Abstract
Background
Neurological Examination Abnormalities (NEA, often called “neurological soft signs”) have been observed in early schizophrenia and may be heritable. We investigated the prevalence, and neurocognitive and psychopathological correlates of NEA among offspring of schizophrenia patients who are at increased genetic risk for this illness.
Methods
Neurological examinations were conducted on high risk (HR, n = 74) and healthy comparison subjects (HS, n = 86), using the Heinrichs–Buchanan scale. Cognitive–perceptual (CogPer) and repetitive motor (RepMot) subscores, and total NEA scores were computed. All HR and HS were assessed using K-SADS/SCID for diagnoses. Schizotypy was measured using the Magical Ideation and the Perceptual Aberration subscales (Chapman scale), attention using Continuous Performance Test (CPT-IP) and executive functions using the Wisconsin Card Sorting Test (WCST).
Results
CogPer (F(1,160) = 7.14, p = 0.008) but not RepMot NEA scores were higher in HR subjects compared to HS after controlling for age and sex. CogPer NEA scores were higher in HR subjects with axis I psychopathology compared to those without (F(2,170)–6.41, p = 0.002). HR subjects had higher schizotypy scores (composite of the magical ideation and perceptual aberration scales) (F(1,141) = 23.25, p = 0.000004). Schizotypy scores were negatively correlated with sustained attention and executive functions. In addition, schizotypy was positively correlated with CogPer NEA scores.
Conclusions
Young relatives at increased genetic risk for schizophrenia show more frequent NEA. CogPer but not RepMot NEA scores were elevated, consistent with our prior observation of CogPer NEA being relatively specific for schizophrenia. The observed relationships between NEA, cognitive impairments, schizotypy and axis I disorders suggest that NEA may characterize a subgroup of HR offspring at an elevated risk for psychopathology.
Keywords: Schizophrenia, Psychotic disorders, High risk, Neurological examination abnormalities, Neurological soft signs, Schizotypy, Cognitive functions, Neuropsychology, Endophenotype
1. Introduction
Neurological Examination Abnormalities (NEA), also called ‘soft’ neurological signs, are observed in a substantial portion of patients with schizophrenia. These are subtle neurological abnormalities comprising impairments in motor function and sensory integration, and persistence of primitive reflexes. NEA have been well documented in first episode antipsychotic naïve (Sanders et al., 1994; Venkatasubramanian et al., 2003), and treated subjects with schizophrenia (Chen et al., 2005), and may be more prominent in schizophrenia compared to other psychiatric disorders both among those with adult onset and adolescent onset (Heinrichs and Buchanan, 1988; Keshavan et al., 2003a; Woods et al., 1986; Zabala et al., 2006). NEA may also distinguish those at genetic risk for schizophrenia from those at risk for other mental disorders (Schubert and McNeil, 2004, 2005). Syndromal heterogeneity within schizophrenia may be related to NEA (Arango et al., 2000; Tosato and Dazzan, 2005). In addition, cognitive deficits are related to NEA in schizophrenia (Arango et al., 1999; Sanders et al., 2004) and among family members of subjects with schizophrenia (Hyde et al., 2007; Sanders et al., 2006). The significance of NEA is further highlighted by their association with poor premorbid function (Quitkin et al., 1976), earlier onset of the illness (Torrey, 1980), cognitive impairment (Arango et al.,1999; Flashman et al.,1996; Mohr et al., 1996), ventricular enlargement (Mohr et al., 1996) and poor long term outcome (Bombin et al., 2005;Heinrichs and Buchanan,1988; Johnstone et al.,1990; Mohr et al.,1996; Torrey, 1980).
The etiology of NEA is uncertain although they point to abnormal neurodevelopmental trajectory with genetic underpinnings. In support of this proposition, NEA have been documented among subjects genetically at risk for developing schizophrenia (Gourion et al., 2003; Kinney et al., 1999; Schubert and McNeil, 2005; Woods et al., 1986) but who have not yet manifested the clinical symptoms of psychosis. Interestingly, in a study on a small sample of young healthy adolescents, first episode schizophrenia and non-schizophrenia patients, an inverse correlation of NEA scores with age among the healthy adolescents, a trend for inverse correlation among non-schizophrenia patients and no correlation with age among the schizophrenia patients was observed (Zabala et al., 2006). These observations suggest that NEA may be developmentally mediated, and tend to decrease in frequency as the brain matures. Such a process may be impaired among those at genetic risk for developing schizophrenia. NEA and the pathophysiology of schizophreniamay share a common genetic background that affects the neurodevelopmental trajectory. A recent preliminary study on eight multiplex multigenerational families with at least two members with schizophrenia in each family provides suggestive evidence for heritability of certain NEA (Sanders et al., 2006). The same authors observed that the heritable NEA correlated with many neurocognitive impairments that were found to be heritable in a larger set of multiplex multigenerational families (Gur et al., 2007). However, so far no specific genetic variant has been associated with NEA.
We have used an abbreviated version of the Neurological Evaluation Scale (NES) (Buchanan and Heinrichs, 1989). The abbreviated version resulted from inter-rater reliability studies (Sanders et al., 1998) and factor analyses (Keshavan et al., 2003a; Sanders et al., 2005; Sanders et al., 2000). Principal factors showed differences in relationships with diagnosis (Keshavan et al., 2003a), cognition (Sanders et al., 2004) and neuroanatomy (Keshavan et al., 2003a). We have found that cognitive-perceptual tasks are specifically impaired in schizophrenia among the psychotic disorders, are more strongly related with cognitive functioning, and are uniquely related to heteromodal cortex volume.
In this study, we examined whether the presence of NEA would identify young relatives at even higher risk for developing schizophrenia. As a first step in that direction, our goal was to examine whether NEA was associated with increased risk for axis I disorders and for schizophrenia spectrum psychopathology among offspring of schizophrenia patients. Based on prior results, we hypothesized that: (a) the NEA associated with cognitive domains will be increased among the offspring of schizophrenia patients compared to healthy controls, and (b) the cognitive NEA will be associated with increased frequency of psychopathology among offspring of schizophrenia patients. In addition, we also hypothesized that the cognitive NEA and schizotypy scores will be correlated with neuropsychological measures.
