treated or treated with IVTT BIM or PUMA, followed by alkali extraction. The alkali-sensitive supernatant (S) and alkali-resistant pellet (P) fractions were analyzed by anti-BAX immunoblots. The numbers shown denote the percent of BAX quantified by densitometry.
The α1 helix of BAX controls the engagement of the α9 helix into the dimerization pocket
The observation that the N-terminal exposure of BAX initiates its activation and dictates mitochondrial targeting prompted us to explore how the N-terminal exposure of BAX regulates its mitochondrial targeting. Given that the α9 helix of BAX occupies its hydrophobic pocket formed by BH1-3 domains to prevent mitochondrial targeting and dimerization (Suzuki et al., 2000), one testable thesis is that the N-terminus stabilizes the engagement of the α9 helix in the hydrophobic pocket to keep BAX in an inactive conformation. To test this hypothesis, we developed an in vitro system to recapitulate the intramolecular interaction between the hydrophobic pocket of BAX and the C-terminal α9 transmembrane domain (TM). We found that HA-tagged BAXΔC could co-precipitate the α9 helix of BAX fused to the C-terminus of GST using anti-HA immunoprecipitation in the presence of CHAPS (Fig. 4A). S184V mutation within the α9 helix abolished its association with BAXΔC, suggesting that the α9 helix of BAX S184V no longer stays in the pocket and becomes available to insert into the MOM. Importantly, deletion of the α1 helix in BAXΔC (BAXΔNΔC) disrupted the interaction between the α9 helix and the hydrophobic pocket, indicating that the N-terminal α1 helix stabilizes the binding of the α9 helix to the pocket (Fig. 4A). These data are supported by a previous report demonstrating that N-terminally deleted BAX could target to mitochondria constitutively (Goping et al., 1998).
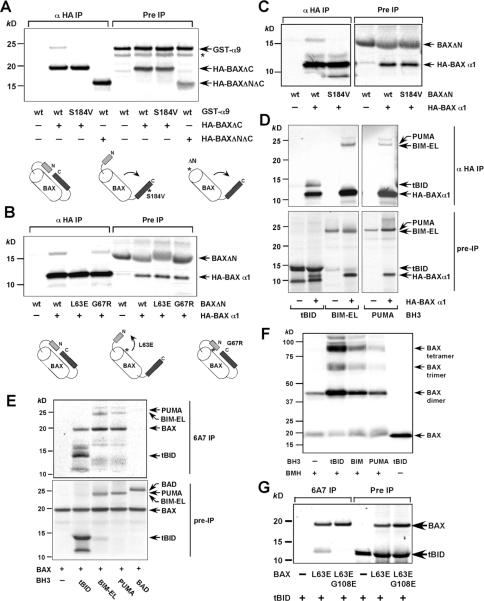
Figure 4. Activation of BAX can be dissected into two sequential steps, mitochondrial targeting and homo-oligomerization, both of which require activator BH3s.
(A) The α1 helix of BAX keeps the α9 helix engaged in the dimerization pocket. Radiolabeled IVTT HA-tagged BAXΔC or HA-tagged BAXΔNΔC in combination with GST-α9 or GST-α9 S184V were subjected to anti-HA immunoprecipitation in 1 % CHAPS. Immunoprecipitates and pre-IP input were analyzed by Nu-PAGE and autoradiography. Asterisk denotes the degradation products.
(B) The L63E mutation in BAX disrupts the binding of the α1 helix to the rest of the protein, resulting in N-terminal exposure and mitochondrial targeting. Radiolabeled IVTT HA3-tagged BAX α1 helix in combination with BAXΔN wt, L63E or G67R were subjected to anti-HA immunoprecipitation in 1 % CHAPS. Immunoprecipitates and pre-IP input were analyzed by Nu-PAGE and autoradiography.
(C) The S184V mutation in BAX destabilizes the binding of the α1 helix to the rest of the protein. Radiolabeled IVTT HA3-tagged BAX α1 helix in combination with BAXΔN wt or S184V were subjected to anti-HA immunoprecipitation in 1 % CHAPS. Immunoprecipitates and pre-IP input were analyzed by Nu-PAGE and autoradiography.
(D) tBID, BIM, and PUMA bind to the α1 helix of BAX. Radiolabeled IVTT HA3-tagged BAX α1 helix in combination with tBID, BIM, or PUMA were subjected to anti-HA immunoprecipitation in 1 % CHAPS. Immunoprecipitates and pre-IP input were analyzed by Nu-PAGE and autoradiography.
(E) tBID, BIM, and PUMA, but not BAD, induce the N-terminal exposure of BAX and remain associated with the N-terminally exposed BAX. Radiolabeled IVTT BAX incubated with tBID, BIM, PUMA, or BAD were immunoprecipitated with the 6A7 antibody in 1 % CHAPS. Immunoprecipitates and pre-IP input were analyzed by Nu-PAGE and autoradiography.
