Abstract
Background
Postnatal health sequelae associated with low birth weight have been attributed to ‘poor fetal growth’ from inferred adverse prenatal environments; risks augmented by infant growth rates. Identifying prenatal growth-restricting events is essential to clarify pathways and mechanisms of fetal growth.
Aim
The specific aim of this investigation was to examine whether an episode of preterm labor may compromise fetal growth.
Subjects and methods
Fetal size at the end of the second trimester and birth were compared among a sample of women with uncomplicated pregnancies (n=3167) and those who experienced preterm labor (<37 weeks) and subsequently delivered at term (>=37 weeks, n=147). Fetal weight was estimated from ultrasound measures and changes in weight standard scores across the third trimester investigated significant centile-crossing (> 0.67 standard deviation score change).
Results
Fetuses delivered at term after an episode of preterm labor were smaller at birth relative to their peers than at the end of the second trimester, and were 47% more likely to experience clinically significant downward centile crossing (p<0.05) than their peers (OR 1.47, 95% CI 1.04-2.07)
Conclusion
An episode of preterm labor may signal an adverse prenatal environment for term-delivered neonates. Epidemiologically silent events in the natural history of pregnancy are an understudied source of fetal growth compromise as inferred by small birth size among peers.
Keywords: Catch-down growth, fetal growth, preterm labor
Introduction
Postnatal health sequelae associated with low birth weight have been attributed to ‘poor fetal growth’ from inferred adverse prenatal environments. The evidence for specific growth restricting events during the course of pregnancy that may contribute to fetal growth faltering is less well characterized. The identification of prenatal growth-restricting events is essential to better understand pathways and mechanisms of fetal growth.
Centile crossing, both upwards (“catch-up” growth) and downwards (“catch-down” growth), has been used to describe growth rate changes with respect to reference growth charts after growth-constraining (Prader et al. 1963) or growth-promoting (Tanner et al. 1975, Hunziker et al. 1986, Stenhouse et al., 2006) factors have been removed. Percentile-crossing has been used more generally in studies assessing postnatal growth patterns (e.g., Smith et al., 1976, Ong et al. 2000, Mei et al. 2004), and, less frequently, in studies of prenatal growth (Stratton et al. 1995, Verkauskiene et al. 2007). Reports of decrements in standard scores following the onset of identifiable growth insults are rare (Viner et al. 1999) and undocumented during fetal development.
Recognition of specific insults and the mechanisms through which they contribute to fetal growth impairment is a valuable aid in the discrimination of causality, and in the broadening of our understanding of genetic and environmental influences on fetal growth, keys to the implementation of customized strategic interventions. To accomplish this, an evidence-based paradigm of developmental growth physiology and a re-definition of the fetus within human biology are required. This includes moving forward from the current view of fetal growth, predominantly a “medicalized” and partitioned biological phenomenon. Fetal growth and well-being are traditionally monitored first by obstetricians and maternal-fetal medicine specialists, as indicators of successful pregnancy. Subsequently, after birth, perinatologists, neonatologists and pediatricians apply their unique expertise and contiguous, but distinctive conceptual frameworks to what should be considered, instead, as a single and ongoing process of early human growth.
Identifying analytic methods to investigate growth patterns in clinically ascertained data is of paramount importance in moving beyond inferred intrauterine growth reduction based on a categorical outcome variable commonly used to represent poor fetal growth: size at birth below the tenth percentile, one metric identifying neonates as small for gestational age (SGA). A number of investigators have previously questioned the extent to which fetal growth restriction that stops short of a small for gestational age (SGA) outcome should also be of clinical concern. Conflicting conclusions have been reported (e.g., Altman and Hytten 1989, Danielian et al. 1992, Stratton et al. 1995) and it is challenging to draw a single viewpoint from these studies because they were not uniform in their design.
Recent studies, assessing fetal growth by serial ultrasound examinations repeated at four week intervals, have identified potential consequences for postnatal health in terms of body composition and hormonal status associated with a decline in fetal growth velocity (Hemachandra and Klebanoff 2006, Verkauskiene et al. 2007), regardless of the final birth weight. These studies highlight the importance of investigating the dynamics of fetal growth patterns, while reiterating the importance of methodological considerations necessary in assessing incremental growth changes (Cameron et al. 2005). Negative growth trajectories for the fetus may signal the presence of a potentially hostile prenatal environment, just as negative growth trajectories in postnatal life are recognized as markers of adverse environments (Cameron 2007).
The case of preterm labor
Preterm labor is defined in the presence of signs and symptoms of labor (uterine contractions and cervical changes) occurring prior to 37 gestational weeks. An episode of preterm labor can resolve within relatively short time as active labor leads to preterm birth, or, alternatively, an event of preterm labor may arrest and the pregnancy continues to deliver the preterm labor-exposed fetus at term. Spontaneous preterm labor carries significant health consequences in terms of neonatal morbidity and mortality as it is responsible for about 45% of preterm births, which are approximately 5 to 12% of pregnancies presently in developed countries (Goldenberg 2008). Preterm labor is one of the great obstetrical syndromes (Romero et al., 2006b), and while different etiologies, including vascular disease, infection or inflammation, stress and uterine over-distension may contribute to its onset, in many cases, no specific etiology has been identified to date.