2. Methods
2.1. Subjects
A series of subjects who were deemed to be at an elevated risk for developing schizophrenia due to family history (HR, n = 74) and matched healthy control subjects (HS, n = 86) were recruited. The HR subjects were slightly younger (mean age, 15.09 ± 3.62 years) than the HS (mean age 16.18 ± 4.32 years; t = 1.73, p = 0.086). The gender distribution (HR, male 34, female 40; HS, male 41, female 45; χ2 = 0.05, p = 0.83, NS) between the groups did not differ significantly. HR subjects were defined as those between the ages of 10 and 25 years who had at least one parent with schizophrenia, schizoaffective or schizophreniform disorder as defined in the DSM IV. HR subjects with a lifetime history of schizophrenia or schizoaffective disorder, mental retardation per DSM IV, significant current or previous head injury, medical or neurological illnesses were excluded. Subjects with current substance use disorder were excluded from the study. Healthy control subjects similar in age and gender distribution were recruited through local advertisements from the same geographical region as the HR subjects. After fully explaining the study procedures an informed consent was obtained from all subjects. For subjects below the age of 18 years we obtained consent from a parent or guardian, and informed assent from the participants. All study procedures were approved by the University of Pittsburgh Institutional Review Board.
2.2. Assessment of psychopathology
All HS and HR subjects were assessed by using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) (First, 1997), supplemented by the Behavioral Disorders sections of the K-SADS (Kaufman et al., 2000). Diagnoses among the parents were ascertained using the SCID-I. We ascertained the diagnoses using DSM-IV criteria at consensus conference meetings attended by senior clinicians (MSK, KMP, DM). Schizotypy was measured using the Chapman's Magical Ideation and the Perceptual Aberration scales (Chapman et al., 1978; Eckblad and Chapman, 1983). Large scale adult and adolescent studies of the Chapman scales show that these scales have robust psychometric properties and confirm their reliability and validity (Horan et al., 2008; Keshavan et al., 2003b; Lin et al., 2007). Composite schizotypy scores were calculated as an average of the Chapman's magical ideation and perceptual aberration scale scores.
2.3. Neuropsychological evaluation
Attention was evaluated using Continuous Performance Test-Identical Pair version (CPT-IP) (Cornblatt et al., 1989). Both visual d' and β values were obtained. We focused on visual d' in this manuscript because this measure assesses ability to identify targets rather than the conservativeness of these judgments that discriminated subjects with schizophrenia from the HS (Keshavan et al., 2003a). Executive functions were evaluated using the Wisconsin Card Sorting Test (WCST). Number of perseverative errors and concept formation were included in the analyses as measures of executive function. All evaluations were done by experienced evaluators (DD and GT).
2.4. Assessment of NEA
NEA were evaluated using the modified Buchanan–Heinrichs scale (Buchanan and Heinrichs, 1989). Previous inter-rater reliability analyses reduced the original scale to 13 items (Sanders et al., 1998). Principal component analyses identified four factors, namely repetitive motor (fist-ring, fist-edge-palm, alternating fist-palm, dysdiadochokinesis), cognitive–perceptual abnormalities (memory, audiovisual integration, right-left orientation, face-hand test, rhythm tapping reproduction), balancing tasks (Romberg, tandem gait), and the palmomental reflex. Of them, the cognitive–perceptual (CogPer) and repetitive-motor (RepMot) domains showed the highest factor loadings and validity (Keshavan et al., 2003a; Sanders et al., 2004). Therefore, we computed CogPer, RepMot and total NEA scores (13 items) (Sanders et al., 2000).
2.5. Statistical analyses
We first compared NEA, neuropsychological measures and schizotypy scores between the HR and HS in separate MANCOVA models using age and sex as covariates. The effect sizes for such models are provided as partial η2 . For all between-group differences, effect sizes were independently calculated and provided as Cohen's d and r. Next, using partial correlation tests including age as a covariate, we examined the correlation of NEA scores with schizotypy, attention and executive function scores within each group. Finally, we examined the NEA scores within the HR group between those with axis I psychopathology and those without.
3. Results
3.1. NEA scores
A MANCOVA model consisting of the study groups, NEA total scores, average CogPer and average RepMot scores was significant (Wilk's λ = 0.94, F = 3.31, p = 0.022) after controlling for age and gender. The effect size of the study group in the model was modest . It was interesting to note that age had a larger effect size whereas gender contributed minimally across the groups. Age correlated with NEA scores in both the groups but there were no differential effect of age on NEA scores in either group.
Univariate tests for between-subjects effects revealed that the total scores on the 13 item NEA scale (HR, mean 0.52 ± 0.35; HS, 0.42 ± 0.30; ANCOVA F(1,160) = 1.71, p = 0.19, NS) and RepMot scores (HR, 0.57 ± 0.55; HS, 0.54 ± 0.52) (F(1, 160) = 0.33, p = 0.57, NS) were not significantly different between the two groups. However, HR subjects scored approximately twice as high as the healthy control subjects on average CogPer NEA scores (HR, 0.48 ± 0.52, 95%CI 0.33–0.50; HS, 0.26 ± 0.37, 95%CI 0.18–0.35; ANCOVA, F(1, 160) = 7.14, p = 0.008) with a medium effect size (Cohen's d = 0.49; r = 0.24). Since CogPer set of NEA are closely related to IQ, covarying the IQ would reduce the significance. In our dataset, IQ was strongly correlated with CogPer NEA after covarying for age and gender (partial r = −0.47, p = 0.0001) but not RepMot (partial r = −0.13, p = 0.31) as expected among HR whereas the relationship was relatively weak among HS (CogPer, partial r = −0.25, p = 0.02; RepMot, partial r = −0.11, p = 0.3). We conducted a Univariate General Linear Model ANCOVA of average CogPer scores weighted for IQ scores and covarying for age and gender comparing HR with HS. Since we did not have IQ measures on four subjects, the sample for this particular analysis consisted of 85 HS and 71 HR. The results suggest a trend toward significance (F(1, 155) = 2.78, p = 0.098).