(F) tBID, BIM, and PUMA induce the homo-oligomerization of BAX S184V. Mitochondria isolated from DKO MEFs reconstituted with BAX S184V were incubated with IVTT tBID, BIM, or PUMA for 30 min and then treated with BMH crosslinker. The BAX oligomers were detected by an anti-BAX immunoblot.
(G) BH1 domain is required for N-terminally exposed BAX to interact with tBID. Radiolabeled IVTT BAX L63E or BAX L63E/G108E incubated with tBID were immunoprecipitated with the 6A7 antibody in 1 % CHAPS. Immunoprecipitates and pre-IP input were analyzed by Nu-PAGE and autoradiography.
The aforementioned findings led to our next hypothesis–exposure of the α1 helix results in the disengagement of the α9 helix and subsequent mitochondrial translocation. To test this hypothesis, we developed another in vitro system to detect the intramolecular interaction between the α1 helix of BAX and BAXΔN with deletion of the N-terminal 37 amino acid residues. HA-tagged α1 helix of BAX co-precipitated BAXΔN using anti-HA immunoprecipitation in the presence of CHAPS (Fig. 4B). Of note, NP-40 disrupted this interaction, consistent with the notion that NP-40 induces the conformational changes of BAX (Fig. S5). The NMR structure of BAX reveals that the α2 helix (BH3 domain) of BAX is in close proximity to its α1 helix (Suzuki et al., 2000). This prompted us to investigate whether the mitochondria-targeted BAX α2 helix L63E mutant might destabilize the interaction between the α1 helix of BAX and BAXΔN, resulting in secondary disengagement of the α9 helix. Indeed, the α1 helix failed to interact with BAXΔN containing L63E mutation (Fig. 4B). By contrast, another BH3 domain mutation G67R had no effect on this interaction and BAX G67R was cytosolic (Fig. 4B). These findings provide mechanistic basis explaining how a mutation at the BH3 domain affects mitochondrial targeting. Moreover, S184V mutation abrogated the interaction between the α1 helix and BAXΔN, consistent with the observation that BAX S184V readily exposed its N-terminus (Fig. 4C).
tBID, BIM, and PUMA induce the N-terminal exposure of BAX and remain associated with the N-terminally exposed BAX through the BH1 domain
As the α1 helix of BAX controls the pivotal step of conformational changes, we next investigated whether activator BH3s initiate BAX activation by binding directly to the α1 helix of BAX. Indeed, HA-tagged α1 helix of BAX co-precipitated tBID, BIM, and PUMA, but not BAD or BMF in vitro (Fig. 4D and Fig. S6). These findings are supported by our recently solved NMR structure of BAX complexed with BIM BH3 peptide in which the α1 and α6 helices of BAX were involved in the binding with BIM (Gavathiotis et al., 2008). Furthermore, tBID, BIM, and PUMA, but not BAD or BMF, could directly induce the N-terminal exposure of BAX in vitro (Fig. 4E and Fig. S7). Co-incubation of BAX with tBID, BIM, and PUMA led to the exposure of α1 helix of BAX that could be immunoprecipitated by the 6A7 antibody (Fig. 4E). Remarkably, tBID, BIM, and PUMA were co-precipitated with the N-terminally exposed BAX using the 6A7 antibody (Fig. 4E). Since the α1 helix is the epitope recognized by the 6A7 antibody, the binding between BH3s and the α1 helix of BAX must be transient, otherwise activated BAX would not be pulled down by the 6A7 antibody. As BH3s remain associated with the N-terminally exposed BAX, they must bind to a region other than the α1 helix. The second interaction is probably essential for the subsequent activation of BAX to induce homo-oligomerization since the N-terminal exposure of BAX only contributes to mitochondrial targeting. The N-terminally exposed BAX S184V mutant did not undergo homo-oligomerization until it was activated by tBID, BIM, and PUMA (Fig. 4F). Of note, inactivator BH3s such as BAD or BMF failed to induced homo-oligomerization of BAX S184V mutant (Fig. S8). To identify the second interaction site between N-terminally exposed BAX and BH3s, we reason that the canonical dimerization pocket is the most likely candidate since it is no longer blocked by the α9 helix and the BH1 domain has been demonstrated to mediate the interaction between BAX and BID (Wang et al., 1996). Since BH1 domain mutant of BAX (G108E) failed to undergo N-terminal exposure triggered by BH3s, we introduced L63E mutation on top of G108E to bypass the first activation step. Indeed, BAX L63E/G108E mutant constitutively exposed its N-terminus and targeted to the mitochondria (data not shown). Importantly, BAX L63E/G108E failed to co-precipitate tBID using the 6A7 antibody (Fig. 4G). Furthermore, BAX G108E failed to interact with tBID in NP-40 which induced its N-terminal exposure (Fig. S9). Together, our data discover dynamic interactions between activator BH3s and BAX.