Espinoza et al. (2007), in a retrospective cohort study including 849 patients hospitalized between 20 and 36 weeks of gestation with a diagnosis of preterm labor, have recently reported that an episode of preterm labor among those who delivered at term, is a risk factor for the birth of a small for gestational age neonate (birth weight <10th percentile for gestational age). This risk was positively correlated with the interval between the episode of preterm labor and delivery with an odds ratio of 2.22 (95% CI, 1.28-3.85) to deliver an SGA neonate (after controlling for confounding variables). Of note, placentas from patients who delivered at term had a significantly higher frequency of fetal or maternal vascular lesions (without histologic evidence of inflammation) than those who delivered preterm (p=0.01). These observations were novel and suggested that insults to the fetoplacental unity, such as compromised supply line (e.g., vascular abnormality), may be resolved by either irreversible preterm parturition or restriction of fetal growth.
Why are neonates born at term after an episode of preterm labor more likely to be small relative to their peers? Three alternatives include: 1) It is possible that the “preterm labor event” itself constitutes an insult to fetal growth, altering the subsequent fetal growth rate and trajectory; 2) It is possible that an altered fetal growth pattern predates, contributes to and eventually culminates in the phenotype of preterm labor; 3) It is possible that one of the underlying causes of preterm labor provokes both the event and the subsequent growth insult. Since no serial ultrasound assessments of fetal size were available for the original analysis (Espinoza et al. 2007), it was not possible to further investigate these alternatives.
The specific aim of this investigation was to examine whether an episode of preterm labor may be a fetal growth insult that subsequently results in less growth than would otherwise have been expected. A sample of fetuses from pregnancies uncomplicated by preterm labor serves as an internal control for fetuses from pregnancies complicated by preterm labor who delivered at term.
Methods
Study design
This is a longitudinal nested cohort study comparing fetal size and growth trajectories between the end of the second trimester and birth, among fetuses of women with uncomplicated, singleton pregnancies and those who experienced preterm labor and subsequently delivered at term (>37 weeks).
Study population
A total of 4115 pregnant women were enrolled at the time of their first prenatal visit (<22 weeks of gestation) at the Sotero del Rio Hospital in Puento Alto, Santiago, Chile. Exclusion critera were: previous chronic disease (diabetes, chronic hypertension, cardiac insufficiency), chronic conditions requiring treatment (e.g., asthma treated with steroids), evident fetal anomalies and fetal demise at the time of enrollment. Pregnancies were dated according to the last menstrual period (LMP) or by sonographic criteria if there was a discrepancy greater than one week between the gestational age derived by the menstrual period and fetal biometry at the first ultrasound. Participants provided written informed consent for data collection under protocols approved by the Institutional Review Boards of the Sotero del Rio Hospital and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute of Health, Department of Health and Human Services (NIH/DHHS).
Clinical definitions
Patients were considered to have a normal pregnancy if they did not have obstetrical, medical or surgical complications of pregnancy, and delivered a term (>=37 weeks) neonate. Preterm labor occurred in 9.9% of the sample (409/4115) and was defined as the presence of regular uterine contractions (at least four in 30 minutes) and progressive cervical change (cervical dilation of >=1 cm while under observation or cervical effacement of >=50%), occurring prior to 37 weeks of gestation.
Fetal weight assessment
All pregnant women participating in the study underwent serial fetal ultrasound examinations. Biometric fetal growth parameters, including the biparietal diameter, femur diaphyseal length and abdominal circumference were measured at each ultrasound assessment and were used for the estimation of fetal weight according to the Hadlock et al. formula (Hadlock et al. 1985). Multiple sonographers contributed to the collection of the ultrasound data. The inter- and intra-observer disagreement in the sonographic estimation of fetal weight ranges from +/- 3 % (Anderson et al. 2007).
Study aims and statistical analysis
Fetal growth assessment
In order to ascertain if an episode of preterm labor was a fetal growth insult, it was important to evaluate the fetuses prior to the preterm labor event. Sample-specific standard scores for estimated fetal weight were calculated, stratified by sex, and percentiles of birth weight for gestational age were derived by comparison with the Chilean national reference, which is based on more than two million singleton births between 22 and 42 weeks during 1993 and 2000, stratified by sex (González et al. 2004). Group differences were assessed by the Mann-Whitney test.
If an episode of preterm labor was a growth insult, a decline in the subsequent growth rate of exposed fetuses was predicted to result in reduced incremental growth and, therefore, standard scores across the third trimester.
Growth trajectory definition
“Clinically significant” changes in growth trajectories were investigated. A change in reference-based standard scores greater than +/- 0.67 (after Ong et al. 2000) has been previously proposed to be useful for this purpose, a criterion selected as it represents the width of percentile bands on growth charts (distance between the 3rd, 10th, 25th, 50th, 75th, 90th and 97th percentiles) and, thus, corresponds to the clinically salient event of an individual changing one major growth reference percentile (Cole 1994). This approach was run separately with a 0.73 standard deviation score criterion to incorporate a consideration of errors inherent in the estimation of fetal weight (Gardosi 1994) at 24 gestational weeks of age in this sample, or 0.06 standard scores, a value less than the distance between all contiguous growth percentiles at this age.