3.2. Neuropsychological evaluations
Mean IQ of HR subjects (98.31 ± 14.84) showed a trend toward significance relative to that of the healthy control subjects (110.79 ± 13.21) after controlling for age and socioeconomic status (F(1,178) = 3.41, p = 0.07). Using MANCOVA, we examined sustained attention and executive functions as measured using CPT-IP and WCST. A model comprising visual d' on CPT-IP, numbers of perseverative errors and concept formation scores on WCST was significant (Wilk's λ = 0.92, F = 3.28, p = 0.024; . Tests of between subjects effects using univariate tests showed that the visual d' was significantly lower and number of perseverative errors was significantly higher among the HR subjects compared to HS. Table 1 shows the mean, SD, 95%CI and the test statistics, significance and the effect size of each measure.
Table 1.
Differences in neuropsychological performances among the HR and HS
| Cognitive measure | HS (Mean ± SD) | 95% CI | HR (Mean ± SD) | 95% CI | F(1,118) | p | Effect size | |
|---|---|---|---|---|---|---|---|---|
| d | r | |||||||
| Visual d' (CPT-IP) | 1.56 ± 0.85 | 1.30–1.72 | 1.05 ± 0.87 | 0.89–1.34 | 6.13 | 0.015 | 0.59 | 0.28 |
| WCST Perseverative errors | 11.90 ± 8.48 | 9.98–14.48 | 16.34 ± 9.42 | 13.55–18.36 | 4.95 | 0.028 | 0.49 | 0.24 |
| WCST concept formation | 69.21 ± 12.47 | 65.81–72.85 | 67.77 ± 15.94 | 63.89–71.37 | 0.42 | 0.52 | 0.10 | 0.05 |
Abbr: HS: Healthy Subjects; HR: High Risk offspring subjects; CPT-IP, Continuous Performance Test-Identical Pairs; WCST, Wisconsin Card Sorting Test.
3.3. Psychopathology scores
As hypothesized, the schizotypy scores (composite of Chapman's magical ideation and perceptual aberration scores) were elevated among the HR subjects (mean score, 0.15 ± 0.01, 95%CI 0.13–0.18) compared to healthy controls (mean score, 0.07 ± 0.01, 95%CI 0.05–0.09) (ANCOVA, F(1,141) = 23.25, p = 0.000004; Cohen's d = 7.99; r = 0.97). The schizotypy scores were not correlated with either the CogPer or RepMot NEA scores (Table 2).
Table 2.
Correlations between NEA, psychopathology and neuropsychological measures
| Schizotypy scores |
Average CogPer NEA scores |
Average RepMot NEA scores |
Visual d' | Perseverative errors (WCST) |
Concept formation (WCST) |
Category completed (WCST) |
||
|---|---|---|---|---|---|---|---|---|
| Schizotypy scores | r | 1.000 | .129 | .070 | −.322 | .310 | −.346 | −.303 |
| p | . | .357 | .618 | .019* | .024* | .011** | .027* | |
| Av CogPer NEA scores | r | .129 | 1.000 | .302 | −.271 | .272 | −.164 | −.186 |
| p | .357 | . | .028* | .049* | .048* | .241 | .182 | |
| Av RepMot NEA scores | r | .070 | .302 | 1.000 | −.170 | .239 | .036 | −.197 |
| p | .618 | .028 | . | .224 | .085 | .798 | .156 | |
| Visual d' | r | −.322 | −.271 | −.170 | 1.000 | −.226 | .209 | .196 |
| p | .019* | .049* | .224 | . | .104 | .134 | .160 | |
| Perseverative errors (WCST) | r | .310 | .272 | .239 | −.226 | 1.000 | −.682 | −.811 |
| p | .024* | .048* | .085 | .104 | . | .000 | .000 | |
| Concept formation (WCST) | r | −.346 | −.164 | .036 | .209 | −.682 | 1.000 | .722 |
| p | .011** | .241 | .798 | .134 | .000 | . | .000 |
Nominally significant.
Survived Bonferroni correction for multiple testing.
3.4. Associations among NEA, neuropsychological performance and psychopathology
Among the HR subjects, attentional performance (visual d') negatively correlated with CogPer NEA (partial correlation, rp = −0.27, p = 0.049) but not with RepMot NEA (rp = −0.17, p = 0.22, NS). Executive functions as measured by the number of perseverative errors on the Wisconsin Card Sorting Test (WCST) positively correlated with CogPer (partial correlation, rp = 0.27, p = 0.048) but not with RepMot NEA (rp = 0.24, p = 0.09). Concept formation was not correlated with either CogPer or RepMot NEA scores. These differences did not sustain corrections for multiple testing using Bonferroni method (effective number of tests = 6; 2 NEA measures and 3 cognitive measures). No significant correlations among these variables were observed among healthy control subjects. Schizotypy scores were negatively correlated with visual d' (r = −0.32, p = 0.019), concept formation on WCST (r = 0.35, p = 0.011), and positively correlated with the perseverative errors on WCST (r = 0.31, p = 0.024) after controlling for age (Table 2). After applying Bonferroni correction for multiple tests (effective number of tests = 3; 1 schizotypy score and 3 cognitive measures), concept formation remained significant whereas number of perseverative errors and visual d' retained trends toward significance.