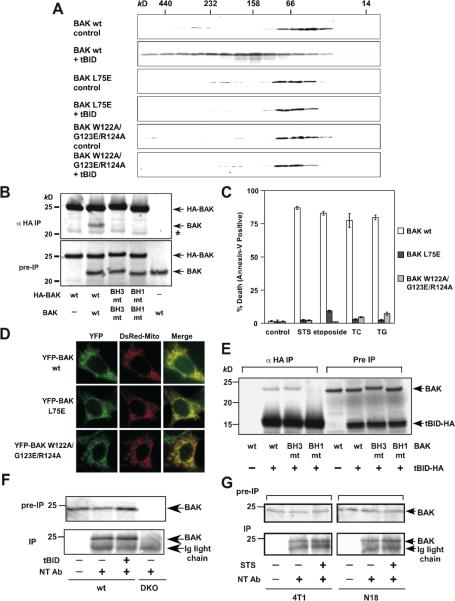
The BH1 and BH3 domains are essential for the assembly of higher-ordered BAK oligomers and the proapoptotic activity of BAK
To investigate the importance of BH1 and BH3 domains for the pro-apoptotic activity of BAK, W122A/G123E/R124A (BH1) and L75E (BH3) mutants of BAK were generated. These two mutants failed to form higher-ordered oligomers in response to tBID (Fig. 5A). In addition, HA-tagged BH1 or BH3 mutant was unable to co-precipitate non-tagged corresponding mutant by anti-HA immunoprecipitation (Fig. 5B). Accordingly, they were unable to induce apoptosis following intrinsic death signals when they were reconstituted in DKO cells (Fig. 5C). Of note, these two mutants localized at the mitochondria like wt BAK (Fig. 5D). Similar to the N-terminally exposed BAX, the BH1 domain of BAK was involved in its binding to tBID (Fig. 5E). Although the N-terminal exposure of human BAK following apoptotic signals was suggested by a conformation-specific antibody (Ab-1) using FACS analysis (Griffiths et al., 1999), mutagenesis studies indicated that it neither correlated with dimerization nor was required for the proapoptotic activity (Dewson et al., 2008). Intriguingly, the epitope recognized by Ab-1 is not clear since it was generated using the C-terminal truncated human BAK protein and Ab-1 could not detect mouse BAK even by immunoblots. The enhanced detection of BAK by Ab-1 or Ab-2 during apoptosis may represent a more global conformational change rather than a simple exposure of the α1 helix of BAK. To address the exposure of the α1 helix in mouse BAK, we performed immunoprecipitation using antibody specific for the α1 helix of BAK (NT), which immunoprecipitated comparable amounts of mouse BAK before or after tBID activation in MEFs (Fig. 5F). Comparable amounts of BAK were immunoprecipitated before or after staurosporine treatment in two additional mouse cell lines 4T1 (breast cancer) and N18 (neuroblastoma) (Fig. 5G). These data suggest that the integral mitochondrial membrane protein BAK constitutively exposes its N-terminal α1 helix, reminiscent to mitochondrially translocated BAX.
Figure 5. BH1 and BH3 domains are required for the homo-oligomerization and proapoptotic activity of BAK.
(A) BH1 and BH3 domain mutants of BAK fail to undergo homo-oligomerization in response to tBID. Mitochondria isolated from DKO MEFs reconstituted with wt or mutant BAK were treated with recombinant tBID (1 ng/μl) and solubilized in 2 % CHAPS buffer. Protein lysates (200 μg) were subjected to Superdex 200 (HR 10/30) gel filtration chromatography and fractions were analyzed by anti-BAK immunoblots.
(B) BH1 and BH3 domain mutants of BAK fail to form homo-dimers. Radiolabeled IVTT N-terminal HA3-tagged wt or mutant BAK plus non-tagged counterparts were immunoprecipitated with anti-HA antibody. Immunoprecipitates and pre-IP input were analyzed by Nu-PAGE and autoradiography. Asterisk denotes degradation products.
(C) BH1 and BH3 domain mutants of BAK fail to trigger apoptosis. DKO MEFs reconstituted with wt or mutant BAK were treated with staurosporine (18 hr), etoposide (18 hr), tunicamycin (30 hr) or thapsigargin (30 hr) to induce apoptosis. Cell death was quantified by Annexin-V. Data shown are mean ± s.d. from three independent experiments.
(D) BH1 and BH3 domain mutants of BAK are localized at the mitochondria. Fluorescence microscopy of DKO MEFs stably reconstituted with YFP-tagged wt or mutant BAK followed by retroviral transduction of DsRed-Mito.
(E) BH1 domain is required for BAK to interact with tBID. Radiolabeled IVTT BAK wt, BAK BH3 mt (L75E) or BH1 mt (W122A/G123E/R124A) incubated with tBID-HA were immunoprecipitated with anti-HA antibody. Immunoprecipitates and pre-IP input were analyzed by Nu-PAGE and autoradiography.
(F) BAK constitutively exposes its N-terminal α1 helix. Mitochondria isolated from wt MEFs, mock treated or treated with tBID, were lysed in 1% CHAPS and then immunoprecipitated with the α1 helix specific anti-BAK antibody (NT). Immunoprecipitates and pre-IP input were analyzed by anti-BAK (G23) immunoblots. Mitochondria isolated from DKO MEFs serve as a negative control.