Chi square tests investigated group differences in the proportions of individuals experiencing the different growth trajectories (increases, decreases and no change in standard scores). Logistic regression explored covariates that may have contributed to individual variability, including maternal size, primiparity and smoking, and fetal sex (conditioned on estimated fetal weight at 24 weeks). The odds ratio for downward centile-crossing associated with preterm labor and the interaction between preterm labor and length of gestation was also assessed.
All variables were assessed for normality (Shapiro-Francia). Kruskal-Wallis analysis tested the hypothesis that continuous maternal characteristic variables were drawn from the same population and Pearson chi square compared the categorical variables. All analyses employed Stata Release 9 (StataCorp 2005) and probabilities less than 0.05 are reported as significant.
Results
In the present sample, 45% of the pregnancies experiencing preterm labor continued to term, statistics similar to those found in other samples (Goldenberg et al. 1998). Ultrasound assessment at a median age of 24.3 gestational weeks (24-24.9, interquartile) provided a maximum sample size for longitudinal comparison with birth outcome and captured the exposed fetuses prior to preterm labor in 94% of the cases. A total sample of 3314 maternal-fetal pairs contributed to the present analysis: 3167 fetuses from uncomplicated pregnancies and 147 from those complicated by an episode of preterm labor, delivering at term. Among the fetuses delivered from pregnancies complicated by preterm labor, the time between an episode of preterm labor and birth was a median of 6.3 weeks (4.2-9.7 interquartile; 0-22.9, range).
Maternal size variables were non-normally distributed. No significant differences in maternal size, parity or self-reported intake of alcohol or cigarettes (expressed either as daily consumption [cigarettes/day, drinks/day] or presence/absence) distinguished the women in the study sample by time of labor and there were no significant effects of fetal sex on the likelihood of preterm labor (Table 1).
TABLE 1. Maternal characteristics and fetal sex distribution among study groups. Data are expressed as median (interquartile) for continuous variables.
| Uncomplicated | Preterm labor Term birth | P value | |
|---|---|---|---|
| Maternal weight | 59 (52-66) | 58 (50-64) | 0.11 |
| Maternal height | 1.56 (1.52-1.6) | 1.56 (1.52-1.59) | 0.23 |
| Maternal BMI | 23.9 (21.8-26.7) | 23.5 (21.3-26.3) | 0.31 |
| Maternal age | 25 (21-30) | 23 (20-30) | 0.13 |
| Maternal parity | 35.4% | 35.7% | 0.87 |
| Fetal sex (m) | 51.6 % | 51.0 % | 0.89 |
Medical history identified that four women who delivered at term after experiencing preterm labor experienced gestational diabetes (4/147). Amniocentesis was performed on 44 of the women who experienced preterm labor in this study sample; none had evidence of infection (positive culture and/or elevated white blood cell counts).
The mean and standard deviation of term birth weight in the study sample differed little from the reference population (3410, SD 427 vs. 3400, SD 410, respectively, González et al. 2004). Fetuses born of pregnancies complicated by preterm labor were born at significantly earlier gestational ages than those born of uncomplicated pregnancies (Table II). Gestational age-based reference weights were not significantly different among fetuses born at term of uncomplicated pregnancies from those complicated by preterm labor at either 24 gestational weeks or birth. By late second trimester (median gestational age 24.3 weeks), male fetuses were larger than females in both groups, effects that remained significant at birth among the neonates from uncomplicated pregnancies only (Table II).
TABLE 2.
Fetal size, and ages at assessment and preterm labor for the study sample. Significant sex differences were found for both groups at 24 weeks, and among neonates from uncomplicated pregnancies at birth. Gestational ages at birth were younger for neonates delivered from pregnancies after preterm labor. Percentiles are sex- and age-adjusted with respect to the Chilean national reference (González et al. 2004). Data are medians (interquartile range) of percentiles (weight), weeks (age).
| Group (n) | EFW | Gestational Age at EFW | Birth Weight | Gestational Age at birth | Gestational Age at preterm labor |
|---|---|---|---|---|---|
| Uncomplicated (n=3167) |
55 (37-71) |
24.3 (24 - 24.9) |
47 (23- 70) |
39.7 (38.9 - 40.4) |
|
|
*Males (n=1633) |
57 (41-75) |
52 (27-76) |
|||
| Females (n=1534) |
52 (33-68) |
40 (18-65) |
|||
|
| |||||
| Preterm labor, term birth (n=147) |
57 (39-74) |
24.4 (24 -24.9) |
41 (25-67) |
*39 (38.1 - 39.7) |
32.4 (29.6-34.7) |
|
*Males (n=75) |
67 (42-77) |
||||
| Females (n=72) |
53 (38-65) |
||||
p<0.05;
EFW=Estimated fetal weight; data are medians (interquartile range) of percentiles (weight), weeks (age).
Growth trajectories
The fetuses delivered at term from pregnancies complicated by preterm labor were relatively smaller compared to their peers at birth than they had been at the end of the second trimester (Figure 1). Analysis identified that a greater proportion of fetuses delivered at term after an episode of preterm labor experienced downward centile-crossing during the third trimester (p=0.05) compared to those from uncomplicated pregnancies, with an odds ratio of 1.47 (95% CI, 1.04-2.07, p=0.03) (Tables III and IV). By contrast, a greater proportion of individuals among the uncompromised group experienced upward crossing of growth centiles during the same time interval (p=0.02, Table III). Fetuses from pregnancies complicated by preterm labor were 43% less likely to experience upward centile-crossing (OR 0.57, 95% CI, 0.33-0.97, p=0.04) than their peers from uncomplicated pregnancies. The results did not alter in a repeated analysis aimed to account for potential errors of estimated fetal weight (+/-0.73 standard scores).