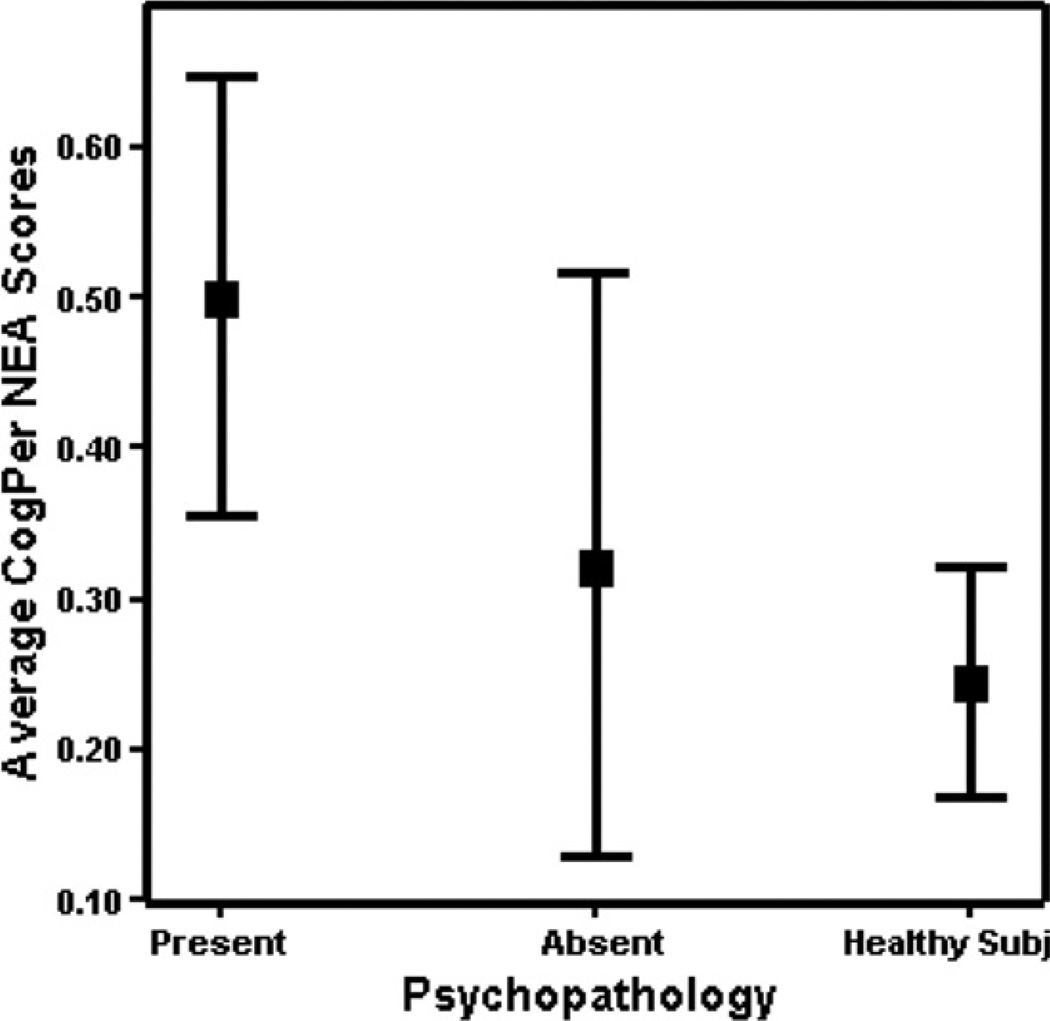
We divided the HR group into those with axis I psychopathology (HR + P, n = 48) and those without axis I psychopathology (HR − P, n = 24). Among the HR subjects, about 33% of subjects had no DSM IV axis I psychopathology whereas the other 67% had at least one diagnosable axis I disorder. Axis I diagnoses included mood disorders (26%), and ADHD (15%). A MANCOVA model consisting of CogPer and RepMot NEA scores was significant across HS, HR + P and HR − P (Wilk's λ = 0.91, F = 3.80, p = 0.005, . Univariate tests of between-subjects effects revealed that the CogPer NEA scores were significantly different across the groups (F(2,170) = 6.41, p = 0.002). Posthoc Bonferroni tests revealed that the HR + P group showed significantly elevated CogPer NEA scores compared to both HR − P (HR + P, 0.50 ± 0.52; HR − P, 0.32 ± 0.50, p = 0.04; Cohen's d = 0.35, r = 0.17) and was even higher compared to HS ((HR + P, 0.50 ± 0.52; HS, 0.24 ± 0.37, p = 0.002; Cohen's d = 0.58, r = 0.28). RepMot NEA scores were not different across the groups (Fig. 1). However, HR − P and HS did not differ significantly in the CogPer NEA scores. Next, we compared HR + P and HR − P on average CogPer scores weighted for IQ because IQ could explain part of the variance in the difference. After covarying for age and gender, we observed that average CogPer scores weighted for IQ were significantly elevated among HR + P compared to HR − P (F(1,77) = 4.58, p = 0.036). In addition, HR + P showed higher average CogPer scores weighted for IQ compared to HS (F(2,167) = 3.50, p = 0.033, Posthoc Bonferroni HR + P > HS, p = 0.054).
Fig. 1.
Average Cognitive–Perceptual NEA scores across HR subjects with axis I psychopathology (HR + P), without psychopathology (HR − P) and healthy subjects. Lines represent 95% confidence interval and the box represents the means.
We examined the neuropsychological performance across these groups. A MANCOVA model consisting of the groups (HR + P, HR − P and HS), and cognitive measures (visual d', perseverative errors and concept formation scores) was significant (Wilk's λ = 0.89, F = 2.66, p = 0.016, . Univariate ANCOVA tests between-subjects effects showed that visual d' was significantly different across these groups (F(2,137) = 6.40, p = 0.002) but not perseverative errors or concept formation scores. Posthoc Bonferroni tests showed that the HR + P performed worse on sustained attention measures compared to HS (p = 0.002). The differences between HR − P and HR + P, and HR − P and HS were not different.
4. Discussion
Our observations suggest that young relatives at increased genetic risk for schizophrenia show more frequent NEA, more prominently CogPer compared to RepMot NEA. These results are consistent with our previous observation of CogPer NEA being more prominent among subjects with schizophrenia relative to those with non-schizophrenic psychotic disorders and healthy subjects (Keshavan et al., 2003a). Increased CogPer NEA in the HR + P group, and an association between schizotypy and neuropsychological performance suggest that these measures may share deficits in the same neural substrates. After weighting the CogPer scores with IQ measures, although the HR − HS differences became relatively weaker, as expected, the differences between the HR + P and HR − P on the one hand, and HR + P and HS on the other persisted. Because IQ performance is compromised in schizophrenia and in individuals at risk, especially on tests of “fluid” intelligence, and the CogPer dimension is by definition related to general cognitive ability, partialling out variance associated with IQ is a reasonable strategy and we pursued it in this study. However, it rather has serious risk of throwing the baby out with the bathwater as happened here because the magnitude of illness effects can become markedly underestimated. In addition, demonstration of heritability of attention, certain executive functions, and NEA suggest that these abnormalities may share common etiologic and perhaps genetic underpinnings (Gur et al., 2007; Sanders et al., 2006). In addition, schizotypy has been reported to be heritable (Lin et al., 2007; Linney et al., 2003) and associated with several schizophrenia risk gene variants (Ma et al., 2007; Schurhoff et al., 2007; Stefanis et al., 2007). Therefore, individuals with higher CogPer NEA scores, schizotypy, the presence of axis I psychopathology, and impaired sustained attention and executive functions may forma distinct subgroup of offspring of schizophrenia or schizoaffective disorder patients with an elevated risk for axis I psychopathology.