(G) BAK constitutively exposes its N-terminal α1 helix. 4T1 or N18 cells before or after staurosporine treatment were lysed in 1% CHAPS and then immunoprecipitated with the α1 helix specific anti-BAK antibody (NT). Immunoprecipitates and pre-IP input were analyzed by anti-BAK (G23) immunoblots.
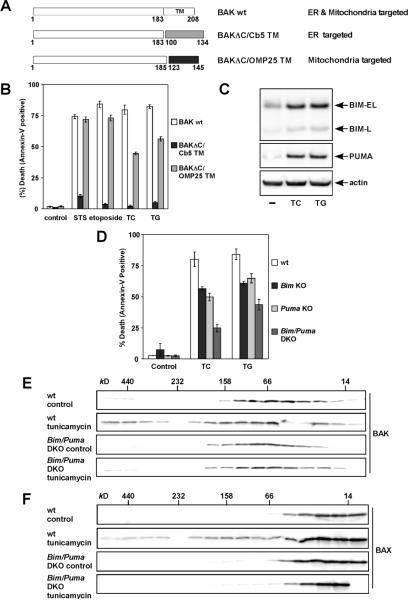
Death signals initiated from ER require mitochondria- but not ER-localized BAK to execute apoptosis
BAX and BAK not only control the mitochondrial gateway of apoptosis but also regulate ER calcium homeostasis (Scorrano et al., 2003; Zong et al., 2003). Since BAK is an integral membrane protein that resides at both mitochondria and ER (Scorrano et al., 2003; Zong et al., 2003), we generated BAK chimera proteins that were specifically targeted to ER or mitochondria to determine the contribution of ER calcium regulation to apoptosis controlled by BAK. The transmembrane domain of BAK was replaced by heterologous ER or mitochondrial targeting signals derived from cytochrome b5 and OMP25, respectively (Fig. 6A) (Shirane and Nakayama, 2003; Zhu et al., 1996). The restricted localization of BAKΔC/Cb5 at ER and that of BAKΔC/OMP25 at mitochondria were confirmed by fusing these chimera proteins with YFP (Fig. S10). The proapoptotic activity of these BAK mutants was assessed by reconstituting these BAK mutants in DKO cells. Interestingly, targeting of BAK to the mitochondria restored the apoptotic response of DKO cells not only to staurosporine (a general kinase inhibitor) and etoposide (DNA damage) but also to ER stress induced by tunicamycin and thapsigargin (Fig. 6B). By contrast, ER-targeted BAK failed to rescue the apoptotic defect of DKO cells even to ER stress (Fig. 6B). The minor difference in activating apoptosis between wt BAK and BAKΔC/OMP25 is likely due to the lower protein expression level of BAKΔC/OMP25 which was less stable (Fig. S1). These data clearly indicate that death signals emanated from ER also converge on mitochondrial BAK to execute apoptosis.
Figure 6. BIM and PUMA activate mitochondrial BAK and BAX to execute apoptosis upon ER stress.
(A) Schematic of the mitochondria- and ER-targeted BAK chimera mutants.
(B) Mitochondria- but not ER-targeted BAK is required for apoptosis triggered by intrinsic death signals. DKO MEFs reconstituted with wt or mutant BAK were treated with staurosporine (9 hr), etoposide (18 hr), tunicamycin (30 hr) or thapsigargin (30 hr) to induce apoptosis.
(C) BIM and PUMA are induced by ER stress. DKO MEFs untreated or treated with tunicamycin or thapsigargin for 18 hr were lysed and analyzed by anti-BIM and PUMA immunoblots.
(D) BIM and PUMA are required for ER stress-induced apoptosis. Wild-type, Bim KO, Puma KO, or Bim/Puma DKO MEFs were treated with tunicamycin or thapsigargin for 30 hr.
(E) Deficiency of Bim and Puma prevents ER stress-induced BAK homo-oligomerization. Wild-type or Bim/Puma DKO MEFs were untreated or treated with tunicamycin for 30 hr. Protein lysates were subjected to Superdex 200 (HR10/30) gel filtration chromatography. Fractions were analyzed by anti-BAK immunoblots.
(F) Deficiency of Bim and Puma prevents ER stress-induced BAX homo-oligomerization. The blots shown in (E) were stripped and reprobed with anti-BAX antibody.
Data shown in (B) and (D) are mean ± s.d. from three independent experiments. Cell death was quantified by Annexin-V.
ER stress induces BIM and PUMA to activate BAX/BAK-dependent mitochondrial death program
Since BAK needs to be activated by BH3s, we envision that activator BH3s must be induced by ER stress to provide the missing link that interconnects ER stress signaling and the BAX/BAK-dependent mitochondrial death program. Indeed, ER stress induced BIM and PUMA proteins (Fig. 6C). Consequently, Bim or Puma single knockout cells were resistant to ER stress-induced apoptosis and double deficiency of Bim and Puma DKO cells provided further protection (Fig. 6D). Importantly, ER stress-induced homo-oligomerization of BAX or BAK was blocked by the deficiency of Bim and Puma, supporting that BIM and PUMA are required to activate BAX/BAK upon ER stress (Fig. 6, E and F). Collectively, these findings exemplify the essence of BAX/BAK activation driven by activator BH3s in the context of normal cell death signaling cascades.