FIGURE 1. Change in size relative to peers across the third trimester.
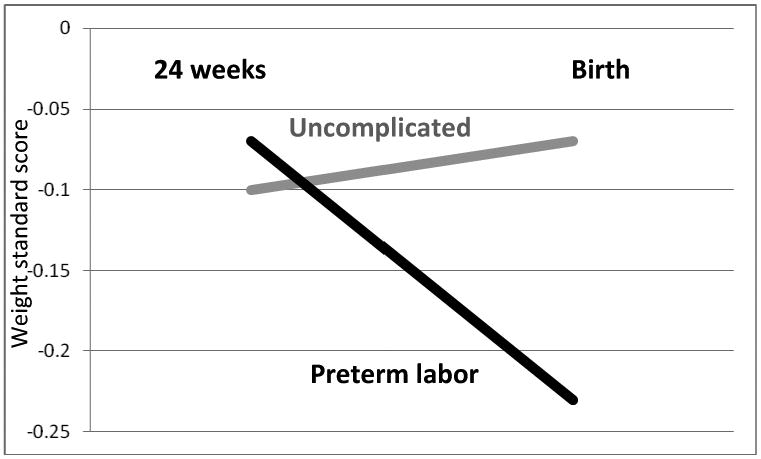
Fetuses delivered at term from pregnancies complicated by preterm labor (median gestational age of 32.4 weeks, interquartile range, 29.6-34.7weeks) were relatively smaller than their peers at birth compared to that at the end of the second trimester (median age 24.3 gestational weeks). Estimated fetal weight standard scores at 24 weeks were sex-stratified, internal z-scores; birth standard scores represent the sex-stratified population reference to correct for significant gestational age at birth effects (González et al., 2004).
TABLE 3.
Individual growth trajectories. Proportion of individuals whose fetal growth trajectories were characterized by upward centile-crossing, downward centile- crossing and no change in weight standard scores between the end of the second trimester and birth (defined as a change of > 0.67 standard score, < -0.67 standard score and a change>=-0.67 and <=0.67 standard scores, respectively, after Ong et al., 2000). The proportion of fetuses experiencing downward centile-crossing was greatest among those who experienced preterm labor (p=0.05). The proportion of fetuses experiencing upward centile-crossing was greater among the fetuses delivered of uncomplicated pregnancies (p=0.02).
| Uncomplicated | Preterm labor | |
|---|---|---|
| Upward centile- crossing | 18% * | 10% |
| Downward centile-crossing | 30% | 39% * |
| No change | 51% | 51% |
p≤0.056.
TABLE 4. Odds ratios for downward centile-crossing.
| Odds Ratio | Standard error | z | P value | 95% CI | |
|---|---|---|---|---|---|
| Uncomplicated pregnancies | |||||
|
| |||||
| 1Maternal Height | 0.05 | 0.03 | -4.3 | 0.001 | 0.01- 0.19 |
| 1Maternal Weight | 0.97 | 0.004 | -7.3 | 0.001 | 0.96- 0.98 |
| 1Maternal BMI | 0.94 | 0.01 | -5.89 | 0.001 | 0.92- 0.96 |
| Maternal smoking | 1.27 | 0.15 | 1.96 | 0.05 | 1.00- 1.61 |
| Primiparity | 1.56 | 0.13 | 5.4 | 0.001 | 1.33- 1.84 |
| Sex (female) | 1.41 | 0.11 | 4.3 | 0.001 | 1.21- 1.65 |
| 2 Time to birth | 0.80 | 0.03 | -6.23 | 0.001 | 0.74-0.86 |
|
| |||||
| Preterm labor | |||||
|
| |||||
| 1.47 | 0.26 | 2.19 | 0.03 | 1.04-2.07 | |
Tertiles;
gestational age at birth minus gestational age at 24 week measurement.
In order to gain some insight into sources of individual variability in growth patterns, logistic regression quantified the odds ratios of growth covariates and growth trajectory patterns (Table IV). Controlling for estimated fetal weight at the end of the second trimester, covariates associated with an increased odds ratio of subsequent downward centile-crossing among the uncomplicated pregnancies included small maternal size (height, weight and BMI, entered individually due to significant inter-measure correlation), maternal smoking and primiparity, and female sex; increasing time between the late second trimester assessment and birth was associated with decreased odds ratios. None of these factors were significant among the term-delivered fetuses of mothers who experienced preterm labor.
Discussion
By birth, the fetuses from pregnancies complicated by preterm labor had declined in size relative to their peers from unaffected pregnancies. These observations suggest that preterm labor may not be a benign feature in the natural history of pregnancy for the fetus with continued intrauterine coexistence, with repercussions for growth that accrue with time. This was reflected in the significant interaction between time to delivery and risk for downward centile-crossing among the fetuses from pregnancies experiencing preterm labor. It is notable that the protective effects from maternal size and multiparity afforded the fetuses delivered from uncomplicated pregnancies did not extend to the fetuses from mothers who experienced an episode of preterm labor, further suggesting the latter event as a source of growth compromise. While positing that growth was less than would otherwise have been expected across a time span coincident with the preterm labor event contributes some insight into the question of why neonates born after an episode of preterm labor might be small relative to their peers, this analysis does not clarify causality. It cannot be excluded that an altered growth biology predated and contributed to the preterm labor with a consequent growth decline, or that a common factor was the determinant of both the preterm labor and declining growth rate phenotype.