The significance of our approach is that the NEA are observed within the framework of developmental trajectory in a cohort who is genetically at an elevated risk for developing schizophrenia. We noted that age was significantly correlated with NEA scores in both HR and HS; the strength of association was not different between the groups. A prior study on a small cohort of adolescent first episode psychosis subjects observed negative correlation with NEA among HS but not among subjects with psychotic disorders (Zabala et al., 2006). These observations along with reports of stability of NEA among persons with schizophrenia (Chen et al., 2005; Emsley et al., 2005) suggest that NEA may attenuate until the onset of the clinical syndrome after which NEA may become stable. Such speculations can be resolved by longitudinal observations on a cohort of HR subjects. Besides, the genetic HR approach, in contrast to clinical HR studies, offers a unique advantage of measuring the potential phenotypic markers well before the onset of psychotic symptoms. In the clinical HR approach, subjects are defined by the presence of sub-threshold symptoms of schizophrenia along with decline in functioning (Yung and McGorry, 1996). Such individuals are likely to be heterogeneous with, perhaps, both the genetic and environmental factors already set in motion, making it more difficult to parse each other. Nevertheless, this strategy provides valuable information on neurobiological changes proximal to the onset of clinical symptoms. Examining the genetic HR individuals could identify phenotypic markers that may be more strongly related to the underlying genetic factors or to an interaction between genetic and environmental factors than to the environmental factors alone. In this regard a recent exploratory study observed that many tasks in the RepMot category of NEA showed high heritability whereas only one test in the CogPer domain (audio-visual integration) showed significant heritability (Sanders et al., 2006). Other studies reported modest heritability estimates (Egan et al., 2001; Hyde et al., 2007).
We did not observe an association of CogPer with cognitive performance. An earlier study reported association of CogPer NEA scores with cognitive performance among persons with first episode psychoses (Sanders et al., 2004). In another study, many RepMot NEA were correlated with neuropsychological performance but not CogPer (Sanders et al., 2006). In that study, NEA were studied individually, using continuous data (completion time, number of errors, etc.) that showed greater variability than the ordinal data used in this and most NEA studies. The continuous data generated by RepMot tasks has much more variability than CogPer data, so the stronger heritability and correlations with neurocognitive measures found in that study may have been due to differences in the statistical power of the variables. Such differences may also be due to the sample size and the type of sample studied in these two studies. In our sample, age was significantly correlated with NEA scores and cognitive performance in both groups. After partialing out the effects of age, we did not observe such correlations. This may be because there is no real correlation between these measures among HR subjects or because the effect may be small that our sample was inadequately powered.
The derived group of NEAs employed in this study putatively map onto a broad range of regions in the frontal, parietal and temporal lobes. Therefore, measures of NEA may not specifically test key regions of interest that are thought to regulate cognitive domains. Additionally, associations between cognitive domains and brain regions are complex (Demakis, 2004). Therefore, NEA may not reliably point to a network that could be associated with cognitive impairment. However, neurobiological dysfunction underlying the NEA is proposed to be due to a failure of integration within/between sensory and repetitive motor systems and subcortical structural abnormalities (Dazzan and Murray, 2002). In the same study, frontal release signs correlated with perseverative errors on Wisconsin Card Sorting Test suggesting that these signs may be frontal cortical in origin. Previously we observed that CogPer NEA scores correlated with the volume of heteromodal association cortex whereas RepMot NEA associated with caudate and cerebellum suggesting a relatively specific regional structural alteration associated with NEA categories (Keshavan et al., 2003a).
CogPer NEA may be a useful intermediate phenotype. This and previous studies by other groups have observed increased frequency of NEA among first-degree relatives of schizophrenia patients (Cantor-Graae et al., 2000; Cantor-Graae et al., 1994; Ismail et al., 1998a). The heritable nature of some of the NEA (Egan et al., 2001; Hyde et al., 2007; Sanders et al., 2006) and increased frequency among patients with schizophrenia are observed (Ismail et al., 1998a; Keshavan et al., 2003a; Venkatasubramanian et al., 2003). Some NEA have been associated with specific regions in the brain suggesting neurobiological correlations for these markers (Dazzan et al., 2006, 2004; Keshavan et al., 2003a; Thomann et al., 2008). In addition, these abnormalities are observed before the onset of the psychotic symptoms and are relatively stable over the course of the illness suggesting that these traits may be state independent (Chen et al., 2005; Emsley et al., 2005; Heinrichs and Buchanan, 1988; Ismail et al., 1998a,b). Of further clinical significance is that they are non-invasive, inexpensive, quantifiable and reliable phenotypic markers. Taken together, NEA appears to meet several criteria suggested by Gottesman and Gould (2003) such as segregation among schizophrenia subjects, heritability, state independence, familial association and co-segregation with relatives of subjects with schizophrenia (Chan and Gottesman, 2008). Studies such as this could help further examine whether these markers predict the onset of the disease, which has been proposed as another criteria for an endophenotype (Almasy and Blangero, 2001).
The association of NEA scores with axis I psychopathology on the one hand, and schizotypy and cognitive impairment on the other is intriguing. Unraveling this clustering of heritable traits could be critical to understanding the nature of genetic pathology. A constellation of heritable intermediate phenotypes (Prasad and Keshavan, 2008) among those genetically at risk to develop schizophrenia could help characterize a genetic ultra high risk relative. Identifying and longitudinally following up such groups could potentially yield valuable data on the evolution of pathophysiology, the intermediate phenotypes and the clinical phenotype that may help in designing strategies for early detection, and perhaps prevention. Further, our observation of this conglomeration of CogPer NEA, schizotypy, cognitive impairments, and axis I psychopathology among offspring of schizophrenia patients points to a “broad spectrum” schizophrenia-related pre-psychotic phenotypic complex that could aid in prospective identification of future development of schizophrenia in these individuals. A systematic examination of this “broad spectrum” schizophrenia-related pre-psychotic phenotypic complex along with other heritable phenotypes associated with schizophrenia among subjects at an elevated risk for schizophrenia using a longitudinal design could provide clues to understanding the genetic basis.