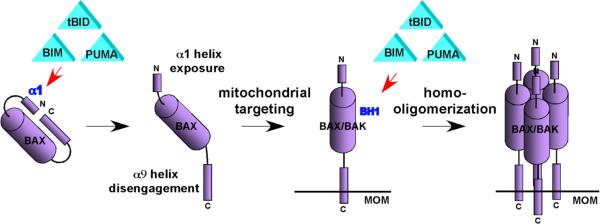
DISCUSSION
How the death effectors BAX and BAK are activated to trigger the mitochondria-dependent death program remains as one of the most vigorously debated topics in apoptosis research (Chipuk and Green, 2008; Youle and Strasser, 2008). Here, we propose a stepwise activation model of BAX and BAK driven by activator BH3s, tBID, BIM, and PUMA (Fig. 7 and table S1). We demonstrated that the α1 helix of BAX stabilizes and thereby controls the engagement of the α9 helix in the dimerization pocket. tBID, BIM, and PUMA bind transiently to the α1 helix of BAX to unleash auto-inhibition which allows for the structural reorganization by exposing both N- and C-termini. The C-terminal transmembrane domain hence becomes available for insertion into the MOM. tBID, BIM, and PUMA remain associated with the N-terminally exposed BAX to drive the homo-oligomerization of mitochondrially localized BAX. BAK, an integral mitochondrial membrane protein, constitutively exposes its α1 helix and requires tBID, BIM, and PUMA to trigger its homo-oligomerization. The homo-oligomerization of BAX or BAK appears to involve the interaction between the BH3 domain of one molecule and the canonical dimerization pocket of the other since mutations in either BH1 or BH3 domain abolish their homo-oligomerization, the same strategy utilized for the heterodimerization between BH3s and anti-apoptotic BCL-2 members (Sattler et al., 1997; Walensky, 2006). Previous mutagenesis studies often involve overexpression of mutant BAX or BAK in wt cells such that BH3 but not BH1 mutants were defective in triggering apoptosis (Chittenden et al., 1995; Simonen et al., 1997). Recently, the importance of BH1 and BH3 domains for the homo-oligomerization of BAX and BAK was reported (Dewson et al., 2008; George et al., 2007).
Figure 7. Schematic depicts the model of activation of BAX and BAK driven by “activator” BH3s.
The “indirect” activation model of BAX/BAK is proposed based on the lack of strong and stable interaction between BH3s and BAX/BAK (Willis et al., 2007). Our study reveals the dynamic nature of the interaction between BH3s and BAX because the conformational changes of BAX apparently involve a continuous, step-by-step structural reorganization, which helps explain the difficulty in detecting these interactions. BH3s first attack the α1 helix of BAX and then interact with BH1 domain once the N- and C-termini are exposed. Importantly, BH3s stay associated with BAX in the “transition state” conformation. This is for the first time that stable interaction between BH3s and BAX, at close to 1:1 stoichiometry, could be captured in solution (Fig. 4E). The reason why this interaction could be readily detectable is likely due to the block of subsequent conformational changes that requires the presence of lipid microenvironment since the N-terminally exposed BAX is supposed to insert into the MOM. An “embedded together” model has been proposed to emphasize the importance of lipid in the activation of BAX that was facilitated by cardiolipin (Leber et al., 2007). In addition, it was reported that unidentified MOM proteins assist tBID to activate BAX in the formation of lipidic pore (Schafer et al., 2009). The identification of the canonical dimerization pocket as the second interaction site helps reconcile with previous mutagenesis study demonstrating the involvement of BH1 domain in the binding between BAX and BID (Wang et al., 1996). BH1 domain is also required for BAK to interact with tBID. However, this interaction should be “hit-and-run” in nature since the same pocket is also utilized for homo-oligomerization. If BH3s continue to occupy the pocket, it is impossible for BAX or BAK to undergo homo-oligomerization. Indeed, it was reported that tBID was not co-eluted with higher-ordered BAX oligomers by gel filtration upon TNFα induced apoptosis (Sundararajan and White, 2001). On the other hand, BH3s binds tightly to the pocket of anti-apoptotic BCL-2 members such that anti-apoptotic BCL-2 members do not homo-oligomerize (Fig. S11). Moreover, the fact that BCL-2 was not co-eluted with the higher-ordered BAX/BAK oligomers suggest that BCL-2 is unlikely to inhibit activated BAX/BAK directly (Fig. S11). Anti-apoptotic BCL-2 members function like “decoy” death receptors that form inert stable complexes with BH3s but are incapable to assemble the death machinery that permeabilizes the MOM.