The documentation of preterm labor as a specific growth insult identifiable through altered growth trajectories among fetuses who remain in the prenatal environment reported from this Chilean data base support the cross-sectional clinical observations from Michigan (Espinoza et al. 2007). The details of what makes the post-preterm labor uterine experience adverse remain to be clarified. Previous work has identified an increased frequency of fetal and/or maternal vascular lesions in placenta associated with fetal growth restriction (e.g., Sheppard and Bonnar 1976) as well as those experiencing preterm labor and delivery (Kim et al. 2003). More specifically, an increased frequency of maternal and/or fetal placental lesions was found among term pregnancies following preterm labor compared to those who delivered preterm (Espinoza et al. 2007), implicating risk at the maternal/fetal interface that accrues over time as the supply line is compromised.
Whether there are significant health effects associated with prenatal downward centile-crossing as observed in this sample are unclear. The risks attendant with fetal growth faltering exceed those identifiable at the time of birth (Stratton et al., 1995). The present observations serve as an indicator of the need for further evaluation of preterm labor sequelae, specifically, and for understanding sources of suboptimal fetal growth more broadly. This event in the natural history of pregnancy emerges as one of what may be numerous epidemiologically silent events that affect pregnancy outcome in terms of neonatal size. Clarification of such relationships may begin to fill in the broad descriptive details of the associations between the lower end of the normal birth weight range and the often described health sequelae (Barker 2006). This case serves as an example of what may be elucidated from a synergy between clinical data and traditional auxological approaches in further understanding the predictive descriptions between birth weight and later health.
A challenge for research aiming to clarify the fetal-to-infant growth trajectory includes measurement metrics and references. Debate ensues regarding the best-practice use of reference data for assessing fetal weight (fetal vs neonatal weight, Ehrenkranz 2007, internal vs population and conditional vs unconditional, Royston 1995; Gardosi 2004). In the present analysis, downward centile-crossing was identified based on estimated fetal weight standard scores that were internally-based, and birth weight standard scores that were population-based (both sex-stratified). The former was chosen to avoid the pitfalls of using a reference for estimated fetal weights based on birth weights of very premature infants (e.g., Ehrenkranz 2007) and the latter to correct for the variability in gestational age at birth in the sample. The clinically-significant downward centile-crossing growth trajectories were based on the population level reference framework used in practice. The analysis is based on the proposition that the birth weight vs fetal weight effects apply to all individuals in the sample without bias towards outcome category and with the benefit of permitting a comparison among study peers in the context of their larger population. Further work addressing growth patterns based on customized assessments awaits their development in this population.
A benefit to this analysis was the natural history design which permitted an unbiased identification of preterm labor episodes and a clear temporal sequence for the investigation of growth trajectories before and after the event among a large number of pregnancies. Conceptualizing growth rate in terms of peer-related trajectories across a single time span has several methodological advantages: In the present study, it provided an approach for examining whether fetal growth might be less than expected in the context of preterm labor. More generally, it permits the researcher to take advantage of clinical data sets that are rich in information regarding the fetal environment, but are often collected according to measurement protocols that are insufficiently regular for the statistical requirements of longitudinal analyses.
The observation of downward centile-crossing in the present sample of Chilean fetuses exposed to preterm labor serves as an example of how fetal growth faltering may be identified in the context of adverse prenatal environments of numerous etiologies. The approach may be constructive for future investigation of what to date are primarily putative relationships between fetal and infant growth. Declining fetal growth trajectories are a likely precursor to growth patterns of early infancy with a high probability of contributing mechanistically to the risk-laden associations between early infant growth rates and later body composition (Cameron et al., 2003) and hormonal and metabolic profiles (Soto et al. 2003, 2008, Ong et al. 2004 a,b, Kajante et al.), as previously suggested for endocrine pathways (Ben-Shlomo et al. 2003). Thus, the identification of individuals who have experienced downward centile - crossing prenatally, while not necessarily predictive of immediate birth outcome compromise (Stratton et al. 1995), may serve as a marker for later health risks.
Previous work has focused on postnatal centile-crossing, tracking growth adjustment that occurs after birth, particularly during the first year of life when infants, released from the effects of the prenatal environment, shift up (catch-up) and down (catch-down) across growth reference percentiles as they fine-tune their growth rates in the postnatal environment and become canalized into their genetic potential for growth (e.g., Smith et al. 1976, Mei et al. 2004, Völkl et al. 2006). The prenatal precursors for these patterns have received less attention. The present data suggest this may be a logical future course of investigation. The working assumption is that the catch-up growth of early infancy follows fetal growth faltering, implied by the relationship between upward centile-crossing and small birth size, for example (e.g., Ong et al., 2000).