In summary, our study observes a subgroup of neurological examination abnormalities to be elevated among offspring of subjects with schizophrenia/schizoaffective disorder. Such elevated CogPer NEA scores are even more prominent among those with an axis I psychopathology. An elevated frequency of NEA among subjects at risk for schizophrenia and those at risk for affective illnesses (Schubert and McNeil, 2005;Woods et al., 1986) suggest possible common trajectories for these disorders. Further, CogPer NEA show nominal associations with cognitive impairments, cognitive impairments are associated with schizotypy, but there is no correlation between the latter and the NEA scores; this may suggest a pathway from NEA to schizotypy through cognitive functioning. We are at present conducting a pathway analysis using these, and other clinical and neurobiological variables.
Acknowledgment
Authors acknowledge the assistance provided by Ms. Melissa Ziegler who was initially involved in the data collection.
Role of the funding source
This work was funded by MH64023 and NARSAD Established Investigator Award (MSK), MH72995 (KMP), MH64023 (JS), MH68680 (VD). The funding agencies did not have any further role in the design or execution of the study, analyses and preparation of the manuscript.
Footnotes
Contributors
MSK, KMP, VD and JS designed the study and wrote the protocol. KMP, MSK, and RS did the literature search and analyses of the existing literature. JM and DM managed the database. KMP and MSK did the statistical analyses. KMP, MSK, RS and JS interpreted the results of the analyses. KMP wrote the manuscript that was edited, modified and revised by MSK, RS, JS, DM, JM and DD. All authors contributed to and approved the final manuscript.
Conflict of interest
All authors declare no conflict of interest with the design, collection, analyses and interpretation of the results of this manuscript.
References
- Almasy L, Blangero J. Endophenotypes as quantitative risk factors for psychiatric disease: rationale and study design. Am. J. Med. Genet. 2001;105(1):42–44. [PubMed] [Google Scholar]
- Arango C, Bartko JJ, Gold JM, Buchanan RW. Prediction of neuropsychological performance by neurological signs in schizophrenia. Am. J. Psychiatry. 1999;156(9):1349–1357. doi: 10.1176/ajp.156.9.1349. [DOI] [PubMed] [Google Scholar]
- Arango C, Kirkpatrick B, Buchanan RW. Neurological signs and the heterogeneity of schizophrenia. Am. J. Psychiatry. 2000;157(4):560–565. doi: 10.1176/appi.ajp.157.4.560. [DOI] [PubMed] [Google Scholar]
- Bombin I, Arango C, Buchanan RW. Significance and meaning of neurological signs in schizophrenia: two decades later. Schizophr. Bull. 2005;31(4):962–977. doi: 10.1093/schbul/sbi028. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Heinrichs DW. The Neurological Evaluation Scale (NES): a structured instrument for the assessment of neurological signs in schizophrenia. Psychiatry Res. 1989;27(3):335–350. doi: 10.1016/0165-1781(89)90148-0. [DOI] [PubMed] [Google Scholar]
- Cantor-Graae E, McNeil TF, Rickler KC, Sjostrom K, Rawlings R, Higgins ES, Hyde TM. Are neurological abnormalities in well discordant monozygotic co-twins of schizophrenic subjects the result of perinatal trauma? Am. J. Psychiatry. 1994;151(8):1194–1199. doi: 10.1176/ajp.151.8.1194. [DOI] [PubMed] [Google Scholar]
- Cantor-Graae E, Ismail B, McNeil TF. Are neurological abnormalities in schizophrenic patients and their siblings the result of perinatal trauma? Acta Psychiatr. Scand. 2000;101(2):142–147. doi: 10.1034/j.1600-0447.2000.90054.x. [DOI] [PubMed] [Google Scholar]
- Chan RC, Gottesman Neurological soft signs as candidate endophenotypes for schizophrenia: a shooting star or a Northern star? Neurosci. Biobehav. Rev. 2008;32(5):957–971. doi: 10.1016/j.neubiorev.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, Raulin ML. Body-image aberration in schizophrenia. J. Abnorm. Psychology. 1978;87(4):399–407. doi: 10.1037//0021-843x.87.4.399. [DOI] [PubMed] [Google Scholar]
- Chen EY, Hui CL, Chan RC, Dunn EL, Miao MY, Yeung WS, Wong CK, Chan WF, Tang WN. A 3-year prospective study of neurological soft signs in first-episode schizophrenia. Schizophr. Res. 2005;75(1):45–54. doi: 10.1016/j.schres.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Lenzenweger MF, Erlenmeyer-Kimling L. The continuous performance test, identical pairs version: II. Contrasting attentional profiles in schizophrenic and depressed patients. Psychiatry Res. 1989;29(1):65–85. doi: 10.1016/0165-1781(89)90188-1. [DOI] [PubMed] [Google Scholar]
- Dazzan P, Murray RM. Neurological soft signs in first-episode psychosis: a systematic review. Br. J. Psychiatr., Suppl. 2002;43:s50–s57. doi: 10.1192/bjp.181.43.s50. [DOI] [PubMed] [Google Scholar]
- Dazzan P, Morgan KD, Orr KG, Hutchinson G, Chitnis X, Suckling J, Fearon P, Salvo J, McGuire PK, Mallett RM, Jones PB, Leff J, Murray RM. The structural brain correlates of neurological soft signs in AESOP first-episode psychoses study. Brain. 2004;127(Pt 1):143–153. doi: 10.1093/brain/awh015. [DOI] [PubMed] [Google Scholar]
- Dazzan P, Morgan KD, Chitnis X, Suckling J, Morgan C, Fearon P, McGuire PK, Jones PB, Leff J, Murray RM. The structural brain correlates of neurological soft signs in healthy individuals. Cereb. Cortex. 2006;16(8):1225–1231. doi: 10.1093/cercor/bhj063. [DOI] [PubMed] [Google Scholar]