Since the α1 helix plays a pivotal role in initiating BAX activation, it is conceivable that inhibitors of BAX activation likely bind to the α1 helix to keep BAX inactive, exemplified by humanin (Guo et al., 2003). On the contrary, VDAC2 binds to the dimerization pocket of BAK to keep BAK in check (Cheng et al., 2003). In the absence of VDAC2, BAK exists as a monomer yet displaying conformation-specific proteolysis sensitivity comparable to activated BAK (Cheng et al., 2003). However, its full activation, i.e. homo-oligomerization, is still dependent on activator BH3s (Cheng et al., 2003). By analogy, VDAC2 functions as a brake controlling the BAK-centered death machinery, whereas activator BH3s provide the driving force. Our recent genetic study provided in vivo evidence demonstrating a critical VDAC2-BAK axis in regulating the negative selection and survival of thymocytes (Ren et al., 2009).
Both multidomain proapoptotic and anti-apoptotic BCL-2 members reside at the ER to affect ER Ca2+ store. Interestingly, BCL-2 and BCL-XL remain capable of regulating ER Ca2+ even in Bax/Bak DKO cells (Oakes et al., 2005). These data position anti-apoptotic BCL-2 proteins downstream of BAX/BAK in maintaining ER Ca2+ homeostasis, a hierarchy opposite to that for the control of mitochondrial apoptosis. We found that mitochondria- but not ER-targeted BAK is indispensable for ER stress-induced apoptosis in which BIM and PUMA function as sentinels interconnecting upstream death signals and downstream death program. BIM was induced by ER stress through CHOP-mediated transcriptional regulation and dephosphorylation-dependent stabilization (Puthalakath et al., 2007), whereas how PUMA is regulated by ER stress remains unclear. The role of BID in ER stress-induced apoptosis was uncovered recently in that BID was cleaved by Caspase-2 upon ER stress (Upton et al., 2008), which helps explain why double deficiency of Bim and Puma provides less apoptotic block than the deletion of Bax and Bak.
In summary, our data propose a stepwise model of BAX/BAK activation that integrates available structural and functional analyses and resolves previously elusive hypothesis. Our study clearly demonstrates the essential axis of activator BH3s-BAX/BAK in mitochondrial apoptosis.
EXPERIMENTAL PROCESURES
Plasmid construction and retrovirus production
Indicated Bcl-2 members were cloned into pSG5 (Stratagene), pCDNA3 (Invitrogen), or the retroviral expression vector MSCV-IRES-GFP (pMIG) or MSCV-Puro (Clontech). All the constructs were confirmed by DNA sequencing. The production of retroviruses was described previously (Cheng et al., 2001).
Cell culture and viability assay
The MEFs utilized were SV40-transformed. Reconstitution of BAX or BAK into DKO cell was achieved by retroviral transduction of BAX-IRES-GFP or BAK-IRES-GFP, followed by MoFlo (DaykoCytomation) sorting for GFP-positive cells. Cell death was quantified by Annexin-V-Cy3 (BioVision) staining, followed by flow cytometric analyses using a FACS Caliber (BD Bioscience) and CellQuest Pro software. P values for statistical analyses were obtained using Student's t test.
Mitochondria isolation, alkaline extraction of BAX, Protein Cross-Linking, and gel filtration chromatography
Mitochondria isolation and cross-linking of BAX were performed as described previously (Cheng et al., 2001; Kim et al., 2006). The chromatographic step of Superdex 200 (HR 10/30, GE-Amersham) was performed on an automatic fast protein liquid chromatography (AKTApurifier, GE-Amersham). The column was equilibrated with 2% CHAPS buffer (2% CHAPS, 300 mM NaCl, 0.2 mM DTT, 20 mM HEPES pH 7.5) and calibrated with thyroglobulin (669 kD), ferritin (440 kD), catalase (232 kD), aldolase (158 kD), bovine serum albumin (66 kD), and cytochrome c (14 kD). Protein lysates (200 μg) were loaded onto the column, eluted at a flow rate of 0.3 ml/min. Fractions of 0.6 ml were collected, precipitated by trichloroacetic acid, and analyzed by 8–16% SDS-PAGE (Bio-Rad). For alkaline extraction, mitochondria were resuspended in 0.1 M Na2CO3 (pH 11.5) for 30 min on ice. Supernatant (alkali-sensitive) and pellet (alkali-resistant) fractions were separated by centrifugation at 75,000 rpm for 10 min at 4 °C.
Immunoblot analysis and immunoprecipitation
Cell or mitochondrial lysates were resolved by NuPAGE (Invitrogen) gels and transferred onto PVDF (Immobilon-P, Millipore) followed by antibody detection using enhanced chemiluminescence method (Western Lightening, Perkin Elmer) and LAS-300 Imaging system (FUJIFILM Life Science). In vitro transcription and translation (IVTT) reactions were performed using TNT T7 Quick Coupled Transcription/Translation System or Wheat Germ Extract System (Promega). Immunoprecipitation (IP) of IVTT proteins was described previously (Kim et al., 2006). To detect BAX or BAK homo-dimers, IP was performed using 0.2% NP40 isotonic buffer (0.2% NP40, 142.5 mM KCl, 5 mM MgCl2, 1 mM EGTA, 20 mM HEPES at pH 7.5). To detect intramolecular interaction of BAX, IP was performed using 1 % CHAPS buffer (1 % CHAPS, 142.5 mM KCl, 2 mM CaCl2, 20 mM Tris-Cl, pH 7.4). Anti-6A7 IP was performed using 1 % CHAPS buffer.