For theoretical perspective, a comparison of the third trimester growth trajectory patterns found in the present study with those previously reported for the first six postnatal months (Mei et al. 2004) illustrates the importance of considering the continuity of growth patterns from pre- to postnatal life within individuals in future studies. The data source for the postnatal study were from the California Child Health and Development Study (Van den Berg et al., 1988), with a mean birth weight similar to the present sample (3359, sd 496 vs 3410, sd 427, respectively), analyzed as they crossed major growth reference percentiles of the United States CDC growth charts (Kuczmarski et al., 2002). It is important to note that a comparison between the Chilean national fetal growth percentiles and previously published data from fetuses aged 22 to 42 weeks from healthy pregnancies in California (Williams et al., 1982) found no significant differences (González et al., 2004). With these caveats, it is notable that in the present study, proportionately more fetal trajectories were characterized by downward centile-crossing and no significant shifts in peer-related size by comparison with the data from early infancy, in which the largest proportion of infants expressed upward centile-crossing (Figure 2). Taken together, these data suggest that it is possible to track growth within individuals across the birth rubicon to address the numerous speculations regarding causal effects. To date, an evidence-based illustration has been lacking.
FIGURE 2. Fetal and infant growth trajectory patterns compared.
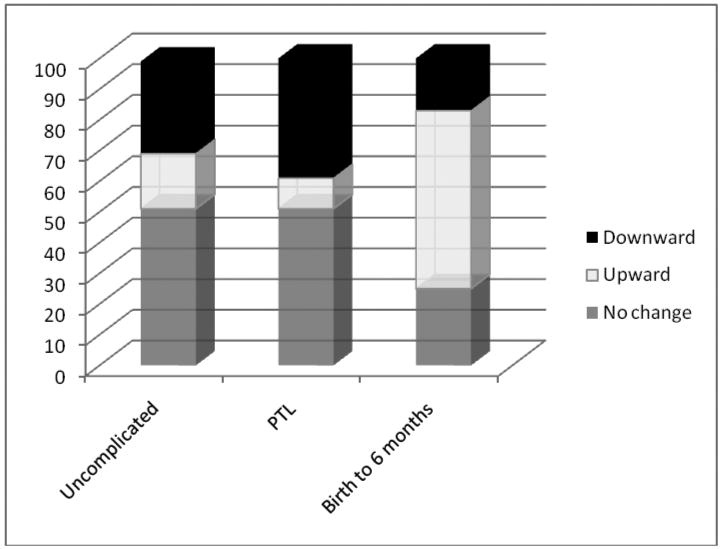
Proportions of individual growth trajectories characterized by upward centile-crossing, downward centile-crossing and no significant change in standard scores across the third trimester (defined as >0.67,<-0.67, >-0.67 and <0.67 standard scores, respectively, or the distance between major growth percentiles) among the present fetal study sample of term births from pregnancies with uncomplicated term labor and those complicated by preterm labor (PTL) compared to proportions of individual growth trajectories among a sample of infants assessed during their first six postnatal months from Mei et al., (2004), data from the California Health and Development Study (Van den Berg et al., 1988). The definitions for growth trajectories among the infant sample were based on infants crossing one or more major growth percentile on the United States CDC growth charts (Kuczmarski et al. 2000).
Conclusions
Further work directed at bridging the fetal/infant growth trajectory will contribute to a better understanding of the determinants of early growth and subsequent health. The present observations direct attention to the investigation of specific events identifiable during pregnancy that contribute to fetal growth rate and, thereby, birth size. The clarification of these relationships may begin to fill in the broad descriptive details of the often-described sequelae of birth size. To the extent that pregnancy-related events reflect a genetic influence (e.g., Goddard et al. 2007), there may be associations between maternal genetics and fetal outcomes mediated through reproductive experiences. Increased understanding of fetal growth will benefit from an integration of the breadth and depth of clinically-oriented data, basic science evidence (Romero et al. 2006a) and the substantial toolkit of traditional auxology, and will expand our understanding of the early biology of human variability.