- Demakis GJ. Frontal lobe damage and tests of executive processing: a meta-analysis of the category test, stroop test, and trail-making test. J. Clin. Exp. Neuropsychol. 2004;26(3):441–450. doi: 10.1080/13803390490510149. [DOI] [PubMed] [Google Scholar]
- Eckblad M, Chapman LJ. Magical ideation as an indicator of schizotypy. J. Consult. Clin. Psychol. 1983;51(2):215–225. doi: 10.1037//0022-006x.51.2.215. [DOI] [PubMed] [Google Scholar]
- Egan MF, Hyde TM, Bonomo JB, Mattay VS, Bigelow LB, Goldberg TE, Weinberger DR. Relative risk of neurological signs in siblings of patients with schizophrenia. Am. J. Psychiatry. 2001;158(11):1827–1834. doi: 10.1176/appi.ajp.158.11.1827. [DOI] [PubMed] [Google Scholar]
- Emsley R, Turner HJ, Oosthuizen PP, Carr J. Neurological abnormalities in first-episode schizophrenia: temporal stability and clinical and outcome correlates. Schizophr. Res. 2005;75(1):35–44. doi: 10.1016/j.schres.2004.06.014. [DOI] [PubMed] [Google Scholar]
- First MB. The Structured Clinical Interview for DSM-IV for Axis I disorders: Clinical Version, Administration Booklet. Washington, DC.: American Psychiatric Press; 1997. [Google Scholar]
- Flashman LA, Flaum M, Gupta S, Andreasen NC. Soft signs and neuropsychological performance in schizophrenia. Am. J. Psychiatry. 1996;153(4):526–532. doi: 10.1176/ajp.153.4.526. [DOI] [PubMed] [Google Scholar]
- Gottesman, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am. J. Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gourion D, Goldberger C, Bourdel MC, Bayle FJ, Millet B, Olie JP, Krebs MO. Neurological soft-signs and minor physical anomalies in schizophrenia: differential transmission within families. Schizophr. Res. 2003;63(1–2):181–187. doi: 10.1016/s0920-9964(02)00333-x. [DOI] [PubMed] [Google Scholar]
- Gur RE, Nimgaonkar VL, Almasy L, Calkins ME, Ragland D, Pogue-Geile MF, Kanes S, Blangero J, Gur RC. Neurocogntive endophenotypes in a multiplex multigenerational family study of schizophrenia. Am. J. Psychiatry. 2007;164(5):813–819. doi: 10.1176/ajp.2007.164.5.813. [DOI] [PubMed] [Google Scholar]
- Heinrichs DW, Buchanan RW. Significance and meaning of neurological signs in schizophrenia. Am. J. Psychiatry. 1988;145(1):11–18. doi: 10.1176/ajp.145.1.11. [DOI] [PubMed] [Google Scholar]
- Horan WP, Reise SP, Subotnik KL, Ventura J, Nuechterlein KH. The validity of Psychosis Proneness Scales as vulnerability indicators in recent-onset schizophrenia patients. Schizophr. Res. 2008;100(1–3):224–236. doi: 10.1016/j.schres.2007.12.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde TM, Goldberg TE, Egan MF, Lener MC, Weinberger DR. Frontal release signs and cognition in people with schizophrenia, their siblings and healthy controls. Br. J. Psychiatry. 2007;191:120–125. doi: 10.1192/bjp.bp.106.026773. [DOI] [PubMed] [Google Scholar]
- Ismail B, Cantor-Graae E, McNeil TF. Neurological abnormalities in schizophrenic patients and their siblings. Am. J. Psychiatry. 1998a;155(1):84–89. doi: 10.1176/ajp.155.1.84. [DOI] [PubMed] [Google Scholar]
- Ismail BT, Cantor-Graae E, Cardenal S, McNeil TF. Neurological abnormalities in schizophrenia: clinical, etiological and demographic correlates. Schizophr. Res. 1998b;30(3):229–238. doi: 10.1016/s0920-9964(97)00150-3. [DOI] [PubMed] [Google Scholar]
- Johnstone EC, Macmillan JF, Frith CD, Benn DK, Crow TJ. Further investigation of the predictors of outcome following first schizophrenic episodes. Br. J. Psychiatry. 1990;157:182–189. doi: 10.1192/bjp.157.2.182. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent DA, Ryan ND, Rao U. K-Sads-Pl. J. Am. Acad. Child Adolesc. Psych. 2000;39(10):1208. doi: 10.1097/00004583-200010000-00002. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Sanders RD, Sweeney JA, Diwadkar VA, Goldstein G, Pettegrew JW, Schooler NR. Diagnostic specificity and neuroanatomical validity of neurological abnormalities in first-episode psychoses. Am. J. Psychiatry. 2003a;160(7):1298–1304. doi: 10.1176/appi.ajp.160.7.1298. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Sujata M, Mehra A, Montrose DM, Sweeney JA. Psychosis proneness and ADHD in young relatives of schizophrenia patients. Schizophr. Res. 2003b;59(1):85–92. doi: 10.1016/s0920-9964(01)00400-5. [DOI] [PubMed] [Google Scholar]
- Kinney DK, Yurgelun-Todd DA, Woods BT. Neurologic signs of cerebellar and cortical sensory dysfunction in schizophrenics and their relatives. Schizophr. Res. 1999;35(2):99–104. doi: 10.1016/s0920-9964(98)00121-2. [DOI] [PubMed] [Google Scholar]
- Lin CC, Su CH, Kuo PH, Hsiao CK, Soong WT, Chen WJ. Genetic and environmental influences on schizotypy among adolescents in Taiwan: a multivariate twin/sibling analysis. Behav. Genet. 2007;37(2):334–344. doi: 10.1007/s10519-006-9104-5. [DOI] [PubMed] [Google Scholar]
- Linney YM, Murray RM, Peters ER, MacDonald AM, Rijsdijk F, Sham PC. A quantitative genetic analysis of schizotypal personality traits. Psychol. Med. 2003;33(5):803–816. doi: 10.1017/s0033291703007906. [DOI] [PubMed] [Google Scholar]
- Ma X, Sun J, Yao J, Wang Q, Hu X, Deng W, Sun X, Liu X, Murray RM, Collier DA, Li T. A quantitative association study between schizotypal traits and COMT, PRODH and BDNF genes in a healthy Chinese population. Psychiatry Res. 2007;153(1):7–15. doi: 10.1016/j.psychres.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Mohr F, Hubmann W, Cohen R, Bender W, Haslacher C, Honicke S, Schlenker R, Wahlheim C, Werther P. Neurological soft signs in schizophrenia: assessment and correlates. Eur. Arch. Psychiatry Clin. Neurosci. 1996;246(5):240–248. doi: 10.1007/BF02190275. [DOI] [PubMed] [Google Scholar]