Fluorescence microscopy
Fluorescence images were acquired with a SPOT camera (Diagnostics Instruments) mounted on an Olympus IX51 microscope (Olympus).
Supplementary Material
ACKNOWLEDGEMENTS
We thank Hsiu-Fang Chen for technical assistance and Dr. Richard Youle for providing 6A7 antibody. This work was supported by grants to E.H.C. from the NCI/NIH (K01CA98320 & R01CA125562) and the Searle Scholars Program, and to G. P. Z. from the NIH (R01GM083159 and P30CA21765).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Cartron PF, Gallenne T, Bougras G, Gautier F, Manero F, Vusio P, Meflah K, Vallette FM, Juin P. The first alpha helix of Bax plays a necessary role in its ligand-induced activation by the BH3-only proteins Bid and PUMA. Mol Cell. 2004;16:807–818. doi: 10.1016/j.molcel.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Cartron PF, Priault M, Oliver L, Meflah K, Manon S, Vallette FM. The N-terminal end of Bax contains a mitochondrial-targeting signal. J Biol Chem. 2003;278:11633–11641. doi: 10.1074/jbc.M208955200. [DOI] [PubMed] [Google Scholar]
- Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, Letai A. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science. 2003;301:513–517. doi: 10.1126/science.1083995. [DOI] [PubMed] [Google Scholar]
- Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer SJ. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008;18:157–164. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittenden T, Flemington C, Houghton AB, Ebb RG, Gallo GJ, Elangovan B, Chinnadurai G, Lutz RJ. A conserved domain in Bak, distinct from BH1 and BH2, mediates cell death and protein binding functions. Embo J. 1995;14:5589–5596. doi: 10.1002/j.1460-2075.1995.tb00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, Maundrell K, Antonsson B, Martinou JC. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J Cell Biol. 1999;144:891–901. doi: 10.1083/jcb.144.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewson G, Kratina T, Sim HW, Puthalakath H, Adams JM, Colman PM, Kluck RM. To trigger apoptosis, Bak exposes its BH3 domain and homodimerizes via BH3:groove interactions. Mol Cell. 2008;30:369–380. doi: 10.1016/j.molcel.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Gavathiotis E, Suzuki M, Davis ML, Pitter K, Bird GH, Katz SG, Tu HC, Kim H, Cheng EH, Tjandra N, Walensky LD. BAX activation is initiated at a novel interaction site. Nature. 2008;455:1076–1081. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George NM, Evans JJ, Luo X. A three-helix homo-oligomerization domain containing BH3 and BH1 is responsible for the apoptotic activity of Bax. Genes Dev. 2007;21:1937–1948. doi: 10.1101/gad.1553607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goping IS, Gross A, Lavoie JN, Nguyen M, Jemmerson R, Roth K, Korsmeyer SJ, Shore GC. Regulated targeting of BAX to mitochondria. J Cell Biol. 1998;143:207–215. doi: 10.1083/jcb.143.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths GJ, Dubrez L, Morgan CP, Jones NA, Whitehouse J, Corfe BM, Dive C, Hickman JA. Cell damage-induced conformational changes of the pro-apoptotic protein Bak in vivo precede the onset of apoptosis. J Cell Biol. 1999;144:903–914. doi: 10.1083/jcb.144.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B, Zhai D, Cabezas E, Welsh K, Nouraini S, Satterthwait AC, Reed JC. Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature. 2003;423:456–461. doi: 10.1038/nature01627. [DOI] [PubMed] [Google Scholar]
- Hsu YT, Youle RJ. Nonionic detergents induce dimerization among members of the Bcl-2 family. J Biol Chem. 1997;272:13829–13834. doi: 10.1074/jbc.272.21.13829. [DOI] [PubMed] [Google Scholar]
- Kim H, Rafiuddin-Shah M, Tu HC, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat Cell Biol. 2006;8:1348–1358. doi: 10.1038/ncb1499. [DOI] [PubMed] [Google Scholar]
- Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 2000;7:1166–1173. doi: 10.1038/sj.cdd.4400783. [DOI] [PubMed] [Google Scholar]
- Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, Newmeyer DD. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, Green DR, Newmeyer DD. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–342. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- Leber B, Lin J, Andrews DW. Embedded together: the life and death consequences of interaction of the Bcl-2 family with membranes. Apoptosis. 2007;12:897–911. doi: 10.1007/s10495-007-0746-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- Lindsten T, Ross AJ, King A, Zong W-X, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, et al. The combined functions of proapoptotic Bcl-2 family members Bak and Bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell JF, Billen LP, Bindner S, Shamas-Din A, Fradin C, Leber B, Andrews DW. Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell. 2008;135:1074–1084. doi: 10.1016/j.cell.