Acknowledgments
This research was supported by the Intramural Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS
Literature Cited
- Altman DG, Hytten FE. Intrauterine growth retardation: let's be clear about it. Br J Obstet Gynaecol. 1989;96:1127–1132. doi: 10.1111/j.1471-0528.1989.tb03185.x. [DOI] [PubMed] [Google Scholar]
- Anderson NG, Jolley IJ, Wells JE. Sonographic estimation of fetal weight: comparison of bias, precision and consistency using 12 different formulae. Ultrasound Obstet Gynecol. 2007;30:173–179. doi: 10.1002/uog.4037. [DOI] [PubMed] [Google Scholar]
- Barker DG. Adult consequences of fetal growth restriction. Clin Obstet Gynecol. 2006;49:270–283. doi: 10.1097/00003081-200606000-00009. [DOI] [PubMed] [Google Scholar]
- Ben-Shlomo Y, Holly J, McCarthy A, Savage P, Davies D, Gunnell D, Davey Smith G. An investigation of fetal, postnatal and childhood growth with insulin-like growth factor I and binding protein 3 in adulthood. Clinical Endocrinol. 2003;59:366–373. doi: 10.1046/j.1365-2265.2003.01857.x. [DOI] [PubMed] [Google Scholar]
- Cameron N. Growth patterns in adverse environments. Am J Hum Biol. 2007;19:615–621. doi: 10.1002/ajhb.20661. [DOI] [PubMed] [Google Scholar]
- Cameron N, Pettifor J, De Wet T, Norris S. The relationship of rapid weight gain in infancy to obesity and skeletal maturity in childhood. Obesity Research. 2003;11:457–460. doi: 10.1038/oby.2003.62. [DOI] [PubMed] [Google Scholar]
- Cameron N, Preece MA, Cole TJ. Catch-up growth or regression to the mean? Recovery from stunting revisited. Am J Hum Biol. 2005;17:412–417. doi: 10.1002/ajhb.20408. [DOI] [PubMed] [Google Scholar]
- Cole TJ. Do growth chart centiles need a face lift? BMJ. 1994;308:641–642. doi: 10.1136/bmj.308.6929.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielian PJ, Allman ACJ, Steer PJ. Is obstetric and neonatal outcome worse in fetuses who fail to reach their own growth potential? Br J Obstet Gynaecol. 1992;99:452–454. doi: 10.1111/j.1471-0528.1992.tb13779.x. [DOI] [PubMed] [Google Scholar]
- Ehrenkranz RA. Estimated fetal weights versus birth weights: should the reference intrauterine growth curves based on birth weights be retired? Arch Dis Child Fetal Neonatal Ed. 2007;92:F161–2. doi: 10.1136/adc.2006.109439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza J, Kusanovic JP, Kim CJ, Kim YM, Kim JS, Hassan SS, Gotsch F, Goncalves LF, Erez O, Friel L, Soto E, Romero R. An episode of preterm labor is a fisk factor for the birth of a small-for-gestational-age neonate. Am J Obstet Gynecol. 2007;196:574.e1–5. doi: 10.1016/j.ajog.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardosi J. Is obstetric and neonatal outcome worse in fetuses who fail to reach their own growth potential? Br J Obstet Gynaecol. 1994;101:827–832. doi: 10.1111/j.1471-0528.1994.tb11957.x. [DOI] [PubMed] [Google Scholar]
- Gardosi J. Customized fetal growth standards: rationale and clinical application. Semin Perinatol. 2004;28:33–40. doi: 10.1053/j.semperi.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Goddard KA, Tromp G, Romero R, Olson JM, Lu Q, Xu Z, Parimi N, Nien JK, Gomez R, Behnke E, Solari M, Espinoza J, Santolaya J, Chaiworapongsa T, Lenk GM, Volkenant K, Anant MK, Salisbury BA, Carr J, Lee MS, Vovis GF, Kuivaniemi H. Candidate-gene association study of mothers with pre-eclampsia, and their infants, analyzing 775 SNPs in 190 genes. Hum Hered. 2007;63:1–16. doi: 10.1159/000097926. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González RP, Gomez RM, Castro RS, Nien JK, Merino PO, Etchegaray AB, Carstens MR, Medina LH, Viviani PG, Rojas IT. A national birth weight distribution curve according to gestational age in Chile from 1993 to 2000. Rev Med Chil. 2004;132(10):1155–1165. doi: 10.4067/s0034-98872004001000001. [DOI] [PubMed] [Google Scholar]
- Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body and femur measurements—a prospective study. Am J Obstet Gynecol. 1985;151(3):333–337. doi: 10.1016/0002-9378(85)90298-4. [DOI] [PubMed] [Google Scholar]
- Hemachandra AH, Klebanoff MA. Use of serial ultrasound to identify periods of fetal growth restriction in relation to neonatal anthropometry. Am J Hum Biol. 2006;18:791–797. doi: 10.1002/ajhb.20552. [DOI] [PubMed] [Google Scholar]
- Hunziker U, Largo R, Zachmann M, Prader A. Compensatory maturational deceleration of “catch-down growth” in patients with congenital adrenal hyperplasia after delayed initiation of therapy. Eur J Pediatr. 1986;144:550–553. doi: 10.1007/BF00496033. [DOI] [PubMed] [Google Scholar]
- Kajante E, Barker DJ, Osmond C, Forsen T, Eriksson JG. Growth before 2 years of age and serum lipids 60 years later:the Helsinki Birth Cohort Study. Int J Epidemiol. 2008;37:280–289. doi: 10.1093/ije/dyn012. [DOI] [PubMed] [Google Scholar]
- Kim YM, Bujold E, Chaiworapongsa T, Gomez R, Yoon BH, Thaler HT, Rotmensch S, Romero R. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2003;189:1063–1069. doi: 10.1067/s0002-9378(03)00838-x. [DOI] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Guo SS, et al. CDC growth charts for the United States: methods and development. Vital Health Stat 11. 2000;2002:1–190. [PubMed] [Google Scholar]