- Prasad KM, Keshavan MS. Structural cerebral variations as useful endophenotypes in Schizophrenia: do they help construct “extended endophenotypes”? Schizophr. Bull. 2008;34(4):774–790. doi: 10.1093/schbul/sbn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quitkin F, Rifkin A, Klein DF. Neurologic soft signs in schizophrenia and character disorders. Organicity in schizophrenia with premorbid asociality and emotionally unstable character disorders. Arch. Gen. Psychiatry. 1976;33(7):845–853. doi: 10.1001/archpsyc.1976.01770070075008. [DOI] [PubMed] [Google Scholar]
- Sanders RD, Keshavan MS, Schooler NR. Neurological examination abnormalities in neuroleptic-naive patients with first-break schizophrenia: preliminary results. Am. J. Psychiatry. 1994;151(8):1231–1233. doi: 10.1176/ajp.151.8.1231. [DOI] [PubMed] [Google Scholar]
- Sanders RD, Forman SD, Pierri JN, Baker RW, Kelley ME, Van Kammen DP, Keshavan MS. Inter-rater reliability of the neurological examination in schizophrenia. Schizophr. Res. 1998;29(3):287–292. doi: 10.1016/s0920-9964(97)00103-5. [DOI] [PubMed] [Google Scholar]
- Sanders RD, Keshavan MS, Forman SD, Pieri JN, McLaughlin N, Allen DN, van Kammen DP, Goldstein G. Factor structure of neurologic examination abnormalities in unmedicated schizophrenia. Psychiatry Res. 2000;95(3):237–243. doi: 10.1016/s0165-1781(00)00176-1. [DOI] [PubMed] [Google Scholar]
- Sanders RD, Schuepbach D, Goldstein G, Haas GL, Sweeney JA, Keshavan MS. Relationships between cognitive and neurological performance in neuroleptic-naive psychosis. J. Neuropsychiatry Clin. Neurosci. 2004;16(4):480–487. doi: 10.1176/jnp.16.4.480. [DOI] [PubMed] [Google Scholar]
- Sanders RD, Allen DN, Forman SD, Tarpey T, Keshavan MS, Goldstein G. Confirmatory factor analysis of the Neurological Evaluation Scale in unmedicated schizophrenia. Psychiatry Res. 2005;133(1):65–71. doi: 10.1016/j.psychres.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Sanders RD, Joo YH, Almasy L, Wood J, Keshavan MS, Pogue-Geile MF, Gur RC, Gur RE, Nimgaonkar VL. Are neurologic examination abnormalities heritable? A preliminary study. Schizophr. Res. 2006;86(1–3):172–180. doi: 10.1016/j.schres.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Schubert EW, McNeil TF. Prospective study of neurological abnormalities in offspring of women with psychosis: birth to adulthood. Am. J. Psychiatry. 2004;161(6):1030–1037. doi: 10.1176/appi.ajp.161.6.1030. [DOI] [PubMed] [Google Scholar]
- Schubert EW, McNeil TF. Neuropsychological impairment and its neurological correlates in adult offspring with heightened risk for schizophrenia and affective psychosis. Am. J. Psychiatry. 2005;162(4):758–766. doi: 10.1176/appi.ajp.162.4.758. [DOI] [PubMed] [Google Scholar]
- Schurhoff F, Szoke A, Chevalier F, Roy I, Meary A, Bellivier F, Giros B, Leboyer M. Schizotypal dimensions: an intermediate phenotype associated with the COMT high activity allele. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2007;144B(1):64–68. doi: 10.1002/ajmg.b.30395. [DOI] [PubMed] [Google Scholar]
- Stefanis NC, Trikalinos TA, Avramopoulos D, Smyrnis N, Evdokimidis I, Ntzani EE, Ioannidis JP, Stefanis CN. Impact of schizophrenia candidate genes on schizotypy and cognitive endophenotypes at the population level. Biol. Psychiatry. 2007;62(7):784–792. doi: 10.1016/j.biopsych.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Thomann PA, Wustenberg T, Santos VD, Bachmann S, Essig M, Schroder J. Neurological soft signs and brain morphology in first-episode schizophrenia. Psychol. Med. 2008:1–9. doi: 10.1017/S0033291708003656. [DOI] [PubMed] [Google Scholar]
- Torrey EF. Neurological abnormalities in schizophrenic patients. Biol. Psychiatry. 1980;15(3):381–388. [PubMed] [Google Scholar]
- Tosato S, Dazzan P. The psychopathology of schizophrenia and the presence of neurological soft signs: a review. Curr. Opin. Psychiatry. 2005;18(3):285–288. doi: 10.1097/01.yco.0000165599.90928.c7. [DOI] [PubMed] [Google Scholar]
- Venkatasubramanian G, Latha V, Gangadhar BN, Janakiramaiah N, Subbakrishna DK, Jayakumar PN, Keshavan MS. Neurological soft signs in never-treated schizophrenia. Acta. Psychiatr. Scand. 2003;108(2):144–146. doi: 10.1034/j.1600-0447.2003.00113.x. [DOI] [PubMed] [Google Scholar]
- Woods BT, Kinney DK, Yurgelun-Todd D. Neurologic abnormalities in schizophrenic patients and their families. I. Comparison of schizophrenic, bipolar, and substance abuse patients and normal controls. Arch. Gen. Psychiatry. 1986;43(7):657–663. doi: 10.1001/archpsyc.1986.01800070043006. [DOI] [PubMed] [Google Scholar]
- Yung AR, McGorry PD. The prodromal phase of first-episode psychosis: past and current conceptualizations. Schizophr. Bull. 1996;22(2):353–370. doi: 10.1093/schbul/22.2.353. [DOI] [PubMed] [Google Scholar]
- Zabala A, Robles O, Parellada M, Moreno DM, Ruiz-Sancho A, Burdalo M, Medina O, Arango C. Neurological soft signs in adolescents with first episode psychosis. Eur. Psychiatry. 2006;21(5):283–287. doi: 10.1016/j.eurpsy.2005.09.006. [DOI] [PubMed] [Google Scholar]