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Nechushtan A, Smith CL, Hsu YT, Youle RJ. Conformation of the Bax C-terminus regulates subcellular location and cell death. Embo J. 1999;18:2330–2341. doi: 10.1093/emboj/18.9.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes SA, Scorrano L, Opferman JT, Bassik MC, Nishino M, Pozzan T, Korsmeyer SJ. Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2005;102:105–110. doi: 10.1073/pnas.0408352102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthalakath H, O'Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Reed JC. Proapoptotic multidomain Bcl-2/Bax-family proteins: mechanisms, physiological roles, and therapeutic opportunities. Cell Death Differ. 2006;13:1378–1386. doi: 10.1038/sj.cdd.4401975. [DOI] [PubMed] [Google Scholar]
- Ren D, Kim H, Tu HC, T.D. W, J.K. F, J.A. R, S.J. K, Hsieh JJ, Cheng EH. The VDAC2-BAK rheostat controls thymocyte survival. Sci Signal. 2009 doi: 10.1126/scisignal.2000274. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Korsmeyer SJ, Schlesinger PH. BAX-dependent transport of cytochrome c reconstituted in pure liposomes. Nat Cell Biol. 2000;2:553–555. doi: 10.1038/35019596. [DOI] [PubMed] [Google Scholar]
- Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, Yoon HS, Shuker SB, Chang BS, Minn AJ, et al. Structure of Bcl-xLBak peptide complex: recognition between regulators of apoptosis. Science. 1997;275:983–986. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- Schafer B, Quispe J, Choudhary V, Chipuk JE, Ajero TG, Du H, Schneiter R, Kuwana T. Mitochondrial outer membrane proteins assist Bid in Bax-mediated lipidic pore formation. Mol Biol Cell. 2009;20:2276–2285. doi: 10.1091/mbc.E08-10-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinzel A, Kaufmann T, Schuler M, Martinalbo J, Grubb D, Borner C. Conformational control of Bax localization and apoptotic activity by Pro168. J Cell Biol. 2004;164:1021–1032. doi: 10.1083/jcb.200309013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- Shirane M, Nakayama KI. Inherent calcineurin inhibitor FKBP38 targets Bcl-2 to mitochondria and inhibits apoptosis. Nat Cell Biol. 2003;5:28–37. doi: 10.1038/ncb894. [DOI] [PubMed] [Google Scholar]
- Simonen M, Keller H, Heim J. The BH3 domain of Bax is sufficient for interaction of Bax with itself and with other family members and it is required for induction of apoptosis. Eur J Biochem. 1997;249:85–91. doi: 10.1111/j.1432-1033.1997.t01-1-00085.x. [DOI] [PubMed] [Google Scholar]
- Sundararajan R, White E. E1B 19K blocks Bax oligomerization and tumor necrosis factor alpha-mediated apoptosis. J Virol. 2001;75:7506–7516. doi: 10.1128/JVI.75.16.7506-7516.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Youle RJ, Tjandra N. Structure of Bax: coregulation of dimer formation and intracellular localization. Cell. 2000;103:645–654. doi: 10.1016/s0092-8674(00)00167-7. [DOI] [PubMed] [Google Scholar]
- Upton JP, Austgen K, Nishino M, Coakley KM, Hagen A, Han D, Papa FR, Oakes SA. Caspase-2 cleavage of BID is a critical apoptotic signal downstream of endoplasmic reticulum stress. Mol Cell Biol. 2008;28:3943–3951. doi: 10.1128/MCB.00013-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walensky LD. BCL-2 in the crosshairs: tipping the balance of life and death. Cell Death Differ. 2006;13:1339–1350. doi: 10.1038/sj.cdd.4401992. [DOI] [PubMed] [Google Scholar]
- Walensky LD, Pitter K, Morash J, Oh KJ, Barbuto S, Fisher J, Smith E, Verdine GL, Korsmeyer SJ. A stapled BID BH3 helix directly binds and activates BAX. Mol Cell. 2006;24:199–210. doi: 10.1016/j.molcel.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Wang K, Gross A, Waksman G, Korsmeyer SJ. Mutagenesis of the BH3 domain of BAX identifies residues critical for dimerization and killing. Mol Cell Biol. 1998;18:6083–6089. doi: 10.1128/mcb.18.10.6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Yin XM, Chao DT, Milliman CL, Korsmeyer SJ. BID: a novel BH3 domain-only death agonist. Genes Dev. 1996;10:2859–2869. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–2933. [PubMed] [Google Scholar]
- Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A, Ashiya M, Thompson CB, Korsmeyer SJ. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 2000;14:2060–2071. [PMC free article] [PubMed] [Google Scholar]
- Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, Ierino H, Lee EF, Fairlie WD, Bouillet P, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- Zhu W, Cowie A, Wasfy GW, Penn LZ, Leber B, Andrews DW. Bcl-2 mutants with restricted subcellular location reveal spatially distinct pathways for apoptosis in different cell types. Embo J. 1996;15:4130–4141. [PMC free article] [PubMed] [Google Scholar]
- Zong WX, Li C, Hatzivassiliou G, Lindsten T, Yu QC, Yuan J, Thompson CB. Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis. J Cell Biol. 2003;162:59–69. doi: 10.1083/jcb.200302084. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.