- Mei Z, Grummer-Strawn LM, Thompson D, Dietz WH. Shifts in percentiles of growth during early childhood: Analysis of longitudinal data from the California Child Health and Development Study. Pediatrics. 2004;113(6):e617–e627. doi: 10.1542/peds.113.6.e617. [DOI] [PubMed] [Google Scholar]
- Ong KKL, Ahmed ML, Emmett PM, Preece MA, Dunger DB the Avon Longitudinal Study of Pregnancy and Childhood Study Team. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ. 2000;320:967–971. doi: 10.1136/bmj.320.7240.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong KK, Petry CJ, Emmett PM, Sandhu MS, Kiess W, Hales CN, Ness AR, Dunger DB the ALSPAC study team. Insulin sensitivity and secretion in normal children related to size at birth, postnatal growth, and plasma insulin-like growth factor-1 levels. Diabetologia. 2004a;7:1064–1070. doi: 10.1007/s00125-004-1405-8. [DOI] [PubMed] [Google Scholar]
- Ong KK, Potau N, Petry CJ, Jones R, Ness AR, the Avon Longitudinal Study of Parents and Children (ALSPAC) Study Team. Honour JW, De Zegher F, Ibanez L, Dunger DB. Opposing influences of prenatal and postnatal weight gain on adrenarche in normal boys and girls. J Clin Endocrinol Metab. 2004b;89:2647–2651. doi: 10.1210/jc.2003-031848. [DOI] [PubMed] [Google Scholar]
- Prader A, Tanner JM, van Harnack GA. Catch-up growth following illness or starvation. J Pediatr. 1963;62:646–659. doi: 10.1016/s0022-3476(63)80035-9. [DOI] [PubMed] [Google Scholar]
- Romero R, Espinoza J, Gotsch F, Kusanovic JP, Friel LA, Erez O, Mazaki-Tovi S, Than NG, Hassan S, Tromp G. The use of high-dimensional biology (genomics, transcriptomics, proteomics, and metabolomics) to understand the preterm parturition syndrome. BJOG. 2006a;113 3:118–135. doi: 10.1111/j.1471-0528.2006.01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M. The preterm parturition syndrome. BJOG. 2006b 3:17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royston P. Calculation of unconditional and conditional reference intervals for foetal size and growth from longitudinal measurements. Stat Med. 1995;14:1417–1436. doi: 10.1002/sim.4780141303. [DOI] [PubMed] [Google Scholar]
- Sheppard BL, Bonnar J. The ultrastructure of the arterial supply of the human placenta in pregnancy complicated by fetal growth restriction. Br J Obstet Gynaecol. 1976;88:695–705. doi: 10.1111/j.1471-0528.1976.tb00781.x. [DOI] [PubMed] [Google Scholar]
- Smith DW, Truog W, Rogers JE, et al. Shifting linear growth during infancy: illustration of genetic factors in growth from fetal life through infancy. J Pediatr. 1976;89:225–230. doi: 10.1016/s0022-3476(76)80453-2. [DOI] [PubMed] [Google Scholar]
- Soto N, Bazaes RA, Pena V, Salazar T, Avila A, Iniguez G, Ong KK, Dunger DD, Mericq MV. Insulin sensitivity and secretion are related to catch-up growth in small-for-gestational age infants at age 1 year: Results from a prospective cohort. J Clin Endocrinol Metab. 2003;88:3645–3650. doi: 10.1210/jc.2002-030031. [DOI] [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release 9. College Station, TX: StataCorp LP; 2005. [Google Scholar]
- Stenhouse E, Wright DE, Hattersley AT, Millward BA. Maternal glucose levels influence birthweight and ‘catch up’ and ‘catch-down’ growth in a large contemporary cohort. Diabet Med. 2006;23:1207–1212. doi: 10.1111/j.1464-5491.2006.01964.x. [DOI] [PubMed] [Google Scholar]
- Stratton JF, Scanaill SN, Stuart B, Turner MJ. Are babies of normal birth weight who fail to reach their growth potential as diagnosed by ultrasound at increased risk? Ultrasound Obstet Gynecol. 1995;5:114–118. doi: 10.1046/j.1469-0705.1995.05020114.x. [DOI] [PubMed] [Google Scholar]
- Tanner JM, Lejarraga H, Cameron N. The natural history of the Silver-Russell Syndrome: a longitudinal study of thirty-one cases. Pediatr Res. 1975;9:611–623. doi: 10.1203/00006450-197508000-00001. [DOI] [PubMed] [Google Scholar]
- Van den Berg BJ, Christianson RE, Oechsli FW. The California Child Health and Development Studies of the School of Public Health, University of California at Berkeley. Paediatr Perinat Epidemiol. 1988;2:265–282. doi: 10.1111/j.1365-3016.1988.tb00218.x. [DOI] [PubMed] [Google Scholar]
- Verkauskiene R, Beltrand J, Claris O, Chevenne D, Deghmoun S, Dorgeret S, Alison M, Gaucherand P, Sibony O, Lévy-Marchal C. Impact of fetal growth restriction on body composition and hormonal status at birth in infants of small and appropriate weight for gestational age. Eur J Endocrinol. 2007;157:605–612. doi: 10.1530/EJE-07-0286. [DOI] [PubMed] [Google Scholar]
- Viner RM, Forton JTM, Cole TJ, Clark IH, Noble-Jamieson G, Barnes ND. Growth of long term survivors of liver transplantation. Arch Dis Child. 1999;80:235–240. doi: 10.1136/adc.80.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Völkl TMK, Haas B, Beier C, Simm D, Dörr HG. Catch-down growth during infancy of children born small (SGA) or appropriate (AGA) for gestational age with short-statured parents. J Pediatr. 2006;148:747–752. doi: 10.1016/j.jpeds.2006.01.034. [DOI] [PubMed] [Google Scholar]
- Williams RL, Creasy RK, Cunningham GC, Hawes WE, Norris FD, Tashiro M. Fetal growth and perinatal viability in California. Obstet Gynecol. 1982;59:624–632. [PubMed] [Google Scholar]


